Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrochemical copolymerization of 3,4-ethylenedioxythiophene and dithienothiophene: influence of feed ratio on electrical, optical and electrochromic properties†
Rashi Kediaab,
Manisha Khatakab,
Manisha Balkhandiaab and
Asit Patra *ab
*ab
aPhotovoltaic Metrology Section, Advanced Materials & Device Metrology Division, CSIR-National Physical Laboratory, Dr K. S. Krishnan Marg, New Delhi, 110012, India. E-mail: apatra@nplindia.org
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India
First published on 8th April 2024
Abstract
Designing a copolymer is an efficient and alternative method to generate new chemical and physical properties compared to parent homopolymers without complex synthesis and structural modification. We herein report the electrochemical deposition of copolymer using two monomers 3,4-ethylenedioxythiophene (EDOT) and dithieno[3,2-b:2′,3′-d]thiophene (DTT). Three different copolymers P[EDOT-co-DTT] were synthesized by using different feed ratios of monomers (EDOT and DTT molar ratios in solution are 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in acetonitrile containing 0.1 M tetrabutylammonium perchlorate (TBAClO4) as a supporting electrolyte. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and UV-vis-NIR spectroscopy were employed to characterize the obtained copolymers. Energy dispersive X-ray spectroscopy (EDX) analysis was used to estimate the composition of EDOT and DTT units in copolymers. The electrochemical and morphological properties were analyzed using cyclic voltammetry (CV) and field emission scanning electron microscopy (FESEM). In situ spectroelectrochemistry and electrochromic studies were performed to investigate the optical and switching properties of the resultant copolymers. The homopolymers poly(3,4-ethylenedioxythiophene) (PEDOT) and polydithieno[3,2-b:2′,3′-d]thiophene (PDTT) were also prepared using similar electrochemical conditions and made comparisons where applicable. Computational calculations were done to understand the structure and energy levels of these polymers. It was found that these copolymers P[EDOT-co-DTT] show new properties as compared to homopolymers PEDOT and PDTT for organic electronic applications. Interesting to note that the resultant copolymers display the property of tunable electrochromism with improved transmittance and redox color change between the neutral and oxidized states.
2) in acetonitrile containing 0.1 M tetrabutylammonium perchlorate (TBAClO4) as a supporting electrolyte. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and UV-vis-NIR spectroscopy were employed to characterize the obtained copolymers. Energy dispersive X-ray spectroscopy (EDX) analysis was used to estimate the composition of EDOT and DTT units in copolymers. The electrochemical and morphological properties were analyzed using cyclic voltammetry (CV) and field emission scanning electron microscopy (FESEM). In situ spectroelectrochemistry and electrochromic studies were performed to investigate the optical and switching properties of the resultant copolymers. The homopolymers poly(3,4-ethylenedioxythiophene) (PEDOT) and polydithieno[3,2-b:2′,3′-d]thiophene (PDTT) were also prepared using similar electrochemical conditions and made comparisons where applicable. Computational calculations were done to understand the structure and energy levels of these polymers. It was found that these copolymers P[EDOT-co-DTT] show new properties as compared to homopolymers PEDOT and PDTT for organic electronic applications. Interesting to note that the resultant copolymers display the property of tunable electrochromism with improved transmittance and redox color change between the neutral and oxidized states.
1. Introduction
Conjugated polymers have drawn significant attention in the field of optoelectronics applications due to their tunable properties and versatile fabrication approach.1–4 They have been widely used in organic solar cells, energy storage, chemical sensors, electrochromic devices, organic light-emitting diodes, actuators, field effect transistors and so forth.5–11 Synthesis of new monomer units is a common approach for the modification of chemical and physical properties of conjugated polymers such as solubility, stability, optical and electrical, etc., but it requires multi-step synthesis, time-consuming and costly process. Alternatively, various types of conjugated polymers, such as copolymer, donor–acceptor, block, end-group functional polymers, etc., have been designed and synthesized to achieve desirable chemical and physical properties for organic electronic applications.12,13 Among them, copolymerization of two distinct monomer units is a simple method to generate new properties compared to parent homopolymers without complex synthesis.14,15 Considerable efforts have been made to synthesize copolymers in which two or more suitably functionalized monomers are copolymerized together in search of intensified properties to those of the individual homopolymers.16–20Substituted polythiophenes, especially 3,4-ethylenedioxythiophene (EDOT) based polymer, has been of particular research interest because of captivating properties of PEDOT such as high conductivity, superior environmental stability, low oxidation potential, fast switching between conducting doped and insulating undoped form, high transparency in the oxidized state.21 However, the low band gap of PEDOT allows the color change from dark blue to light blue only upon oxidation which confines the application of homopolymer PEDOT in some cases.22 Further, the higher-lying HOMO energy level of PEDOT leads to oxidization under ambient conditions. Precisely for PEDOT-based copolymers, Yi-Jie et al. showed the copolymerization of EDOT with pyrrole, which displays a wide range of color variation from purple-red, brick-red, and dark-grey to light blue.23 Camurlu and coworkers reported the copolymers between EDOT and 1-(perfluorophenyl)-2,5-di(thiophen-2-yl)-1H-pyrrole (FPTPy) to achieve desired electrochromic properties with multiple color variations and good switching times.24 Hu et al. reported the electrochemical synthesis of copolymer from EDOT and perylene units, which showed four color changes from reddish brown to light green at the different doped states.25 Feng et al. electrosynthesized multielectrochromic copolymer of 1-(3-methylthiophen-2-yl) pyrene and EDOT to fabricate electrochromic device based on copolymers which showed better properties than corresponding homopolymers.26 The copolymerization between EDOT and pyrene resulted in good thermal stability and smooth morphology.27
On the other hand, polydithieno[3,2-b:2′,3′-d]thiophene (PDTT) has fused aromatic rings, which may lead to a better conjugation in the polymeric backbone for intermolecular charge transfer.28 Polymer PDTT shows a band gap at 1.96 eV with p-doping behaviour and exhibits visible colour change between the undoped and doped forms.29–31 The colours of the neutral and oxidized PDTT film are red-orange and blue-grey, respectively. However, the electrochromic behaviour of PDTT film is difficult to be exploited mainly due to the degradation of the film on repetitive colour switching due to the instability of the oxidized state.32
Generally, the properties of copolymers mainly depend on the nature of precursors, along with the minor effect of polymerization technique and reaction conditions. In this connection, it is expected that electro-copolymerization between EDOT and DTT might produce new and improved properties, which could overcome the limitation of corresponding homopolymers. Electrochemical synthesis is an efficient one-step approach to produce copolymer films.33 The advantages of electrochemical deposition are (i) small amounts of precursor are required and high purity polymer films are obtained (ii) the technique is fast and environmentally friendly (iii) electrochemical reactions are carried out at room temperature, (iv) controllable parameters such as the thickness of film, deposition time, etc.34–36 Moreover, an effective electrochemical copolymerization occurs when the oxidation potentials of two monomers are close to each other.37,38 Herein, both monomers EDOT (oxidation potential 1.11 V) and DTT (oxidation potential 1.07 V) could be oxidized simultaneously within the same potential range and react with each other to form copolymers.
In continuation of our work on the conjugated copolymers,39–44 three copolymers based on PEDOT and PDTT were synthesized by using different feed ratios of monomers EDOT and DTT as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. Copolymer films were electrodeposited on the ITO-coated glass surface by electrochemical oxidation of monomers EDOT and DTT, via electro-copolymerization method in 0.1 M TBAClO4/MeCN solution. The resulting copolymer films were characterized by Fourier transform infrared (FTIR) and Raman spectroscopy. The elemental composition of copolymer films was examined by using an energy-dispersive X-ray (EDX) spectrophotometer. The electrochemical and morphological properties were analyzed using cyclic voltammetry (CV) and field emission scanning electron microscopy (FESEM). The optoelectronic properties of the prepared copolymer films were examined via spectroelectrochemistry in 0.1 M TBAClO4/MeCN solution. Computational studies were carried out to understand the theoretical optimized geometry, HOMO, LUMO and band gap of the copolymer P[EDOT-co-DTT]. Finally, the electrochromic properties of the obtained copolymer films were examined, and the result shows the property of tunable electrochromism with improved transmittance and redox color change between the neutral and oxidized states.
2, respectively. Copolymer films were electrodeposited on the ITO-coated glass surface by electrochemical oxidation of monomers EDOT and DTT, via electro-copolymerization method in 0.1 M TBAClO4/MeCN solution. The resulting copolymer films were characterized by Fourier transform infrared (FTIR) and Raman spectroscopy. The elemental composition of copolymer films was examined by using an energy-dispersive X-ray (EDX) spectrophotometer. The electrochemical and morphological properties were analyzed using cyclic voltammetry (CV) and field emission scanning electron microscopy (FESEM). The optoelectronic properties of the prepared copolymer films were examined via spectroelectrochemistry in 0.1 M TBAClO4/MeCN solution. Computational studies were carried out to understand the theoretical optimized geometry, HOMO, LUMO and band gap of the copolymer P[EDOT-co-DTT]. Finally, the electrochromic properties of the obtained copolymer films were examined, and the result shows the property of tunable electrochromism with improved transmittance and redox color change between the neutral and oxidized states.
2. Experimental section
2.1 Materials
3,4-Ethylenedioxythiophene (EDOT, 97%), dithieno[3,2-b:2′,3′-d]thiophene (DTT, 97%) and tetrabutylammonium perchlorate (TBAClO4, for electrochemical analysis ≥99.0%) were purchased from Sigma-Aldrich. Both EDOT and DTT were used as received while TBAClO4 was oven-dried at 60 °C for 12 h before use. Acetonitrile (MeCN, 99%) was purchased from Finar Chemicals, which was purified over calcium chloride by refluxing and distillation methods. The working electrode indium tin oxide coated glass (ITO, Rs < 10 Ω sq−1) was purchased from Shilpa Enterprises, Nagpur, India and was cleaned with deionized water and acetone, respectively. Potassium bromide (KBr, 99.5%) was bought from Sisco Research Laboratories Pvt. Ltd India. Other chemicals were used as received without further purification if otherwise stated.2.2 Electrochemical copolymerization
Electrochemical synthesis of copolymers based on PEDOT and PDTT was performed via the potentiodynamic process in a one-compartment cell equipped with three electrodes. ITO-coated glass (dimension: 7 mm × 50 mm × 1.1 mm, Rs < 10.0 Ω sq−1), gold (Au) wire and silver (Ag) wire were used as a working, counter and reference electrode, respectively. All three electrodes were immersed in MeCN solution containing 0.1 M TBAClO4 as a supporting electrolyte. The two monomers EDOT and DTT were added to the above solution in various feed ratios (by mole) i.e., 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively, to synthesize three different copolymers P1(2
2, respectively, to synthesize three different copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), individually (Table S1†). Before electro-copolymerization, the resultant solutions were deoxygenated by purging N2 gas for 5 min. At scan rate of 100 mV s−1 and in the potential range of −0.8 to 1.5 V, the corresponding copolymers P1(2
2), individually (Table S1†). Before electro-copolymerization, the resultant solutions were deoxygenated by purging N2 gas for 5 min. At scan rate of 100 mV s−1 and in the potential range of −0.8 to 1.5 V, the corresponding copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively, were electrodeposited on the surface of ITO-coated glass substrate. The obtained copolymer films were rinsed in MeCN to remove unreacted monomers and residual electrolytes. For comparison purposes, homopolymers PEDOT and PDTT were prepared by electrochemical polymerization of monomer EDOT and DTT respectively under similar conditions as described above for copolymerization.
2), respectively, were electrodeposited on the surface of ITO-coated glass substrate. The obtained copolymer films were rinsed in MeCN to remove unreacted monomers and residual electrolytes. For comparison purposes, homopolymers PEDOT and PDTT were prepared by electrochemical polymerization of monomer EDOT and DTT respectively under similar conditions as described above for copolymerization.
2.3 Equipments and characterizations
Electrochemical copolymerization and the electrochemical measurements were carried out by cyclic voltammetry on Metrohm Autolab, PGSTAT204 potentiostat equipped with NOVA software. A conventional single-compartment, three-electrode system was employed for electrochemical setup with Ag wire as reference, Au wire as counter and ITO-coated glass slide as working electrode. The reference electrode was calibrated externally by adding 0.001 M solution of ferrocene in a degassed electrolytic solution of 0.1 M TBAClO4/MeCN. The oxidation peak of the ferrocene/ferrocenium (Fc/Fc+) redox couple was found to be 0.4 V vs. Ag/Ag+. All the electrochemical investigations were performed at the room temperature. For the structural characterization, as prepared copolymer samples were dispersed (by scratching from the ITO surface) in potassium bromide (KBr) to make pellets for FTIR analysis and then spectra were recorded on a PerkinElmer Spectrum-2 spectrophotometer in the wavenumber range of 4000 to 400 cm−1. The Raman spectra of the resultant copolymer films were obtained by using a T64000 triple Raman spectrometer with a 514 nm laser. The absorption spectra of the monomers solution having different feed ratios of EDOT and DTT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by mole) were recorded before and after the electro-copolymerization process by using a UV-1800 Shimadzu spectrophotometer over the wavelength range from 200 to 1100 nm. The surface morphology of the copolymer films was analysed by field emission scanning electron microscope (FESEM, Zeiss Supra 40VP), connected to a dispersive energy X-ray (EDX) microanalyzer for the elemental analysis at an accelerating voltage of 5.0 kV. Before the characterizations, the electrodeposited copolymer films on ITO-coated glass were freely removed from the electrochemical cell and rinsed in MeCN to wipe out unreacted monomers and residual electrolytes. Further, as-prepared copolymer films were dried under N2 atmosphere. Prior to FESEM analysis, the dried films were mounted on copper stubs and sputter-coated with an ultrathin layer of gold to avoid charging during the measurements. In situ spectroelectrochemical measurements were conducted in a monomer-free electrolytic solution (0.1 M TBAClO4/MeCN) using a quartz cuvette by applying the different potentials. The optical band gap (Eg,opt) of the copolymers were calculated from the onset absorption wavelength of the copolymer, obtained by extrapolating the linear portion of the curve to the wavelength axis (X-axis). Electrochromic properties of the copolymer films were investigated by using the chronoamperometry technique at λmax in the potential range from −1.0 to 1.0 V.
2 by mole) were recorded before and after the electro-copolymerization process by using a UV-1800 Shimadzu spectrophotometer over the wavelength range from 200 to 1100 nm. The surface morphology of the copolymer films was analysed by field emission scanning electron microscope (FESEM, Zeiss Supra 40VP), connected to a dispersive energy X-ray (EDX) microanalyzer for the elemental analysis at an accelerating voltage of 5.0 kV. Before the characterizations, the electrodeposited copolymer films on ITO-coated glass were freely removed from the electrochemical cell and rinsed in MeCN to wipe out unreacted monomers and residual electrolytes. Further, as-prepared copolymer films were dried under N2 atmosphere. Prior to FESEM analysis, the dried films were mounted on copper stubs and sputter-coated with an ultrathin layer of gold to avoid charging during the measurements. In situ spectroelectrochemical measurements were conducted in a monomer-free electrolytic solution (0.1 M TBAClO4/MeCN) using a quartz cuvette by applying the different potentials. The optical band gap (Eg,opt) of the copolymers were calculated from the onset absorption wavelength of the copolymer, obtained by extrapolating the linear portion of the curve to the wavelength axis (X-axis). Electrochromic properties of the copolymer films were investigated by using the chronoamperometry technique at λmax in the potential range from −1.0 to 1.0 V.
3. Result and discussion
3.1 Electrochemical copolymerization
The electrochemical synthesis route of copolymer P[EDOT-co-DTT] is illustrated in Scheme 1. Three different feed ratios of monomers (molar ratio of EDOT and DTT) i.e., 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in 0.1 M TBAClO4/MeCN were used to obtain three different copolymers films on the ITO-coated glass slide and are denoted as P1(2
2 in 0.1 M TBAClO4/MeCN were used to obtain three different copolymers films on the ITO-coated glass slide and are denoted as P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. The electrochemical copolymerization curves of monomers with various feed ratios as 2
2), respectively. The electrochemical copolymerization curves of monomers with various feed ratios as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
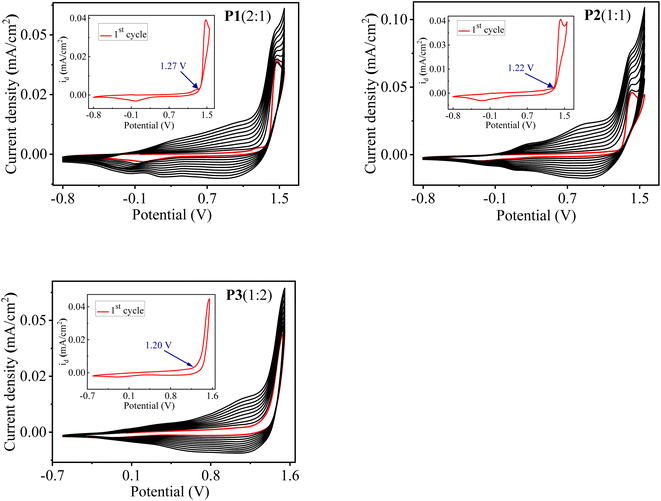
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is shown in Fig. 1. The first cycle corresponds to the irreversible oxidation of monomers and is also depicted in the inset graph of Fig. 1. The onset oxidation potential (Eonset) of monomers was found to be 1.27, 1.22 and 1.20 V, corresponding to three different feed ratios of monomers as 2
2 is shown in Fig. 1. The first cycle corresponds to the irreversible oxidation of monomers and is also depicted in the inset graph of Fig. 1. The onset oxidation potential (Eonset) of monomers was found to be 1.27, 1.22 and 1.20 V, corresponding to three different feed ratios of monomers as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. It is worth noticing that Eonset of monomers is higher than those of individual monomer EDOT (1.11 V) and DTT (1.07 V), indicating the existence of interaction between the monomers in 0.1 M TBAClO4/MeCN solution. Eonset of the individual monomer EDOT and DTT were extracted from their electropolymerization curves, as illustrated in Fig. S1.† It is well-known that a successful electrochemical copolymerization occurs when the Eonset of different monomers are close to each other.22,35 In our case, it was found that the difference in the Eonset of the EDOT (1.11 V) and the DTT (1.07 V) is as low as 0.04 V, which suggests the feasibility of electro-copolymerization. From this point, the monomers EDOT and DTT are revealed to get oxidized within the same potential range and radical cations of both monomers might form simultaneously on the ITO surface where they can react with each other and form a copolymer.
2, respectively. It is worth noticing that Eonset of monomers is higher than those of individual monomer EDOT (1.11 V) and DTT (1.07 V), indicating the existence of interaction between the monomers in 0.1 M TBAClO4/MeCN solution. Eonset of the individual monomer EDOT and DTT were extracted from their electropolymerization curves, as illustrated in Fig. S1.† It is well-known that a successful electrochemical copolymerization occurs when the Eonset of different monomers are close to each other.22,35 In our case, it was found that the difference in the Eonset of the EDOT (1.11 V) and the DTT (1.07 V) is as low as 0.04 V, which suggests the feasibility of electro-copolymerization. From this point, the monomers EDOT and DTT are revealed to get oxidized within the same potential range and radical cations of both monomers might form simultaneously on the ITO surface where they can react with each other and form a copolymer.
As shown in Fig. 1, from the second cycle onwards, a new reversible redox couple appears, indicating the deposition of polymer on the ITO electrode and denotes the redox behaviour of the electrodeposited polymers. The current density increases with the increasing number of cycles, which shows the gradual deposition of well-adhered, conducting, insoluble polymer film on the ITO surface and suggests an effective copolymerization process. Moreover, the increment between consecutive cycles and redox behaviour of the copolymers is completely different from those of their parent homopolymers PEDOT and PDTT, which confirms the formation of polymers consisting of both EDOT and DTT units.
3.2 Absorption spectra of monomers before and after polymerization
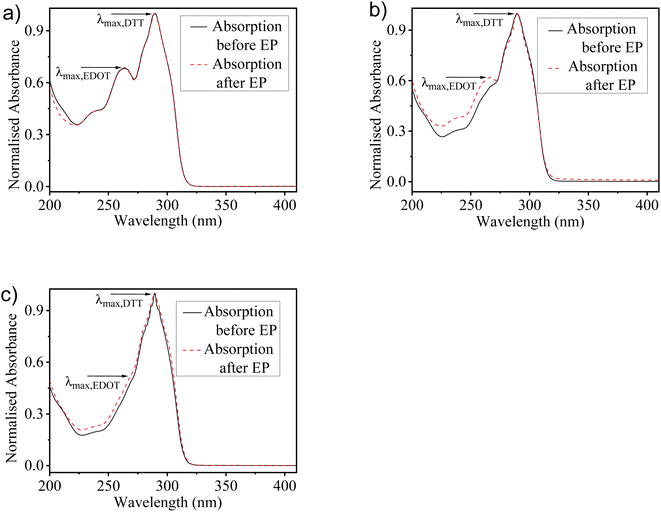
The optical spectra of monomers EDOT and DTT show the absorption maxima peaks at 257 nm and 289 nm, respectively in the MeCN solution (Fig. S2†). To understand the relative consumption of monomers EDOT and DTT units in solutions used for electro-copolymerization, UV-visible absorption spectra of the solutions before and after electrochemical deposition of copolymers were recorded. All the proposed feed ratios of EDOT and DTT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were used for the preparation of three different solutions in MeCN. Fig. 2 represents the absorption spectra of monomers solution with the different feed ratios of EDOT
2) were used for the preparation of three different solutions in MeCN. Fig. 2 represents the absorption spectra of monomers solution with the different feed ratios of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTT as 2
DTT as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively before and after the electro-copolymerization process. It was observed that the absorption spectra show pronounced differences by varying the feed ratios in the copolymerization mixtures. The monomers solution prepared with a 2
2, respectively before and after the electro-copolymerization process. It was observed that the absorption spectra show pronounced differences by varying the feed ratios in the copolymerization mixtures. The monomers solution prepared with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition, having a higher concentration of EDOT shows a well-defined peak of EDOT and another peak for DTT, which indicates the occurrence of both EDOT and DTT units in the solution. While the solution having a 1
1 composition, having a higher concentration of EDOT shows a well-defined peak of EDOT and another peak for DTT, which indicates the occurrence of both EDOT and DTT units in the solution. While the solution having a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of EDOT
1 ratio of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTT shows a less intense peak for EDOT, whereas, in a 1
DTT shows a less intense peak for EDOT, whereas, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, the EDOT peak almost diminishes. Correspondingly, the characteristic peak of DTT gets enhanced as the ratio of DTT units increases in the solution. Moreover, the absorption spectra before and after the electro-copolymerization process reveal that the copolymers have maintained the composition of both monomer units as the feed ratio used for copolymerization. However, discrepancies have been found as the slight difference in the absorption spectra before and after the electro-copolymerization process. Further, it is worth noticing that the spectral change upon monomers incorporation does not indicate any chemical changes. Therefore, this shows that no chemical reaction takes place between EDOT and DTT and does not have any influence on the molecular structure during the electrochemical deposition of copolymer films.
2 ratio, the EDOT peak almost diminishes. Correspondingly, the characteristic peak of DTT gets enhanced as the ratio of DTT units increases in the solution. Moreover, the absorption spectra before and after the electro-copolymerization process reveal that the copolymers have maintained the composition of both monomer units as the feed ratio used for copolymerization. However, discrepancies have been found as the slight difference in the absorption spectra before and after the electro-copolymerization process. Further, it is worth noticing that the spectral change upon monomers incorporation does not indicate any chemical changes. Therefore, this shows that no chemical reaction takes place between EDOT and DTT and does not have any influence on the molecular structure during the electrochemical deposition of copolymer films.
3.3 FTIR spectra
To verify the presence of PEDOT as well as PDTT in the obtained copolymer films, FTIR spectroscopy were carried out. For FTIR investigation, copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were blended with anhydrous KBr and pressed into a pellet by hydraulic press for the measurement. For comparison purposes, FTIR spectra of their homopolymers PEDOT and PDTT were also recorded and is represented in Fig. S3(i).† Fig. 3 presents the FTIR spectra of PEDOT, P1(2
2) were blended with anhydrous KBr and pressed into a pellet by hydraulic press for the measurement. For comparison purposes, FTIR spectra of their homopolymers PEDOT and PDTT were also recorded and is represented in Fig. S3(i).† Fig. 3 presents the FTIR spectra of PEDOT, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1
1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT in the neutral state. As seen from the spectrum of pure PEDOT in the neutral state [Fig. 3a], the bands at 1658, 1518, 1470 and 1338, 1210 cm−1 are attributed to the stretching vibration modes of C
2) and PDTT in the neutral state. As seen from the spectrum of pure PEDOT in the neutral state [Fig. 3a], the bands at 1658, 1518, 1470 and 1338, 1210 cm−1 are attributed to the stretching vibration modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C in the thiophene rings, respectively.24,34,45 While, the bands at 1144 and 1090 cm−1 originate from the stretching modes of C–O–C in the ethylenedioxy group46,47 and the bands at 982, 935, 842 and 695 cm−1 are assigned to the vibration from C–S bond in the thiophene ring of PEDOT.48,49 In the spectrum of pure PDTT [Fig. 3e], the vibration of C–S bond is reflected at 803 cm−1 and the bands at 1095 and 1126 cm−1 correspond to the C–S–C bond stretch. Whereas, the vibration of C
C and C–C in the thiophene rings, respectively.24,34,45 While, the bands at 1144 and 1090 cm−1 originate from the stretching modes of C–O–C in the ethylenedioxy group46,47 and the bands at 982, 935, 842 and 695 cm−1 are assigned to the vibration from C–S bond in the thiophene ring of PEDOT.48,49 In the spectrum of pure PDTT [Fig. 3e], the vibration of C–S bond is reflected at 803 cm−1 and the bands at 1095 and 1126 cm−1 correspond to the C–S–C bond stretch. Whereas, the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C are observed at ≈1380, 1465 and 1630 cm−1, respectively.
C and C–C are observed at ≈1380, 1465 and 1630 cm−1, respectively.
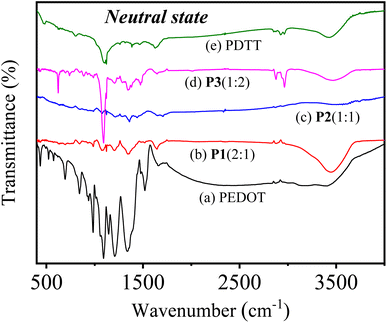 | ||
Fig. 3 FTIR spectra of (a) PEDOT, (b) P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (c) P2(1 1), (c) P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (d) P3(1 1), (d) P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (e) PDTT in the neutral form. 2) and (e) PDTT in the neutral form. | ||
Compared with the corresponding homopolymers, the FTIR spectra of the neutral state of three copolymers P[EDOT-co-DTT] in Fig. 3(b–d) exhibits the above-mentioned characteristic bands of PEDOT and PDTT, suggesting the presence of both EDOT and DTT units in the formation of copolymers. Next, it is evident that the FTIR spectrum of P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resembles more with pure PEDOT due to the higher ratio of EDOT units in the polymer matrix. As the feed ratio of EDOT is decreased in P2(1
1) resembles more with pure PEDOT due to the higher ratio of EDOT units in the polymer matrix. As the feed ratio of EDOT is decreased in P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the bands of PEDOT become less intense and the spectrum depicts broad bands with intermediate characteristics bands of both the homopolymers. On the other hand, in P3(1
1), the bands of PEDOT become less intense and the spectrum depicts broad bands with intermediate characteristics bands of both the homopolymers. On the other hand, in P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) a sharp peak appears at 1090 cm−1 which corresponds to C–S–C bond of PDTT, which indicates that the resulting copolymer film contains a higher ratio of DTT unit. These observations confirm that diverse feed ratios of monomers have resulted in the formation of three different copolymers, indicating the significant impact of feed ratios of monomers. Moreover, the resemblance of the obtained FTIR spectrum of all three copolymers with homopolymers indicates that copolymers have been successfully prepared via the electro-copolymerization method. Despite the difficulty of the detailed quantitative analysis due to the broadness and peak overlaps, the resulting FTIR absorption spectra clearly show that the peak intensity at correlating C–S stretch increases with the incorporation of more DTT units. Further, similar observations have been found in the oxidized state of the homopolymers and copolymers and are represented in Fig. S3(ii),† which confirms the corresponding polymers are copolymers, not the blend of PEDOT and PDTT.
2) a sharp peak appears at 1090 cm−1 which corresponds to C–S–C bond of PDTT, which indicates that the resulting copolymer film contains a higher ratio of DTT unit. These observations confirm that diverse feed ratios of monomers have resulted in the formation of three different copolymers, indicating the significant impact of feed ratios of monomers. Moreover, the resemblance of the obtained FTIR spectrum of all three copolymers with homopolymers indicates that copolymers have been successfully prepared via the electro-copolymerization method. Despite the difficulty of the detailed quantitative analysis due to the broadness and peak overlaps, the resulting FTIR absorption spectra clearly show that the peak intensity at correlating C–S stretch increases with the incorporation of more DTT units. Further, similar observations have been found in the oxidized state of the homopolymers and copolymers and are represented in Fig. S3(ii),† which confirms the corresponding polymers are copolymers, not the blend of PEDOT and PDTT.
3.4 Raman spectra
Raman spectroscopy was performed on electrodeposited copolymer films to further examine the composition and structure of P[EDOT-co-DTT] with different feed ratios of monomers EDOT and DTT units. Consequently, as-prepared thin films of different copolymers i.e., P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
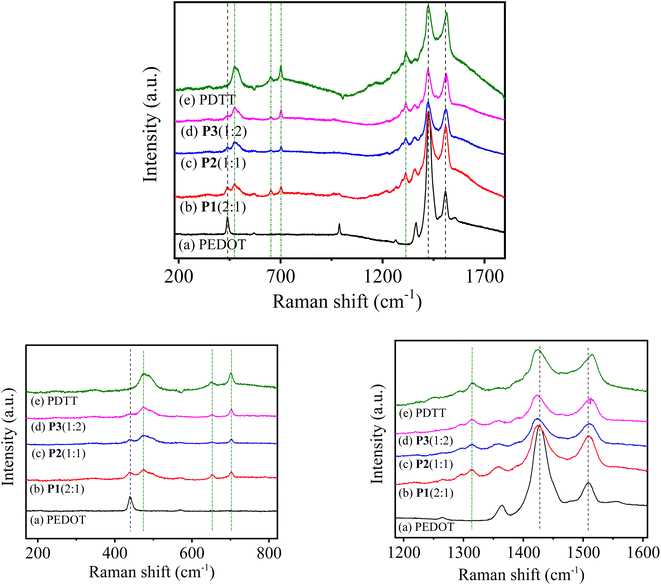
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) on ITO-coated glass were directly subjected for the characterization. The spectral changes upon dissimilar monomers incorporation have been interpreted by correlating the band characteristics of the resultant copolymers with individual homopolymers (PEDOT and PDTT). Thus, the Raman spectra of homopolymers (PEDOT and PDTT) were also recorded under similar conditions. Fig. 4 represents the Raman spectra of PEDOT, P1(2
2) on ITO-coated glass were directly subjected for the characterization. The spectral changes upon dissimilar monomers incorporation have been interpreted by correlating the band characteristics of the resultant copolymers with individual homopolymers (PEDOT and PDTT). Thus, the Raman spectra of homopolymers (PEDOT and PDTT) were also recorded under similar conditions. Fig. 4 represents the Raman spectra of PEDOT, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1
1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT, respectively from the wavenumber 180 to 1800 cm−1. The homopolymer PEDOT shows strong Raman bands near 438, 985, 1364, 1427 (very strong), 1508 cm−1 along with weak bands around 567, 1265 cm−1. While pure PDTT spectra present strong bands at 475, 1423, 1515 cm−1 along with medium bands around 650, 700, and 1315 cm−1. In the expanded spectra (1200–1600 cm−1, Fig. 4) of pure PEDOT, a strong peak at ∼1427 and 1507 cm−1 is the characteristics peak of symmetric and asymmetric Cα = Cβ stretching, respectively with a shoulder peak at approximately 1363 cm−1, which corresponds to Cβ = Cβ stretching.50,51 Whereas, pure PDTT exhibits most prominent peaks at 1423 (symmetric Cα = Cβ stretching), 1515 (asymmetric Cα = Cβ stretching) and 1315 cm−1 (Cβ = Cβ stretching).
2) and PDTT, respectively from the wavenumber 180 to 1800 cm−1. The homopolymer PEDOT shows strong Raman bands near 438, 985, 1364, 1427 (very strong), 1508 cm−1 along with weak bands around 567, 1265 cm−1. While pure PDTT spectra present strong bands at 475, 1423, 1515 cm−1 along with medium bands around 650, 700, and 1315 cm−1. In the expanded spectra (1200–1600 cm−1, Fig. 4) of pure PEDOT, a strong peak at ∼1427 and 1507 cm−1 is the characteristics peak of symmetric and asymmetric Cα = Cβ stretching, respectively with a shoulder peak at approximately 1363 cm−1, which corresponds to Cβ = Cβ stretching.50,51 Whereas, pure PDTT exhibits most prominent peaks at 1423 (symmetric Cα = Cβ stretching), 1515 (asymmetric Cα = Cβ stretching) and 1315 cm−1 (Cβ = Cβ stretching).
On comparing the different copolymers spectra with the homopolymers, copolymers P[EDOT-co-DTT] exhibit similar peak patterns of both the homopolymer PEDOT and PDTT, indicating the presence of both EDOT and DTT units in the resultant copolymers, formed with different feed ratios of monomers. It was observed that the spectra of the obtained copolymers show pronounced differences by varying the monomers compositions. For instance, in the P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) spectrum in Fig. 4b, all the vibrational modes are shifted to a lower frequency compared to homopolymer PEDOT, which is expected because of the incorporation of DTT units in the copolymer. The downshift of the Cα = Cβ peak observed in copolymer films from 1426 [in P1(2
1) spectrum in Fig. 4b, all the vibrational modes are shifted to a lower frequency compared to homopolymer PEDOT, which is expected because of the incorporation of DTT units in the copolymer. The downshift of the Cα = Cβ peak observed in copolymer films from 1426 [in P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] to 1422 cm−1 [in P3(1
1)] to 1422 cm−1 [in P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)], indicates that the effective conjugation length has been increased, which is consistent with the increased number of DTT units in P3(1
2)], indicates that the effective conjugation length has been increased, which is consistent with the increased number of DTT units in P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The Cβ = Cβ peaks of copolymers, however, were shifted to ≈1358 cm−1 for P1(2
2). The Cβ = Cβ peaks of copolymers, however, were shifted to ≈1358 cm−1 for P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ≈1357 cm−1 for P2(1
1) and ≈1357 cm−1 for P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Further, no significant shifts were observed in expanded spectra of polymers ranging wavenumber from 180 to 820 cm−1. The vibrational assignment from the Raman spectra of P[EDOT-co-DTT] copolymers support the wavenumber assignment interpreted from the FTIR of the copolymers. Hence, from the FTIR and Raman spectra, we proposed that the P[EDOT-co-DTT] copolymer with three different compositions has been electrodeposited successfully on the ITO surface.
2). Further, no significant shifts were observed in expanded spectra of polymers ranging wavenumber from 180 to 820 cm−1. The vibrational assignment from the Raman spectra of P[EDOT-co-DTT] copolymers support the wavenumber assignment interpreted from the FTIR of the copolymers. Hence, from the FTIR and Raman spectra, we proposed that the P[EDOT-co-DTT] copolymer with three different compositions has been electrodeposited successfully on the ITO surface.
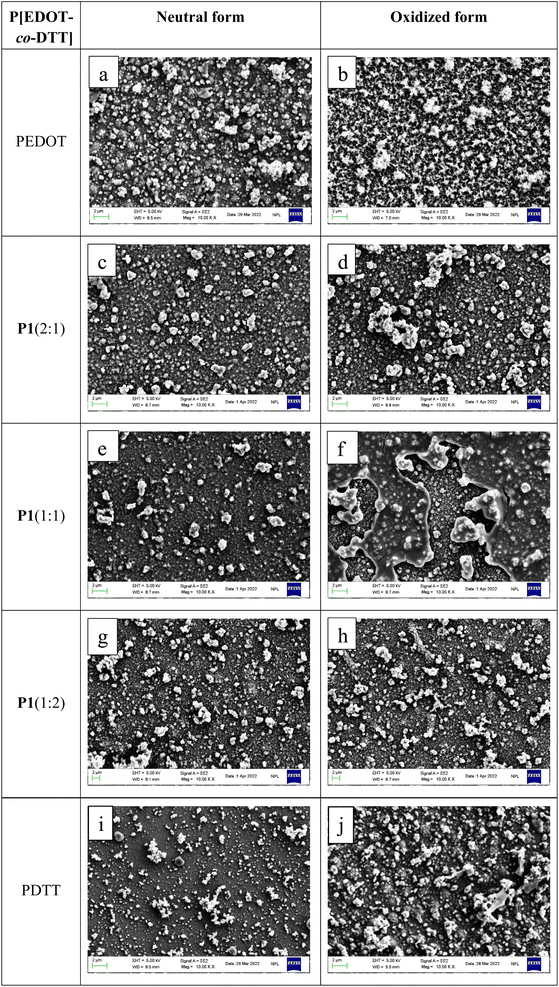
3.5 Elemental analysis
To quantitatively investigate the atomic compositions of the resultant copolymer films prepared from various feed ratios of EDOT and DTT, EDX analyses were performed. Both the neutral and oxidized forms of three different P[EDOT-co-DTT] films were examined by the EDX test. Table S2† depicts the field image and its corresponding EDX maps of three main peaks (C, O and S atoms) for P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively in neutral as well as in oxidized state. The summarized atomic composition derived from EDX data is represented in Table 1, suggesting the formation of different copolymers with various compositions of the monomers. On moving from the EDX spectrum of P1(2
2), respectively in neutral as well as in oxidized state. The summarized atomic composition derived from EDX data is represented in Table 1, suggesting the formation of different copolymers with various compositions of the monomers. On moving from the EDX spectrum of P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to P3(1
1) to P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), a significant increase in sulphur peak with atomic composition increasing from 8.91% to 14.41% in neutral form and 11.21% to 13.64% in oxidized form was observed. Simultaneously, the decrease in oxygen peak (17.35% to 15.23% in neutral and 23.41% to 20.42% in oxidized state), confirms the incorporation of more DTT units in P3(1
2), a significant increase in sulphur peak with atomic composition increasing from 8.91% to 14.41% in neutral form and 11.21% to 13.64% in oxidized form was observed. Simultaneously, the decrease in oxygen peak (17.35% to 15.23% in neutral and 23.41% to 20.42% in oxidized state), confirms the incorporation of more DTT units in P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), as compared to P1(2
2), as compared to P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Whereas, from the EDX spectrum of P2(1
1). Whereas, from the EDX spectrum of P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the intermediate outcomes were observed for sulphur and oxygen content, which is consistent with the feed ratio of 1
1), the intermediate outcomes were observed for sulphur and oxygen content, which is consistent with the feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for EDOT and DTT. These observations show that copolymers P[EDOT-co-DTT] with different feed ratios of EDOT and DTT were successfully electrodeposited on the ITO-coated glass surface.
1 for EDOT and DTT. These observations show that copolymers P[EDOT-co-DTT] with different feed ratios of EDOT and DTT were successfully electrodeposited on the ITO-coated glass surface.
| P[EDOT-co-DTT] | Elements of P[EDOT-co-DTT] | |||||
|---|---|---|---|---|---|---|
| In neutral form | In oxidized form | |||||
| C% | O% | S% | C% | O% | S% | |
P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
73.74 | 17.35 | 8.91 | 65.38 | 23.41 | 11.21 |
P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
72.79 | 16.11 | 11.10 | 74.26 | 16.73 | 9.01 |
P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
70.36 | 15.23 | 14.41 | 65.94 | 20.42 | 13.64 |
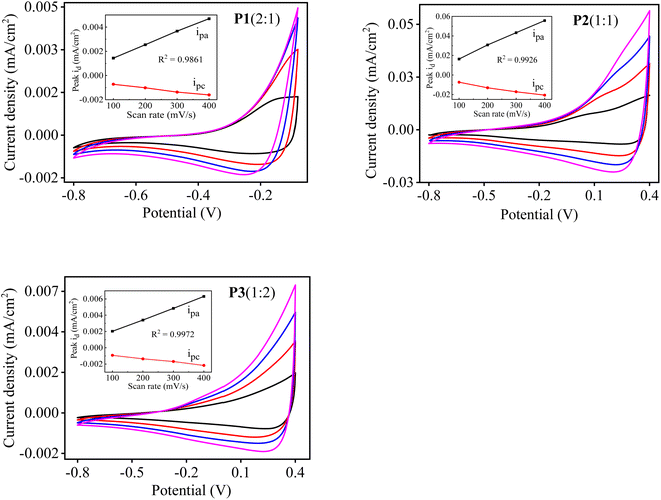
3.6 Redox properties of P[EDOT-co-DTT] copolymer films
The electrochemical behaviour of the obtained copolymer films, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were investigated by CV at the different scan rates of 100, 200, 300 and 400 mV s−1 in a single compartment three-electrode cell. P[EDOT-co-DTT] with various feed ratios of EDOT and DTT as 2
2) were investigated by CV at the different scan rates of 100, 200, 300 and 400 mV s−1 in a single compartment three-electrode cell. P[EDOT-co-DTT] with various feed ratios of EDOT and DTT as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively were electrodeposited on the Pt electrode by sweeping the potential from −0.8 to 1.5 V for four cycles. For CV measurements, the polymer-coated Pt was used as a working electrode, Ag wire as a reference and Au wire as a counter electrode in a monomer-free solution of MeCN containing 0.1 M TBAClO4. Fig. 5 shows the cyclic voltagramms of the different copolymers, i.e., P1(2
2, respectively were electrodeposited on the Pt electrode by sweeping the potential from −0.8 to 1.5 V for four cycles. For CV measurements, the polymer-coated Pt was used as a working electrode, Ag wire as a reference and Au wire as a counter electrode in a monomer-free solution of MeCN containing 0.1 M TBAClO4. Fig. 5 shows the cyclic voltagramms of the different copolymers, i.e., P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. All three copolymer films show a reversible redox process between the anodic and cathodic peak potentials at different scan rates, suggesting a typical electrochemical behaviour of conjugated polymers. It was observed that the copolymers P[EDOT-co-DTT] prepared with different feed ratios show diverse electrical properties. For example, the oxidation onset potential (Eoxonset) of the copolymers were found to be −0.41, −0.21 and −0.16 V corresponding to P1(2
2), respectively. All three copolymer films show a reversible redox process between the anodic and cathodic peak potentials at different scan rates, suggesting a typical electrochemical behaviour of conjugated polymers. It was observed that the copolymers P[EDOT-co-DTT] prepared with different feed ratios show diverse electrical properties. For example, the oxidation onset potential (Eoxonset) of the copolymers were found to be −0.41, −0.21 and −0.16 V corresponding to P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. For comparison purposes, the CV of homopolymers were also recorded under similar conditions and is presented in the ESI Fig. S4.† It is evident that Eoxonset values of the copolymers are the intermediate values of the homopolymers, PEDOT (−0.62 V) and PDTT (0.21 V), which shows that the different copolymers of P[EDOT-co-DTT] have well-defined and intermediate redox properties. Further, the Eoxonset values were used to calculate the experimental HOMO energy level of the polymers and are summarized in Table 2. Compared to homopolymers, the HOMO energy level of the prepared copolymer films was found to be in between PEDOT and PDTT. The HOMO values of P[EDOT-co-DTT] were calculated to be −3.99, −4.19 and −4.24 eV for P1(2
2), respectively. For comparison purposes, the CV of homopolymers were also recorded under similar conditions and is presented in the ESI Fig. S4.† It is evident that Eoxonset values of the copolymers are the intermediate values of the homopolymers, PEDOT (−0.62 V) and PDTT (0.21 V), which shows that the different copolymers of P[EDOT-co-DTT] have well-defined and intermediate redox properties. Further, the Eoxonset values were used to calculate the experimental HOMO energy level of the polymers and are summarized in Table 2. Compared to homopolymers, the HOMO energy level of the prepared copolymer films was found to be in between PEDOT and PDTT. The HOMO values of P[EDOT-co-DTT] were calculated to be −3.99, −4.19 and −4.24 eV for P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. This interprets that different feed ratios of monomers have a significant effect on the HOMO level of the polymers and thus by varying the feed ratios, redox properties can be tuned for various applications.
2), respectively. This interprets that different feed ratios of monomers have a significant effect on the HOMO level of the polymers and thus by varying the feed ratios, redox properties can be tuned for various applications.
| Polymers | Eoxonset (V) | HOMOa (eV) | Epa (V) | Epc (V) | Eb1/2 (V) |
|---|---|---|---|---|---|
| a HOMO = −(4.8 − EFc/Fc+ + Eoxonset) eV, where EFc/Fc+ is found to be 0.4 V vs. Ag/Ag+ and Eb1/2 is the half-wave potential of the first oxidation and reduction process and is calculated as E1/2 = (Epa + Epc)/2. | |||||
| PEDOT | −0.77 | −3.63 | 0.11 | −0.61 | −0.25 |
P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−0.41 | −3.99 | −0.13 | −0.19 | −0.16 |
P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−0.21 | −4.19 | 0.27 | 0.22 | 0.24 |
P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
−0.16 | −4.24 | 0.37 | 0.26 | 0.32 |
| PDTT | 0.21 | −4.61 | 0.96 | 0.82 | 0.89 |
For a better understanding of the influence of monomers feed ratio on the electrical properties of prepared polymers, CV curves of homopolymers and copolymers were drawn together. Fig. 6 depicts the normalized CV curves of PEDOT, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1
1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT, respectively at the scan rate of 100 mV s−1. It is worth noticing that redox peaks of P1(2
2) and PDTT, respectively at the scan rate of 100 mV s−1. It is worth noticing that redox peaks of P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) which contains more EDOT units is nearer to PEDOT and as the concentration of EDOT decreases in P2(1
1) which contains more EDOT units is nearer to PEDOT and as the concentration of EDOT decreases in P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and even less in P3(1
1) and even less in P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), a positive shift of peaks was observed. The positive shift of P3(1
2), a positive shift of peaks was observed. The positive shift of P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) is due to more DTT units incorporated into the copolymer chain, which correlates well with the elemental analysis results for P3(1
2) is due to more DTT units incorporated into the copolymer chain, which correlates well with the elemental analysis results for P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
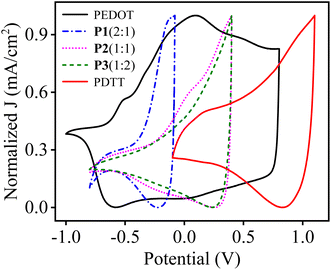 | ||
Fig. 6 Normalised CV curves of PEDOT, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1 1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1 1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT, respectively at the scan rate of 100 mV s−1. 2) and PDTT, respectively at the scan rate of 100 mV s−1. | ||
Next, in all three copolymers, the peak current density response increases with an increase in the scan rate. The anodic peak potential (Epa) and cathodic peak potential (Epc) show a linear dependence as a function of scan rate, as depicted in the inset graph of Fig. 5. This also demonstrates that the electrochemical process of the copolymers is reversible and not diffusion-limited even at higher scan rates.52 Additionally, the Epa and Epc were used to determine the half-wave potential (E1/2) of the resultant copolymers. As can be seen in Fig. 5, when the feed ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the copolymer P1(2
1, the copolymer P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) shows Epa at 0.13 V and Epc at 0.19 V at the scan rate of 100 mV s−1. As the feed ratio is changed to 1
1) shows Epa at 0.13 V and Epc at 0.19 V at the scan rate of 100 mV s−1. As the feed ratio is changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the copolymer P2(1
1, the copolymer P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) shows Epa and Epc at 0.27 and 0.22 V, respectively. When the feed ratio becomes 1
1) shows Epa and Epc at 0.27 and 0.22 V, respectively. When the feed ratio becomes 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the copolymer P3(1
2, the copolymer P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) presents Epa at 0.37 V and Epc at 0.26 V. Based on this, E1/2 values of each copolymer were calculated and is summarized in Table 2. Compared to homopolymers PEDOT (E1/2 ∼ 0.09 V) and PDTT (E1/2 ∼ 0.86 V), the copolymers P[EDOT-co-DTT] have in-between E1/2 values, which confirms the intermediate electrical characteristics of the resultant copolymers. This further depicts the importance of the feed ratio of monomers during the electropolymerisation process.
2) presents Epa at 0.37 V and Epc at 0.26 V. Based on this, E1/2 values of each copolymer were calculated and is summarized in Table 2. Compared to homopolymers PEDOT (E1/2 ∼ 0.09 V) and PDTT (E1/2 ∼ 0.86 V), the copolymers P[EDOT-co-DTT] have in-between E1/2 values, which confirms the intermediate electrical characteristics of the resultant copolymers. This further depicts the importance of the feed ratio of monomers during the electropolymerisation process.
3.7 Absorption and spectroelectrochemical properties
UV-visible absorption spectra of homopolymers (PEDOT and PDTT) and their copolymers P[EDOT-co-DTT] prepared with different feed ratios of EDOT and DTT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) on ITO-coated glass slides were recorded over the wavelength range from 300 to 900 nm. Fig. 7 depicts UV-visible spectra of homopolymers and the copolymers P1(2
2) on ITO-coated glass slides were recorded over the wavelength range from 300 to 900 nm. Fig. 7 depicts UV-visible spectra of homopolymers and the copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in the neutral state. The obtained copolymers show one strong peak with maximum absorbance wavelength (λmax) at 520, 505 and 490 nm corresponding to P1(2
2) in the neutral state. The obtained copolymers show one strong peak with maximum absorbance wavelength (λmax) at 520, 505 and 490 nm corresponding to P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively and are attributed to the π–π* electronic transition from the valence band to the conduction band in conjugated polymers. In contrast, the homopolymers PEDOT and PDTT show characteristic absorption peaks at about 610 and 440 nm, respectively. The diverse and in-between λmax values of P[EDOT-co-DTT] films show the significance of the feed ratio of monomers during the electropolymerisation process. It is worth noticing that compared to pure PEDOT, all the copolymers exhibit a continuous blue shift of the absorption peak as the feed ratio of EDOT
2), respectively and are attributed to the π–π* electronic transition from the valence band to the conduction band in conjugated polymers. In contrast, the homopolymers PEDOT and PDTT show characteristic absorption peaks at about 610 and 440 nm, respectively. The diverse and in-between λmax values of P[EDOT-co-DTT] films show the significance of the feed ratio of monomers during the electropolymerisation process. It is worth noticing that compared to pure PEDOT, all the copolymers exhibit a continuous blue shift of the absorption peak as the feed ratio of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTT decreases, implying the increase of DTT units in the polymer chain, and further confirming the occurrence of copolymerization and as well by the results of elemental analysis.
DTT decreases, implying the increase of DTT units in the polymer chain, and further confirming the occurrence of copolymerization and as well by the results of elemental analysis.
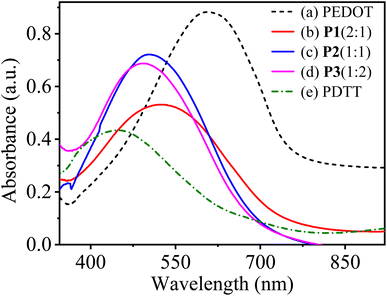 | ||
Fig. 7 UV-visible absorption spectra of (a) PEDOT, (b) P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (c) P2(1 1), (c) P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (d) P3(1 1), (d) P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (e) PDTT electrodeposited on ITO-coated glass in the neutral state. 2) and (e) PDTT electrodeposited on ITO-coated glass in the neutral state. | ||
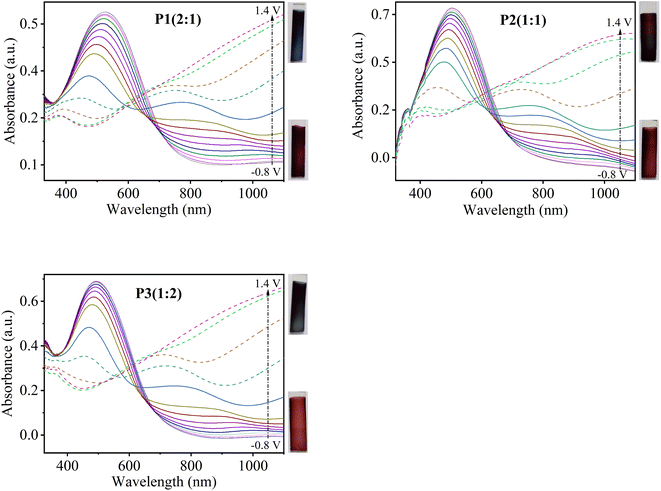
To further examine the effect of monomers feed ratio on the optoelectronic properties of the prepared copolymer films, in situ spectroelectrochemical investigations were performed. In situ UV-visible absorption spectra of copolymer films deposited on ITO-coated glass slides were recorded in the potential range from −0.8 to 1.4 V in a monomer-free solution of 0.1 M TBAClO4 in MeCN. The change in absorbance spectra of P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a function of different applied potentials is shown in Fig. 8. In neutral state (undoped form, −0.8 V), P[EDOT-co-DTT] films exhibit a broad peak at their respective λmax (520, 505 and 490 nm for P1(2
2) as a function of different applied potentials is shown in Fig. 8. In neutral state (undoped form, −0.8 V), P[EDOT-co-DTT] films exhibit a broad peak at their respective λmax (520, 505 and 490 nm for P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively) which relates to π–π* electronic transition. As the potential is increased, the films get oxidized and the intensity of π–π* transition decreases and a new absorption peak appears around 700 nm and 1050 nm which is due to the formation of polaron and bipolarons, respectively. P[EDOT-co-DTT] formed with a different feed ratio of EDOT
2), respectively) which relates to π–π* electronic transition. As the potential is increased, the films get oxidized and the intensity of π–π* transition decreases and a new absorption peak appears around 700 nm and 1050 nm which is due to the formation of polaron and bipolarons, respectively. P[EDOT-co-DTT] formed with a different feed ratio of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTT show significantly different isosbestic points when the potential was applied up to 0.8 V as illustrated in Fig. 8.
DTT show significantly different isosbestic points when the potential was applied up to 0.8 V as illustrated in Fig. 8.
It is well-known that PEDOT shows blue and transmissive grey colour in neutral and oxidized states, respectively. While PDTT shows red-orange in undoped form and blue-grey colour in doped form. Interestingly, it was found that the resultant copolymers show different colour in neutral as well as in oxidized state, compared to homopolymers, PEDOT and PDTT. Table S3† illustrates the photographs of copolymers P[EDOT-co-DTT] electrodeposited onto ITO-coated glass from various feed ratios of EDOT and DTT as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in both neutral and oxidized states. P1(2
2 in both neutral and oxidized states. P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) shows wine colour at −0.8 V and blue colour at 1.4 V while, P2(1
1) shows wine colour at −0.8 V and blue colour at 1.4 V while, P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displays brick-red and brown colour at neutral and oxidized states, respectively. For P3(1
1) displays brick-red and brown colour at neutral and oxidized states, respectively. For P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the colours are red-orange to blue-grey at the neutral and oxidized state. This interprets that different feed ratios of EDOT and DTT enrich the colour range of P[EDOT-co-DTT] copolymers, which could have significant applications in optoelectronic devices. The optical band-gap energy (Eg) of the different copolymer films, P1(2
2), the colours are red-orange to blue-grey at the neutral and oxidized state. This interprets that different feed ratios of EDOT and DTT enrich the colour range of P[EDOT-co-DTT] copolymers, which could have significant applications in optoelectronic devices. The optical band-gap energy (Eg) of the different copolymer films, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were calculated to be 1.67 eV (λonset ∼ 740 nm), 1.71 eV (λonset ∼ 725 nm) and 1.75 eV (λonset ∼ 705 nm), respectively, which are higher than that of PEDOT (1.6 eV) and lower than PDTT (1.94 eV). This infers that electro-copolymerization with different feed ratios of EDOT and DTT has positively resulted in the tuning of bandgap energy of the resultant copolymers, which is suitable for applications like organic light light-emitting diodes and sensors. Table 3 summarizes the maximum absorption wavelength (λmax), low-energy absorption edge wavelength (λonset), optical band-gap energy (Eg), full width at half maxima (FWHM) of PEDOT, copolymers P1(2
2) were calculated to be 1.67 eV (λonset ∼ 740 nm), 1.71 eV (λonset ∼ 725 nm) and 1.75 eV (λonset ∼ 705 nm), respectively, which are higher than that of PEDOT (1.6 eV) and lower than PDTT (1.94 eV). This infers that electro-copolymerization with different feed ratios of EDOT and DTT has positively resulted in the tuning of bandgap energy of the resultant copolymers, which is suitable for applications like organic light light-emitting diodes and sensors. Table 3 summarizes the maximum absorption wavelength (λmax), low-energy absorption edge wavelength (λonset), optical band-gap energy (Eg), full width at half maxima (FWHM) of PEDOT, copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1
1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT.
2) and PDTT.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1
1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT
2) and PDTT
| Polymers | λmaxa (nm) | λonsetb (nm) | Egc (eV) | FWHMd (nm) [eV] |
|---|---|---|---|---|
| a Wavelength at the maximum absorbance.b Wavelength at the onset of π–π* electronic transition.c Optical band gap calculated from the equation: Eg = 1240/λonset.d FWHM values of absorption peak in nm and values in bracket are in eV. | ||||
| PEDOT | 610 | 775 | 1.60 | 228 [0.81] |
P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
520 | 740 | 1.67 | 198 [0.92] |
P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
505 | 725 | 1.71 | 185 [0.91] |
P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
490 | 688 | 1.80 | 155 [0.79] |
| PDTT | 440 | 640 | 1.94 | 111 [0.61] |
3.8 Morphological properties
To examine the surface morphology of the resultant polymers, FESEM analysis was performed in the neutral as well as in the oxidized state of the copolymer films. P[EDOT-co-DTT] copolymer films with various feed ratios of monomers (EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTT as 2
DTT as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively) were electrodeposited on ITO substrate by potentiodynamic method in 0.1 M TBAClO4/MeCN in the potential range −0.8 to 1.5 V. All the films were rinsed in acetonitrile to remove residual electrolytes, monomers and dried under nitrogen before analysis. For comparison purposes, FESEM analysis of homopolymers PEDOT and PDTT was also carried out. Fig. 9 represents the FESEM micrographs of PEDOT, P1(2
2, respectively) were electrodeposited on ITO substrate by potentiodynamic method in 0.1 M TBAClO4/MeCN in the potential range −0.8 to 1.5 V. All the films were rinsed in acetonitrile to remove residual electrolytes, monomers and dried under nitrogen before analysis. For comparison purposes, FESEM analysis of homopolymers PEDOT and PDTT was also carried out. Fig. 9 represents the FESEM micrographs of PEDOT, P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3(1
1), P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and PDTT. The images on the left side (a, c, e, g and i) show the neutral form, whereas, right-side (b, d, f, h and j) shows the oxidized form of the respective conjugated polymers. We found that PEDOT exhibits an accumulation of clusters of globules in a neutral state (Fig. 9a) and loose spongy network structure in oxidized form (Fig. 9b), which correlates well with the earlier reported results in organic solution.53 While neutral PDTT (Fig. 9i) shows a smooth surface with small-sized particles all over the surface and oxidized PDTT (Fig. 9h) depicts higher surface coverage with an ordered arrangement. It is worth noticing that the micrograph of copolymers reveals significantly different surface morphology from the two corresponding homopolymers. We have found that depending upon the monomers' feed ratios, the surface morphologies of copolymer films varied meaningfully both in neutral as well as in oxidized state. The copolymer P1(2
2) and PDTT. The images on the left side (a, c, e, g and i) show the neutral form, whereas, right-side (b, d, f, h and j) shows the oxidized form of the respective conjugated polymers. We found that PEDOT exhibits an accumulation of clusters of globules in a neutral state (Fig. 9a) and loose spongy network structure in oxidized form (Fig. 9b), which correlates well with the earlier reported results in organic solution.53 While neutral PDTT (Fig. 9i) shows a smooth surface with small-sized particles all over the surface and oxidized PDTT (Fig. 9h) depicts higher surface coverage with an ordered arrangement. It is worth noticing that the micrograph of copolymers reveals significantly different surface morphology from the two corresponding homopolymers. We have found that depending upon the monomers' feed ratios, the surface morphologies of copolymer films varied meaningfully both in neutral as well as in oxidized state. The copolymer P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the neutral form shows globules dispersed all over the surface, while the oxidized form shows smaller granules, which is very different from PEDOT. In contrast, the surface of P2(1
1) in the neutral form shows globules dispersed all over the surface, while the oxidized form shows smaller granules, which is very different from PEDOT. In contrast, the surface of P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) film displays non-uniform-sized particles distributed all over the surface both in neutral and oxidized states. P3(1
1) film displays non-uniform-sized particles distributed all over the surface both in neutral and oxidized states. P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) displays a homogeneous distribution as the neutral form reveals a porous and rough morphology, while the oxidized form, films revealed a more compact and rougher surface. These variations in the surface morphology of the different copolymers coincide well with the above-mentioned outcomes from CV (good redox activity) and spectroelectrochemistry.
2) displays a homogeneous distribution as the neutral form reveals a porous and rough morphology, while the oxidized form, films revealed a more compact and rougher surface. These variations in the surface morphology of the different copolymers coincide well with the above-mentioned outcomes from CV (good redox activity) and spectroelectrochemistry.
3.9 Computational studies
Theoretically, the optical and electronic properties of the molecules were studied through density functional theory (DFT) with the help of electronic distribution patterns of Frontier Molecular Orbitals (FMOs). For DFT calculations, basis set 6-31G(d) was used with the B3LYP level of theory to analyse the effect of the basis set on optoelectronic properties which were further compared with our experimental results. The calculated value of HOMO, LUMO and band gap for the homopolymers and copolymer is illustrated in Table 4. The HOMO levels of homopolymers are at −3.51 eV for PEDOT and −4.62 eV for PDTT, whereas LUMO levels are at −1.66 and −2.66 eV for PEDOT and PDTT, respectively. It was found that the HOMO energy of P[EDOT-co-DTT] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of EDOT and DTT is −4.27 eV, while LUMO is at −2.28 eV, which is in-between values of their corresponding homopolymers. Experimental HOMO energy values obtained from the onset of oxidation peaks in CV curves correlate well with the theoretical data for polymers, as illustrated in Table 4. If a correction value (ΔE) 0.02 eV is applied to correlate the experimental and theoretical values. The DFT optimized structure and electronic distribution patterns of the homopolymers, and copolymer P[EDOT-co-DTT] are shown in Fig. 10.
1 ratio of EDOT and DTT is −4.27 eV, while LUMO is at −2.28 eV, which is in-between values of their corresponding homopolymers. Experimental HOMO energy values obtained from the onset of oxidation peaks in CV curves correlate well with the theoretical data for polymers, as illustrated in Table 4. If a correction value (ΔE) 0.02 eV is applied to correlate the experimental and theoretical values. The DFT optimized structure and electronic distribution patterns of the homopolymers, and copolymer P[EDOT-co-DTT] are shown in Fig. 10.
| Polymers | HOMO (eV) | LUMO (eV) | Eg(calc.)a (eV) | Eg(exp.)b (eV) | ΔEc (eV) |
|---|---|---|---|---|---|
| a Band gap calculated from Eg(calc) = (LUMO – HOMO) eV.b Experimental band gaps from the onset of the absorption spectra in the neutral state.c Difference in energy is calculated by ΔE = Eg(calc.) − Eg(exp). | |||||
| PEDOT | −3.51 | −1.66 | 1.85 | 1.60 | 0.25 |
| P[EDOT-co-DTT] | −4.27 | −2.28 | 1.99 | 1.71 | 0.28 |
| PDTT | −4.62 | −2.66 | 1.96 | 1.94 | 0.02 |
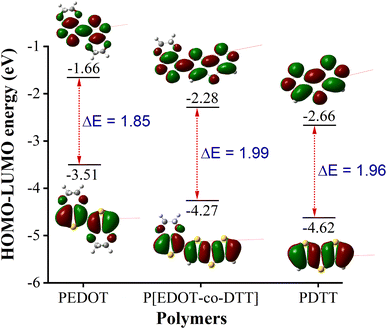 | ||
| Fig. 10 Optimized ground state frontier molecular HOMO and LUMO orbitals of PEDOT, P[EDOT-co-DTT] and PDTT. | ||
3.10 Electrochromic properties of P[EDOT-co-DTT] films
To investigate the switching property and ability to exhibit striking colour change between its neutral and oxidized state, electrochromic switching studies were carried out. Accordingly, we have considered the three different copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to determine the electrochromic characteristics such as optical contrast (ΔT%), response time and coloration efficiency (CE) for the different copolymer films. The copolymer films were electrodeposited on ITO and the double potential step chronoamperometry technique was used to test the switching ability between its neutral and oxidized state with a change in transmittance at a fixed wavelength. All the copolymer films were switched between −1.0 and 1.0 V at a regular interval of 5 s, and the percentage transmittance (% T) of the copolymers was at λmax and 1050 nm. Considering the impact of feed ratios of monomers, the ΔT% between the redox states of the copolymers at their respective λmax was calculated to be 38% (520 nm), 24% (505 nm) and 32% (490 nm) for P1(2
2) to determine the electrochromic characteristics such as optical contrast (ΔT%), response time and coloration efficiency (CE) for the different copolymer films. The copolymer films were electrodeposited on ITO and the double potential step chronoamperometry technique was used to test the switching ability between its neutral and oxidized state with a change in transmittance at a fixed wavelength. All the copolymer films were switched between −1.0 and 1.0 V at a regular interval of 5 s, and the percentage transmittance (% T) of the copolymers was at λmax and 1050 nm. Considering the impact of feed ratios of monomers, the ΔT% between the redox states of the copolymers at their respective λmax was calculated to be 38% (520 nm), 24% (505 nm) and 32% (490 nm) for P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) respectively (Fig. 11a, c and e). The corresponding chronoamperograms showing current consumption during the electrochromic switching of 0.1 M MeCN/TBAClO4 electrolyte solution are shown in Fig. S5.† At the higher wavelength of 1050 nm, the ΔT% was found to be 51%, 50% and 54% for the corresponding copolymers P1(2
2) respectively (Fig. 11a, c and e). The corresponding chronoamperograms showing current consumption during the electrochromic switching of 0.1 M MeCN/TBAClO4 electrolyte solution are shown in Fig. S5.† At the higher wavelength of 1050 nm, the ΔT% was found to be 51%, 50% and 54% for the corresponding copolymers P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and is shown in Fig. 11b, d and f, respectively. Conversely, the lower ΔT% were recorded for the homopolymers PEDOT as well as PDTT under similar conditions and is illustrated in the Fig. S6.† Next, the optical response time of P1(2
2) and is shown in Fig. 11b, d and f, respectively. Conversely, the lower ΔT% were recorded for the homopolymers PEDOT as well as PDTT under similar conditions and is illustrated in the Fig. S6.† Next, the optical response time of P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be 1.8 s from reduced to oxidized state and 1.4 s from oxidized to reduced state at 520 nm. We have observed that the copolymer P3(1
1) was found to be 1.8 s from reduced to oxidized state and 1.4 s from oxidized to reduced state at 520 nm. We have observed that the copolymer P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) has a fast response when compared with the P1(2
2) has a fast response when compared with the P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P2(1
1) and P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), as represented in Table 5. The faster-switching response of P3(1
1), as represented in Table 5. The faster-switching response of P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) film may be attributed due to the faster dopant ion diffusion during the redox process, which is due to the introduction of more DTT units into the polymer backbone. Then, the coloration efficiency (CE) at the higher wavelength of 1050 nm was calculated to be 78.0, 105.1 and 122.8 cm2 C−1 for P1(2
2) film may be attributed due to the faster dopant ion diffusion during the redox process, which is due to the introduction of more DTT units into the polymer backbone. Then, the coloration efficiency (CE) at the higher wavelength of 1050 nm was calculated to be 78.0, 105.1 and 122.8 cm2 C−1 for P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P2(1
1), P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3(1
1) and P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. Consequently, P3(1
2), respectively. Consequently, P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) shows comparatively high CE values in comparison to P1(2
2) shows comparatively high CE values in comparison to P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P2 (1
1) and P2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for all the respective wavelengths, making them suitable for electrochromic devices such as smart windows, mirrors.
1) for all the respective wavelengths, making them suitable for electrochromic devices such as smart windows, mirrors.
| Copolymers | Wavelength (nm) | Optical contrast ΔT (%) | Response timea (s) | Injected charge density (μC cm−2) | CEb (cm2 C−1) | |
|---|---|---|---|---|---|---|
| τoxi | τred | |||||
| a Response time calculated at 90% of a full switch from neutral to oxidized state (τoxi)and vice versa for τred.b Coloration efficiency (CE) is calculated by CE = log(Toxi/Tred)/injected charge density, where Toxi and Tred refer to transmittance in the oxidized and neutral state, respectively. | ||||||
| PEDOT | 610 | 28 | 2.4 | 2.1 | 3.85 | 63.9 |
| 1050 | 39 | 2.2 | 2.2 | 3.15 | 65.8 | |
P1(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
520 | 38 | 1.8 | 1.4 | 5.28 | 72.4 |
| 1050 | 51 | 2.1 | 2.1 | 4.50 | 78.0 | |
P2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
505 | 24 | 1.6 | 1.4 | 4.68 | 70.7 |
| 1050 | 50 | 1.9 | 1.6 | 3.89 | 105.1 | |
P3(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
490 | 32 | 1.6 | 1.2 | 4.07 | 80.7 |
| 1050 | 54 | 1.5 | 1.1 | 3.64 | 122.8 | |
| PDTT | 475 | 27 | 1.1 | 1.0 | 2.62 | 74.7 |
| 1050 | 40 | 1.2 | 1.1 | 2.46 | 112.7 | |
4. Conclusions
In summary, a straightforward methodology for the synthesis of complex conjugated materials from low-cost monomer units with controlled stoichiometric ratios has been developed. Thus, three different copolymers P[EDOT-co-DTT] films were deposited on ITO-coated glass using three different feed ratios of monomers via an electro-copolymerization method in 0.1 M TBAClO4/MeCN solution. The synthesized copolymers were well characterized by FTIR, Raman spectroscopy and UV-visible spectroscopy and confirmed that the obtained copolymers contain both EDOT and DTT units. EDX analysis shows that the ratio of EDOT and DTT units in copolymers changes as the feed ratio of EDOT and DTT varies during polymerization. We have observed that feed ratios of monomers significantly affect the electrical, optical and morphological properties of the copolymer films. The electrochemical and spectroelectrochemical properties of all three copolymer films were in between their parent homopolymers. Fine-tuning in the band gap with HOMO and LUMO energy levels was achieved by tailoring the feed ratios of monomers in the copolymerization mixture. The obtained copolymer films exhibit smooth morphology and tunable electrochromism, which can be extended for the fabrication of electrochromic devices. In addition, the DFT calculations supported our experimental findings. Thus, copolymers P[EDOT-co-DTT] can be considered as a good candidate for organic electronic applications such as organic photovoltaics, organic light-emitting diodes, etc. The copolymers display the property of tunable electrochromism with improved transmittance and redox color change between the neutral and oxidized states, hence finding the applications for electrochromic devices such as smart windows, mirrors, and displays. Further additional monomer units in a complex conjugated system for the tailoring of the next-generation optoelectronic materials are under process in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. K. and M. K. acknowledge Council of Scientific & Industrial Research (CSIR), New Delhi, India and M. B. acknowledges University Grants Commission (UGC), New Delhi, India, for their fellowship. We are grateful to Dr Naval Kishore (CSIR-NPL) for FESEM characterizations.References
- I. F. Perepichka and D. F. Perepichka, Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics. 2 Volume Set, John Wiley & Sons, New York, 2009 Search PubMed.
- T. M. Swager, 50th Anniversary Perspective: Conducting/Semiconducting Conjugated Polymers. A Personal Perspective on the Past and the Future, Macromolecules, 2017, 50, 4867–4886 CrossRef CAS.
- K. Wang, K. Amin, Z. An, Z. Cai, H. Chen, H. Chen, Y. Dong, X. Feng, W. Fu, J. Gu and Y. Han, Advanced functional polymer materials, Mater. Chem. Front., 2020, 4, 1803–1915 RSC.
- A. Patra, M. Bendikov and S. Chand, Poly(3,4-ethylenedioxyselenophene) and Its Derivatives: Novel Organic Electronic Materials, Acc. Chem. Res., 2014, 47, 1465–1474 CrossRef CAS PubMed.
- M. Li, Y. Sheynin, A. Patra and M. Bendikov, Tuning the Electrochromic Properties of Poly(alkyl-3,4-ethylenedioxyselenophenes) Having High Contrast Ratio and Coloration Efficiency, Chem. Mater., 2009, 21, 2482–2488 CrossRef CAS.
- J. Zhang, Z. Chen, X.-Y. Wang, S.-Z. Guo, Y.-B. Dong, G.-A. Yu, J. Yin and S.-H. Liu, Redox-modulated near-infrared electrochromism, electroluminochromism, and aggregation-induced fluorescence change in an indolo [3, 2-b] carbazole-bridged diamine system, Sens. Actuators, B, 2017, 246, 570–577 CrossRef CAS.
- M. Li, A. Patra, Y. Sheynin and M. Bendikov, Hexyl-derivatized poly (3, 4-ethylenedioxyselenophene): novel highly stable organic electrochromic material with high contrast ratio, high coloration efficiency, and low-switching voltage, Adv. Mater., 2009, 21, 1707–1711 CrossRef CAS.
- S. T. Aslan, D. Cevher, E. Bolayır, G. H. Ozsoy, Y. A. Udum, E. Yıldırım, L. Toppare and A. Cirpan, Synthesis of selenophene substituted benzodithiophene and fluorinated benzothiadiazole based conjugated polymers for organic solar cell applications, Electrochim. Acta, 2021, 398, 139298 CrossRef CAS.
- E. A. Yilmaz, M. Yasa, A. Cirpan and L. Toppare, A follow-up investigation: Organic solar cells based on chalcogenophene-Thieno[3,4-c]pyrrole-4,6-dione-chalcogenophene containing random conjugated polymers, J. Electroanal. Chem., 2023, 932, 117213 CrossRef CAS.
- C. Tong, A. Goyal, D. Wang and J. Gao, Visualizing the Effects of Salt Concentration in Planar Polymer Light-Emitting Electrochemical Cells, Electrochim. Acta, 2022, 423, 140574 CrossRef CAS.
- A. Patra, R. Kumar and S. Chand, Selenium-Containing π-Conjugated Polymers for Organic Solar Cells, Isr. J. Chem., 2014, 54, 621–641 CrossRef CAS.
- V. Agrawal, Shahjad, D. Bhardwaj, R. Bhargav, G. D. Sharma, R. K. Bjardwaj, A. Patra and S. Chand, Morphology and doping level of electropolymerized biselenophene-flanked 3, 4-ethylenedioxythiophene polymer: effect of solvents and electrolytes, Electrochim. Acta, 2016, 192, 52–60 CrossRef CAS.
- D. Bhardwaj, S. Gupta, P. Yadav, R. Bhargav and A. Patra, All Conjugated Poly (3-hexylthiophene)-block-poly (hexyl-3, 4-ethylenedioxythiophene) Copolymers, ChemistrySelect, 2017, 2, 9557–9562 CrossRef CAS.
- I. D. Brotherston, D. S. Mudigonda, J. M. Osborn, J. Belk, J. Chen, D. C. Loveday, J. L. Boehme, J. P. Ferraris and D. L. Meeker, Tailoring the electrochromic properties of devices via polymer blends, copolymers, laminates and patterns, Electrochim. Acta, 1999, 44, 2993–3004 CrossRef CAS.
- Z. Zhao-yang, T. Yi-jie, X. Xiao-qian, Z. Yong-jiang, C. Hai-feng and Z. Wen-wei, Multicolor electrochromism of low-bandgap copolymers based on pyrrole and 3,4-ethylenedioxythiophene: Fine-tuning colors through feed ratio, J. Appl. Polym. Sci., 2013, 129, 1506–1512 CrossRef.
- G. Inzelt, M. Pineri, J. W. Schultze and M. A. Vorotyntsev, Electron and proton conducting polymers: recent developments and prospects, Electrochim. Acta, 2000, 45, 2403–2421 CrossRef CAS.
- V. V. Abalyaeva, M. N. Efimov, O. N. Efimov, G. P. Karpacheva, N. N. Dremova, E. N. Kabachkov and D. G. Muratov, Electrochemical synthesis of composite based on polyaniline and activated IR pyrolyzed polyacrylonitrile on graphite foil electrode for enhanced supercapacitor properties, Electrochim. Acta, 2020, 354, 136671 CrossRef CAS.
- A. Yildirim, S. Tarkuç, M. Ak and L. Toppare, Syntheses of electroactive layers based on functionalized anthracene for electrochromic applications, Electrochim. Acta, 2008, 53, 4875–4882 CrossRef CAS.
- R. M. G. Rajapakse, N. H. Attanayake, D. Karunathilaka, A. E. Steen, N. I. Hammer, D. R. Strongin and D. L. Watkins, Advances in electro-copolymerization of NIR emitting and electronically conducting block copolymers, J. Mater. Chem. C, 2019, 7, 3168–3172 RSC.
- M. Balkhandia, R. Kedia, M. Khatak, N. Chaudhary and A. Patra, Tailoring the properties in conjugated copolymer P(EDOS-co-EDOT): Electrochemical polymerization and role of heteroatom, Electrochim. Acta, 2023, 66, 143002 CrossRef.
- A. Elschner, S. Kirchmeyer, W. Lovenich, U. Merker and K. Reuter, PEDOT: Principles and Applications of an Intrinsically Conductive Polymer, CRC press, 2010 Search PubMed.
- B. Zhuang, X. Wang, Q. Zhang, J. Liu, Y. Jin and H. Wang, Nanoengineering of poly (3, 4-ethylenedioxythiophene) for boosting electrochemical applications, Sol. Energy Mater. Sol. Cells, 2021, 232, 111357 CrossRef CAS.
- T. Yi-Jie, C. Hai-Feng, Z. Wen-Wei and Z. Zhao-Yang, Electrosynthesis and characterizations of a multielectrochromic copolymer based on pyrrole and 3, 4-ethylenedioxythiophene, J. Appl. Polym. Sci., 2013, 127, 636–642 CrossRef.
- P. Camurlu, E. Şahmetlioğlu, E. Şahin, İ. M. Akhmedov, C. Tanyeli and L. Toppare, Fine tuning of color via copolymerization and its electrochromic device application, Thin Solid Films, 2008, 516, 4139–4144 CrossRef CAS.
- B. Hu, Y. Zhang, X. Lv, M. Ouyang, Z. Fu and C. Zhang, Electrochemical and electrochromic properties of a novel copolymer based on perylene and EDOT, Opt. Mater., 2012, 34, 1529–1534 CrossRef CAS.
- G. Q. Feng, Z. Wang, Q. X. Gao, S. Chen and X. L. Xu, Electrosyntheses and characterizations of a new multielectrochromic copolymer of 1-(3-methylthiophen-2-yl) pyrene and 3, 4-ethylenedioxythiophene, Int. J. Electrochem. Sci., 2014, 9, 5820–5836 CrossRef.
- C. Zhang, Y. Xu, N. Wang, Y. Xu, W. Xiang, M. Ouyang and C. Ma, Electrosyntheses and characterizations of novel electrochromic copolymers based on pyrene and 3,4-ethylenedioxythiophene, Electrochim. Acta, 2009, 55, 13–18 CrossRef CAS.
- E. B. Sevinis, C. Sahin, M. E. Cinar, M. S. Eroglu and T. Ozturk, Copolymers possessing dithienothiophene and boron for optoelectronic applications, Polym. Eng. Sci., 2016, 56, 1390–1398 CrossRef CAS.
- P. Di Marco, M. Mastragostino and C. Taliani, Optical, electrical and electrochemical characterization of the doped polydithienothiophene, Mol. Cryst. Liq. Cryst., 1985, 118, 241–244 CrossRef CAS.
- C. Arbizzani, M. Catellani, M. Mastragostino and M. Cerroni, A spectroelectrochemical study of poly (dithienothiophenes), J. Electroanal. Chem., 1997, 423, 23–28 CrossRef CAS.
- P. Buttol, M. Mastragostino, S. Panero and B. Scrosati, The electrochemical characteristics of a polydithienothiophene electrode in lithium cells, Electrochim. Acta, 1986, 31, 783–788 CrossRef CAS.
- R. Kedia, M. Balkhandia, M. Khatak, N. Chaudhary and A. Patra, Electrochemical, optical and electrochromic properties of fused polydithienothiophene: A stable material with reversible doping, Synth. Met., 2023, 299, 117478 CrossRef CAS.
- C. Li, H. Bai and G. Shi, Conducting polymer nanomaterials: electrosynthesis and applications, Chem. Soc. Rev., 2009, 38, 2397–2409 RSC.
- C. Gu, N. Huang, Y. Chen, L. Qin, H. Xu, S. Zhang, F. Li, Y. Ma and D. Jiang, π-conjugated microporous polymer films: designed synthesis, conducting properties, and photoenergy conversions, Angew. Chem., 2015, 127, 13798–13802 CrossRef.
- K. Lin, C. Li, W. Tao, J. Huang, Q. Wu, Z. Liu, Y. Zhang, D. Wang and X. Liu, Electrochemical synthesis and electro-optical properties of dibenzothiophene/thiophene conjugated polymers with stepwise enhanced conjugation lengths, Front. Chem., 2020, 8, 819 CrossRef CAS PubMed.
- Y. Yuan and A. Lei, Is electrosynthesis always green and advantageous compared to traditional methods?, Nat. Commun., 2020, 11, 1–3 CrossRef PubMed.
- G. Nie, L. Qu, Y. Zhang, J. Xu and S. Zhang, Electrochemical copolymerization of 3, 4-ethylenedioxythiophene and 5-methylindole and characterizations of the copolymers, J. Appl. Polym. Sci., 2008, 109, 373–381 CrossRef CAS.
- Y. J. Tao, H. F. Cheng, W. W. Zheng, Z. Y. Zhang and D. Q. Liu, Electrosynthesises and characterizations of copolymers based on pyrrole and 3, 4-ethylenedioxythiophene in aqueous micellar solution, Synth. Met., 2012, 162, 728–734 CrossRef CAS.
- A. Patra, Y. H. Wijsboom, G. Leitus and M. Bendikov, Tuning the band gap of low-band-gap polyselenophenes and polythiophenes: The effect of the heteroatom, Chem. Mater., 2011, 23, 896–906 CrossRef CAS.
- S. Singhal, P. Yadav, S. Naqvi, S. Gupta and A. Patra, Donor–acceptor–donor copolymers with 3, 4-ethylenedioxythiophene moiety: electropolymerization and effect on optoelectronic and electrochromic properties, ACS Omega, 2019, 4, 3484–3492 CrossRef CAS PubMed.
- E. Poverenov, N. Zamoshchik, A. Patra, Y. Ridelman and M. Bendikov, Unusual doping of donor–acceptor-type conjugated polymers using lewis acids, J. Am. Chem. Soc., 2014, 136, 5138–5149 CrossRef CAS PubMed.
- S. Singhal and A. Patra, Benzothiadiazole bridged EDOT based donor–acceptor polymers with tuneable optical, electrochemical, morphological and electrochromic performance: effects of solvents and electrolytes, Phys. Chem. Chem. Phys., 2020, 22, 14527–14536 RSC.
- A. Mishra, S. Gupta and A. Patra, Synthesis and properties of 3, 4-dioxythiophene and 1, 4-dialkoxybenzene based copolymers via direct C-H arylation: Dopant-free hole transport material for perovskite solar cells, J. Polym. Sci., 2022, 60, 975–984 CrossRef CAS.
- D. Bhardwaj, S. Gupta, P. Yadav, R. Bhargav and A. Patra, All Conjugated Poly (3-hexylthiophene)-block-poly (hexyl-3, 4-ethylenedioxythiophene) Copolymers, ChemistrySelect, 2017, 2, 9557–9562 CrossRef CAS.
- L. Sunghwan and K. K. Gleason, Enhanced optical property with tunable band gap of cross-linked PEDOT copolymers via oxidative chemical vapor deposition, Adv. Funct. Mater., 2015, 25, 85–93 CrossRef.
- C. Xinfeng, J. Zhao, Y. Fu, C. Cui and X. Zhang, Electrosynthesis and characterization of a multielectrochromic copolymer of tris [4-(2-thienyl) phenyl] amine with 3, 4-ethylenedioxythiophene, J. Electrochem. Soc., 2012, 160, G6 Search PubMed.
- N. Guangming, L. Qu, J. Xu and S. Zhang, Electrosyntheses and characterizations of a new soluble conducting copolymer of 5-cyanoindole and 3, 4-ethylenedioxythiophene, Electrochim. Acta, 2008, 53, 8351–8358 CrossRef.
- Y. Ma, F. Zhao and B. Zeng, Electrodeposition of poly (3, 4-ethylenedioxythiophene) on a stainless steel wire for solid phase microextraction and GC determination of some esters with high boiling points, Talanta, 2013, 104, 27–31 CrossRef CAS PubMed.
- Z. Zhao-yang, T. Yi-jie, X. Xiao-qian, Z. Yong-jiang, C. Hai-feng and Z. Wen-wei, Electrosynthesises and characterizations of copolymers based on thiophene and 3, 4-ethylenedioxythiophene in boron trifluoride diethyl etherate, Synth. Met., 2012, 162, 2176–2181 CrossRef.
- S. Kulandaivalu, Z. Zainal and Y. Sulaiman, A new approach for electrodeposition of poly (3, 4-ethylenedioxythiophene)/polyaniline (PEDOT/PANI) copolymer, Int. J. Electrochem. Sci., 2015, 10, 8926–8940 CrossRef CAS.
- W. W. Chiu, J. Travaš-Sejdić, R. P. Cooney and G. A. Bowmaker, Studies of dopant effects in poly (3, 4-ethylenedi-oxythiophene) using Raman spectroscopy, J. Raman Spectrosc., 2006, 37, 1354–1361 CrossRef CAS.
- J. Zhang, H. Hu, P. Wang, C. Zhang, M. Wu and L. Yang, A stable biosensor for organophosphorus pesticide detection based on chitosan modified graphene, Biotechnol. Appl. Biochem., 2022, 69, 567–575 CrossRef CAS PubMed.
- E. Poverenov, M. Li, A. Bitler and M. Bendikov, Major effect of electropolymerization solvent on morphology and electrochromic properties of PEDOT films, Chem. Mater., 2010, 22, 4019–4025 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08729h |
| This journal is © The Royal Society of Chemistry 2024 |