Open Access Article
Open Access ArticleSynthesis of new DOPO derivatives and investigation of their synergistic effect with APP–PEI on the flame retardancy of epoxy composite†
Cong Trinh Duc a,
Linh Chi Nguyena,
Phuc Ban Vana,
Ha Thanh Nguyen
a,
Linh Chi Nguyena,
Phuc Ban Vana,
Ha Thanh Nguyen ab,
Tuyet Anh Dang Thi
ab,
Tuyet Anh Dang Thi ab,
Giang Le-Nhat-Thuy
ab,
Giang Le-Nhat-Thuy ab,
Quynh Giang Nguyen Thi
ab,
Quynh Giang Nguyen Thi a,
Phuong Hoang Thia,
Tuan Anh Nguyen
a,
Phuong Hoang Thia,
Tuan Anh Nguyen a,
Quang Vinh Tran
a,
Quang Vinh Tran ab,
Hung Tran Quang
ab,
Hung Tran Quang ab,
Mai Ha Hoang
ab,
Mai Ha Hoang ab and
Tuyen Nguyen Van
ab and
Tuyen Nguyen Van *ab
*ab
aInstitute of Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam. E-mail: ngvtuyen@hotmail.com; ngvtuyen@ich.vast.vn
bGraduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
First published on 9th February 2024
Abstract
Epoxy resin has been extensively used in many industrial and daily applications due to its unique properties. However, the high flammability of epoxy has limited its further development. DOPO derivatives, which are organophosphorus compounds, are highly effective components of flame retardant epoxy composites due to their good compatibility with the resin and their lower toxicity compared to halogenated compounds. This study synthesized sixteen new DOPO derivatives, characterizing their chemical structures with NMR spectroscopy. The combination of synthesized DOPO derivatives and APP–PEI (ammonium polyphosphate–polyethyleneimine) has shown a synergistic effect on enhancing the flame retardancy of epoxy resin with the UL-94 V-0 rating and the LOI value of 28.6%. Moreover, the epoxy composites displayed relatively high mechanical performance with the impact strength of 26–28 kJ m−2.
1 Introduction
Epoxy resin is classified as a thermosetting polymer and has found widespread use in many industrial and daily applications with some strong points such as good mechanical strength, chemical, moisture and corrosion resistance, versatile formulation and cost-effectiveness.1 However, it is noteworthy that epoxy exhibits high flammability and emits significant amounts of heat and smoke when exposed to flames.2 Consequently, flame retardants for epoxy resin are of the most significant concern.Halogen-based flame retardants possess excellent flame retardancy, but at present, they have been prohibited in some countries due to their toxicity towards human health and the environment.3 Organophosphorus-based flame retardants have proved to be promising alternatives. A typical example is DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide), which is widely utilized as a flame retardant in commercial applications.2 Nevertheless, DOPO can react with epoxy resin.4 In order to get comparable results with that obtained with halogen-based flame retardants, a large amount of DOPO is necessary.5 However, it should be noted that the presence of DOPO in epoxy resin has been found to have damaging impacts on its mechanical properties.5 As a result, scientists employ the DOPO derivatives that exhibit synergistic effects, leading to a reduction in the amount of flame retardant necessary.4,6–10 The achieved results were beneficial: by incorporating flame retardant chemicals at a concentration of less than 10 wt%, the epoxy resin composite successfully met the requirements of the UL-94 V-0 test. Additionally, in numerous cases, the composite exhibited a limiting oxygen index (LOI) above 30%. For instance, Chuanbai Yu et al. reported a novel compound named DDPPM which enhanced the self-extinguish properties of epoxy resin.8 Other DOPO-based compounds in Zaisheng Cai's study were incorporated to epoxy resin at 10 wt%, the modified epoxy resin displayed V-0 rating and LOI value of 36.1%.11 When epoxy resin was treated with 4.7 wt% DOP-DDM, V-0 rating and 33.5 LOI value were achieved.12 A DOPO derivative from the study of Shi-bin Nie et al. was employed as flame retardant for epoxy composite. The results showed a significant improvement in LOI rating (30.0%) with 5 wt% of the flame retardant, while the peak heat release rate, total heat release, and total smoke production decreased by 25.1, 18.7 and 13.9%, respectively.4
The flame resistance of APP–PEI (ammonium polyphosphate–polyethyleneimine) when combined with epoxy resin was investigated and reported by Wang et al. in 2016.13,14 With 15 wt% of APP–PEI, the epoxy resin qualified for V-0 by UL-94 test and the LOI value reached 29.5%, indicating a high resistance to combustion. Notably, no dripping was seen during the test. Furthermore, there was a significant decrease in both the total heat release and total smoke production, with reductions of 76.1% and 70.5%, respectively. Songqi Ma et al. studied the synergistic effect of APP and three different organophosphorus derivatives to improve the flame retardancy of epoxy resin.15 In order to achieve a V-0 rating in the UL-94 test, a large amount of ammonium polyphosphate (APP) was required (15 wt%). However, the high concentration of APP resulted in a reduction of the performance of epoxy resin. By employing this combination, the loading of APP was decreased to a range of 7–9 wt% while maintaining the mechanical properties of the materials.
Herein, our study presented a variety of DOPO derivatives, which were examined in combination with APP–PEI, focusing on their flame retardant properties. The chemical structures of these compounds were analyzed using 1H and 13C-NMR spectra. The flame retardancy of the epoxy resin was determined via the UL-94 vertical burning test and limiting oxygen index test. The mechanical stability of the epoxy composites was examined based on the Izod impact strength test.
2 Results and discussion
2.1. Synthesis of DOPO derivatives
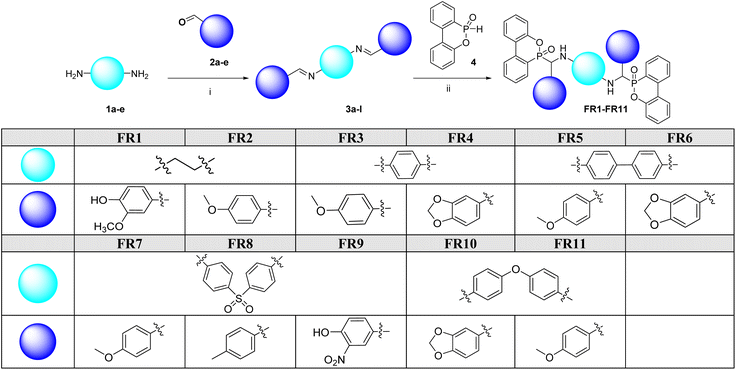
A two-step Pudovik reaction was applied to achieve compound FR1–FR11 (Scheme 1). In the first step, diamines (1a–e) reacted with aromatic aldehydes (2a–e) to yield the imine intermediates (3a–l). The intermediates were supplied to reactions with DOPO to achieve compounds FR1–FR11.Compounds FR12–FR14 were synthesized using the phospha-Michael addition method, employing dimaleimides (6a–c) and DOPO as reactants (Scheme 2). The product was obtained within the range of good to excellent yield.
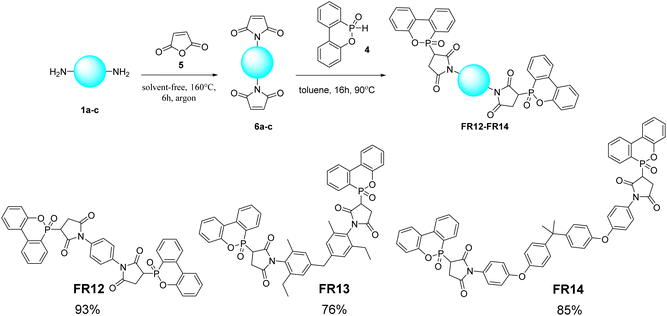 | ||
| Scheme 2 General synthesis process and structure of compounds FR12–FR14. Reagents and conditions: 6a–6c (1 eq.), DOPO (2 eq.), toluene, 16 hours, 90 °C. | ||
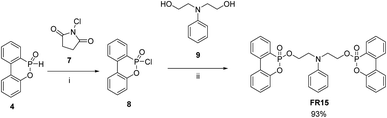
The synthesis of FR15 involves the chlorination of DOPO in toluene utilizing N-chlorosuccinimide, subsequently followed by the reaction of the resulting DOPO–Cl 8 with 2,2′-(phenylimino)diethanol 9 in THF, catalyzed by triethylamine (Scheme 3).
FR16 was achieved via a hydrophosphination reaction of alkynes using DOPO 4 and 3,5-dimethylhex-1-yn-3-ol 10 as reactants (Scheme 4). The reaction was carried out under conditions with the presence of oxygen and heating at a temperature of 100 °C for 22 hours. The product is then purified to obtain FR16 in 45%.
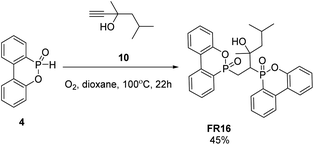 | ||
| Scheme 4 Synthesis process and structure of compound FR16. Reagents and conditions: 4 (3 eq.), 10 (1 eq.), O2, dioxane, 100 °C, 22 hours, 45%. | ||
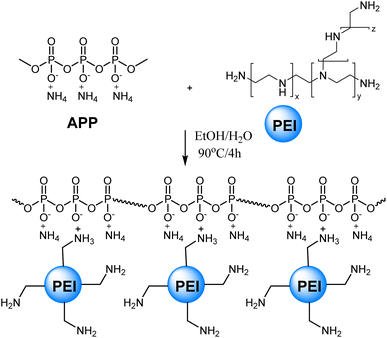
2.2. Synthesis of APP–PEI
APP–PEI was synthesized via reaction of PEI and APP in an EtOH/H2O solvent mixture under continuous nitrogen flow (Scheme 5).2.3. Flammability and mechanical strength
Insufficient flame retardancy was achieved for EP/10FR11 by employing a 10 wt% FR11, an organic flame retardant DOPO derivative. It was confirmed by the failure in both UL-94 and LOI tests. Nevertheless, incorporating 5 wt% APP–PEI with 5 wt% of different FRs exhibited a distinct synergistic effect on enhancing flame retardancy. EP/5APP–PEI/5FR3, EP/5APP–PEI/5FR5, EP/5APP–PEI/5FR7, EP/5APP–PEI/5FR9, EP/5APP–PEI/5FR10, and EP/5APP–PEI/5FR11 samples obtained a V-1 rating. Notably, EP/5APP–PEI/5FR10 and EP/5APP–PEI/5FR11 were recognized as self-extinguishing materials due to their exceptional fire resistance, as they achieved a V-0 rating with a LOI value above 28%.
In this study, the DOPO compounds were linked via various bridging groups, including ethylene (FR1 and FR2), phenylene (FR3 and FR4), biphenyl (FR5 and FR6), sulfonyl diphenyl (FR7, FR8, and FR9), and diphenyl ether (FR10 and FR11). Table 1 shows that FR1 and FR2, containing ethylene bridging groups, exhibited insufficient flame retardancy, failing the UL-94 and LOI tests, indicating that the linkage containing ethylene group was not feasible. In contrast, samples with other bridging groups demonstrated improved flame retardancy, with FR10 and FR11, containing O in the bridging group, showing the best performance.
| Entry | Composite samples | t1a (s) | t2b (s) | UL-94 rating | LOI (%) | Impact strength (kJ m−2) |
|---|---|---|---|---|---|---|
| a Average self-extinguishing time after the first ignition.b Average self-extinguishing time after the second ignition.c NQ: not qualified. | ||||||
| 1 | Epoxy resin (EP) | — | — | NQc | 19.2 | 42.18 |
| 2 | EP/10APP–PEI | 18 | 21 | V-1 | 25.8 | 17.76 |
| 3 | EP/10FR11 | 17 | 44 | NQ | — | 38.66 |
| 4 | EP/5APP–PEI/5FR1 | 18 | 37 | NQ | — | 26.14 |
| 5 | EP/5APP–PEI/5FR2 | 19 | 36 | NQ | — | 26.37 |
| 6 | EP/5APP–PEI/5FR3 | 7 | 24 | V-1 | 26.2 | 25.62 |
| 7 | EP/5APP–PEI/5FR4 | 12 | 32 | NQ | — | 25.94 |
| 8 | EP/5APP–PEI/5FR5 | 5 | 19 | V-1 | 27.4 | 27.08 |
| 9 | EP/5APP–PEI/5FR6 | 32 | 26 | NQ | — | 26.43 |
| 10 | EP/5APP–PEI/5FR7 | 6 | 24 | V-1 | 26.0 | 26.72 |
| 11 | EP/5APP–PEI/5FR8 | 9 | 28 | V-1 | 25.6 | 25.87 |
| 12 | EP/5APP–PEI/5FR9 | 8 | 20 | V-1 | 26.2 | 23.34 |
| 13 | EP/5APP–PEI/5FR10 | 4 | 6 | V-0 | 28.4 | 27.84 |
| 14 | EP/5APP–PEI/5FR11 | 3 | 4 | V-0 | 28.6 | 27.32 |
| 15 | EP/5APP–PEI/5FR12 | 28 | 36 | NQ | — | 26.72 |
| 16 | EP/5APP–PEI/5FR13 | 8 | 22 | V-1 | 27.0 | 26.41 |
| 17 | EP/5APP–PEI/5FR14 | 16 | 26 | V-1 | 26.4 | 26.05 |
| 18 | EP/5APP–PEI/5FR15 | 28 | 32 | NQ | — | 27.56 |
| 19 | EP/5APP–PEI/5FR16 | 26 | 38 | NQ | — | 27.64 |
The effect of the substituent on the phenyl moieties (depicted by the dark blue sphere in Scheme 2).
When considering the same bridging groups between two secondary amine moieties discussed before, the different structures of substituents on the phenyl moieties of FRs had specific effects when used as flame retardant additives. It became evident when comparing samples, specifically EP/5APP–PEI/5FR3 with EP/5APP–PEI/FR4, and EP/5APP–PEI/5FR5 with EP/5APP–PEI/5FR6. These observations highlighted the influence of the substituent on the phenyl groups of FRs. The correlation between the chemical structure and fire resistance of these samples indicated that EP/5APP–PEI/5FR3 and EP/5APP–PEI/5FR5, which contained methoxy groups, exhibited higher values of V-1 and LOI compared to EP/5APP–PEI/5FR4 and EP/5APP–PEI/5FR6, which featured a methylenedioxy phenyl ring substituent (and failed to satisfy the requirements of the UL-94 and LOI testing methods). This correlation was valid when comparing EP/5APP–PEI/5FR10 with EP/5APP–PEI/5FR11.
FR10 and FR11 demonstrated higher efficiency in the EP/5APP–PEI/5FRs composition, as indicated by the results of UL-94 and LOI tests. In terms of the correlation between chemical structure and flame retardancy, the diphenyl ether-linked component demonstrated the highest level of flame retardancy. The presence of substituents in the phenyl group did not significantly impact this particular case. This result could be explained by the fact that phenolic compounds have good free radical scavenging ability, thus helping to increase the fire resistance of the epoxy composite.
2.4. Surface morphology and thermal properties of the composites
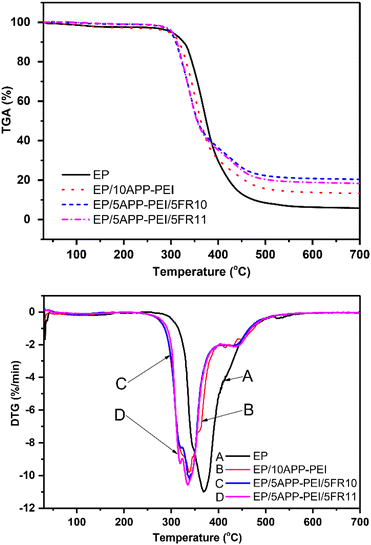
Fig. 1 and Table 2 illustrated the thermal decomposition behavior of the original epoxy resin (EP) with a decomposition temperature of 368.7 °C. However, the incorporation solely of 10 wt% APP–PEI (EP/10APP–PEI) caused the material's decomposition temperature to significantly decrease to 337.3 °C. The result indicated that APP–PEI promoted the early decomposition of the composite. The introduction of APP–PEI into the polymer matrix formed a protective char layer at low temperatures, preventing direct flame exposure and enhancing the material's flame resistance.| Sample | Tmax (°C) | Residual solid content at 700 °C (%) |
|---|---|---|
| EP | 368.7 | 5.801 |
| EP/10APP–PEI | 337.3 | 13.223 |
| EP/5APP–PEI/5FR10 | 336.5 | 20.376 |
| EP/5APP–PEI/5FR11 | 334.7 | 18.281 |
It had been observed that the blending of either 5 wt% FR10 (EP/5APP–PEI/5FR10) or 5 wt% FR11 (EP/5APP–PEI/5FR11) as a replacement for 5 wt% APP–PEI resulted in a slight reduction in the decomposition temperatures of these samples compared to that of the EP/10APP–PEI sample. The findings suggested that the flame-retardant mechanisms of FR10 and FR11 generated an effective char layer, meanwhile the APP–PEI mechanism failed to form a similarly effective char layer. FR10 and FR11 exhibited comparable behavior to DOPO, concerning their role in the gaseous phase during combustion. These flame retardants were designed to increase the amount of DOPO by combining two DOPO moieties into molecules. Additionally, the presence of the bridging group structures and phenyl moieties substituents assisted in improving the efficacy of DOPO. The residue content at 700 °C for EP/5APP–PEI/5FR10 (20.376%) and EP/5APP–PEI/5FR11 (18.281%) compared to EP/10APP–PEI (13.233%) demonstrated the higher efficiency of these flame retardants. The flame retardant additives exhibited the ability to scavenge free radicals, thereby synergistically enhancing the flame resistance of the epoxy composite material.
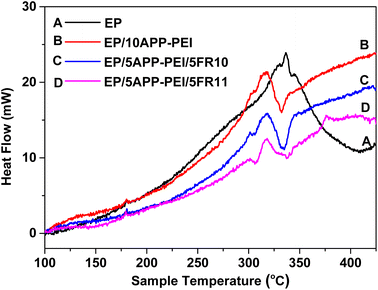
The DSC thermogram of pure epoxy resin displays a major exothermic peak at 337 °C (Fig. 2). It can be observed that pure epoxy almost burns in the temperature range from 200–400 °C, which demonstrates the flammability of epoxy. The incorporation of APP–PEI, FR10 or FR11 into epoxy resin results in slower thermal oxidative decomposition and less heat release. EP/10APP–PEI, EP/5APP–PEI/5FR10 and EP/5APP–PEI/5FR11 show endothermic peaks at 332 °C, 335 °C and 337 °C, respectively. These endothermic peaks, caused by the thermal decomposition of APP–PEI, FR10 or FR11, contribute to the flame retardancy of the composites.
The SEM images of the composites in Fig. 3 clearly illustrated the dispersion of flame retardants in the epoxy matrix. Fig. 3a depicted a relatively flat surface with cracks on the original epoxy resin. After incorporating 10 wt% of APP–PEI, the relatively large APP–PEI particles aggregated on the epoxy matrix (Fig. 3b and c). The APP–PEI agglomeration was likely responsible for the notable decrease in the impact strength of the EP/10APP–PEI material. SEM images of EP/5APP–PEI/5FR10 (Fig. 3d) and EP/5APP–PEI/5FR11 (Fig. 3e) exhibited clearly different surfaces. The distribution of APP–PEI particles was more homogeneous, interspersed with FR particles. The investigation above demonstrated that the most effective flame retardant consisted of two DOPO moieties connected by a bridging diphenyl ether group and possessing a methoxy substituent on the phenyl moiety (EP/5APP–PEI/5FR11). Nevertheless, the flame resistance of the EP/5APP–PEI/5FR10 and EP/5APP–PEI/5FR11 samples was very comparable. In the meantime, the EP/5APP–PEI/5FR10 sample exhibited only slight improvements in mechanical and thermal properties compared to the EP/5APP–PEI/5FR11 sample. According to these findings, both EP/5APP–PEI/5FR10 and EP/5APP–PEI/5FR11 exhibited great potential as flame retardants.
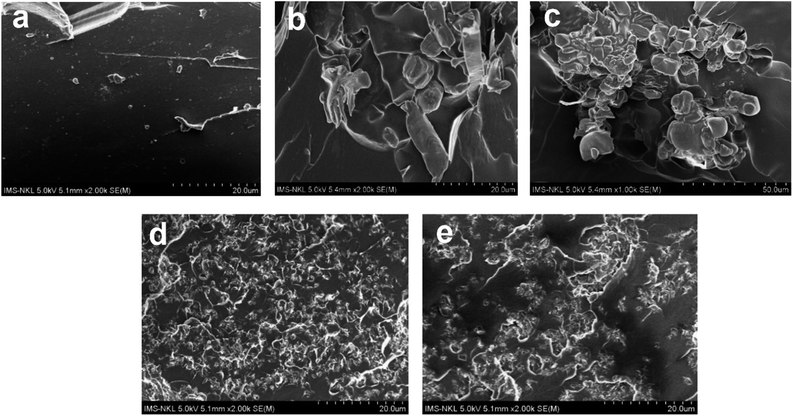 | ||
| Fig. 3 SEM images of the composites. EP (a); EP/10APP–PEI (b and c); EP/5APP–PEI/5FR10 (d); EP/5APP–PEI/5FR11 (e). | ||
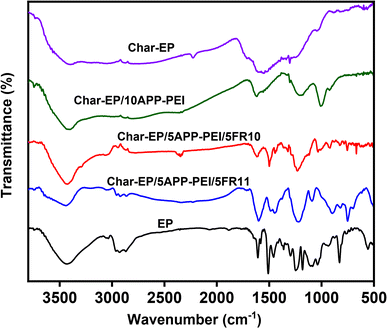
The char product obtained from the TGA characterization process of the samples analyzed by FTIR spectroscopy reveals the protective effects on the base epoxy resin of fire retardant additives. The FTIR spectra are depicted in Fig. 4. The FTIR spectrum of the char–EP sample indicates that during the analysis process using the TGA method under N2 flow, the epoxy resin underwent thermal decomposition to yield char products with a structure resembling activated carbon obtained at a temperature of 700 °C, characterized by distinctive peaks corresponding to O–H bonds at around 3400 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds at around 1500 cm−1, and C–O–H bonds at approximately 900 cm−1.16 Upon incorporating fire retardant additives into the epoxy resin, the fire resistance of the composites significantly increased, as evidenced by the LOI and UL-94 results shown in Table 1. The FTIR spectra of the char residues corresponded to the composites (char–EP/10APP–PEI, char–EP/5APP–PEI/5FR10 and char–EP/5APP–PEI/5FR11 samples) also demonstrate a clear increase in the sharpness of characteristic bands for functional groups of the original epoxy resin (EP sample), in the order of char–EP/10APP–PEI > char–EP/5APP–PEI/5FR10 > char–EP/5APP–PEI/5FR11. These results distinctly demonstrate the protective capabilities of the APP–PEI and DOPO-derivatives flame retardant additives in combination with epoxy resin, forming an intumescent char layer on the surface to insulate the material from surrounding heat sources.17,18 Among these, the 5APP–PEI/5FR11 additive system exhibited the most effective performance among the studied flame retardant systems.
C bonds at around 1500 cm−1, and C–O–H bonds at approximately 900 cm−1.16 Upon incorporating fire retardant additives into the epoxy resin, the fire resistance of the composites significantly increased, as evidenced by the LOI and UL-94 results shown in Table 1. The FTIR spectra of the char residues corresponded to the composites (char–EP/10APP–PEI, char–EP/5APP–PEI/5FR10 and char–EP/5APP–PEI/5FR11 samples) also demonstrate a clear increase in the sharpness of characteristic bands for functional groups of the original epoxy resin (EP sample), in the order of char–EP/10APP–PEI > char–EP/5APP–PEI/5FR10 > char–EP/5APP–PEI/5FR11. These results distinctly demonstrate the protective capabilities of the APP–PEI and DOPO-derivatives flame retardant additives in combination with epoxy resin, forming an intumescent char layer on the surface to insulate the material from surrounding heat sources.17,18 Among these, the 5APP–PEI/5FR11 additive system exhibited the most effective performance among the studied flame retardant systems.
The obtained results have demonstrated that the newly developed fire retardant system comprising of APP–PEI combined with novel DOPO derivatives, for the first time applied on epoxy resin, has effectively enhanced the flame retardancy of the epoxy composite material. The uniform distribution of additives within the epoxy matrix not only enhances the synergistic effect of the two additives but also mitigates the reduction in the impact strength of the flame-retardant epoxy composite material.
3 Experimental
3.1. Materials
Chemicals and solvents were purchased from AK Scientific, Inc. (USA) and Sigma-Aldrich (Merck, Germany) and used without further purification.3.2. Synthesis of APP–PEI
APP–PEI was synthesized according to the reported procedure.13,14 14.0 g PEI was added to a mixture of 400 ml ethanol and 20 ml distilled water in a three-necked round-bottom flask. The mixture was stirred under nitrogen for 30 minutes before adding 20.0 g of APP. Then, the mixture was stirred at 90 °C under nitrogen for 4 hours. After cooling the mixture to room temperature, the precipitation was collected by filtration, washed with ethanol and dried under vacuum to obtain the final product with the yield of 65%.Successful APP–PEI preparation was confirmed by FTIR analysis. Characteristic peaks were observed at 3058 cm−1 (N–H amine salt), 1689 cm−1 (N–H), 1613 cm−1 (N–H), 1466 cm−1 (C–H alkane), 1442 cm−1 (C–H alkane), 1252 cm−1 (C–N amine), 1198 cm−1 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1015 cm−1 (C–N amine).
O), and 1015 cm−1 (C–N amine).
3.3. Synthesis of DOPO derivatives
Other DOPO-based flame retardants (FR2–FR11) were synthesized using the above procedure.
6,6′-((Ethane-1,2-diylbis(azanediyl))bis((4-methoxyphenyl)methylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR2 was synthesized according to general procedure in Scheme 1 with ethylenediamine (0.8 g, 13.31 mmol, 1 eq.), anisaldehyde (3.99 g, 29.3 mmol, 2.2 eq.) and DOPO (5.14 g, 23.75 mmol, 2.2 eq.) to afford compound FR2 as a white solid (7.4 g, 94%). Compound FR2: white solid; mp 223 °C; 1H NMR (600 MHz, CDCl3) δ 8.07–7.93 (m, 1H), 7.93–7.85 (m, 1H), 7.85–7.74 (m, 2H), 7, 74–7.54 (m, 5H), 7.5 2–7.40 (m, 1H), 7.40–7.30 (m, 1H), 7.30–7.24 (m, 3H), 7.24–6.96 (m, 6H), 6.96–6.89 (m, 1H), 6.89–6.69 (m, 4H), 6.61–6.45 (m, 3H), 4.20–4.01 (m, 1H), 3.99–3.80 (m, 1H), 3.80–3.73 (m, 3H), 3.73–3.64 (m, 5H); 13C NMR (126 MHz, DMSO) δ 205.41, 131.24, 129.24, 124.85, 124.73, 114.17, 113.19, 113.00, 78.77, 78.25, 55,30, 54.78, 39.58, 39.2 3, 30.06; FT-IR (KBr): νmax/cm−1 766, 834, 1032, 1057, 1092, 1134, 1187, 1255, 1289, 1382, 1448, 1515, 1609, 3071.
6,6′-((1,4-Phenylenebis(azanediyl))bis((4-methoxyphenyl)methylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR3 was synthesized according to general procedure in Scheme 1 with p-phenylenediamine (1 g, 9.25 mmol, 1 eq.), anisaldehyde (2.8 g, 20.34 mmol, 2.2 eq.) and DOPO (4.14 g, 19.16 mmol, 2.2 eq.) to afford compound FR3 (6 g, 89%) as a white solid. Compound FR3: white solid; mp 238 °C; 1H NMR (600 MHz, DMSO) δ 8.23–8.06 (m, 3H), 7.99 (dd, J = 11.8, 7.7 Hz, 1H), 7.74 (q, J = 8.4 Hz, 1H), 7.71–7.64 (m, 2H), 7.55–7.48 (m, 1H), 7.45–7.34 (m, 3H), 7.29 (dtd, J = 15.4, 7.6, 1.4 Hz, 2H), 7.26–7.16 (m, 3H), 7.10 (ddd, J = 8.2, 3.7, 1.3 Hz, 1H), 6.98 (ddt, J = 8.1, 6.4, 1.8 Hz, 1H), 6.85–6.78 (m, 2H), 6.78–6,72 (m, 2H), 6.30 (dd, J = 22.7, 2.3 Hz, 3H), 5.84 (dt, J = 14.5, 7.2 Hz, 1H), 5, 40 (dd, J = 10.2, 5.0 Hz, 1H), 5.18 (dd, J = 17.6, 10.5 Hz, 1H), 4.82–4.70 (m, 1H), 3.73–3.65 (m, 5H); 13C NMR (151 MHz, DMSO) δ 158.67, 158.60, 148.82, 148.77, 138.69, 138.59, 135.12, 133.47, 133.07, 131.74, 131,58, 131.52, 130.67, 130.43, 129.77, 129.61, 129.48, 129.45, 128.83, 128.31, 128.23, 128.13, 126.99, 126.82, 125.69, 125.42, 124.59, 124.45, 124.18, 123.97, 123.90, 123.74, 123.42, 121.77, 121.72, 120,14, 119.90, 119.86, 114.93, 114.90, 114.09, 113.42, 56.76, 56.03, 54.94, 54.92, 40.04; FT-IR (KBr): νmax/cm−1 761, 776, 808, 821, 920, 1025, 1116, 1178, 1192, 1230, 1256, 1305, 1429, 1446, 1474, 1512, 1582, 1607, 3384.
6,6′-((1,4-Phenylenebis(azanediyl))bis(benzo[d][1,3]dioxol-5-ylmethylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR4 was synthesized according to general procedure in Scheme 1 with p-phenylenediamine (1 g, 9.25 mmol, 1 eq.), piperonal (3 g, 20.34 mmol, 2.2 eq.) and DOPO (4.1 g, 18.91 mmol, 2.2 eq.) to afford compound FR4 (6.6 g, 95%) as a white solid. Compound FR4: white solid; mp 236 °C; 1H NMR (600 MHz, DMSO) δ 8.26–8.10 (m, 4H), 8.04–7.95 (m, 1H), 7.81–7.66 (m, 2H), 7, 52 (tq, J = 6.9, 3.9 Hz, 1H), 7.46–7.33 (m, 2H), 5.51–5.15 (m, 1H), 4.87–4, 77 (m, 1H), 3.32 (s, 4H); 13C NMR (126 MHz, DMSO) δ 148.77, 147.05, 146.65, 135.18, 133.52, 131.54, 130.66, 130.45, 128.95, 128.83, 128,34, 128.24, 128.13, 125.68, 125.41, 124.58, 124.46, 124.15, 123.97, 123.89, 123.23, 122.03, 121.74, 121.66, 120.17, 119.88, 114.99, 108.56, 107.61, 100.84, 57.06, 56.19; FT-IR (KBr): νmax/cm−1 751, 784, 812, 930, 1039, 1117, 1205, 1232, 1256, 1443, 1487, 1515, 1583, 1595, 1608, 2892, 3066 (bw), 3297.
6,6′-(([1,1′-Biphenyl]-4,4′-diylbis(azanediyl))bis((4-methoxyphenyl)methylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR5 was synthesized according to general procedure in Scheme 1 with benzidine (1.5 g, 8.14 mmol, 1 eq.), anisaldehyde (2.44 g, 17.91 mmol, 2.2 eq.) and DOPO (3.62 g, 16.74 mmol, 2.2 eq.) to afford compound FR5 (5.5 g, 85%) as a white solid. Compound FR5: white solid; mp 221 °C; 1H NMR (600 MHz, DMSO) δ 8.17 (ddt, J = 14.6, 8.1, 3.1 Hz, 5H), 8.06 (td, J = 11.1, 6.3 Hz, 2H), 7.82–7.65 (m, 3H), 7.55 (tt, J = 7.5, 3.4 Hz, 2H), 7.49–7.39 (m, 2H), 7 0.38–7.27 (m, 6H), 7.21–6.97 (m, 6H), 6.92–6.80 (m, 4H), 6.73–6.62 (m, 6H), 6.28–5.40 (m, 1H), 5.03 (td, J = 13.1, 5.5 Hz, 2H), 3.78–3.68 (m, 6H); 13C NMR (126 MHz, DMSO) δ 158.77, 148.90, 148.83, 145.25, 135.29, 135.24, 133.62, 131.67, 130.74, 130.51, 129.68, 129.56, 128.39, 128.29, 126.66, 125.85, 125.73, 124.66, 123.97, 123.07, 121.71, 121.63, 119.86, 114.06, 113.48, 55.96, 55.10, 54.97, 54.84 FT-IR (KBr): νmax/cm−1 754, 777, 835, 920, 1029, 1116, 1175, 1206, 1233, 1431, 1475, 1508, 1582, 1610, 3291.
6,6′-(([1,1′-Biphenyl]-4,4′-diylbis(azanediyl))bis(benzo[d][1,3]dioxol-5-ylmethylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR6 was synthesized according to general procedure in Scheme 1 with benzidine (1.5 g, 8.14 mmol, 1 eq.), piperonal (2.69 g, 17.91 mmol, 2.2 eq.) and DOPO (3.61 g, 16.68 mmol, 2.2 eq.) to afford compound FR6 (6.2 g, 93%) as a white solid. Compound FR6: white solid; mp 249 °C; 1H NMR (600 MHz, DMSO) δ 8.24–8.13 (m, 6H), 8.05 (ddd, J = 11.8, 7.6, 3.9 Hz, 2H), 7.83–7.66 (m, 4H), 7.56 (tt, J = 7.3, 3.2 Hz, 2H), 7.45 (ddddd, J = 18.5, 12.7, 10.6, 8,0, 3.2 Hz, 4H), 7.35–7.23 (m, 3H), 7.18 (dt, J = 8.2, 2.0 Hz, 1H), 7.14–6, 97 (m, 11H), 6.92–6.84 (m, 1H), 6.84–6.75 (m, 5H), 6.68 (tdt, J = 15.4, 10.2, 6.0 Hz, 7H), 6.23 (dt, J = 10.0, 4.1 Hz, 1H), 6.02–5.92 (m, 6H), 5.56–5.41 (m, 1H), 5.06 (dtd, J = 12.7, 6.9, 3.3 Hz, 2H), 3.35 (s, 5H); 13C NMR (126 MHz, DMSO) δ 148.94, 148.87, 148.72, 148.65, 147.10, 146.76, 145.32, 145.20, 135.35, 135.30, 135,20, 133.68, 133.29, 131.64, 131.57, 130.74, 130.53, 130.20, 129.80, 128.87, 128.78, 128.43, 128.33, 125.91, 125.72, 125.49, 124.65, 124.56, 124.00, 123.93, 123.86, 123.49, 122.94, 122.09, 121.66, 121,58, 120.18, 119.88, 119.83, 114.17, 114.07, 108.75, 108.61, 107.71, 100.92, 56.38, 55.53, 55.05; FT-IR (KBr): νmax/cm−1 754, 813, 930, 1040, 1117, 1233, 1255, 1445, 1486, 1502, 1612, 3285.
6,6′-(((Sulfonylbis(4,1-phenylene))bis(azanediyl))bis((4-methoxyphenyl)methylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR7 was synthesized according to general procedure in Scheme 1 with diamino diphenylsulfone (1.5 g, 6.04 mmol, 1 eq.), anisaldehyde (1.8 g, 13.29 mmol, 2.2 eq.) and DOPO (2.75 g, 12.71 mmol, 2.2 eq.) to afford compound FR7 (5.0 g, 94%) as a white solid. Compound FR7: white solid; mp 344 °C; 1H NMR (600 MHz, CDCl3) δ 8.01 (m, 1H), 7.80 (m, 4H), 7.58 (m, 2H), 7.40 (m, 5H), 7.18 (m, 7H), 7.01 (m, 1H), 6.90 (m, 2H), 6.70 (m, 2H), 6.60 (m, 2H), 6.50–6.34 (m, 3H), 6.07 (m, 1H), 5.90 (m, 1H), 4.73 (m, 1H), 4.67 (m, 1H), 3.73 (s, 3H), 3, 59 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 159.72, 159.57, 149.96, 149.88, 149.33, 149.28, 149.21, 149.15, 136.37, 136.32, 136,29, 136.24, 134.08, 134.00, 131.85, 131.08, 131.02, 130.99, 130.93, 130.71, 129.16, 129.13, 129.08, 129.04, 128.84, 128.81, 128.77, 128.74, 128.69, 128.61, 128.17, 128.09, 125.26, 125.16, 125.03, 124,83, 123.74, 123.67, 123.61, 123.54, 122.95, 122.17, 122.04, 121.97, 121.89, 121.81, 120.98, 120.45, 114.21, 114.09, 113.25, 57.27, 56.59, 56.10, 55.44, 55.27, 55.24; FT-IR (KBr): νmax/cm−1 754, 835, 921, 1030, 1105, 1144, 1248, 1284, 1332, 1431, 1447, 1477, 1509, 1595, 2839, 2924, 3065, 3164, 3286.
6,6′-(((Sulfonylbis(4,1-phenylene))bis(azanediyl))bis(p-tolylmethylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR8 was synthesized according to general procedure in Scheme 1 with diamino diphenylsulfone (1.5 g, 6.04 mmol, 1 eq.), 4-methylbenzaldehyde (1.6 g, 13.29 mmol, 2.2 eq.) and DOPO (2.63 g, 12.15 mmol, 2.2 eq.) to afford compound FR8 (4.4 g, 90%) as a white solid. Compound FR8: white solid; mp 298 °C; 1H NMR (600 MHz, CDCl3) δ 7.95 (m, 2H), 7.55 (m, 5H), 7.25 (m, 3H), 7.12 (m, 2H), 6.90 (m, 3H), 6.59 (m, 1H), 6.45 (m, 2H), 5.70 (m, 1H), 4.70 (m, 1H), 2.24 (ddd, J = 34, 1, 8.6, 1.9 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 150.52, 149.80, 149.31, 149.13, 138.36, 136.39, 134.11, 133.98, 131.85, 131.80, 131,30, 131.04, 130.69, 130.39 (d, J = 8.2 Hz), 129.45, 129.35, 129.27, 129.25, 128.90, 128.85, 128.13, 128.04, 127.79, 125.17, 125.06, 124.76, 123.76, 123.59, 123.52, 122.10, 121.73, 120.92, 120.55, 120.51, 114.12, 114.06, 114.04, 113.35, 113.29, 113.23, 57.82, 56.48, 55.83, 21.12, 21.08; FT-IR (KBr): νmax/cm−1 755, 828, 921, 1104, 1143, 1204, 1234, 1280, 1331, 1431, 1445, 1475, 1509, 1624, 2857, 2920, 3060, 3347.
6,6′-(((Sulfonylbis(4,1-phenylene))bis(azanediyl))bis((4-hydroxy-3-nitrophenyl)methylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR9 was synthesized according to general procedure in Scheme 1 with diamino diphenylsulfone (1.5 g, 6.04 mmol, 1 eq.), 4-hydroxy-3-nitro benzaldehyde (2.2 g, 13.29 mmol, 2.2 eq.) and DOPO (2.7 g, 12.48 mmol, 2.2 eq.) to afford compound FR9 (5.3 g, 95%) as a yellow solid. Compound FR9: yellow solid; mp 205 °C; 1H NMR (600 MHz, DMSO) δ 11.01 (s, 3H), 8.18–8.07 (m, 5H), 8.04 (q, J = 2.6 Hz, 2H), 7.94 (d, J = 2.3 Hz, 1H), 7.90–7.83 (m, 1H), 7.78 (dt, J = 9.6, 4.8 Hz, 2H), 7.74–7.64 (m, 3H), 7.62 (dq, J = 11.2, 3.9 Hz, 3H), 7.54 (dt, J = 8.7, 1.9 Hz, 1H), 7.50–7.31 (m, 9H), 7.31–7.19 (m, 5H), 7.15–6.96 (m, 5H), 6.80 (dd, J = 9.2, 3.1 Hz, 5H), 5.84 (dd, J = 17.3, 9.6 Hz, 2H), 5.44 (dd, J = 16.4, 10.0, 3.2 Hz, 1H); 13C NMR (126 MHz, DMSO) δ 170.30, 151.96, 149.08, 148.68, 148.60, 136.11, 135.81, 135.42, 135.14, 134.96, 134,07, 133.65, 131.73, 130.69, 130.57, 130.36, 129.76, 128.37, 128.26, 128.09, 125.61, 125.47, 125.18, 124.93, 124.62, 123.91, 122.47, 121.53, 121.29, 121.21, 120.06, 119.03, 118.95, 112.90, 78.66, 59,73, 5 3,70, 52,86; FT-IR (KBr): νmax/cm−1 758, 826, 924, 1105, 1147, 1234, 1294, 1431, 1477, 1536, 1595, 1629, 1730, 2853, 1924, 3434.
6,6′-(((Oxybis(4,1-phenylene))bis(azanediyl))bis(benzo[d][1,3]dioxol-5-ylmethylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR10 was synthesized according to general procedure in Scheme 1 with diamino diphenyl ether (1.5 g, 7.49 mmol, 1 eq.), piperonal (2.5 g, 16.48 mmol, 2.2 eq.) and DOPO (3.3 g, 15.16 mmol, 2.2 eq.) to afford compound FR10 (5.6 g, 91%) as a white solid. Compound FR10: white solid; mp 233 °C; 1H NMR (600 MHz, DMSO) δ 8.22–8.13 (m, 6H), 8.00 (dd, J = 11.8, 7.4 Hz, 2H), 7.75 (dt, J = 22.5, 7.8 Hz, 4H), 7.56 (td, J = 7.5, 3.2 Hz, 2H), 7.45 (dq, J = 27.9, 9.8 Hz, 4H), 7.30 (qd, J = 7.3, 4.7 Hz, 3H), 7.16 (d, J = 8.1 Hz, 1H), 7.06–6.96 (m, 5H), 6.84–6.75 (m, 5H), 6.62 (dd, J = 21.7, 8.8 Hz, 6H), 6.53–6.39 (m, 9H), 6.00–5.93 (m, 6H), 5.39 (dd, J = 17.9, 10.3 Hz, 1H), 5.04–4.89 (m, 2H), 2.54–2.44 (m, 5H); 13C NMR (126 MHz, DMSO) δ 148.89, 147.11, 146.77, 133.70, 130.73, 128.71, 128.41, 125.73, 124.64, 123.94, 123, 84, 122.09, 119.83, 118.69, 118.55, 114.76, 108.64, 107.71, 100.93, 56.76, 55.90; FT-IR (KBr): νmax/cm−1 754, 920, 1041, 1117, 1229, 1444, 1501, 1595, 1607, 2881, 3064, 3290.
6,6′-(((Oxybis(4,1-phenylene))bis(azanediyl))bis((4-methoxyphenyl)methylene))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR11 was synthesized according to general procedure in Scheme 1 with diamino diphenyl ether (1.5 g, 7.49 mmol, 1 eq.), anisaldehyde (2.24 g, 16.48 mmol, 2.2 eq.) and DOPO (3.3 g, 15.12 mmol, 2.2 eq.) to afford compound FR11 (5.4 g, 90%) as a white solid. Compound FR11: white solid; mp 232 °C; 1H NMR (600 MHz, DMSO) δ 8.24–8.09 (m, 9H), 8.05–8.00 (m, 3H), 7.80–7.64 (m, 6H), 7, 60–7.52 (m, 3H), 7.47–7.38 (m, 4H), 7.38–7.24 (m, 10H), 7.21–7.10 (m, 1H), 7.04 (ddd, J = 8.2, 4.9, 2.0 Hz, 3H), 6.95–6.70 (m, 8H), 6.69–6.57 (m, 8H), 6.55–6.42 (m, 10H), 6.06 (td, J = 10.2, 5.4 Hz, 1H), 5.49–5.31 (m, 1H), 5.10–4.88 (m, 3H), 3.72 (s, 8H), 3.71 (d, J = 2.9 Hz, 3H); 13C NMR (126 MHz, DMSO) δ 158.80, 158.78, 149.12, 149.01, 148.94, 148.87, 148.77, 142.47, 142.36, 135.36, 135,31, 133.62, 133.21, 131.75, 1 31.68, 130.72, 130.49, 129.76, 129.60, 129.56, 129.44, 128.37, 128, 27, 126.78, 126.61, 126.59, 125.72, 125.46, 124.64, 12 4.51, 123.99, 123.92, 123.65, 123.06, 121.90, 121.74, 121.66, 121.56, 120.17, 119.90, 119.85, 118.68, 118.60, 118.53, 118.45, 114.68, 113.49, 56,39, 55.53, 55.34, 54.98, 54.84; FT-IR (KBr): νmax/cm−1 760, 781, 824, 921, 1033, 1117, 1230, 1305, 1430, 1476, 1498, 1511, 1608, 3318.
Procedure for the synthesis of 1,1′-(1,4-phenylene)bis(3-(6-oxidodibenzo[c,e][1,2]oxaphosphinin-6-yl)pyrrolidine-2,5-dione) FR12 (Scheme 2): p-phenylenediamine (1.5 g, 13.87 mmol, 1 eq.) and maleimide (6.8 g, 69.4 mmol, 5 eq.) were mixed together in a 40 ml flask filled with argon. The mixture was heated at 160 °C for six hours. The product yielded was washed with NaHCO3 solution 1 M. The precipitated solid was dried in a vacuum to yield the intermediate 6a (3.5 g, 13.05 mmol, 1 eq.), which was reacted with DOPO (5.64 g, 26.1 mmol, 2 eq.) in 15 ml toluene (90 °C, 16 hours) to achieve compound FR12 as a yellow solid (8.5 g, 93%). Compound FR12: yellow solid; mp 206 °C; 1H NMR (600 MHz, DMSO) δ 8.34–6.93 (m, 10H), 4.27–4.00 (m, 1H), 3.33–3.10 (m, 2H); 13C NMR (151 MHz, DMSO) δ 166.73, 163.02, 154.13, 137.33, 134.49, 134.13, 131.55, 131.20, 131.04, 130.72, 130.41, 130.32, 129.14, 128.87, 128.78, 128.18, 125.77, 125.29, 125.21, 124.42, 124.36, 121.43, 120.30, 120.26, 120.03, 118.82, 115.67, 40.04, 21.01 FT-IR (KBr): νmax/cm−1 755, 841, 969, 1007, 1197, 1323, 1405, 1510, 1627, 1701, 1900, 3084, 3189, 3280.
In the case of DOPO derivatives FR13 and FR14 (Scheme 2), the reactions started from the commercial intermediate (6b–c). The intermediate 6b (5 g, 11.3 mmol, 1 eq.) and DOPO (4.89 g, 22.6 mmol, 2 eq.) were mixed in a 40 ml flask filled with toluene (15 ml). After 16 hours at 90 °C, the reaction yielded compound FR13 as a white solid (7.51 g, 76%). Compound FR14 (7.47 g, 85%) was achieved following the same procedure from the intermediate 6c (5 g, 8.76 mmol, 1 eq.) and DOPO (3.79 g, 17.53 mmol, 2 eq.).
1,1′-(Methylenebis(2-ethyl-6-methyl-4,1-phenylene))bis(3-(6-oxidodibenzo[c,e][1,2]oxaphosphinin-6-yl)pyrrolidine-2,5-dione) FR13: white solid; mp 145 °C; 1H NMR (600 MHz, CDCl3) δ 8.11–7.81 (m, 5H), 7.72 (q, J = 8.5 Hz, 2H), 7.62–7.43 (m, 2H), 7.38 (q, J = 7.8 Hz, 2H), 7.33–7.12 (m, 4H), 7.04–6.77 (m, 3H), 3.84 (t, J = 17.0 Hz, 2H), 3.63–3.41 (m, 2H), 3.10 (ddtd, J = 28.5, 19.4, 10.4, 4.4 Hz, 1H), 2.47 (p, J = 7.8 Hz, 1H), 2.35–2.18 (m, 2H), 2.18–2.08 (m, 1H), 2.04–1.78 (m, 3H), 1.37–1.10 (m, 2H), 1.10–0.73 (m, 3H); 13C NMR (126 MHz, CDCl3) δ 173.94, 173.90, 171.72, 171.69, 171.67, 170.51, 148.83, 148.75, 148.69, 142.24, 142,07, 142.06, 141.99, 141.87, 141.25, 141.23, 141.20, 136.52, 136.35, 136.29, 136.21, 136.14, 135.41, 135.39, 134.56, 134.55, 134.53, 134.33, 132.59, 132.53, 132.46, 131.12, 131.09, 131.07, 131.04, 130,98, 129.50, 129.45, 129.36, 129.32, 129.06, 129.01, 128.96, 128.87, 128.82, 128.76, 128.71, 128.54, 127.81, 127.77, 127.59, 127.54, 127.50, 127.28, 127.26, 127.22, 126.97, 125.60, 125.52, 125.32, 125,29, 125.23, 125.20, 124.15, 124.07, 123.99, 122.72, 122.60, 122.56, 122.51, 122.48, 121.77, 121.70, 121.62, 121.52, 120.77, 120.72, 120.18, 120.13, 77.27, 65.27, 41.95, 41.87, 41.47, 41.35, 41,29, 41,23, 41,15, 40,72, 29,73, 29,22 FT-IR (KBr): νmax/cm−1 755, 918, 1117, 1191, 1236, 1377, 1447, 1477, 1594, 1712, 1779, 2927, 2968, 3469.
1,1′-(((Propane-2,2-diylbis(4,1-phenylene))bis(oxy))bis(4,1-phenylene))bis(3-(6-oxidodibenzo[c,e][1,2]oxaphosphinin-6-yl)pyrrolidine-2,5-dione) FR14: yellow solid; mp 150 °C; 1H NMR (600 MHz, CDCl3) δ 8.09 (ddt, J = 13.4, 7.6, 1.4 Hz, 1H), 8.04–7.90 (m, 5H), 7.77 (tdt, J = 8.3, 7.3, 1.2 Hz, 2H), 7.58 (dtdd, J = 21.7, 7.5, 3.2, 1.0 Hz, 2H), 7, 45–7.34 (m, 2H), 7.34–7.22 (m, 6H), 7.22–7.16 (m, 4H), 7.15–7.09 (m, 2H), 7.05–6.99 (m, 2H), 6.97–6.84 (m, 7H), 3.68 (dddd, J = 15.5, 10.1, 4.0, 1.6 Hz, 1H), 3.58–3.36 (m, 3H), 3.17–2.99 (m, 2H), 1.68 (t, J = 3.4 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 173.70, 173.65, 171.58, 170.41, 157.94, 157.77, 154.09, 149.12, 146.29, 136.56, 136,29, 134.54, 134.52, 132.33, 132.25, 131.14, 131.05, 129.02, 128.91, 128.89, 128.78, 128.20, 128.18, 127.88, 127.68, 125.93, 125.82, 125.56, 125.51, 125.34, 124.12, 124.05, 123.98, 120.47, 120.19, 120,14, 119.06, 118.97, 118.70, 118.61, 77.25, 42.25, 42.13, 41.94, 41.41, 41.22, 31.00, 29.60, 29.25; FT-IR (KBr): νmax/cm−1 755, 833, 922, 1013, 1082, 1117, 1172, 1240, 1386, 1431, 1448, 1500, 1595, 1715, 1782, 2967, 3064, 3476.
The synthesis procedure of 6,6′-(((phenylazanediyl)bis(ethane-2,1-diyl))bis(oxy))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR15: DOPO (4 g, 18.5 mmol, 1 eq.) and N-chlorosuccinimide (2.97 g, 22.2 mmol, 1.2 eq.) were dissolved in toluene (10 ml) in a 40 ml flask. The reaction was cooled down to 5 °C using an ice bath. After 6 hours, the reaction mixture was filtered with toluene to remove the white solid of N-succinimide. The toluene solvent was dried in vacuo to yield the intermediate 8 (3.8 g, 17.58 mmol) as a light yellow oil. The intermediate 7 (3.8 g, 17.58 mmol, 1 eq.) was dissolved in THF (5 ml), then compound 9 (1.59 g, 8.79 mmol, 0.5 eq.) and triethylamine (1.8 g, 17.58 mmol, 1 eq.) were mixed in THF (5 ml) and dropped slowly in the solution of compound 8. After 12 hours, the synthesis mixture was filtered with ethyl acetate to remove the white solid. The mixture solvent was dried in a vacuum to yield a light yellow oil, which was then further purified in column chromatography (dichloromethane/methanol 100/1) to give compound FR15 as a colorless liquid (5 g, 93%). Compound FR15: colorless liquid; 1H NMR (600 MHz, CDCl3) δ 7.96–7.80 (m, 6H), 7.69 (ddt, J = 8.3, 6.8, 1.3 Hz, 2H), 7.45 (ttd, J = 7.6, 3.8, 1.0 Hz, 2H), 7.31 (dtt, J = 8.1, 6.9, 1.4 Hz, 2H), 7.27–7, 19 (m, 2H), 7.12–7.02 (m, 4H), 6.65 (tt, J = 7.3, 1.0 Hz, 1H), 6.37 (dq, J = 7, 8, 1.1 Hz, 2H), 4.07 (dddt, J = 11.1, 9.4, 6.1, 2.4 Hz, 4H), 3.36–3.22 (m, 4H); 13C NMR (151 MHz, CDCl3) δ 149.81, 149.75, 146.16, 136.95, 136.91, 133.59, 130.60, 130.19, 130.13, 129.34, 128.36, 128.25, 125.23, 125.08, 124.80, 124.13, 124.04, 122.55, 122.49, 122.47, 121.29, 120.18, 120.14, 117.09, 111.87, 68.18, 63.02, 63.01, 62.98, 62.96, 50.92, 50.89, 50.87, 50.85; FT-IR (KBr): νmax/cm−1 743, 1432, 1451, 1475, 1506, 1518, 1560, 1652, 1700, 2320, 2355, 2954, 3064.
The synthesis procedure of 6,6′-(3-hydroxy-3,5-dimethylhexane-1,2-diyl)bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) FR16: in a 40 ml flask, DOPO (15.42 g, 71.3 mmol, 3 eq.) was mixed with 3,5-dimethylhex-1-yn-3-ol (3 g, 23.7 mmol, 1 eq.) in dioxane (15 ml). Oxygen was supplied to the reaction from balloons. After 22 hours at 100 °C, the reaction solvent was removed in vacuum to yield a yellow liquid, which was further purified in column chromatography (dichloromethane/methanol 100/1) to yield compound FR16 as a yellow liquid (5.98 g, 45%). Compound FR16: yellow liquid; 1H NMR (600 MHz, CDCl3) δ 8.06 (ddd, J = 12.6, 7.6, 1.4 Hz, 1H), 7.99–7.86 (m, 6H), 7.72–7.62 (m, 2H), 7.50–7.43 (m, 2H), 7.33 (ddt, J = 8.3, 7.4, 1.1 Hz, 3H), 7.25–7.16 (m, 5H), 4.39–4.20 (m, 2H), 3.99 (dt, J = 10.4, 5.1 Hz, 1H), 3.87–3.51 (m, 10H), 3.50–3.40 (m, 3H); 13C NMR (151 MHz, CDCl3) δ 149.51, 149.45, 149.39, 149.33, 136.39, 136.35, 136.18, 136.14, 133.78, 133.76, 133, 66, 133.64, 131.95, 131.88, 131.34, 131.27, 130.67, 130.56, 129.11, 128.60, 128.51, 128.48, 128.40, 126.97, 125.00, 124.93, 124.60, 124.56, 123.51, 123.45, 123.39, 122.57, 122.33, 121.83, 121.80, 121.76, 121.73, 121.66, 121.56, 120.91, 120.39, 120.35, 120.12, 120.08, 73.02, 72.88, 70.97, 70.27, 70.23, 70.20, 69.76, 69.01, 61.28, 61.16, 29.69; FT-IR (KBr): νmax/cm−1 754, 912, 1117, 1200, 1431, 1447, 1477, 1594, 2867, 2958, 3063, 3214.
3.4. Incorporation of flame retardants into epoxy resin
Epoxy resin LR 385, APP–PEI, and DOPO derivatives (FR1–FR16) on different ratios (Table 3) were mixed together at 60 °C for 30 minutes. Let the mixtures cool down to room temperature. A hardener LH-368 curing agent was added to the dispersed mixtures. The mixtures were stirred for more minutes until they homogenized. Then the mixtures were poured into the molds and cured for 8 hours to obtain the research samples.| Entry | Composite samples | Epoxy resin (g) | Hardener (g) | APP–PEI (g) | DOPO derivatives (g) |
|---|---|---|---|---|---|
| 1 | EP | 7.56 | 3.24 | — | — |
| 2 | EP/10APP–PEI | 7.56 | 3.24 | 1.2 | — |
| 3 | EP/10FR11 | 7.56 | 3.24 | — | 1.2 |
| 4 | EP/5APP–PEI/5FR1 | 7.56 | 3.24 | 0.6 | 0.6 |
| 5 | EP/5APP–PEI/5FR2 | 7.56 | 3.24 | 0.6 | 0.6 |
| 6 | EP/5APP–PEI/5FR3 | 7.56 | 3.24 | 0.6 | 0.6 |
| 7 | EP/5APP–PEI/5FR4 | 7.56 | 3.24 | 0.6 | 0.6 |
| 8 | EP/5APP–PEI/5FR5 | 7.56 | 3.24 | 0.6 | 0.6 |
| 9 | EP/5APP–PEI/5FR6 | 7.56 | 3.24 | 0.6 | 0.6 |
| 10 | EP/5APP–PEI/5FR7 | 7.56 | 3.24 | 0.6 | 0.6 |
| 11 | EP/5APP–PEI/5FR8 | 7.56 | 3.24 | 0.6 | 0.6 |
| 12 | EP/5APP–PEI/5FR9 | 7.56 | 3.24 | 0.6 | 0.6 |
| 13 | EP/5APP–PEI/5FR10 | 7.56 | 3.24 | 0.6 | 0.6 |
| 14 | EP/5APP–PEI/5FR11 | 7.56 | 3.24 | 0.6 | 0.6 |
| 15 | EP/5APP–PEI/5FR12 | 7.56 | 3.24 | 0.6 | 0.6 |
| 16 | EP/5APP–PEI/5FR13 | 7.56 | 3.24 | 0.6 | 0.6 |
| 17 | EP/5APP–PEI/5FR14 | 7.56 | 3.24 | 0.6 | 0.6 |
| 18 | EP/5APP–PEI/5FR15 | 7.56 | 3.24 | 0.6 | 0.6 |
| 19 | EP/5APP–PEI/5FR16 | 7.56 | 3.24 | 0.6 | 0.6 |
3.5. Characterization
The evaluation of IR spectrum was carried out on PerkinElmer device with KBr pellets (the frequency range: 4000–400 cm−1). The structure of DOPO derivatives was elucidated by 1H and 13C-NMR spectra on a Bruker 600 MHz spectrometer with DMSO-d6 and CDCl3 as solvents. The surface morphology of the composites was observed on a field emission scanning electron microscope (FE-SEM, Hitachi S-4800, Japan) operating at 5 kV. Using a thermogravimeter (LABSYS Evo STA, Setaram, France), thermogravimetric analysis (TGA) was carried out from room temperature to 700 °C with a rate of 10 °C min−1 under nitrogen environment, differential scanning calorimetry (DSC) was performed from room temperature to 450 °C with a rate of 10 °C min−1 under oxygen air atmosphere.The flammability of epoxy composites was evaluated by the UL-94 vertical burning test. The UL-94 burning rating was measured on HT-4326 device (Shenzhen Meiju Gao Testing Equipment Co., Ltd.) according to the ASTM D3801-2010 standard, with the sample size of 125 mm × 13 mm × 3.2 mm. The achieved data was sorted into four levels: V-0, V-1, V-2 and not qualified. According to ASTM D2863-97, the limiting oxygen index (LOI) values of the composites were determined by an oxygen index flammability tester (Yasuda 214, Japan).
The mechanical stability of epoxy resin incorporated with flame retardants was evaluated by the Izod impact strength test on TM2101-T5 device (China) with standard samples of 75 mm × 13 mm × 3.2 mm and the impact velocity of 3.5 m s−1.
4 Conclusions
In conclusion, the combination of APP–PEI with novel DOPO derivatives has shown a synergistic effect in enhancing the flame retardant characteristics of epoxy resin. Moreover, the obtained composite has maintained or improved its mechanical performance compared to when APP–PEI is used alone. The most effective flame retardant organic materials possess a structure including a diphenyl ether bridging core. EP/5APP–PEI/5FR10 and EP/5APP–PEI/5FR11 are the best options with UL-94 V-0 rating and LOI values of 28.4% and 28.6%, respectively. The flame retardancy mechanism is achieved through a synergistic effect of radical trapping DOPO derivatives and char-forming APP–PEI. This approach overcomes the limitations of high loadings when used individually. These findings provide useful insights for the future development of flame retardant epoxy composites.Author contributions
Cong Trinh Duc: investigation: evaluation of the flammability and mechanical strength properties of epoxy composites. Linh Chi Nguyen, Phuc Ban Van, and Quynh Giang Nguyen Thi: investigation: synthesis of DOPO derivatives. Ha Thanh Nguyen: investigation: characterization of derivatives, writing – review & editing. Tuyet Anh Dang Thi and Giang Le-Nhat-Thuy: investigation: characterization of derivatives. Phuong Hoang Thi, Tuan Anh Nguyen, and Quang Vinh Tran: investigation: synthesis of composites. Hung Tran Quang: investigation: writing – original draft. Mai Ha Hoang: investigation: evaluation of thermal properties of the composites. Tuyen Nguyen Van: project administration: conceptual design, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Vietnam Academy of Science and Technology (Grant No. TĐPCCC.03/21-23).Notes and references
- H. Q. Pham and M. J. Marks, Epoxy Resins, in Ullmann's Encyclopedia of Industrial Chemistry, 2005 Search PubMed.
- A. Bifulco, C. Varganici, L. Rosu, F. Mustata, D. Rosu and S. Gaan, Recent advances in flame retardant epoxy systems containing non-reactive DOPO based phosphorus additives, Polym. Degrad. Stab., 2022, 200, 109962 CrossRef CAS.
- S. Huo, P. Song, B. Yu, S. Ran, V. S. Chevali, L. Liu, Z. Fang and H. Wang, Phosphorus-containing flame retardant epoxy thermosets: recent advances and future perspectives, Prog. Polym. Sci., 2021, 114, 101366 CrossRef CAS.
- W. Peng, S. Nie, Y. Xu and W. Yang, A tetra-DOPO derivative as highly efficient flame retardant for epoxy resins, Polym. Degrad. Stab., 2021, 193, 109715 CrossRef CAS.
- A. Schäfer, S. Seibold, O. Walter and M. Döring, Novel high Tg flame retardancy approach for epoxy resins, Polym. Degrad. Stab., 2008, 93, 557–560 CrossRef.
- M. Li, X. Hao, M. Hu, Y. Huang, Y. Qiu and L. Li, Synthesis of bio-based flame-retardant epoxy co-curing agent and application in wood surface coating, Prog. Org. Coat., 2022, 167, 106848 CrossRef CAS.
- J. Wang, H. Tang, X. Yu, J. Xu, Z. Pan and H. Zhou, Reactive organophosphorus flame retardant for transparency, low-flammability, and mechanical reinforcement epoxy resin, J. Appl. Polym. Sci., 2021, 138, 50536 CrossRef CAS.
- P. Zhao, W. Rao, H. Luo, L. Wang, Y. Liu and C. Yu, Novel organophosphorus compound with amine groups towards self-extinguishing epoxy resins at low loading, Mater. Des., 2020, 193, 108838 CrossRef CAS.
- J. Zhou, Z. Heng, H. Zhang, Y. Chen, H. Zou and M. Liang, High residue bio-based structural–functional integration epoxy and intrinsic flame retardant mechanism study, RSC Adv., 2019, 9, 41603–41615 RSC.
- T. Chen, X. Chen, M. Wang, P. Hou, C. Jie, J. Li, Y. Xu, B. Zeng and L. Dai, A novel halogen-free co-curing agent with linear multi-aromatic rigid structure as flame-retardant modifier in epoxy resin, Polym. Adv. Technol., 2018, 29, 603–611 CrossRef CAS.
- P. Wang, F. Yang, L. Li and Z. Cai, Flame-retardant properties and mechanisms of epoxy thermosets modified with two phosphorus-containing phenolic amines, J. Appl. Polym. Sci., 2016, 133, 43953 CrossRef.
- Z. Liu, Z. Yu, T. Qiaolin, Z. Kaixin, D. Weihao, Z. Lewen, W. Rong, C. Jin, D. Jingjing, L. Wang, W. Qiwei, C. Mingjun and L. Zhiguo, Highly efficient flame-retardant and transparent epoxy resin, Polym. Adv. Technol., 2021, 32, 2940–2952 CrossRef CAS.
- Y. Tan, Z. B. Shao, L. X. Yu, Y. J. Xu, W. H. Rao, L. Chen and Y. Z. Wang, Polyethyleneimine modified ammonium polyphosphate toward polyamine-hardener for epoxy resin: thermal stability, flame retardance and smoke suppression, Polym. Degrad. Stab., 2016, 131, 62–70 CrossRef CAS.
- H. T. Doan, N. T. Nguyen, N. T. Hac, P. T. L. Huong, M. H. Hoang, Q. A. Ngo, V. T. Nguyen and V. Q. Tran, Synthesis and synergistic effect of cuprous(I) oxide nanoparticles and polyethyleneimine modified ammonium polyphosphate on enhancing the flame resistance of epoxy resin, Mater. Res. Express, 2023, 10, 115002 CrossRef.
- X. Xu, S. Wang, S. Ma, W. Yuan, Q. Li, J. Feng and J. Zhu, Vanillin-derived phosphorus-containing compounds and ammonium polyphosphate as green fire-resistant systems for epoxy resins with balanced properties, Polym. Adv. Technol., 2019, 30, 264–278 CrossRef CAS.
- C. Achmad, A. Widi, A. Venitalitya, T. N. Dinda and R. Nur, Removal of methyl violet dye via adsorption using activated carbon prepared from Randu sawdust (Ceiba pentandra), IOP Conf. Ser. Earth Environ. Sci., 2018, 167, 012013 CrossRef.
- K. S. Lim, S. T. Bee, L. T. Sin, T. T. Tee, C. T. Ratnam, H. David and A. R. Rahm, A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites, Composites, Part B, 2016, 84, 155–174 CrossRef CAS.
- Y. K. Chen, Q. X. Lu, G. Zhong, H. G. Zhang, M. F. Chen and C. P. Liu, DOPO-based curing flame retardant of epoxy composite material for char formation and intumescent flame retardance, J. Appl. Polym. Sci., 2021, 138(9), 49918 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00051j |
| This journal is © The Royal Society of Chemistry 2024 |