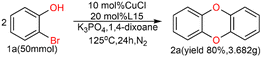Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterocycle compounds synthesized by amide ligand-promoted copper salt catalyzed construction of C–O(S) bonds†
Ruiting Yina,
Hailong Liu *b,
Xue Yanga,
Xiaoyu Zhou
*b,
Xue Yanga,
Xiaoyu Zhou b,
Xia Chenb and
Shenmin Lia
b,
Xia Chenb and
Shenmin Lia
aSchool of Environmental and Chemical Engineering, Dalian University, Dalian 116622, Liaoning Province, China
bSchool of Chemical and Materials Engineering, Liupanshui Normal College, Guizhou Province 553004, China. E-mail: hailongliu@lpssy.edu.cn
First published on 26th March 2024
Abstract
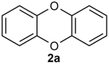
We introduce a mild method for the ligand-promoted copper-catalyzed coupling of 2-halophenol to construct DBDO using cost-effective copper salts, ligands, and alkaline reagents. This method cleverly makes 2-bromophenol complete the Ullman reaction twice, achieves efficient C–O(S) bond coupling and intermolecular cyclization, and yields high amounts of oxygen(sulfur)-containing six-membered ring products. Less reactive 2-chlorophenol was also applied in this catalytic system. The application range of the copper-amide catalytic system was further expanded. Moreover, the success of a gram-scale reaction demonstrated that this operationally simple process is scalable.
Introduction
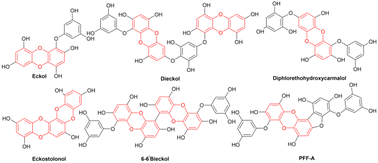
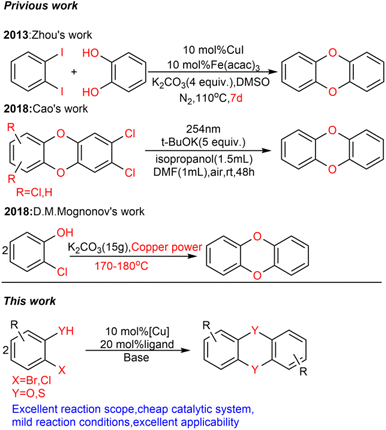
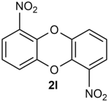
The dibenzoxin group is an important fragment of a bioactive and effective drug molecule in seaweed extract.1 In recent years, it has been found that seaweed extract shows important biological activities, such as neuroprotection,2 hypoglycemic activity,3 antiviral properties (SARS-CoV-2, SARS-CoV-3CLpro)4–6 and the inhibition of the HIV-1 strain.7Thus, the dibenzoxin group has great potential in future drug research (Scheme 1).Accordingly, the development of synthetic methods for the construction of dibenzo-p-dioxins (DBDO) and their derivatives has been an area of intense research. At present, the synthesis protocols of DBDO are limited by low yields, harsh reaction conditions, and no substrate scope. In 1972, Albert E. Pohland8 and co-workers obtained a series of chlorinated DBDO, which was prepared for use as standards in the development of analytical methodology and for use in toxicological studies. In 2005, Catherine S. Evans9 and co-workers obtained DBDO with a low yield. In 2007, Jae-Yong Ryu10 and co-workers obtained DBDO in a 69% yield, but it needed to be carried out at a high temperature of 400 °C. In 2013, Zhou11 and co-workers used 1,2-diiodobenzene and diphenol as substrates and catalyzed by CuI/Fe(acac)3 under 110 °C and nitrogen atmosphere for 7 days to obtain six-membered ring compounds of DBDO with a yield of 12–18%. In 2018, Cao12 and co-workers reduced 2,3-dichlorodibenzo [b,e][1,4] dioxin and 2,3,7,8-tetrachlorodibenzo [b,e][1,4] dioxin (TCDD) by UV(254 nm) irradiation in a strong alkaline environment with yields of 81% and 83%, respectively. In 2018, D. M. Mognonov13 and co-workers found that in the presence of anhydrous potassium carbonate and ultrafine copper powder, o-chlorophenol was thermally condensed for 6–8 hours to obtain DBDO at 170–180 °C and recrystallized from benzene with a yield of 45% (Scheme 2).
Transition metal-catalyzed C–O bond coupling of aryl halides with alcohols to synthesize aryl ethers has become a very common method. However, the high cost of palladium and phosphine ligands hinder their development. MacMillan14 and co-workers used a more expensive ruthenium catalyst to complete their research, and Stradiotto15 and co-workers used nickel instead of palladium but it required pairing with phosphine ligands. The copper(I)-catalyzed Ullmann-type reaction developed by Ma and co-workers16 is one of the most effective and important synthetic strategies to construct C–C and C-heteroatom bonds, which is widely used in the synthesis of bioactive molecules, natural products and functional materials. However, to the best of our knowledge, the synthesis of DBDO and its derivatives catalyzed by copper salt has not yet been reported.
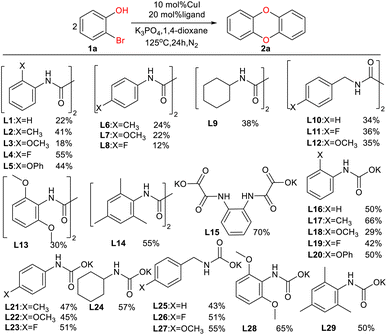
In order to fill this gap, we envisaged the use of appropriate ligands and reaction conditions to effectively synthesize DBDO. Therefore, we systematically studied many oxalate diamide ligands that showed excellent activity in promoting the arylation of other nucleophilic reagents catalyzed by Cu17 and tried to use Cu salts as catalyst precursors with amides and amide salts as ligands to promote the C–O bond binding of halogenated phenols. It was found that with the help of amide salt ligands, Cu-catalyzed 2-bromophenols to form DBDO can be carried out under relatively mild reaction conditions. Here, we explored the suitable ligands and reaction conditions.
Results and discussion
As shown in Table 1, we selected CuI-catalyzed coupling of 2-bromophenol as a model reaction to optimize the reaction conditions of amide ligands. It was found that more than ten examples gave much lower yields, but the catalytic efficiency of L15 was the best. Therefore, we subsequently used L15 for futher optimization.As shown in Table 2, the anion of copper salts was found to be important for this reaction. Our copper salt screening investigations demonstrated that CuCl was suitable for our system, affording 2a in 85% yield (Table 2, entries 1, 5–9). K3PO4 can provide a suitable alkaline environment for the coupling of 2-bromophenol. After a series of solvents were tried, it was found that 1,4-dioxane showed a prominent advantage as a solvent (Table 2, entries 13–17) (see ESI† for detailed optimization procedures).
| Entrya | Deviation from standard conditions | Yieldb (%) |
|---|---|---|
| a The general conditions are as follows: 1a (2.0 mmol), CuCl (0.1 mmol), L15 (0.2 mmol), K3PO4 (3.0 mmol), 1,4-dioxane (3.0 mL).b Determined by gas chromatography using n-decane as the internal standard.c Isolated yield. | ||
| 1 | None | 85(82)c |
| 2 | CuCl (20 mol%) | 84 |
| 3 | CuCl (5 mol%) | 76 |
| 4 | No CuCl | 0 |
| 5 | CuI instead of CuCl | 70 |
| 6 | Cu2O instead of CuCl | 45 |
| 7 | CuBr instead of CuCl | 58 |
| 8 | Cu(OAc)2 instead of CuCl | 61 |
| 9 | Cu(OTf)2 instead of CuCl | 49 |
| 10 | L15 (40 mol%) | 79 |
| 11 | No L15 | 5 |
| 12 | No K3PO4 | 10 |
| 13 | DMSO instead of 1,4-dioxane | 13 |
| 14 | DMF instead of 1,4-dioxane | 10 |
| 15 | Toluene instead of 1,4-dioxane | 50 |
| 16 | H2O instead of 1,4-dioxane | 0 |
| 17 | EtOH instead of 1,4-dioxane | 38 |
| 18 | 90 °C instead of 125 °C | 67 |
| 19 | Air instead of N2 | 10 |
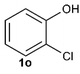
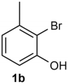
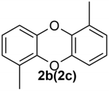
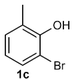
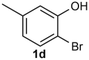
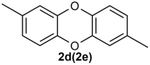
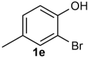
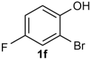
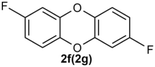
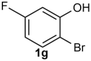
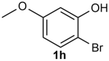
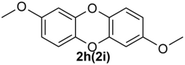
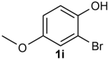
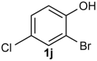
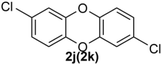
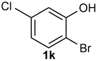
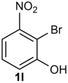
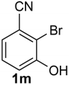
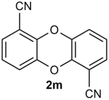
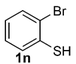
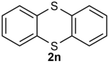
Under the optimized reaction conditions, we set out to investigate the scope of the reaction with respect to o-halo(thio)phenols (Table 3). 2-Bromophenol with methyl substituent could proceed smoothly and gave the products in good yields (Table 3, entries 3–6). Moreover, the steric hindrance affected the outcome of the reaction to a certain extent. When –CH3 was present ortho to the reactive site, the yields of the reaction decreased slightly (Table 3, entries 3 and 4 vs. entries 5 and 6). The electronic properties of the substituents have no obvious effect on the reaction results. Electron-rich and electron-deficient substrates all afforded the expected products in good yields (Table 3, entries 7–12). Unfortunately, nitro and cyano-substituted 2-bromophenols (1l and 1m) have poor performance in the coupling reaction, which might be due to the electron-withdrawing nature of the nitro and cyano substituents. To our delight, we tried to apply the above experimental scheme to the C–S bond coupling reaction of 2-bromothiophenol and received good feedback to obtain the target product thianthrene in 75% yield. It is proved that L15 is not only an effective ligand that can efficiently promote copper salt-catalyzed C–O bond coupling and complete cyclization but also has good potential in promoting C–S bond coupling reaction. Aryl chlorides remain an uncommon reagent in coupling reactions because of the low reactivity of the relatively inert C–Cl bond. However, because of their lower cost and wider diversity, aryl chlorides are always attractive substrates instead of their bromo counterparts. We were pleased to find that o-chlorophenol was also reactive under the established catalytic conditions, albeit with a low yield (Table 3, entry 2).
A scaled-up experiment (Scheme 3) was performed on a gram-scale reaction under the same conditions. Scaled up by 25 times to 50 mmol, the reaction proceeded as expected to give the desired product 2a in 80% isolated yield, demonstrating the practicality of this method.
In this study, DBDO was formed by C–O bond binding of 2-bromophenol twice, so we propose that the coupling reaction might undergo a typical Ullmann reaction, as described in (Scheme 4) The catalytic cycle was completed under the catalysis of CuCl and L15.
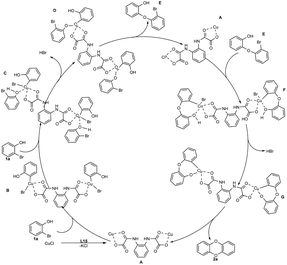 | ||
| Scheme 4 Possible mechanism for the formation of DBDO by CuCl/L15-catalyzed coupling of 2-bromophenol. | ||
Conclusions
In summary, we have presented a convenient and mild method for ligand-promoted copper-catalyzed coupling of 2-halophenol to construct DBDO. We utilized cost-effective and readily available copper salts, ligands and alkaline reagents to achieve C–O(S) bond coupling and facilitate intermolecular cyclization. High yields of oxygen-containing six-membered ring products were obtained, effectively promoting the cross-coupling of C–S bonds and completing the construction of sulfur-containing six-membered rings. Furthermore, the less reactive 2-chlorophenol could also be employed in this catalytic system.Author contributions
Ruiting Yin performed the experiments and analysed the data. Ruiting Yin and Hailong Liu designed the study and supervised the project. Ruiting Yin wrote the manuscript. All authors had approved the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the Natural Science Foundation of Guizhou Province (No. qiankehejichu-ZK[2023]zhongdian048), the Foundation of Guizhou Educational Committee (No. qianjiaoji[2023]088), High-level Talents Research Start-up Fund (LPSSYKYJJ201909), Liupanshui Normal University Scientific Research and Cultivation Projects (LPSSYLPY202227), Guizhou Provincial Key Laboratory of Coal Clean Utilization (qiankehepingtairencai [2020]2001) and the Science and Technology Innovation Team at Liupanshui Normal University (LPSSYKJTD201904) for financial support.Notes and references
- D. K. Rajan, K. Mohan, S. Zhang and A. R. Ganesan, Biomed. Pharmacother., 2021, 142, 111988 CrossRef CAS PubMed.
- Y. Shi and H. Qi, Food Rev. Int., 2023, 39, 1137–1156 CrossRef.
- S. Agarwal, V. Singh and K. Chauhan, Crit. Rev. Food Sci. Nutr., 2023, 63, 5739–5770 CrossRef PubMed.
- J. Qiao, Y.-S. Li, R. Zeng, F.-L. Liu, R.-H. Luo, C. Huang, Y.-F. Wang, J. Zhang, B. Quan, C. Shen, X. Mao, X. Liu, W. Sun, W. Yang, X. Ni, K. Wang, L. Xu, Z.-L. Duan, Q.-C. Zou, H.-L. Zhang, W. Qu, Y.-H.-P. Long, M.-H. Li, R.-C. Yang, X. Liu, J. You, Y. Zhou, R. Yao, W.-P. Li, J.-M. Liu, P. Chen, Y. Liu, G.-F. Lin, X. Yang, J. Zou, L. Li, Y. Hu, G.-W. Lu, W.-M. Li, Y.-Q. Wei, Y.-T. Zheng, J. Lei and S. Yang, Science, 2021, 371, 1374–1378 CrossRef CAS PubMed.
- J.-Y. Park, J. H. Kim, J. M. Kwon, H.-J. Kwon, H. J. Jeong, Y. M. Kim, D. Kim, W. S. Lee and Y. B. Ryu, Bioorg. Med. Chem., 2013, 21, 3730–3737 CrossRef CAS PubMed.
- M. Aatif, G. Muteeb, A. Alsultan, A. Alshoaibi and B. Y. Khelif, Mar. Drugs, 2021, 19, 242 CrossRef CAS PubMed.
- F. Karadeniz, K.-H. Kang, J. W. Park, S.-J. Park and S.-K. Kim, Biosci., Biotechnol., Biochem., 2014, 78, 1151–1158 CrossRef CAS PubMed.
- A. E. Pohland and G. C. Yang, J. Agric. Food Chem., 1972, 20, 1093–1099 CrossRef CAS PubMed.
- C. S. Evans and B. Dellinger, Environ. Sci. Technol., 2005, 39, 2128–2134 CrossRef CAS PubMed.
- J.-Y. Ryu, Chemosphere, 2008, 71, 1100–1109 CrossRef CAS PubMed.
- Q. Zhou, L. Su, T. Jiang, B. Zhang, R. Chen, H. Jiang, Y. Ye, M. Zhu, D. Han, J. Shen, G. Dai and Z. Li, Tetrahedron, 2014, 70, 1125–1132 CrossRef CAS.
- D. Cao, C. Yan, P. Zhou, H. Zeng and C.-J. Li, Chem. Commun., 2019, 55, 767–770 RSC.
- D. M. Mognonov, O. Z. Ayurova, O. V. Il'ina and V. V. Khakhinov, Russ. Chem. Bull., 2018, 67, 1903–1907 CrossRef CAS.
- J. A. Terrett, J. D. Cuthbertson, V. W. Shurtleff and D. W. C. MacMillan, Nature, 2015, 524, 330–334 CrossRef CAS PubMed.
- P. M. MacQueen, J. P. Tassone, C. Diaz and M. Stradiotto, J. Am. Chem. Soc., 2018, 140, 5023–5027 CrossRef CAS PubMed.
- (a) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450–1460 CrossRef CAS PubMed; (b) J. Jia, C. Jiang, X. Zhang, Y. Jiang and D. Ma, Tetrahedron Lett., 2011, 52, 5593–5595 CrossRef CAS; (c) X. Yang, Y. Zhang and D. Ma, Adv. Synth. Catal., 2012, 354, 2443–2446 CrossRef CAS; (d) W. Zhou, M. Fan, J. Yin, Y. Jiang and D. Ma, J. Am. Chem. Soc., 2015, 137, 11942–11945 CrossRef CAS PubMed; (e) M. Fan, W. Zhou, Y. Jiang and D. Ma, Angew. Chem., Int. Ed., 2016, 55, 6211–6215 CrossRef CAS PubMed; (f) Y. Chen, L. Xu, Y. Jiang and D. Ma, Angew. Chem., Int. Ed., 2021, 60, 7082–7086 CrossRef CAS PubMed; (g) J. Huang, T. Li, X. Lu and D. Ma, Angew. Chem., Int. Ed., 2023, e202315994 Search PubMed.
- Q. Cai and W. Zhou, Chin. J. Chem., 2020, 38, 879–893 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00701h |
| This journal is © The Royal Society of Chemistry 2024 |