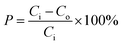Open Access Article
Open Access ArticleEnhanced reduction of COD in water associated with natural gas production using iron-based nanoparticles†
Moataz Elsaeed Selima,
Magdi E. Khalifaa,
Fawaz A. Agizahb,
Eman M. Mostafa c and
Fathi S. Awad
c and
Fathi S. Awad *ad
*ad
aChemistry Department, Faculty of Science, Mansoura University, Mansoura 35516, Egypt. E-mail: fathyawad949@yahoo.com; fathysamy@mans.edu.eg; Tel: +201000166374
bRashid Petroleum Company, Cairo, Egypt
cProduction Department, Egyptian Petroleum Research Institute, Cairo, Egypt
dChemistry Department, Faculty of Science, New Mansoura University, New Mansoura City, 35712, Egypt
First published on 10th April 2024
Abstract
The natural gas production industry faces the problem of the proper disposal of produced water and its treatment with significantly advanced technologies to meet the minimum quality standard for irrigation activities, commercial purposes, and consumption by living organisms. This study describes an effective method for reducing the COD (chemical oxygen demand) content in formation water using different metal oxide nanoparticles such as iron oxide (FO), iron zinc oxide (FZO), and iron vanadium oxide (FVO) nanoparticles. These nanoparticles were synthesized and fully characterized using powder X-ray diffraction (XRD) analysis, Fourier transform infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (SEM), energy dispersive X-ray (EDX) analysis, dynamic light scattering particle size (DLS) analysis and zeta potential analysis. The experimental results revealed that the maximum reduction of COD content was 42.18% using FVO nanoparticles with a dose of 3 g L−1 at 25 °C and pH = 6. Compared to commercial products [Redoxy and Oxy(OXYSORB)], the synthesized FO, FZO, and FVO nanoparticles demonstrated their superiority by achieving excellent results in decreasing the COD content of wastewater associated with natural gas production by more than 86%. This study introduces a promising technique for decreasing the COD content using metal oxide nanoparticles, which are eco-friendly, bio-safe, cheap, and nontoxic materials, and improving the quality of wastewater associated with natural gas production for its safe disposal through sewage and treatment plants.
1. Introduction
Water is the most necessary resource on the planet, and there is no life for humans or any living organism without it. Water has several unique characteristics that make it a precious resource. Today, finding pure water sources is a complicated global problem. Due to an increase in global urbanization and industrialization activities, a large amount of formation water is generated, and this formation water is disposed without proper management and treatment processes.1 One of these industries is natural gas and oil production, which is facing the problem of disposing the generated formation water and treating it with significantly advanced technologies to meet the minimum quality standard for irrigation activities, commercial purposes, and consumption by living organisms.2–5 During natural gas exploration and production, the produced fluids consist of natural gas and gas condensates, which are physically separated from formation water using advanced techniques, and the volume of produced formation water depends on the nature and the age of the gas-producing wells.6 Wastewater associated with petroleum industries (gas/oil) is considered a useless product generated from oil and gas reservoirs. Formation water is in direct contact with exploration petroleum products such as gas and oil in the reservoirs, wells, or surface pipelines. The general properties (chemical and physical) of formation water disposal in the petroleum industry vary greatly depending on the reservoir type (e.g., oil, gas, or coal), the geographic location of the field, geologic formation, and the produced hydrocarbon products (e.g., heavy oil, medium oil, light oil, lean gas, and rich gas). The lifetime of the proposed reservoir is the most critical factor for determining the characteristics and volume of formation water associated with petroleum industries, in which the small amount of formation water products at the beginning of the production from the reservoir increases with time as the reservoir becomes older.7Formation water produced in the natural gas industry is considered contaminated water compared with ground and surface water, which contains various organic species such as dissolved and dispersed hydrocarbons, phenols, and aromatic compounds, in addition to inorganic matter such as minerals, gases, heavy metals and biological contaminants such as SRB (sulfate reducing bacteria).7 The composition of formation water associated with natural gas production depends on the geological age and location, field position, type of production wells, and chemicals used during the production process, such as corrosion inhibitors, methanol, mono ethylene glycol, and biocides.8,9 Also, it has abnormal values in salinity, alkalinity, Chemical Oxygen Demand (COD), and Biochemical Oxygen Demand (BOD). Discharging the formation water in natural water sources without effective treatment will be toxic to living organisms and have harmful impacts on the environment.2,10–13 Formation water produced by the petroleum industry contains large quantities of pollutants that can badly affect the components of the environment, including human health, different water resources, air, crop production, aquatic life, etc. In addition, formation water from gas production is more toxic than that produced from oil because it contains high contents of hydrocarbons such as benzene, toluene, and xylene, which causes many problems such as dehydration and death of plants due to high salinity and damage to aquatic species because of reducing oxygen level (high levels of COD and BOD), scale problem and environmental impact due to chemical additives like corrosion inhibitors, methanol and biocides.14,15
The COD value of the wastewater is the most critical parameter for ensuring the quality of wastewater for disposal and the monitoring/control of discharge. The COD is an indicator of the concentration of organic constituents present in the wastewater.16,17 In recent years, the importance of treatment and reusing the formation water has increased by using advanced treatment techniques for reducing the values of COD, BOD5, oil and grease, and TSS to acceptable levels for using water for different purposes. In the petroleum industry, the removal of hydrocarbon components is the main aim in the treatment of formation water associated with the petroleum industry. There are many techniques (chemical, physical and biological) that are used in formation water treatment, such as chlorine and its derivatives, ultraviolet light,18 boiling, low-frequency ultrasonic irradiation,17,19 distillation, reverse osmosis, water sediment filters (fiber and ceramic), activated carbon, solid block, pitcher and faucet-mount filters, bottled water, ion exchange water softeners, flotation, coagulation and ozonisation. There are many factors in choosing the most effective method and the suitable materials for formation water treatment, such as its efficiency, reuse of the materials used, environmentally friendly materials, and cost-effectiveness.20–22 Although chemical and physical methods are preferred, they have some disadvantages, such as the high cost of treatment and massive, hazardous sludge produced from chemical treatment.23,24
In the recent century, nanotechnology has become the most unique and advanced technology that has helped humans solve several problems related to the environment and industry. This technology has many technical applications in science,25 such as nano system physics,26 nano chemistry,27 nanomaterials science, nanobiology,28 nanoelectronics,25 nano processing25 and nano mechanics.25 Nanomaterials are defined as materials that have dimensions less than 100 nm.29,30 Due to their existence on the nanoscale, nanomaterials have unique properties better than those of microscale materials, such as adsorption, small size, high surface area, reactivity, and catalytic activities.20,31 Nanomaterials have many applications in different fields, such as pharmaceuticals, medicine, photocatalysts, formation water treatment, removal of pollutants, and sensors.29 Many nanomaterials, such as titanium oxide nanoparticles, iron oxide nanoparticles, silver nanoparticles, zinc oxide nanoparticles, vanadium oxide nanoparticles, and copper nanoparticles,32,33 are used in formation water treatment.
In this research, metal oxide nanoparticles such as iron oxide (FO), iron zinc oxide (FZO), and iron vanadium oxide (FVO) nanoparticles were used to reduce the COD content in the formation water. The factors affecting the process of decreasing COD content using the metal oxide nanoparticles were studied to reach optimum conditions to achieve the maximum reduction of COD content, and these factors are nanoparticle concentration, temperature, and pH. These metal oxide nanoparticles are synthesized using the green synthesis method. The advantages of the green synthesis method are using eco-friendly, bio-safe, cheap, and nontoxic materials, availability of starting materials, and an effortless synthesis method. The pollutants and waste from this technique are the lowest waste compared to other synthesis techniques. This study introduces a promising, effective method to decrease the COD content and improve the quality of wastewater associated with natural gas production for disposal in sewage treatment plants. It reveals that using nanomaterials in wastewater treatment is an effective and distinctive method compared to other traditional treatment methods because of their unique properties, such as high surface area, small size, surface modifiability, magnetic properties, and excellent biocompatibility.
2. Materials and experimental
2.1. Materials and reagents
Iron chloride (FeCl3 and FeCl2), plant extract (green tea extract), sodium hydroxide (NaOH), distilled water, ammonium vanadate, anhydrous zinc chloride, and ammonia solution were obtained from Sigma-Aldrich. Commercial products [Redoxy and Oxy(OXYSORB)] were purchased from Watch Water company. Industrial formation water was obtained from a natural gas production company mercury(II)sulfate was obtained from scholar company.The samples of the formation water (2 L) were collected from the sampling point of the industrial formation water tank at the natural gas production plant. The collection of samples was carried out using suitable glass containers and standard sampling procedures. All chemicals used in this research were of analytical grade and had the highest purity. All solutions were prepared in distilled water and made on each experimental day.
2.2. Experimental
where P(%) is the removal percentage of COD; Co is the initial concentration of COD (mg L−1); Ci is the final concentration of COD (mg L−1).
3. Results and discussion
3.1. Characterization of iron oxide (FO), iron zinc oxide (FZO) and iron vanadium oxide (FVO) nanoparticles
The results of the XRD analysis of FO nanomaterials showed the peaks of the corresponding XRD patterns which are referenced to the following compounds, as shown in Fig. 1A. The hexagonal hematite Fe2O3 shows diffraction peaks at 24.120°, 33.077°, 35.627°, 40.820°, 49.401°, 53.945°, 57.429°, 62.381°, 63.993°, 71.661° and 75.445° corresponding to (012), (104), (110), (113), (024), (122), (214), (030), (1010) and (220) patterns, respectively. The cubic magnetite Fe3O4 shows diffraction peaks at 18.292, 21.152° and 35.439° corresponding to (111), (002), and (113) patterns, respectively.36,37 Fig. S4† shows the ICSD of the above compounds.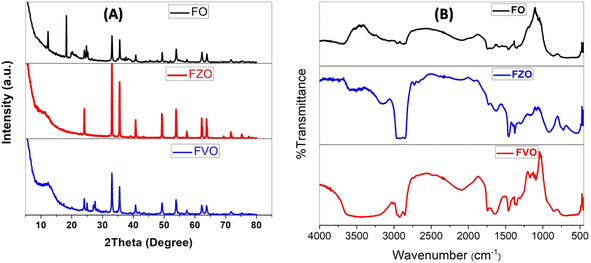 | ||
| Fig. 1 XRD analysis (A) and FT-IR spectra (B) of iron oxide (FO), iron zinc oxide (FZO), and iron vanadium oxide (FVO) nanoparticles. | ||
The XRD analysis of FZO nanomaterials showed many peaks of XRD patterns related to the following compounds as shown in Fig. 1A: hexagonal hematite Fe1.766O3 shows diffraction peaks at 24.120°, 33.077°, 35.627°, 39.129°, 40.820°, 43.488°, 49.401, 53.945°, 57.429°, 62.381°, 63.993°, 69.406° and 71.661° corresponding to (012), (104), (110), (006), (113), (202), (024), (116), (122), (030), (208) and (1010) patterns, respectively, cubic magnetite Fe3O4 shows diffraction peaks at 35.509°, 43.157° and 75.162° corresponding to (113), (004), and (226) patterns, respectively, hexagonal hematite Fe36O36 shows diffraction peaks at 32.958°, 38.240°, 51356°, 54.250°, 58.856°, 61.513°, 62.384, 69.129°, 72.2.385°, and 74.789° corresponding to (110), (012), (202), (211), (300), (113), (122), (220), (104), and (131) patterns, respectively, cubic magnetite Fe3O4 shows diffraction peaks at 35.659°, 43.341°, 53.778°, and 57.331° corresponding to (113), (004), (224), and (115) patterns, respectively, cubic magnetite Fe2.937O4 shows diffraction peaks at 35.279°, and 62.241° corresponding to (113), and (044) patterns, respectively, and ZnO shows diffraction peaks at 31.9°,34.6° and 36.2° corresponding to (100), (002) and (101) patterns, respectively.37,38 Fig. S5† shows the ICSD of the above compounds.
The XRD analysis of FVO nanomaterials indicated many peaks of XRD patterns related to the existing compounds as shown in Fig. 1A: hexagonal hematite (water containing) H0.99Fe1.67O3 shows diffraction peaks at 24.113°, 33.080°, 35.605°, 40.806°, 43.465°, 49.386°, 53.945°, 57.413°, 62.354°, 63.950°, 71.694° and 75.392° corresponding to (012), (104), (110), (113), (202), (024), (116), (018), (214), (030), (1010) and (220) patterns, respectively, orthorhombic vanadium oxide hydroxide H0.3O2V1 shows diffraction peaks at 27.585°, 55.473°, and 75.303° corresponding to (110), (211), and (320) patterns, respectively, tetragonal vanadium oxide hydroxide O0.532V1 shows diffraction peaks at 40.019° and 43.497° corresponding to (011) and (110) patterns, respectively, tetragonal vanadium oxide O2V16 shows diffraction peaks at 40.675° and 42.734° corresponding to (042), and (440) patterns, respectively, and monoclinic vanadium oxide O7V3 shows diffraction peaks at 24.926° and 31.261° corresponding to (111), and (206) patterns, respectively.29,32 Fig. S6† shows the ICSD of the above compounds. The average sizes of the nanoparticles have been estimated from the full width at half maximum (FWHM) of the diffraction peak using the Debye-Sherrer formula according to the equation
D = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
Fig. 2B illustrates the FTIR spectrum of FO, FZO, and FVO nanoparticles. In the FTIR spectrum of FO nanoparticles, the absorption bands are observed from the FTIR spectrum at 3439 cm−1, 469.582 cm−1, 436.798 cm−1, and 421.37 cm −1. A broad band appears at 3439 cm−1 due to –OH stretching, and the Fe–O bond vibration of Fe3O4 nanoparticles is at 469.582 cm−1. The low-frequency band at 436.798 cm−1 can be related to the Fe–O deformation in the octahedral region of hematite. A peak at 421.37 cm −1 shows the Fe2O3 bending vibration.34,35 From the FTIR spectrum of FZO nanoparticles, the absorption band is observed at 3498 cm−1, 539.971 cm−1, 480.188 cm−1, 469.582 cm−1, 436.798 cm−1, and 420 cm −1. A broad band appears at 3498 cm−1 due to –OH stretching, and the Fe–O bond vibration of Fe3O4 nanoparticles is at 469.582 cm−1. The low-frequency band at 436.798 cm−1 can be related to the Fe–O deformation in the octahedral region of hematite. The peak at 421.37 cm −1 shows the Fe2O3 bending vibration. The strong peaks at 539.971 and 480.188 cm−1 correspond to the stretching vibrations of Zn–O bands, which indicates that the samples are well crystallized.35,36 From the FTIR spectrum of FVO nanoparticles, the absorption bands are observed at 3436.53 cm−1, 1024.98, 468.617 cm−1, 436.798 cm−1, and 420.4 cm −1. A broad band appears at 3436.53 cm−1 due to –OH stretching, and the Fe–O bond vibration of Fe3O4 nanoparticles is at 468.617 cm−1. The low-frequency band at 436.798 cm−1 can be related to the Fe–O deformation in the octahedral region of hematite. The peak at 420.4 cm −1 shows vs. (symmetric stretching) modes of V–O–V vibration and the peak observed around 1024.98 cm−1 is attributed to the terminal oxygen vibration mode (V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).39,40
O).39,40
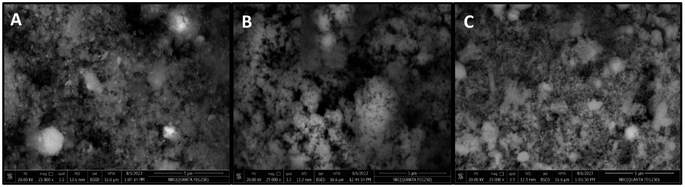 | ||
| Fig. 2 SEM images of iron oxide (FO) (A), iron zinc oxide (FZO) (B), and iron vanadium oxide (FVO) nanoparticles. | ||
The SEM images (Fig. 2) showed the morphology and the successful synthesis of FO, FZO, and FVO nanoparticles. The particle sizes of FO, FZO, and FVO nanoparticles are 254 nm, 290 nm, and 232 nm, respectively.
The EDX results showed the successful synthesis of FO, FZO, and FVO nanoparticles. From the EDX spectrums, the elemental analysis of samples reveals that iron oxide (FO) nanoparticles contain 39.28% iron weight and 60.72% oxygen weight as shown in Fig. S1(A),† iron zinc oxide (FZO) nanoparticles contain 84.16% iron weight, 9.75% oxygen weight and 6.09% zinc weight as shown in Fig. S1(B)† and iron vanadium oxide (FVO) nanoparticles contain 70.56% iron weight, 13.25% oxygen weight and 16.19% vanadium weight as shown in Fig. 3. The EDX analysis confirms the presence of FO, FZO, and FVO nanoparticles.
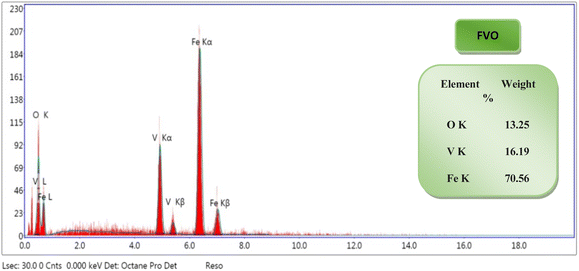 | ||
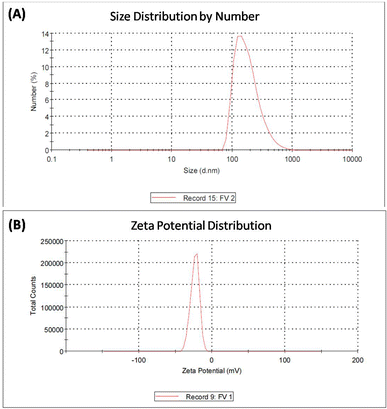
| Fig. 3 EDX analysis of iron vanadium oxide (FVO) nanoparticles. The results of DLS analysis for FO, FZO, and FVO nanoparticles are presented in Fig. 4A and S2,† including size distribution by number. The mean hydrodynamic sizes of iron oxide (FO), iron zinc oxide, and iron vanadium oxide (FVO) nanoparticles are 426.4 nm, 487.7822 nm, and 330.9 nm, respectively. | ||
Fig. S3† shows the zeta potential of FO nanoparticles and FZO nanoparticles. The zeta potential value of iron oxide (FO) nanoparticles is −21.6 mV, the zeta potential value of iron zinc oxide (FZO) nanoparticles is 14.1 mV, and the zeta potential value of iron vanadium oxide (FVO) nanoparticles is −23.6 mV as shown in Fig. 4B. The zeta potential value far from zero indicates that the particles disperse well in the media since the electrostatic repulsive force among the particles is significant. Thus, the particles have high aqueous stability. The nanoparticle dispersion with a value of zeta potential far from zero indicated stable or relatively high monodispersion, while that with a value close to zero indicated poor monodispersion. The particles with the zeta potential close to zero agglomerate to minimize Gibbs free energy. Surface modification by zinc or vanadium could prevent particle agglomeration and enable the dispersion of individual particles in the media.41
3.2. COD removal results
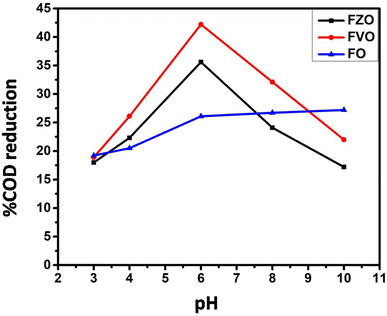
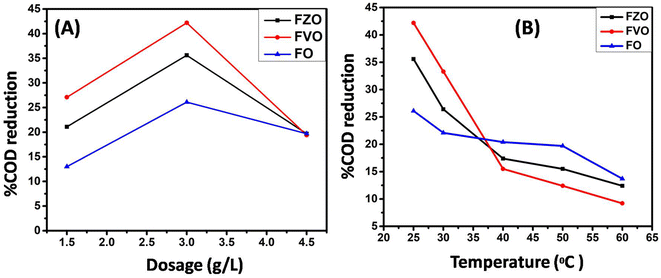
The effect of pH value on reducing COD content in formation water associated with natural gas production was studied by using FO, FZO, and FVO at different solution pH values (3, 4, 6, 8, and 10) and different doses of nanoparticles (1.5, 3.0, and 4.5 g L−1) at room temperature. The effect of pH on the adsorption behavior of different nanomaterials (FO, FZO, and FVO) for reducing the COD content is shown in Table S1† and Fig. 5. The optimum solution pH was 6 for FVO and FZO and 8 for FO, which achieved the highest adsorption capacity for reducing the COD. The pH of the solution regulates the adsorption capacity because it affects the adsorbent's surface characteristics. The adsorption capacity for nanomaterials decreased at low values (acidic medium) because positively charged hydrogen ions and organic contaminants repel each other electrostatically. When there is a significant concentration of H+, organic contaminants are substituted by protons at the adsorbent site, which decreases the removal efficiency. The stability of iron oxides decreases in an acidic medium. However, the adsorption capacity of nanoparticles improved as the pH values were raised from 3 to 6. Since fewer hydrogen ions were present at this pH, the functional groups on the adsorbent binding site were free to bond with organic contaminants and potentially produce organic pollutant–chelate complex. The percentage of COD content removal reduced at high pH levels (pH > 6). The competition between OH− ions and organic pollutants for adsorption sites on the adsorbent surface causes a decrease in organic pollutant removal at a pH above the ideal pH.42The effect of adsorbent dosage on reducing COD content by three different nanoparticles FO, FZO, and FVO in formation water associated with natural gas production was studied by carrying out experiments with different doses (1.5, 3.5, and 4.5 g L−1) at pH 6 and 25 °C. The effect of adsorbent dosage on the adsorption behavior of FO, FZO, and FVO nanomaterials for reducing COD content is shown in Table S2† and Fig. 6A. The COD removal efficiency increased with increasing adsorbent doses (1.5 to 3 g L−1) because more adsorption sites were available on the adsorbent surface, increasing surface area and adsorption capacity. However, the COD removal efficiency decreased at the adsorbent dose of 4.5 g L−1 because of the aggregation of nanomaterials, which reduced the total surface area and adsorption capacity. The maximum COD content reduction was achieved at 3 g L−1 for FO, FZO, and FVO nanomaterials.43–45
The effect of temperature value on reducing COD content by three different nanoparticles (FO, FZO, and FVO) in formation water associated with natural gas production was studied by carrying out experiments at different temperatures (25 °C, 30 °C, 40 °C, 50 °C, and 60 °C), 3 g L−1 dosage and pH 6. The effect of temperature on the adsorption behavior using different nanomaterials (FO, FZO, and FVO) for reducing COD content is shown in Table S3† and Fig. 6B. The highest COD removal was at 25 °C for all nanomaterials. The efficiency of reducing COD content decreased with increasing the temperature above 25 °C. So, the nature of the adsorption is exothermic.46 The temperature scale was chosen to match and reflect the conditions of normal operations at natural gas production sites, which are limited between 25 °C and 60 °C. In addition to increasing temperatures, it will represent an economic burden and a high significant cost. The use of heat exchangers on site to change and control temperatures could be an essential objective to apply. The primarily goal is to treat the wastewater operationally and economically appropriately.
The effect of commercial products on % COD reduction was studied by experimenting with two commercial products (Redox and Oxy) at different dosages (1.5, 3.0, 4.5, 100.0, 200.0, and 300.0 g L−1). The results revealed that the maximum COD reduction (22.12%) was obtained using the commercial product Redoxy at 300 g L−1, 25 °C, and pH = 10. In comparison, 0.0% COD reduction is achieved using the commercial product Oxy under the same experimental conditions, as shown in Table S3.† Therefore, FO, FZO, and FVO nanomaterials are highly efficient in reducing COD content in formation water samples compared to commercial materials. This is because they work at any pH value, are environmentally friendly, and can reduce COD content by more than 86% when compared to commercial products, which can only be used in alkaline medium (pH = 10). Fig. 7 compares the commercial products and nanoparticles (FO, FZO, and FVO).
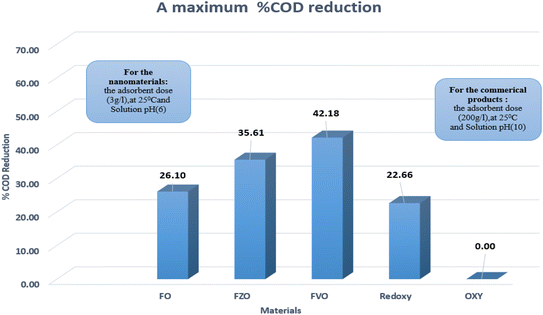 | ||
| Fig. 7 The maximum % COD reduction for commercial products (Redoxy and Oxy) and synthesized nanomaterials (FO, FZO, and FVO). | ||
3.3. The mechanism of COD removal in oilfield water using nanoparticles (FO, FZO, and FVO)
The oxidation power of the used nano compounds (according to the oxidation efficiency) in decreasing COD content varies and can be arranged as follows: FVO > FZO > FO > Redox > Oxy. For FVO nanoparticles, their power of decreasing COD content could be explained by the zeta potential value of −23.6 mV, in which the electrostatic repulsive force among the particles is largest and those particles have the most considerable aqueous stability compared to the zeta values for the other nanoparticles (FO and FZO). Additionally, the FVO NPs have high adsorption capacity due to their small particle size (232 nm) compared to other nanoparticles, which increases their surface area and efficiency in removal and decreases the COD content. The FVO is the most effective nanoparticle. Moreover, the photocatalytic behavior of FVO is mostly the effective force in the photocatalytic degradation of the organic pollutants in water formation. Thus, FVO can have a higher photo catalyst absorption of visible light than the other two compounds, FZO and FO.47,48Iron oxides mainly acted as a photocatalyst, while organic pollutants existing in the formation water, like mono ethylene glycol and methanol, which are the leading cause of high COD content, will generate electron–hole pairs, and the heterogeneous iron oxide–glycol/methanol medium (system) could exhibit a robust ligand-to-metal charge transformation ability as described below. Firstly, glycol/methanol can be adsorbed by iron oxide particles to form iron oxide–glycol/methanol complexes on the surface in solution, which are much more photoactive than other Fe3+ species, with the generation of methanol radical and glycol radical. Then a rapid decarboxylation is followed, and glycol radical is transformed into carbon-centered radical 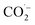 , and then further transformed into superoxide ion
, and then further transformed into superoxide ion 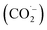 radical, which produces H2O2 and O2 by disproportion. H2O2 plays an essential role in the photocatalytic degradation process. The photo-Fenton-like system (Scheme 1) can be considered a promising and effective method for the photocatalytic degradation of organic pollutants and achieves excellent results in removing organic contaminants.48,49
radical, which produces H2O2 and O2 by disproportion. H2O2 plays an essential role in the photocatalytic degradation process. The photo-Fenton-like system (Scheme 1) can be considered a promising and effective method for the photocatalytic degradation of organic pollutants and achieves excellent results in removing organic contaminants.48,49
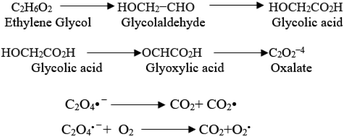 | ||
| Scheme 1 The photo-Fenton-like system is an effective method for photocatalytic degradation of organic pollutants. | ||
Generally, the oxidation power of the used iron oxide nanomaterial compounds varied widely in the purification of the industrial formation water from one to another due to the influence of the physical properties of contaminants that existed in the water rather than the properties of nanomaterials themselves, such as the size of nanomaterials, adsorption characters of the NMs, mechanism of the nanomaterials and photocatalytic behaviors. Therefore, the difference in the size of Fe3O4 nano adsorbents was favorable for the diffusion of metal ions from water solution onto the active sites of the adsorbent surface of nanomaterials. Fe3O4 nanomaterials are adequate for rapidly removing and decreasing metal ions from industrial water effluents.48,50,51
The adsorption mechanism of contaminants from industrial water by modified iron oxide nanoparticles includes surface site binding, magnetic selective adsorption, electrostatic interaction, and a combination of modified ligands. Thus, the addition of specific metal ions of heavy transition metals to the nanomaterials can achieve high efficiency in the oxidation operation of COD content existing in the formation water. The adsorption of organic contaminant species that existed in the formation water took place via surface exchange reactions until the surface functional sites were fully occupied, and the other contaminants could diffuse into the adsorbent for further interactions with the functional groups in the nanoparticles. So, modification and chemical treatment using NMs can be widely used to enhance the adsorption capability target. The important information obtained from this research is a precious aspect that is overcoming the toxicity of magnetic nano compounds generally synthesized by nanotechnology. The synthesis of nontoxic nanomaterials via green chemistry almost diminishes the environmental pollution as much as possible. It gives a better understanding of the potential hazards that can be mitigated using magnetic nanomaterials, especially in water and wastewater treatment.
4. Conclusion
This study described a promising attempt to reduce the COD levels in the formation water associated with natural gas production using iron-based nanoparticles such as iron oxide (FO) NPs, iron zinc oxide (FZO) NPs and iron vanadium oxide (FVO) NPs. The synthesized FO, FZO, and FVO nanoparticles were characterized by different techniques such as XRD, FT-IR, SEM, EDX, DLS, and zeta potential analysis. Many factors such as concentration, pH, and temperature are studied to get optimum conditions to achieve the most efficiency of % COD reduction using nanoparticles synthesized by a simple method in one step. The results illustrated that the optimum conditions for achieving the maximum COD content reduction by different nanomaterials (FO, FZO, and FVO) are 3.0 g L−1 adsorbent dose, 25 °C, and pH (6). The highest percentages of COD content reduction are 26.10% for FO, 35.61% for FZO, and 42.18% for FVO. Compared to commercial products (Redoxy and Oxy), the synthesized FO, FZO, and FVO nanoparticles have demonstrated their superiority by achieving excellent results in decreasing the COD content of formation water by more than 86%. With a COD reduction rate of up to 42.18% compared to other materials, iron vanadium oxide (FVO) is considered the best nanomaterial utilized overall. The chemical removal of COD using green synthesized nanomaterials was one of the biggest challenges in selecting and applying it to wastewater treatment operations. However, the obtained 42.18% COD removal represents an excellent result for COD removal from the chemical treatment plant objective point of view; the additional requirements for treatment could be achieved by the suitable chemical treatment and/or powerful oxidation operations such as ozonisation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This paper is based upon work supported by mansoura university research fund.References
- M. Amjad, S. Hussain, K. Javed, A. R. Khan and M. Shahjahan, World, 2020, 5, 34–40 Search PubMed
.
- S. Varjani, R. Joshi, V. K. Srivastava, H. H. Ngo and W. Guo, Environ. Sci. Pollut. Res., 2020, 27, 27172–27180 CrossRef CAS PubMed
.
- W.-W. Li and H.-Q. Yu, Biotechnol. Adv., 2011, 29, 972–982 CrossRef CAS PubMed
.
- X. Zhang, W. He, L. Ren, J. Stager, P. J. Evans and B. E. Logan, Bioresour. Technol., 2015, 176, 23–31 CrossRef CAS PubMed
.
- S. Varjani, G. Kumar and E. R. Rene, J. Environ. Manage., 2019, 232, 505–513 CrossRef CAS PubMed
.
- S. Shokrollahzadeh, F. Golmohammad, N. Naseri, H. Shokouhi and M. Arman-Mehr, Procedia Eng., 2012, 42, 942–947 CrossRef
.
- M. Nasiri and I. Jafari, Period. Polytech., Chem. Eng., 2017, 61, 73–81 CAS
.
- N. Mintcheva, G. Gicheva and M. Panayotova, Pollutants, 2022, 2, 234–251 CrossRef
.
- J. Lu, X. Wang, B. Shan, X. Li and W. Wang, Chemosphere, 2006, 62, 322–331 CrossRef CAS PubMed
.
- F. P. Perera, D. Tang, S. Wang, J. Vishnevetsky, B. Zhang, D. Diaz, D. Camann and V. Rauh, Environ. Health Perspect., 2012, 120, 921–926 CrossRef CAS PubMed
.
- J. Jasmine and S. Mukherji, J. Environ. Manage., 2015, 149, 118–125 CrossRef CAS PubMed
.
- C. Thakur, V. C. Srivastava, I. D. Mall and A. D. Hiwarkar, Clean: Soil, Air, Water, 2018, 46, 1700624 Search PubMed
.
- S. J. Varjani, R. R. Joshi, P. Senthil Kumar, V. K. Srivastava, V. Kumar, C. Banerjee and R. Praveen Kumar, Waste bioremediation, 2018, pp. 185–199 Search PubMed
.
- G. Zafra, A. Moreno-Montaño, Á. E. Absalón and D. V. Cortés-Espinosa, Environ. Sci. Pollut. Res., 2015, 22, 1034–1042 CrossRef CAS PubMed
.
- S. J. Varjani, E. Gnansounou and A. Pandey, Chemosphere, 2017, 188, 280–291 CrossRef CAS PubMed
.
- K. Sheth and K. V. Italia, Int. j. innov. res. sci. technol., 2017, 4, 56–59 Search PubMed
.
- D. K. Tiwari, J. Behari and P. Sen, World Appl. Sci. J., 2008, 3, 417–433 Search PubMed
.
- R. L. Droste and R. L. Gehr, Theory and practice of water and wastewater treatment, John Wiley & Sons, 2018 Search PubMed
.
- S. K. Gupta, J. Behari and K. K. Kesari, Asian J. Water, Environ. Pollut., 2006, 3, 101–105 CAS
.
- P. Jangid and M. P. Inbaraj, Mater. Today: Proc., 2021, 43, 2877–2881 CAS
.
- I. Oller, S. Malato and J. Sánchez-Pérez, Sci. Total Environ., 2011, 409, 4141–4166 CrossRef CAS PubMed
.
- L. Zhang and M. Fang, Nano Today, 2010, 5, 128–142 CrossRef CAS
.
- X. Dai, J. Fang, L. Li, Y. Dong and J. Zhang, Int. J. Environ. Res. Public Health, 2019, 16, 3223 CrossRef CAS PubMed
.
- A. Fakhru'l-Razi, A. Pendashteh, L. C. Abdullah, D. R. A. Biak, S. S. Madaeni and Z. Z. Abidin, J. Hazard. Mater., 2009, 170, 530–551 CrossRef PubMed
.
- M. Amil Usmani, I. Khan, A. H. Bhat, R. S. Pillai, N. Ahmad, M. K. Mohamad Haafiz and M. Oves, Curr. Org. Synth., 2017, 14, 206–226 CrossRef
.
- M. Super, H. D. V. Heese, D. MacKenzie, W. Dempster, J. Du Plessis and J. Ferreira, Water Res., 1981, 15, 1265–1270 CrossRef CAS
.
- J. N. Kostraba, E. C. Gay, M. Rewers and R. F. Hamman, Diabetes Care, 1992, 15, 1505–1508 CrossRef CAS PubMed
.
- M. M. Dorsch, R. K. Scragg, A. J. McMichael, P. A. Baghurst and K. F. Dyer, Am. J. Epidemiol., 1984, 119, 473–486 CrossRef CAS PubMed
.
- O. Aremu, C. Akintayo, E. Naidoo, S. Nelana and O. Ayanda, Int. J. Environ. Sci. Technol., 2021, 1–20 Search PubMed
.
- K. Gupta, S. Bhattacharya, D. Chattopadhyay, A. Mukhopadhyay, H. Biswas, J. Dutta, N. R. Ray and U. C. Ghosh, Chem. Eng. J., 2011, 172, 219–229 CrossRef CAS
.
- M. E. Doyle, Nanotechnology: a Brief Literature Review, Food Research Institute, UW-Madison, 2006 Search PubMed
.
- E. A. S. Dimapilis, C.-S. Hsu, R. M. O. Mendoza and M.-C. Lu, Sustainable Environ. Res., 2018, 28, 47–56 CrossRef CAS
.
- P. R. S. Baabu, H. K. Kumar, M. B. Gumpu, J. Babu K, A. J. Kulandaisamy and J. B. B. Rayappan, Materials, 2022, 16, 59 CrossRef PubMed
.
- N. Priya, K. Kaur and A. K. Sidhu, Front. nanotechnol., 2021, 3, 655062 CrossRef
.
- E. M. Mostafa and E. Amdeha, Environ. Sci. Pollut. Res., 2022, 29, 69861–69874 CrossRef CAS PubMed
.
- A. Vakili, A. Zinatizadeh, Z. Rahimi, S. Zinadini, P. Mohammadi, S. Azizi, A. Karami and M. Abdulgader, J. Cleaner Prod., 2023, 382, 134899 CrossRef CAS
.
- O. Bundit and K. Wongsaprom, J. Phys.: Conf. Ser., 2018, 1144, 012044 CrossRef
.
- P. Lahure, P. Salunke, R. Soliwal, A. Yadav, S. Tripathi and A. Koser, Int. J. Sci. Res. Phys. Appl. Sci., 2015, 3, 32–33 Search PubMed
.
- M. Farahmandjou and F. Soflaee, Phys. Chem. Res., 2015, 3, 191–196 CAS
.
- A. B. Habtemariam and M. Maaza, 2022.
- N. Chandrasekar, K. Kumar, K. S. Balasubramnian, K. Karunamurthy and R. Varadharajan, Dig. J. Nanomater. Biostructures., 2013, 8, 265 Search PubMed
.
- H. T. Ha, P. T. Phong and T. D. Minh, J. Anal. Methods Chem., 2021, 2021, 1–9 CrossRef PubMed
.
- P. Kongsune, S. Rattanapan and R. Chanajaree, Groundw. Sustain. Dev., 2021, 12, 100524 CrossRef
.
- F. S. Awad, K. M. AbouZied, A. M. Bakry, W. M. Abou El-Maaty, A. M. El-Wakil and M. S. El-Shall, J. Mater. Sci., 2021, 56, 7982–7999 CrossRef CAS
.
- F. S. Awad, K. M. AbouZied, W. M. Abou El-Maaty, A. M. El-Wakil and M. S. El-Shall, Arabian J. Chem., 2020, 13, 2659–2670 CrossRef CAS
.
- H. I. Adegoke, F. AmooAdekola, O. S. Fatoki and B. J. Ximba, Korean J. Chem. Eng., 2014, 31, 142–154 CrossRef CAS
.
- P. Xu, G. M. Zeng, D. L. Huang, C. L. Feng, S. Hu, M. H. Zhao, C. Lai, Z. Wei, C. Huang and G. X. Xie, Sci. Total Environ., 2012, 424, 1–10 CrossRef CAS PubMed
.
- N. J. Bandara, J. P. A. Hettiaratchi, S. Wirasinghe and S. Pilapiiya, Environ. Monit. Assess., 2007, 135, 31–39 CrossRef PubMed
.
- X. Zhang, X. Dong, H. Huang, Y. Liu, W. Wang, X. Zhu, B. Lv, J. Lei and C. Lee, Appl. Phys. Lett., 2006, 89, 051101 CrossRef
.
- S. Hu and L. V. Wang, Front. Neuroenerg., 2010, 2, 1560 Search PubMed
.
- M. Ozmen, K. Can, G. Arslan, A. Tor, Y. Cengeloglu and M. Ersoz, Desalination, 2010, 254, 162–169 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: EDX analysis of iron oxide (FO) and iron zinc oxide (FZO) (Fig. S1). DLS analysis of iron oxide (FO) and iron zinc oxide (FZO) (Fig. S2). Zeta potential analysis of iron oxide (FO) and iron zinc oxide (FZO) (Fig. S3). The effect of pH upon % COD reduction at different dosages of nanoparticles (Table S1). The effect of nanomaterial dosage (upon % COD reduction (Table S2). The effect of temperature upon % COD reduction (Table S3). The effect of different commercial products upon % COD (Table S4). The components of oilfield water (Table S5). The ICSD of compounds existed in FO nanomaterials (Fig. S4). The ICSD of compounds existed in FZO nanomaterials (Fig. S5). The ICSD of compounds existed in FVO nanomaterials (Fig. S6). See DOI: https://doi.org/10.1039/d4ra00888j |
| This journal is © The Royal Society of Chemistry 2024 |