Open Access Article
Open Access ArticleArtificial vitriols: a contemporary interpretation of historical ingredients†
Giacomo Montanari *a,
Marianna Marchini
*a,
Marianna Marchini b,
Matteo Martelli
b,
Matteo Martelli a and
Lucia Maini
a and
Lucia Maini *b
*b
aDepartment of Philosophy, University of Bologna, Italy. E-mail: giacomo.montanari14@unibo.it
bDepartment of Chemistry “G. Ciamician”, University of Bologna, Italy. E-mail: l.maini@unibo.it
First published on 8th July 2024
Abstract
“Vitriol” is a term that appeared during the Middle-Ages to indicate a wide range of ingredients widely used both in medicinal and alchemical recipes. Green, blue, or white vitriols are easily associated with iron(II), copper(II), and zinc sulphate respectively thanks to the historical sources composed in the time period when the ancient and modern nomenclatures overlapped. However, other colours of vitriols are attested throughout history, such as yellow, red, or black. The identification of these compounds is significantly less straightforward, and often chalked up to Decknamen (code names) or unspecified impure ores. Moreover, from several sources it is apparent that some of these compounds are artificial, or at least the result of technical operations rather than mineral ores used as they are. By thermal manipulation of iron(II) sulphate, we managed to identify several compounds that fit with historical descriptions, which were later characterized through XRPD. Moreover, by using a Kofler bench and variable temperature XRPD, we were able to further investigate the transitions between these phases.
Introduction
The understanding of alchemical practices begins with the understanding of the reagents that were used in them. This poses a complex problem of identification, especially for the compounds and operations that were considered common knowledge at that time, and therefore not described in great detail. It is therefore essential to combine the philological analysis of the surviving recipes with a modern chemical discussion, to achieve a more trustful translation and to reach a deeper understanding of the history of chemistry.1,2Vitriols are a family of compounds widely discussed in alchemy and medicine since their early days,3 and therefore used for a wide variety of applications, ranging from “transmuting” (dyeing) metals4 to the treatment of blood loss.5
Even though these compounds are known and used since antiquity, the Latin word vitriolum appears only in the XIII century,6 and it was used alongside several other terms, sometimes classical in origin (such as atramentum),7 but often showing influences from Arabic traditions (such as azeg or zegi, from the Arabic zāj),8 all of which remain in the proto-chemical language up to the eighteenth century.9
The word vitriolum itself describes the appearance of these compounds, that are said to be “glass-like”, meaning “crystalline and transparent”.
With the advent of a more modern understanding and classification of chemicals, the term vitriol became a synonym for sulphate, and with this meaning it is present in texts dating up to the early twentieth century.10,11
To differentiate between different types of vitriols, it was common practice to define them by their colour. Thus, expressions such as green, blue, or white vitriol that were already common during the Renaissance, remained in use throughout the development of modern chemistry, allowing us to correlate them with iron, copper and zinc sulphate respectively. Moreover, they are easily related to natural ores which allow us to identify the composition and associate both a chemical formula and a crystal structure to it.
Green vitriol, usually identified with the mineral melanterite, is composed of iron sulphate heptahydrate (FeSO4·7H2O). It appears as a pale green crystalline powder, that undergoes dehydration when left in a hot and dry environment.3,10
Blue vitriol has been identified as chalcanthite, a bright blue mineral composed of copper sulphate pentahydrate (CuSO4·5H2O).10
The identification of white vitriol is less established, but is commonly accepted as a form of zinc sulphate, possibly goslarite (ZnSO4·7H2O).10
However, several other colours are associated with vitriols in ancient and early modern alchemical literature, such as yellow, red, and black.4,12,13 The identification of these compounds is often the subject of speculation, and preconceived ideas about them can lead to incorrect conclusions when interpreting alchemical recipes. This is particularly relevant when discussing any ingredient that is man-made to some extent, rather than a natural mineral.
A yellow, man-made vitriol is an ingredient only occasionally mentioned in alchemical texts. For instance, George Ripley, an English alchemist from the XV century,14 includes it in some of his recipes, and Johnson in his lexicon speaks of an atramentum citrinum (yellow vitriol), but he doesn't offer any subsequent elucidation on the matter. Moreover, the yellowness of vitriol is also described with the Latin adjective flavus, with expressions such as atramentum flavum and a vitriolum flavum. However, the former often refers to a yellow ink15 and the latter seems to refer to a natural ore.16,17
From this few and sparse indications, however, yellow vitriol appears to be the result of a heat treatment of a sulphate, either of iron or copper, as it will be shown in the following sections.
Red vitriol can be even more elusive. The reddening of vitriol is tangentially mentioned in the Antidotarium Nicholai (see Text 1 in the ESI†)‡, and this compound is sometimes called colchotar,9,10,18 as well as vitriolum rubeum or rubificatum18 (red or reddened vitriol), or equivalent expression in other languages (such as vitriuolo rubificato§).
According to historical sources, this red vitriol could be prepared by heating green vitriol18 (sometimes already partially calcined to whiteness or yellowness¶) at high temperature, or alternatively by collecting what remained after the distillation of vitriol to make sulphuric acid (oil of vitriol). Similarly, Jonhson stated that ut vitriolum, quod si fixetur, colchotar vocatur (thus vitriol, when it is fixed [after distillation], is called colchotar). These two methods are presented as equivalent.
This is consistent with modern chemical knowledge, since heating Fe(II) sulphate above 500 °C in the presence of atmospheric oxygen, induces oxidation to Fe(III) and then decomposition to iron oxide and SO3, that can be used to make sulphuric acid under the right conditions:3,19
| 4FeSO4 + O2 → 2Fe2O(SO4)2 | (1) |
| 3Fe2O(SO4)2 → Fe2(SO4)3 + Fe2O3 | (2) |
| Fe2(SO4)3 → Fe2O3 + SO3 | (3) |
Others proposed that red vitriol can also be made from a mixture of copper and iron sulphates.20 These minerals are often found together in ores,21 making it possible that they were worked together.
The issue regarding black vitriol has another layer of complexity: even though an atramentum nigrum (black vitriol) is mentioned in the Latin translation of Rhazes' work (the Liber Secretorum de voce Bubacaris), this seems to be a natural ore (of uncertain identification, possibly the same called sory by Dioscorides) rather than a man-made compound.22 Moreover, the understanding of this topic is often muddled by the tendency of some scholars (especially in the past) to use “black vitriol” a translation for instances of ancient terminology even when there is no explicit mention of colour in the original text.
Therefore, it is possible that this is just a different way to call iron(II) sulphate (or possibly other iron-based minerals), as it was used to make black inks and dyes, for instance the so-called iron gall ink,22 in which iron sulphate and tannic acid from gall nuts react to form an insoluble black complex such as Fe(III) gallate.23
For the scope of this paper, however, we entertained also the possibility that this compound may be the product of high temperature decomposition of iron sulphate.
Therefore, the aim of this paper is to give an overview of the yellow, and red vitriols, in relation to their production, manipulation and use in the early period of the development of chemical knowledge. In particular, we describe the solid-state phase conversion following heat treatments, which are not always described in the contemporary literature, followed by a comprehensive characterization of the present phases as well as colour and habit.
The article aims to provide a useful starting point both for historians of chemistry looking to shed some light on alchemical practices and for modern chemists looking to reclaim a once-common material knowledge which is now being lost.
Results and discussion
To prepare the vitriols, we tried to stay as close as possible to the indications provided in historical sources, when available. To solve an ambiguity regarding the yellow vitriol in particular, we tested two different approaches, as explained in the relevant section. We focused especially on the indications provided by George Ripley regarding its preparation.Furthermore, as red and “black” vitriol are made in a similar fashion, and appears to be closely related in terms of chemical composition and crystallographic phase, they are discussed together. For the preparation of red vitriol, we focused on the recipe provided in the Antidotarium Collegii Medicorum Bononiensis (see Text 2 in the ESI†), due to its clarity and consistency with older, and less clear, sources.
In Table 1 the phases of starting materials and final products are listed, with the associated mineral names and ICDD references used for identification.
| Phase | Mineral name | ICDD PDF |
|---|---|---|
| a IS(OH) notation refers only to this paper. | ||
| FeSO4·7H2O | Melanterite | 01-072-1106 |
| FeSO4·4H2O | Rozenite | 01-073-1428 |
| FeSO4·H2O | Szomolnokite | 00-045-1365 |
| FeIIFeIII(SO4)2(OH)·nH2O | IS(OH)a | 00-053-1056 |
| CuSO4·5H2O | Chalcanthite | 00-008-0089 |
| Fe2O3 | Hematite | 00-033-0664 |
| Fe2(SO4)3 | Mikasaite | 00-033-0679 |
| (Fe,Cu)SO4·H2O | Poitevinite | 01-083-0079 |
Yellow vitriol
The historical sources lack clear indications on the preparation of yellow vitriol. One of the few indications is given by Ripley, who only prescribes to “dry Roman vitriol over fire”.25Moreover, there is an ambiguity regarding the identification of “Roman” vitriol, as it is defined as a copper or iron compound in different sources.10,26
Considering the uncertainty regarding what Ripley may have meant by “drying”, we devised two methods to prepare it:
(i) A “dry method”, by placing the sulphate in an alumina crucible and heating it at about 200 °C for about 30 minutes; (ii) a “wet method”, by dissolving the sulphate in a suitable amount of water and evaporating the water by boiling until dry.
Due to the ambiguity of the attribution of the “Roman” vitriol we tested both iron and copper sulphate with the two methods.
Neither method supports the identification of “Roman vitriol” with copper sulphate or as a mineral based mainly on copper, since the “dry method” only resulted in the dehydration of copper sulphate pentahydrate to its anhydrous form (which is white), and the “wet method” only resulted in the recrystallization of copper sulphate pentahydrate (which is blue).
When the “dry method” is applied to melanterite (iron sulphate), after 30 min at 200 °C a yellow powder is obtained which is composed of FeIIFeIII(SO4)2(OH)·nH2O, namely IS(OH).
The presence of Fe(III) ions was confirmed by dissolving a small amount of the sample in water and adding a few crystals of KSCN. The resulting solution turned blood-red due to the formation of a Fe(III) thiocyanate complex.27
The variable temperature X-ray powder diffraction (VTXRPD) scans, collected while heating a sample of melanterite, show the phase transitions in detail (Fig. 1): melanterite is fully converted in rozenite at 60 °C, with szomolnokite that begin forming above 75 °C. IS(OH) forms only when the sample is kept at 150 °C for a prolonged period of time. It should be noted that anhydrous iron sulphate was not identified at any point.
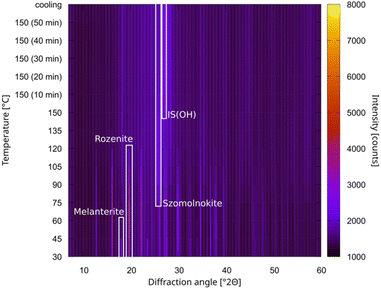 | ||
| Fig. 1 VTXRPD scan map obtained upon heating IS7 up to 150 °C; the primary peaks of each identified phase are highlighted. The phase identification and the Rietveld refinement are summarized in the ESI (Fig. S1).† | ||
To better appreciate the colour change, we followed the heating process on the Kofler bench. The iron(II) sulphate heptahydrate initially turns white due to dehydration, then depending on the temperature begins to turn to brown and then yellow.
The final colour depends of the temperature: up to 80 °C the powder is mainly white, while in the range 80–150 °C is mainly brown and in the range 150–265 °C is mainly yellow; the colours are stable for at least 90 minutes (see Fig. 2).
 | ||
| Fig. 2 Kofler bench of IS7 sample over 120 min; x axis reproduces the non-linear scale of the bench; the bars on top represent the intervals at which samples were collected for XRPD. | ||
The sample was divided into five roughly equally sized parts, as shown in Fig. 2.
The samples (A–E) collected from the bench were analyzed by XRPD (Fig. S2†), from which we found that sample A is a mixture of rozenite and szomolnokite, B is composed of szomolnokite alone, C contains primarily IS(OH) with a small amount of szomolnokite, while D and E are composed solely of IS(OH). These results are consistent with the VTXRPD scan.
Even when the “wet method” is applied to melanterite a yellow powder is obtained (Fig. 3). It should be noted that the solution of melanterite is pale green which turns bright yellow when heated, due to the partial oxidation of Fe(II) to Fe(III) (Fig. S6†). The recovered powder is consistent with melanterite, despite its yellow colour (Fig. S3†), which means that the powder is mainly Fe(II) sulphate with a small amount of Fe(III), giving the different colour.
The presence of the Fe(III) was easily confirmed by dissolving a small amount of the yellow vitriol in distilled water and by adding KSCN which turned the solution in blood red.
TGA analyses in air of the starting melanterite and the yellow melanterite show that both samples undergo a continuous mass loss from 30 to 200 °C, corresponding to the loss of six and five water molecules respectively, in the range 200–560 °C the oxidation of the iron and the release of the last water molecules occurs in several steps,28,29 while the last weight loss, above 560 °C, corresponds to the release of SO3 and formation of Fe2O3, confirmed by XRPD and the red colour of both sample after the analysis (Fig. S4 and Table S2†).
Red vitriol
In order to make reddish vitriol, the Antidotarium Collegii Medicorum Bononiensis prescribes to take whitened vitriol (i.e. green vitriol that is heated until it dehydrates and turns white) and to “treat it with a reverberating fire, until it acquires a dark red colour” (Text S2†).Therefore, we took a sample of iron(II) sulphate heptahydrate and heated it over a Bunsen burner in a closed alumina crucible for 20 minutes, resulting in the formation of a bright red powder (Fig. 4), consistent with the historical recipe and description. This sample was then characterized by XRPD and was identified as a mixture of Fe(III) oxide (hematite) and anhydrous Fe(III) sulphate (mikasaite), 68.2 ± 0.7% hematite and 31.8 ± 0.7% mikasaite by Rietveld refinement. On the other hand, when iron sulphate heptahydrate was heated using a muffle furnace at 900 °C overnight, resulted in an apparently black powder, that turns dark red if ground using mortar and pestle: this was identified as pure hematite (Fe2O3, see Fig. S5†). It's worth noting that there is no ambiguity on ascertain colchotar as the product of calcination of green vitriol and, when cholcotar is mentioned in the primary sources we discussed, there is no doubt that it refers to hematite.
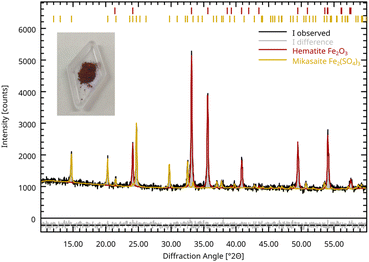 | ||
| Fig. 4 XRPD pattern of red vitriol sample obtained by Bunsen calcination; hematite (red) and mikasaite (yellow) phases are shown. Inset: red vitriol sample. For the Rietveld refinement were used the structures listed in Table S1;† the calculated Rwp for the refinement was calculated at 2.23%. | ||
Furthermore, to test the hypothesis that red vitriol could be obtained from a mixture of copper and iron sulphate,20 we also dissolved three different weight ratios of a mixture of the two sulphates (see Table 2), and then boiled the solution until dry. These samples were then characterized by XRPD to identify the phases present.
The X-ray powder pattern of R1 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of iron and copper sulphate) is attributed to poitevinite ((Cu,Fe)SO4·H2O) with no other phases, whereas the diffraction patterns of both R2 and R3 show copper sulphate mixed with the poitevinite. Moreover, only R1 resulted in reddish powder, while the other two resulted in colours veering toward yellow.
1 ratio of iron and copper sulphate) is attributed to poitevinite ((Cu,Fe)SO4·H2O) with no other phases, whereas the diffraction patterns of both R2 and R3 show copper sulphate mixed with the poitevinite. Moreover, only R1 resulted in reddish powder, while the other two resulted in colours veering toward yellow.
Poitevinite forms only if the mixture is quickly recrystallized by heating, as slow recrystallization by water evaporation at room temperature leads to the two sulphates forming separate crystalline forms (melanterite and chalcanthite), either from the physical mixture of the two sulphates or from the recrystallization of poitevinite itself.
Materials and methods
Green and blue vitriols
The recipes for artificial yellow or red vitriol start from or refer to green or possibly blue vitriol. These compounds are commonly identified with iron(II) sulphate and copper(II) sulphate, respectively.10Iron sulphate heptahydrate was purchased from Sigma-Aldrich. By XRPD analysis it was confirmed that its diffraction pattern is consistent with melanterite.
Copper sulphate pentahydrate was purchased from Sigma-Aldrich. By XRPD analysis it was confirmed that its diffraction pattern is consistent with chalcanthite.
Powder X-ray diffraction
All XRPD measurements were carried out using a Panalytical X'Pert Pro automated diffractometer equipped with an X'Celerator detector in a Bragg–Brentano configuration, Cu-K radiation.The variable temperature X-ray diffractograms were acquired with the Panalytical X'Pert Pro diffractometer equipped with an Anton Paar TTK 450 system for measurements at a controlled temperature. IS7 was heated from 30 °C to 150 °C in 15 °C steps and its XRPD patterns collected. Then it was kept at 150 °C collecting a scan every 10 minutes for 60 minutes, with the last scan collected during cooldown.
Phase identification was performed using X'Pert HighScore Plus, and the PDF 2 Release 2004 database.24 When relevant, a quantitative analysis was performed by Rietveld refinement.
Rietveld refinements and data visualization was performed using Profex.30 Starting structures for refinement were taken from the Crystallography Open Database,31 reference codes for the entries used are reported in Table S1.†
Thermal gravimetric analysis
The TGA analyses were performed under air flow (60 mL min−1), using a TA Instruments TGA Q500, in an open pan, and with a scan rate of 10 °C min−1, from 30 °C to 700 °C.Kofler bench
A Wagner & Munz Kofler bench was used to heat an iron sulphate sample from RT to over 250 °C over the span of two hours to monitor the effects of temperature and time. The sample was placed along the cold bench, which was then turned on and the changes in the sample were monitored for 120 minutes. This bench model requires 40 minutes to reach working temperatures from RT, after that the heating is isothermal.Conclusions
The importance of vitriols in historical recipes cannot be overstated. For this reason, it is particularly important to identify them in modern chemical terms, in particular in the case of man-made vitriols such as yellow and red vitriols.We tested different hypothesis on the process to obtain yellow vitriols, with different starting materials and different procedures. We exclude that the yellow vitriol could be obtained from copper sulphate minerals since the final powders were not yellow. When melanterite is used as the starting material, a yellow powder can be obtained either by the “dry” and “wet methods”. The “dry method” consist of simply heating the melanterite which turns white and then brownish, due to dehydration, and then yellow as it begins to oxidize. The yellow powder is a mixed Fe(II) and Fe(III) sulphate IS(OH), a phase that is not known to correspond to any natural mineral, and can be obtained at relatively low temperatures given sufficient time. When the “wet” method is used, a yellow “melanterite” is obtained, while the X-ray powder pattern is consistent with the presence of FeSO4·7H2O, the final colour is due to the presence of Fe(III) ions in the structure.
It is worth noting that the powders obtained in the different methods can be reasonably be called “yellow vitriol” as they have similar colourations and, since they contain sulphate ions, both can be used in the production of sulphuric acid although they are not the same crystal phase.
When melanterite is heated above 500 °C, it decomposes into a red powder that is mainly composed of hematite but contains also a significant amount of Fe(III) sulphate, while at higher temperature (900 °C) only hematite is present. Therefore, even though this is called “red vitriol”, this compound does not contain sulphates and has a different chemical behaviour.
However, it is possible to make a reddish compound (poitevinite – (Fe,Cu)SO4·H2O) by the “wet method” that still fits the description of a “red vitriol”, and it has been successfully used in replicating historical recipes.20 Again, different procedures lead to quite different products from the chemical point of view, even if they share the same colour. Unfortunately, the replications did not dispel any doubts and lead to a univocal answer, but rather showed that colour alone, which is one of the few indicators of the ancients, cannot be discriminating on the crystalline phase and that the correct attribution needs to be confirmed with further tests. In the case of ‘yellow vitriol’, the different preparations always lead to the formation of a sulphate, thus a potential reagent for the production of sulphuric acid. In the case of ‘red vitriol’, on the other hand, two very different products were obtained: haematite vs. poitevinite, with only the latter retaining the sulphate ion.
These observations are of particular importance when interpreting historical recipes: some caveats should be kept in mind when a historical recipe is “translated” in modern chemical terms to avoid this risk of oversimplification presuming a full equivalence between reagents.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This paper is also the result of a project funded by the Italian Ministry of University and Research (MUR) under the FARE program (ID R18W2STNE2 – AlchemEast in the West. Graeco–Arabic Alchemy in Western Europe, PI: Matteo Martelli). We would like to thank Emma Contini for performing the TGA analyses.Notes and references
- M. Marchini, M. Gandolfi, L. Maini, L. Raggetti and M. Martelli, Proc. Natl. Acad. Sci. U. S. A., 2022, 119 DOI:10.1073/pnas.2123171119.
- H. Chang, Sci. Educ., 2011, 20, 317–341 CrossRef.
- V. Karpenko and J. A. Norris, Chem. Listy, 2002, 96, 997–1005 CAS.
- M. Mertens, Les Alchimistes Grecs: Mémoires Authentiques, Les Belles Lettres, Paris, 1995 Search PubMed.
- L. Y. Beck, Pedanius Dioscorides of Anazarbus De Materia Medica, Olms – Weidmann, Hildesheim, Zürich, New York, 2020 Search PubMed.
- A. Blaise, Lexicon Latinitatis Medii Aevi [Entry: Uitriolum], Brepols Editores pontificii, Turnhout, 2013 Search PubMed.
- H. Rackham, Pliny the Elder, Natural History. 1: Preface and Books 1–2, Harvard Univ. Press, Cambridge (MA), 1938 Search PubMed.
- F. Käs, Die Mineralien in der Arabischen Pharmakognosie, Harrassowitz Verlag, Wiesbaden, 2010 Search PubMed.
- W. Johnson, Lexicon chymicum [entries: azag, zegi, calcata, cooptatione], Nealand, London, 1652 Search PubMed.
- A. Campana, Farmacopea Ferrarese del dottore Antonio Campana [Entries: Vetriolo Verde, Vetriolo Blu, Vetriolo Bianco], Piatti, Florence, 1808 Search PubMed.
- Direzione Generale della Sanità Pubblica, Farmacopea Ufficiale del Regno d'Italia [Entries: Vetriolo Verde, Vetriolo Blu], Regia Tipografia delle Mantellate, Rome, 1902 Search PubMed.
- R. Duval, J. Asiat., 1893, 2, 290–361 Search PubMed.
- M. Berthelot, Collection Des Anciens Alchimistes Grecs, Forgotten Books, London, 2018 Search PubMed.
- J. M. Rampling, Ambix, 2008, 55, 189–208 CrossRef CAS PubMed.
- S. Maffei, G. B. Piazzetta, F. Zucchi and G. B. Parone, Istoria Teologica Delle Dottrine e Delle Opinioni Corse ne' Cinque Primi Secoli Della Chiesa in Proposito Della Divina Grazia, del Libero Arbitrio, e Della Predestinazione, Gianbattista Parone Stampatore Episcopale, Verona, 1742, pp. 204–213 Search PubMed.
- J. S. Schröter, Mineralogisches und bergmännisches wörterbuch, Varrentrap und Wenner, Frankfurt, 1789 Search PubMed.
- E. Castell, Lexicon Heptaglotton [Entry: qlq], Roycroft, London, 1669 Search PubMed.
- AAVV, Antidotarium Collegii Medicorum Bononiensis, Pezzana, Venice, 1783 Search PubMed.
- P. K. Gallagher, D. W. Johnson and F. Schrey, J. Am. Ceram. Soc., 1970, 53, 666–670 CrossRef CAS.
- L. Raggetti, J. Islam. Manuscripts, 2019, 10, 201–239 Search PubMed.
- J. W. Anthony, R. A. Bideaux, K. W. Bladh and M. C. Nichols, Handbook of Mineralogy, Mineralogical Society of America, https://www.handbookofmineralogy.org/, accessed March, 2023 Search PubMed.
- H. E. Stapleton, Mem. Asiat. Soc. Bengal, 1927, 8, 317–428 CAS.
- M. J. Melo, V. Otero, P. Nabais, N. Teixeira, F. Pina, C. Casanova, S. Fragoso and S. O. Sequeira, Heritage Sci., 2022, 10 DOI:10.1186/s40494-022-00779-2.
- S. Gates-Rector and T. Blanton, Powder Diffr., 2019, 34, 352–360 CrossRef CAS.
- J. M. Rampling, Ambix, 2024, 71, 73–97, DOI:10.1080/00026980.2024.2309059.
- Oxford English Dictionary [Entry: Roman Vitriol], https://www.oed.com/, accessed November, 2022 Search PubMed.
- A. Peloso, Analisi Chimica Qualitativa Inorganica. 1, Libreria Cortina, Padua, 1991 Search PubMed.
- N. Kanari, N.-E. Menad, E. Ostrosi, S. Shallari, F. Diot, E. Allain and J. Yvon, Metals, 2018, 8, 1084 CrossRef CAS.
- T. Wang, K. A. Debelak and J. A. Roth, Thermochim. Acta, 2007, 462, 89–93 CrossRef CAS.
- N. Döbelin and R. Kleeberg, J. Appl. Crystallogr., 2015, 48, 1573–1580, DOI:10.1107/S1600576715014685.
- S. Grazulis, D. Chateigner, R. T. Downs, A. T. Yokochi, M. Quiros, L. Lutterotti, E. Manakova, J. Butkus, P. Moeck and A. Le Bail, J. Appl. Crystallogr., 2009, 42, 726–729, DOI:10.1107/S0021889809016690.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01896f |
| ‡ In the Antidotarium is mentioned in passing as a way to prove that a different ingredient was adulterated by adding (green) vitriol to it. |
| § See for example Florence, MS BNCF, Fondo Nazionale, II–III 305, ff 58r–60r. |
| ¶ In the cited source the wording is unclear: the starting material is called “vitriolum calcinatum ad albedinem” (vitriol calcined until white), however in the same recipe this ingredient is then described as “subviridem”, which can be understood as yellowish brown. |
| This journal is © The Royal Society of Chemistry 2024 |