Open Access Article
Open Access ArticleRational synthesis of 3D coral-like ZnCo2O4 nanoclusters with abundant oxygen vacancies for high-performance supercapacitors†
Yanlei Bia,
Huiqing Fan *a,
Chuansen Hua,
Ru Wanga,
Lujie Niua,
Guangwu Wenb and
Luchang Qinc
*a,
Chuansen Hua,
Ru Wanga,
Lujie Niua,
Guangwu Wenb and
Luchang Qinc
aSchool of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo 255000, China. E-mail: huiqingfan@yeah.net; hqfan@sdut.edu.cn
bSchool of Materials Science and Engineering, Shandong University of Technology, Zibo 255000, China
cDepartment of Physics and Astronomy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3255, USA
First published on 11th April 2024
Abstract
Transition metal oxides with high theoretical capacitance are regarded as desired electrode materials for supercapacitors, however, the poor conductivity and sluggish charge transfer kinetics constrain their electrochemical performance. The three-dimensional (3D) coral-like ZnCo2O4 nanomaterials with abundant oxygen vacancies were synthesized through a facile hydrothermal method and chemical reduction approach. The introduced oxygen vacancies can provide more active sites and lower the energy barrier, thereby facilitating the kinetics of surface reactions. Furthermore, the abundant oxygen vacancies in metal oxides can function as shallow donors to facilitate charge carrier diffusion, resulting in a faster ion diffusion rate and superior electrochemical conductivity. The electrochemical performance of ZnCo2O4 was optimized by the introduction of oxygen vacancies. The ZnCo2O4 nanoclusters, reduced by 0.5 M NaBH4 (ZnCo2O4-0.5), exhibit a specific capacitance of 2685.7 F g−1 at 1 A g−1, which is nearly twice that of the pristine ZnCo2O4 (1525.7 F g−1 at 1 A g−1). The ZnCo2O4-0.5 exhibits an excellent rate capacity (81.9% capacitance retention at 10 A g−1) and a long cycling stability (72.6% specific capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 3 A g−1). Furthermore, the asymmetric supercapacitor (ASC, ZnCo2O4-0.5 nanoclusters//active carbon) delivers a maximum energy density of 50.2 W h kg−1 at the power density of 493.7 W kg−1 and an excellent cycling stability (75.3% capacitance retention after 3000 cycles at 2 A g−1), surpassing the majority of previously reported ZnCo2O4-based supercapacitors. This work is important for revealing the pivotal role of implementing the defect engineering regulation strategy in achieving optimization of both electrochemical activity and conductivity.
000 cycles at 3 A g−1). Furthermore, the asymmetric supercapacitor (ASC, ZnCo2O4-0.5 nanoclusters//active carbon) delivers a maximum energy density of 50.2 W h kg−1 at the power density of 493.7 W kg−1 and an excellent cycling stability (75.3% capacitance retention after 3000 cycles at 2 A g−1), surpassing the majority of previously reported ZnCo2O4-based supercapacitors. This work is important for revealing the pivotal role of implementing the defect engineering regulation strategy in achieving optimization of both electrochemical activity and conductivity.
1. Introduction
With the growing ubiquity of unmanned intelligent systems and portable wearable devices, contemporary society necessitates an ever-increasing amount of energy.1 To mitigate the prevailing energy scarcity, extensive research is being conducted on energy storage devices that exhibit remarkable attributes such as substantial capacity and fast-charging abilities.2 Compared to lithium-ion batteries, which are costly and pose safety concerns due to the formation of lithium dendrites during charging and discharging processes,3 supercapacitors (SCs) emerge as ideal candidates for electrochemical energy storage devices owing to their exceptional power density, rapid recharge capabilities, prolonged lifespan, and environmental friendliness.4 Nevertheless, the limited energy density of SCs significantly hinders their practical utilization.5 The objective is to facilitate the commercialization of SCs; considerable attempts have pursued exploration of electrode materials possessing high theoretical capacity and electrochemical activity.6Up to now, the spinel cobalt-based oxide has garnered significant attention in the realm of electrode materials research.7 In comparison to simple monometallic oxides,8 various ternary metal oxides (e.g., ZnCo2O4,9 NiCo2O4,10 and CuCo2O4 (ref. 11)) possess notable advantages, including high theoretical capacitance, multiple oxidation states, and abundant reserves on Earth, thereby exhibiting outstanding electrochemical properties.12 However, the poor intrinsic conductivity and sluggish charge transfer kinetics of ZnCo2O4 significantly compromise its specific capacitance,13 which falls far below the theoretical value and fails to meet expectations. Extensive research has been implemented to overcome these bottlenecks, including the design of 3D structures and composites with high electrical conductivity materials (e.g., MXenes14 and graphene11). Li et al. synthesized the hierarchical cactus-like ZnCo2O4 films on nickel foam (NF) via 3D structure design, thereby increasing the electrode/electrolyte interface area and demonstrating an exceptionally high specific capacity of 1115.7 F g−1 at a current density of 1 A g−1.15 The investigation conducted by Bhagwan et al. focused on reducing the electron/ion diffusion length of ZnCo2O4 nanoparticles through nanostructure engineering, which exhibited a specific capacitance of 843 F g−1 at 1 A g−1 and experienced only an 8.5% capacity loss after undergoing 2000 cycles.16 Nevertheless, the existing strategies can only enhance the electrochemical performance of ZnCo2O4 materials to a certain extent without altering their inherent properties,17 the research on SCs has yet to meet the anticipated advancements.
Defect engineering of electrode materials has emerged as a highly effective strategy in the realm of electrochemical energy storage.18 Numerous studies have proved that the defect energy level could induce impurity states in the bandgap and serve as shallow donors to increase the concentration of carriers, thereby enhancing the intrinsic conductivity of electrode materials.19 Incorporating oxygen vacancies into metal oxides can disrupt the metal–oxygen bonds and intrinsically change the electronic structure of metallic oxides, leading to an increased number of delocalized electrons and enhanced charge transport capacity. Furthermore, oxygen vacancies can function as electroactive sites,20 reducing the activation energy of pseudocapacitive reactions, accelerating surface redox reactions, and greatly boosting electrochemical performance.21 Liu et al. successfully synthesized the long-range disordered MoO2 with rich oxygen vacancies, exhibiting an impressive specific capacity of 1631.3 mA h g−1 at a current density of 0.2 A g−1 and demonstrating excellent rate capability (592.6 mA h g−1 at 8.0 A g−1).22
The strategies for inducing oxygen vacancies primarily revolve around hydrogen treatment,23 post-annealing in oxygen-deficient atmospheres,24 ion doping,25 or electron irradiation.26 However, the aforementioned strategies often exhibit drawbacks such as high risks of hydrogen explosion, elevated temperature conditions, low efficiency, and intricate experimental procedures, thereby impeding the implementation of introducing oxygen vacancies in transition metal oxides. Compared to the above strategies, chemical reduction is a straightforward method with a gentle and controllable reaction process. The relative content of oxygen vacancies can be effectively modulated by manipulating the reaction temperature, duration, and reducing agent dosage.27,28 The utilization of NaBH4 as a reducing agent for the chemical reduction method has garnered significant attention in introducing oxygen vacancies in transition metal oxides. Under the influence of NaBH4 aqueous solution, the reducing molecule initially adsorbs onto the surface of the metal oxide, facilitating the reduction of partial metal ions within the oxide to a lower oxidation state.29 Subsequently, electron transfer enables the capture of an oxygen atom from surface oxygen,30 resulting in the formation of oxygen vacancies. The process of NaBH4 reduction is governed by the following mechanism:31,32
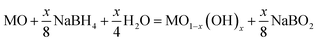 | (1) |
Unfortunately, there are rare reports involving the application of defect engineering and structure design to ZnCo2O4 materials. Therefore, developing facile and efficient strategies for the introduction of oxygen vacancies into 3D ZnCo2O4 electrode materials is still an ideal strategy for achieving enhanced electrochemical performance.
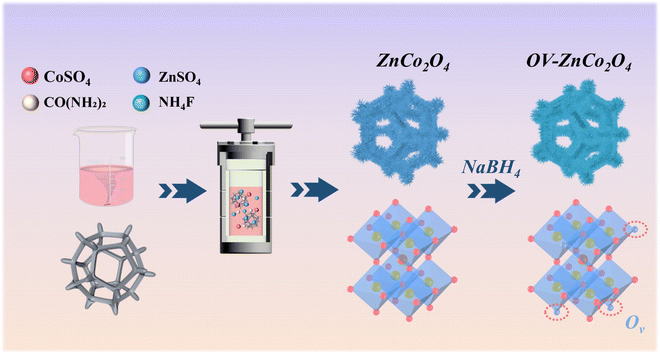
Herein, the novel coral-like ZnCo2O4-0.5 nanoclusters were synthesized utilizing a straightforward and secure methodology. The ZnCo2O4 nanoclusters firmly adhere to the NF substrate through a one-step hydrothermal process followed by a subsequent calcination step, eliminating the need for conductive agents and binders. Afterwards, NaBH4 was utilized as a reducing agent for obtaining the final product. In addition, by optimizing the concentration of NaBH4 and fine-tuning the reduction time, the resulting electrode material exhibits higher conductivity, multiple active sites, and remarkable mechanical robustness. Furthermore, the introduction of oxygen vacancies into ZnCo2O4 is successfully achieved through this simple and efficient method, while preserving its original morphology and structure to the greatest extent. Benefiting from the aforementioned profitable properties, the resultant coral-like ZnCo2O4-0.5 nanoclusters exhibit a specific capacitance of 2685.7 F g−1 at a current density of 1 A g−1, representing an approximately twofold increase compared to that of the untreated ZnCo2O4. Moreover, the assembled ASC device of ZnCo2O4-0.5 nanoclusters//active carbon (AC) delivers a superb energy density of 50.2 W h kg−1 at a power density of 493.7 W kg−1 and shows satisfactory cycling performance (75.32% capacitance retention after 3000 cycles at 2 A g−1).
2. Experimental section
2.1. Chemicals
The reagents used in this research are of analytical grade. Sinopharm Chemical Reagent supplied zinc sulfate heptahydrate (ZnSO4·7H2O, ≥99.5%), cobalt sulfate heptahydrate (CoSO4·7H2O, ≥98.5%), ammonium fluoride (NH4F, ≥96.0%) and urea (CH4N2O, ≥98.5%). The experiments were conducted using deionized water.2.2. Synthesis of pristine ZnCo2O4 and ZnCo2O4 with abundant oxygen vacancies (OV-ZnCo2O4)
The Ni foam (NF) underwent sequential pretreatment with acetone and dilute hydrochloric acid initially, followed by multiple rinses using deionized (DI) water and ethanol. Afterward, it was dried at 60 °C for 12 hours to obtain the pristine NF substrate.In a typical preparation of the ZnCo2O4, 6 mmol ZnSO4·7H2O, 12 mmol CoSO4·7H2O, 36 mmol NH4F, and 90 mmol CH4N2O in 200 mL DI water were mixed to obtain a homogeneous solution. The obtained mixture was stirred for 15 minutes to yield a transparent pink solution, which was subsequently transferred into a 50 mL Teflon-lined stainless steel autoclave. And then the NF substrate (effective reaction surface area: 1 × 2 cm2) was immersed in the reactant solution contained within the Teflon-lined vessel. The autoclave was subsequently placed in a blast oven and subjected to heating at 120 °C for 5 hours. Following cooling of the autoclave to ambient temperature, the substrate containing the precursor underwent sequential rinsing with deionized water and anhydrous ethanol. The precursor was ultimately subjected to calcination at 300 °C for 3 hours in the air using a muffle furnace with a heating rate of 2 °C min−1, resulting in the formation of the ZnCo2O4 material. The NF coated with ZnCo2O4 was immersed in NaBH4 solutions of varying concentrations (0.3 M, 0.5 M, 0.8 M, and 1 M) for 20 minutes, followed by rinsing with DI water and ethanol, subsequently dried at 60 °C for 12 hours under vacuum. The final products were named ZnCo2O4-0.3, ZnCo2O4-0.5, ZnCo2O4-0.8, and ZnCo2O4-1.0, respectively. The areal mass loading of active materials on Ni foam substrate was ∼1.08 mg cm−2. In addition, Table S1† shows the weight variations of Ni foam after different treatments during the experiment.
2.3. Material characterization
The crystal structure was analyzed by X-ray powder diffraction (XRD Rigaku, Japan, D/max-2200PC diffractometer with Cu Kα). Field emission scanning electron microscope (SEM, Gemini 300-71-31) and high-resolution electron microscope (TEM, FEI Talos F200x) were utilized to investigate the morphology and compositions of the as-synthesized materials. The chemical composition and surface electronic states were characterized by XPS (with an X-ray source of Thermo Scientific K-Alpha America). The Bruker EMXplus-6/1 EPR spectrometer detected the EPR signal. The specific surface area and the corresponding pore size distribution were evaluated by Brunauer–Emmett–Teller (BET, NOVA 4000e).2.4. Electrochemical characterization
To investigate the impact of oxygen vacancies introduction on electrochemical performance, the as-prepared ZnCo2O4 and OV-ZnCo2O4 samples were analyzed using an electrochemical workstation (CHI 660E, Chenhua, China) by a three-electrode system in 6 M KOH electrolyte. The as-prepared electroactive materials were employed directly as the working electrode, and an Ag/AgCl electrode and a platinum foil were utilized as the reference electrode and counter electrode, respectively. Cyclic voltammetry (CV), galvanostatic charging-discharging (GCD), and electrochemical impedance spectroscopy (EIS) were carried out at room temperature to test electrochemical properties. The specific capacitance (Csp) of the as-prepared electrode was calculated by the following equation:
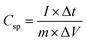 | (2) |
2.5. Fabrication of ZnCo2O4-0.5//AC ASC device
An ASC device was assembled using the as-prepared ZnCo2O4-0.5 materials as the positive electrode, AC as the negative electrode, and aqueous 6 M KOH as the electrolyte. Moreover, the balance of charges (q+ = q−) was considered to determine the most optimal mass ratio for the positive and negative electrodes as follows:
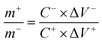 | (3) |
The energy density (E, W h kg−1) and power density (P, W kg−1) can be calculated by the following equation:
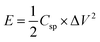 | (4) |
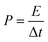 | (5) |
3. Results and discussion
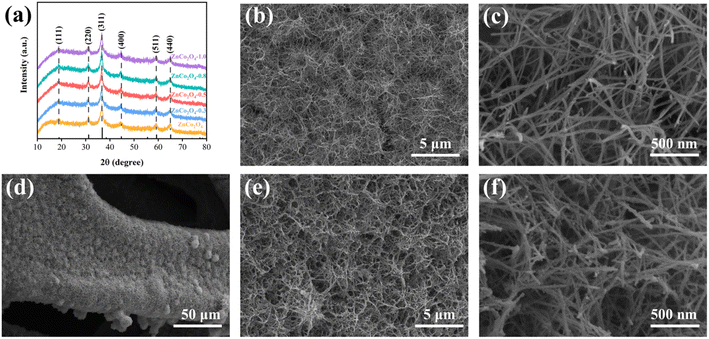
The synthetic process of the 3D coral-like ZnCo2O4-0.5 nanoclusters with abundant oxygen vacancies is shown in Scheme 1. Firstly, hydrothermal and calcination processes are carried out to facilitate the growth of ZnCo2O4 on the NF skeleton. Afterwards, the pristine ZnCo2O4 are immersed in NaBH4 solutions with varying concentrations (0.3 M, 0.5 M, 0.8 M, and 1.0 M) and the resulting products are subsequently dried at 60 °C for 12 hours under vacuum.To examine the impact of introducing oxygen vacancies on the crystalline structure, the X-ray diffraction (XRD) patterns of ZnCo2O4 and OV-ZnCo2O4 (with different NaBH4 concentrations) were shown in Fig. 1a, the same diffraction peaks of all samples situated at 19.0°, 31.2°, 36.8°, 59.3°, and 65.2° are exclusively indexed to the (111), (220), (311), (511), and (440) planes of cubic ZnCo2O4 phase (JCPDS card No. 23-1390).33 The aforementioned results indicate that the crystal structure has no obvious change during the NaBH4 reduction process.34 The XRD patterns were analyzed with correlational Rietveld refinement to characterize the structure of the synthesized materials (Fig. S1–S2 and Table S2†). Compared with that of pristine ZnCo2O4, the crystallinity of the ZnCo2O4-0.5 decreased. The Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of ZnCo2O4 and ZnCo2O4-0.5 is 1
O ratio of ZnCo2O4 and ZnCo2O4-0.5 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.90 and 1
3.90 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.66, respectively, indicating that the formation of oxygen vacancies in ZnCo2O4-0.5 material.24,35,36
3.66, respectively, indicating that the formation of oxygen vacancies in ZnCo2O4-0.5 material.24,35,36
The structures and morphologies of pristine ZnCo2O4 and ZnCo2O4-0.5 are investigated by SEM images. Fig. 1b and c depict SEM images of pristine ZnCo2O4, the nanowires have uniform growth on the surface of NF substrate with an average width of approximately 15 nm and length of 2 μm. Fig. 1d–f present SEM images of ZnCo2O4-0.5 samples. After the reduction treatment, it can be observed in Fig. 1d that the ZnCo2O4-0.5 nanowires uniformly adhered to the NF substrate. Fig. 1e indicates the morphology of ZnCo2O4-0.5, remains largely consistent with that of the pristine ZnCo2O4, the nanowires intersect with each other, resulting in the formation of 3D coral-like ZnCo2O4-0.5 nanoclusters. Additionally, the surface of nanowires shows an increased roughness after undergoing reduction treatment (Fig. 1f). The 3D coral-like ZnCo2O4-0.5 nanoclusters with rougher surface not only enhance the material's surface area and facilitate electrode–electrolyte contact but also provide additional active sites, thereby accelerating the redox reaction rate and optimizing its electrochemical performance.37 Additionally, SEM images of OV-ZnCo2O4 materials obtained with varying concentrations of NaBH4 solution are presented in Fig. S3,† the morphology of nanoclusters is preserved after reduction by 0.3 M NaBH4 in ZnCo2O4-0.3. When the concentration was increased to 0.8 M or 1 M, ZnCo2O4-0.8 and ZnCo2O4-1.0 could still partially maintain the original structure. However, due to its limited mechanical adhesion, the active material detaches from the NF substrate and compromises the integrity of the electrode structure, consequently leading to a reduction in electrochemical capacity.
The microstructure of ZnCo2O4-0.5 was further evidenced through TEM images. Fig. 2a provides further support for the 3D coral-like ZnCo2O4-0.5 nanoclusters are composed of interwoven nanowires. As shown in the HRTEM image (Fig. 2b), the well-defined lattice fringes of 0.241 nm, 0.278 nm, and 0.459 nm are assigned to the (111), (220), and (311) planes of the cubic ZnCo2O4 phase, respectively.38 As shown in Fig. 2c, the selected area electron diffraction (SAED) pattern also follows the XRD results, several diffraction rings can be indexed to the (220), (311), and (511) facets of the spinel ZnCo2O4 phase.39 The STEM image and energy dispersive spectroscopy (EDS) mapping characterization in Fig. 2d demonstrate a homogeneous distribution of Co, Zn, and O throughout the representative ZnCo2O4-0.5.
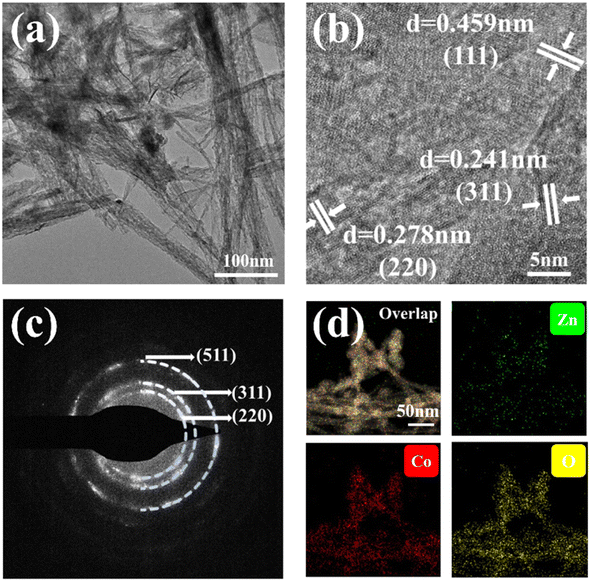 | ||
| Fig. 2 (a) TEM image of ZnCo2O4-0.5 nanoclusters. (b) HRTEM image, (c) SAED image, and (d) TEM elemental mapping analysis image of ZnCo2O4-0.5 nanoclusters. | ||
The specific surface area and pore size distribution were investigated through the analysis of N2 adsorption–desorption isotherms. As shown in Fig. S4a,† both pristine ZnCo2O4 and ZnCo2O4-0.5 display a characteristic IV isotherm with an H3-type hysteresis loop, indicating their mesoporous properties.40 The specific surface area of ZnCo2O4 and ZnCo2O4-0.5 were calculated by the BET method, revealing that the latter exhibited a significantly higher specific surface area (116.1 m2 g−1) compared to pristine ZnCo2O4 (99.1 m2 g−1). Moreover, the pore size distribution was depicted in Fig. S4b,† it has been determined that the average pore diameter of ZnCo2O4-0.5 (10.1 nm) surpasses that of ZnCo2O4 (8.7 nm). The larger specific surface area and mesoporous structure of ZnCo2O4-0.5 provide more active sites for redox reactions and enhance the penetration of electrolyte into the electrode materials.41
To reveal the surface chemical composition and electronic states of the pristine ZnCo2O4 and ZnCo2O4-0.5 nanoclusters, the XPS analysis was conducted. The wide-scan XPS spectra in Fig. 3a confirm the existence of Zn, Co, O, and Ni in both of them. The Ni component was derived from the NF substrate and the C 1s was used to rectify the binding energy.42 The Co 2p XPS spectra are presented in Fig. 3b. The two prominent peaks for pristine ZnCo2O4 were located at 780.1 eV and 794.9 eV, while that of ZnCo2O4-0.5 at 780.8 and 796.1 eV, which corresponded to the Co 2p3/2 and Co 2p1/2 spin–orbit components, respectively.43 The fitted results indicate that the distinct peaks for ZnCo2O4 at 779.8 and 794.8 eV were attributed to Co3+, while the peaks at 781.8 and 796.8 eV correspond to Co2+. With regard to the ZnCo2O4-0.5, the binding energies located at 779.9 and 796.1 eV are corresponded to Co3+, and at 781.8 and 798.1 eV are associated with Co2+, the Co 2p spectrum exhibits a slight blue shift which further indicates the reduction reaction.44 The intensities of the Co2+ peaks increased for ZnCo2O4-0.5 in comparison with that of pristine ZnCo2O4, accompanied by a decrease in the Co3+ peaks. This observation indicates that the reduction treatment promotes the conversion of high-valence Co3+ cations to lower-valence Co2+ cations while also promoting the generation of oxygen vacancies.45 By quantifying the peak area percentage of ZnCo2O4 and ZnCo2O4-0.5, the atomic ratio of Co2+/Co3+ on the surface changes from 0.54 to 1.22, which further indicates the partial reduction of Co3+ to Co2+. Moreover, the observed satellite peaks for both ZnCo2O4 and ZnCo2O4-0.5 detected at 775.6, 786.2, and 802.7 eV exhibit specific characteristics associated with Co2+, the introduction of oxygen vacancies leads to the conversion of Co3+ into Co2+, resulting in more intense satellite peaks for the ZnCo2O4-0.5.36 As for the O 1s high-resolution level spectra (Fig. 3c), three characteristic peaks for pristine ZnCo2O4 were located at 529.6, 531.4, and 533.1 eV, while that of ZnCo2O4-0.5 at 529.3, 531.4, and 533.8 eV, which attributed to the presence of the lattice oxygen (OL), oxygen vacancy (OV), and physico- and chemi-sorbed water (OW), respectively.46 After the NaBH4 treatment, the intensity of the lattice oxygen bond peak weakened, while there was an increase in the intensity of the oxygen vacancy peak. Based on the percentage of OV peaks area of ZnCo2O4 and ZnCo2O4-0.5, the proportion of OV increases from 44.8% to 73.6%, which further confirms the increase in oxygen vacancy content. As shown in Fig. 3d, two types of zinc species (Zn 2p3/2 and Zn 2p1/2) are observed, with characteristic spin–orbit peaks at 1021.7 and 1044.8 eV corresponding to each type respectively.47 The spectra indicate the presence of Zn(II) oxidation state for pristine ZnCo2O4 and ZnCo2O4-0.5. Therefore, based on XPS analysis, a significant alteration in the electron valence state of ZnCo2O4 is observed after reduction treatment, which supports the idea that the increased concentration of oxygen vacancies enhances the electrochemical performance of supercapacitors.48
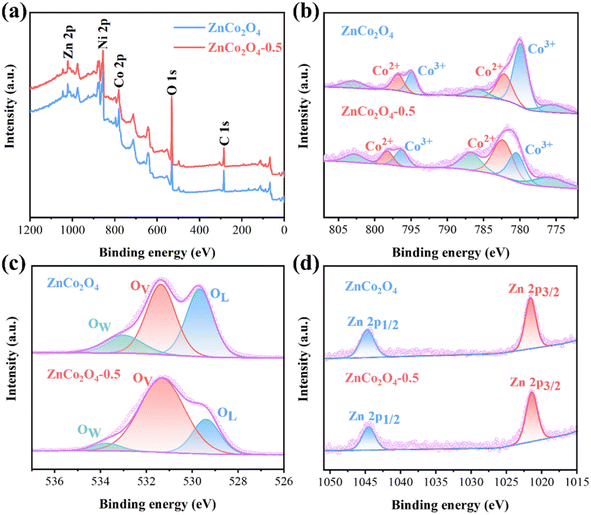 | ||
| Fig. 3 XPS spectra of pristine ZnCo2O4 and ZnCo2O4-0.5 nanoclusters: (a) wide-scan, (b) Co 2p, (c) O 1s and (d) Zn 2p. | ||
To provide additional evidence for the existence of oxygen vacancies, the electron paramagnetic resonance (EPR) spectra are performed in Fig. S5.† The spectra of ZnCo2O4 and ZnCo2O4-0.5 samples exhibit a symmetrical EPR signal at g ∼ 2.000 and g ∼ 2.002, respectively, which can be explained by the electron trapped in the oxygen vacancy.49 The ZnCo2O4-0.5 has a higher intensity of EPR signal than pristine ZnCo2O4, revealing that the NaBH4 reduction treatment instigates the formation of rich oxygen vacancies. Based on the analysis of the EPR signal, the oxygen vacancy concentrations of ZnCo2O4 and ZnCo2O4-0.5 are calculated to be 2.823 × 1011 spins per mm3 and 4.580 × 1011 spins per mm3, respectively. The results demonstrated the successful introduction of oxygen vacancies following the reduction treatment, thereby substantiating the XPS-based conclusion.35
To investigate the impact of oxygen vacancies introduction on electrochemical performance, a three-electrode device was fabricated using ZnCo2O4 and ZnCo2O4-0.5 as working electrodes, while a 6 M KOH aqueous solution was employed as the electrolyte.
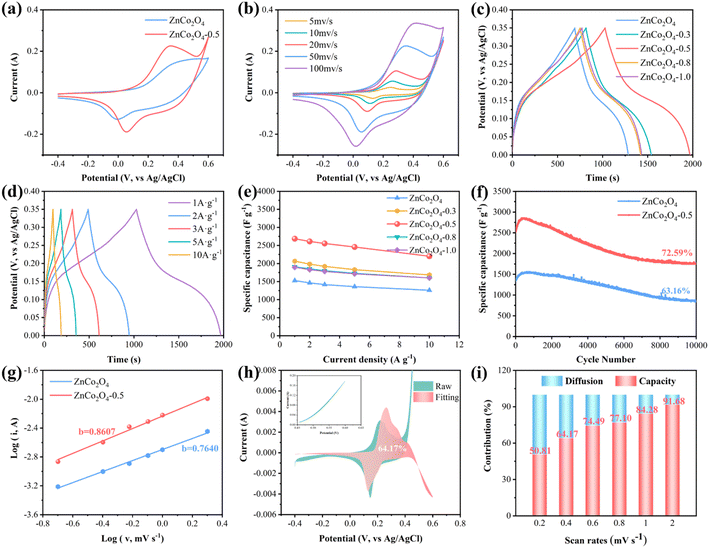
Fig. 4a shows CV curves at a scan rate of 50 mV s−1. Both ZnCo2O4 and ZnCo2O4-0.5 can observe a pair of well-defined redox peaks, which are indicative of the characteristic pseudo-capacitance behavior exhibited by electrode materials.50 The above redox reactions are mainly based on the following Faraday redox reactions:16,51,52
| ZnCo2O4 + H2O + OH− ↔ 2CoOOH + ZnOOH + e− | (6) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (7) |
The CV curve exhibited a single pair of redox peaks due to the synergistic effect exerted by the redox reactions of M–O and M–O–OH (where M represents Zn and Co ions) associated with OH−.53 Simultaneously, the reduction of ZnCo2O4 results in the appearance of a cathodic peak and the oxidation of both CoOOH and ZnOOH leads to an anodic peak, which aligns with the previous research conducted on the ZnCo2O4 materials.38,54
Additionally, compared with the pristine ZnCo2O4, the CV curve of ZnCo2O4-0.5 exhibits a larger integral area and higher peak current density due to the enhanced adsorption capacity of OH− resulting from an increased number of oxygen vacancies on the material surface.55 Evidently, the synergistic effect of oxygen vacancies and 3D nanostructures leads to an accelerated redox reaction rate and consequently, improved charge storage capability. Fig. 4b and S6a† present CV curves of the ZnCo2O4-0.5 and ZnCo2O4 at scan rates varying from 5 to 100 mV s−1 in a potential range of −0.4–0.6 V, respectively. With the increase in scan rate, a slight shift occurs in the position of both the anode and cathode peaks due to the rapid redox reaction at the electrode–electrolyte interface while the overall shape of the CV curve remained unaltered, representing the typical Faraday reaction and reversible redox reaction of the electrode material.56
The capacity optimization of ZnCo2O4 with the introduction of oxygen vacancies was further explored by galvanostatic charge–discharge (GCD) tests at 1 A g−1 within the potential range from 0 to 0.35 V presented in Fig. 4c. The discharge time of ZnCo2O4-0.5 is longer than that of others, indicating its superior electrochemical capacity. The calculated specific capacitance of ZnCo2O4-0.5 is 2685.7 F g−1 at 1 A g−1, which significantly surpasses that of the ZnCo2O4 (1525.7 F g−1). Moreover, as shown in Fig. 4d and S6b,† the GCD curves of ZnCo2O4-0.5 and ZnCo2O4 demonstrate a distinct voltage plateau accompanied by nonlinear and symmetrical characteristics, thereby providing additional substantiation for the pseudo-capacitance behavior with excellent reversibility.57 As summarized in Fig. 4e, the specific capacitance of the ZnCo2O4 samples after reduction treatment using different concentrations of NaBH4 at different current densities was calculated from the GCD curves. The electrochemical performance of ZnCo2O4 samples treated with 0.5 M NaBH4 was superior compared to other conditions. The specific capacitance of the ZnCo2O4-0.5 electrode is 2685.7, 2611.4, 2554.3, 2457.1, and 2200.2 F g−1 at 1, 2, 3, 5, and 10 A g−1, respectively. The noteworthy observation is that the specific capacitance of the ZnCo2O4 is merely 1525.7, 1462.9, 1414.3, 1357.1, and 1257.1 F g−1 even under identical current densities. The rate capability of ZnCo2O4-0.5 is commendable, as it demonstrates a capacity retention of 83.7% even under an increased current density of 10 A g−1. This superior electrochemical performance is attributed to the presence of richness-enabled oxygen vacancies on the surface, which can provide more active sites and lead to fast transmission of electrons and ions.11 The CV curves of bare NF substrate and ZnCo2O4-0.5 at 50 mV s−1 are shown in Fig.S7a.† By incorporating the CV curves, the contribution of the NF substrate to the electrochemical reaction is considered negligible, as it accounts for only 1.07% of the ZCO-0.5 area. The GCD curves and specific capacitance of the NF substrate are also presented in Fig. S7b and S8,† respectively. The calculated specific capacitance of the NF substrate is 21.2 F g−1 at 1 A g−1. The aforementioned results indicate that the contribution of NF substrate to the overall capacitance can be disregarded.
As shown in Fig. 4f, the cycling stability of the ZnCo2O4-0.5 and ZnCo2O4 electrodes was investigated by conducting repetitive galvanostatic charge–discharge tests within the voltage range of 0 to 0.35 V, at a high current density of 3 A g−1 for a total of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which serves as a crucial indicator for evaluating the commercial applications of supercapacitors. The specific capacitance of both ZnCo2O4 and ZnCo2O4-0.5 electrodes exhibited a marginal increase in the initial stage, which can be attributed to the ongoing activation process.58 The cycling stability of ZnCo2O4 was found to be insufficient, with only 63.16% of the initial capacitance retained after 10
000 cycles, which serves as a crucial indicator for evaluating the commercial applications of supercapacitors. The specific capacitance of both ZnCo2O4 and ZnCo2O4-0.5 electrodes exhibited a marginal increase in the initial stage, which can be attributed to the ongoing activation process.58 The cycling stability of ZnCo2O4 was found to be insufficient, with only 63.16% of the initial capacitance retained after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. However, subsequent chemical reduction resulted in an improvement in cycling stability to 72.59%, indicating the enhanced cycling stability of ZnCo2O4-0.5 can be attributed to the introduction of oxygen vacancies. The excellent cycling stability of ZnCo2O4-0.5 electrode is ascribed to the reversible electrochemical reaction and robust mechanical stability. A comparison of the electrochemical performance with the reported ZnCo2O4 electrodes is shown in Table S3.†
000 cycles. However, subsequent chemical reduction resulted in an improvement in cycling stability to 72.59%, indicating the enhanced cycling stability of ZnCo2O4-0.5 can be attributed to the introduction of oxygen vacancies. The excellent cycling stability of ZnCo2O4-0.5 electrode is ascribed to the reversible electrochemical reaction and robust mechanical stability. A comparison of the electrochemical performance with the reported ZnCo2O4 electrodes is shown in Table S3.†
The superior electrochemical performance of ZnCo2O4-0.5 is attributed to the following reasons:
(1) The oxygen vacancies can serve as active sites involved in the Faraday redox reaction, facilitating the adsorption of OH− species onto the surface of ZnCo2O4-0.5 nanoclusters, thereby accelerating the kinetics of surface reactions and reducing the energy barrier.59
(2) The presence of oxygen vacancies facilitates the removal of oxygen atoms from Zn–O and Co–O bonds, leading to electron migration from the oxygen 2p orbitals towards Zn2+, Co3+, and O atoms, increasing the degree of electron delocalization. The enhancement in the quantity of free electrons significantly enhances the intrinsic electrical conductivity of the ZnCo2O4-0.5 nanoclusters.46
(3) The introduction of oxygen vacancies optimizes the electronic structure of ZnCo2O4-0.5. The upward shift of the valence band and the downward shift of the conduction band effectively reduce the path length for electron transport processes in the bandgap, thereby enhancing the electronic conductivity of ZnCo2O4-0.5 nanoclusters.60
(4) Oxygen vacancies can also act as shallow donors, enhancing the diffusion of charge carriers and optimizing the electrochemical performance of ZnCo2O4-0.5 nanoclusters.61
(5) The ZnCo2O4-0.5 nanoclusters exhibit a distinctive coral-like 3D hierarchical porous structure, comprising micropores and mesopores, thereby providing enhanced electroactive surface area and effectively mitigating volume changes during charge and discharge processes.62 The NF substrate functions as a conductive grid, facilitating supplementary pathways for charge transfer.63
The intrinsic electrical properties of ZnCo2O4 and ZnCo2O4-0.5 electrodes were further investigated via electrochemical impedance spectroscopy (EIS) tests, in the frequency range of 100 kHz to 0.01 Hz. The EIS curves (Fig. S9†) exhibit distinct characteristics: the low-frequency region demonstrates a slope indicative of ion diffusion resistivity between the electrolyte and electrode,64 and the high-frequency region displays a semi-circle, while the semi-circle diameter representing Faraday charge transfer resistance (Rct).65 Additionally, the intercept on the real axis indicates the inherent resistance of the electrochemical system (Rs), which encompasses the intrinsic resistance of the active material, ionic resistance of electrolyte, and contact resistance between current collector and active material.66 The ZnCo2O4-0.5 exhibits a steeper slope in the low-frequency region compared to ZnCo2O4, implying an enhanced electron diffusion rate and reduced electrolyte diffusion resistance.67 The fitting value of Rs decreased from 0.59 to 0.40 Ω, and Rct decreased from 2.84 to 1.28 Ω after the introduction of oxygen vacancies. The EIS results imply that the introduction of oxygen vacancies enhances the original conductivity of pristine ZnCo2O4 electrode materials, while the 3D coral-like microstructure facilitates charge transfer and accelerates redox reaction kinetics.
To better investigate the impact of oxygen vacancies on the kinetic behavior and charge storage mechanism of ZnCo2O4-0.5, the mathematical relationship between the peak current (i) versus the scan rate (v), as obtained from the analysis of the CV curves under low current density, can be described by the following equation:68
| i = avb | (8) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = b i = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + log v + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a
| (9) |
| i = k1v + k2v1/2 | (10) |
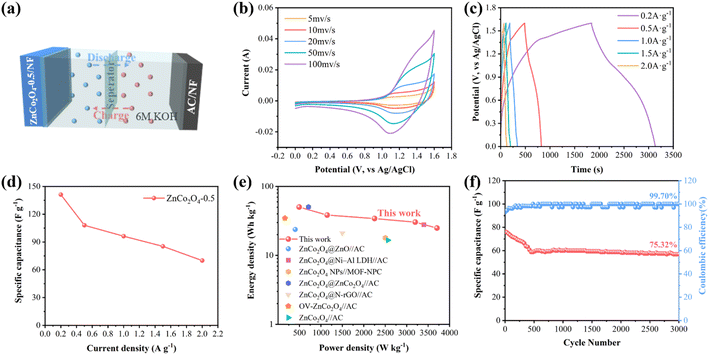
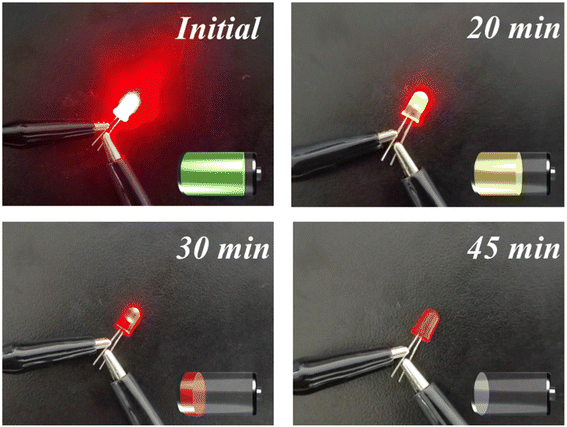
To investigate the electrochemical performance of ZnCo2O4-0.5 in practical applications, the ZnCo2O4-0.5 nanoclusters//AC ASC device was fabricated with ZnCo2O4-0.5 nanoclusters as the positive electrode, AC as the negative electrode, and 6 M KOH as the electrolyte, respectively (Fig. 5a). When the scan rate is set to 5 mV s−1, the CV curves of ZnCo2O4-0.5 and AC are illustrated in Fig. S11a.† Specifically, the shape of the CV curves represents the electrical double layer capacitance (EDLC) behavior of AC in the range of −1–0 V, while the obvious redox peaks of ZnCo2O4-0.5 in the range of 0–0.6 V indicate that the capacitance is derived from pseudo-capacitor behavior.73 The utilization of distinct electrode materials for the positive and negative electrodes effectively expands the voltage range of the ASC device.74 The CV curves of the ZnCo2O4-0.5 nanoclusters//AC ASC device obtained at different voltage windows are presented in Fig. S11b.† When the voltage is increased to 1.8 V, polarization effects during the charge and discharge process cause distortion in the CV curve, therefore, the optimal operating potential is 0–1.6 V.15 The CV curves of the ZnCo2O4-0.5 nanoclusters//AC ASC device at different scan rates are shown in Fig. 5b, and the shape of CV curve is well retained as the scan rate increases. The well-defined voltage plateau and nearly symmetrical charge–discharge curves in Fig. 5c further indicate the exceptional electrochemical properties, which are following the CV analysis. As summarized in Fig. 5d, the specific capacitance of the ZnCo2O4-0.5 nanoclusters//AC ASC device at different current densities calculated from the GCD curves are 141.1, 108.1, 96.3, 85.3, and 70.0 F g−1 at 0.2, 0.5, 1.0, 1.5, and 2 A g−1 respectively. In contrast, the specific capacitance of the ZnCo2O4 nanoclusters//AC ASC device is only 102.6 F g−1 at 0.2 A g−1. The lifespan parameter plays a crucial role in the practical implementation. The long-term cycling stability of the ZnCo2O4-0.5 nanoclusters//AC device was also tested for 3000 continuous cycles at the constant current density of 2 A g−1, which shows that the remarkable cycling performance with about 75.32% specific capacitance retention of its initial value after 3000 cycles and over as high as 99.70% coulombic efficiency retention (Fig. 5f). The initial stage may exhibit a weak capacity attenuation, which can be attributed to the insufficient adhesion of certain AC or ZnCo2O4-0.5 material on the NF substrate due to inherent imperfections in the coating process.75 It's worth noting that energy density and power density are two crucial parameters for assessing the commercial application viability of ASC devices. As shown in Fig. 5e, the ZnCo2O4-0.5 nanoclusters//AC ASC device reaches a maximum energy density of 50.2 W h kg−1 at a power density of 493.7 W kg−1 and remains 24.9 W h kg−1 at 3705.9 W kg−1, which is comparable to or approaching that most previously reported ZnCo2O4-based ASC device, such as ZnCo2O4@ZnO//AC (23.77 W h kg−1 at 399.98 W kg−1),76 ZnCo2O4@Ni-Al LDH//AC (27.84 W h kg−1 at 3400 W kg−1),77 ZnCo2O4 NPs//MOF-NPC (18 W h kg−1 at 2500 W kg−1),78 ZnCo2O4@ZnCo2O4//AC (50.41 W h kg−1 at 710.49 W kg−1),79 ZnCo2O4@N-rGO//AC (21 W h kg−1 at 1500 W kg−1),80 ZnCo2O4/NiCo2O4 @GO//AC (50.8 W h kg−1 at 800 W kg−1),81 OV-ZnCo2O4//AC (34.6 W h kg−1 at 160 W kg−1),82 ZnCo2O4//AC (16.63 W h kg−1 at 2561 W kg−1),83 thereby highlighting the optimization of electrochemical performance by oxygen vacancies introduction. Additionally, the practical application of ASC devices utilizing ZnCo2O4-0.5 nanoclusters as a positive electrode assembly was further investigated. As shown in Fig. 6 two coin batteries connected in series could illuminate a LED bulb (2 V, 20 mA) and sustain brightness for over 30 minutes.
4. Conclusion
In conclusion, the synthesis of ZnCo2O4 was achieved through a straightforward and efficient hydrothermal and calcination procedure. Furthermore, by investigating the optimal reduction concentration of NaBH4, 3D coral-like ZnCo2O4-0.5 nanomaterials with abundant oxygen vacancies were synthesized while preserving the original morphology of ZnCo2O4. Compared to the ZnCo2O4 (1525.7 F g−1), the specific capacitance of the ZnCo2O4-0.5 electrode achieves 2685.7 F g−1 at the current density of 1 A g−1, exhibiting an outstanding rate capability (maintaining more than 83.71% of its capacitance at 10 A g−1). The constructed ZnCo2O4-0.5 nanoclusters//AC ASC device delivers a maximum energy density of 50.2 W h kg−1 at the power density of 493.7 W kg−1, demonstrating inspiring cycling stability. The findings robustly confirm that the strategic integration of oxygen vacancies in metal oxides constitutes a novel and efficient method to enhance electrochemical performance.Author contributions
Yanlei Bi: conceptualization, methodology, formal analysis, writing – original draft. Huiqing Fan: conceptualization, project administration, funding acquisition, writing – review & editing. Chuansen Hu: methodology, formal analysis. Ru Wang: methodology, formal analysis. Lujie Niu: writing – review & editing. Guangwu Wen: supervision. Lu-Chang Qin: supervision, validation, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2019BB036).References
- X. Zou, C. Dong, Y. Jin, D. Wang, L. Li, S. Wu, Z. Xu, Y. Chen, Z. Li and H. Yang, Colloids Surf., A, 2023, 672, 131715 CrossRef CAS.
- Y. Wang, X. Wu, Y. Han and T. Li, J. Energy Storage, 2021, 42, 103053 CrossRef.
- Y. Sun, Y. Jin, Z. Jiang and L. Li, Eng. Failure Anal., 2023, 149, 107259 CrossRef CAS.
- N. Roy, K. S. Kumar, B. D. P. Raju, A. M. Karami, G. R. Reddy, H. R. Barai and S. W. Joo, Colloids Surf., A, 2024, 685, 133240 CrossRef CAS.
- F. Naseri, S. Karimi, E. Farjah and E. Schaltz, Renewable Sustainable Energy Rev., 2022, 155, 111913 CrossRef CAS.
- A. Joseph and T. Thomas, Prog. Solid State Chem., 2022, 68, 100381 CrossRef CAS.
- W. Guo, D.-F. Chai, J. Li, J. Lv, J. Wang, D. Guo, G. Sui and J. Xing, J. Energy Storage, 2022, 52, 104849 CrossRef.
- J. Li, W. Jiang and D. Wang, Colloids Surf., A, 2023, 658, 130750 CrossRef CAS.
- T. Ramachandran and F. Hamed, J. Phys. Chem. Solids, 2024, 188, 111915 CrossRef CAS.
- T. Ramachandran, F. Hamed, R. K. Raji, S. M. Majhi, D. Barik, Y. A. Kumar, R. M. Jauhar, M. P. Pachamuthu, L. Vijayalakshmi and S. Ansar, J. Phys. Chem. Solids, 2023, 180, 111467 CrossRef CAS.
- Y. Feng, W. Liu, Y. Wang, W. Gao, J. Li, K. Liu, X. Wang and J. Jiang, J. Power Sources, 2020, 458, 228005 CrossRef CAS.
- R. Jiang, S. Wang, M. Du, L. Zhang, J. Cao, Y. Zeng, M. Zhang and Y. Sui, Colloids Surf., A, 2023, 675, 132042 CrossRef CAS.
- L. Yuan, Y. Liu, N. Xin and R. He, J. Energy Storage, 2022, 52, 104727 CrossRef.
- T. Ramachandran, F. Hamed, Y. A. Kumar, R. K. Raji and H. H. Hegazy, J. Energy Storage, 2023, 73, 109299 CrossRef.
- S. Li, H. Fan, Y. Yang, Y. Bi, G. Wen and L.-C. Qin, J. Alloys Compd., 2022, 920, 165861 CrossRef CAS.
- J. Bhagwan, S. Khaja Hussain and J. S. Yu, J. Alloys Compd., 2020, 815, 152456 CrossRef CAS.
- M. Isacfranklin, S. Daphine, R. Yuvakkumar, L. Kungumadevi, G. Ravi, A. G. Al-Sehemi and D. Velauthapillai, Ceram. Int., 2022, 48, 24745–24750 CrossRef CAS.
- Y. Bai, Y. Ma, S. Zheng, C. Zhang, C. Hu, B. Liang, Y. Xu, G. Huang and R. Yang, Colloids Surf., A, 2022, 647, 129064 CrossRef CAS.
- S. Meena, K. S. Anantharaju, S. Malini, A. Dey, L. Renuka, S. C. Prashantha and Y. S. Vidya, Ceram. Int., 2021, 47, 14723–14740 CrossRef CAS.
- Y. Wu, Y. Wang, P. Zhu, X. Ye, R. Liu and W. Cai, Appl. Surf. Sci., 2022, 606, 154863 CrossRef CAS.
- Z. Xu, J. Jiang, M. Wang, J. Wang, Y. Tang, S. Li and J. Liu, Sep. Purif. Technol., 2023, 304, 122055 CrossRef CAS.
- R. Liu, J. Feng, R. Tang and T. Meng, Chem. Eng. J., 2023, 468, 143766 CrossRef CAS.
- Y. Wang, X. Xiao, Q. Li and H. Pang, Small, 2018, 14, e1802193 CrossRef PubMed.
- J. Yuan, W. Feng, Y. Zhang, J. Xiao, X. Zhang, Y. Wu, W. Ni, H. Huang and W. Dai, Adv. Mater., 2024, 36, e2303845 CrossRef PubMed.
- Y. Liu, Z. Ma, N. Xin, Y. Ying and W. Shi, J. Colloid Interface Sci., 2021, 601, 793–802 CrossRef CAS PubMed.
- X. Zhang, X. Liu, Y. Zeng, Y. Tong and X. Lu, Small Methods, 2020, 4, 190823 Search PubMed.
- S. Khaja Hussain and J. H. Bang, Phys. Chem. Chem. Phys., 2023, 25, 11892–11907 RSC.
- Q. Song, S. Zhou, S. Wang, S. Li, L. Xu and J. Qiu, Chem. Eng. J., 2023, 461, 142033 CrossRef CAS.
- L. Fu, S. Zhou, M. Xiang, J. Yang, W. Fan, Z. Yang and J. Ou, J. Electroanal. Chem., 2022, 921, 116650 CrossRef CAS.
- G. Zhuang, Y. Chen, Z. Zhuang, Y. Yu and J. Yu, Sci. China Mater., 2020, 63, 2089–2118 CrossRef CAS.
- J. Kumar, H. J. Jung, R. R. Neiber, R. A. Soomro, Y. J. Kwon, N. U. Hassan, M. Shon, J. H. Lee, K. Y. Baek and K. Y. Cho, Int. J. Energy Res., 2022, 46, 7055–7081 CrossRef CAS.
- X. Wei, C. Chen, X. Z. Fu and S. Wang, Adv. Energy Mater., 2023, 14, 2303027 CrossRef.
- H. Fan, H. Di, Y. Bi, R. Wang, G. Wen and L. C. Qin, RSC Adv., 2024, 14, 650–661 RSC.
- J. Yue, G. Lu, P. Zhang, Y. Wu, Z. Cheng and X. Kang, Colloids Surf., A, 2019, 569, 10–17 CrossRef CAS.
- A. Zhang, R. Gao, L. Hu, X. Zang, R. Yang, S. Wang, S. Yao, Z. Yang and H. Hao, Chem. Eng. J., 2021, 417, 129186 CrossRef CAS.
- Q. Ma, F. Cui, J. Zhang, X. Qi and T. Cui, Appl. Surf. Sci., 2022, 578, 152001 CrossRef CAS.
- B. Chettiannan, S. Mathan, G. Arumugam, A. Srinivasan and R. Rajendran, J. Energy Storage, 2024, 77, 110008 CrossRef.
- B. Naresh, C. Kuchi, S. K. Kummara, O. R. Ankinapalli and P. S. Reddy, Synth. Met., 2023, 293, 117283 CrossRef CAS.
- X. Li, M. Zhang, L. Wu, Q. Fu and H. Gao, J. Alloys Compd., 2019, 773, 367–375 CrossRef CAS.
- G. M. Tomboc, H. S. Jadhav and H. Kim, Chem. Eng. J., 2017, 308, 202–213 CrossRef CAS.
- M. Yuan, Z. Sun, Z. Wu, D. Wang, H. Yang, C. Nan, H. Li, W. Zhang and G. Sun, J. Colloid Interface Sci., 2022, 608, 1384–1392 CrossRef CAS PubMed.
- D. A. Alshammari, I. A. Ahmed, S. D. Alahmari, M. Abdullah, S. Aman, N. Ahmad, A. M. A. Henaish, Z. Ahmad, H. M. T. Farid and Z. M. El-Bahy, J. Energy Storage, 2024, 75, 109886 CrossRef.
- C. Zhang, L. Lu, S. Hao, S. Fang, Q. Sui, J. Li, Y. Zou, F. Xu, L. Sun, C. Xiang and J. Xie, J. Energy Storage, 2024, 77, 109983 CrossRef.
- Z. Wu, X. Hu, C. Cai, Y. Wang, X. Li, J. Wen, B. Li and H. Gong, J. Colloid Interface Sci., 2024, 657, 75–82 CrossRef CAS PubMed.
- C. Wang, G. Sui, D. Guo, J. Li, Y. Zhuang, W. Guo, Y. Zhou, X. Yang and D.-F. Chai, J. Energy Storage, 2022, 49, 104083 CrossRef.
- D. Yan, W. Wang, X. Luo, C. Chen, Y. Zeng and Z. Zhu, Chem. Eng. J., 2018, 334, 864–872 CrossRef CAS.
- P. Suggana, E. P. Kumar, K. Chandrasekhar Reddy, M. V. Lakshmaiah, S. W. Joo and G. R. Reddy, Colloids Surf., A, 2023, 669, 131423 CrossRef CAS.
- D. Cai, Q. Cao, J. Du, Y. Liu, F. Bu, Y. Yan, X. Lu, Q. Xia, D. Zhou and Y. Xia, Batteries Supercaps, 2021, 5, 1–11 Search PubMed.
- Y. Zheng, H. Che, A. Liu, X. Liu, T. Tian, Z. Guo, J. Mu, X. Zhang, Y. Wang and Z. Zhang, Colloids Surf., A, 2023, 675, 132029 CrossRef CAS.
- J. Xu, Z. Meng, Z. Hao, X. Sun, H. Nan, H. Liu, Y. Wang, W. Shi, H. Tian and X. Hu, J. Colloid Interface Sci., 2022, 609, 878–889 CrossRef CAS PubMed.
- H. Yu, H. Zhao, Y. Wu, B. Chen and J. Sun, J. Phys. Chem. Solids, 2020, 140, 109385 CrossRef CAS.
- Y. Shang, T. Xie, Y. Gai, L. Su, L. Gong, H. Lv and F. Dong, Electrochim. Acta, 2017, 253, 281–290 CrossRef CAS.
- G. R. Reddy, G. R. Dillip, T. V. M. Sreekanth, R. Rajavaram, B. D. P. Raju, P. C. Nagajyothi and J. Shim, Appl. Surf. Sci., 2020, 529, 147123 CrossRef CAS.
- A. J. C. Mary and A. C. Bose, Appl. Surf. Sci., 2018, 449, 105–112 CrossRef CAS.
- X. Xu, H. Lu, D. Xu, P. Zhou, Y. Ying, L. Li and Y. Liu, Appl. Surf. Sci., 2023, 614, 156174 CrossRef CAS.
- B. Zhang, L. Gao, H. Bai, Y. Li, B. Jia, X. Zhou, A. Li and L. Li, J. Alloys Compd., 2023, 934, 167979 CrossRef CAS.
- J. Ye, X. Zhai, L. Chen, W. Guo, T. Gu, Y. Shi, J. Hou, F. Han, Y. Liu, C. Fan, G. Wang, S. Peng and X. Guo, J. Energy Chem., 2021, 62, 252–261 CrossRef CAS.
- S. Chen, Q. Yang, J. Shi, Y. Ying and Y. Liu, Colloids Surf., A, 2022, 635, 128078 CrossRef CAS.
- J. Zhao, G. Lu, Y. Wu, P. Zhang, J. Yue, Z. Cheng, J. Zhang and X. Kang, Colloids Surf., A, 2020, 603, 125254 CrossRef CAS.
- D. Zu, H. Wang, S. Lin, G. Ou, H. Wei, S. Sun and H. Wu, Nano Res., 2019, 12, 2150–2163 CrossRef CAS.
- R. Wei, Y. Lu and Y. Xu, Sci. China: Chem., 2021, 64, 1826–1853 CrossRef CAS.
- Y. Li, J. Mei, L. Wu, Q. Xu and Z. Li, Int. J. Hydrogen Energy, 2024, 49, 67–80 CAS.
- K. Krishnamoorthy, G. K. Veerasubramani, S. Radhakrishnan and S. J. Kim, Chem. Eng. J., 2014, 251, 116–122 CrossRef CAS.
- M. Xu, M. Sun, S. u. Rehman, K. Ge, X. Hu, H. Ding, J. Liu and H. Bi, Chin. Chem. Lett., 2021, 32, 2027–2032 CrossRef CAS.
- J. Wang, Y. Luo, T. Xu, Z. Guo, G. Chen, Y. Ren, Y. Xue, N. Cai, H. Li and F. Yu, J. Ind. Eng. Chem., 2023, 118, 70–77 CrossRef CAS.
- X. Fu, Z. Zhang, Y. Zheng, J. Lu, S. Cheng, J. Su, H. Wei and Y. Gao, J. Colloid Interface Sci., 2024, 653, 1272–1282 CrossRef CAS PubMed.
- C. Ryu, H. M. Do and J. B. In, Appl. Surf. Sci., 2024, 643, 158696 CrossRef CAS.
- H. Wang, N. Mi, S. Sun, W. Zhang and S. Yao, J. Alloys Compd., 2021, 869, 159294 CrossRef CAS.
- P. Qi, H. Wang, Y. Lu, M. Chen, G. Liu, W. Li, C. Huang and Y. Tang, J. Alloys Compd., 2022, 895, 162535 CrossRef CAS.
- J. Sun, Y. Wang, J. Zhou, K. Chen, K. Tao, W. Zhao and L. Han, Inorg. Chem., 2022, 61, 4283–4291 CrossRef CAS PubMed.
- S. Xiong, X. Lin, S. Liu, S. Weng, S. Jiang, Y. Jiao, Y. Xu and J. Cheng, Vacuum, 2020, 182, 109692 CrossRef CAS.
- X. Wei, X. Zhou, L. Li, W. Feng and H. Wu, Appl. Surf. Sci., 2023, 613, 155959 CrossRef CAS.
- S. Wei, C. Wan, L. Zhang, X. Liu, W. Tian, J. Su, W. Cheng and Y. Wu, Chem. Eng. J., 2022, 429, 132242 CrossRef CAS.
- T. Ramachandran, S. S. Sana, K. D. Kumar, Y. A. Kumar, H. H. Hegazy and S. C. Kim, J. Energy Storage, 2023, 73, 109096 CrossRef.
- J. Li, R. Lian, J. Wang, S. He, S. P. Jiang and Z. Rui, Electrochim. Acta, 2020, 331, 135395 CrossRef CAS.
- Q. Ma, F. Cui, J. Zhang and T. Cui, J. Colloid Interface Sci., 2023, 629, 649–659 CrossRef CAS PubMed.
- X. Bai, D. Cao and H. Zhang, Ceram. Int., 2019, 45, 14943–14952 CrossRef CAS.
- D. He, Y. Gao, Y. Yao, L. Wu, J. Zhang, Z. H. Huang and M. X. Wang, Front. Chem., 2020, 8, 719 CrossRef CAS PubMed.
- P. Sivakumar, L. Kulandaivel, J. Park, C. J. Raj, R. Manikandan and H. Jung, J. Alloys Compd., 2023, 952, 170042 CrossRef CAS.
- G. Vignesh, R. Ranjithkumar, P. Devendran, N. Nallamuthu, S. Sudhahar and M. Krishna Kumar, Mater. Sci. Eng., B, 2023, 290, 116328 CrossRef CAS.
- X. Chen, N. Xin, Y. Li, C. Sun, L. Li, Y. Ying, W. Shi and Y. Liu, J. Mater. Sci. Technol., 2022, 127, 236–244 CrossRef CAS.
- K. Xiang, D. Wu, Y. Fan, W. You and D. Zhang, Chem. Eng. J., 2021, 425, 130583 CrossRef CAS.
- B. Guan, D. Guo, L. Hu, G. Zhang, T. Fu, W. Ren, J. Li and Q. Li, J. Mater. Chem. A, 2014, 2, 16116–16123 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00927d |
| This journal is © The Royal Society of Chemistry 2024 |