Open Access Article
Open Access ArticleQuantification of the composition of pyrolysis oils of complex plastic waste by gas chromatography coupled with mass spectrometer detector†
A. Serras-Malillos,
B. B. Perez-Martinez,
A. Iriondo,
E. Acha *,
A. Lopez-Urionabarrenechea and
B. M. Caballero
*,
A. Lopez-Urionabarrenechea and
B. M. Caballero
Chemical and Environmental Engineering Department, Faculty of Engineering of Bilbao, University of the Basque Country (UPV/EHU), Plaza Ingeniero Torres Quevedo, 1, 48013-Bilbao, Spain. E-mail: esther.acha@ehu.eus
First published on 25th March 2024
Abstract
Waste valorisation through pyrolysis generates solid, liquid and gaseous fractions that need to be deeply characterised in order to try to recover secondary raw materials or chemicals. Depending on the waste and the process conditions, the liquid fraction obtained (so-called pyrolysis oil) can be very complex. This work proposes a method to quantitatively measure the composition of pyrolysis oils coming from three types of polymeric waste: (1) plastic packaging from sorting plants of municipal solid waste, (2) plastic rich fractions rejected from sorting plants of waste of electrical and electronic equipment and (3) end-of-life carbon/glass fibre reinforced thermoset polymers. The proposed methodology uses a gas chromatography (GC) coupled with mass spectrometer detector (MS) analytical technique, a certified saturated alkanes' mix, an internal standard and fourteen model compounds. Validation of the methodology concluded that the average relative error was between −59 wt% and +62 wt% (with standard deviations between 0 wt% and 13 wt%). Considering that the state-of-the-art scenario to quantify complex plastic pyrolysis oils as a whole is almost none and that they are usually evaluated only qualitatively based on the area percentage of the GC-MS chromatograms, the presented quantification methodology implies a clear step forward towards complex pyrolysis oil compositional quantification in a cost-effective way. Besides, this quantification methodology enables determining what proportion is being detected by GC-MS with respect to the total oil. Finally, the presented work includes all the Kováts RI for complex temperature-program gas chromatography of all the signals identified in the analysed pyrolysis oils, to be readily available to other researchers towards the identification of chemical compounds in their studies.
1. Introduction
Thermal and chemical recycling processes are being promoted in recent years in order to valorise plastic waste that is difficult to recycle mechanically. The pyrolysis process, which has been studied for several decades to recycle thermosets, thermoplastics and complex mixtures of plastics and other materials, is currently receiving unusual interest from industry and administration.1 It is possible to return to the economy certain valuable products from non-recyclable waste that currently litters landfills around the world.2,3 Pyrolysis generates three product fractions: solid, liquid and gas. When pyrolysing plastic (or plastic rich) waste, the liquid fraction (commonly known as pyrolysis oils) is usually the main and target product. It consists of a complex mixture of hydrocarbons of a broad range of carbon atoms, which, depending on the waste pyrolysed, may be accompanied by other heteroatomic organic chemicals (mainly nitrogen, sulphur, oxygen, halogens and metals) that could be considered pollutants.4M. Kusenberg et al. presented a detailed review of the most common analytical techniques used to chemically characterise waste plastic pyrolysis oils.5 As they reported, chromatography stands out as the most frequently used method for oil compositional analysis thanks to its versatility, as different detectors can be coupled and trace compounds down to ppb-s can be detected. The thermal conductivity detector (TCD) or the flame ionisation detector (FID) are reported to be currently the widest used in chromatography configurations.5–7 In the case of the mass spectrometry detector (MS), the main advantage lies in its ability to identify unknown compounds. Indeed, TCD and FID could provide better signal quality results, but using these detectors would require prior knowledge of the nature of the chemicals present in pyrolysis oils, because identification is based on the comparison between the retention time of each chemical present in the oil and that of a standard substance. This is not an easy task, or even possible, when dealing with pyrolysis oils from real waste where thousands of chemical compounds could coexist as it has been reported for liquids obtained from the pyrolysis of biomass8,9 or from petroleum fractions.10 Combining the GC technique with mass spectrometry (MS) detectors could be a way to address coelution and complexity of the samples.11 Gas chromatography coupled with mass spectrometry (GC-MS) provides a qualitative compositional analysis, based on the correspondence between the measured mass spectra and those available in the MS libraries12 for volatile and semi-volatile compounds (typically weights up to 220 Da and/or boiling points below 350 °C), being possible to detect hundreds of compounds.6,8,13 However, chromatographic techniques combined with additional detectors (GC-MS/FID, GCxGC/FID/MS and high-pressure liquid chromatography) are claimed as the most appropriate to characterise and quantify compounds in pyrolysis oils, based on a recent review about characterisation of pyrolysis oils from plastics.11 Other powerful techniques such as gel permeation chromatography, ultraviolet-visible spectroscopy, Fourier-transform infrared spectroscopy, nuclear magnetic resonance and high resolution mass spectrometry are also mentioned as necessary in order to have a complete overview of the oil composition. These spectroscopic techniques have been successfully applied to classical petrochemical hydrocarbon streams (i.e. gasoline or diesel fuels). However, they are not enough if a detailed molecular characterisation is pursued and compositional differences exist between these fuels and pyrolysis oils from plastic waste. Despite the undoubtedly promising contribution to this research field of the mentioned techniques (with special mention to GCxGC), their use may not be as widespread as GC-MS yet, probably, due to the higher technical complexity as well as their higher cost.
Concerning quantitative composition of pyrolysis oils, during all these years the scientific community has normally used the “peak area percent” as the standard indicator for composition when using GC-MS.14–21 However, the area percentage does not provide real information on the amount, because the signal intensity is not directly proportional to the concentration. Indeed, aspects such as the molecular weight of the species, the polarity, the selected chromatographic column, the volatility and the temperature influence the signal obtained. This “peak area percent” could be enough to be able to compare the compositional variations generated by the change in operating conditions and to know the approximate composition (aromatics, paraffins, olefins and others) of the oils that were proposed for use as alternative fuels or refinery blending mixtures.6 However, individual quantification of each compound requires a laborious calibration procedure that is usually not cost-effective due to the large number, variability and diversity of chemicals implied.7,13,22,23 A method is therefore needed that strikes a compromise between the cost in time/effort and the quality of the information obtained.
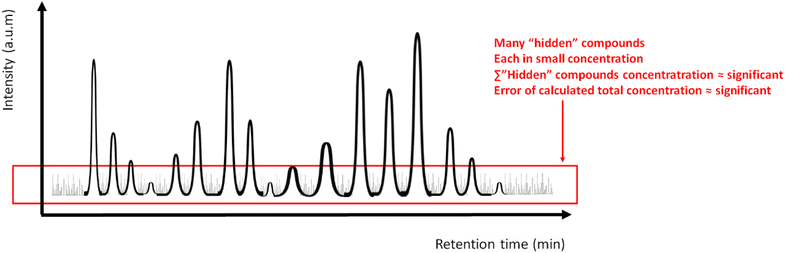
Apart from the challenge of quantification, there are also difficulties in identifying some chemicals present in pyrolysis oils. In fact, it is very common for a proportion, sometimes not negligible, of these oils to be defined as “unidentified”. In this respect, it is possible to use mass spectral libraries such as those of NIST/EPA/NIH for identification.24 However they are only partially useful if a complete identification and quantification of all peaks present is pursued.22 The use of retention indices is the easiest way to identify chemicals because they perform better in peak identification than retention times (RT), due to their lesser dependency on GC parameters.25 More precisely, the Kováts Retention Index (Kováts RI), defined as the relative retention time normalised to the near eluting n-alkanes,26 is the most accepted one.12,22 Updated databases (i.e. NIST 17 GC Method/Retention Index Library)27,28 and specific software (e.g. MassFinder 4 (ref. 26)) do exist, where compounds and RI are related. What is missing is the availability of a systematic assignment of the Kováts RI to pyrolysis oils in research works, in order to feed and complete a global database where the identification of compounds based on this index would be simple and useful for both the scientific community and the industry.
This paper presents a methodology to identify and quantify by GC-MS the composition of pyrolysis oils produced from different kinds of real complex plastic waste: mixed plastics from packaging, mixed plastics from electronics and fibre reinforced thermosets. The idea is to provide with a better solution to all those research groups who analyse pyrolysis oils by GC-MS and report their results in area%.29,30 The proposed methodology was successfully applied to twelve pyrolysis oils coming from the three mentioned plastic groups. The results of three of the pyrolysis oils have been explicitly included in this text and the results of the remaining nine have been included in the ESI† to limit the length of this paper. The non-isothermal Kováts RI (modification introduced by Van den Dool and Kratz)31 of all the chemicals identified in the oils analysed are also included. The aim is to contribute to the existing databases28 and to facilitate the identification of chemicals for the scientific community working on the pyrolysis of waste plastics.
2. Materials and methods
2.1. Pyrolysis oils
Three types of plastic waste were used as feedstock for the pyrolysis oil generation: post-consumer plastic packaging waste (Packaging), plastic fraction rejected from waste of electric and electronic equipment (WEEE) and fibre reinforced plastic waste (FRP). The intention was to produce pyrolysis oils of different chemical characteristics, in order to design a method that would be versatile. Thus, the so-called Packaging sample was a reject from the planar fraction obtained in mechanical–biological treatment plants of municipal solid waste and consisted mainly of polyolefins (polyethylene and polypropylene), which normally produces alkane and olefin-rich hydrocarbon mixtures in oils, and polystyrene. The WEEE sample was the plastic reject from a WEEE management and recycling plant and in this case consisted mainly of styrenic plastics (polystyrene, acrylonitrile-butadiene-styrene), which generate oils of aromatic nature. At last, the FRP waste was an end-of-life glass fibre reinforced polyester thermoset resin from the nautical sector, which produces oxygenated and aromatic oils with a significant proportion of unidentified compounds, some of them including heteroatoms like nitrogen and halogens. In addition to this particular FRP waste, the method was also tested with oils from other glass and carbon fibre reinforced plastic waste, both based on polyester and epoxy. This information can be found in Table S1 of the ESI.†Waste samples of ca. 100 g were pyrolysed in each case in a laboratory installation composed of a stainless steel non-stirred tank reactor electrically heated. The condensable compounds present in the generated pyrolysis vapours were cooled down and collected as liquids. As in the case of sample selection, oils from pyrolysis processes of different operating conditions were also selected. More precisely, for the post-consumer plastic packaging a single-step pyrolysis treatment was performed using a heating rate of 40 °C min−1 up to 640 °C without carrier gas but with pure N2 purge. Regarding the WEEE sample, the selected oil came from a stepwise pyrolysis process (heating rate of 15 °C min−1, 1 h isothermal step at 300 °C and a later heating till 500 °C) carried out with a continuous N2 gas flow of 1 L min−1 (at 20 °C and 1 bar). In addition, before the condensation of oils, the generated pyrolysis volatiles were further thermally cracked at 400 °C in a fixed bed reactor in series, where a halogen adsorbent was placed. In this case, the proposed methodology was also applied for oils coming from the same sample (WEEE), but processed at different operating conditions (see Table S1 in ESI†). At last, the oil coming from the FRP waste sample (as well as those included in the Table S1†) was obtained through a single-step pyrolysis treatment using a heating rate of 3 °C min−1 up to 500 °C without N2 purge nor inert carrier gas. Table 1 shows the summary of the qualitative composition by GC-MS (following the chromatographic programme described in Section 2.3) for the three pyrolysis oils analysed in the present work and employed as use-case (Packaging, WEEE and FRP).
| No. | RT (min) | Compound | Formula | CAS number | Area% |
|---|---|---|---|---|---|
| “Packaging”: plastic packaging from sorting plants | |||||
| 1 | 3.6 | n-Hexane | C6H14 | 110-54-3 | 1.1 |
| 2 | 3.7 | 1-Hexene | C6H12 | 592-41-6 | 0.8 |
| 3 | 3.8 | n-Heptane | C7H16 | 142-82-5 | 0.4 |
| 4 | 4.0 | 1-Heptene | C7H14 | 592-76-7 | 1.0 |
| 5 | 7.9 | Toluene | C7H8 | 108-88-3 | 1.9 |
| 6 | 8.1 | 1-Octene | C8H16 | 111-66-0 | 1.8 |
| 7 | 8.3 | Water | H2O | 7732-18-5 | 0.7 |
| 8 | 9.1 | Cyclooctene | C8H14 | 931-88-4 | 0.1 |
| 9 | 9.9 | Ethylbenzene | C8H10 | 100-41-4 | 2.8 |
| 10 | 12.9 | Styrene | C8H8 | 100-42-5 | 38.1 |
| 11 | 14.5 | Alpha-methylstyrene | C9H10 | 98-83-9 | 3.6 |
| 12 | 15.4 | 1-Undecene | C11H22 | 821-95-4 | 1.3 |
| 13 | 17.1 | 5-Dodecene, (E)- | C12H24 | 7206-16-8 | 1.1 |
| 14 | 24.5 | 5-Octadecene, (E)- | C18H36 | 7206-21-5 | 0.5 |
| 15 | 25.7 | Nonadecane | C19H40 | 629-92-5 | 0.2 |
| 16 | 26.7 | 1-Nonadecene | C19H38 | 18435-45-5 | 0.8 |
| 17 | 27.8 | Eicosane | C20H42 | 112-95-8 | 0.4 |
| 18 | 28.7 | Cycloeicosane | C20H40 | 296-56-0 | 0.9 |
| 19 | 29.6 | Heneicosane | C21H44 | 629-94-7 | 0.8 |
| 20 | 30.4 | 10-Heneicosene (c,t) | C21H42 | 95008-11-0 | 1.1 |
| 21 | 31.2 | Docosane | C22H46 | 629-97-0 | 0.9 |
| 22 | 31.8 | Benzene, 1,1′-(1,3-propanediyl)bis- | C15H16 | 1081-75-0 | 0.7 |
| 23 | 31.9 | 1-Docosene | C22H44 | 1599-67-3 | 1.4 |
| 24 | 32.6 | Tricosane | C23H48 | 638-67-5 | 1.1 |
| 25 | 33.2 | 9-Tricosene, (Z)- | C23H46 | 27519-02-4 | 1.2 |
| 26 | 33.8 | Tetracosane | C24H50 | 646-31-1 | 1.0 |
| 27 | 34.5 | Cyclotetracosane | C24H48 | 297-03-0 | 1.2 |
| 28 | 35.0 | Pentacosane | C25H52 | 629-99-2 | 1.3 |
| 29 | 35.6 | Z-12-Pentacosene | C25H50 | — | 1.1 |
| 30 | 36.9 | 9-Hexacosene | C26H52 | 71502-22-2 | 1.4 |
| 31 | 37.5 | Heptacosane | C27H56 | 593-49-7 | 0.8 |
| 32 | 38.9 | Octacosane | C28H58 | 630-02-4 | 0.7 |
| 33 | 39.8 | Cyclooctacosane | C28H56 | 297-24-5 | 0.9 |
| 34 | 40.6 | Nonacosane | C29H60 | 630-03-5 | 0.7 |
| 35 | 41.7 | Z-14-Nonacosane | C29H58 | — | 0.8 |
| 36 | 44.1 | Cyclotriacontane | C30H60 | 297-35-8 | 0.7 |
| 37 | 45.2 | Triacontane | C30H62 | 638-68-6 | 0.5 |
| Unknown | 24.2 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| “WEEE”: plastic fraction from waste of electric and electronic equipment | |||||
| 1 | 6.1 | Benzene | C6H6 | 71-43-2 | 0.3 |
| 2 | 7.2 | 2-Propenenitrile | C3H3N | 107-13-1 | 0.3 |
| 3 | 7.4 | 2-Butenoic acid, methyl ester | C5H8O2 | 18707-60-3 | 0.3 |
| 4 | 8.1 | Toluene | C7H8 | 108-88-3 | 9.2 |
| 5 | 10.2 | Ethylbenzene | C8H10 | 100-41-4 | 16.4 |
| 6 | 11.3 | Benzene, (1-methylethyl) | C9H12 | 98-82-8 | 2.7 |
| 7 | 12.1 | Benzene, propyl | C9H12 | 103-65-1 | 0.6 |
| 8 | 13.2 | Styrene | C8H8 | 100-42-5 | 42.4 |
| 9 | 14.7 | Alpha-methylstyrene | C9H10 | 98-83-9 | 11.8 |
| 10 | 25.8 | Naphthalene, 1-methyl | C11H10 | 90-12-0 | 0.1 |
| 11 | 25.9 | Phenol, 2,6-dimethyl | C8H10O | 576-26-1 | 0.6 |
| 12 | 27.9 | Phenol | C6H6O | 108-95-2 | 8.2 |
| 13 | 29.4 | Phenol, 2,3-dimethyl- | C8H10O | 526-75-0 | 0.8 |
| 14 | 30.1 | Benzenebutanenitrile | C10H11N | 2046-18-6 | 1.2 |
| 15 | 30.9 | Phenol, 3-ethyl | C8H10O | 620-17-7 | 0.4 |
| 16 | 31.5 | Phenol, 4-(1-methylethyl) | C9H12O | 99-89-8 | 3.1 |
| 17 | 32.5 | Phenol, p-tert-butyl | C10H14O | 98-54-4 | 0.7 |
| 18 | 34.7 | p-Isopropenylphenol | C9H10O | 4286-23-1 | 0.5 |
| Unknown | 0.6 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| “FRP”: fibre reinforced polyester resin based plastic waste | |||||
| 1 | 4.3 | Propanal | C3H6O | 123-38-6 | 1.9 |
| 2 | 6.1 | Benzene | C6H6 | 71-43-2 | 2.3 |
| 3 | 7.7 | 1,3-Dioxolane, 2-ethyl-4-methyl- | C6H12O2 | 4359-46-0 | 4.4 |
| 4 | 8.2 | Toluene | C7H8 | 108-88-3 | 2.8 |
| 5 | 10.2 | Ethylbenzene | C8H10 | 100-41-4 | 8.6 |
| 6 | 11.3 | Benzene, (1-methylethyl)- | C9H12 | 98-82-8 | 1.4 |
| 7 | 13.2 | Styrene | C8H8 | 100-42-5 | 19.9 |
| 8 | 32.2 | Benzene, 1,1′-(1,3-propanediyl)bis- | C15H16 | 1081-75-0 | 11.2 |
| 9 | 34.6 | Benzoic acid | C7H6O2 | 65-85-0 | 9.4 |
| 10 | 35.1 | Bicyclo[4.2.1]nona-2,4,7-triene, 7-phenyl- | C15H14 | — | 3.7 |
| Unknown | 34.3 | ||||
2.2. Commercial reagents
The development of the analytical quantification methodology proposed in this work required the use of the following chemical compounds or mixtures:(a) Mixture of saturated C7–C30 alkanes (alkane-mix) with reference number 49451-U from Supelco. This is a certified reference material with a concentration of 1000 μg mL−1 for each of the 23 components, which are dissolved in hexane. See Table S2 of the ESI.†
(b) 1-Propanol (purity 99.8%, CAS number 71-23-8, provided by Supelco) used as internal standard (IS).
(c) Six model compounds: cyclohexane (purity 99.5%, CAS number 110-82-7, supplied by PanReac-AppliChem), benzene (purity 99.8%, CAS number 71-43-2, supplied by Sigma-Aldrich), o-xylene (purity 99.0%, CAS number 95-47-6, supplied by Supelco), quinoline (purity 96.0%, CAS number 91-22-5, supplied by Honeywell Riedel-de Haën), m-cresol (purity 99.0%, CAS number 108-39-4, supplied by Sigma-Aldrich) and ethyl 4-aminobenzoate (purity 98.0%, CAS number 94-09-7, supplied by Thermo Scientific).
(d) Additional compounds needed to complete the calibration at low retention times: n-heptane (purity 99.0%, CAS number 142-82-5, supplied by PanReac-AppliChem) and cyclopentyl methyl ether (CPME) (purity 99.9%, CAS number 5614-37-9, supplied by Sigma-Aldrich).
(e) Chromatographic grade tetrahydrofuran (THF) solvent (purity 99.9%, product code 361736, supplied by PanReac-AppliChem).
(f) Chemical compounds used for the methodology validation: phenol (purity 99.5%, CAS number 108-95-2, supplied by Sigma-Aldrich), toluene (purity 99.5%, CAS number 108-88-3, supplied by Sigma-Aldrich), ethylbenzene (purity 99.0%, CAS number 100-41-4, supplied by Sigma-Aldrich), styrene (purity 99.9%, CAS number 100-42-5, supplied by Supelco), benzenebutanenitrile (purity 99.0%, CAS number 2046-18-6, supplied by Sigma-Aldrich). Additionally, nine of the alkanes contained in the alkane-mix that were not used as calibration compounds were used as validation compounds: nonadecane, eicosane, heneicosane, tricosane, tetracosane, pentacosane, heptacosane, nonacosane and tricontane. The selection of alkanes from the alkane-mix to be used in the calibration process will be further described in Section 3.1.
2.3. GC-MS analysis
The pyrolysis oils were prepared for analysis by diluting ca. 0.4 g of the oil in the case of “Packaging”, ca. 0.1 g and 0.01 g for “WEEE” and “WEEE (1/10)”, respectively, and around 0.1 g and 1 g in the case of “FRP” and “FRP (×10)”, respectively, in 10 mL of a THF solution (Table 2) that already included 1-propanol as internal standard (IS). A constant concentration of the IS was employed to work with a signal area in the same order of magnitude of the signals obtained in the analysed pyrolysis oils chromatograms and to minimise the instrumental variabilities. The samples were analysed on an Agilent GC-MS system with a gas chromatograph (6890) using a J&W DB-FFAP capillary column (60 m × 0.320 mm × 0.25 μm) coupled to a mass spectrometer (5973). Injector temperature was set at 300 °C. The analysis started at 40 °C for 5 minutes, ramped up by 8 °C min−1 to 150 °C and held at 150 °C for 5 minutes. It was then heated to 240 °C at 8 °C min−1 and maintained for 10 minutes. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 split was used with 1.5 mL per min helium as carrier gas flow employing an injection volume of 0.5 μL. The electron ionisation (EI) was set to 70 eV and the source temperature was set to 230 °C. The mass spectrometer measured from 0 min to 10 min in a range of 10–120 m/z with a threshold of 150 and 10.68 scans per s, and from minute 10 onwards in a range of 33–442 m/z with a threshold of 150 and 3.67 scans per s. The initial mass acquisition range (RT: 0–10 min) upper limit was set just six mass-charge units, 120 m/z, above the molecular weight of n-octane (114 Da), the highest molecular weight compound that was expected to appear in the studied oils at low retention times. The second mass acquisition range (RT: 10–47 min) mass-charge limit was set, in its lower limit 33 m/z, to avoid contamination from ions that can enter the analysis from the air dragged during the injection, nitrogen (28 Da) and oxygen (32 Da), and its upper limit corresponded to the highest molecular weight compound of the calibration mix, triacontane (C30, 442 Da). The difference in the mass acquisition range frequencies was set by the detector limitations for scanning a wide range of masess, as for a wider range of masses the scans per second decreases. The commercial NIST-08 mass spectral library was used for identification of the compounds. The acceptance criterion for identification was set at a minimum match quality of 85%. Each sample was analysed in triplicate in order to ensure the repeatability of the measurements.
15 split was used with 1.5 mL per min helium as carrier gas flow employing an injection volume of 0.5 μL. The electron ionisation (EI) was set to 70 eV and the source temperature was set to 230 °C. The mass spectrometer measured from 0 min to 10 min in a range of 10–120 m/z with a threshold of 150 and 10.68 scans per s, and from minute 10 onwards in a range of 33–442 m/z with a threshold of 150 and 3.67 scans per s. The initial mass acquisition range (RT: 0–10 min) upper limit was set just six mass-charge units, 120 m/z, above the molecular weight of n-octane (114 Da), the highest molecular weight compound that was expected to appear in the studied oils at low retention times. The second mass acquisition range (RT: 10–47 min) mass-charge limit was set, in its lower limit 33 m/z, to avoid contamination from ions that can enter the analysis from the air dragged during the injection, nitrogen (28 Da) and oxygen (32 Da), and its upper limit corresponded to the highest molecular weight compound of the calibration mix, triacontane (C30, 442 Da). The difference in the mass acquisition range frequencies was set by the detector limitations for scanning a wide range of masess, as for a wider range of masses the scans per second decreases. The commercial NIST-08 mass spectral library was used for identification of the compounds. The acceptance criterion for identification was set at a minimum match quality of 85%. Each sample was analysed in triplicate in order to ensure the repeatability of the measurements.
| Name | ID#a | Pyrolysis oil weight (g) | Dissolution final volume (mL) | Total pyrolysis oil concentration (μg mL−1) |
|---|---|---|---|---|
| a Identification number used in the complete pyrolysis oil list in the ESI Table S1. | ||||
| Packaging | ID12 | 0.3544 | 10 mL | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
| WEEE | ID6 | 0.1004 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
|
| WEEE (1/10) | ID6 diluted | 0.0102 | 1020 | |
| FRP | ID1 | 0.0982 | 9820 | |
| FRP (×10) | ID1 concentrated | 1.0020 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
|
On the other hand, the use of split type injection generates discrimination by molecular mass. This problem can be solved using other types of injections, such as on-column injection, headspace sampling, splitless injection, etc. However, regarding these mentioned injection possibilities, it was not possible to adopt them due to the available GC-MS configuration. In fact, the complexity of the samples analysed and the different concentration in some of their components (as high as 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and as low as 450
000 and as low as 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (area units)) required the use of a split injection. Nonetheless, the molecular mass discrimination problem was addressed in this work by improving the heat transfer ahead of entering the column.32,33 For that purpose, a deactivated glass-wool packed liner (Agilent 5183-4647) was selected to try to minimize the loss of volatile compounds during the injection.
000 (area units)) required the use of a split injection. Nonetheless, the molecular mass discrimination problem was addressed in this work by improving the heat transfer ahead of entering the column.32,33 For that purpose, a deactivated glass-wool packed liner (Agilent 5183-4647) was selected to try to minimize the loss of volatile compounds during the injection.
To assess the possible differences between split and splitless injections due to molecular mass discrimination, the alkanes calibration mix used during this work was analysed by the chromatographic method proposed in this work and by an analogue with a splitless injection. The results, presented in Table S3 in ESI,† revealed that no discrimination was occurring during the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 split injection.
15 split injection.
3. Results and discussion
The state of the art calculates concentration of pyrolysis oils coming from plastic waste assuming equivalence of peak area percentage to weight percentage of compounds. The present approach introduces different response factors at different retention time intervals. In order to approach further accuracy of the quantitative method, a correction factor was used at each selected retention time intervals.3.1. Development of the quantification method
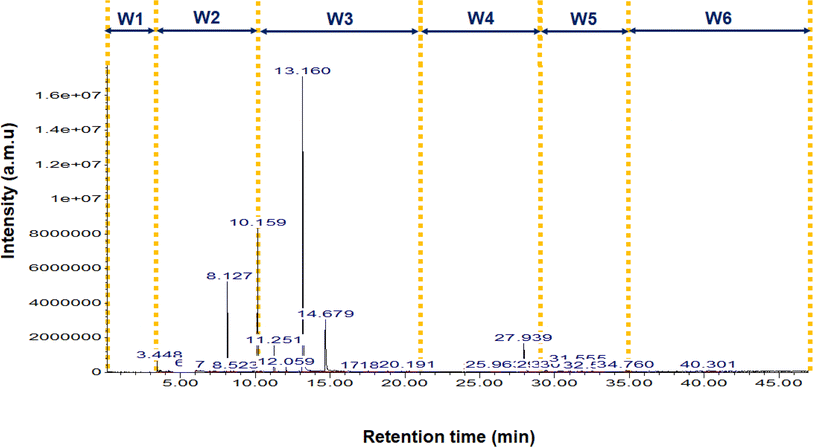
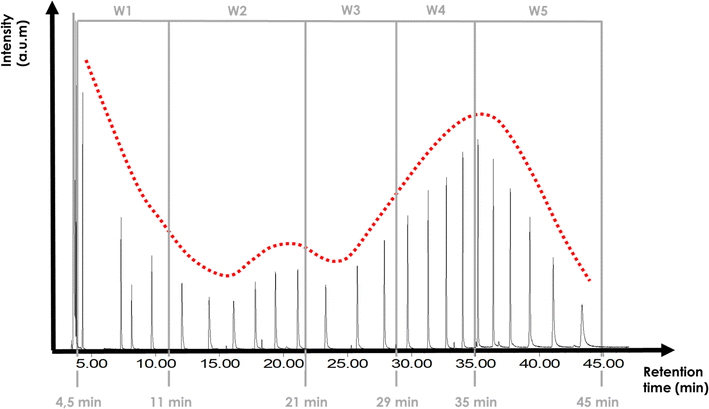
The first step of the quantification method was to divide the chromatogram total duration into smaller time sections which were named as windows (W), strategy that has been previously reported in the quantification procedure of bio-oils.34 The reason to dissect the chromatogram into smaller sections was to try to minimize the impact of all the aspects that have an influence on the area of the signals (i.e. molecular weight, polarity, solvent, chromatographic column). For the purpose of this paper, six windows were defined and applied to all the analysed pyrolysis oils: W1 from 0 min to 4.5 min, W2 from 4.5 min to 11 min, W3 from 11 min to 21 min, W4 from 21 min to 29 min, W5 from 29 min to 35 min and W6 from 35 min to 47 min. The selection of the number of windows was done as a compromise between introducing more than one calibration standard for the complete duration of the chromatogram and the minimisation of the proposed methodology's tediousness when applying it. The time interval duration was adapted to adjust the validation compounds that we selected. Fig. 1 shows the chromatogram of “WEEE” liquid (ID6) with the windows retention time range limited with yellow vertical lines to illustrate the followed procedure.The second step was to calibrate the certified saturated n-alkanes mix (hereafter the alkane-mix). Fig. 2 shows the chromatographic response (signal intensity vs. retention time) of the alkanes of the alkane-mix for a concentration of 1000 μg per mL per alkane. A red line has been added to the graph to highlight the variability of peak signal intensity in function of retention time for different alkane compounds with the same concentration. The approach of dissecting the chromatogram in windows and the use of one calibration compound per window seeks to capture better the response factor variation along the chromatogram typically found when using mass selective detectors. Calibration was performed with four different concentrations, dissolving the mix in THF. In addition to the alkane-mix, the compounds heptane and cyclopentyl methyl ether (CPME) were also employed to have other compounds at low retention times, due to the poor solubility of the low retention time compounds from the alkane-mix (see Fig. S1†). The four calibration concentrations used were: (a) alkane-mix in THF: 900 μg mL−1, 700 μg mL−1, 500 μg mL−1 and 300 μg mL−1, (b) heptane in THF: 873 μg mL−1, 679 μg mL−1, 485 μg mL−1 and 291 μg mL−1 and (c) CPME in THF: 837 μg mL−1, 651 μg mL−1, 465 μg mL−1 and 279 μg mL−1.
In this calibration process a response factor (RF) was obtained by linear regression between the peak area and concentration for each alkane of the alkane-mix, for heptane and for CPME. Details of the calibration for all compounds, response factors and the R2 linear fitting quality can be reviewed in Table 3. Eqn (1) shows the determination of the mentioned response factors obtained in the calibration process, where “alkane” applies to every alkane present in the alkane-mix, to heptane and to CPME. Note that the area of the alkane is referred to the area normalised to the internal standard (IS) area which has been employed to minimise the variability of the measured peak areas due to instrumental errors. Integrated areas were determined considering the total ion chromatogram (TIC).
 | (1) |
| Compound | Formula | RT (min) | RF | R2 |
|---|---|---|---|---|
| a Poor solubility. | ||||
| Heptane | C7H16 | 3.51 | 43.2 | 0.9823 |
| Cyclopentyl methyl ether (CPME) | C6H12O | 6.03 | 35.5 | 0.9915 |
| Octanea | C8H18 | 4.31 | 28.4 | 0.9982 |
| Nonanea | C9H20 | 7.31 | 25.7 | 0.9956 |
| Decanea | C10H22 | 9.71 | 25.5 | 0.9957 |
| Undecanea | C11H24 | — | — | — |
| Dodecanea | C12H26 | 12.06 | 25.7 | 0.991 |
| Tridecanea | C13H28 | 14.18 | 25.6 | 0.9936 |
| Tetradecane | C14H30 | 16.10 | 23.9 | 0.9909 |
| Pentadecane | C15H32 | 17.80 | 23.1 | 0.9911 |
| Hexadecane | C16H34 | 19.37 | 21.3 | 0.9801 |
| Heptadecane | C17H36 | 21.11 | 19.2 | 0.991 |
| Octadecane | C18H38 | 23.30 | 19.6 | 0.9924 |
| Nonadecane | C19H40 | 25.77 | 18.0 | 0.9866 |
| Eicosane | C20H42 | 27.88 | 16.8 | 0.9912 |
| Heneicosane | C21H44 | 29.70 | 15.5 | 0.9897 |
| Docosane | C22H46 | 31.30 | 14.2 | 0.9899 |
| Tricosane | C23H48 | 32.72 | 14.0 | 0.9919 |
| Tetracosane | C24H50 | 34.01 | 13.3 | 0.9891 |
| Pentacosane | C25H52 | 35.19 | 13.0 | 0.9828 |
| Hexacosane | C26H54 | 36.39 | 12.6 | 0.9865 |
| Heptacosane | C27H56 | 37.72 | 12.2 | 0.9896 |
| Octacosane | C28H58 | 39.26 | 11.0 | 0.9902 |
| Nonacosane | C29H60 | 41.07 | 11.6 | 0.9934 |
| Triacontane | C30H48 | 43.32 | 12.5 | 0.9989 |
The third step was to select, between the chemicals listed in Table 3, a representative calibration substance per window (hereafter calibration compounds). The aim was that the concentration of all substances belonging to the same window of the chromatogram was calculated using the response factor of the corresponding representative substance. For that, selected chemicals from the alkane-mix + heptane and CPME were: heptane in W1, CPME in W2, pentadecane in W3, octadecane in W4, docosane in W5 and octacosane in W6. These calibration compounds were selected trying to choose the compound located in the centre of each corresponding window. The fourth step was to establish a correction factor (CF) to calculate the concentration of such substances that could be present in pyrolysis oils but could be of very different chemical nature compared to the calibration compounds (alkanes + CMPE), for instance, naphthenic, aromatic and heteroatomic organic compounds. For that, six chemicals (hereafter model compounds) were selected: cyclohexane in W1, benzene in W2, o-xylene in W3, quinolone in W4, m-cresol in W5 and ethyl 4-aminobenzoate in W6. The correction factor (CF) calculation in each window is described in eqn (2), showing that the area of the model compound selected per window is divided by the area of the calibration compound per window. This CF value will be applied to all the detected signals in the chromatogram. Table 4 compiles the obtained results. Note that the area of the representative substance and the area of the model compound is referred to the area normalised to the IS area and it is calculated as the average of three measurements as aforementioned. Integrated areas were determined considering the total ion chromatogram (TIC).
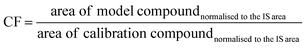 | (2) |
| Compound | Type | Window (W) | Conc. (μg mL−1) | Normalised average area (NAA) | CF = NAA of MC/NAA of CC | RF per window |
|---|---|---|---|---|---|---|
| a Calculated using the response factor from the calibration (Table 3). | ||||||
| Cyclohexane | MC | 1 | 490 | 13.9 | 1.2 | 43.2 |
| Heptane | CC | 490 | 11.3a | |||
| Benzene | MC | 2 | 540 | 12.5 | 0.8 | 35.5 |
| CPME | CC | 540 | 15.2a | |||
| o-Xylene | MC | 3 | 500 | 26.1 | 1.2 | 23.1 |
| Pentadecane | CC | 500 | 21.6a | |||
| Quinoline | MC | 4 | 670 | 38.4 | 1.1 | 19.6 |
| Octadecane | CC | 670 | 34.2a | |||
| m-Cresol | MC | 5 | 430 | 18.4 | 0.6 | 14.2 |
| Docosane | CC | 430 | 30.2a | |||
| Ethyl 4-aminobenzoate | MC | 6 | 480 | 21.4 | 0.5 | 11.0 |
| Octacosane | CC | 480 | 43.7a | |||
The fifth and last step consisted of calculating the concentration of the compounds detected in the chromatograms of the pyrolysis oils, as described in eqn (3). For that, the window to which each compound belonged was initially defined according to its retention time (RT). Then, the area of the compound was multiplied by the response factor (RF) (eqn (1)) per window and by the correction factor (CF) (eqn (2)) of that window too. Note that the area of the compound is referred to the area normalised to the IS area. Integrated areas were determined considering the total ion chromatogram (TIC).
| [Compound] = area of compoundnormalised to the IS area × CF × RF | (3) |
3.2. Determination of the relative error of the proposed quantification methodology
The proposed quantification methodology was evaluated through comparison with conventional calibration of fourteen chemical compounds (five of aromatic nature and nine of aliphatic nature) present in the pyrolysis oils (hereafter the validation compounds). Validation compounds were present in all the pyrolysis oils studied in this work and they were calibrated following the conventional procedure: preparing known concentrations of the compounds, analysing them in the GC-MS, and getting a response factor from the comparison of concentration and areas. Aromatic nature validation compounds were: toluene, ethylbenzene, styrene, phenol and benzenebutanenitrile, and aliphatic nature ones were: nonadecane, eicosane, heneicosane, tricosane, tetracosane, pentacosane, heptacosane, nonacosane and triacontane. The selection of the validation compounds was made trying to have them distributed along the complete duration of the chromatogram. In Table 5 the validation compounds are listed, together with the window of the chromatograph (W) in which each compound appears, the calibration concentration range, the response factor (RF) obtained per compound through conventional calibration and the R2 linear fitting quality. It can be observed that the conventional calibration was successfully performed with all the validation compounds, with a R2 value higher than 0.95.| W | Compound | Concentration (μg mL−1) | RF | R2 |
|---|---|---|---|---|
| Aromatic compounds | ||||
| 2 | Toluene | 170; 350; 650; 880; 900; 1200; 4270 | 20.4 | 0.9500 |
| 2 | Ethylbenzene | 270; 590; 900; 1212; 4640 | 20.2 | 0.9918 |
| 3 | Styrene | 240; 670; 800; 1520; 4508 | 17.5 | 0.9874 |
| 4 | Phenol | 300; 590; 930; 1230 | 27.8 | 0.9947 |
| 5 | Benzenebutanenitrile | 90; 330; 840 | 18.0 | 0.9983 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Aliphatic compounds | ||||
| 4 | Nonadecane | 300; 500; 700; 900 | 18.0 | 0.9866 |
| 4 | Eicosane | 300; 500; 700; 900 | 16.8 | 0.9912 |
| 5 | Heneicosane | 300; 500; 700; 900 | 15.5 | 0.9897 |
| 5 | Tricosane | 300; 500; 700; 900 | 14.0 | 0.9919 |
| 5 | Tetracosane | 300; 500; 700; 900 | 13.3 | 0.9891 |
| 6 | Pentacosane | 300; 500; 700; 900 | 13.0 | 0.9828 |
| 6 | Heptacosane | 300; 500; 700; 900 | 12.2 | 0.9896 |
| 6 | Nonacosane | 300; 500; 700; 900 | 11.6 | 0.9934 |
| 6 | Triacontane | 300; 500; 700; 900 | 12.5 | 0.9989 |
The validation of the proposed quantification methodology was done based on the absolute and relative errors obtained applying eqn (4) and (5), respectively. The prepared concentration (named as [ ]prepared (μg mL−1)) refers to the concentrations prepared for conventional calibration with the validation compounds, while the calculated concentration (named as [ ]calculated (μg mL−1)) refers to the concentrations calculated by applying the quantification methodology proposed in this work.
| Absolute error (μg mL−1) = [ ]prepared (μg mL−1) − [ ]calculated (μg mL−1) | (4) |
| Relative error (wt%) = 100 × absolute error (μg mL−1)/[ ]prepared (μg mL−1) | (5) |
Additionally, the concentrations of these validation compounds were also calculated following the proposed quantification methodology, but without applying the correction factor (CF). This was done in order to assess the impact of the nature of the quantified compounds and whether there was a need to implement a correction factor (CF) through the use of model compounds. Finally, the concentration of these validation compounds was also calculated considering that the area percentage was equal to weight percentage. The aim was to assess the hypothesis that the area percentage was equal to the weight percentage, and whether this could be an acceptable approach when only the order of magnitude of the concentration of the chemical compounds in the pyrolysis oils is pursued. In Fig. 3, the relative errors of these three quantification approaches are graphically represented (including average value and standard deviation). The average and the standard deviation values have been calculated considering the three pyrolysis oils used as use-case in this work (Packaging, WEEE, FRP) and the two additional liquid dissolutions (WEEE (1/10), FRP (×10)), as well as the additional nine pyrolysis oils studied and included in the ESI.† See Tables S4–S6† for detailed numerical data of the quantification of validation compounds for all the tested pyrolysis oils.
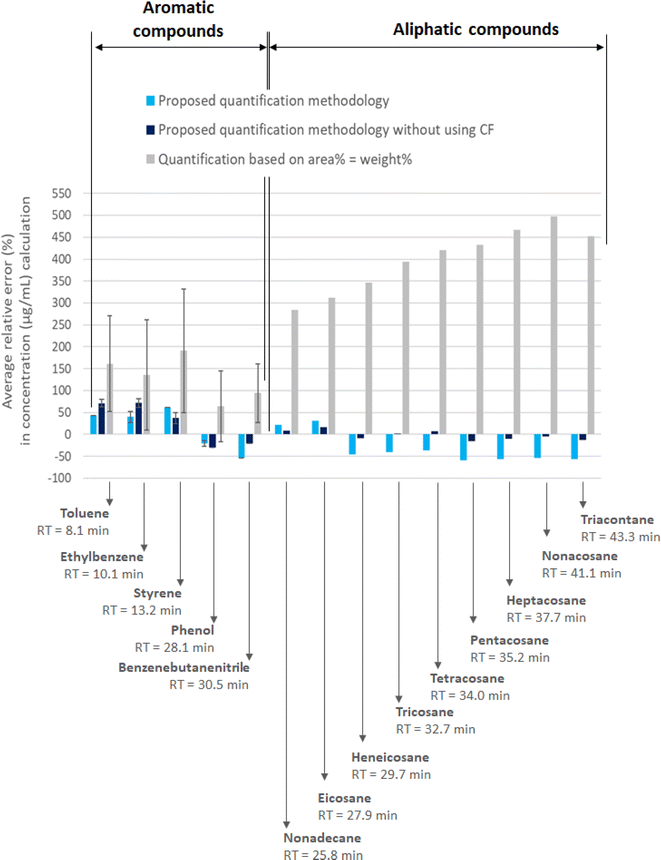
On the one hand, as observed in Fig. 3, the average relative error (wt%) when it is assumed that the area percentage was equivalent to the weight percentage (grey-colour bars) shows that lack of repeatability and high relative errors were generated for both aromatic and aliphatic compounds, although the latter showed the highest ones. This was an expected result based on the aspect of Fig. 2 where different areas are visually clear for same mass concentrations. Regarding the relative error with aromatic validation compounds was between 95 wt% to 191 wt% and the standard deviation between 67 wt% and 141 wt%. Regarding aliphatic validation compounds, no standard deviation is included because only one of the liquid-type (“Packaging” liquid) showed aliphatic compounds in it. In this case, aliphatic compounds showed relative errors between 284 wt% and 498 wt%. Therefore, in the light of these results, this quantification strategy (peak area% = weight%) can be considered useless from a quantification point of view, even for having an idea of the order of magnitude of the concentration of compounds in pyrolysis oils. This is an important remark because, as stated in the introduction of the article, the determination of the composition of pyrolysis oils through the area% obtained by GC-MS is a common and widespread practice in the scientific community.
In contrast, interesting results were observed when applying the proposed quantification methodology of this work, using the correction factor (light-blue-colour bars) and without using it (dark-blue-colour bars). Although the average relative errors were not negligible (they were between +62 wt% and −59 wt% using the CF and between +72 wt% and −29 wt% without using the CF) for all-type compounds, they were definitely lower than the peak area% approach. Moreover, taking into account that the currently employed procedures and standards for the quantification of organic compounds in bio-oils by GC-MS analytical technique assume variabilities around 20%,35–37 it could be considered that the relative error of the proposed quantification methodology application fell within the assumable deviation for complex liquids as such. Also, regarding the standard deviation registered for the aromatic compounds, they could be considered more stable results due to its lower variability (between 0 wt% and +13 wt%). Surprisingly, the application of the quantification methodology without using the correction factor (CF) showed very similar results to those obtained when including it. It seemed that the adjustment proposed for the alkane-mix response factor by using model compounds only reduces the quantification error for the compounds appearing at the initial part of the chromatogram (i.e. toluene and ethylbenzene). Meaning that, the determination of correction factors could be avoided with the consequent simplification of the proposed methodology. Obviously, results related to the quantification of aliphatic compounds without using the CFs showed much lower relative errors because there is no need to adjust the response factor of the alkane mix in this case.
3.3. Quantification methodology application for concentration calculation
The proposed quantification methodology was applied to all the detected signals in the chromatograms of the studied pyrolysis oils following the steps described in Section 3.1. As a result, the concentration (in μg mL−1) of each of the signals detected was obtained. Quantification results for each signal peak for the three use-case pyrolysis liquids (Packaging, WEEE and FRP) are included in Tables 6, 7 and 8, respectively, while the remaining nine pyrolysis oils, “WEEE (1/10)” and “FRP (×10)” can be found in the ESI (from Tables S7–S17†). The following information is included in the tables: (a) number of signal, (b) retention time (RT), (c) window (W), (d) compound name (in case quality match of the MS library search was equal or above 85%), (e) integrated area, (f) area percentage, (g) normalised area to the internal standard, (h) calculated concentration in μg mL−1 (calculated as normalised area of the compound multiplied by the correction factor (CF) and by the response factor (RF)), (i) calculated concentration in μg mL−1 (calculated as normalised area of the compound without (w/o) multiplying by the correction factor (CF) and multiplying by the response factor (RF)), (j) concentration in wt% (having used the CF), and (k) concentration in wt% (w/o using the CF). At the end of each table, the sum of the concentration of all the detected signals are included (in μg mL−1 and wt% referred to the total concentration of the oils described in Table 2). These values are further discussed in the next Section 3.4 where the influence of sample dilution, complexity of the oil composition and GC-MS analytical technique's limitations are related to the obtained results. Additionally, the total sum of the proportion of identified and unknown signals are also included. These results will be referred in Section 3.5, where Kováts Retention Index (KRI) is suggested as an additional compound identification strategy.| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) |
|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT (min) | W | Compound | Area | Area% | Norm. area | [ ] with CF (μg mL−1) | [ ] w/o CF (μg mL−1) | [ ] with CF (wt%) | [ ] w/o CF (wt%) |
| 1 | 3.464 | 1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 342![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
1.0 | 5.2 | 276.1 | 225.1 | 0.8 | 0.6 | |
| 2 | 3.515 | 1 | 815![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 406 |
0.1 | 0.3 | 16.9 | 13.8 | 0.0 | 0.0 | |
| 3 | 3.545 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019 019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872 872 |
0.1 | 0.4 | 21.1 | 17.2 | 0.1 | 0.0 | |
| 4 | 3.608 | 1 | Hexane | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 719 719![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 271 |
1.1 | 5.7 | 304.6 | 248.3 | 0.9 | 0.7 |
| 5 | 3.672 | 1 | 1-Hexene | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 791 791![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387 387 |
0.8 | 4.2 | 223.3 | 182.1 | 0.6 | 0.5 |
| 6 | 3.731 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 341![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 412 412 |
0.1 | 0.5 | 27.8 | 22.6 | 0.1 | 0.1 | |
| 7 | 3.831 | 1 | Heptane | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 339 339![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 563 |
0.4 | 2.1 | 110.5 | 90.1 | 0.3 | 0.3 |
| 8 | 3.951 | 1 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 408![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 157 157 |
0.3 | 1.3 | 70.5 | 57.5 | 0.2 | 0.2 | |
| 9 | 3.997 | 1 | 1-Heptene | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 024 024 |
1.0 | 5.1 | 269.1 | 219.4 | 0.8 | 0.6 |
| 10 | 4.142 | 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 091 091![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 863 |
0.2 | 0.8 | 43.3 | 35.3 | 0.1 | 0.1 | |
| 11 | 4.210 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 277 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 172 |
0.1 | 0.5 | 26.4 | 21.5 | 0.1 | 0.1 | |
| 12 | 4.263 | 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386 386 |
0.2 | 0.9 | 48.6 | 39.6 | 0.1 | 0.1 | |
| 13 | 4.364 | 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487 |
0.2 | 0.8 | 44.6 | 36.3 | 0.1 | 0.1 | |
| 14 | 4.422 | 1 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 572 572![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 419 |
0.4 | 2.2 | 115.3 | 94.0 | 0.3 | 0.3 | |
| 15 | 4.494 | 1 | 839![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 856 856 |
0.1 | 0.3 | 17.4 | 14.2 | 0.0 | 0.0 | |
| 16 | 6.071 | 2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889 889![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821 821 |
1.3 | 6.6 | 192.4 | 233.9 | 0.5 | 0.7 | |
| 17 | 6.192 | 2 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 349 |
0.3 | 1.3 | 39.0 | 47.4 | 0.1 | 0.1 | |
| 18 | 6.295 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258 258![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 393 |
0.1 | 0.5 | 14.3 | 17.4 | 0.0 | 0.0 | |
| 19 | 6.898 | 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 174 |
0.2 | 0.9 | 27.2 | 33.1 | 0.1 | 0.1 | |
| 20 | 7.233 | 2 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283 283![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845 845 |
0.4 | 2.1 | 60.2 | 73.2 | 0.2 | 0.2 | |
| 21 | 7.928 | 2 | Toluene | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 266![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
1.9 | 9.9 | 288 | 349.9 | 0.8 | 1.0 |
| 7.920 | 2 | 1-Propanol (IS) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 527 |
|||||||
| 22 | 8.121 | 2 | 1-Octene | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 863![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 565 565 |
1.8 | 9.3 | 271.9 | 330.4 | 0.8 | 0.9 |
| 23 | 8.317 | 2 | Water | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 625![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 645 |
0.7 | 3.8 | 109.7 | 133.3 | 0.3 | 0.4 |
| 24 | 9.137 | 2 | Cyclooctene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 431 431![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585 585 |
0.1 | 0.6 | 16.3 | 19.8 | 0.0 | 0.1 |
| 25 | 9.946 | 2 | Ethylbenzene | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 223 |
2.8 | 14.5 | 423 | 514.2 | 1.2 | 1.5 |
| 26 | 10.343 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958 958![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923 |
0.1 | 0.8 | 22.3 | 27.1 | 0.1 | 0.1 | |
| 27 | 11.000 | 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 761 761![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669 669 |
0.3 | 1.5 | 40.9 | 34.0 | 0.1 | 0.1 | |
| 28 | 11.331 | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 049 049![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.1 | 0.4 | 11.4 | 9.5 | 0.0 | 0.0 | |
| 29 | 11.821 | 3 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 514![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524 524 |
0.3 | 1.8 | 49.1 | 40.8 | 0.1 | 0.1 | |
| 30 | 12.917 | 3 | Styrene | 498![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 974 974![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 981 981 |
38.1 | 194.9 | 5430 | 4504.3 | 15.3 | 12.7 |
| 31 | 13.570 | 3 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357 357![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 266 |
0.5 | 2.5 | 69.2 | 57.4 | 0.2 | 0.2 | |
| 32 | 13.651 | 3 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 693![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 308 |
0.6 | 3.0 | 83.7 | 69.4 | 0.2 | 0.2 | |
| 33 | 14.363 | 3 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708 708![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589 589 |
0.4 | 2.2 | 62.1 | 51.5 | 0.2 | 0.1 | |
| 34 | 14.544 | 3 | Alpha-methylstyrene | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
3.6 | 18.4 | 512.7 | 425.3 | 1.4 | 1.2 |
| 35 | 15.260 | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676 676![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 598 598 |
0.2 | 1.0 | 29.1 | 24.2 | 0.1 | 0.1 | |
| 36 | 15.446 | 3 | 1-Undecene | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651 651 |
1.3 | 6.4 | 178.7 | 148.2 | 0.5 | 0.4 |
| 37 | 16.198 | 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 898![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 518 |
0.3 | 1.5 | 42.4 | 35.2 | 0.1 | 0.1 | |
| 38 | 16.298 | 3 | 925![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401 401 |
0.1 | 0.4 | 10.1 | 8.4 | 0.0 | 0.0 | |
| 39 | 16.325 | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958 958![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 906 |
0.1 | 0.8 | 21.3 | 17.7 | 0.1 | 0.0 | |
| 40 | 16.996 | 3 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 306 306 |
0.4 | 2.0 | 55.6 | 46.1 | 0.2 | 0.1 | |
| 41 | 17.145 | 3 | 5-Dodecene, (E)- | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 322 322![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 563 |
1.1 | 5.6 | 155.9 | 129.3 | 0.4 | 0.4 |
| 42 | 17.299 | 3 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196 196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 556 |
0.3 | 1.6 | 45.7 | 37.9 | 0.1 | 0.1 | |
| 43 | 17.843 | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 634![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
0.2 | 1.0 | 28.7 | 23.8 | 0.1 | 0.1 | |
| 44 | 18.056 | 3 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 261![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 108 |
0.3 | 1.7 | 46.4 | 38.5 | 0.1 | 0.1 | |
| 45 | 18.536 | 3 | 817![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489 489 |
0.1 | 0.3 | 8.9 | 7.4 | 0.0 | 0.0 | |
| 46 | 18.690 | 3 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335 335 |
1.6 | 8.4 | 232.8 | 193.1 | 0.7 | 0.5 | |
| 47 | 19.388 | 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 805 805![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
0.3 | 1.5 | 41.4 | 34.4 | 0.1 | 0.1 | |
| 48 | 19.574 | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 621 621 |
0.2 | 1.0 | 28.6 | 23.7 | 0.1 | 0.1 | |
| 49 | 19.832 | 3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243 243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 637 637 |
0.8 | 4.0 | 111.5 | 92.5 | 0.3 | 0.3 | |
| 50 | 20.104 | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 261![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
0.2 | 0.9 | 24.6 | 20.4 | 0.1 | 0.1 | |
| 51 | 20.267 | 3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626 626 |
1.2 | 6.0 | 167.5 | 138.9 | 0.5 | 0.4 | |
| 52 | 21.097 | 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 944 944![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792 792 |
0.1 | 0.8 | 16.7 | 14.9 | 0.0 | 0.0 | |
| 53 | 21.264 | 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 441![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 849 849 |
0.2 | 1.0 | 20.9 | 18.7 | 0.1 | 0.1 | |
| 54 | 22.006 | 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 368 368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896 896 |
0.1 | 0.5 | 11.7 | 10.5 | 0.0 | 0.0 | |
| 55 | 22.134 | 4 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 552 |
0.7 | 3.6 | 79.3 | 70.7 | 0.2 | 0.2 | |
| 56 | 24.513 | 4 | 5-Octadecene, (E)- | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 398 398 |
0.5 | 2.4 | 53.5 | 47.7 | 0.2 | 0.1 |
| 57 | 25.633 | 4 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 191 191![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 486 |
0.2 | 1.2 | 27.4 | 24.4 | 0.1 | 0.1 | |
| 58 | 25.742 | 4 | Nonadecane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 856 856 |
0.2 | 0.9 | 19.5 | 17.4 | 0.1 | 0.0 |
| 59 | 26.738 | 4 | 1-Nonadecene | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 156![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
0.8 | 4.0 | 87.1 | 77.7 | 0.2 | 0.2 |
| 60 | 27.722 | 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 942![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
0.2 | 1.1 | 25.2 | 22.5 | 0.1 | 0.1 | |
| 61 | 27.790 | 4 | Eicosane | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 636 636![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 173 |
0.4 | 1.8 | 39.8 | 35.4 | 0.1 | 0.1 |
| 62 | 28.306 | 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 505![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 186 |
0.1 | 0.6 | 12.9 | 11.5 | 0.0 | 0.0 | |
| 63 | 28.673 | 4 | Cycloeicosane | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 601![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 316 316 |
0.9 | 4.5 | 99.5 | 88.7 | 0.3 | 0.3 |
| 64 | 29.566 | 5 | Heneicosane | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 082 082![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
0.8 | 4.3 | 36.2 | 61.6 | 0.1 | 0.2 |
| 65 | 30.373 | 5 | 10-Heneicosene (c,t) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178 178 |
1.1 | 5.5 | 45.8 | 77.9 | 0.1 | 0.2 |
| 66 | 31.152 | 5 | Docosane | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 086 |
0.9 | 4.5 | 38.0 | 64.6 | 0.1 | 0.2 |
| 67 | 31.474 | 5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272 272![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
0.2 | 0.9 | 7.4 | 12.6 | 0.0 | 0.0 | |
| 68 | 31.755 | 5 | Benzene, 1,1′-(1,3-propanediyl)bis- | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 525![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 223 |
0.7 | 3.3 | 27.9 | 47.4 | 0.1 | 0.1 |
| 69 | 31.873 | 5 | 1-Docosene | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 701 701![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131 131 |
1.4 | 7.3 | 61.2 | 103.9 | 0.2 | 0.3 |
| 70 | 32.562 | 5 | Tricosane | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 634![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
1.1 | 5.7 | 47.9 | 81.3 | 0.1 | 0.2 |
| 71 | 32.861 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 304 304![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927 927 |
0.1 | 0.5 | 4.3 | 7.3 | 0.0 | 0.0 | |
| 72 | 33.219 | 5 | 9-Tricosene, (Z)- | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528 528![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 469 469 |
1.2 | 6.1 | 50.8 | 86.3 | 0.1 | 0.2 |
| 73 | 33.318 | 5 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388 388 |
2.5 | 12.5 | 105.0 | 178.5 | 0.3 | 0.5 | |
| 74 | 33.495 | 5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757 757![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
0.4 | 2.2 | 18.8 | 32.0 | 0.1 | 0.1 | |
| 75 | 33.839 | 5 | Tetracosane | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 419![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 496 496 |
1.0 | 5.2 | 43.9 | 74.6 | 0.1 | 0.2 |
| 76 | 34.030 | 5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 663![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006 006 |
0.3 | 1.4 | 12.0 | 20.4 | 0.0 | 0.1 | |
| 77 | 34.460 | 5 | Cyclotetracosane | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199 199![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 698 698 |
1.2 | 5.9 | 49.7 | 84.5 | 0.1 | 0.2 |
| 78 | 34.642 | 5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
0.3 | 1.3 | 10.9 | 18.6 | 0.0 | 0.1 | |
| 79 | 34.737 | 5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 565 565![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549 549 |
0.3 | 1.4 | 11.7 | 19.8 | 0.0 | 0.1 | |
| 80 | 35.036 | 6 | Pentacosane | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376 376![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798 |
1.3 | 6.8 | 36.5 | 74.5 | 0.1 | 0.2 |
| 81 | 35.648 | 6 | Z-12-Pentacosene | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821 821![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 715 715 |
1.1 | 5.8 | 31.2 | 63.6 | 0.1 | 0.2 |
| 82 | 36.282 | 6 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 397 397![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 205 |
1.6 | 8.4 | 45.0 | 91.8 | 0.1 | 0.3 | |
| 83 | 36.903 | 6 | 9-Hexacosene | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 731 731 |
1.4 | 7.1 | 38.1 | 77.8 | 0.1 | 0.2 |
| 84 | 37.496 | 6 | Heptacosane | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 |
0.8 | 4.3 | 23.4 | 47.7 | 0.1 | 0.1 |
| 85 | 37.632 | 6 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 997 |
0.3 | 1.7 | 9.3 | 19.1 | 0.0 | 0.1 | |
| 86 | 38.253 | 6 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117 |
1.1 | 5.8 | 31.0 | 63.2 | 0.1 | 0.2 | |
| 87 | 38.765 | 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303 303![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777 777 |
0.2 | 0.9 | 4.8 | 9.9 | 0.0 | 0.0 | |
| 88 | 38.919 | 6 | Octacosane | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933 933![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 266 |
0.7 | 3.5 | 18.8 | 38.3 | 0.1 | 0.1 |
| 89 | 39.110 | 6 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 892 |
0.2 | 1.2 | 6.7 | 13.7 | 0.0 | 0.0 | |
| 90 | 39.830 | 6 | Cyclooctacosane | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409 409![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 498 498 |
0.9 | 4.8 | 26.1 | 53.2 | 0.1 | 0.2 |
| 91 | 40.619 | 6 | Nonacosane | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456 456![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 695 |
0.7 | 3.7 | 19.9 | 40.6 | 0.1 | 0.1 |
| 92 | 40.863 | 6 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 086![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 064 064 |
0.2 | 1.2 | 6.5 | 13.2 | 0.0 | 0.0 | |
| 93 | 41.738 | 6 | Z-14-Nonacosane | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295 295![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018 018 |
0.8 | 4.0 | 21.6 | 44.2 | 0.1 | 0.1 |
| 94 | 42.191 | 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 425![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 556 |
0.2 | 0.9 | 5.1 | 10.4 | 0.0 | 0.0 | |
| 95 | 42.667 | 6 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 853 853 |
0.6 | 2.9 | 15.7 | 32.1 | 0.0 | 0.1 | |
| 96 | 43.025 | 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 518![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568 568 |
0.1 | 0.6 | 3.2 | 6.5 | 0.0 | 0.0 | |
| 97 | 44.058 | 6 | Cyclotriacontane | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 564 |
0.7 | 3.5 | 18.9 | 38.6 | 0.1 | 0.1 |
| 98 | 45.213 | 6 | Triacontane | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 978![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 784 784 |
0.5 | 2.3 | 12.6 | 25.6 | 0.0 | 0.1 |
| 99 | 45.671 | 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 051![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 534 534 |
0.2 | 0.8 | 4.3 | 8.8 | 0.0 | 0.0 | |
| 100 | 46.954 | 6 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 024 024 |
0.3 | 1.4 | 7.3 | 14.9 | 0.0 | 0.0 | |
| Total detected signals | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 019 019 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 508 508 |
33.9 | 32.5 | ||||||
| Identified signals | 9242 | 8748 | 26 | 25 | ||||||
| Unknown signals | 2777 | 2760 | 7.8 | 7.9 | ||||||
| Unknown% = 100 × unknown signals/total detected signals | 23.1 | 24.0 | 23.0 | 24.3 | ||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) |
|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT (min) | W | Compound | Area | Area% | Norm. area | [ ] with CF (μg mL−1) | [ ] w/o CF (μg mL−1) | [ ] with CF (wt%) | [ ] w/o CF (wt%) |
| 1 | 6.074 | 2 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 533![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 137 |
0.3 | 0.9 | 26.4 | 32.1 | 0.3 | 0.3 | |
| 2 | 7.160 | 2 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 571![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 959 |
0.3 | 0.9 | 26.7 | 32.4 | 0.3 | 0.3 | |
| 3 | 7.375 | 2 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894 894 |
0.3 | 1.3 | 36.5 | 44.4 | 0.4 | 0.4 | |
| 8.146 | 2 | 1-Propanol (IS) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 909![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 636 636 |
|||||||
| 4 | 8.136 | 2 | Toluene | 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 315![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 966 966 |
9.2 | 32.8 | 958.3 | 1164.7 | 9.5 | 11.6 |
| 5 | 10.162 | 2 | Ethylbenzene | 228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 793 793![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549 549 |
16.4 | 58.5 | 1707.7 | 2075.4 | 17.0 | 20.7 |
| 6 | 11.255 | 3 | Benzene, (1-methylethyl) | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591 591![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 576 576 |
2.7 | 9.6 | 268.1 | 222.4 | 2.7 | 2.2 |
| 7 | 12.061 | 3 | Benzene, propyl | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
0.6 | 2.1 | 57.7 | 47.9 | 0.6 | 0.5 |
| 8 | 13.163 | 3 | Styrene | 592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 506 506![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672 672 |
42.4 | 151.6 | 4223.4 | 3503.5 | 42.1 | 34.9 |
| 9 | 14.689 | 3 | Alpha-methylstyrene | 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 247 247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 441 |
11.8 | 42.3 | 1178.2 | 977.4 | 11.7 | 9.7 |
| 10 | 18.772 | 3 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 816![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 613 613 |
0.4 | 1.5 | 41.4 | 34.3 | 0.4 | 0.3 | |
| 11 | 25.755 | 4 | Naphthalene, 1-methyl | 998![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 436 436 |
0.1 | 0.3 | 5.6 | 5.0 | 0.1 | 0.0 |
| 12 | 25.934 | 4 | Phenol, 2,6-dimethyl | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 049 049 |
0.6 | 2.2 | 49.0 | 43.7 | 0.5 | 0.4 |
| 13 | 27.923 | 4 | Phenol | 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078 078 |
8.2 | 29.3 | 643.1 | 573.2 | 6.4 | 5.7 |
| 14 | 29.368 | 5 | Phenol, 2,3-dimethyl- | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638 |
0.8 | 2.9 | 24.1 | 41.0 | 0.2 | 0.4 |
| 15 | 30.086 | 5 | Benzenebutanenitrile | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073 073![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734 |
1.2 | 4.1 | 34.6 | 58.8 | 0.3 | 0.6 |
| 16 | 30.943 | 5 | Phenol, 3-ethyl | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 332![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 514 |
0.4 | 1.3 | 11.3 | 19.1 | 0.1 | 0.2 |
| 17 | 31.504 | 5 | Phenol, 4-(1-methylethyl) | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139 139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 189 189 |
3.1 | 11.0 | 92.4 | 157.1 | 0.9 | 1.6 |
| 18 | 32.471 | 6 | Phenol, p-tert-butyl | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589 589 |
0.7 | 2.3 | 12.6 | 25.7 | 0.1 | 0.3 |
| 19 | 34.711 | 6 | Isopropenylphenol | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303 303 |
0.5 | 1.9 | 10.1 | 20.6 | 0.1 | 0.2 |
| 20 | 40.301 | 6 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013 013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 257 257 |
0.2 | 0.8 | 4.4 | 9.0 | 0.0 | 0.1 | |
| Total detected signals | 9411.7 | 9087.7 | 93.7 | 90.5 | ||||||
| Identified signals | 9276.2 | 8935.5 | 92.3 | 89.0 | ||||||
| Unknown signals | 135.5 | 152.2 | 1.4 | 1.5 | ||||||
| Unknown% = 100 × unknown signals/total detected signals | 1.4 | 1.7 | 1.5 | 1.7 | ||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) |
|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT (min) | W | Compound | Area | Area% | Norm. area | [ ] with CF (μg mL−1) | [ ] w/o CF (μg mL−1) | [ ] with CF (wt%) | [ ] w/o CF (wt%) |
| 1 | 3.454 | 1 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 268 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 546 546 |
1.4 | 1.6 | 84.4 | 68.8 | 0.9 | 0.7 | |
| 2 | 3.615 | 1 | 867![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 527 |
0.3 | 0.3 | 17.1 | 14.0 | 0.2 | 0.1 | |
| 3 | 3.844 | 1 | 510![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681 681 |
0.2 | 0.2 | 10.1 | 8.2 | 0.1 | 0.1 | |
| 4 | 4.268 | 1 | Propanal | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878 878![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 351 351 |
1.9 | 2.2 | 116.2 | 94.7 | 1.2 | 1.0 |
| 5 | 6.069 | 2 | Benzene | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294 294![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 525 |
2.3 | 2.7 | 79.4 | 96.4 | 0.8 | 1.0 |
| 6 | 6.295 | 2 | 255![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963 963 |
0.1 | 0.1 | 2.8 | 3.4 | 0.0 | 0.0 | |
| 7 | 6.402 | 2 | 374![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
0.1 | 0.1 | 4.1 | 4.9 | 0.0 | 0.1 | |
| 8 | 7.724 | 2 | 1,3-Dioxolane, 2-ethyl-4-methyl- | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667 667![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 087 087 |
4.4 | 5.1 | 148.7 | 180.7 | 1.5 | 1.8 |
| 8.150 | 2 | 1-Propanol (IS) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681 681![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 511 |
|||||||
| 9 | 8.146 | 2 | Toluene | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941 941![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 908 908 |
2.8 | 3.3 | 97.3 | 118.2 | 1.0 | 1.2 |
| 10 | 8.682 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 571![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 214 |
0.5 | 0.6 | 17.1 | 20.8 | 0.2 | 0.2 | |
| 11 | 9.930 | 2 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 393![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889 889 |
1.1 | 1.3 | 36.9 | 44.9 | 0.4 | 0.5 | |
| 12 | 10.184 | 2 | Ethylbenzene | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058 058![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 674 674 |
8.6 | 10.1 | 294.4 | 357.8 | 3.0 | 3.6 |
| 13 | 10.577 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 091 091![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676 676 |
0.3 | 0.4 | 11.9 | 14.4 | 0.1 | 0.1 | |
| 14 | 11.277 | 3 | Benzene, (1-methylethyl)- | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 288![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 186 |
1.4 | 1.6 | 44.6 | 37.0 | 0.5 | 0.4 |
| 15 | 11.634 | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 036 036 |
0.4 | 0.5 | 13.5 | 11.2 | 0.1 | 0.1 | |
| 16 | 12.098 | 3 | 681![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 737 737 |
0.2 | 0.3 | 7.1 | 5.9 | 0.1 | 0.1 | |
| 17 | 12.786 | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503 503 |
0.4 | 0.4 | 11.5 | 9.5 | 0.1 | 0.1 | |
| 18 | 13.203 | 3 | Styrene | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539 539![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229 229 |
19.9 | 23.3 | 649.8 | 539.1 | 6.6 | 5.5 |
| 19 | 14.733 | 3 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018 018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 066 066 |
2.9 | 3.4 | 93.7 | 77.7 | 1.0 | 0.8 | |
| 20 | 20.372 | 3 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 577 577 |
1.4 | 1.6 | 44.8 | 37.2 | 0.5 | 0.4 | |
| 21 | 24.526 | 4 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587 587![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 552 |
2.7 | 3.2 | 70.3 | 62.7 | 0.7 | 0.6 | |
| 22 | 28.102 | 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 419![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 221 221 |
0.8 | 0.9 | 19.8 | 17.7 | 0.2 | 0.2 | |
| 23 | 32.230 | 5 | Benzene, 1,1′-(1,3-propanediyl)bis- | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 313 |
11.2 | 13.2 | 110.3 | 187.4 | 1.1 | 1.9 |
| 24 | 33.750 | 5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
1.7 | 2.0 | 16.6 | 28.2 | 0.2 | 0.3 | |
| 25 | 34.563 | 5 | Benzoic acid | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741 741 |
9.4 | 11.1 | 92.5 | 157.3 | 0.9 | 1.6 |
| 26 | 35.076 | 5 | Bicyclo[4.2.1]nona-2,4,7-triene, 7-phenyl- | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 146 |
3.7 | 4.3 | 36.3 | 61.8 | 0.4 | 0.6 |
| 27 | 35.760 | 6 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 771 771![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 207 |
6.3 | 7.4 | 39.7 | 81.0 | 0.4 | 0.8 | |
| 28 | 38.217 | 6 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 998 998![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 932 |
6.4 | 7.5 | 40.2 | 81.9 | 0.4 | 0.8 | |
| 29 | 40.642 | 6 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 308 |
3.6 | 4.2 | 22.6 | 46.1 | 0.2 | 0.5 | |
| 30 | 46.039 | 6 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 575![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
3.7 | 4.3 | 23.2 | 47.4 | 0.2 | 0.5 | |
| Total detected signals | 2256.8 | 2516.3 | 23.0 | 25.6 | ||||||
| Identified signals | 1669.5 | 1830.4 | 17 | 18.6 | ||||||
| Unknown signals | 587.3 | 685.9 | 6.0 | 7.0 | ||||||
| Unknown% = 100 × unknown signals/total detected signals | 26.0 | 27.3 | 26.1 | 27.3 | ||||||
From the quantification of the oils the following aspects could be highlighted. For Packaging, around the 33 wt% of the total concentration was detected by GC-MS and styrene was by far the main identified (12–15 wt%). This fact is related to the presence of polystyrene plastic in the original pyrolysed waste, whose monomer styrene is one of the major products when thermally cracking it.38 Among the remaining detected signals, all presented weight percentages were below the 2 wt%. Regarding the composition, this oil was characterised by a mixture of aromatic and aliphatic compounds. In the case of FRP, similar overall quantification rate was obtained; 23–26 wt% of the total concentration was detected by GC-MS. In this case too, styrene was the compound with the highest yield (ca. 5 wt%) followed by other aromatic compounds such as ethylbenzene (ca. 3 wt%) and toluene (ca. 1.5 wt%). The aromatic nature of FRP oil was expected because polyester is an aromatic resin and additionally, styrene and styrene derivatives are typically used as reactive diluents in the manufacturing process of polyester thermoset resins.39–41 Therefore, it is expected to recover them when thermally degrading it.42–47 Finally, WEEE oil showed a much higher quantification yield for the detected compounds by GC-MS (around the 90 wt% of the total concentration). Moreover, a very interesting composition was obtained for this upgraded WEEE oil where around the 80 wt% of the total identified composition was made up by styrene (∼35–42 wt%), ethylbenzene (∼17–21 wt%), alpha-methylstyrene (∼10–12 wt%), toluene (∼10–12 wt%) and phenol (∼6 wt%); all of these chemicals with high market value. The remaining weight percentage was related to other phenolic compounds and aromatic hydrocarbons, mainly, although one nitrogenous compound and some unidentified ones were also detected.
3.4. Influence of the sample dissolution and complex nature in the obtained quantification results
Due to the high quantity of compounds present in the studied pyrolysis oils and the presence of unknown compounds, it was not possible to calibrate all the detected signals to determine the quantification error for each of the studied oils. However, it was possible to compare the experimentally prepared concentration of the studied pyrolysis oils (see Table 2) with the total concentration of the pyrolysis oils determined by applying the proposed quantification methodology (included as “total value” in the last row of each table in Section 3.3). For that, it was assumed that the total concentration quantified through GC-MS for each pyrolysis oil was equal to the sum of the concentrations of all the detected signals in the chromatograms of each pyrolysis oil.Fig. 4 compiles together the numerical values of the total concentrations for the three pyrolysis oils used as used-case in Section 3.3 (Packaging, WEEE and FRP) jointly with two additional liquid dissolutions (FRP (×10) and WEEE (1/10)), listed also in Table 2, with the aim to assess the influence of the prepared pyrolysis liquids' concentration in the obtained results. On the upper part of the figure, the absolute and the relative error were included (calculated based on (eqn (3)) and (eqn (4)), respectively) for the total concentration calculated experimentally and quantified based on the proposed methodology (for the blue colour bars). On the bottom of the figure, the number of detected signals per liquid were included together with the grade of sample concentration and the reference to the pyrolysis operating condition classifying the oils as raw and upgraded. Finally, in pink colour the total concentration of the validation compounds (prepared experimentally in dark pink and quantified applying the proposed methodology in light pink) were also added. The aim of showing together these four total concentrations' values was to bear in mind the capacity of the proposed methodology to quantify fairly well validation compounds (as explained in Section 3.2). Tables S18 and S19† include the detailed numerical data of Fig. 4.
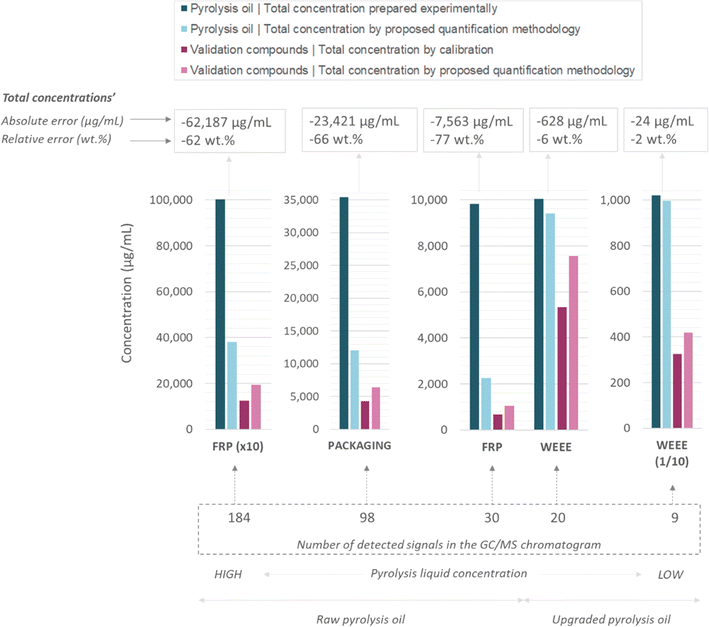 | ||
| Fig. 4 Total concentration (μg mL−1) of the use-case pyrolysis oils and of the validation compounds (experimental results vs. proposed quantification method results). | ||
A remarkable aspect in Fig. 4 was related to the difference observed between the values obtained for the experimentally prepared samples (dark blue) and the values calculated applying the proposed quantification methodology (light blue). On one side, FRP (×10), Packaging and FRP samples presented significant differences. This fact confirms that a small part of raw pyrolysis oils (less than 40 wt% of the total sample in this case) were being detected by GC-MS. This result was somehow foreseeable, as the fraction of volatile and semi-volatile compounds that could be detected by GC-MS related to the total quantity of the raw pyrolysis liquid has been reported to be only a small part (prior to the application of upgrading processes).6–8,13 On the other side, WEEE and WEEE (1/10) liquids showed very little difference between the prepared total concentration and the quantified one by the proposed method. This is most probably explained by the fact that these pyrolysis liquids were processed further; they were the outcome of an in-line upgrading process (step-wise pyrolysis plus adsorption step as mentioned in Section 2.1) of the volatiles generated during the pyrolysis process.
Fig. 4 shows also two additional pink-colour bars. On one hand, dark-pink colour bars correspond to the sum of the concentration of the validation compounds calculated by conventional calibration process. And, on the other hand, light-pink colour bars, show the sum of the concentration of the calibration compounds calculated applying the proposed quantification methodology. In all cases, light-pink bars show higher values than dark-pink bars, meaning there was some over-estimation in the concentration quantification. However, this was consistent with the relative errors reported for each validation compound in Fig. 3 in Section 3.2. On the one side, for the aromatic validation compounds tested, it could be inferred that for retention times below 28.1 min (RT for phenol), the proposed quantification methodology overestimated the concentrations, while above this retention time there was underestimation. On the other side, for the saturated alkane validation compounds, a similar trend was observed, with the exception of tricosane (RT = 32.7 min) and tetracosane (RT = 34.0 min) where a slight overestimation could be appreciated. Note that regarding alkanes, results without applying the correction factor were considered. This explains why the total concentration of the validation compounds estimated using the proposed quantification methodology was overestimated. The validation compounds present in the samples FRP, Packaging and WEEE were majorly present below the retention time 28.1 min. This means that the quantification error included in the quantification of these samples was mainly affected by overestimation errors.
Apart from that, results showed in Fig. 4 illustrates that the proposed quantification methodology was able to quantify the concentration of the compounds fairly well, independently of the type and dilution of the sample. Lastly, it was also noteworthy for FRP (×10), Packaging and FRP, the imbalance in the values obtained for the difference in the total concentration values for the pyrolysis liquid as a whole (blue-colour bars) and the difference in the total concentration values for the validation compounds (pink-colour bars). Blue-colour bars differ much more between them (dark-blue vs. light-blue) than the pink-colour bars (dark-pink vs. light-pink). This is not the case for WEEE and WEEE (1/10) due to the stated reasons.
The number of detected signals in GC-MS chromatograms seem to be influenced by the sample dilution. The obtained results indicated that, on the one hand, when the concentration of the sample FRP was increased from 9820 μg mL−1 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μg mL−1, the number of signals detected in the chromatogram increased too, from 30 to 184. However, the total concentration calculated applying the proposed methodology remained under-quantified (14
200 μg mL−1, the number of signals detected in the chromatogram increased too, from 30 to 184. However, the total concentration calculated applying the proposed methodology remained under-quantified (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 μg mL−1 vs. 100
910 μg mL−1 vs. 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μg mL−1) with a −85 wt% of relative error. This result showed that, even though an increase in the concentration of the analysed pyrolysis oil by a tenfold enabled the detection of 154 new chemical compounds by the GC-MS, the quantification of the total concentration of this pyrolysis oil applying the proposed quantification methodology was again much smaller than the one prepared experimentally. Therefore, it could be inferred that the total number of compounds in the pure pyrolysis oil could be much higher and that many of them would appear in such small quantity that they were not detected.
200 μg mL−1) with a −85 wt% of relative error. This result showed that, even though an increase in the concentration of the analysed pyrolysis oil by a tenfold enabled the detection of 154 new chemical compounds by the GC-MS, the quantification of the total concentration of this pyrolysis oil applying the proposed quantification methodology was again much smaller than the one prepared experimentally. Therefore, it could be inferred that the total number of compounds in the pure pyrolysis oil could be much higher and that many of them would appear in such small quantity that they were not detected.
Notwithstanding the fact that the proposed quantification methodology's error limits the precision of the calculated concentration values, there could be other reasons that lead to obtain very low total concentration values for some of the pyrolysis oils, mainly for Packaging and FRP. These high quantification differences were thought to be related to the incapacity of the GC-MS technique (in SCAN mode) to depict all the existing chemical compounds in the generated chromatograms. The reasons for not being able to capture all the compounds were thought to be four: (a) the incapacity to detect non-volatiles and high-molecular weight compounds using the GC-MS technique as stated in the previous section, (b) the presence of many chemical compounds in low concentrations, (c) the need to dilute the oil samples for their analysis leading to reduce the original concentration of the compounds to be identified and (d) the sensitivity limit of the GC-MS analytical technique. Fig. 5 schematically illustrates this last hypothesis where many chemical compounds may be lost in the base line noise of the GC-MS chromatogram, and ignored in the quantification process, due to their low concentration. This idea is consistent with the number of detected signals in the GC-MS chromatograms for each analysed pyrolysis oil samples (Fig. 4). It could be hinder that it was directly related to the pyrolysis liquid concentration analysed, as the higher the concentration of the pyrolysis liquid in the solvent, the higher the number of detected compounds in the GC-MS chromatogram (FRP (×10) vs. FRP, WEEE vs. WEEE (1/10), and FRP (×10) > Packaging > FRP > WEEE > WEEE (1/10)). According to these results, it could be said that chemical compounds were lost in the base line of the GC-MS analytical technique when their concentration was low, leading to identify less volatile or semi-volatile compounds than those present in the liquids.
On the other hand, if the concentration of the sample WEEE was decreased from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 μg mL−1 to 1020 μg mL−1, the number of detected signals decreased consistently (from 20 to 9 chemical compounds). However, in this case the total concentration calculated applying the proposed methodology was fairly good (996 μg mL−1 vs. 1020 μg mL−1). This result might be explained by the combination of two facts. On the one hand, the 11 signals that were not detected in the least concentrated sample (WEEE (1/10)) could represent a small weight percentage of the total sample. If so, they would impact mildly in the total concentration value. On the other hand, as showed in Section 3.2, the error added by the proposed quantification methodology could also provoke some over-estimation in the few detected signals. Finally, as mentioned before in Section 3.4, another plausible explanation for this low quantification relative errors could be related to the probably less complex nature of these WEEE liquids, what could also be inferred by the fewer number of compounds detected and shown previously in Table 7 compared to Packaging (Table 6) and FRP (Table 8), as well as the higher total concentration of the calibrated compounds (in dark pink in Fig. 4). This is consistent with the difference in the number of detected compounds between FRP and WEEE (30 vs. 20, respectively). They differed in number even though the same pyrolysis liquid concentration was prepared in both cases (around 10
040 μg mL−1 to 1020 μg mL−1, the number of detected signals decreased consistently (from 20 to 9 chemical compounds). However, in this case the total concentration calculated applying the proposed methodology was fairly good (996 μg mL−1 vs. 1020 μg mL−1). This result might be explained by the combination of two facts. On the one hand, the 11 signals that were not detected in the least concentrated sample (WEEE (1/10)) could represent a small weight percentage of the total sample. If so, they would impact mildly in the total concentration value. On the other hand, as showed in Section 3.2, the error added by the proposed quantification methodology could also provoke some over-estimation in the few detected signals. Finally, as mentioned before in Section 3.4, another plausible explanation for this low quantification relative errors could be related to the probably less complex nature of these WEEE liquids, what could also be inferred by the fewer number of compounds detected and shown previously in Table 7 compared to Packaging (Table 6) and FRP (Table 8), as well as the higher total concentration of the calibrated compounds (in dark pink in Fig. 4). This is consistent with the difference in the number of detected compounds between FRP and WEEE (30 vs. 20, respectively). They differed in number even though the same pyrolysis liquid concentration was prepared in both cases (around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1). In this case, complexity of the liquid composition should be considered together with the pyrolysis operation conditions. On the one hand, FRP was a pyrolysis liquid coming from a polyester based glass fibre reinforced plastic end-of-life waste. Due to the complex nature of polyester thermosetting resins,40,48,49 it was expected that the applied thermal treatment (single-step pyrolysis at 3 °C min−1 heating rate up to 500 °C without N2 gas flow) would generate higher quantity of chemical compounds. On the other hand, even though the plastic fraction from WEEE could be considered as complex as the plastic from FRPs, the pyrolysis treatment carried out to WEEE sample provoked higher cracking of the chemical compounds generated during the pyrolysis. In the case of WEEE, continuous N2 gas flow was included in addition to a step-wise pyrolysis followed by a thermal cracking step in a fixed bed reactor in series, where an adsorbent upgraded the quality of the pyrolysis oil (as briefly described in Table S1†). Therefore, the higher number of detected signals for FRP compared to WEEE in the GC-MS chromatograms was consistent with the operating conditions of the pyrolysis process employed with each sample.
000 μg mL−1). In this case, complexity of the liquid composition should be considered together with the pyrolysis operation conditions. On the one hand, FRP was a pyrolysis liquid coming from a polyester based glass fibre reinforced plastic end-of-life waste. Due to the complex nature of polyester thermosetting resins,40,48,49 it was expected that the applied thermal treatment (single-step pyrolysis at 3 °C min−1 heating rate up to 500 °C without N2 gas flow) would generate higher quantity of chemical compounds. On the other hand, even though the plastic fraction from WEEE could be considered as complex as the plastic from FRPs, the pyrolysis treatment carried out to WEEE sample provoked higher cracking of the chemical compounds generated during the pyrolysis. In the case of WEEE, continuous N2 gas flow was included in addition to a step-wise pyrolysis followed by a thermal cracking step in a fixed bed reactor in series, where an adsorbent upgraded the quality of the pyrolysis oil (as briefly described in Table S1†). Therefore, the higher number of detected signals for FRP compared to WEEE in the GC-MS chromatograms was consistent with the operating conditions of the pyrolysis process employed with each sample.
3.5. Kováts retention index calculation for temperature-programmed gas chromatography (Van den Dool and Kratz formula)
Depending on the complexity of the generated pyrolysis liquids (raw vs. upgraded) the proportion of the oil composition detected by GC-MS could be lower or higher. In the same way, among these detected compounds, the capacity to correctly identify chemical compounds by the MS library differs from oil to oil. In the presented work, in order to accept a compound as correctly identified by the MS library, the minimum quality match was set in 85%. Based on this criteria, 20% of the detected signals by the GC-MS were poorly identified in the case of Packaging (Table 6) and FRP (Table 8) and, therefore, no compound name was assigned to them. In contrast, it was possible to identify the remaining 80% of the detected signals. The case of WEEE sample was very different. In this case, the rate of unknown compounds was below the 2% (Table 7), which is consistent with the aspects previously discussed regarding the simpler composition of the upgraded pyrolysis oils.In this sense, Kováts Retention Index (KRI) can be employed as a complementary information to identify the unknown compounds present in the pyrolysis oils. The use of the homologous series of n-alkanes is based on the knowledge that under isothermal conditions the retention times increase exponentially. This KRI relates the logarithm of the retention time value and the number of carbons of each alkane. The Kováts RI of these reference alkanes are calculated, by definition, multiplying the number of carbons of the alkane by 100 (for any stationary phase and at any column temperature). However, when non-isothermal chromatographic conditions are used, the modification introduced by Van den Dool and Kratz (eqn (6)) is a more appropriate approach.31
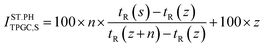 | (6) |
Fig. 6 shows the linear regression between the non-isothermal Kováts RI of all the alkanes present in the employed alkane-mix and their retention times (RTs) for the temperature programme defined in Section 2.3. The linear regression equations fitting the n-alkanes' mix is shown in eqn (7)–(11).
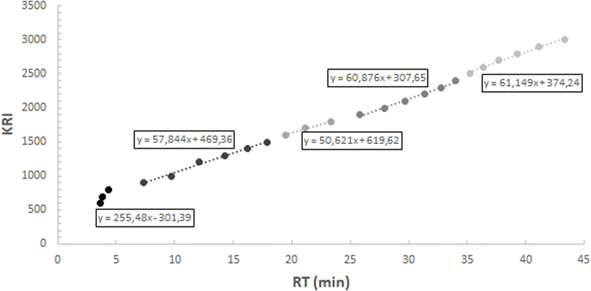 | ||
| Fig. 6 Kováts Retention Index (KRI) in function of retention time (RT) and heating segment for the n-alkanes in the employed alkane-mix. | ||
Isothermal 40 °C:
| KRI (y axis) = 255.48 × RT (x axis) − 301.39 (R2 = 0.9465) | (7) |
Non-isothermal 40–150 °C:
| KRI (y axis) = 57.844 × RT (x axis) + 469.36 (R2 = 0.9923) | (8) |
Isothermal 150 °C:
| KRI (y axis) = 50.621 × RT (x axis) + 619.62 (R2 = 0.9958) | (9) |
Non-isothermal 150–240 °C:
| KRI (y axis) = 60.876 × RT (x axis) + 307.65 (R2 = 0.9918) | (10) |
Isothermal 240 °C:
| KRI (y axis) = 61.149 × RT (x axis) + 374.24 (R2 = 0.9864) | (11) |
In this way, identification of chemical compounds defined in the MS library with a low match quality level might be possible. The Kováts RI of all the signals detected on the chromatograms of the pyrolysis oils were determined following this procedure Table S20† compiles the KRI and the identified compound for all the analysed liquids to make them available to other researchers/databases who may find this information useful to identify their own compounds in their pyrolysis oils (taking into account the chromatographic column and temperature programme implemented, described in Section 2.3).
4. Conclusions
This paper presents an easy methodology that makes it possible to quantify the concentration of all the detected compounds by GC-MS, present in pyrolysis oils coming from streams of complex plastic waste from diverse nature. The quantification error is between +62 wt% and −59 wt% with a standard deviation between 0 wt% and + 13 wt%. So far, the state of the art for quantification of the concentration of compounds by GC-MS can be considered the one carried out for bio-oils (pyrolysis liquids generated from biomass) which reported variabilities around the 20%. Therefore, the error presented by the proposed quantification methodology would be in the same order of magnitude. The proposed quantification methodology provides a quantitative idea of the composition of the compounds present in the oils compared to the current situation, where results from the analysis of complex plastic pyrolysis oils using GC-MS are almost exclusively published and evaluated using the area percentage of identified compounds without questioning the representativeness of the obtained results. Moreover, the presented work also includes the evaluation of what would be the quantification error assuming that the area percentage was equal to the weight percentage of the compounds. As a result, it has been concluded that the average relative error for the validation compounds (between 284 wt% and 498 wt%) is higher than with the proposed quantification methodology. But, more importantly, the standard deviation is much higher (between 67% and 147%), meaning that reliable results could not be expected from this assumption. Definitively, the proposed quantification methodology is seen as a feasible approach to be easily implemented. It might not accurately determine every chemical compound present in the pyrolysis oil mixture when the complexity of the pyrolysis oils is high. However, it does provide a quantitative overview of the concentration of the main detected compounds by the GC-MS as well as a quantification value (as a whole) of those compounds that are not being visualised in the GC-MS chromatogram due to their low individual concentration, non-volatility or high molecular weight. Moreover, the difference between the value of the total concentration of the experimentally prepared pyrolysis oils and the value of the total concentration calculated applying the proposed quantification methodology could be an indicator of the complexity of the pyrolysis oil being analysed, by suggesting the presence of more or less chemical compounds.Author contributions
A. Serras-Mallillos: conceptualization, investigation, methodology, writing, original draft preparation, B. B. Perez-Martínez: investigation, writing, reviewing and editing, A. Iriondo: supervision, reviewing and editing, E. Acha: funding acquisition, supervision, reviewing and editing, A. López-Urionabarrenechea: funding acquisition, supervision, writing, reviewing and editing, B. M. Caballero: funding acquisition, project administration, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Innovation of Spain [grant number PID2019-110770RB-I00/AEI/10.13039/501100011033]; the Basque Government [grant number IT1554-22 and ELKARTEK 2023 Program (NEOPLAST 2 Project, Reference KK-2023/00060)]. The authors thank for the technical and human support provided by SGiker (UPV/EHU, ERDF, EU). Authors are especially grateful to Luis Bartolomé from SGIker for the technical and human support provided.References
- I. S. Lase, D. Tonini, D. Caro, P. F. Albizzati, J. Cristóbal, M. Roosen, M. Kusenberg, K. Ragaert, K. M. Van Geem, J. Dewulf and S. De Meester, How much can chemical recycling contribute to plastic waste recycling in Europe? An assessment using material flow analysis modeling, Resour., Conserv. Recycl., 2023, 192, 1–19, DOI:10.1016/j.resconrec.2023.106916.
- T. Xayachak, N. Haque, R. Parthasarathy, S. King, N. Emami, D. Lau and B. K. Pramanik, Pyrolysis for plastic waste management: An engineering perspective, J. Environ. Chem. Eng., 2022, 10, 108865, DOI:10.1016/j.jece.2022.108865.
- S. Das, C. Liang and J. B. Dunn, Plastics to fuel or plastics: Life cycle assessment-based evaluation of different options for pyrolysis at end-of-life, Waste Manag., 2022, 153, 81–88, DOI:10.1016/j.wasman.2022.08.015.
- M. Kusenberg, M. Roosen, A. Zayoud, M. R. Djokic, H. Dao Thi, S. De Meester, K. Ragaert, U. Kresovic and K. M. Van Geem, Assessing the feasibility of chemical recycling via steam cracking of untreated plastic waste pyrolysis oils: Feedstock impurities, product yields and coke formation, Waste Manag., 2022, 141, 104–114, DOI:10.1016/j.wasman.2022.01.033.
- M. Kusenberg, A. Eschenbacher, M. R. Djokic, A. Zayoud, K. Ragaert, S. De Meester and K. M. Van Geem, Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate?, Waste Manag., 2022, 138, 83–115, DOI:10.1016/j.wasman.2021.11.009.
- M. Staš, D. Kubička, J. Chudoba and M. Pospíšil, Overview of analytical methods used for chemical characterization of pyrolysis bio-oil, Energy Fuels, 2014, 28, 385–402, DOI:10.1021/ef402047y.
- M. Staš, M. Auersvald, L. Kejla, D. Vrtiška, J. Kroufek and D. Kubička, Quantitative analysis of pyrolysis bio-oils: A review, TrAC, Trends Anal. Chem., 2020, 126, 115857, DOI:10.1016/j.trac.2020.115857.
- M. Staš, J. Chudoba, M. Auersvald, D. Kubička, S. Conrad, T. Schulzke and M. Pospíšil, Application of orbitrap mass spectrometry for analysis of model bio-oil compounds and fast pyrolysis bio-oils from different biomass sources, J. Anal. Appl. Pyrolysis, 2017, 124, 230–238, DOI:10.1016/j.jaap.2017.02.002.
- M. Staš, J. Chudoba, D. Kubička, J. Blažek and M. Pospíšil, Petroleomic Characterization of Pyrolysis Bio-oils: A Review, Energy Fuels, 2017, 31, 10283–10299, DOI:10.1021/acs.energyfuels.7b00826.
- A. G. Marshall and R. P. Rodgers, Petroleomics: Chemistry of the underworld, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18090–18095, DOI:10.1073/pnas.0805069105.
- D. Zanella, M. Romagnoli, S. Malcangi, M. Beccaria, T. Chenet, C. De Luca, F. Testoni, L. Pasti, U. Visentini, G. Morini, A. Cavazzini and F. A. Franchina, The contribution of high-resolution GC separations in plastic recycling research, Anal. Bioanal. Chem., 2023, 415, 2343–2355, DOI:10.1007/s00216-023-04519-8.
- S. Đurović, Application of the GC/MS technique in environmental analytics: Case of the essential oils, Green Sustainable Process for Chemical and Environmental Engineering and Science: Analytical Techniques for Environmental and Industrial Analysis, 2020, pp. 197–208, DOI:10.1016/B978-0-12-821883-9.00005-9.
- A. Undri, M. Abou-Zaid, C. Briens, F. Berruti, L. Rosi, M. Bartoli, M. Frediani and P. Frediani, A simple procedure for chromatographic analysis of bio-oils from pyrolysis, J. Anal. Appl. Pyrolysis, 2015, 114, 208–221, DOI:10.1016/j.jaap.2015.05.019.
- D. K. Ratnasari, M. A. Nahil and P. T. Williams, Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils, J. Anal. Appl. Pyrolysis, 2017, 124, 631–637, DOI:10.1016/j.jaap.2016.12.027.
- N. Miskolczi, F. Buyong, A. Angyal, P. T. Williams and L. Bartha, Two stages catalytic pyrolysis of refuse derived fuel: Production of biofuel via syncrude, Bioresour. Technol., 2010, 101, 8881–8890, DOI:10.1016/j.biortech.2010.06.103.
- N. Miskolczi, N. Borsodi, F. Buyong, A. Angyal and P. T. Williams, Production of pyrolytic oils by catalytic pyrolysis of Malaysian refuse-derived fuels in continuously stirred batch reactor, Fuel Process. Technol., 2011, 92, 925–932, DOI:10.1016/j.fuproc.2010.12.012.
- A. López, I. de Marco, B. M. Caballero, M. F. Laresgoiti and A. Adrados, Pyrolysis of municipal plastic wastes: Influence of raw material composition, Waste Manag., 2010, 30, 620–627, DOI:10.1016/j.wasman.2009.10.014.
- I. S. C. Carregosa, J. de C. Carregosa, W. R. Silva, T. M. Santos and A. Wisniewski, Thermochemical conversion of aquatic weed biomass in a rotary kiln reactor for production of bio-based derivatives, J. Anal. Appl. Pyrolysis, 2023, 173, 106048, DOI:10.1016/j.jaap.2023.106048.
- S. I. Okopi, J. Wang, W. Kong, Z. Yu, E. A. Ndudi, L. Che, Z. Gu and F. Xu, Valorization of food waste impurities by catalytic co-pyrolysis for production of pyrolysis oil with high energy potential, J. Anal. Appl. Pyrolysis, 2023, 170, 105918, DOI:10.1016/j.jaap.2023.105918.
- S. Vichaphund, P. Wimuktiwan, C. Soongprasit, K. Soongprasit, S. Phetchcrai, R. Sirichaivetkul and D. Atong, Study on pyrolysis behavior of polymeric coated aluminium scrap using TGA and Py-GC/MS, J. Anal. Appl. Pyrolysis, 2023, 172, 105982, DOI:10.1016/j.jaap.2023.105982.
- Z. Fu, F. Hua, S. Yang, H. Wang and Y. Cheng, Evolution of light olefins during the pyrolysis of polyethylene in a two-stage process, J. Anal. Appl. Pyrolysis, 2023, 169, 105877, DOI:10.1016/j.jaap.2023.105877.
- M. R. Djokic, T. Dijkmans, G. Yildiz, W. Prins and K. M. Van Geem, Quantitative analysis of crude and stabilized bio-oils by comprehensive two-dimensional gas-chromatography, J. Chromatogr. A, 2012, 1257, 131–140, DOI:10.1016/j.chroma.2012.07.035.
- P. Wenig and J. Odermatt, Efficient analysis of Py-GC/MS data by a large scale automatic database approach: An illustration of white pitch identification in pulp and paper industry, J. Anal. Appl. Pyrolysis, 2010, 87, 85–92, DOI:10.1016/j.jaap.2009.10.007.
- P. Ausloss, C. L. Clifton, S. G. Lias, A. I. Mikaya, S. E. Stein, D. V. Tchekhovskoi, O. D. Sparkman, V. Zaikin and D. Zhu, The Critical Evaluation of a Comprehensive Mass Spectral Library, J. Am. Soc. Mass Spectrom., 1999, 10, 287–299 CrossRef PubMed.
- T. O. Melo, F. A. Marques and F. A. Hansel, Pyrolysis-gas chromatography–mass spectrometry Kováts retention index of pyrolysis products of lignocellulosic materials, J. Anal. Appl. Pyrolysis, 2017, 126, 332–336, DOI:10.1016/j.jaap.2017.05.013.
- S. C. Detlev Hochmuth, Mass Finder 4, 2010, https://massfinder.com/wiki/MassFinder_4#Retention_index_feature Search PubMed.
- N. Mass Spectrometry Data Center, NIST 17 GC Method/Retention Index Library, 2020, https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:ridatabase Search PubMed.
- V. I. Babushok, P. J. Linstrom, J. J. Reed, I. G. Zenkevich, R. L. Brown, W. G. Mallard and S. E. Stein, Development of a database of gas chromatographic retention properties of organic compounds, J. Chromatogr. A, 2007, 1157, 414–421, DOI:10.1016/j.chroma.2007.05.044.
- H. Burdová, D. Pilnaj and P. Kuráň, Application of low-energy-capable electron ionization with high-resolution mass spectrometer for characterization of pyrolysis oils from plastics, J. Chromatogr. A, 2023, 1711, 1–10, DOI:10.1016/j.chroma.2023.464445.
- M. S. Klippel and M. F. Martins, Physicochemical assessment of waxy products directly recovered from plastic waste pyrolysis: Review and synthesis of characterization techniques, Polym. Degrad. Stab., 2022, 204, 1–17, DOI:10.1016/j.polymdegradstab.2022.110090.
- G. Castello, Retention index systems: Alternatives to the n-alkanes as calibration standards, J. Chromatogr. A, 1999, 842, 51–64, DOI:10.1016/S0021-9673(98)00989-3.
- M. Trass, Split Injections in Gas Chromatography: How to Reduce Inlet Discrimination by Using a Liner with Glass Wool, 2008, pp. 1–2, https://www.phenomenex.com Search PubMed.
- D. Rood, Gas chromatography problem solving and troubleshooting, J. Chromatogr. Sci., 2004, 42, 506–507, DOI:10.1093/chromsci/42.1.54.
- J. H. Marsman, J. Wildschut, F. Mahfud and H. J. Heeres, Identification of components in fast pyrolysis oil and upgraded products by comprehensive two-dimensional gas chromatography and flame ionisation detection, J. Chromatogr. A, 2007, 1150, 21–27, DOI:10.1016/j.chroma.2006.11.047.
- E. Christensen and J. Ferrell, Quantification of Semi-volatile Oxygenated Components of Pyrolysis Bio-Oil by Gas Chromatography/Mass Spectrometry (GC/MS), 2016, pp. 1–10, https://www.nrel.gov/publications Search PubMed.
- ASTM, ASTM D7544-12(2017) Standard Specification for Pyrolysis Liquid Biofuel, 2012, p. 5, DOI:10.1520/D7544-12R17.
- EPA, Method 8270D Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry, US EPA Arch, 2014, https://archive.epa.gov/epa/sites/production/files/2015-12/documents/8270d.pdf Search PubMed.
- Y. Liu, J. Qian and J. Wang, Pyrolysis of polystyrene waste in a fluidized-bed reactor to obtain styrene monomer and gasoline fraction, Fuel Process. Technol., 2000, 63, 45–55, DOI:10.1016/S0378-3820(99)00066-1.
- A. Kandelbauer, G. Tondi, O. C. Zaske and S. H. Goodman, Unsaturated Polyesters and Vinyl Esters, Elsevier Inc., 3rd edn, 2014, DOI:10.1016/B978-1-4557-3107-7.00006-3.
- A. A. Athawale and J. A. Pandit, Unsaturated Polyester Resins, Blends, Interpenetrating Polymer Networks, Composites, and Nanocomposites: State of the Art and New Challenges, Elsevier Inc., 2019, DOI:10.1016/B978-0-12-816129-6.00001-6.
- P. Penczek, P. Czub and J. Pielichowski, Unsaturated polyester resins: Chemistry and technology, Adv. Polym. Sci., 2005, 184, 1–95, DOI:10.1007/b136243.
- N. Grittner, W. Kaminsky and G. Obst, Fluid bed pyrolysis of anhydride-hardened epoxy resins and polyether-polyurethane by the Hamburg process, J. Anal. Appl. Pyrolysis, 1993, 25, 293–299, DOI:10.1016/0165-2370(93)80048-5.
- A. Torres, I. De Marco, B. M. Caballero, M. F. Laresgoiti, M. A. Cabrero and M. J. Chomón, GC-MS analysis of the liquid products obtained in the pyrolysis of fibre-glass polyester sheet moulding compound, J. Anal. Appl. Pyrolysis, 2001, 58–59, 189–203, DOI:10.1016/S0165-2370(00)00122-4.
- F. A. López, M. I. Martín, F. J. Alguacil, J. M. Rincón, T. A. Centeno and M. Romero, Thermolysis of fibreglass polyester composite and reutilisation of the glass fibre residue to obtain a glass-ceramic material, J. Anal. Appl. Pyrolysis, 2012, 93, 104–112, DOI:10.1016/j.jaap.2011.10.003.
- L. Giorgini, C. Leonardi, L. Mazzocchetti, G. Zattini, M. Cavazzoni, I. Montanari, C. Tosi and T. Benelli, Pyrolysis of fiberglass/polyester composites: Recovery and characterization of obtained products, FME Trans., 2016, 44, 405–414, DOI:10.5937/fmet1604405G.
- S. R. Naqvi, H. M. Prabhakara, E. A. Bramer, W. Dierkes, R. Akkerman and G. Brem, A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy, Resour., Conserv. Recycl., 2018, 136, 118–129, DOI:10.1016/j.resconrec.2018.04.013.
- S. M. Seraji, P. Song, R. J. Varley, S. Bourbigot, D. Voice and H. Wang, Fire-retardant unsaturated polyester thermosets: The state-of-the-art, challenges and opportunities, Chem. Eng. J., 2022, 430, 132785, DOI:10.1016/j.cej.2021.132785.
- S. V. Levchik and E. D. Weil, Thermal decomposition, combustion and flame-retardancy of epoxy resins - A review of the recent literature, Polym. Int., 2004, 53, 1901–1929, DOI:10.1002/pi.1473.
- T. Takeichi and N. Furukawa, Epoxy Resins and Phenol-Formaldehyde Resins, Elsevier B.V., 2012, DOI:10.1016/B978-0-444-53349-4.00157-6.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00226a |
| This journal is © The Royal Society of Chemistry 2024 |