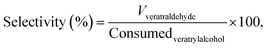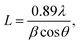DOI:
10.1039/D4RA00846D
(Paper)
RSC Adv., 2024,
14, 18093-18102
Liquid phase selective oxidation of veratryl alcohol to veratraldehyde using pure and Mg-doped copper chromite catalysts
Received
11th February 2024
, Accepted 30th April 2024
First published on 5th June 2024
Abstract
Mg-doped copper chromite (CuCr2O4) nanocomposites were synthesised through conventional technique. The pure and doped CuCr2−xMgx O4 (x = 0.00–0.1, 0.2 and 0.3%) nanocomposites were characterized in terms of their morphology, crystal structure, surface area and catalytic performance. The chemical composition of CuCr2−xMgx O4 was confirmed via FT-IR. The formation of pure and doped catalysts was validated by XRD results. TEM/SEM confirmed the formation of CuCr2−xMgxO4 nanoparticles. Mg-doped samples possess a high specific surface area compared to pure CuCr2O4. Thus, the effects of temperature, solvent, time, oxidant and the amount of catalyst on the oxidation of veratryl alcohol were reported. Furthermore, detailed mechanisms of the catalytic oxidation of veratryl alcohol as well as the reusability and stability of the nanomaterial were investigated. The resulting composites were shown to be effective heterogeneous catalysts for the oxidation of veratryl alcohol.
1. Introduction
In the field of heterogeneous catalysis, sunlight-activated materials are necessary. Because nanotechnology enables control over their size and morphology, there is a correlation between their increased efficiency and these developments. In the field of catalysis, ternary metal oxides with a spinel structure (AB2O4) have demonstrated exceptional properties. Thus, metal oxides were located around the octahedral (B site) and tetrahedral (A site) positions, and the quantities, type of oxides and the location of metal cations in the crystalline structure significantly affect the chemical–physical properties of chromite materials.1,2 At present, chromite materials with spinel structures are present with a face-centered lattice with the space group Fd3m, whereas Cr3+ cations are in octahedral positions and Cu2+ cations occupy tetrahedral positions.3 Chromium-based catalysts exhibit good resistance to atmospheric effects, high thermal stability, optical properties, resistance to chemical corrosion, photocatalysis, catalysis etc. Beyond that, it is interesting to see numerous applications for ceramic materials.4 The doping of divalent Mg2+ metal ions is primarily incorporated into A-site cations in the synthesized copper chromite materials. The stoichiometry of chemical compositions and the controlled crystallite size of chromite materials prepared via the chemical method were found to be extremely favorable for achieving good-quality products.5 Copper chromates were synthesized using various methods, such as sol–gel, ceramic,6 microwave and co-precipitation techniques.7 New chemical methods, such as hydrothermal, sol–gel/citrate,8 conventional (low-temperature method), and low-temperature solution synthesis methods, were also developed.9
Mg2+ doped chromite based catalysts were used in the selective oxidation reaction. Also, chromite based materials were more useful in industrial and technological applications.10 Selective oxidation of veratryl alcohol to carbonyl compounds (aldehyde to acid) is highly challenging.11 Selective oxidation of veratryl alcohol is employed to produce several industrially valuable chemicals, such as those associated with the food and fragrance industries; veratraldehyde is a highly valued chemical that is derived from crude oil and via the methylation of vanillin. It is interesting to note that the industry that utilizes veratraldehyde spends as much as 100 times more on bio-based veratraldehyde than on crude oil-based veratraldehyde.12–14 Herein, we synthesized citrate CuCr2O4 and Mg-doped CuCr2O4 via a conventional combustion route, followed by their morphological and structural characterisation. The chemical–physical properties of the catalysts are characterised using multiple techniques. The resulting composites were shown to be effective heterogeneous catalysts for the oxidation of veratryl alcohol.
2. Experimental
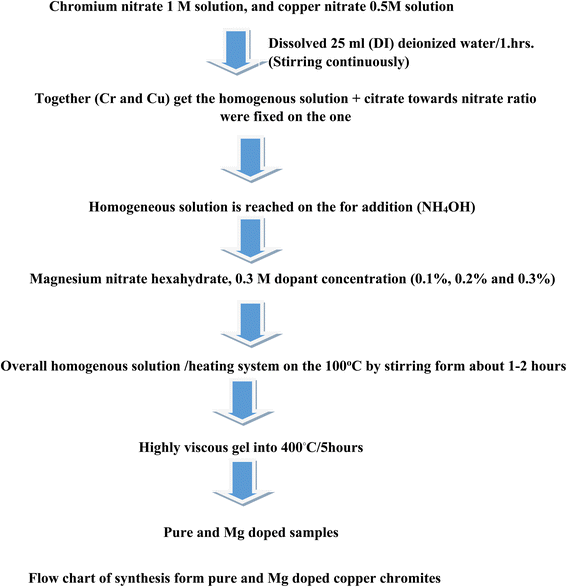
At present, (CuCr2O4) copper chromite spinel oxides were (flow chart 1) prepared via the conventional combustion route. The following chemicals, such as citric acid C6H8O7 (used as fuel), chromium nitrate Cr (NO3)3·9H2O, copper nitrate Cu (NO3)2·6H2O and magnesium nitrate Mg (NO3)2·6H2O were used without purification. First stage (I): chromium nitrate 1 M solution and copper nitrate 0.5 M solution were prepared separately in 25 mL (DI) deionized water. Second stage (II): Cr and Cu solutions were mixed and stirred continuously for 1 h to obtain a homogenous solution. Third stage (III): An aqueous solution of 5 M citric acid was prepared in DI water, and the citrate to nitrate ratio was fixed at one. Then, the obtained solution (citrate) is dropped into the nitrate solution under continuous stirring, and the homogeneous solution is neutralized with the addition of ammonium hydroxide (NH4OH). Following this, different amounts (0.1%, 0.2%, and 0.3%) of magnesium nitrate hexahydrate (0.3 M) were introduced into the aforementioned solution. Fourth stage (IV): the overall homogenous solution was evaporated using a heating system; it was kept on a hot plate at 100 °C with intensive stirring for about 1–2 hours. Thus, a highly viscous gel was obtained. Fifth stage (V): the extremely viscous gel was then transferred to a muffle furnace, where self-ignition was initiated at an experimental temperature of 400 °C/5 hours. The samples (Table 1) of the (0.0) undoped CuCr2O4 and (0.1 to 0.3) from Mg-doped CuCr2O4 were labelled (a) and (b) to (d).
Table 1 Sample code and sample composition with their abbreviations
| Sample code |
Sample composition |
| a |
CuCr2O4 |
| b |
Mg (0.1%) CuCr2O4 |
| c |
Mg (0.2%) CuCr2O4 |
| d |
Mg (0.3%) CuCr2O4 |
2.1. Instrumental
X-ray diffraction analysis was carried out using a Rigaku DMAX-3A powder diffractometer with CuKα radiation at a wavelength of 1.54 Å to investigate the crystalline phase and the various structural parameters of the prepared samples. These patterns of XRD were scanned in a 2θ range of 20° to 80° with a scanning step size of 0.05°. The morphology was characterized via field-emission scanning electron microscopy (FESEM) (SEM, QUANTA FEG 250, USA) and transmission electron microscopy (TEM). The specific surface areas (BET) of the products were tested using a Micromeritics ASAP 2020 apparatus. Fourier-transform infrared (FTIR) spectra were recorded using a thermo scientific Nicolet iS10 instrument in an in situ Harrick IR cell.
2.2. Catalytic test
Catalytic oxidation of alcohol was carried out on a batch reactor functioning under atmospheric conditions. 10 mmol of (CH3CN, DMF and CHCl3), 10 mmol of veratryl alcohol (at a molar ratio of H2O2/alcohol of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and 10 mmol of oxidant (TBHP/H2O2) was added along with 0.1 to 0.3 g of the pure and Mg-doped copper chromites, and the fillings were heated to 100 °C at different reaction times for 12 h at altered time intervals in a three-necked round bottom flask equipped with a reflux condenser and the thermometer.
1), and 10 mmol of oxidant (TBHP/H2O2) was added along with 0.1 to 0.3 g of the pure and Mg-doped copper chromites, and the fillings were heated to 100 °C at different reaction times for 12 h at altered time intervals in a three-necked round bottom flask equipped with a reflux condenser and the thermometer.
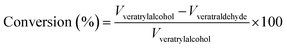
From these values, the overall conversion of the reactant veratryl alcohol and the selectivity of the product (3) was estimated.
3. Results and discussion
3.1. XRD pattern of the Mg-doped CuCr2O4 nanocomposites
The XRD measurements were performed to determine the effectiveness of the production on the pure and Mg-doped CuCr2O4. As shown in Fig. 1, the obtained XRD peaks are indexed as undoped and Mg-CuCr2O4 with a cubic structure formation. As confirmed by a standard card (JCPDS No. 34-0424), these XRD peaks conform to undoped and Mg-CuCr2O4, matching well with the standard. As the amount of Mg doping increased from x = 0 to 0.1, 0.2, and 0.3%, the samples showed only copper chromite's phase, and the peak intensities were comparable with the pure CuCr2O4 sample. This indicates the integrity of the Mg dopant within the copper chromite crystal structure. XRD patterns ranging from 35.5° to 37.5° were assigned to the 111 plane, which has the highest intensity. Furthermore, the primary peaks at 2θ-values adhering to the (111) and (311) planes show a small change as the doping concentration of Mg quantity increases. The XRD peaks of the samples progressively changed between low and high angles, which correlated with the extension (Table 2) of the lattice parameters, cell volume (Å3) and d spacing values. Thus, ionic radii of Mg2+ = 86 pm, Cr3+ = 75.5 pm, and Cu2+ = 77 pm were compared. Mg-doped copper chromites have smaller crystallite sizes than pure copper chromites due to the substitution of the lattice ions by the Mg2+ ions and their entry into interstitial locations.15,16
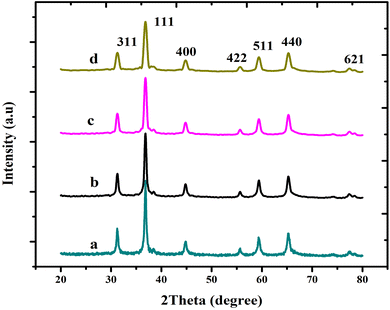 |
| | Fig. 1 X-ray diffraction pattern of (a) CuCr2O4 and (b–d) Mg-doped CuCr2O4. | |
Table 2 Crystallite size, lattice parameters and lattice strain of copper chromite and Mg-doped copper chromates
| Samples |
Crystallite size (nm) |
Lattice parameters |
Strain |
| a = b (Å) |
c (Å) |
| a |
43.12 |
5.9832 |
3.9810 |
3.234 |
| b |
38.45 |
5.9833 |
3.9811 |
3.091 |
| c |
31.45 |
5.9835 |
3.9833 |
2.981 |
| d |
29.98 |
5.9837 |
3.9835 |
2.567 |
The average crystallite size was determined using the Scherrer formula:17
where
L is the mean crystal size (Å) and
λ is the wavelength of the X-ray source (1.5404 Å), thus, in the XRD pattern,
θ and
β correspond to the (radians) diffraction angle of the observed peaks and the full width at half maximum (FWHM), respectively. 0.89 is the Scherrer constant. Thus, for peak position, FWHM can be acquired with two Gaussian curves by fitting the measured peaks. The monochromatic Cu Kα radiation was used a X-ray source. Based on the results of X-ray diffraction, the decrease in crystallite size from (a) pure CuCr
2O
4 43.12 nm, Mg-doped CuCr
2O
4 samples (b–d) 38.45 nm, 31.45 to 29.98 nm could be linked to maximizing the concentrations of dopant.
3.2. FTIR spectra
Fourier transform infrared (FTIR) spectroscopy was recorded for the four undoped samples (Fig. 2a and b) and Mg-CuCr2O4 around room temperature from 400 to 4000 cm−1. Thus, two sharp bands for the different absorption peaks observed around 561 and 475 cm−1 lower frequency band (ν2) correspond to the vibration of –Mg2+–Cr3+–O2+ by sites, indicating the formation of octahedrons and tetrahedrons, respectively (Table 3). The bands around 560–470 cm−1 correspond to the intrinsic stretching vibration of metal ions at the tetrahedral site, and the band in the range of 510–550 cm−1 is attributed to the M–Mg–O bond vibration. These two bands positioned a high-frequency band (ν1) at 500 and 678 cm−1 characteristic of CuCr2O4 spinel. The FT-IR spectra exhibited two characteristic absorption bands at 600 and 900 cm−1, corresponding to the tetrahedral and octahedral formations, respectively. Thus, both bands similarly correspond to the asymmetric stretching vibrations of Cr–O–Cu–Mg. In general, Mg substitution via transition metal ions, such as Cu2+, leads to a shift in the frequency bands and is due to the variation of CrO4 bond length.18,19
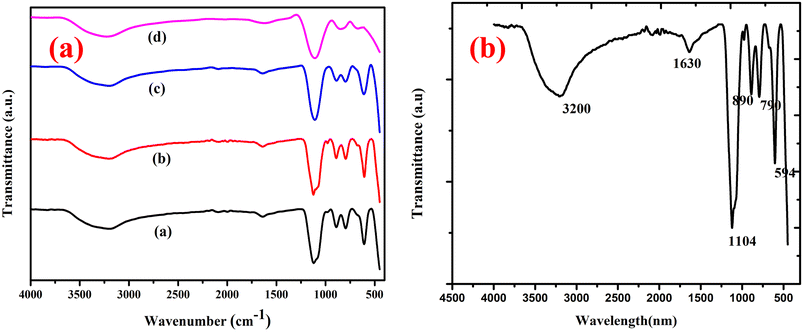 |
| | Fig. 2 (a) FTIR spectra of (a) CuCr2O4 and (b–d) Mg-doped CuCr2O4; (b) FTIR spectra of pure CuCr2O4. | |
Table 3 FTIR modes (υ1 and υ2) of copper chromite and Mg-doped copper chromite
| Samples |
Wave number υ1 (cm−1) |
Wave number υ2 (cm−1) |
| a |
613, 801 |
898, 1125 |
| b |
608, 799 |
894, 1122 |
| C |
605, 796 |
890, 1120 |
| D |
661, 856 |
1118 |
3.3. Morphology
SEM images and micrographs of the undoped and Mg-CuCr2O4 compositions (x = 0.00, 0.1, 0.02 and 0.03%) are demonstrated in Fig. 3a–d. The SEM images and micrographs show irregularly shaped large grains of undoped and smaller grains of Mg-CuCr2O4. In the arrangement within the SEM results, the samples have an extensive particle distribution and present strong agglomerations; hence, a measurement from the particle size was not possible. However, the obtained results give some statistical arrangements of Mg–Cu–Cr–O. As can be observed, SEM images detect particularly pure and doped sample differences between XRD crystallite sizes and particle sizes, as shown in Table 4. The crystallite size determined using the XRD approach was found to be correct, however the grain size assessed using the SEM analysis had broader values and may have measurement errors. In our studies, CuCr2O4 nanocomposites having a cluster-like structure with sizes ranging from 100 to 500 nm made by identical particles. Pure and doped samples both have discrete particle-like appropriate interfaces in CuCr2O4 nanocomposites. In addition, it is noticed that the structure can be obtained at lower synthesis temperatures from the conventional method.20 Thus, TEM was used to control the morphologies and particle size of copper chromite and the Mn doping copper chromite. As illustrated in Fig. 4a–d, spherical particles and regular structures are present in the doped samples. TEM images for CuCr2O4 particles were obtained and the particle-like shape changed to form spherical particles, as concentration on the Mg2+ is improved.
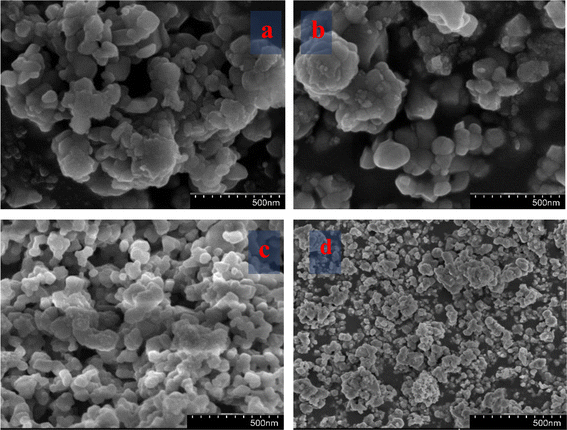 |
| | Fig. 3 SEM showing (a) CuCr2O4 and (b–d) Mg-doped CuCr2O4. | |
Table 4 Difference between the calculated crystallite sizes (XRD) of samples and the grain sizes (SEM) of copper chromite and Mg-doped copper chromites
| Samples |
Crystallite size from XRD (nm) |
Particle size from SEM (nm) |
| a |
43.12 |
49–53 |
| b |
38.45 |
43–39 |
| c |
31.45 |
40–31 |
| D |
29.98 |
39–29 |
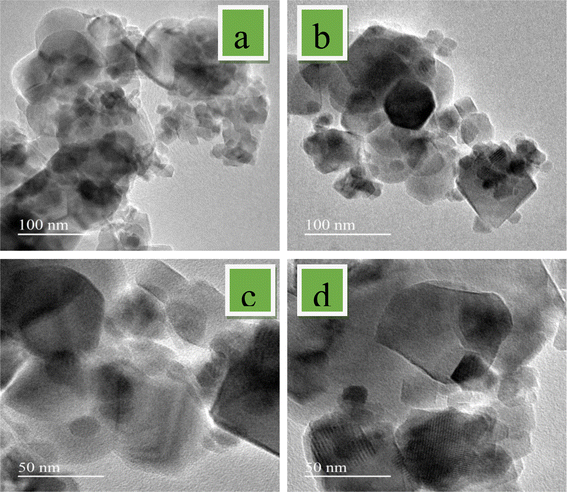 |
| | Fig. 4 TEM images of (a) CuCr2O4 and (b–d) Mg-doped CuCr2O4. | |
3.4. Surface area analysis
Thus, a specific surface area can be used from the BET equation to determine the amount of nitrogen absorbed into copper chromite and the Mn doping copper chromite. The H–K method was used to obtain the average pore diameter, and the t-plot method was employed to obtain the total pore volume. In terms of catalytic activity, surface area is an important parameter when evaluating potential catalytic applications involving catalytic materials such as copper chromite and Mg-doped copper chromite. Table 5 displays the pore volume (Vp), specific surface area (SBET), pore radius (Rp), and crystallite size (nm). The copper chromite and Mg-doped copper chromite samples increase the surface area for converted catalytic applications because they demonstrate increasing contact between alcohol reacting molecules and aldehyde from the active sites.21,22 For all the samples, this was followed by the type III isotherms with the hysteresis loop at the high P/P0 and illuminating the representative mesoporous material.23 The system, copper chromite and the Mg-doped copper chromite of the plot on the amount of gas adsorbed as a function of the relative pressure are exposed altogether as a type-III (a–d) and with hysteresis loop, as shown in Fig. 5a.
Table 5 BET surface area, average pore diameter, pore volume and crystallite size (nm) of CuCr2O4 and Mg-doped CuCr2O4
| Parameters |
a |
b |
c |
d |
| SBET (m2 g−1) |
38.2 |
40.2 |
57.8 |
63.4 |
| Smic (m2 g−1) |
2.9 |
5.1 |
7.5 |
9.2 |
| Smeso (m2 g−1) |
35.3 |
35.1 |
50.0 |
54.1 |
| Total pore volume (cm3 g−1) |
0.1 |
0.1 |
0.1 |
0.2 |
| Micro pore volume (cm3 g−1) |
0.0 |
0.0 |
0.1 |
0.1 |
| Meso pore volume (cm3 g−1) |
0.1 |
0.1 |
0.0 |
0.1 |
| Average pore diameter (nm) |
13.6 |
14.1 |
17.9 |
19.8 |
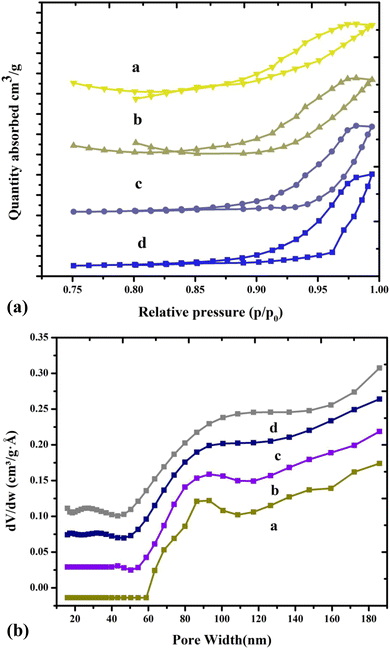 |
| | Fig. 5 (a) N2 adsorption/desorption isotherms of (a) pure and (b–d) Mg-doped copper chromite. (b) Pore size distributions of (a) pure and (b–d) Mg-doped copper chromites. | |
When magnesium is added, the bulk copper chromite phase grows more slowly, increasing the surface area, improving porosity, and creating micropores. Because of nonisomorphic substitution, the addition of magnesium to the copper chromite phase matrix causes a decrease in grain size, which results in an increase in surface area. The three components of Mg, Cr, and Cu can form pores in their matrices, leading to the production of pores of varying sizes. The reduction in grain size and the non-isomorphic substitution of Mg in the copper chromite matrix resulted in the formation of significant micropores, which is why the average pore diameter for the Mg-doped sample decreased. Thus, the addition of Mg, which simultaneously increases the mesopores and introduces micropores, increases the catalytic activity of copper chromites.24,25
4. Catalytic activity of selective oxidation
4.1. Effect of temperatures
The effect of temperature on selectivity towards veratryl aldehyde and the conversion of veratryl alcohol were studied in the range of 60–140 °C, as presented in Fig. 6. The selectivity (80%) of veratryl aldehyde decreases with increasing temperature, which is particularly due to the over-oxidation of veratryl aldehyde to veratric acid. However, conversion of veratryl alcohol increased from 60 to 80% with an increase in the temperature from 60 to 140 °C.26–28 This system increased the reaction temperature, which was important for the decrease in the oxygen, resulting in unfavorable product formation. The optimum temperature is 100 °C.
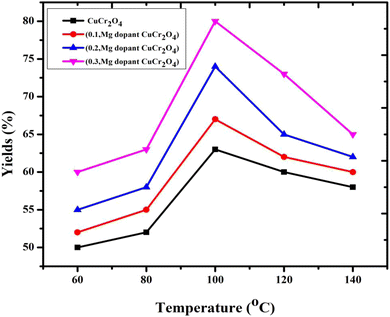 |
| | Fig. 6 Catalytic activity of the catalysts versus different reaction temperatures in the oxidation of veratryl alcohol. Reaction conditions: pure and doped samples: 0.3 g; alcohol: 10 mmol; acetonitrile: 10 mmol; H2O2: 10 mmol; time: 12 h; temperature: 60 to 140 °C. | |
4.2. Amount of catalyst
The effect of catalyst amount (0.1 g to 0.5 g) on the selectivity of veratryl aldehyde and the conversion of veratryl alcohol into by-products formation were studied and the results are presented in Fig. 7. The catalytic activity is closely related to the following two factors: the copper chromite amount of catalyst for the active sites Cu and Cr and the size for O2−. The doping concentration of Mg was determined to ensure appropriate active Mg sites; however, smaller Mg particles are usually obtained. Therefore, the optimal pure and doping copper chromites are 0.3 g because they give the best yield among others. However, the amount of catalyst interaction with the catalyst regenerates the catalyst surface, causing the product molecules to desorb and encouraging additional oxidation.
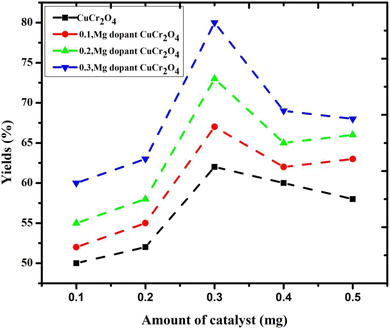 |
| | Fig. 7 Yield of the product via the selective oxidation of veratryl alcohol using different catalysts. Reaction conditions: pure and doped samples: 0.1 to 0.5 g; alcohol: 10 mmol; acetonitrile: 10 mmol; H2O2: 10 mmol; time: 12 h; temperature: 100 °C. | |
4.3. Effect of reaction time
This conversion of veratryl alcohol and the selective formation of product are observed at different reaction time intervals of 0.5, 1, 2, 4, 6, 8, 10, 12 and 14 h. As demonstrated in Fig. 8, 11% veratryl alcohol conversion and 50% veratryl aldehyde selectivity are obtained at 0.5 h; after allowing the reaction for 10 h, 80% yields were formed. The main product was veratryl aldehyde with veratryl acid as a by-product. Based on the catalytic reaction route, to increase the conversion of aldehyde, the reaction time needs to be prolonged. Thus, the catalytic activity was achieved with a reaction time of 12 h and a high yield was obtained, and the time was increased as the products formed.29 Thus, an optimum catalytic reaction time of 12 h was selected.
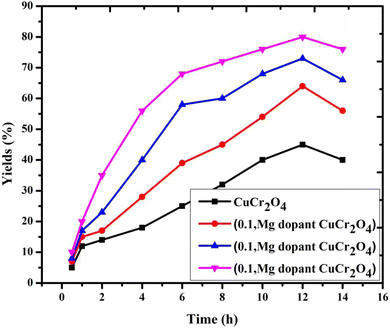 |
| | Fig. 8 Catalytic activity of the catalysts versus different reaction times for the selective oxidation of veratryl alcohol. Reaction conditions: pure and doped samples: 0.3 g; alcohol: 10 mmol; acetonitrile: 10 mmol; H2O2: 10 mmol; time: 0 to 16 h; temperature: 100 °C. | |
4.4. Effect of solvents
The catalytic performance was evaluated in several solvents, such as chloroform (CHCl3), acetonitrile (CH3CN) and dimethyl formamide (DMF), for the conversion of veratryl alcohol to veratryl aldehyde. Thus, a solvent plays an important role in the reaction induced by the alcohol conversion of an aldehyde decomposed for the by-products. Consequently, a higher yield for acetonitrile than for dimethyl formamide and chloroform was observed, as listed in Table 6. For this reason, a polar solvent was more easily adsorbed on the catalyst surface, preventing the adsorption of alcohol to aldehyde. Because the substrates and oxidants are more soluble in the solvent, they can more readily bind to the catalyst's active sites. Therefore, acetonitrile was the more active solvent for the catalytic system under study. In the case of CHCl3 and DMF, the yields are lower compared to acetonitrile, might be due to the inefficient hydrogen transfer by solvents, and the incomplete solubility of organic substrates and catalytic systems in the solvents. It turned out that the conversion of alcohol depended on the polarity of the solvent molecules, polarity order from acetonitrile, dimethyl formamide and chloroform. Besides, acetonitrile was selected as the solvent for the catalytic system.
Table 6 Yield of the product in different oxidants obtained via veratryl alcohola
| Samples |
Chloroform |
Dimethyl formamide |
Acetonitrile |
| Reaction conditions: pure and doped samples: 0.3 g; alcohol: 10 mmol; acetonitrile: CHCl3 and DMF 10 mmol; H2O2: 10 mmol; time 12 h: temperature: 100 °C. |
| a |
32 |
40 |
46 |
| b |
45 |
54 |
65 |
| c |
59 |
67 |
78 |
| d |
68 |
73 |
83 |
4.5. Effect of oxidants
Catalytic performance for oxidation as a function of the oxidant played an important role. This catalytic reaction is controlled by the option for the reaction to occur due to the contribution of the oxygen lattice (Fig. 9) from the oxidant. Hereafter, the reaction was successively carried out in the presence of different oxidants, such as TBHP and hydrogen peroxide. TBHP was slightly soluble on the selected organic substrate and the catalytic system. As a result, the yield of veratryl aldehyde is low in all synthesized CuCr2O4 nanomaterials. In the case of H2O2, the solubility on the chosen organic substrate could be higher. In conclusion, H2O2 on acetonitrile was usual because the establishment of the peroxycarboximidic acid was intermediate and the transfer agent was good oxygen. Hence, the choice of the oxidant was hydrogen peroxide.30,31
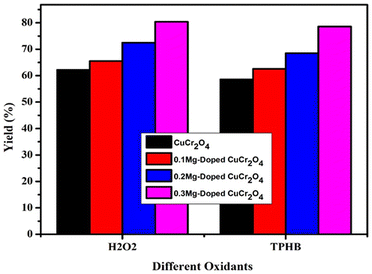 |
| | Fig. 9 Effect of oxidants in the selective oxidation of veratryl alcohol. Reaction conditions: pure and doped samples: 0.3 g; alcohol: 10 mmol; acetonitrile: 10 mmol; TBHP and H2O2: 10 mmol; time: 12 h; temperature: 100 °C. | |
4.6. Mechanism
A proposed mechanism in Scheme 1 suggests that H2O2 molecules undergo homolytic cleavage, resulting in two hydroperoxy radicals. These radicals react with Cr3+ and Cu2+ ions on the surface of CuCr2O4, creating Cr2+ and Cu+ ions and regenerating hydroperoxyl. The hydroperoxy radicals also react with hydroxy radicals on the surface to form water and oxygen. Veratryl alcohol molecules are adsorbed onto the surface of CuCr2O4, forming metal-alkoxide intermediates and adsorbing hydrogen via O–H bond cleavage. The surface O–H group is formed by bonding adsorbed hydrogen with the surface lattice oxygen and coordinating with the carbon atom of the methyl group in the veratryl alcohol molecule. Finally, the C–H bond in the β position is activated, resulting in the formation of veratraldehyde and water. The oxygen adsorbed onto the support surface dissociates into chemisorbed oxygen species and diffuses into the lattice vacancies of the support. This is followed by activation in the oxygen vacancies and conversion into lattice oxygen (O2−). The lattice oxygen further transfers to the surface of CuCr2O4 through oxygen-deficient sites, restoring the interface between the CuCr2O4 active sites.32–35| | |
OH− + H2O2 ↔ HO2− + H2O
| (1) |
| | |
2H2O2 + Cr2+ + Cu+ ↔ Cr3+ + Cu2+ + 2OH˙ + 2OH−
| (2) |
| | |
2HO2− + Cr3+ + Cu2+ ↔ Cr2+ + Cu+ + 2HO2˙
| (3) |
| | |
HO2˙ + OH˙ ↔ H2O + O2
| (4) |
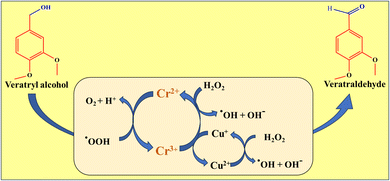 |
| | Scheme 1 Shows the selective oxidation of veratryl alcohol to veratraldehyde. | |
4.7. Recyclability of the catalyst
To examine the recyclability of the catalyst, the oxidation of veratryl alcohol to veratraldehyde was repeated five times with the same catalyst. The percentage conversion of veratraldehyde slightly decreased. The catalysts after the reaction were recovered by centrifugation, washed with ethanol, and then dried and reused for another reaction under the same conditions, as shown in Fig. 10. From this determination, the samples were filtered and the residue was washed several times with acetone and dried at 120 °C in an air oven for 3 h; this was repeated five times under identical catalytic conditions. Consequently, considering the small amount of catalyst used in the reaction, the great loss of catalyst should be due to the process of washing, which could be easily addressed in the large scale industrial production.
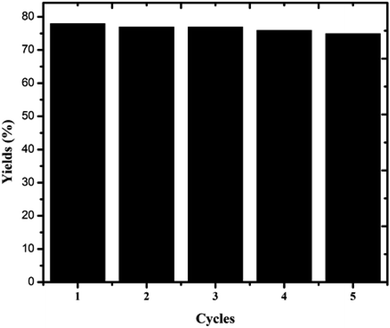 |
| | Fig. 10 Cycling performance of the copper chromite catalysts. | |
During the catalytic oxidation of veratryl alcohol to veratraldehyde, O–H bonds are broken and attended through the formation of hydrogen atoms, which further react with oxygen to generate water to achieve dehydrogenation to produce veratraldehyde. Subsequently, one hydrogen atom in the CHO groups was formed via a broken O–H bond, forming a metal–alcohol salt intermediate. Further, the objectivity of the hydrogen atom from carbon induces the conversion of the alcohol group into an aldehyde.
5. Conclusions
This paper demonstrates a simple method for producing efficient catalysts for the catalytic oxidation of veratryl alcohol to veratraldehyde. The further detachment of a hydrogen atom from carbon causes the alcohol group to be converted into an aldehyde group, resulting in the creation of an intermediate and veratric acid. XRD confirmed the development of the spinel phase. Thus, pure and Mg-doped spinels were found to be highly porous, as confirmed by SEM. Thus, copper chromite nanocomposites with a crystallite size in the range of 43–29 nm were synthesized. FT-IR was used to confirm the formation of the spinel phase. The current technology is applicable to various d and f block metal-supported catalysts for further catalytic processes.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R584), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/122/45.
References
- V. K. Gupta, N. Atar, M. L. Yola, Z. Üstündağ and L. Uzun, A novel magnetic Fe@Au core-shell nanoparticles anchored grapheneoxide recyclable nanocatalyst for the reduction of nitrophenolcompounds, Water Res., 2014, 48, 210–217 CrossRef CAS PubMed
 .
. - R. Saravanan, M. M. Khan, V. K. Gupta, E. Mosquera, F. Gracia, V. Narayanan and A. Stephen, ZnO/Ag/Mn2O3nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and ascorbic acid sensing and antimicrobial activity, RSC Adv., 2015, 5, 34645–34651 RSC
 .
. - V. K. Gupta, I. Ali, T. A. Saleh, M. N. Siddiqui and S. Agarwal, Chromium removal from water by activated carbon developed from waste rubber tires, Environ. Sci. Pollut. Res., 2013, 20, 1261–1268 CrossRef CAS PubMed
 ; S. Sessarego, S. C. G. Rodrigues, Y. Xiao, Q. Lu and J. M. Hill, Phosphonium-enhanced chitosan for Cr(VI) adsorption in waste water treatment, Carbohydr. Polym., 2019, 211, 249–256 CrossRef PubMed
; S. Sessarego, S. C. G. Rodrigues, Y. Xiao, Q. Lu and J. M. Hill, Phosphonium-enhanced chitosan for Cr(VI) adsorption in waste water treatment, Carbohydr. Polym., 2019, 211, 249–256 CrossRef PubMed  .
. - R. J. Bartlett, Chromium cycling in soils and water: Links, gaps, and methods, Environ. Health Perspect., 1991, 92, 17–24 CrossRef CAS PubMed
 .
. - G. Chen, C. Qiao, Y. Wang and J. Yao, Synthesis of magnetic gelatin and its adsorption property for Cr(VI), Ind. Eng. Chem. Res., 2014, 53, 15576–15581 CrossRef CAS
 .
. - B. Qiu, H. Gu, X. Yan, J. Guo, Y. Wang, D. Sun, Q. Wang, M. Khan, X. Zhang, B. L. Weeks, D. P. Young, Z. Guo and S. Wei, Cellulose derived magnetic mesoporous carbon nanocomposites with enhanced hexavalent chromium removal, J. Mater. Chem. A, 2014, 2, 17454–17462 RSC
 .
. - S. S. Banerjee, M. V. Joshi and R. V. Jayaram, Removal of Cr(VI)and Hg(II) from aqueous solutions using fly ash and impregnated flyash, Sep. Sci. Technol., 2005, 39, 1611–1629 CrossRef
 .
. - W. Jiang, Q. Cai, W. Xu, M. Yang, Y. Cai, D. D. Dionysiou and K. E. O'Shea, Cr(VI) adsorption and reduction by humic acid coated on magnetite, Environ. Sci. Technol., 2014, 48, 8078–8085 CrossRef CAS PubMed
 .
. - J. Zhao, Z. Li, J. Wang, Q. Li and X. Wang, Capsular polypyrrolehollow nanofibers: an efficient recyclable adsorbent for hexavalentchromium removal, J. Mater. Chem. A, 2015, 3, 15124–15132 RSC
 .
. - Y. Liu, L. Song, L. Du, P. Gao, N. Liang, S. Wu, T. Minami, L. Zang, C. Yu and X. Xu, Preparationofpolyaniline/emulsionmicrosphere composite for efficient adsorption of organic dyes, Polymers, 2020, 12, 167 CrossRef CAS PubMed
 .
. - N. Ballav, H. J. Choi, S. B. Mishra and A. Maity, Polypyrrole-coatedhalloysite nanotube clay nanocomposite: synthesis, characterization and Cr(VI) adsorption behaviour, Appl. Clay Sci., 2014, 102, 60–70 CrossRef CAS
 .
. - J. Wang, Y. Wen, X. Feng, Y. Song and L. Jiang, Control over the wettability of colloidal crystal films by assembly temperature, Macromol. Rapid Commun., 2006, 27, 188–192 CrossRef CAS
 .
. - A. Rugge, W. T. Ford and S. H. Tolbert, From a colloidal crystal to an interconnected colloidal array: A mechanism for a spontaneous rearrangement, Langmuir, 2003, 19, 7852–7861 CrossRef CAS
 .
. - J. Tan, Y. Song, X. Huang and L. Zhou, Facile functionalization of natural peach gum polysaccharide with multiple amine groups for highly efficient removal of toxic hexavalent chromium (Cr(VI)) ions from water, ACS Omega, 2018, 3, 17309–17318 CrossRef CAS PubMed
 .
. - M. Bhaumik, A. Maity, V. V. Srinivasu and M. S. Onyango, Removal of hexavalent chromium from aqueous solution usingpolypyrrole-polyaniline nanofibers, Chem. Eng. J., 2012, 181–182, 323–333 CrossRef CAS
 .
. - M. Mobarak, E. A. Mohamed, A. Q. Selim, L. Sellaoui, A. B. Lamine, A. Erto, A. Bonilla-Petriciolet and M. K. Seliem, Surfactant−modified serpentine for fluoride and Cr(VI) adsorption in single and binary systems: Experimental studies and theoretical modeling, Chem. Eng. J., 2019, 369, 333–343 CrossRef CAS
 .
. - M. N. Hasan, M. S. Salman, A. Islam, H. Znad and M. M. Hasan, Sustainable composite sensor material for optical cadmium(II) monitoring and capturing from wastewater, Microchem. J., 2021, 161, 105800 CrossRef CAS
 .
. - K. T. Kubra, M. M. Hasan, M. N. Hasan, M. S. Salman, M. A. Khaleque, M. C. Sheikh, A. I. Rehan, A. I. Rasee, R. M. Waliullah, M. E. Awual, M. S. Hossain, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, The heavy lanthanide of Thulium(III) separation and recovery using specific ligand-based facial composite adsorbent, Colloids Surf., A, 2023, 667, 131415 CrossRef CAS
 .
. - M. S. Salman, M. N. Hasan, M. M. Hasan, K. T. Kubra, M. C. Sheikh, A. I. Rehan, R. M. Waliullah, A. I. Rasee, M. E. Awual, M. S. Hossain, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent, J. Mol. Struct., 2023, 1282, 135259 CrossRef CAS
 .
. - A. Somy, M. R. Mehrnia, H. D. Amrei, A. Ghanizadeh and M. Safari, Adsorption of carbon dioxide using impregnated activated carbon promoted by Zinc, Int. J. Greenhouse Gas Control, 2009, 3, 249–254 CrossRef CAS
 .
. - A. K. Kercher and D. C. Nagle, Microstructural evolution during charcoal carbonization by X-ray diffraction analysis, Carbon, 2003, 41, 15–27 CrossRef CAS
 .
. - M. M. Hasan, K. T. Kubra, M. N. Hasan, M. E. Awual, M. S. Salman, M. C. Sheikh, A. I. Rehan, A. I. Rasee, R. M. Waliullah, M. S. Islam, S. Khandaker, A. Islam, M. S. Hossain, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, Sustainable ligand-modified based composite material for the selective and effective cadmium(II) capturing from wastewater, J. Mol. Liq., 2023, 371, 121125 CrossRef CAS
 .
. - M. R. Awual, I. M. M. Rahman, T. Yaita, M. A. Khaleque and M. Ferdows, PH dependent Cu(II) and Pd(II) ions detection and removal from aqueous media by an efficient mesoporous adsorbent, Chem. Eng. J., 2014, 236, 100–109 CrossRef CAS
 .
. - A. Dandekar, R. T. K. Baker and M. A. Vannice, Characterization of activated carbon, graphitized carbon fibers and synthetic diamond powder using TPD and DRIFTS, Carbon, 1998, 36, 1821–1831 CrossRef CAS
 .
. - Z. Oruç, M. Ergüt, D. Uzunoğlu and A. Özer, Green synthesis of biomass-derived activated carbon/Fe-Zn bimetallic nanoparticles from lemon (Citrus limon (L.) Burm. f.) wastes for heterogeneous Fenton-like decolorization of Reactive Red 2, J. Environ. Chem. Eng., 2019, 7, 103231 CrossRef
 .
. - S. Naghash-Hamed, N. Arsalani and S. B. Mousavi, The Catalytic Reduction of Nitroanilines Using Synthesized CuFe2O4 Nanoparticles in an Aqueous Medium, ChemistryOpen, 2022, 11, 1–10 CrossRef PubMed
 .
. - S. Naghash-Hamed, N. Arsalani and S. B. Mousavi, Facile copper ferrite/carbon quantum dot magnetic nanocomposite as an effective nanocatalyst for reduction of para-nitroaniline and ortho-nitroaniline, Nano Futures, 2022, 6, 045003 CrossRef CAS
 .
. - G. I. Danmaliki and T. A. Saleh, Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon, Chem. Eng. J., 2017, 307, 914–927 CrossRef CAS
 .
. - Y. Hu, G. Jiang, G. Xu and X. Mu, Hydrogenolysis of lignin model compounds into aromatics with bimetallic Ru-Ni supported onto nitrogen-doped activated carbon catalyst, Mol. Catal., 2018, 445, 316–326 CrossRef CAS
 .
. - S. Bose, T. Mukherjee and M. Rahaman, Simultaneous adsorption of manganese and fluoride from aqueous solution via bimetal impregnated activated carbon derived from waste tire: Response surface method modeling approach, Environ. Prog. Sustainable Energy, 2021, 40, e13600 CrossRef CAS
 .
. - M. Pirmoradi, N. Janulaitis, R. J. Gulotty Jr and J. R. Kastner, Bi-Metal-Supported Activated Carbon Monolith Catalysts for Selective Hydrogenation of Furfural, Ind. Eng. Chem. Res., 2020, 59, 17748–17761 CrossRef CAS
 .
. - P. Pré, G. Huchet, D. Jeulin, J.-N. Rouzaud, M. Sennour and A. Thorel, A new approach to characterize the nanostructure of activated carbons from mathematical morphology applied to high resolution transmission electron microscopy images, Carbon, 2013, 52, 239–258 CrossRef
 .
. - P. Veerakumar, V. Veeramani, S.-M. Chen, R. Madhu and S.-B. Liu, Palladium Nanoparticle Incorporated Porous Activated Carbon: Electrochemical Detection of Toxic Metal Ions, ACS Appl. Mater. Interfaces, 2016, 8, 1319–1326 CrossRef CAS PubMed
 .
. - Kong, Y.-Y. Xin, B. Li, X.-F. Zhang, Z.-P. Deng, L. H. Huo and S. Gao, Non-enzymatic CuCr2O4/GCE amperometric sensor for high sensing and rapid detection of nM level H2O2, Microchem. J., 2023, 194, 109343 CrossRef CAS
 .
. - T. Tatarchuk, A. Shyichuk, I. Trawczyńska, I. Yaremiy, A. T. Pędziwiatr, P. Kurzydło, B. F. Bogacz and R. Gargula, Spinel cobalt(II) ferrite-chromites as catalysts for H2O2 decomposition: Synthesis, morphology, cation distribution and antistructure model of active centers formation, Ceram. Int., 2020, 46, 27517–27530 CrossRef CAS
 .
.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *e,
A. Rajendranf,
Krishna Kumar Yadav
*e,
A. Rajendranf,
Krishna Kumar Yadav gh,
Ghadah Shukri Albakrii,
Mohamed Abbasj and
Maha Awjan Alreshidik
gh,
Ghadah Shukri Albakrii,
Mohamed Abbasj and
Maha Awjan Alreshidik
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and 10 mmol of oxidant (TBHP/H2O2) was added along with 0.1 to 0.3 g of the pure and Mg-doped copper chromites, and the fillings were heated to 100 °C at different reaction times for 12 h at altered time intervals in a three-necked round bottom flask equipped with a reflux condenser and the thermometer.
1), and 10 mmol of oxidant (TBHP/H2O2) was added along with 0.1 to 0.3 g of the pure and Mg-doped copper chromites, and the fillings were heated to 100 °C at different reaction times for 12 h at altered time intervals in a three-necked round bottom flask equipped with a reflux condenser and the thermometer.

.
.
; S. Sessarego, S. C. G. Rodrigues, Y. Xiao, Q. Lu and J. M. Hill, Phosphonium-enhanced chitosan for Cr(VI) adsorption in waste water treatment, Carbohydr. Polym., 2019, 211, 249–256 CrossRef PubMed
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.