Open Access Article
Open Access ArticleDesign, synthesis and cytotoxic activity of molecular hybrids based on quinolin-8-yloxy and cinnamide hybrids and their apoptosis inducing property†
Dalal Nasser Binjawhar a,
Fawziah A. Al-Salmib,
Ola A. Abu Ali
a,
Fawziah A. Al-Salmib,
Ola A. Abu Ali c,
Maha Ali Alghamdid,
Eman Fayad
c,
Maha Ali Alghamdid,
Eman Fayad d,
Rasha Mohammed Saleeme,
Islam Zaki
d,
Rasha Mohammed Saleeme,
Islam Zaki *f and
N. A. Faroukg
*f and
N. A. Faroukg
aDepartment of Chemistry, College of Science, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
bBiology Department, College of Sciences, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia
cDepartment of Chemistry, College of Science, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia
dDepartment of Biotechnology, College of Sciences, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia
eDepartment of Laboratory Medicine, Faculty of Applied Medical Sciences, Al-Baha University, Al-Baha 65431, Saudi Arabia
fPharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Port Said University, Port Said 42526, Egypt. E-mail: Eslam.Zaki@pharm.psu.edu.eg
gDepartment of Chemistry, Faculty of Science, Port Said University, Port Said 42526, Egypt
First published on 9th April 2024
Abstract
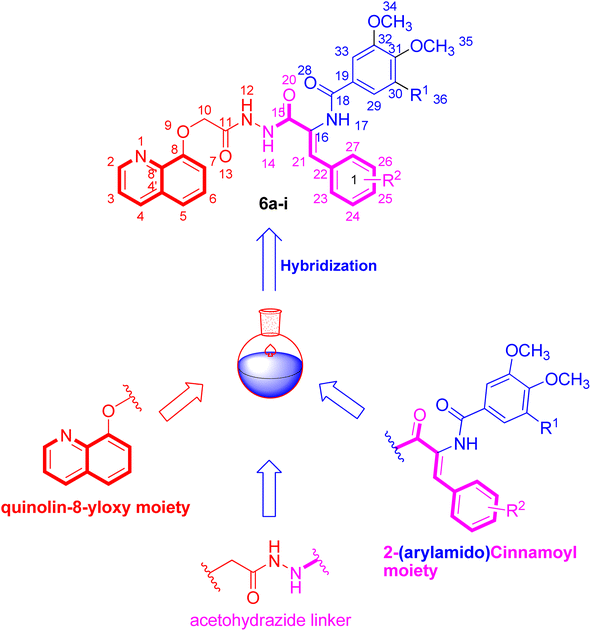
The present work aims at design and synthesis of a congeneric series of small hybrids 5 and 6a–i featuring the privileged quinoline scaffold tethered with 2-(arylamido)cinnamide moiety as potential anticancer tubulin polymerization inhibitors. Most of the synthesized hybrids 5 and 6a–i significantly inhibited the growth of the HepG2 cell line, with IC50 ranged from 2.46 to 41.31 μM. In particular, 2-(3,4,5-trimethoxybenzamido)-4-methoxycinnamide-quinoline hybrid 6e displayed potent IC50 value toward the examined cell line, and hence chosen for further mechanistic investigations. It is noteworthy that the antiproliferative action of compound 6e highly correlated well with its ability to inhibit tubulin polymerization. In addition, the most potent hybrid 6e demonstrated a significant modification in the cellular cycle distribution, in addition to provoke of apoptotic death within the tested HepG2 cell line. Furthermore, the mechanistic approach was confirmed by a substantial upregulation in the quantity of active caspase 9 by 5.81-fold relative to untreated control cells.
1. Introduction
Cancer is a major global health issue defined by accelerated and uncontrolled cellular proliferation of normal cells.1 In addition, cancer is still ranked as one of the leading causes of death worldwide.2 The marketed anticancer chemotherapeutic agents were accompanied with considerable negative impacts due to the dosage and duration of the treatment as well as the resistance developed against them.3 As consequently, the search for new and less unsafe therapeutics with enhanced selectivity toward cancer cells remain a promising research field.4 As a result, the most recent strategies aimed to discover and seek out certain biological markers crucial for cancerous cells such as aberrant, modified or overstated proteins.5 In recent times, tubulin polymerization has been recognized as a significant molecular target for the search and development of anticancer drugs.6 The polymerization of tubulin is necessary to form microtubules that have a crucial role in cellular functions, including the maintenance of cellular structure, intracellular transportation as well as mitotic spindle formation for cell division.7,8 In recent decades, a wide range of natural compounds and synthetic components have been identified as anticancer drugs that interfere with tubulin-microtubule dynamics.9–11 The clinical success of such agents demonstrates that enormous potential of this target to the design and development of new anti-tubulin polymerization compounds as effective targeted anticancer regimens.12Quinoline scaffold (1-aza-naphthalene or benzo[b]pyridine) as an important class of N-biologically active heterocycles, represent a leading and promising heterocyclic pharmacophore.13 The quinoline scaffold is a well-recognized for diverse pharmacological applications, various natural products and bioactive drug compounds incorporate quinoline framework.14,15 Notable medicinal applications associated with the quinoline heterocycle includes anticancer, antiviral, antitubercular and many other biological properties with significant interest in the creation of beneficial quinoline-based anticancer compounds such as Campothecin, Topotecan and Irinotecan.16–20
In parallel, cinnamide pharmacophore is regarded an interesting scaffold in the realm of medicinal and pharmaceutical chemistry.21 Cinnamide-based compounds also displayed a wide plethora of biological properties including anticancer activities via different mechanism of action such as the inhibition of tyrosine kinase, cell cycle arrest and so on.22–26 Moreover, cinnamide moiety is commonly exploited as a potential pharmacologically active component in the construction of tubulin assembly inhibitors.27
Molecular hybridization strategy of a well-known pharmacophores could provide effective in exerting a beneficial role for drug-sensitive cancer and also for drug-resistance cancer.28,29 Incorporation of heterocyclic moiety such as the quinoline moiety into anticancer agents was succeeded as a new strategy to produce hybrid molecules with potent anticancer activity.30 In this regard, we perform the current study to optimize the cytotoxic potential after incorporation of quinoline moiety with cinnamide scaffold and investigate their ability to interfere with tubulin polymerization as well as apoptosis induction.31,32 In addition, the structure activity relationship (SAR) of the target hybrids was achieved through changes in 2-(arylamido) substituents (R1) with either (3,4,5-trimethoxybenzamido) or (3,4-dimethoxybenzamido) groups as well as variation of the type of substituents (R2) on the cinnamide moiety to include electron-donating and electron-withdrawing groups (Fig. 1). All the herein reported quinoline-cinnamide hybrids 5 and 6a–i were screened for their cytotoxic action against liver HepG2 cancerous cell line. The most promising member in the cytotoxicity assay was subsequently investigated for its activity against tubulin inhibitory activity, as well as its effect on cellular cycle distribution and ability to induce apoptosis.
2. Results and discussion
2.1. Chemistry
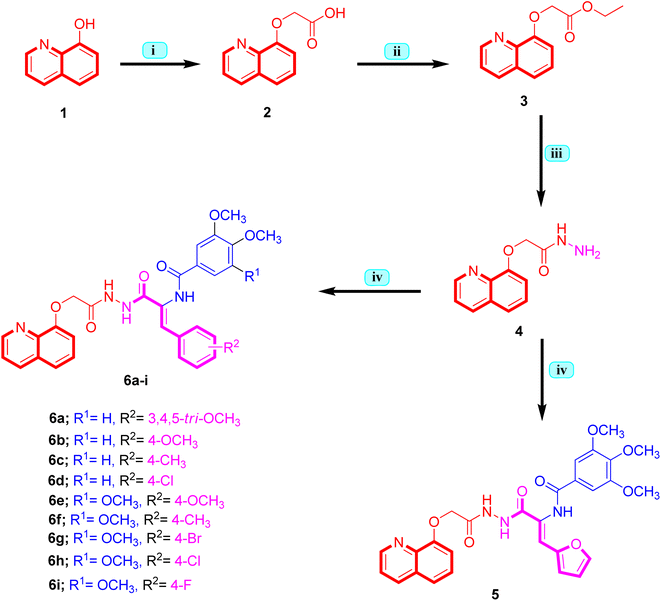
The strategy to synthesize the target quinoline-cinnamide hybrids 5 and 6a–i is depicted in Scheme 1. Initiated from 8-hydroxyquinoline 1, it was reacted with 2-chloroacetic acid in the presence of K2CO3 using dimethylformamide as solvent. The reaction was carried out at reflux temperature to afford 2-(quinoline-8-yloxy)acetic acid 2 that was subjected to esterification reaction in the presence of pure ethanol and concentrated sulfuric acid to get ethyl 2-(quinoline-8-yloxy)acetate 3. Hydrazinolysis of ethyl 2-(quinoline-8-yloxy)acetate 3 with hydrazine hydrate in methanol as solvent, 2-(quinoline-8-yloxy)acetohydrazide 4 was obtained in good yield.33 The key 2-(quinoline-8-yloxy)acetohydrazide intermediate 4 was condensed with appropriate ethyl 2-(arylamido)-cinnamate derivatives to yield the titled quinoline-cinnamide hybrids 5 and 6a–i. During the reaction, thin layer chromatography (TLC) was used in order to optimize the reactions, purity and completeness. The structural assignments to new hybrids were based on the elemental analysis and spectral (1H-NMR and 13C-NMR) methods. 1H-NMR spectrum of quinoline-4-methoxycinnamide hybrid 6b, as representative example, exhibited signals (δ: 10.93, 10.16 and 9.79 ppm) ascribed to the three amide functions appeared as three singlets. In addition, the 1H-NMR spectrum of compound 6b was also characterized by two signals (δ: 8.89 and 8.36 ppm) appeared as doublet and doublet of doublet signals with coupling constant of 11.9, 3.3 and 8.2 Hz, respectively assigned to C2–H and C4–H of quinoline moiety, while the other aromatic protons were observed between δ 7.69–6.96 ppm. Besides, the presence of singlet signal (δ: 7.24 ppm) ascribed to olefinic proton of cinnamide moiety. Moreover, the three methoxy groups contained in 3,4-dimethoxybenzamide and 4-methoxycinnamide moieties of the representative quinoline-4-methoxycinnamide hybrid 6b were observed as three singlet signals (δ: 3.84, 3.83 and 3.76 ppm) integrating three protons each. Furthermore, the signal observed at δ 4.89 ppm pointing to the presence of methylene group in the hybrid compound 6b. The 13C-NMR spectrum of quinoline-4-methoxycinnamide hybrid 6b revealed four upfield signals at δ 68.18, 56.14, 56.07 and 55.68 ppm attributed to one methylene carbon and three methoxy carbons, respectively. Even though the three amide linkers' carbonyl carbons appeared downfield at δ 167.21, 165.90 and 165.03 ppm. The other peaks of carbons were observed at aromatic region in the range δ 160.20–111.35 ppm verifying that the hybrid compound 6b possesses twenty three carbons in.2.2. Biology
| Comp no. | R1 | R2 | IC50 value (μM) |
|---|---|---|---|
| HepG2 | |||
| 5 | — | — | 4.82 ± 0.24 |
| 6a | H | 3,4,5-tri-OCH3 | 6.24 ± 0.36 |
| 6b | H | 4-OCH3 | 15.71 ± 0.61 |
| 6c | H | 4-CH3 | 23.48 ± 0.78 |
| 6d | H | 4-Cl | 26.93 ± 0.74 |
| 6e | OCH3 | 4-OCH3 | 2.46 ± 0.14 |
| 6f | OCH3 | 4-CH3 | 5.35 ± 0.32 |
| 6g | OCH3 | 4-Br | 41.31 ± 0.87 |
| 6h | OCH3 | 4-Cl | 6.79 ± 0.39 |
| 6i | OCH3 | 4-F | 6.32 ± 0.33 |
| Col | — | — | 6.09 ± 0.17 |
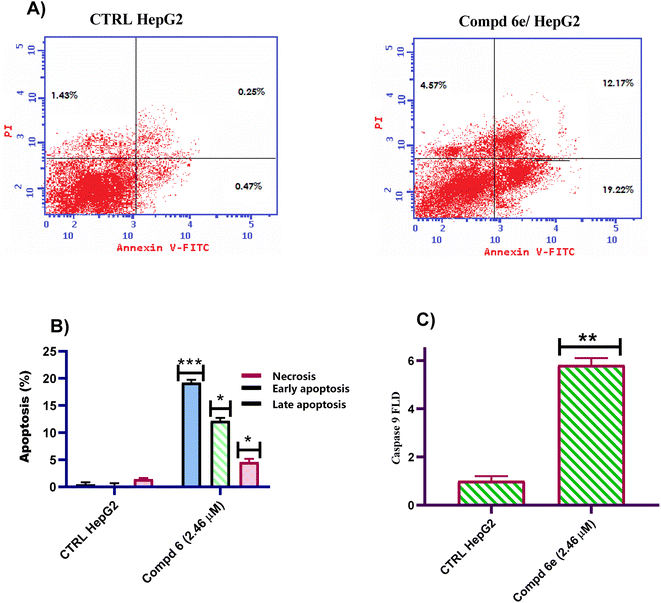
On the other hand, caspase is an important apoptotic protein. Therefore, the impact of quinoline-4-methoxycinnamide 6e on the expression of initiator caspase 9 was measured using qR-PCR method. As can be seen in Fig. 4C, treating HepG2 cells with quinoline-4-methoxycinnamide 6e at the IC50 concentration (2.46 μM) for 48 h, the active caspase 9 level was upregulated. The results demonstrated that quinoline-4-methoxycinnamide 6e boosted the active caspase 9 quantity by 5.81-fold when compared to untreated control leading to cellular apoptosis.
3. Conclusions
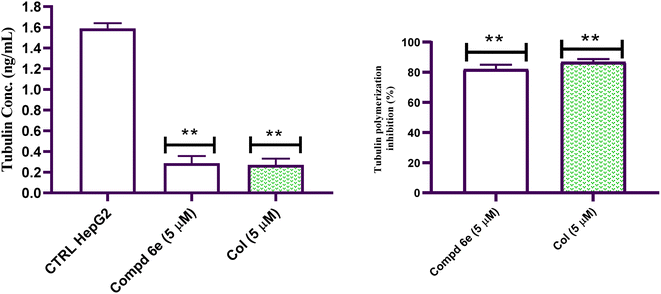
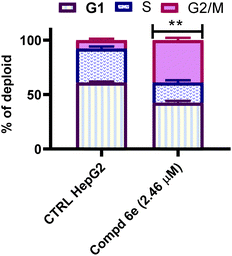
This study represents the synthesis of a congeneric series of quinoline scaffold tethered cinnamide hybrids 5 and 6a–i for the development of novel anticancer agents. All the synthesized hybrids were assessed for their potential cytotoxic action toward the liver (HepG2) cancerous cell line. The tested 2-(arylamido)cinnamide-quinoline derivatives 5 and 6a–i showed moderate to outstanding cytotoxic activity with IC50 values ranging from 2.46–41.31 μM against HepG2 cell line. In particular, 2-(3,4,5-trimethoxybenzamido)-4-methoxycinnamide-quinoline hybrid 6e had the highest IC50 value of 2.46 μM against the HepG2 cell line and outperforming to the reference drug Col (IC50 = 6.09 μM). The findings of tubulin polymerization inhibition assay for 2-(3,4,5-trimethoxybenzamido)-4-methoxycinnamide-quinoline hybrid 6e showed that the tested hybrid inhibited tubulin polymerization with percentage inhibition value of 82.01% which was slightly lower than that of Col (86.79% percent inhibition value). In addition, cell cycle analysis for the most active hybrid 6e revealed a significant drop in the cell distribution at G1 and S phases which was accompanied by marked elevation in the percentage of G/M phase, implying that the cell cycle was primarily halted at the G2/M phase in HepG2 cells. Moreover, an Annexin V-FITC/PI analytical method demonstrated that the tested 2-(3,4,5-trimethoxybenzamido)-4-methoxycinnamide-quinoline hybrid 6e was able to induce 43.60-fold increase in HepG2 cells when compared to untreated control. Furthermore, the 2-(3,4,5-trimethoxybenzamido)-4-methoxycinnamide-quinoline hybrid 6e boosted active caspase 9 level by 5.81-fold compared to control cells. The findings suggested that the antiproliferative activity was caused by suppression of tubulin polymerization and subsequent apoptosis induction. Collectively, these results suggested that 2-(3,4,5-trimethoxybenzamido)-4-methoxycinnamide-quinoline hybrid 6e is a potential lead candidate for future optimization and development as promising liver cancer antitumor agent with tubulin polymerization suppression activity.4. Experimental
4.1. General
4.1.1.1. General procedure for the synthesis of (Z)-aryl-N-(1-aryl-3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyl)hydrazinyl)prop-1-en-2-yl)benzamides 5 and 6a–i. 2-(Quinoline-8-yloxy)acetohydrazide 4 (0.217 g, 1 mmol) and appropriate ethyl 2-(arylamido)-cinnamate derivative (1 mmol) were dissolved in pure ethanol (20 mL) and then glacial acetic acid (10 drops) was added. After refluxing to 18–20 h, the reaction mixture was cooled down to ambient temperature and allowed to precipitate for the entire night. The crude material thus obtained was chromatographed using (CH2Cl2/MeOH 97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to get the required quinoline-cinnamide hybrids.
3) to get the required quinoline-cinnamide hybrids.(Z)-N-(1-(Furan-2-y1)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyl)hydrazinyl)prop-1-en-2-y1)-3,4,5-trimethoxybenzamide (5): Yield: 62%, m.p. 249–251 °C. Analysis: calc. for C28H26N4O8 (546.53): C 61.53, H 4.80, N 10.25%, found: C 61.59, H 4.84, N 10.18%. 1H-NMR (400 MHz, DMSO-d6) δ: 10.43 (d, J = 9.9 Hz, 1H, NH), 10.23 (s, 1H, NH), 9.70 (d, J = 111.6 Hz, 1H, NH), 8.90 (d, J = 3.5 Hz, 1H, C2–H quinoline), 8.36 (d, J = 8.2 Hz, 1H, C4–H quinoline), 7.83 (d, J = 20.4 Hz, 1H, Ar–H), 7.64–7.56 (m, 2H, Ar–H), 7.56–7.50 (m, 1H, Ar–H), 7.39 (s, 2H, Ar–H), 7.29 (d, J = 7.6 Hz, 1H, Ar–H), 7.19 (s, 1H, olefinic CH), 6.77 (d, J = 3.2 Hz, 1H, furan CH), 6.61 (s, 1H, furan CH), 4.89 (s, 2H, OCH2), 3.87 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 166.80, 165.20, 163.68, 153.88, 152.54, 149.49, 149.30, 144.86, 140.34, 139.78, 136.01, 129.11, 128.92, 126.71, 125.80, 121.99, 120.95, 118.21, 114.53, 112.39, 111.64, 105.59, 67.70 (OCH2), 60.12 (OCH3), 56.07 (2OCH3) ppm.
(Z)-3,4-Dimethoxy-N-(3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyl)hydraziny1)-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)benzamide (6a): Yield: 65%, m.p. 233–235 °C. Analysis: calc. for C32H32N4O9 (616.62): C 62.33, H 5.23, N 9.09%, found: C 62.22, H 5.31, N 8.96%. 1H-NMR (400 MHz, DMSO-d6) δ: 10.44 (d, J = 12.1 Hz, 1H, NH), 10.24 (s, 1H, NH), 9.85 (s, 1H, NH), 8.91 (d, J = 2.9 Hz, 1H, C2–H quinoline), 8.36 (d, J = 7.6 Hz, 1H, C4–H quinoline), 7.70 (d, J = 8.6 Hz, 1H, Ar–H), 7.67 (s, 1H, Ar–H), 7.62–7.56 (m, 2H, Ar–H), 7.53 (t, J = 7.9 Hz, 1H, Ar–H), 7.31 (d, J = 4.4 Hz, 2H, Ar–H and olefinic CH), 7.09–7.04 (m, 1H, Ar–H), 6.99 (s, 2H, Ar–H), 4.90 (s, 2H, OCH2), 3.84 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.62 (s, 6H, 2OCH3). 13C-NMR (DMSO, 101 MHz) δ: 167.19, 165.89, 164.75, 154.37, 153.03, 152.16, 149.77, 148.58, 140.26, 138.57, 136.47, 130.97, 129.77, 129.57, 128.40, 127.18, 126.27, 122.46, 121.93, 121.40, 112.11, 111.67, 111.24, 107.64, 68.18 (OCH2), 60.52 (OCH3), 56.12 (OCH3), 56.10 (OCH3), 56.07 (2OCH3).
(Z)-3,4-Dimethoxy-N-(1-(4-methoxypheny1)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyl) hydrazinyl)prop-1-en-2-yl)benzamide (6b): Yield: 69%, m.p. 281–283 °C. Analysis: calc. for C30H28N4O7 (556.57): C 64.74, H 5.07, N 10.07%, found: C 64.61, H 4.90, 10.19.68%. 1H-NMR (400 MHz, DMSO-d6) δ: 10.39 (s, 1H, NH), 10.16 (s, 1H, NH), 9.79 (s, 1H, NH), 8.89 (dd, J = 11.9, 3.3 Hz, 1H, C2–H quinoline), 8.36 (d, J = 8.3 Hz, 1H, C4–H quinoline), 7.69 (t, J = 8.5 Hz, 1H, Ar–H), 7.62 (s, 2H, Ar–H), 7.58 (d, J = 3.9 Hz, 2H, Ar–H), 7.57–7.50 (m, 2H, Ar–H), 7.30 (d, J = 7.5 Hz, 1H, Ar–H), 7.24 (s, 1H, olefinic CH), 7.08 (d, J = 8.4 Hz, 1H, Ar–H), 6.96 (dd, J = 15.9, 8.7 Hz, 2H, Ar–H), 4.89 (s, 2H, OCH2), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.76 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 167.21, 165.90, 165.03, 160.20, 154.36, 152.11, 149.77, 148.63, 140.26, 136.46, 131.72, 130.40, 129.57, 127.18, 127.07, 126.92, 126.44, 122.45, 121.86, 121.39, 114.53, 112.10, 111.80, 111.35, 68.18 (OCH2), 56.14 (OCH3), 56.07 (OCH3), 55.68 (OCH3).
(Z)-3,4-Dimethoxy-N-(3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyphydraziny1)-1-p-tolylprop-1-en-2-yl)benzamide (6c): Yield: 71%, m.p. 275–277 °C. Analysis: calc. for C30H28N4O6 (540.57): C 66.66, H 5.22, N 10.36%, found: C 66.82, H 5.13, N 10.24%. 1H NMR (400 MHz, DMSO-d6) δ: 10.44 (d, J = 14.3 Hz, 1H, NH), 10.16 (d, J = 59.9 Hz, 1H, NH), 9.83 (s, 1H, NH), 9.03–8.83 (m, 1H, C2–H quinoline), 8.36 (d, J = 8.0 Hz, 1H, C4–H quinoline), 7.70–7.64 (m, 1H, Ar–H), 7.60 (d, J = 9.2 Hz, 2H, Ar–H), 7.56 (dd, J = 12.8, 6.1 Hz, 2H, Ar–H), 7.50 (d, J = 8.2 Hz, 2H, Ar–H), 7.30 (d, J = 7.5 Hz, 1H, Ar–H), 7.23 (s, 1H, olefinic CH), 7.18 (d, J = 7.8 Hz, 2H, Ar–H), 7.14–7.05 (m, 1H, Ar–H), 4.90 (s, 2H, OCH2), 3.85 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 2.29 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz) δ: 167.24, 165.92, 164.96, 154.37, 152.14, 149.77, 148.64, 140.26, 139.07, 136.47, 131.68, 130.29, 129.97, 129.62, 129.57, 128.55, 127.19, 126.40, 122.46, 121.87, 121.41, 112.12, 111.79, 111.35, 68.19 (OCH2), 56.14 (OCH3), 56.07 (OCH3), 21.37 (CH3).
(Z)-N-(1-(4-Chloropheny1)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyl)hydrazinyl)prop-1-en-2-y1)-3,4-dimethoxybenzamide (6d): Yield: 74%, m.p. 250–252 °C. Analysis: calc. for C29H25ClN4O6 (560.98): C 62.09, H 4.49, N 9.99%, found: C 61.94, H 4.57, N 10.11%. 1H-NMR (400 MHz, DMSO-d6) δ: 10.44 (s, 1H, NH), 10.30 (s, 1H, NH), 9.88 (s, 1H, NH), 8.91 (dd, J = 4.1, 1.6 Hz, 1H, C2–H quinoline), 8.36 (dd, J = 8.3, 1.6 Hz, 1H, C4–H quinoline), 7.65 (t, J = 7.5 Hz, 1H, Ar–H), 7.61 (s, 1H, Ar–H), 7.58 (d, J = 4.6 Hz, 3H, Ar–H), 7.57–7.52 (m, 1H, Ar–H), 7.52–7.47 (m, 1H, Ar–H), 7.44 (d, J = 8.6 Hz, 2H, Ar–H), 7.29 (d, J = 6.8 Hz, 1H, Ar–H), 7.19 (s, 1H, olefinic CH), 7.08 (d, J = 8.6 Hz, 1H, Ar–H), 4.89 (s, 2H, OCH2), 3.84 (s, 3H, OCH3), 3.82 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 167.23, 165.89, 164.75, 154.38, 152.23, 149.78, 148.66, 140.28, 136.46, 133.63, 133.51, 131.53, 130.12, 129.57, 129.06, 128.38, 127.18, 126.18, 122.45, 121.94, 121.42, 112.15, 111.78, 111.36, 68.20 (OCH2), 56.49 (OCH3), 56.08 (OCH3).
(Z)-3,4,5-Trimethoxy-N-(1-(4-methoxyphenyl)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acetylhydrazinyl)prop-1-en-2-yl)benzamide (6e): Yield: 63%, m.p. 258–260 °C. Analysis: calc. for C31H30N4O8 (586.59): C 63.47, H 5.15, N 9.55%, found: C 63.36, H 5.02, N 9.66%. 1H-NMR (400 MHz, DMSO-d6) δ 10.40 (d, J = 10.5 Hz, 1H, NH), 10.16 (d, J = 15.6 Hz, 1H, NH), 9.89 (s, 1H, NH), 8.89 (dd, J = 13.2, 2.8 Hz, 1H, C2–H quinoline), 8.36 (d, J = 7.3 Hz, 1H, C4–H quinoline), 7.66–7.61 (m, 1H, Ar–H), 7.59 (s, 2H, Ar–H), 7.57 (s, 1H, Ar–H), 7.56–7.50 (m, 1H, Ar–H), 7.38 (s, 2H, Ar–H), 7.30 (d, J = 7.4 Hz, 1H, Ar–H), 7.26 (s, 1H, olefinic CH), 6.98 (dd, J = 14.9, 8.8 Hz, 2H, Ar–H), 4.89 (s, 2H, OCH2), 3.86 (s, 6H, 2OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 169.01, 167.26, 165.78, 164.94, 160.27, 154.37, 153.00, 149.77, 140.81, 140.26, 136.46, 131.77, 130.52, 129.57, 129.26, 127.18, 126.82, 122.45, 121.40, 114.58, 112.09, 106.07, 68.17 (OCH2), 60.58 (OCH3), 56.53 (2OCH3), 55.70 (OCH3).
(Z)-3,4,5-Trimethoxy-N-(3-oxo-3-(2-(2-(quinolin-8-yloxy)acetylhydrazinyl)-1-p-tolylprop-1-en-2-yl)benzamide (6f): Yield: 72%, m.p. 264–266 °C. Analysis: calc. for C31H30N4O7 (570.59): C 65.25, H 5.30, N 9.82%, found: C 65.37, H 5.43, N 9.71%. 1H-NMR (400 MHz, DMSO-d6) δ 10.45 (d, J = 23.0 Hz, 1H, NH), 10.22 (d, J = 27.5 Hz, 1H, NH), 9.93 (s, 1H, NH), 8.96–8.85 (m, 1H, C2–H quinoline), 8.41–8.29 (m, 1H, C4–H quinoline), 7.63–7.56 (m, 2H, Ar–H), 7.55 (s, 1H, Ar–H), 7.53 (d, J = 4.1 Hz, 1H, Ar–H), 7.51 (d, J = 4.0 Hz, 1H, Ar–H), 7.38 (s, 2H, Ar–H), 7.30 (d, J = 7.5 Hz, 1H, Ar–H), 7.25 (s, 1H, olefinic CH), 7.20 (d, J = 7.9 Hz, 2H, Ar–H), 4.90 (s, 2H, OCH2), 3.86 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3), 2.29 (s, 3H, CH3). 13C-NMR (DMSO, 101 MHz) δ: 167.28, 165.80, 164.86, 154.36, 153.01, 149.77, 140.85, 140.25, 139.17, 136.47, 131.58, 130.43, 130.00, 129.67, 129.57, 129.21, 128.36, 127.18, 122.45, 121.41, 112.10, 106.07, 68.18 (OCH2), 60.58 (OCH3), 56.53 (2OCH3), 21.37 (CH3).
(Z)-N-(1-(4-Bromophenyl)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acetyl)hydrazinyl)prop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6g): Yield: 76%, m.p. 279–281 °C. Analysis: calc. for C30H27BrN4O7 (635.46): C 56.70, H 4.28, N 8.82%, found: C 56.84, H 4.22, N 8.93%. 1H-NMR (400 MHz, DMSO-d6) δ 10.50 (d, J = 38.7 Hz, 1H, NH), 10.28 (d, J = 40.5 Hz, 1H, NH), 9.98 (s, 1H, NH), 8.96–8.84 (m, 1H, C2–H quinoline), 8.42–8.29 (m, 1H, C4–H quinoline), 7.62 (d, J = 7.7 Hz, 2H, Ar–H), 7.59 (s, 2H, Ar–H), 7.58–7.54 (m, 2H, Ar–H), 7.52 (d, J = 8.4 Hz, 1H, Ar–H), 7.35 (s, 2H, Ar–H), 7.30 (d, J = 7.5 Hz, 1H, Ar–H), 7.20 (s, 1H, olefinic CH), 4.90 (s, 2H, OCH2), 3.86 (s, 6H, 2OCH3), 3.74 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 167.28, 165.77, 164.66, 154.36, 153.02, 149.77, 140.94, 140.26, 136.47, 133.75, 132.13, 132.05, 131.80, 130.00, 129.57, 128.99, 128.63, 127.18, 122.49, 121.43, 112.13, 106.09, 68.19 (OCH2), 60.58 (OCH3), 56.50 (2OCH3).
(Z)-N-(1-(4-Chlorophenyl)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acety)hydrazinyl)prop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6h): Yield: 71%, m.p. 243–245 °C. Analysis: calc. for C30H27ClN4O7 (591.01): C 60.97, H 4.60, N 9.48%, found: C 61.08, H 4.47, N 9.43%. 1H-NMR (400 MHz, DMSO-d6) δ: 10.52 (d, J = 36.4 Hz, 1H, NH), 10.30 (d, J = 38.4 Hz, 1H, NH), 9.99 (s, 1H, NH), 8.97–8.84 (m, 1H, C2–H quinoline), 8.42–8.30 (m, 1H, C4–H quinoline), 7.70–7.63 (m, 1H, Ar–H), 7.61 (s, 1H, Ar–H), 7.59 (s, 1H, Ar–H), 7.58–7.54 (m, 1H, Ar–H), 7.54–7.50 (m, 1H, Ar–H), 7.46 (d, J = 8.5 Hz, 2H, Ar–H), 7.36 (s, 2H, Ar–H), 7.30 (d, J = 7.5 Hz, 1H, Ar–H), 7.23 (s, 1H, olefinic CH), 4.91 (s, 2H, OCH2), 3.86 (s, 6H, 2OCH3), 3.74 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 171.72, 167.30, 165.80, 164.66, 154.36, 153.03, 149.76, 140.94, 140.25, 136.48, 133.75, 133.40, 131.57, 129.91, 129.57, 129.12, 129.00, 128.62, 127.17, 122.45, 121.43, 112.13, 106.09, 68.20 (OCH2), 60.57 (OCH3), 56.54 (2OCH3).
(Z)-N-(1-(4-Fluorophenyl)-3-oxo-3-(2-(2-(quinolin-8-yloxy)acety)hydrazinyl)prop-1-en-2-yl)-3,4,5-trimethoxybenzamide (6i): Yield: 68%, m.p. 236–238 °C. Analysis: calc. for C30H27FN4O7 (574.56): C 62.71, H 4.74, N 9.75%, found: C 62.64, H 4.68, N 9.90%. 1H-NMR (400 MHz, DMSO-d6) δ 10.46 (d, J = 32.5 Hz, 1H, NH), 10.24 (d, J = 28.5 Hz, 1H, NH), 9.95 (s, 1H, NH), 8.90 (dd, J = 4.1, 1.6 Hz, 1H, C2–H quinoline), 8.37 (dd, J = 8.3, 1.6 Hz, 1H, C4–H quinoline), 7.72 (dd, J = 8.5, 5.8 Hz, 1H, Ar–H), 7.66 (dd, J = 8.5, 5.6 Hz, 2H, Ar–H), 7.59 (dt, J = 8.9, 4.7 Hz, 2H, Ar–H), 7.56–7.50 (m, 1H, Ar–H), 7.36 (s, 2H, Ar–H), 7.33–7.26 (m, 2H, Ar–H), 7.25 (s, 1H, olefinic CH), 4.89 (s, 2H, OCH2), 3.85 (s, 6H, 2OCH3), 3.74 (s, 3H, OCH3). 13C-NMR (DMSO, 101 MHz) δ: 167.27, 165.83, 164.72, 154.38, 153.02, 149.77, 140.90, 140.28, 136.46, 132.19, 132.11, 131.00, 129.57, 129.06, 129.01, 127.17, 122.45, 121.42, 116.19, 115.98, 112.12, 106.08, 68.19 (OCH2), 60.58 (OCH3), 56.49 (2OCH3).
4.2. Biological studies
All experimental procedures used in the biological evaluations were shown in the ESI.†Conflicts of interest
The authors report no conflicts of interest.Acknowledgements
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R155), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.References
- Z. Yu, X. Bai, R. Zhou, G. Ruan, M. Guo, W. Han, S. Jiang and H. Yang, Differences in the incidence and mortality of digestive cancer between Global Cancer Observatory 2020 and Global Burden of Disease 2019, Int. J. Cancer, 2024, 154, 615–625 CrossRef CAS PubMed.
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Cancer statistics, 2024. CA: A Cancer, J. Clin., 2024, 74, 12–49 Search PubMed.
- Brianna and S. H. Lee, Chemotherapy: how to reduce its adverse effects while maintaining the potency?, Med. Oncol., 2023, 40, 88–99 CrossRef CAS PubMed.
- H. Tian, T. Zhang, S. Qin, Z. Huang, L. Zhou, J. Shi, E. C. Nice, N. Xie, C. Huang and Z. Shen, Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies, J. Hematol. Oncol., 2022, 15, 132–149 CrossRef PubMed.
- Q.-Q. Zhou, H.-T. Xiao, F. Yang, Y.-D. Wang, P. Li and Z.-G. Zheng, Advancing targeted protein degradation for metabolic diseases therapy, Pharmacol. Res., 2023, 188, 106627–106642 CrossRef CAS PubMed.
- X. Wang, B. Gigant, X. Zheng and Q. Chen, Microtubule-targeting agents for cancer treatment: Seven binding sites and three strategies, MedComm: Oncol., 2023, 2, e46 CAS.
- A. Falconieri, A. Coppini and V. Raffa, Microtubules as a signal hub for axon growth in response to mechanical force, Biol. Chem., 2024, 405, 67–77 CrossRef CAS PubMed.
- E. D. McKenna, S. L. Sarbanes, S. W. Cummings and A. Roll-Mecak, The Tubulin Code, from Molecules to Health and Disease, Annu. Rev. Cell Dev. Biol., 2023, 39, 331–361 CrossRef CAS PubMed.
- I. Zaki, M. K. Abdelhameid, I. M. El-Deen, A. H. A. Abdel Wahab, A. M. Ashmawy and K. O. Mohamed, Design, synthesis and screening of 1, 2, 4-triazinone derivatives as potential antitumor agents with apoptosis inducing activity on MCF-7 breast cancer cell line, Eur. J. Med. Chem., 2018, 156, 563–579 CrossRef CAS PubMed.
- H. M. Abd El-Lateef, L. M. A. Ghany, R. M. Saleem, A. H. A. Maghrabi, M. A. Y. Alahdal, E. H. K. Ali, B. Y. Beshay, I. Zaki and R. E. Masoud, Design, synthesis and antiproliferative screening of newly synthesized coumarin-acrylamide hybrids as potential cytotoxic and apoptosis inducing agents, RSC Adv., 2023, 13, 32547–32557 RSC.
- M. Podolak, S. Holota, Y. Deyak, K. Dziduch, R. Dudchak, M. Wujec, K. Bielawski, R. Lesyk and A. Bielawska, Tubulin inhibitors. Selected scaffolds and main trends in the design of novel anticancer and antiparasitic agents, Bioorg. Chem., 2024, 143, 107076–107088 CrossRef CAS PubMed.
- M. N. Peerzada, M. S. Dar and S. Verma, Development of tubulin polymerization inhibitors as anticancer agents, Expert Opin. Ther. Pat., 2023, 33, 797–820 CrossRef CAS PubMed.
- O. F. Elebiju, O. O. Ajani, G. O. Oduselu, T. A. Ogunnupebi and E. Adebiyi, Recent advances in functionalized quinoline scaffolds and hybrids—Exceptional pharmacophore in therapeutic medicine, Front. Chem., 2023, 10, 1074331–1074342 CrossRef PubMed.
- P. Yadav and K. Shah, Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry, Bioorg. Chem., 2021, 109, 104639–104653 CrossRef CAS PubMed.
- O. O. Ajani, K. T. Iyaye and O. T. Ademosun, Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs–a review, RSC Adv., 2022, 12, 18594–18614 RSC.
- S. Tyagi, A. Mazumder, R. Kumar, V. Datt, K. Shabana, M. S. Yar and M. J. Ahsan, Synthesis and SAR of Potential Anti-Cancer Agents of Quinoline Analogues: A Review, Med. Chem., 2023, 19, 785–812 CrossRef CAS PubMed.
- N. Chirra, G. Udigala, E. O. Sinegubova, D. Nagineni, R. K. Bollikanda, Y. L. Esaulkova, A. A. Muryleva, V. V. Zarubaev and S. Kantevari, Synthesis and antiviral activity of 2-substituted 4-aminoquinoline and its analogous derivatives, J. Heterocycl. Chem., 2023, 60, 1416–1426 CrossRef CAS.
- C.-X. Liu, X. Zhao, L. Wang and Z.-C. Yang, Quinoline derivatives as potential anti-tubercular agents: Synthesis, molecular docking and mechanism of action, Microb. Pathog., 2022, 165, 105507–105519 CrossRef CAS PubMed.
- S. Sharma, K. Singh and S. Singh, Synthetic Strategies for Quinoline Based Derivatives as Potential Bioactive Heterocycles, Curr. Org. Synth., 2023, 20, 606–629 CrossRef CAS PubMed.
- X. Wang, Y. Zhuang, Y. Wang, M. Jiang and L. Yao, The recent developments of camptothecin and its derivatives as potential anti-tumor agents, Eur. J. Med. Chem., 2023, 260, 115710–115722 CrossRef CAS PubMed.
- N. P. Aijijiyah, F. A. Wati, R. Rahayu, A. Srilistiani, F. Mahzumi, T. Aulia, L. Santoso, E. Pamela, E. Y. Ramadhani, Y. A. Ilfahmi, A. S. Purnomo, S. R. Putra, E. Santoso, S. Ningsih, N. Firdausi and M. Santoso, Synthesis, α-glucosidase inhibitory activity, and molecular docking of cinnamamides, Med. Chem. Res., 2023, 32, 723–735 CrossRef CAS.
- B. Zhang, Z. Xu, Q. Liu, S. Xia, Z. Liu, Z. Liao and S. Gou, Design, synthesis and biological evaluation of cinnamamide-quinazoline derivatives as potential EGFR inhibitors to reverse T790M mutation, Bioorg. Chem., 2021, 117, 105420–105437 CrossRef CAS PubMed.
- K. Donthiboina, P. Anchi, S. Gurram, G. Sai Mani, J. Lakshmi Uppu, C. Godugu, N. Shankaraiah and A. Kamal, Synthesis and biological evaluation of substituted N-(2-(1H-benzo[d]imidazole-2-yl)phenyl)cinnamides as tubulin polymerization inhibitors, Bioorg. Chem., 2020, 103, 104191–104203 CrossRef CAS PubMed.
- S. Sana, V. G. Reddy, T. Srinivasa Reddy, R. Tokala, R. Kumar, S. K. Bhargava and N. Shankaraiah, Cinnamide derived pyrimidine-benzimidazole hybrids as tubulin inhibitors: Synthesis, in silico and cell growth inhibition studies, Bioorg. Chem., 2021, 110, 104765–104778 CrossRef CAS PubMed.
- M. M. Alam, A. H. E. Hassan, K. W. Lee, M. C. Cho, J. S. Yang, J. Song, K. H. Min, J. Hong, D.-H. Kim and Y. S. Lee, Design, synthesis and cytotoxicity of chimeric erlotinib-alkylphospholipid hybrids, Bioorg. Chem., 2019, 84, 51–62 CrossRef CAS PubMed.
- S. M. Aboukhatwa, P. A. Sidhom, A. Angeli, C. T. Supuran and H. O. Tawfik, Terminators or Guardians? Design, Synthesis, and Cytotoxicity Profiling of Chalcone-Sulfonamide Hybrids, ACS Omega, 2023, 8, 7666–7683 CrossRef CAS PubMed.
- X.-J. Fu, J. Huang, N. Li, Y.-H. Liu, Q.-G. Liu, S. Yuan, Y. Xu, Y.-F. Chen, Y.-X. Zhao, J. Song, S.-Y. Zhang and Y.-R. Bai, Design, synthesis and biological evaluation of N-benzylaryl cinnamide derivatives as tubulin polymerization inhibitors capable of promoting YAP degradation with potent anti-gastric cancer activities, Eur. J. Med. Chem., 2023, 262, 115883–115898 CrossRef CAS.
- H. Zhu, R. Zhou, D. Cao, J. Tang and M. Li, A pharmacophore-guided deep learning approach for bioactive molecular generation, Nat. Commun., 2023, 14, 6234–6241 CrossRef CAS PubMed.
- P. Das, S. Ganguly, A. Rosenkranz, B. Wang, J. Yu, S. Srinivasan and A. R. Rajabzadeh, MXene/0D nanocomposite architectures: Design, properties and emerging applications, Mater. Today Nano, 2023, 24, 100428 CrossRef CAS.
- X.-Q. Wang, L.-X. Liu, Y. Li, C.-J. Sun, W. Chen, L. Li, H.-B. Zhang and X.-D. Yang, Design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents, Eur. J. Med. Chem., 2013, 62, 111–121 CrossRef CAS PubMed.
- H. M. A. El-Lateef, R. M. Saleem, M. A. Bazuhair, A. H. A. Maghrabi, E. H. K. Ali, I. Zaki and R. E. Masoud, Design, synthesis and tubulin polymerization inhibition activity of newly synthesized hydrazone-linked to combretastatin analogues as potential anticancer agents, J. Mol. Struct., 2023, 1292, 136190–136207 CrossRef CAS.
- K. O. Mohamed, I. Zaki, I. M. El-Deen and M. K. Abdelhameid, A new class of diamide scaffold: Design, synthesis and biological evaluation as potent antimitotic agents, tubulin polymerization inhibition and apoptosis inducing activity studies, Bioorg. Chem., 2019, 84, 399–409 CrossRef CAS PubMed.
- M. Cacic, M. Trkovnik, F. Cacic and E. Has-Schon, Synthesis and Antimicrobial Activity of Some Derivatives on the Basis (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide), Molecules, 2006, 11, 134–147 CrossRef CAS PubMed.
- Y. Tian, A. Yang, H. Huang, J. Xie, L. Wang, D. Liu, X. Wei, P. Tan, P. Tu, D. Fu and Z. Hu, A novel pyrrolidine-2,5-dione derivative induced G2/M phase arrest and apoptosis of hepatocellular carcinoma HepG2 cells through inhibiting tubulin polymerization, Arabian J. Chem., 2024, 17, 105550–105562 CrossRef CAS.
- J. Sebastian and K. Rathinasamy, Microtubules and Cell Division: Potential Pharmacological Targets in Cancer Therapy, Curr. Drug Targets, 2023, 24, 889–918 CrossRef CAS PubMed.
- L. Huang, Y. Peng, X. Tao, X. Ding, R. Li, Y. Jiang and W. Zuo, Microtubule Organization Is Essential for Maintaining Cellular Morphology and Function, Oxid. Med. Cell. Longevity, 2022, 2022, 1623181–1623194 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01911c |
| This journal is © The Royal Society of Chemistry 2024 |