Open Access Article
Open Access ArticleSurface modification strategies for improved hemocompatibility of polymeric materials: a comprehensive review
Abhishek Bhattacharjee†
a,
Aniruddha Vijay Savargaonkar† b,
Muhammad Tahir†c,
Alina Sionkowska
b,
Muhammad Tahir†c,
Alina Sionkowska c and
Ketul C. Popat
c and
Ketul C. Popat *abd
*abd
aSchool of Advanced Material Discovery, Colorado State University, Fort Collins, CO 80523, USA
bDepartment of Mechanical Engineering, Colorado State University, Fort Collins, CO 80523, USA
cDepartment of Biomaterials and Cosmetic Chemistry, Faculty of Chemistry, Nicolaus Copernicus University, Gagarina 7, 87-100 Torun, Poland
dDepartment of Bioengineering, George Mason University, Fairfax, VA 22030, USA. E-mail: kpopat@gmu.edu
First published on 1st March 2024
Abstract
Polymeric biomaterials are a widely used class of materials due to their versatile properties. However, as with all other types of materials used for biomaterials, polymers also have to interact with blood. When blood comes into contact with any foreign body, it initiates a cascade which leads to platelet activation and blood coagulation. The implant surface also has to encounter a thromboinflammatory response which makes the implant integrity vulnerable, this leads to blood coagulation on the implant and obstructs it from performing its function. Hence, the surface plays a pivotal role in the design and application of biomaterials. In particular, the surface properties of biomaterials are responsible for biocompatibility with biological systems and hemocompatibility. This review provides a report on recent advances in the field of surface modification approaches for improved hemocompatibility. We focus on the surface properties of polysaccharides, proteins, and synthetic polymers. The blood coagulation cascade has been discussed and blood – material surface interactions have also been explained. The interactions of blood proteins and cells with polymeric material surfaces have been discussed. Moreover, the benefits as well as drawbacks of blood coagulation on the implant surface for wound healing purposes have also been studied. Surface modifications implemented by other researchers to enhance as well as prevent blood coagulation have also been analyzed.
1. Introduction
Polymeric materials are a widely used class of materials as biomaterials due their great tunability, and wide spectrum of properties which allows for more versatile applications when compared to metals and ceramics. Ever since their first use in the 1940s, when an off-the-shelf nylon cloth was used for vascular surgery,1 polymers have been in high demand for applications ranging from orthopedic implants to skin grafts to cardiovascular stents. The one common denominator for all the applications, however, is the fact that all types of polymeric biomaterials have to deal with blood. The blood contact is also not something which can be avoided because to implant a biomaterial, a wound must be made, making the contact inevitable. Whenever blood encounters a foreign body, it triggers a cascade of events which starts with protein adsorption and leads to coagulation of blood. Blood coagulation, also known as thrombosis, is a double-edged sword when it comes to wound healing. For certain situations like skin wounds, thrombosis is desirable as it prevents blood loss, whereas for a situation like cardiovascular wounds, thrombosis is undesirable as it can lead to a blocking of the blood vessel leading to multiple complications like aneurysm or cardiac arrest.Strategies to combat thrombosis are categorised mainly in two categories, anti-thrombotic drugs, and surface modifications of implants to facilitate better integration with the body. Anti-thrombotic drugs include blood thinners like aspirin, vorapaxar, clopidogrel etc.2 The surface plays a pivotal role in designing and application of biomaterials as well as in the interaction with blood. In recent years several research papers have been published regarding new methods of surface treatment and modification. From the application point of view of medical sciences, the most important are biocompatibility and hemocompatibility. Interactions of biological cells and blood proteins depend on the structure of the surface, its hydrophilic/hydrophobic character and wettability. Usually, biopolymers are recognized as biocompatible macromolecules. However, the family of biopolymers is huge. Only in the family of polysaccharides, there are several polysaccharides which differ in several properties. A similar situation is with proteins, as many of them are used in medical applications. Considering that there is a big group of synthetic polymers applied in the medical field, biocompatibility and blood compatibility are worth to be discussed and the review of the recent literature seems to be on time. The aim of this paper is to provide a comprehensive review of the surface modifications which will improve the hemocompatibility for polymeric materials. The review begins with an introduction to polymers, both natural as well as synthetic and their fabrication techniques (Section 2 & 3). Then the interaction between the surface and blood (Section 4) is explored; followed by how the polymer surface modifications impact the blood coagulation (Section 6). Benefits and drawbacks of blood coagulation for wound healing were evaluated (Section 7 & 8) followed by conclusions.
2. Natural polymers
2.1. Polysaccharides
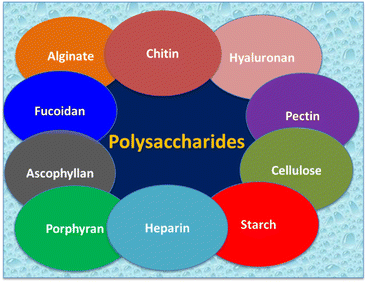
Polymers can be categorized according to their origins, either natural polymers or synthetic polymers.3 Natural polymers being used in biomedical applications mainly are polysaccharides (Fig. 1), and proteins.4 Table 1 summarizes the properties and biomedical applications of polysaccharides. Natural sources of polysaccharides are algae, plants, micro-organisms, and animals.5 Marine algae have been extensively focused on due to their anti-coagulant properties, and anti-coagulant properties are achieved due to the presence of sulphated glycosaminoglycans.6 Numerous polysaccharides derived from marine algae are alginate, fucoidan, ascophyllan, and porphyrin.7 Alginate is an anionic polysaccharide, and it is composed of linear copolymer constituents like (1–4)-linked β-D mannuronic acid (M) and α-L-guluronic acid (G). Alginates having a huge quantity of G blocks represent strong gelling characteristics, and alginates containing a higher amount of M blocks exhibit a viscous nature. The major derivation source of alginate is the cell wall of brown algae.8–10 Alginates show mild gelation as it is crosslinked to divalent cations (Ca2+).11| Polymer | Properties | Biomedical applications | Ref. |
|---|---|---|---|
| Alginate/sodium alginate | Amorphous | Tissue engineering | 11–15 |
| Water soluble | Treatment of skin burn | ||
| Anti-bacterial | Wound dressings | ||
| Biocompatible | |||
| Non-toxic | |||
| Non-immunogenic | |||
| Gelation with divalent | |||
| Fucoidan | Anti-tumor | Delaying the cancer growth cells | 19, 27, 28, 31 and 32 |
| Anti-oxidant | Co-acting agent with cancer drugs | ||
| Anti-coagulant | Wound healing | ||
| Anti-thrombotic | |||
| Anti-viral | |||
| Anti-inflammatory | |||
| Ascophyllan | Anti-bacterial | Cancer treatment | 36 and 37 |
| Anti-oxidant | Infectious diseases | ||
| Starch | Starch sulphate found | Skin tissue engineering | 42, 44 and 45 |
| Anti-coagulant | Wound healing | ||
| Anti-viral | |||
| Pectin | Biocompatible | Wound healing | 47–49 |
| Biodegradable | |||
| Bioactive | |||
| Dextran | Hydrophilic | Scaffold for skin tissue engineering | 55–57 |
| Surface wettable | Wound healing | ||
| Chitosan | Hydrophobic | Cancer treatment | |
| Anti-microbial | Wound healing | 58, 61 and 63 | |
| Sulphate derivative of chitosan | |||
| Anti-coagulant | |||
| Anti-bacterial | |||
| Hyaluronic acid | Anti-viral | 64, 66, 67 and 70 | |
| Non-toxic | Drug delivery | ||
| Biodegradable | Medical imaging | ||
| Non-immunogenic | Tissue engineering | ||
| Anti-cancer | Wound healing | ||
| Anti-microbial | Cardiovascular surgery | 72–74 and 76 | |
| Heparin | Hemocompatible | Cancer treatment | |
| Anti-coagulant | Wound healing |
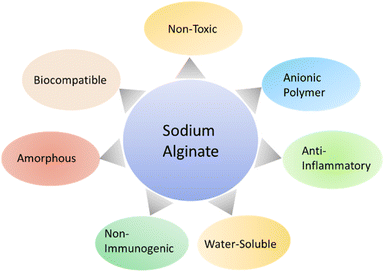
Sodium alginate has a biocompatible, non-toxic, and non-immunogenic nature.12 Sodium alginate is preferred to be used for tissue regeneration.13 Hajiali et al.14 conducted an experiment with electrospun nanofibers of sodium alginate and lavender oil for the treatment of skin burn, and this skin burn happened due to midrange ultraviolet radiation (UVB). It is observed that sodium alginate electrospun nanofibers and lavender oil represented anti-bacterial effect against Staphylococcus aureus, and it also led to the production of pro-inflammatory cytokines in the case of in vivo and in vitro. They also mentioned that sodium alginate presented anti-microbial properties and they observed the prominent anti-inflammatory effect of sodium alginate. Lavender oil showed anti-microbial properties and it also acted to control induced inflammation.
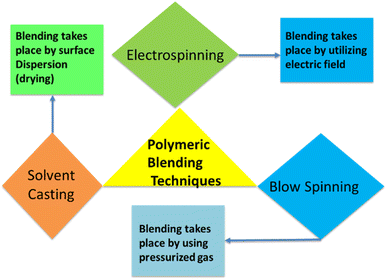
Sodium alginate is an amorphous polymer, and sodium alginate is joined with polyvinyl alcohol blends to enhance the amorphous phases of polymeric blends. It can also lessen the separation phases of polymeric blend sample.15 Sodium alginate and polyvinyl alcohol have been added to the polymeric blend for wound dressing application to enhance the biological, physical, and mechanical properties of wound healing material (Fig. 2). The addition of sodium alginate and polyvinyl alcohol to a polymeric blend is processed by three methods like solvent casting technique, electrospinning, and blow spinning. The solvent casting technique is based on dispersion of polymeric blend on surface, and surface is allowed to dry in ovens or at room temperature. Electrospinning is based on electric field, and it is used to produce fibers with nanoscale dimension and generation of thin, porous membrane. Blow spinning utilized pressurized gas for fiber production.16
Fucoidan is derived from marine algae, and it is composed of sulphated polysaccharides.17,18 Fucoidan performs biological activities like anti-tumor, antioxidant, anti-coagulant, anti-thrombotic, immunoregulatory, anti-viral, and anti-inflammatory.19 Natural polymers did not hold excellent mechanical properties, and synthetic polymers lacked biocompatibility.20 Fucoidan is combined with hydrophobic therapeutic agents to enhance their colloidal stability.21,22 The polymeric blend of fucoidan, polyvinyl alcohol, chitosan, and ampicillin have been prepared as bioartificial polymer material. It is noted that polyvinyl alcohol acted as water resistive, chitosan/fucoidan acted to generate porous microstructure which contributes to grow the cells within the matrix. In terms of cell viability fucoidan, polyvinyl alcohol, and chitosan acted better than polyvinyl alcohol and chitosan.23
Fucoidan with low molecular weight showed anticoagulant properties in the case of in vivo investigations, and anticoagulant effect is evident due to the availability of sulfate contents. Fucoidan having low molecular weight faces issues of degradation and utilization, as it is an easy target of intestinal microorganisms.24–26 Fucoidan found application as effective in delaying cancer cell growths, and it is used as a co-acting agent with anticancer chemotherapeutic drugs.27,28 It is noted that fucoidan did not have direct anti-bacterial effects, but in the case of the culture system fucoidan was used to enhance antibacterial activities against oral bacteria.29,30 High wound healing activity is achieved by low molecular weight fucoidan, even in low concentration (50 mg ml−1).31,32 High molecular weight fucoidan did not have ability to cross lipid bilayer, while low molecular weight fucoidan have ability to pass through lipid bilayer and biological functions are well performed.19,33
Ascophyllan is derived from brown alga Ascophyllum nodosum, and it has complex and heterogeneous sulphated polysaccharide nature.34,35 Ascophyllum nodosum has antibacterial and anti-oxidant properties, as combination of Ascophyllum nodosum and Lithothamnium calcareum have been used which resulted in inflammatory stress properties on pig intestinal cells.36 Ascophyllan contains equal amount of xylose, fucose, and sulfate half-ester groups, having small amounts of glucose, mannose, and galactose.7,35 Ascophyllan has tendency to activate natural killer cells in mice, and it leads to production of IFN-y which shows cytotoxicity against cancer cells. Ascophyllan can be utilized for cancer treatment and infectious diseases as an immunostimulatory molecule.37
Porphyran is extracted from porphyra, and porphyra is most economical available red algae. Porphyran is sulfated galactan mainly composed of 1,4-linked α-L-galactopyranose-6-sulfate (L6S) and 1,3-linked β-D-galactopyranose (G).38 Antioxidation and anti-coagulation properties of porphyran in case of in vitro have been checked, and it was observed that antioxidation properties of porphyran were dependent on degree of substitution while anticoagulation properties were dependent on position of sulphate.39
Polysaccharides based on plant sources are starch, cellulose, and pectin.5,40 Starch is extensively being focused to be used in biomedical applications due to cost effectiveness, ease of availability, and biological value of starch.41 Mistry et al.42 prepared electrospun nanofibers of starch and thermoplastic polyurethane, and electrospun nanofibers were crosslinked with glutaraldehyde. Main objective of crosslinking was to improve water stability and mechanical properties. Crosslinked electrospun nanofibers showed improved wound healing than traditional wound dressing like cotton gauze. Wadke et al.43 fabricated starch/polyvinyl alcohol and silver nanoparticles to produce electrospun nanofibers, and electrospun nanofibers presented anti-bacterial properties. It has potential applications in scaffold for skin tissue engineering and wound healing. Starch sulfate owns biological characteristics like anti-coagulation and anti-viral characteristics.44,45
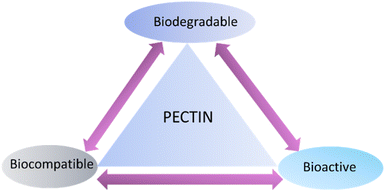
Pectin is also a natural polymer, and it contains huge number of hydroxyl and carboxyl groups. Functional groups present in pectin can interact with metal and organic cations using electrostatic interaction.46 Pectin has found biomedical applications due to biocompatibility, biodegradability, and bioactivity (Fig. 3).47 Pectin/chitosan/ZnO nanoparticles have been prepared by utilizing freeze drying method to get three dimensional porous films. It is found that three dimensional films have shown cell proliferation to enhance wound healing, and anti-microbial properties.48 Song et al.49 designed hydrogel of pectin and chitosan loaded with micelle having a huge amount of ciprofloxacin, and it showed anti-bacterial effect and it promoted wound healing. Hu et al.50 synthesized sulphonated, oxidized pectin having dialdehyde structure, which have presented anti-coagulation properties.
Microorganism origin of polysaccharides is dextran, and animal-derived polysaccharides are chitin, hyaluronic acid, and heparin.51–54 Kenawy et al.55 used electrospinning to fabricate polyvinyl alcohol (10%) and dextran (5%), and obtained nanofibers are crosslinked with sodium ampicillin loaded citric acid (10%). Addition of citric acid enhanced mechanical and thermal stability of nanofibers. As dextran is added to PVA nanofibers, it enhances hydrophilicity, protein adsorption and surface wettability. It also led to improvement in anti-microbial properties, cell viability, and in vitro wound healing as well. Nicu et al.56 prepared cellulose and dextran hydrogels and incorporated with plant active polyphenols to get 3D biocomponent matrices. Incorporation of plant bioactive polyphenols to hydrogel led to anti-inflammation effect, and cellulose-dextran hydrogel has shown cell proliferation without causing cytotoxicity. Pyataev et al.57 noticed that blend of dextran sulphate and doxorubicin presented sensitive behavior to amylase, it also shows anti-coagulant properties in cell culture. It improved efficiency level to treat cancer tumor compared to doxorubicin.
Chitosan is derived from chitin, and it has biocompatible and biodegradable nature. Chitosan has potential applications in field of biomedical applications.58 It is noted that chitosan is used in anti-bacterial wound dressings, and anti-bacterial properties of chitosan have limitations due to water insolubility of chitosan.59 Thi et al.60 prepared polymeric films of pectin and chitosan containing 0%, 10%, 15%, and 20% silver nanoparticles. It was observed that polymeric blend of pectin/chitosan/silver nanoparticles have antibacterial properties to be used in biomedical applications. Zhao et al.61 developed hydrogel of sodium alginate/carboxymethyl chitosan/silver nanoparticles, and this hydrogel presented anti-bacterial properties and biocompatibility for wound healing. Bacterial infection is major reason of inflammation and it cause delay to wound healing. Yu et al.62 fabricated chitosan and poly[2-(methacryloyloxy)ethyl]trimethyl ammonium chloride (PMETAC) to form hydrogel and examine biological activities. It was noticed that this hydrogel had anti-inflammatory, and anti-bacterial properties. Anna et al.63 mentioned that sulphated derivatives of chitosan hold anti-bacterial, anti-viral and also have anti-coagulant properties similar to heparins.
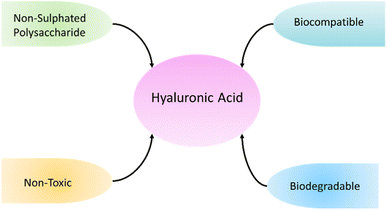
Hyaluronic acid belongs to non sulphated polysaccharides, so it shows non-toxic, non-inflammatory, biocompatible, biodegradable, and non-immunogenic behavior (Fig. 4). Hyaluronic acid has applications in field of drug delivery, medical imaging and tissue engineering.64 Hyaluronic acid is present in articular cartilage, skin, cervix, and glycocalyx of endothelial tissues.65 Kim et al.66 fabricated hyaluronic acid/pectin hydrogel material and have utilized Fe3+ as cross-linking agent. There was a reaction between –COOH functional group and Fe3+, and hydrogel presented wound healing properties (Fig. 5). Hydrogel also holds anti-bacterial properties. Hussein et al.67 fabricated material based on polyvinyl alcohol/hyaluronic acid/cellulose nanocrystals/L-arginine, and this fabricated blend have shown excellent mechanical properties, hemocompatibility, high protein adsorption, and anti-bacterial properties. It is reported that hyaluronic acid oligosaccharides (oHAs) have anti-coagulation and endothelialization properties.68,69 Hyaluronic acid nano-silver composites hold anti-inflammatory, anti-cancer, anti-angiogenic, and anti-microbial properties. Hyaluronic acid silver nanocomposites achieved high level of biocompatibility.70
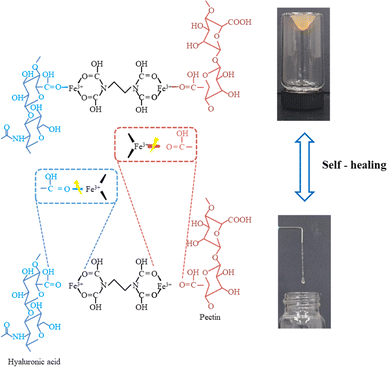 | ||
| Fig. 5 Hyaluronic acid and pectin mechanism and self-healing. Reproduced with permission.66 Copyright 2023, Elsevier. | ||
Heparin is derived from animal source, and it is sulphated polysaccharide.71 Low molecular weight heparin has anti-coagulant properties, and it contains glycosaminoglycan.72 The pair of heparin and protamine is applicable to in vivo cardio-vascular surgery. It is possible due to electrostatic and intermolecular interaction between anionic heparin and cationic protamine. It is noticed that heparin and protamine form complexes with patient blood, which shows their biocompatibility. The anti-cancer efficacy was also improved by using heparin and protamine.73 Nawaz et al.74 loaded heparin to alginate wound dressings, and they concluded that heparin enhanced angiogenic and anti-microbial effect of alginate wound dressings. Alginate and heparin are biocompatible and can be used for wound dressing applications. He et al.75 prepared graphene oxide and heparin hydrogel, and graphene oxide–heparin hydrogel exhibited anti-platelet adhesion, red-blood cells compatibility, hemocompatibility and anti-coagulation behavior. Lee et al.76 conjugated heparin with anti-cancer drug doxorubicin hydrochloride, and conjugated heparin molecule showed decrease in anti-coagulation effect. It also shows cytotoxic effects.
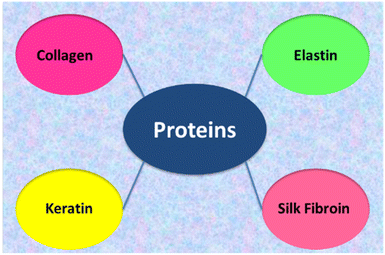
2.2. Proteins
Collagen materials are widely applied in biomedicine because of its biocompatibility.77–81 Collagen can be used in tissue regeneration/engineering, for new materials fabrication, e.g. artificial skin and bone graft substitutes. Collagen-based materials can be prepared as dental or soft tissue implants, artificial tendons and blood vessels. The development of new techniques showed that collagen is good materials for corneal implants, the regeneration of nerves, cartilage, skin and other body organs. Due to its hydrophilic character collagen is employed in hydrogels.82 Collagen hydrogels have been successfully used as three-dimensional substrates for cell culture and have shown promise as scaffolds for engineered tissues and tumours.83 Collagen-based materials can also be obtained by electrospinning.84 A review of recent developments in collagen scaffold fabrication for tissue engineering applications has been presented by Busra et al.85 The significance of collagen in wound dressing management has been discussed in a review paper by Pallaske et al.86 It has been emphasised that collagen plays a significant role in wound healing, and thus, numerous products based on this material have been developed so far. Table 2 summarizes the properties of proteins used in biomedical applications.| Polymer | Properties | Biomedical applications | Ref. |
|---|---|---|---|
| Collagen | Biocompatible | Wound healing | 77–82, 85 and 86 |
| Hydrophilic | Tissue regeneration | ||
| Corneal implants | |||
| Elastin | Hydrophobic | Skin wound treatment | 91, 93 and 94 |
| Bone regeneration | |||
| Keratin | Hydrophobic | 3D scaffold tissue engineering | 97 and 98 |
| Silk fibroin | Biocompatibility | Skin regeneration | 99–103 |
| Mechanically strong | Bone regeneration | ||
| Cartilage tissue regeneration |
Several studies have shown that the direct use of collagen fibre as an adsorbent material can show certain disadvantages, e.g. low adsorption capacity, poor selectivity, or inability to resist high temperatures. Thus, the problems and the technological needs connected to adsorbents based on collagen fibre have been discussed in the literature.87 The modifications of collagen with metal ions, tannin, hyperbranched polymers, and aldehydes etc. may lead to better performance of the material.88–90
Next structural protein applied as biomaterial is elastin (Fig. 6). Elastin is a biopolymer highly crosslinked and insoluble in water due to the presence of specific cross-linkages, desmosine and isodesmosine.91 Due to its insolubility elastin is usually hydrolysed into peptides and polypeptides to get soluble materials. The polypeptides are usually applied to form blends with other biopolymers, for example with collagen.92–96 The combination of collagen with elastin was used for preparation of several biomaterials and also was successfully electrospun to get the skin substitute for skin wounds treatment.93 Collagen, elastin-like peptides can form hydrogels which can be used for drug loading. The drug-loaded collagen–elastin-like peptides hydrogels can be promising in the case of combating bacterial infection and promoting guided bone regeneration.94 The mechanical properties such as stiffness and failure strength of the films depend on solubility of elastin.95
Keratin is a fibrous protein and it is in the animal population the major component of hair, feathers, nails and horns. Keratin, similarly to elastin, is highly crosslinked and insoluble in water, so usually is used in hydrolysed form. Keratin is used mainly with other polymers to form biomaterials. For example, based on collagen and keratin extracts recovered from the leather industry by-products, new biocomposites were made and their specific properties for agricultural and industrial applications were studied.97 The 3D scaffolds based on keratin–collagen blend for biomedical applications have been also proposed.98
Silk fibroin (SF) is a biopolymer produced by the domesticated Bombyx mori silkworm. Silk fibroin can be processed into films and scaffolds to improve regeneration in skin, nerve, bone and cartilage tissue. Silk fibroin shows good biological compatibility and mechanical properties, so it is investigated for many biomedical applications.99–103 Silk fibroin-based materials can be used to improve regeneration of skin, nerve, in bone, and cartilage tissue regeneration.
The recent applications of SF-based materials for small molecule drug delivery, biological drug delivery, gene therapy, wound healing, and bone regeneration have been reviewed by Nguyen et al.104 Silk fibroin can be used for preparation of hydrogels for biomedical applications.105–107 Silk-based materials can be fabricated by electrospinning process and can be used as a wound dressing materials.108–113 Silk fibroin is also widely used for the fabrication of biopolymer blends for biomedical applications.114 In general, the solubilized silk fibroin can be transformed into films, mats, gels, membranes, and also porous scaffolds. All of the above-mentioned form are widely used in biomedical applications. Moreover, silk can be also used together with another polymer forming the polymer blend.115–118 Elahi et al.119 mentioned that pure silk fibroin as a blood contacting material was not a better choice due to lack of good hemocompatibility. The aim of enhanced hemocompatibility of silk fibroin can be achieved by varying the surface properties of silk fibroin. Seib et al.120 have used low molecular weight heparin with silk, and it was proved helpful to increase the hemocompatibility. It played an effective role in maintaining the release of the growth factor which led to promote the human endothelial cells.
3. Synthetic polymers
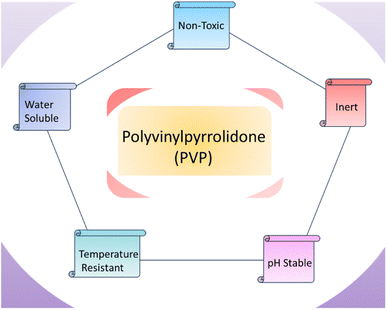
Polyvinyl alcohol is synthetic polymer, and it has applications in biomedical field (Fig. 7). Polyvinyl alcohol was found to be non-toxic and biocompatible to be used to in vivo and in vitro. Polyvinyl alcohol is used to form solid dispersion which is required to enhance solubility of drugs.121 Lu et al.122 fabricated 12% polyvinyl alcohol (PVA) and egg white (EW), and nanofibers were produced by using electrospinning to form membranes. Membranes are prepared by using varying proportions of polyvinyl alcohol and egg white solutions like (60EW/40PVA, 50EW/50PVA, 40EW/60PVA). It is observed that 60EW/40 PVA found effective in case of rat wound healing. Yang et al. prepared polyvinyl alcohol and chitosan hydrogel, and this hydrogel contained 1 and 3% lignin nanoparticles. Hydrogel formation was completed by opting freezing thaw method. It is mentioned that 1% lignin nanoparticles increased mechanical and thermal stability of hydrogel, and lignin nanoparticles with chitosan showed effect against E. coli and S. aureus bacteria. It is suggested that polyvinyl alcohol and chitosan with lignin nanoparticles can be used for biomedical applications, and presence of lignin nanoparticles controlled the deswelling of polyvinyl alcohol in water.123 Sudhakar et al.124 fabricated a tunable hydrophobic bio-membrane by crosslinking PVA with carrageenan via (3-aminopropyl)triethoxysilane (APTES) for enhanced tensile strength. This membrane was found appropriate for dental applications.Polyvinylpyrrolidone (PVP) exhibits water solubility, and PVP is derived from polymerization of n-vinylpyrrolidone. PVP is non-toxic, inert, biodegradable, biocompatible, temperature resistant, and pH stable (Fig. 8). It can be encapsulated to hydrophilic and lipophilic drugs.125 Zhang et al.126 fabricated PVP/chitosan/dihydroquercetin (DHQ) nanofibers film to be examined in wound dressings. Studies related to in vivo and in vitro evaluation have been conducted. It is noted that blend have shown hydrophilicity, good morphology, and thermal stability. MTT cytotoxicity test prevails that nanofibers film is non-toxic. It was found that polymeric blend nanofibrous wound dressing can be effective in wound healing. In vitro evaluation presented anti-bacterial effects against E. coli and S. aureus bacteria. Contardi et al.127 prepared bi-layer blend of polyvinylpyrrolidone and hyaluronic acid to be used in wound dressings. It was observed that bilayer wound dressing of polyvinylpyrrolidone and hyaluronic acid hold excellent anti-microbial properties. Polyvinylpyrrolidone and hyaluronic acid bilayer wound dressing have shown anti-inflammatory effects in diabetic mice model.
Next polymer used in biomedical field is polyurethane. Polyurethane (PU) foam was prepared by solvent casting method, and it was coated with water extract from propolis (WEP). PU/WEP wound dressing presented excellent anti-bacterial properties against E. coli and S. aureus bacteria, and it is due to presence of well-known anti-bacterial agent propolis. PU/WEP wound dressing have been checked in animal model, and it enhanced skin wound healing.128 Rather et al.129 fabricated polyurethane and cellulose acetate electrospun nanofibers, and electrospun nanofiber of cellulose acetate was used to encapsulate rosemary essential oil and adsorbed silver nanoparticles. It showed antibacterial effects, and it also improved hydrophilicity. Nguyen et al.130 fabricated virgin coconut oil with polyurethane/polycaprolactone to produce electrospun nanofibers by electrospinning, and electrospun nanofibers were used to produce membrane. Addition of virgin coconut oil enhanced hydrophobicity of polyurethane/polycaprolactone membrane. The presence of virgin coconut oil improved anti-coagulant, mechanical and anti-thrombotic properties of membrane. Polyurethane is excellent choice for blood contacting devices due to good bio-compatibility.131 Polyurethane has applications in dialysis stents, tissue engineering scaffolds and electrospinning, anti-bacterial surfaces, cardiac valves, vascular prostheses, and in coating for breast implants.132 Barnthip et al.133 fabricated polycaprolactone and cellulose acetate having mixture of fibroin and sericin by using electrospinning. It led to higher elasticity, and it also enhanced cell adhesion. It is suggested to be used in biomedical applications like scaffold for wound healing. Chanda et al.134 carried out electrospinning to fabricate polycaprolactone and chitosan, and then deposited a bilayer of hyaluronic acid over electrospun nanofibers of polycaprolactone and chitosan. It showed reduction in bacterial adhesion, and it is suggested to be used as scaffold for skin tissue engineering. Table 3 summarizes the properties and biomedical applications of synthetic polymers.
| Polymer | Properties | Biomedical applications | Ref. |
|---|---|---|---|
| Polyvinyl alcohol (PVA) | Non-toxic | Wound healing | 121 and 122 |
| Biocompatible | |||
| Hydrophilic | |||
| Polyvinylpyrrolidone (PVP) | Non-toxic | Wound healing | 125 and 126 |
| Biodegradable | |||
| Inert | |||
| Biocompatible | |||
| Temperature resistant pH stable | |||
| Polyurethane (PU) | Biocompatible | Dialysis stent | 131–133, 192–195 |
| Mechanically strong | Cardiac valves | ||
| Vascular prostheses | |||
| Wound healing | |||
| Polyethylene oxide (PEO) | Hemostatic | Wound healing | 183–185 and 208 |
| Blood coagulation | Hemostatic agent | ||
| Anti-thrombotic | |||
| Polyethylene glycol (PEG) | Hemostatic | Wound healing | 154 and 187 |
| Hydrophilicity | |||
| Poly(tetrafluoroethylene) (PTFE) | Inert | Blood contacting implants | 213 |
| Low surface energy | |||
| Low friction | |||
| Hemocompatible | |||
| Poly(vinylidene fluoride) (PVDF) | Thromboresistance | Stents coating | 216 |
| Lowering inflammation | |||
| Poly(L-lactic acid) (PLLA) | Biocompatible | Stents | 147 and 209 |
| Biodegradable |
4. Interaction of blood proteins and cells on polymeric material surfaces
Interaction with blood is one of the first and foremost cascade of events which takes place when a material is implanted into the body. The sequence of events is initiated by the attachment of water molecules which is followed by protein attachment which then attracts cells.135 Hence, to achieve the desired effect from the implants, encouraging or avoiding this cascade is important to its success.4.1. Protein adsorption
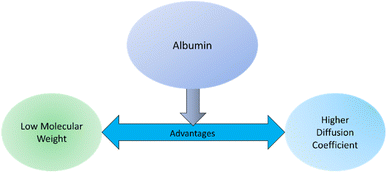
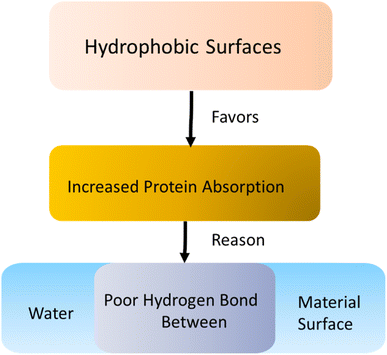
Implant surfaces are foreign materials for the host body. So, immune response happens instantly after materials are implanted to make the implant surface acclimated to the host. The first step of this immune response deals with the blood plasma protein absorption on the material surface. Blood plasma proteins are important message carriers for the host body. Protein absorption involves different mechanisms such as thermodynamic and electrostatic force along with hydrogen and van der Waals interactions.2,136 Primary protein absorption mechanism on surfaces involves hydrophobic thermodynamic interaction.136 The hydrophobic surfaces favor increased amount of protein absorption compared to the hydrophilic surfaces. This is due to the poor hydrogen bond between water and adjacent hydrophobic surfaces along with the release of large entropy for the absorption of hydrophilic protein on the surfaces.136 The secondary interaction involves the electrostatic force coming from the charge difference between the surface and proteins. Proteins generally possess a net negative charge. So, an implant surface having net cationic charge may enhance protein absorption due to the electrostatic attraction force.136 The protein absorption on the surface is often irreversible because of the protein denaturization after being absorbed on the surfaces. Hydrophobic amino acid side chains of the proteins may fold inside their 3-dimensional (3D) structures due to the presence of surrounding water. However, when these proteins are absorbed on a hydrophobic surface, they can unfold and undergoes an irreversible conformational change. For this reason, often biomaterials are designed to either avoid or encourage this irreversible protein adhesion based on their final applications. There are some proteins with hydrophilic amino acid side chains that are attracted to a hydrophilic surface. However, preparation of a hydrophilic surface is often difficult due to several physical property requirements such as glass transition temperature, permeability, conductivity, and method of sterilization.137In blood, the most abundant protein that undergoes absorption on implant surfaces is albumin. Albumin has low molecular weight with a higher diffusion coefficient compared to other blood plasma proteins (Fig. 9). For that reason, albumin is absorbed first on the implant surfaces.136 However, in later stages, albumin is replaced by other high molecular weight proteins such as fibrinogen, immunoglobulin, and factor XII. Fibrinogen is responsible for leukocyte and platelet adhesion on the surfaces. Factor XII is involved in thrombus formation similar to albumin. Additional plasma proteins such as fibronectin, vitronectin, and von Willebrand factor (vWF) are also reported to be capable of mediating platelet adhesion on the surface.138
Another important factor for protein absorption on the implant surfaces are nanoscale surface topography. Recent studies have provided an insight into the relationship between protein absorption kinetics on surfaces having nanoscale features. On the other hand, smooth surfaces avoid protein absorption and thus discourage blood coagulation on the implant surfaces. More on this topic with reported literature are discussed in the later sections of this review.
Protein absorption on implant surfaces plays a critical role in blood coagulation cascade. Implant surfaces should take protein absorption into high consideration because the application of implants is highly dependent on the proteins being absorbed on the surfaces. Implants for blood vessel regeneration or orthopedic implants may avoid blood coagulation. So, a hydrophilic and smooth surface should be prepared. On the other hand, implants for wound closures should encourage blood coagulation and thus have hydrophobic properties and nanostructures to enhance protein absorption.
4.2. Platelet adhesion
Protein absorption on implant surfaces lead to platelet adhesion which is a crucial step of thrombosis. When blood proteins create a clot on the wound surfaces to limit blood loss from vasculatures, the process is called thrombosis. Here, proteins such as fibrinogen and platelets create a clot which requires the adhesion and activation of platelet as plugs to form the clot. The formation of platelet plugs to stop the bleeding is known as primary hemostasis. Blood platelet adhesion is a result of the protein adhesion that was described in the last section. Platelet adhesion is initiated by surface-absorbed proteins such as von Willebrand factor (vWf), fibrinogen, fibronectin, and vitronectin. This binding happens due to the specific adhesion of amino acid sequences in the absorbed proteins and the receptors on platelet surface. Some reports showed that this specific platelet-protein adhesion and subsequent aggregate formation increases on a hydrophobic surface compared to the hydrophilic ones.4.3. Leukocyte adhesion
The next step of blood-material contact is the complement and leukocyte. Leukocytes (monocytes and neutrophils) that support the inflammatory response are activated in this stage.2 Fibrinogen gets converted to fibrin and primarily influence the leukocyte adhesion.139 Leukocytes helps in formation of the macrophages and other giant cells. These cells recruit fibroblasts to form a capsule like structure mainly prepared by fibrous tissue as an inflammatory response to the implanted device.2 In this way, leukocyte adhesion and activation can assist in blood coagulation on the biomaterial implant surfaces.2,1394.4. Platelet–leukocyte interactions
Platelets and leukocytes are two important components of blood which enable the continuance of hemostasis and immune response respectively. When these two components interact, they activate each other which enables them to have a kind of cross talk where platelets influence the immune response and leukocytes play a role in the blood coagulation process.140 This leads to activation of platelets due to leukocytes and increased recruitment of leukocytes due to platelets; this aggregate promotes thrombus formation as observed by Giulio Bizzozero in 1882.1415. Polymer surface properties that influence blood coagulation
All the foreign surfaces which are introduced to the body face a challenge following which the feasibility and success rate of the surface is determined. All the implanted surfaces have to encounter a thromboinflammatory response which can make vulnerable the integrity of the implant.142 This leads to obstruction of the implant surface through blood coagulation which ultimately disables the implant surface from performing the task efficiently. To overcome this obstacle, various modifications are implemented on the surface which will either prevent the cascade from initiating or avoid blood coagulation from occurring.5.1. Surface chemical structure and functional groups
Surface properties are important aspects of polymeric implant materials for biocompatibility. The chemical composition of the functional end groups in a polymeric implant dictates the surface properties and ultimately decides the protein and blood coagulation mechanisms. Several works have been reported recently that modified the surface chemistry of polymeric biomaterials to enhance the protein absorption. Gao et al. (1998)143 reported to modify the surface of poly(glycolic acid) (PGA) scaffold by hydrolysis of the ester bonds on the surface of the material. The modified scaffold surface showed improved serum protein and smooth muscle cell adhesion compared to the unmodified scaffold.5.2. Surface topography
Surface topography and roughness are interesting factors that influence protein and cell adhesion. Topographical features especially the nanoscale topographies on the biomaterial surfaces affect the blood protein and cell adhesion on these surfaces. Micropatterning is a popular method to induce topographical features on polymer surfaces. For instance, Milner and Siedlecki (2007)144 prepared poly(L-lactic acid) (PLLA) samples with ordered 400 nm and 700 nm pillars by replication molding. They assessed human fibroblast adhesion and proliferation on these samples and found that micropatterned surface affect the fibroblast adhesion. Zhou et al. (2013)145 also reported to prepare patterned biomimetic methacrylate polymer brushes to influence protein and neuronal cell adhesion. The patterned surfaces in this case acted like a guide for the protein and cell adhesion. Superhydrophobic polymer deposition via tanfloc/heparin polyelectrolyte multilayers on patterned titania nanotube surfaces was also reported to influence protein and cell adhesion.146 On the other hand, random surface roughness also influences protein and cell adhesion on material surfaces. Xu et al. (2004)147 also used PLLA to prepare substrates having various surface roughness. They found that PLLA substrate prepared by solvent casting that have smooth surface enhanced the functions of endothelial cells. DePalma et al. (1972)148 also showed that rough surfaces tend to attract more protein as well as cells which leads to thrombosis. To overcome this obstacle, various surface treatment techniques are done to smoothen the surface which include mechanical polishing, electropolishing, ultrasonic cleaning and chemical etching.1495.3. Surface energy and wettability
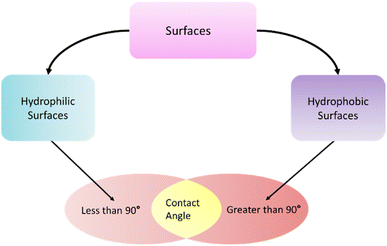
Surface energy and wettability are important parameters for understanding blood coagulation on polymeric biomaterials. Surfaces that have a static contact angle below 90° are referred to hydrophilic surface. On the other hand, hydrophobic surfaces have a static water contact angle of more than 90°.146 Surface energy components drive the wettability of a surface and can be determined using the work proposed by Owens et al. (1969).150 Comelles et al. (2010)151 investigated the surface energy and wettability of three commonly used polymeric biomaterials-poly(methyl methacrylate) (PMMA), polystyrene (PS), and poly(dimethylsiloxane) (PDMS). Their work correlated the lower surface energy to higher serum protein absorption on the surfaces. With increased hydrophobicity, the surface energy of the polymers decreased which in turn increased protein absorption.Surface wettability plays an important role in protein absorption. It has been well established that surfaces with minimum free energy will have minimum cell adhesion, which can be achieved if the surfaces are highly hydrophilic or highly hydrophobic (Fig. 10).152 Hydrophilic polymers have demonstrated a repulsion towards blood protein and cells whereas hydrophobic polymers have demonstrated good hemocompatibility.153 Studies has shown that hydrophobic surfaces allow enhanced protein absorption compared to the hydrophilic surfaces. Water molecules strongly attach to the hydrophilic surfaces, and it is hydrothermally difficult for the proteins to replace the water molecules. But on a hydrophobic surface water is readily displaced by the serum proteins to be readily absorbed.138 So, controlling the surface wettability and energy plays a crucial role in protein absorption and ultimately cell and blood coagulation. For example, poly(ethylene glycol) is a hydrophilic polymer that can be prepared by ethylene glycol or ethylene oxide in aqueous solutions. PEG is commonly used as coatings to impart hydrophilicity on biomaterials. As hydrophilic PEG reduces protein adhesion, they are popular in preparing biocompatible and anti-fouling materials. Krishnan et al. (2006)154 prepared a methyl-terminated self-assembled monolayer surface which was hydrophobic in nature and induced higher adhesion of blood plasma proteins. Hydrophobic polymers are more accepting of proteins like albumin and fibrinogen, which lead to reduced protein adsorption (Fig. 11). Albumin is a highly hydrophobic protein which has shown that it can adhere readily to highly hydrophilic surfaces. Albumin-treated surfaces have shown the resistant to platelet adhesion.155
6. Benefits of enhancing blood coagulation on the implant surface
Blood carries important signalling molecules for the cells to adhere and proliferate to a foreign material. The blood coagulation cascade starts the process of making an implant surface suitable for cell adhesion and proliferation which helps in disease prevention, implant acclimation and improvement in managing health complications. Most of the research work in biomaterial focuses on preventing blood coagulation. This is because blood coagulation is also a process of host body to reject the implant. When protein such as fibrinogen and albumin is absorbed on the surfaces, they create an additional layer on the implant surface to inactivate any harmful release of molecules from the implant. Thus, the implant fails to perform what it was intended for. This is especially important for implants having nanoscale surface characteristics because the cells cannot even connect to the implant surface if the nanoscale structures are covered by the absorbed proteins. However, blood coagulation possesses some merits for the success of implant (Fig. 12). Blood coagulation can help in biological recognition that enhances the biocompatibility of the surfaces.136 Blood carries important cell and signalling molecules which can increase cell adhesion and proliferation on the implant surface. Surfaces having less blood adhesion often suffer from poor integration to the host body environment due to the lack of cell adhesion. The adhered cells also get important cell niche molecules from the blood coagulation. So, preparation of a surfaces that encourage blood coagulation can offer substantial benefits depending upon the implant application. In the following sections, current surface modification strategies for polymeric implant surfaces are reviewed that encourages blood coagulation on the implant surface.7. Polymer surface modification to enhance blood coagulation for wound healing
7.1. Natural biomaterials
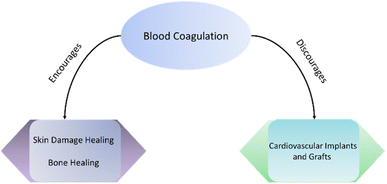
Blood coagulation is an immune response from the host body to protect itself from harmful foreign materials and pathogens such as bacteria, viruses, or fungus. A significant amount of blood serum protein and cell absorption happens on implant materials due to the immune response from the host body. Hence, the implant loses its surface characteristics and fails to achieve its intended biomedical applications. Therefore, majority of biomaterial surface research focuses on discouraging blood coagulation on implant surfaces. However, for several biomedical applications blood coagulation on implant surfaces is indeed an important and significant outcome. Blood can recruit important protein and cells on the implant surfaces to enhance tissue healing. In skin grafts, blood coagulation is a major step for the healing of skin damage. Blood coagulation on bone implants can also improve the bone healing process. On the other hand, blood coagulation should be discouraged on cardiovascular implants and grafts. Despite some interesting applications of blood coagulation on implant surfaces, there remains a lack of focus on this point in the literature. Hence, the one major focus of the current review article is to evaluate existing literature on polymeric surface modifications strategies that encourage blood coagulation.Skin is the outer layer of human body which is a highly flexible and superhydrophobic material. This sheet like structure is capable permitting of gaseous molecules such as oxygen however protects the inner organs or fluids to come out and prevents any pathogen penetration inside the body. Skin is a dynamic tissue that undergoes continuous repair while absorbing all the abuse from outside environment. Hence, there are multitude of blood vessels run through skin. These blood vessels help in blood coagulation and skin repair in case of any damage. The damage repair primarily happens via blood coagulation process that was described earlier in this article. When skin is ruptured, blood vessels also get damaged and results in bleeding complications. Bleeding from the body is highly detrimental and can be deadly if not stopped within a short time. The stoppage of bleeding is known as hemostasis. Several skin grafts are pointed towards hemostasis.
In the history of healthcare, cotton has been widely used as a wound closure or bandage to heal bleeding. Cotton is a cellulosic material which is highly absorbent of blood fluids, thus providing a solid platform for the hemostatic components of blood to adhere, contract and close the wound. Throughout most of human history, cotton has been used as a bandage without any modifications with novel biomaterials or nanocomposites. Cotton bandages are reported to influence platelet plug formation and blood coagulation because of cotton fiber's intrinsic properties such as surface energy, negative surface charge, and hydrophilicity.156 To improve the hydrophilic properties of cotton, conventional methods use wet chemical methods which can be highly energy and water consuming. Recently, plasma treatment approaches are being utilized to replace the conventional methods and save precious water and energy.157,158 The enhanced hydrophilicity of cotton bandages can help in improved blood clotting on the wound. Although cotton bandages improve wound coverage and blood clotting on wound, they often entangle with the tissue surrounding the wound. This creates a complex situation for the patient while removing the bandage causing unnecessary pain and suffering.
Recently, oxidized cellulose (OC) from cotton and oxidized regenerative cellulose (ORC) from wood pulp are used as wound bandages. Due to the oxidative conversion of alcohol at the end of cellulose polymer chain to aldehyde or carboxyl groups, OC and ORC exhibit different physio-chemical and mechanical properties. Their acidic pH helps in platelet activation and blood coagulation, and they are reported to be biodegradable.159 Recently, Lewis et al. (2013)160 compared the hemostasis of regenerative and non-regenerative oxidized cellulose and found superior hemostasis from non-regenerative OC. However, like cotton bandages, OC and ORC also have tissue adherence problem. Tulle gauge is an interesting approach to make the bandages oily for becoming non-adherent for tissues but still possessing the absorbent properties of the cellulose. Tulle gauges are prepared by soaking the cellulosic bandages in parafilm to make the surface hydrophobic.
Another interesting cellulosic material is bacterial cellulose where the source of cellulosic material is Gram-negative bacteria instead of a plant source. Picheth et al. (2017)161 wrote a thorough review of bacterial cellulose (BC) and their use in biomedical applications. Bacterial cellulose can be made into superior wound dressing because of their unique mechanical properties and antimicrobial properties in the wound area.162 BC was also conjugated with other components to impart several biomedical applications such as antibacterial efficacy by doping silver nanoparticle, drug delivery, and drug reservoir.161 Fragoso et al. (2014)163 prepared a sugarcane biopolymer (SCB) which is essentially a bacterial cellulose film to assess the adhesion of mesenchymal stem cells (MSC) on the SCB film. Their report showed that SCB can be utilized as a viable platform for MSC adhesion. Lucena et al. (2015)164 also used a similar film to examine wound healing on induced skin wounds in rats. They reported that after 21 days of post operative process, the film with bacterial cellulose accelerated wound healing significantly compared to the control film having no bacterial cellulose. In another instance, Silveira et al. (2016)165 used bacterial cellulose skin graft to treat tympanic membrane perforation. Their report also showed improved wound closure by using the bacterial cellulose graft.
Fibrin is a protein formed at the end of the coagulation cascade and plays an important role in wound healing and preventing blood loss.166 Fibrin sealants haven been used in different biomedical applications, especially in clinical practices such as cardiovascular, gastrointestinal, pneumothoracic, neuronal, urological, and otolaryngological surgeries.167 Fibrin protein is generally formed by the reaction of thrombin protein on fibrinogen. In fibrin sealants, the reaction between thrombin and fibrinogen is utilized to produce fibrin clot in situ for wound dressing and sealing applications.166,168 There are several commercially available fibrin sealants approved by the U.S. Food and Drug Administration (FDA). Tisseel®169 is the only fibrin sealant that can be used for both hemostasis and sealing applications (Baxter). Artiss®170 from Baxter is used as adhesive which adheres to the wound surface and skin graft to provide a sealing application (Baxter). Both these products use thrombin and fibrinogen to produce fibrin clot in the wound site. Other commercial fibrin sealants for hemostasis are Vitagel™ (Stryker), TachoSil® (Baxter), EVARREST™ (Omrix Biopharmaceuticals).168 Major concern for using fibrin sealant is the immunogenicity and contamination of viral pathogens because of the animal (bovine and porcine) and human pooled blood sources.166 So, recently recombinant protein sources are being investigated to source fibrinogen and thrombin. RECOTHROM from Baxter uses recombinant human thrombin for topical hemostasis use. Wang et al. (2000)171 reported to purify fibrinogen from salmon blood which less prone to viral and bacterial infection. On the other hand, Michaud et al. (2002)172 purified thrombin from salmon source to be used as a fibrin sealant. Another approach to reduce immunogenic reaction and viral contamination is to use low volume plasma to prepare fibrin sealant. CryoSeal® (Asahi Kasei) and Vivostat® (Vivostat) systems utilize this low volume plasma method.166
Collagen is the most abundant proteins in animals, comprises three quarters of the dry skin weight in human, and one of the most prevalent components in the extracellular matrix.173 Since collagen is abundant in human skin, numerous research focused on utilizing collagen for preparing skin graft and blood coagulating biomaterials. It has been reported that activated platelet and vWF secreted from injured endothelium can bind to the exposed collagen, thus helping in primary and secondary hemostasis on biomaterial surface.166 Platelet membrane has specific receptors for collagen type I and type III and undergoes a ligand–receptor interaction to initiate platelet binding and ultimately forming the platelet plug during thrombus formation.174,175 Yang et al., (2004)175 prepared a recombinant human collagen type III-based fibril matrix that could effectively stop bleeding 3 times faster than commercially available collagen hemostat Instat (Ethicon, J & J, USA). Their matrix was also one of the few types III collagen-based hemostat which is better than animal derived type I collagen based biomaterials since platelet aggregation has been reported to be superior on type III collagen compared to the type I.175 Wagner et al. (1996)176 compared several hemostatic agents and reported that collagen-based hemostats performed better than cellulose based hemostats. Also, in their report several collagen-based hemostats were compared and Avitene (a microfibrillar collagen, Davol Inc., USA), Actifoam, and Helistat (Integra LifeSciences, USA) (both are collagen sponges) performed superior to Instat and Surgicel (oxidized regenerated cellulose). Zwischenberger et al. (1999)177 compared Hemostagene and Helistat which are two hemostatic agents on 60 consecutive patients who were undergoing cardiothoracic surgery. They reported successful hemostasis rate of 75% and 77% for Hemostagene and Helistat respectively. Since collagen source is mainly from animal, immunogenic complications have always been a problem with collagen based hemostatic agents. To overcome this, low-immunogenic collagen-derived gelatin has been developed and reported to retain the hemostatic properties of collagen.166 Hajosch et al. (2010)178 prepared a gelatin sponge that exhibited accelerated hemostasis in an in vitro model and also in a young patient suffering from acute bleeding of a pharyngeal angiofibroma. In another work, Xie et al. (2021)179 prepared a gelatin nanofiber sponge utilizing the electrospinning process which exhibited a superior hemostatic property owing to its interesting spongy and interconnected structure. Even though gelatin reduced immunogenic complications, they show poor mechanical properties when in contact with blood.180 To overcome this problem, several composite gelatin hemostatic agents were developed. One of them is FloSeal (Baxter International Inc., IL, USA) which is a composite matrix prepared by gelatin and thrombin. Woodworth et al. (2009)181 prepared a gelatin-thrombin matrix by mixing SURGIFLO™ (Ethicon, J & J, USA) and Thrombin-JMI (King Pharmaceuticals, Bristol, TN) which was proven to effectively control bleeding. Chen et al. (2021)180 used alginate with gelatin to prepare a sponge which was then combined with curcumin loaded electrospun fibers. They reported enhanced hemostatic property of this matrix compared to commercially available hemostatic agents.
7.2. Synthetic biomaterials
Besides the naturally derived biomaterials for skin wound grafts, several synthetic biomaterials have been investigated in recent years for hemostatic applications. One of the popular approaches is to prepare synthetically derived hemostatic material is to conjugate a synthetic polymer with a natural agent such as collagen, gelatin, fibrin, or cellulosic materials. Poly(ethylene oxide) (PEO) is one such common synthetic material. Zhao et al. (2018)182 prepared a nanofibrous membrane by mixing collagen and PEO by electrospinning method. Their material was reported to form 70% blood clot within 5 min. Similarly, Liu et al. (2022)183 prepared a chitosan/PEO nanofiber membrane for rapid hemostasis and accelerated wound healing. Verma et al. (2023)184 prepared a nonwoven hemostatic dressing which was composed of PEO–fibrin and PEO–thrombin microfibers that can facilitate whole blood clotting in less than 30 s. Barba et al. (2018)185 utilized carboxymethyl cellulose, kappa-carrageenan, and PEO to prepare a hemostatic dressing that achieved hemorrhage control in a rat model within 90 s. All these synthetically derived hemostatic materials offered potentially superior hemostasis and hemorrhage control over the traditionally utilized hemostatic agents.Another popular polymer in this regard is poly(ethylene glycol) (PEG). Lewis et al. (2014)186 reported a PEG coated collagen pad (PCC) for improved hemostasis. The PCC offered complete hemostasis when applied to severe arterial bleeds in a heparinized porcine pulmonary segmentectomy model. Zhu et al. (2018)187 prepared a hybrid hydrogel using hyaluronic acid and PEG for improved hemostasis and wound healing.
Poly(vinyl alcohol) (PVA) is another biocompatible synthetically derived polymer that is used in hemostatic and wound healing applications. Zhao et al. (2019)188 reported a hemostatic sponge made from PVA and chitosan (CS). The PVA–CS sponge showed improved blood clotting ability with enhanced blood cell and platelet adhesion and activation when compared to gauge and PVA. Yin et al. (2020)189 also utilized chitosan and PVA to prepare a hemostatic nanofibrous membrane. Besides the improved blood clotting ability, their membrane also showed antibacterial activity which can be helpful to reduce pathogenic infection in the wound graft. Incorporation of nanoparticles in these hemostatic agents also showed improved blood clotting and wound healing capabilities. One such work was reported by Shakiba-Marani and Ehtesabi (2023),190 Their nanocomposite hemostatic sponge was composed of chitosan, PVA, and carbon dot. The nanocomposite sponge showed efficient hemostasis in in vitro and in vivo experiments. Zhang et al. (2022)191 also reported a chitosan/PVA composite with tourmaline nanoparticle for hemostatic applications.
Polyurethane (PU) has also been used for hemostatic applications owing to its superior mechanical properties and biocompatibility. Zou et al. (2022)192 prepared a strong adhesive patch by using bioactive PU and gelatin-methacryloyl. Their material reported to promote organ hemostasis and wound healing by angiogenesis. Recently, Guo et al. (2023)193 also utilized PU to prepare a hemostatic sponge for hemorrhage control. In another work, Lundin et al. (2017)194 used kaolin-PU foam composite for wound healing applications. Liu et al. (2017)195 also prepared a foam based wound healing biomaterial using PU and urea. Broekema et al. (2011)196 utilized PU foam for topical hemostatic application. PU have also been incorporated to hemostatic biomaterials as nanoparticles. Huang et al. (2021)197 reported an anti-inflammatory gelatin based hemostatic agent where PU nanoparticles were incorporated.
8. Drawbacks of blood coagulation on the implant surface for wound healing
The formation of thrombus due to biomaterial is rare but a serious complication in the cardiovascular applications as it can lead to sudden death, chest pain and ischemic electrocardiogram.198 Thrombus formation due to stent has mortality rates of 20–45% whereas its recurrence rates are ∼20%.199,200 To prevent thrombosis, patients are prescribed anti-platelet and/or anticoagulant therapy for varying periods in accordance with the different regulatory bodies. The AHA guideline recommends 3–6 month of anti-platelet therapy whereas the European guidelines advice 6–12 months.201,202 Anti-platelet agents mainly are composed of aspirin and clopidogrel with attempts being made to synthesize biomaterials that release or generate nitric oxide to mimic their effect. Fondaparinux is a synthetic polysaccharide which inhibits Factor Xa and Dabigatran which is an oral thrombin inhibitor, have shown that if the biomaterial-based activation is not blocked, it can lead to adverse outcomes.202 Apart from using the anti-platelet and anticoagulant therapies, modifying the material surface is another approach which is applied to prevent the protein and eventually cell adhesion. The following section talks about the various material properties and surface modifications which will influence the blood coagulation in a negative as well as a positive manner.9. Preventing blood coagulation for wound healing
Preventing blood coagulation in the cardiovascular system is important as formation of a blood clot could prove catastrophic. Ensuring non-thrombogenic and hemocompatible biomaterials for application in the cardiovascular system have been extensively researched and, in this review, we have summarized the research which have used polymers to achieve this goal.9.1. Natural polymers
Heparin after its discovery in 1961 has displayed great anti-coagulation properties as it interacts with thrombin and avoids the amplification of anticoagulant cascade.203 PVC and PTFE surfaces coated with heparin have displayed antithrombogenic activity and prolonged the blood coagulation time.204,205 Heparin immobilized on plasma-polymeric allylamine coating on stainless steel also demonstrated a decrease in adhesion and activation of platelets as well as decreased the activation of fibrinogen.206Albumin is an inert, thromboresistant coating which has displayed significantly lower platelet adhesion to polymer surfaces.207,208 The BioMatrix stent from Biosensors International as well as the Nobori stent from Terumo Corp. were coated with albumin in addition to Biolimus A9™ drug and both have displayed lower blood coagulation compared to a Cypher stent.209
Hyaluronan (HA) is a glycosaminoglycan which possesses anti-thrombotic as well as angiogenic properties as well as inhibits platelet adhesion and activation.210 HA coating on stainless steel resulted in significant reduction of platelet adhesion and activation as compared to uncoated stents.211 HA–collagen nanofibers have demonstrated anti-thrombotic properties as well as reduced platelet adhesion.212
9.2. Synthetic polymers
Poly(tetrafluoroethylene) (PTFE) were used to make the first generation of blood contacting implants due to their natural inertness, low surface energy and low friction. However, blood coagulation was reported on them which encouraged the implementation of surface modifications.213 PTFE has shown enhanced hemocompatibility and lower platelet adhesion and activation when plasma treated with argon and nitrogen gases.214 PTFE has displayed significantly lower platelet adhesion as compared to PET which makes it a useful coating material to stainless steel to lower the surface energy. However, this prevents the endothelial cells from attaching to the surface, hence a layer of fibrinogen is used to counter this issue.215Poly(glycolic acid) (PGA) is a biocompatible and biodegradable polymer which is not toxic even on degradation when in vivo. Gao et al. (1998)143 modified the surface of PGA by hydrolysis of ester bonds on the surface of the material. This modification displayed improved serum protein and smooth muscle cell adhesion as compared to unmodified scaffold.
Fluoropolymer like poly(vinylidene fluoride) (PVDF) have shown to possess inherent properties which contribute to thromboresistance, lowering inflammation. PVDF has been used to coat the stents commercially available from Abbott which have displayed lower blood coagulation which correlates that fluorinated materials are non-thrombogenic.216
Poly(L-lactic acid) (PLLA) is a biocompatible, biodegradable polymer which has a long history of applications in tissue engineering. Hence, PLLA is a polymer of interest for manufacturing and implantation in patients. Currently, commercial stents like Igaki-Tamai stent (Kyoto Medical Planning Co. Ltd.), BVS Stent (Abbott Vascular) are made from PLLA. PLLA surfaces prepared by solvent casting have demonstrated smooth surfaces which enhanced function of endothelial cells.147,209
Nitric oxide (NO) has shown to reduce the thrombus formation in blood vessels and healthy blood vessels have a mechanism wherein NO is released from the vessels itself causing dilation which avoid blood coagulation. NO releasing polymeric coatings composing of poly(vinyl chloride) (PVC) have displayed improved anti-blood coagulating properties as compared to the control polymer.217
Poly(ethylene oxide) (PEO) has demonstrated the lowest levels of cell and protein adhesion among any known polymer. This makes it a great coating for anti-thrombotic application. Hence, surface modification with PEO has been attempted in various ways like covalent grafting, physical adsorption etc.208
Ever since their invention in 1942 and especially after their application in the biomedical industry in 1972, polyurethanes have demonstrated excellent blood compatibility and superior abrasion resistance as well as great thrombus resisting properties.218 Chemical grafting of poly(ethylene glycol) on poly carbonate urethane (PCU) has displayed decreased platelet adhesion and higher hemocompatibility.219 Polyurethane coatings have also demonstrated decreased platelet adhesion and decreased thrombosis.220,221 Poly(carbonate-urea) urethane impregnated with silver nanoparticles have displayed significantly lower platelet activation.222
Poly(ethylene glycol) (PEG) is a hydrophilic polymer that can be prepared by ethylene glycol or ethylene oxide in aqueous solutions. PEG is used to impart hydrophilicity on biomaterials which leads to reduced protein adhesion and makes them popular for preparing biocompatible and anti-fouling materials.154
Poly(2-methoxyethylacrylate) (PMEA) is a hemocompatible, low toxic and easy to manufacture in large quantities polymer. PMEA has demonstrated lower protein adsorption from human plasma as compared to other poly-methacrylates as well as had lower platelet adhesion and activation.223 It has also shown to denature adsorbed proteins and is approved by the US FDA.224
Zwitterion is a substance which has both positive and negative charges within a molecule and has demonstrated anti-fouling and anti-thrombotic effects. Zwitterionic polymers induce a hydration layer on the surface of material which leads to reduced cell and protein adhesion hence, making them hemocompatible (Table 4).225
| Modifier | Surfaces | Results | Ref. |
|---|---|---|---|
| Heparin | Poly(vinyl chloride) (PVC) | Anti-thrombogenic activity | 204 |
| Poly(tetrafluoroethylene) (PTFE) | Prolonged blood coagulation time | 205 | |
| Stainless steel | Decrease in adhesion | 206 | |
| Reduced platelet activation | |||
| Decreased fibrinogen activation | |||
| Hyaluronan | Stainless steel | Decreased platelet adhesion | 211 |
| Decreased platelet activation | |||
| Collagen | Presented anti-thrombotic properties | 212 | |
| Reduced platelet adhesion | |||
| Polyethylene glycol (PEG) | Polycarbonate urethane | Reduced platelet adhesion | 219 |
| Higher hemocompatibility | |||
| Collagen | Improved hemostasis | 186 | |
| Hyaluronic acid | Improved hemostasis | 187 | |
| Wound healing |
10. Conclusions
Blood–material interactions are one of the most critical criteria which determine the success of the implants. It is the case because when an implant is introduced to the body, it encounters a thromboinflammatory cascade that could weaken its integrity and hence, lead to implant failure. Researchers have been trying to improve the hemocompatibility of polymer implants to tailor it to the application. For certain applications like skin tissue, blood coagulation/thrombus formation is a desirable trait to have, whereas; for cardiovascular applications, thrombus formation is not a desirable trait. To design a hemocompatible surface, it is important to understand the thrombus formation cascade. The implant surface properties such as chemistry, wettability, and topography are also significant in the determining the hemocompatibility, hence, various studies have been carried out to develop surfaces with improved hemocompatibility.Different studies reviewed in this manuscript have modified surfaces of polymers to either enhance or prevent blood coagulation based on their application. In addition to these, studies evaluating the effect of changes in surface properties like wettability, chemistry and topography on blood coagulation cascade have been studied. Polymers will be used extensively in future to fabricate implants for various applications and it is important to have the awareness as to which properties need to tailored in what manner in order to achieve the desired purpose.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank National Institute of Biomedical Imaging and Bioengineering (R21EB033511) and Nicholas Copernicus University, Torun, Poland (Excellent Initiative – Research University Programme (IDUB) for funding.References
- B. D. Ratner, Annu. Rev. Biomed. Eng., 2019, 21, 171–191 CrossRef CAS PubMed.
- V. K. Manivasagam, R. M. Sabino, P. Kantam and K. C. Popat, Mater. Adv., 2021, 2, 5824–5842 RSC.
- P. C. Pires, F. Mascarenhas-Melo, K. Pedrosa, D. Lopes, J. Lopes, A. Macário-Soares, D. Peixoto, P. S. Giram, F. Veiga and A. C. Paiva-Santos, Eur. Polym. J., 2023, 187, 111868 CrossRef CAS.
- M. Puertas-Bartolomé, A. Mora-Boza and L. García-Fernández, Polymers, 2021, 13, 1–26 CrossRef PubMed.
- T. Thambi, V. H. G. Phan and D. S. Lee, Macromol. Rapid Commun., 2016, 37, 1881–1896 CrossRef CAS PubMed.
- G. Nayak, S. K. Bhuyan, R. Bhuyan, A. Sahu, A. Kuanar and D. Kar, Univers. J. Public Health, 2022, 10, 15–24 Search PubMed.
- J. Hwang, D. Yadav, P. C. W. Lee and J. O. Jin, Phytother. Res., 2022, 36, 761–777 CrossRef CAS PubMed.
- P. Tennakoon, P. Chandika, M. Yi and W.-K. Jung, iScience, 2023, 26, 106404 CrossRef CAS PubMed.
- A. Stanisci, A. Tøndervik, M. Gaardløs, A. Lervik, G. Skjåk-Bræk, H. Sletta and F. L. Aachmann, ACS Omega, 2020, 5, 4352–4361 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- P. Severino, C. F. da Silva, L. N. Andrade, D. de Lima Oliveira, J. Campos and E. B. Souto, Curr. Pharm. Des., 2019, 25, 1312–1334 CrossRef CAS PubMed.
- A. Ahmad, N. M. Mubarak, F. T. Jannat, T. Ashfaq, C. Santulli, M. Rizwan, A. Najda, M. Bin-Jumah, M. M. Abdel-Daim, S. Hussain and S. Ali, Processes, 2021, 9, 1–27 Search PubMed.
- Q. Wei, J. Zhou, Y. An, M. Li, J. Zhang and S. Yang, Int. J. Biol. Macromol., 2023, 232, 123450 CrossRef CAS PubMed.
- H. Hajiali, M. Summa, D. Russo, A. Armirotti, V. Brunetti, R. Bertorelli, A. Athanassiou and E. Mele, J. Mater. Chem. B, 2016, 4, 1686–1695 RSC.
- H. Albalawi, E. M. Alharbi, A. I. Al-Sulami, N. Al-Qahtani, M. O. Farea and A. Rajeh, Polym. Compos., 2022, 44, 1762–1771 CrossRef.
- M. M. Saraiva, M. d. S. Campelo, J. F. Câmara Neto, M. L. d. C. Gonzaga, M. d. S. R. Bastos, S. d. A. Soares, N. M. P. S. Ricardo, G. S. Cerqueira, R. F. d. C. Leitao and M. E. N. P. Ribeiro, J. Biomed. Mater. Res., Part B, 2023, 111, 220–233 CrossRef CAS PubMed.
- N. V. Dubashynskaya, E. R. Gasilova and Y. A. Skorik, Int. J. Mol. Sci., 2023, 24, 2615 CrossRef CAS PubMed.
- Y. Yao and E. K. F. Yim, Carbohydr. Polym., 2021, 270, 118347 CrossRef CAS PubMed.
- Y. Wang, M. Xing, Q. Cao, A. Ji, H. Liang and S. Song, Mar. Drugs, 2019, 17, 15–17 Search PubMed.
- A. Bernal-Ballen, J. Lopez-Garcia, M. A. Merchan-Merchan and M. Lehocky, Molecules, 2018, 23, 3109 Search PubMed.
- T. L. Ho, C. Mutalik, L. Rethi, H. N. T. Nguyen, P. R. Jheng, C. C. Wong, T. Sen Yang, T. T. Nguyen, B. W. Mansel, C. A. Wang and E. Y. Chuang, Int. J. Biol. Macromol., 2023, 235, 123821 CrossRef CAS PubMed.
- E. D. Obluchinskaya, O. N. Pozharitskaya, E. V Flisyuk and A. N. Shikov, Mar. Drugs, 2021, 19, 643 Search PubMed.
- A. Bernal-ballen and K. Ozaltin, Polymers, 2019, 11, 1325 CrossRef CAS PubMed.
- Y. Qi, L. Wang, Y. You, X. Sun, C. Wen, Y. Fu and S. Song, Foods, 2022, 11, 822 Search PubMed.
- Z. Zhu, Q. Zhang, L. Chen, S. Ren, P. Xu, Y. Tang and D. Luo, Thromb. Res., 2010, 125, 419–426 CrossRef CAS PubMed.
- S. Kwang, H. Jee and J. Soo, Food Chem. Toxicol., 2012, 50, 4468–4478 CrossRef PubMed.
- J. Jin, D. Yadav, K. Madhwani, N. Puranik and V. Chavda, Molecules, 2022, 27, 6032 CrossRef CAS PubMed.
- F. Atashrazm, R. M. Lowenthal, G. M. Woods, A. F. Holloway and J. L. Dickinson, Mar. Drugs, 2015, 13, 2327–2346 CrossRef CAS PubMed.
- J. H. Fitton, D. N. Stringer and S. S. Karpiniec, Mar. Drugs, 2015, 13, 5920–5946 Search PubMed.
- K. Lee, M. Jeong, S. Choi, S. Na, J. Cha and M. I. C. Mbc, Arch. Oral Biol., 2012, 58, 482–492 CrossRef PubMed.
- V. E. Suprunchuk, Carbohydr. Res., 2019, 485 Search PubMed.
- J. H. Park, S. H. Choi, S. J. Park, Y. J. Lee, J. H. Park, P. H. Song, C. M. Cho, S. K. Ku and C. H. Song, Mar. Drugs, 2017, 15, 112 Search PubMed.
- Y. Wang, Q. Wang, X. Han, Y. Ma and Z. Zhang, Food Funct., 2021, 12, 3820–3830 RSC.
- W. Zhang, J. Du, Z. Jiang, T. Okimura, T. Oda, Q. Yu and J. Jin, Mar. Drugs, 2014, 12, 4148–4164 CrossRef PubMed.
- S. N. Akayasu, R. S. Oegima, K. Y. Amaguchi and T. O. Da, Biosci., Biotechnol., Biochem., 2009, 73, 961–964 Search PubMed.
- S. Frazzini, E. Scaglia, M. D. Anno, S. Reggi, D. Lanzoni, C. Angelo, S. Rossi, L. Rossi, S. Panseri and C. Giromini, Antioxidants, 2022, 11, 992 Search PubMed.
- W. Zhang, T. Okimura, T. Oda and J. Jin, Mar. Drugs, 2019, 17, 197 Search PubMed.
- Y. Qiu, H. Jiang, L. Fu, F. Ci and X. Mao, Food Chem., 2021, 349, 129209 CrossRef CAS PubMed.
- Z. Zhang, Q. Zhang, J. Wang, H. Song, H. Zhang and X. Niu, Carbohydr. Polym., 2010, 79, 1124–1129 CrossRef CAS.
- A. Basu, K. R. Kunduru, E. Abtew and A. J. Domb, Bioconjugate Chem., 2015, 26, 1396–1412 Search PubMed.
- T. Hemamalini, V. Rengaswami and G. Dev, Int. J. Biol. Macromol., 2018, 106, 712–718 CrossRef CAS PubMed.
- P. Mistry, R. Chhabra, S. Muke, A. Narvekar and S. Sathaye, Mater. Sci. Eng., C, 2021, 119, 111316 CrossRef CAS PubMed.
- P. Wadke, R. Chhabra, R. Jain and P. Dandekar, Surf. Interfaces, 2017, 8, 137–146 Search PubMed.
- K. Zeng, T. Groth and K. Zhang, ChemBioChem, 2019, 20 Search PubMed.
- G. Liu, G. Borjihan, H. Baigude, H. Nakashima and T. Uryu, Polym. Adv. Technol., 2003, 14, 471–476 Search PubMed.
- J. Li, Z. Yang, T. Ding, Y. Song, H. Li, D. Li, S. Chen and F. Xu, Carbohydr. Polym., 2022, 276, 118789 Search PubMed.
- S. Groult, S. Buwalda and T. Budtova, Eur. Polym. J., 2021, 149, 110386 Search PubMed.
- A. S. Soubhagya, A. Moorthi and M. Prabaharan, Int. J. Biol. Macromol., 2020, 157, 135–145 CrossRef CAS PubMed.
- K. Song, Y. Hao, Y. Liu, R. Cao, X. Zhang, S. He, J. Wen, W. Zheng, L. Wang and Y. Zhang, Carbohydr. Polym., 2023, 300, 120272 CrossRef CAS PubMed.
- M. Hu, X. Peng, S. Shi, C. Wan, C. Cheng, N. Lei and X. Yu, J. Mater. Chem. B, 2022, 10, 8218–8234 Search PubMed.
- J. Yang and S. Wang, Gels, 2023, 9, 138 CrossRef CAS PubMed.
- H. Li, Y. Wang, P. Zhao, L. Guo, L. Huang, X. Li and W. Gao, Carbohydr. Polym., 2023, 313, 120746 CrossRef CAS PubMed.
- S. A. Ganie, L. J. Rather and Q. Li, J. Polym. Environ., 2023, 31, 13–35 CrossRef CAS.
- B. Zhang, D. Shi, M. Li, F. Shi and L. Chi, Carbohydr. Polym., 2023, 301, 120303 CrossRef CAS PubMed.
- E. R. S. Kenawy, E. A. Kamoun, M. S. Eldin, H. M. A. Soliman, S. H. EL-Moslamy, E. M. El-Fakharany and A. baset M. Shokr, Arabian J. Sci. Eng., 2023, 48, 205–222 CrossRef CAS.
- R. Nicu, D. E. Ciolacu, A. Petrovici, D. Rusu, M. Avadanei, A. C. Mihaila, E. Butoi and F. Ciolacu, Int. J. Mol. Sci., 2023, 24, 4213 CrossRef CAS PubMed.
- N. A. Pyataev, P. S. Petrov, O. V Minaeva, M. N. Zharkov, O. A. Kulikov, A. V Kokorev, E. P. Brodovskaya, I. A. Yurlov, I. V Syusin, A. V Zaborovskiy and L. A. Balykova, Polymers, 2019, 11, 921 CrossRef CAS PubMed.
- N. Desai, D. Rana, S. Salave, R. Gupta, P. Patel and B. Karunakaran, Pharmaceuticals, 2023, 15, 1313 CrossRef CAS PubMed.
- Y. Kong, W. Zhang, T. He, X. Yang, W. Bi, J. Li, W. Yang and W. Chen, Carbohydr. Polym., 2023, 304, 120485 CrossRef CAS PubMed.
- T. Thi, T. Nguyen, N. Thi, K. Tran and T. Quoc, Alexandria Eng. J., 2023, 69, 419–430 CrossRef.
- L. Zhao, Z. Feng, Y. Lyu, J. Yang, L. Lin, H. Bai, Y. Li, Y. Feng and Y. Chen, Int. J. Biol. Macromol., 2023, 230, 123231 CrossRef CAS PubMed.
- Q. Yu, Y. Yan, J. Huang, Q. Liang, J. Li, B. Wang, B. Ma, A. Bianco, S. Ge and J. Shao, Int. J. Biol. Macromol., 2023, 231, 123149 CrossRef CAS PubMed.
- A. Smola-dmochowska, K. Lewicka, A. Macyk and P. Rychter, Int. J. Mol. Sci., 2023, 24, 7473 CrossRef CAS PubMed.
- S. Tiwari and P. Bahadur, Int. J. Biol. Macromol., 2019, 121, 556–571 CrossRef CAS PubMed.
- A. Sionkowska and M. Gadomska, Molecules, 2020, 25, 4035 CrossRef CAS PubMed.
- N. Kim, P. Chandika, S. Kim, D. Won, W. Sun, I. Choi, S. Gil, Y. Kim and W. Jung, Polymers, 2023, 271, 125808 CrossRef CAS.
- Y. Hussein, E. M. El-fakharany, E. A. Kamoun, S. A. Loutfy, R. Amin, T. H. Taha, S. A. Salim and M. Amer, Int. J. Biol. Macromol., 2020, 164, 667–676 CrossRef CAS PubMed.
- L. Kang, W. Jia, M. Li, Q. Wang, C. Wang and Y. Liu, Carbohydr. Polym., 2019, 223, 115106 CrossRef CAS PubMed.
- T. Chuang and K. S. Masters, Biomaterials, 2009, 30, 5341–5351 CrossRef CAS PubMed.
- K. Dykas, D. Felkle, K. Karnas, G. Khachatryan, J. Duli and A. Karewicz, Materials, 2022, 15, 234 Search PubMed.
- N. Abdullah Al, V. Ignjatovic, P. Monagle, J. Tsanaktsidis, G. Vamvounis and V. Ferro, Biomacromolecules, 2020, 21, 1009–1021 CrossRef PubMed.
- A. Vitiello and F. Ferrara, Cardiovasc. Drugs Ther., 2023, 37, 277–281 CrossRef CAS PubMed.
- Y. Hata, H. Miyazaki, M. Ishihara and S. Nakamura, Polymers, 2022, 14, 932 CrossRef CAS PubMed.
- A. Nawaz, S. Z. Safi, S. Sikandar, R. Zeeshan, S. Zulfiqar, N. Mehmood, H. M. Alobaid, F. Rehman, M. Imran, M. Tariq, A. Ali, T. Bin Emran and M. Yar, Materials, 2022, 15, 6683 CrossRef CAS PubMed.
- C. He, Z. Shi, L. Ma, C. Cheng, C. Nie, M. Zhou and C. Zhao, J. Mater. Chem. B, 2015, 3, 592–602 RSC.
- J. Lee, S. Yang, J. Lee, H. Lim, S. Lee, T. Kang, J. Lim, Y. J. Kim and J. Park, Carbohydr. Polym., 2023, 120930 CrossRef CAS PubMed.
- R. J. Warth and W. G. Rodkey, Arthrosc. – J. Arthrosc. Relat. Surg., 2015, 31, 927–941 CrossRef PubMed.
- A. Mandal, S. Panigrahi and C. Zhang, Biol. Eng. Trans., 2010, 2, 63–88 Search PubMed.
- A.-M. Gaspar-Pintiliescu, A. Stanciuc and O. Craciunescu, Int. J. Biol. Macromol., 2019, 138, 854–865 CrossRef CAS PubMed.
- Y. Xiaoyue, C. Tang, Q. Yuan, Q. Yuan, Z. Gu, Z. Li and Y. Hu, Curr. Org. Chem., 2016, 20, 1797–1812 CrossRef.
- X. Wang, W. Xiaoqin, L. Ren, T. Qiang, P. Guo and F. Zhang, J. Soc. Leather Technol. Chem., 2015, 99, 216–222 Search PubMed.
- S. Mitura, A. Sionkowska and A. Jaiswal, J. Mater. Sci.: Mater. Med., 2020, 31, 1–14 CrossRef PubMed.
- E. E. Antoine, P. P. Vlachos and M. N. Rylander, Tissue Eng., Part B, 2014, 20, 683–692 CrossRef CAS PubMed.
- Z. Bazrafshan and G. K. Stylios, Int. J. Biol. Macromol., 2019, 129, 693–705 CrossRef CAS PubMed.
- M. F. M. Busra and Y. Lokanathan, Curr. Pharm. Biotechnol., 2019, 20, 992–1003 Search PubMed.
- F. Pallaske, A. Pallaske, K. Herklotz and J. Boese-Landgraf, J. Wound Care, 2018, 27, 692–702 CrossRef PubMed.
- Q. Taotao, L. Xiaoning, R. Longfang and W. Xuechuan, J. Soc. Leather Technol. Chem., 2016, 100, 109–113 Search PubMed.
- M. C. Gomez-Guillen, B. Gimenez, M. E. Lopez-Caballero and M. P. Montero, Food Hydrocolloids, 2011, 25, 1813–1827 CrossRef CAS.
- A. Sionkowska, S. Skrzyński, K. Śmiechowski and A. Kołodziejczak, Polym. Adv. Technol., 2017, 28, 4–9 CrossRef CAS.
- M. I. Avila Rodríguez, L. G. Rodríguez Barroso and M. L. Sánchez, J. Cosmet., Dermatol., 2018, 17, 20–26 CrossRef PubMed.
- L. B. Dyksterhuis, C. Baldock, D. Lammie, T. J. Wess and A. S. Weiss, Matrix Biol., 2006, 25, S17 CrossRef.
- A. J. Ryan and F. J. O'Brien, Biomaterials, 2015, 73, 296–307 CrossRef CAS PubMed.
- J. Jimenez Vazquez and E. San Martín Martínez, J. Mater. Res., 2019, 34, 2819–2827 CrossRef CAS.
- P. Pal, Q. C. Nguyen, A. H. Benton, M. E. Marquart and A. V. Janorkar, Macromol. Biosci., 2019, 19, 1–13 CrossRef.
- D. V. Bax, H. E. Smalley, R. W. Farndale, S. M. Best and R. E. Cameron, Acta Biomater., 2019, 86, 158–170 CrossRef CAS PubMed.
- D. Miranda-Nieves and E. L. Chaikof, ACS Biomater. Sci. Eng., 2017, 3, 694–711 CrossRef CAS PubMed.
- M.-D. Niculescu, D.-G. Epure, M. Lasoń-Rydel, C. Gaidau, M. Gidea and C. Enascuta, Eurobiotech J., 2019, 3, 160–166 CrossRef.
- S. Balaji, R. Kumar, R. Sripriya, U. Rao, A. Mandal, P. Kakkar, P. N. Reddy and P. K. Sehgal, Polym. Adv. Technol., 2012, 23, 500–507 CrossRef CAS.
- Q. Lv, Q. Feng, K. Hu and F. Cui, Polymers, 2005, 46, 12662–12669 CrossRef CAS.
- S. Sofia, M. B. McCarthy, G. Gronowicz and D. L. Kaplan, J. Biomed. Mater. Res., 2001, 54, 139–148 CrossRef CAS PubMed.
- J. Péreź-Rigueiro, C. Viney, J. Llorca and M. Elices, J. Appl. Polym. Sci., 1998, 70, 2439–2447 CrossRef.
- N. Vachiraroj, J. Ratanavaraporn, S. Damrongsakkul, R. Pichyangkura, T. Banaprasert and S. Kanokpanont, Int. J. Biol. Macromol., 2009, 45, 470–477 CrossRef CAS PubMed.
- A. R. Murphy and D. L. Kaplan, J. Mater. Chem., 2009, 19, 6443–6450 RSC.
- T. P. Nguyen, Q. V. Nguyen, V. H. Nguyen, T. H. Le, V. Q. N. Huynh, D. V. N. Vo, Q. T. Trinh, S. Y. Kim and Q. Van Le, Polymers, 2019, 11, 1–25 CAS.
- P. G. Chao, S. Yodmuang, X. Wang, L. Sun, D. L. Kaplan and G. Vunjak-Novakovic, J. Biomed. Mater. Res., Part B, 2010, 95, 84–90 CrossRef PubMed.
- Y. Wang, H. J. Kim, G. Vunjak-Novakovic and D. L. Kaplan, Biomaterials, 2006, 27, 6064–6082 CrossRef CAS PubMed.
- U. J. Kim, J. Park, C. Li, H. J. Jin, R. Valluzzi and D. L. Kaplan, Biomacromolecules, 2004, 5, 786–792 CrossRef CAS PubMed.
- Y. Yu, S. Hua, M. Yang, Z. Fu, S. Teng, K. Niu, Q. Zhao and C. Yi, RSC Adv., 2016, 6, 110557–110565 RSC.
- N. Kasoju and U. Bora, Adv. Healthcare Mater., 2012, 1, 393–412 CrossRef CAS PubMed.
- D. Jao, X. Mou and X. Hu, J. Funct. Biomater., 2016, 7, 22 CrossRef PubMed.
- K. M. Babu, in Woodhead Publishing Series in Textiles, Elsevier Ltd, Amsterdam, The Netherlands, 2nd edn, 2019, pp. 129–142 Search PubMed.
- S. M. Yukseloglu, N. Sokmen and S. Canoglu, Microelectron. Eng., 2015, 146, 43–47 CrossRef CAS.
- K. Numata and D. L. Kaplan, Adv. Drug Delivery Rev., 2010, 62, 1497–1508 CrossRef CAS PubMed.
- S. Grabska-zielińska and A. Sionkowska, Materials, 2021, 14, 1510 CrossRef PubMed.
- L. Liu, S. Zhang and J. Y. Huang, Sci. China: Technol. Sci., 2019, 62, 919–930 CrossRef CAS.
- D. Chouhan and B. B. Mandal, Acta Biomater., 2020, 103, 24–51 CrossRef CAS PubMed.
- A. Tuwalska, S. Grabska-Zielińska and A. Sionkowska, Polymers, 2022, 14, 1343 CrossRef CAS PubMed.
- A. Sionkowska, Prog. Polym. Sci., 2021, 122, 101452 CrossRef CAS.
- M. Fazley Elahi, G. Guan and L. Wang, Rev. Adv. Mater. Sci., 2014, 38, 148–159 Search PubMed.
- F. P. Seib, M. Herklotz, K. A. Burke, M. F. Maitz, C. Werner and D. L. Kaplan, Biomaterials, 2014, 35, 83–91 CrossRef CAS PubMed.
- M. Antunes-ricardo, P. Martínez-morales and G. Rivera-hern, Int. J. Pharm., 2021, 600, 120478 CrossRef PubMed.
- T. Lu, Q. Zou, K. Zhu, D. Yuan, M. Ma and C. Ye, J. Polym. Res., 2021, 28, 1–15 CrossRef.
- W. Yang, E. Fortunati, F. Bertoglio, J. S. Owczarek, G. Bruni, M. Kozanecki and J. M. Kenny, Carbohydr. Polym., 2018, 181, 275–284 CrossRef CAS PubMed.
- M. P. Sudhakar and P. Bargavi, Bioresour. Technol. Rep., 2023, 21, 101344 CrossRef CAS.
- M. Kurakula and G. S. N. K. Rao, J. Drug Delivery Sci. Technol., 2020, 60, 102046 CrossRef CAS PubMed.
- J. Zhang, K. Chen, C. Ding, S. Sun, Y. Zheng, Q. Ding, B. Hong and W. Liu, Int. J. Biol. Macromol., 2022, 206, 591–604 CrossRef CAS PubMed.
- M. Contardi, M. Summa, P. Picone, O. R. Brancato, M. Di Carlo, R. Bertorelli and A. Athanassiou, Pharmaceutics, 2022, 14, 483 CrossRef CAS PubMed.
- D. Khodabakhshi, A. Eskandarinia, A. Kefayat and M. Ra, Colloids Surf., B, 2019, 178, 177–184 CrossRef CAS PubMed.
- A. Hamid Rather, R. Saleem, T. Umair, M. Rafiq, A. H. Jadhav, P. M. Srinivasappa, A. Abdal-hay, P. Sultan, S. Rather, J. Macossay and F. A. Sheikh, Int. J. Biol. Macromol., 2023, 226, 690–705 CrossRef PubMed.
- H. Nguyen, N. Nguyen, N. Tran, T. Nguyen and Q. Nguyen, Mater. Lett., 2022, 320, 132403 CrossRef CAS.
- P. Li, W. Cai, K. Wang, L. Zhou, S. Tang, Y. Zhao, X. Li and J. Wang, Smart Mater. Med., 2022, 3, 361–373 CrossRef.
- L. Rusu, L. C. Ardelean, A. Jitariu, C. A. Miu and C. G. Streian, Polymers, 2020, 12, 1197 CrossRef CAS PubMed.
- N. Barnthip, J. Teeka, P. Kantha, S. Teepoo and W. Damjuti, Sci. Rep., 2022, 12, 22370 CrossRef CAS PubMed.
- A. Chanda, J. Adhikari, A. Ghosh, S. Roy, S. Thomas, P. Datta and P. Saha, Int. J. Biol. Macromol., 2018, 116, 774–785 CrossRef CAS PubMed.
- T. G. P. Galindo, Y. Chai and M. Tagaya, J. Nanomater., 2019, 6495239 CAS.
- D. L. Elbert and J. A. Hubbell, Annu. Rev. Mater. Sci., 1996, 26, 365–394 CrossRef CAS.
- J. Kuchinka, C. Willems, D. V. Telyshev and T. Groth, Bioengineering, 2021, 8, 215 CrossRef CAS PubMed.
- L. C. Xu, J. W. Bauer and C. A. Siedlecki, Colloids Surf., B, 2014, 124, 49–68 CrossRef CAS PubMed.
- M. Gorbet, C. Sperling, M. F. Maitz, C. A. Siedlecki, C. Werner and M. V. Sefton, Acta Biomater., 2019, 94, 25–32 CrossRef CAS PubMed.
- W. C. Schrottmaier, M. Mussbacher, M. Salzmann and A. Assinger, Atherosclerosis, 2020, 307, 109–120 CrossRef CAS PubMed.
- P. Mazzarello, A. L. Calligaro and A. Calligaro, Nat. Rev. Mol. Cell Biol., 2001, 2, 776–781 CrossRef CAS PubMed.
- J. N. Kizhakkedathu and E. M. Conway, Blood, 2022, 139, 1987–1998 CrossRef CAS PubMed.
- J. Gao, L. Niklason and R. Langer, J. Biomed. Mater. Res., 1998, 42(3), 417–424 CrossRef CAS.
- K. R. Milner and C. A. Siedlecki, J. Biomed. Mater. Res., Part A, 2007, 82, 80–91 CrossRef PubMed.
- Z. Zhou, P. Yu, H. M. Geller and C. K. Ober, Biomacromolecules, 2013, 14, 529–537 CrossRef CAS PubMed.
- R. M. Sabino, K. Kauk, L. Y. C. Madruga, M. J. Kipper, A. F. Martins and K. C. Popat, J. Biomed. Mater. Res., Part A, 2020, 108, 992–1005 CrossRef CAS PubMed.
- C. Xu, F. Yang, S. Wang and S. Ramakrishna, J. Biomed. Mater. Res., Part A, 2004, 71, 154–161 CrossRef PubMed.
- V. A. DePalma, R. E. Baier, J. W. Ford, V. L. Gott and A. Furuse, J. Biomed. Mater. Res., 1972, 6, 37–75 CrossRef CAS PubMed.
- F. Nazneen, G. Herzog, D. W. M. Arrigan, N. Caplice, P. Benvenuto, P. Galvin and M. Thompson, J. Biomed. Mater. Res., Part B, 2012, 100, 1989–2014 CrossRef PubMed.
- D. K. Owens and R. C. Wendt, J. Appl. Polym. Sci., 1969, 13, 1741–1747 CrossRef CAS.
- J. Comelles, M. Estévez, E. Martínez and J. Samitier, Nanomedicine, 2010, 6, 44–51 CrossRef CAS PubMed.
- Y. Ikada, M. Suzuki and Y. Tamada, Polymers as Biomaterials, 1984, pp. 135–147 Search PubMed.
- E. J. Brisbois, H. Handa, M. E. Meyerhoff, E. J. Brisbois, H. Handa and M. E. Meyerhoff, Advanced Polymers in Medicine, 2015, pp. 481–511 Search PubMed.
- A. Krishnan, P. Cha, Y. H. Liu, D. Allara and E. A. Vogler, Biomaterials, 2006, 27, 3187–3194 CrossRef CAS PubMed.
- J. D. Andrade and V. Hlady, Adv. Polym. Sci., 1987, 1–63 Search PubMed.
- J. V. Edwards and N. Prevost, J. Funct. Biomater., 2011, 2, 391–413 CrossRef CAS PubMed.
- K. Vinisha Rani, B. Sarma and A. Sarma, Diamond Relat. Mater., 2018, 84, 77–85 CrossRef CAS.
- K. N. Pandiyaraj and V. Selvarajan, J. Mater. Process. Technol., 2008, 199, 130–139 CrossRef CAS.
- S. Dan Dimitrijevich, M. Tatarko, R. W. Gracy, C. B. Linsky and C. Olsen, Carbohydr. Res., 1990, 195, 247–256 CrossRef PubMed.
- K. M. Lewis, D. Spazierer, M. D. Urban, L. Lin, H. Redl and A. Goppelt, Eur. J. Surg., 2013, 45, 213–220 CrossRef CAS PubMed.
- G. F. Picheth, C. L. Pirich, M. R. Sierakowski, M. A. Woehl, C. N. Sakakibara, C. F. de Souza, A. A. Martin, R. da Silva and R. A. de Freitas, Int. J. Biol. Macromol., 2017, 104, 97–106 CrossRef CAS PubMed.
- W. Czaja, A. Krystynowicz, S. Bielecki and R. M. Brown, Biomaterials, 2006, 27, 145–151 CrossRef CAS PubMed.
- A. S. Fragoso, M. B. Silva, C. P. De Melo, J. L. A. Aguiar, C. G. Rodrigues, P. L. De Medeiros, J. F. Branco Junior, C. A. S. Andrade and M. D. L. Oliveira, J. Mater. Sci.: Mater. Med., 2014, 25, 229–237 CrossRef CAS PubMed.
- M. T. de Lucena, M. R. de Melo Júnior, M. M. de Melo Lira, C. M. M. B. de Castro, L. A. Cavalcanti, M. A. de Menezes, F. C. M. Pinto and J. L. de Andrade Aguiar, J. Mater. Sci.: Mater. Med., 2015, 26, 1–6 CrossRef PubMed.
- F. C. A. Silveira, F. C. M. Pinto, S. S. Caldas Neto, M. C. Leal, J. Cesário and J. L. A. Aguiar, Braz. J. Otorhinolaryngol, 2016, 82, 203–208 CrossRef PubMed.
- D. S. A. Hickman, C. L. Pawlowski, U. D. S. Sekhon, J. Marks and A. Sen Gupta, Adv. Mater., 2018, 30, 1700859 CrossRef PubMed.
- D. M. Albala, Cardiovasc. Surg., 2003, 11, 5–11 CrossRef PubMed.
- S. P. Mandell and N. S. Gibran, Expert Opin. Biol. Ther., 2014, 14, 821–830 CrossRef PubMed.
- Tisseel [Fibrin Sealant]|Baxter, https://www.baxter.com/healthcare-professionals/surgical-care/tisseel-fibrin-sealant, accessed 4 September 2023.
- Artiss Fibrin Sealant|Baxter Healthcare, https://advancedsurgery.baxter.com/artiss, accessed 4 September 2023.
- L. Z. Wang, J. Gorlin, S. E. Michaud, P. A. Janmey, R. P. Goddeau, R. Kuuse, R. Uibo, D. Adams and E. S. Sawyer, Thromb. Res., 2000, 100, 537–548 CrossRef CAS PubMed.
- S. E. Michaud, L. Z. Wang, N. Korde, R. Bucki, P. K. Randhawa, J. J. Pastore, H. Falet, K. Hoffmeister, R. Kuuse, R. Uibo, J. Herod, E. Sawyer and P. A. Janmey, Thromb. Res., 2002, 107, 245–254 CrossRef CAS PubMed.
- M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 938–958 CrossRef PubMed.
- M. Moroi and S. M. Jung, Thromb. Haemostasis, 1997, 78, 439–444 CrossRef CAS.
- C. Yang, P. Hillas, J. Tang, J. Balan, H. Notbohm and J. Polarek, J. Biomed. Mater. Res., Part B, 2004, 69, 18–24 CrossRef CAS PubMed.
- W. R. Wagner, J. M. Pachence, J. Ristich and P. C. Johnson, J. Surg. Res., 1996, 66, 100–108 CrossRef CAS PubMed.
- J. B. Zwischenberger, R. L. Brunston, J. R. Swann and V. R. Conti, J. Investig. Surg., 1999, 12, 101–106 CrossRef CAS PubMed.
- R. Hajosch, M. Suckfuell, S. Oesser, M. Ahlers, K. Flechsenhar and B. Schlosshauer, J. Biomed. Mater. Res., Part B, 2010, 94, 372–379 CrossRef PubMed.
- X. Xie, D. Li, Y. Chen, Y. Shen, F. Yu, W. Wang, Z. Yuan, Y. Morsi, J. Wu and X. Mo, Adv. Healthcare Mater., 2021, 10, 2100918 CrossRef CAS PubMed.
- K. Chen, H. Pan, Z. Yan, Y. Li, D. Ji, K. Yun, Y. Su, D. Liu and W. Pan, Int. J. Biol. Macromol., 2021, 182, 1339–1350 CrossRef CAS PubMed.
- B. A. Woodworth, R. K. Chandra, J. D. LeBenger, B. Ilie and R. J. Schlosser, Am. J. Otolaryngol., 2009, 30, 49–53 CrossRef CAS PubMed.
- X. Zhao, J. Gao, X. Hu, H. Guo, F. Wang, Y. Qiao and L. Wang, Appl. Sci., 2018, 8, 1226 CrossRef.
- T. Liu, Z. Zhang, J. Liu, P. Dong, F. Tian, F. Li and X. Meng, Int. J. Biol. Macromol., 2022, 217, 998–1011 CrossRef CAS PubMed.
- S. K. Verma, H. Yaghoobi, L. Kreplak and J. P. Frampton, Adv. Mater. Interfaces, 2023, 10, 2202119 CrossRef CAS.
- B. J. D. Barba, C. T. Aranilla, L. S. Relleve, V. R. C. Cruz, J. R. Vista and L. V. Abad, Radiat. Phys. Chem., 2018, 144, 180–188 CrossRef CAS.
- K. M. Lewis, D. Spazierer, P. Slezak, B. Baumgartner, J. Regenbogen and H. Gulle, J. Biomater. Appl., 2014, 29, 780–788 CrossRef CAS PubMed.
- J. Zhu, F. Li, X. Wang, J. Yu and D. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 13304–13316 CrossRef CAS PubMed.
- Y. F. Zhao, J. Y. Zhao, W. Z. Hu, K. Ma, Y. Chao, P. J. Sun, X. B. Fu and H. Zhang, J. Mater. Chem. B, 2019, 7, 1855–1866 RSC.
- M. Yin, Y. Wang, Y. Zhang, X. Ren, Y. Qiu and T. S. Huang, Carbohydr. Polym., 2020, 232, 115823 CrossRef CAS PubMed.
- R. Shakiba-Marani and H. Ehtesabi, Int. J. Biol. Macromol., 2023, 224, 831–839 CrossRef CAS PubMed.
- H. Zhang, Y. Xu, Y. Lei, X. Wen and J. Liang, Mater. Lett., 2022, 324, 132718 CrossRef CAS.
- F. Zou, Y. Wang, Y. Zheng, Y. Xie, H. Zhang, J. Chen, M. I. Hussain, H. Meng and J. Peng, Bioact. Mater., 2022, 17, 471–487 CAS.
- W. Guo, B. Zhao, M. Shafiq, X. Yu, Y. Shen, J. Cui, Y. Chen, P. Cai, Z. Yuan, M. EL-Newehy, H. EL-Hamshary, Y. Morsi, B. Sun, J. Pan and X. Mo, Regener. Biomater., 2023, 10, rbad019 CrossRef CAS PubMed.
- J. G. Lundin, C. L. McGann, G. C. Daniels, B. C. Streifel and J. H. Wynne, Mater. Sci. Eng., C, 2017, 79, 702–709 CrossRef CAS PubMed.
- X. Liu, Y. Niu, K. C. Chen and S. Chen, Mater. Sci. Eng., C, 2017, 71, 289–297 CrossRef CAS PubMed.
- F. I. Broekema, W. Van Oeveren, J. Zuidema, S. H. Visscher and R. R. M. Bos, J. Mater. Sci.: Mater. Med., 2011, 22, 1081–1086 CrossRef CAS PubMed.
- A. P. H. Huang, D. M. Lai, Y. H. Hsu, H. H. Tsai, C. Y. Su and S. hui Hsu, Mater. Sci. Eng., C, 2021, 121, 111799 CrossRef CAS PubMed.
- B. E. Claessen, J. P. S. Henriques, F. A. Jaffer, R. Mehran, J. J. Piek and G. D. Dangas, JACC: Cardiovascular Interventions, 2014, 7, 1081–1092 CrossRef PubMed.
- R. Reejhsinghani and A. S. Lotfi, Vasc. Health Risk Manage., 2015, 11, 93 Search PubMed.
- T. Gori, A. Polimeni, C. Indolfi, L. Räber, T. Adriaenssens and T. Münzel, Nat. Rev. Cardiol., 2018, 16, 243–256 CrossRef PubMed.
- D. S. Ong and I. K. Jang, Nat. Rev. Cardiol., 2015, 12, 325–336 CrossRef PubMed.
- I. H. Jaffer, J. C. Fredenburgh, J. Hirsh and J. I. Weitz, J. Thromb. Haemostasis, 2015, 13, S72–S81 CrossRef PubMed.
- P. Qi, M. F. Maitz and N. Huang, Surf. Coat. Technol., 2013, 233, 80–90 CrossRef CAS.
- K. Christensen, R. Larsson, H. Emanuelsson, G. Elgue and A. Larsson, Biomaterials, 2001, 22, 349–355 CrossRef CAS PubMed.
- Z. Zha, Y. Ma, X. Yue, M. Liu and Z. Dai, Appl. Surf. Sci., 2009, 256, 805–814 CrossRef CAS.
- Z. Yang, J. Wang, R. Luo, M. F. Maitz, F. Jing, H. Sun and N. Huang, Biomaterials, 2010, 31, 2072–2083 CrossRef CAS PubMed.
- K. Kottke-Marchant, J. M. Anderson, Y. Umemura and R. E. Marchant, Biomaterials, 1989, 10, 147–155 CrossRef CAS PubMed.
- S. W. Jordan and E. L. Chaikof, J. Vasc. Surg., 2007, 45, A104–A115 CrossRef PubMed.
- B. O'Brien, H. Zafar, A. Ibrahim, J. Zafar and F. Sharif, Ann. Biomed. Eng., 2016, 44, 523–535 CrossRef PubMed.
- T. W. Chuang and K. S. Masters, Biomaterials, 2009, 30, 5341–5351 CrossRef CAS PubMed.
- S. Verheye, C. P. Markou, M. Y. Salame, B. Wan, S. B. King, K. A. Robinson, N. A. F. Chronos and S. R. Hanson, Arterioscler., Thromb., Vasc. Biol., 2000, 20, 1168–1172 CrossRef CAS PubMed.
- W. Jia, L. Liu, M. Li, Y. Zhou, H. Zhou, H. Weng, G. Gu, M. Xiao and Z. Chen, Acta Biomater., 2022, 153, 287–298 CrossRef CAS PubMed.
- K. S. Lavery, C. Rhodes, A. Mcgraw and M. J. Eppihimer, Adv. Drug Delivery Rev., 2017, 112, 2–11 CrossRef CAS PubMed.
- N. P. Rhodes, D. J. Wilson and R. L. Williams, Biomaterials, 2007, 28, 4561–4570 CrossRef CAS PubMed.
- G. Mani, M. D. Feldman, D. Patel and C. M. Agrawal, Biomaterials, 2007, 28, 1689–1710 CrossRef CAS PubMed.
- S. L. Chin-Quee, S. H. Hsu, K. L. Nguyen-Ehrenreich, J. T. Tai, G. M. Abraham, S. D. Pacetti, Y. F. Chan, G. Nakazawa, F. D. Kolodgie, R. Virmani, N. N. Ding and L. A. Coleman, Biomaterials, 2010, 31, 648–657 CrossRef CAS PubMed.
- T. C. Major, D. O. Brant, M. M. Reynolds, R. H. Bartlett, M. E. Meyerhoff, H. Handa and G. M. Annich, Biomaterials, 2010, 31, 2736–2745 CrossRef CAS PubMed.
- M. Szycher, J. Biomater. Appl., 1988, 3, 297–402 CrossRef CAS PubMed.
- Y. Feng, H. Zhao, M. Behl, A. Lendlein, J. Guo and D. Yang, J. Mater. Sci.: Mater. Med., 2013, 24, 61–70 CrossRef CAS PubMed.
- T. Peng, P. Gibula, K. De Yao and M. F. A. Goosen, Biomaterials, 1996, 17, 685–694 CrossRef CAS PubMed.
- A. B. Fontaine, K. Koelling, J. Clay, D. G. Spigos, S. Dos Passos, G. Christoforidis, G. Hinkle, T. Hill, J. Cearlock and R. Pozderac, J. Vasc. Interv. Radiol., 1994, 5, 567–572 CrossRef CAS PubMed.
- A. De Mel, K. Chaloupka, Y. Malam, A. Darbyshire, B. Cousins and A. M. Seifalian, J. Biomed. Mater. Res., Part A, 2012, 100, 2348–2357 CrossRef PubMed.
- M. Tanaka, T. Motomura, M. Kawada, T. Anzai, Y. Kasori, T. Shiroya, K. Shimura, M. Onishi and A. Mochizuki, Biomaterials, 2000, 21, 1471–1481 CrossRef CAS PubMed.
- E. J. Brisbois, H. Handa, M. E. Meyerhoff, E. J. Brisbois, · H. Handa and M. E. Meyerhoff, Advanced Polymers in Medicine, 2015, pp. 481–511 Search PubMed.
- C. M. Lim, M. X. Li and Y. K. Joung, Adv. Exp. Med. Biol., 2020, 1250, 189–198 CrossRef CAS PubMed.
Footnote |
| † These authors have contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2024 |