Open Access Article
Open Access ArticleThe preparation of gallic acid-grafted cationic chitosan as effective salt-tolerant flocculants
Jingxuan Liua,
Hongyu Zhaob,
Mengyue Wangc,
Wenzheng Banc,
Xi Lu*d and
Bin Yan *c
*c
aXinjiang Xinchun Petroleum Development Co., Ltd, Sinopec, Dongying, Shandong 257000, China
bPetroleum Exploration Department of SINOPEC ShengLi Oilfield, Dongying 257200, China
cCollege of Biomass Science and Engineering, Sichuan University, Chengdu, 610065, China. E-mail: yanbinscu@126.com
dOil Production Engineering Division, Sinopec Petroleum Exploration and Production Research Institute, Beijing, 100083, China. E-mail: luxi.syky@sinopec.com
First published on 8th May 2024
Abstract
In this work, gallic acid was successfully grafted onto quaternary aminated chitosan to prepare a high efficiency cationic flocculant. The mechanism of flocculation and different influencing factors were studied in detail. The prepared flocculant only needs 60 mg L−1 to achieve a 98.7% and 94.5% removal rate on methyl blue (MB) and Congo red (CR), respectively. The high removal rate (93.2%) of a CR-MB mixed dye also confirms the universality of flocculation. In addition, kaolin as a simulated suspended solid was removed at a rate of 97% in the experiment at a dosage of 3 mg L−1. A zeta potential test showed that it worked best when the potential of the flocculation system was zero; this was because an electrostatic balance was reached between the flocculant and pollutant. Importantly, the three-functional molecules can provide more possibilities to form hydrogen bonds with water molecules, which is conducive to the stretching of flocculant molecular chains in salt water. The flocculant maintained a high stability in four different salt environments and has a positive industrial application significance. Furthermore, the flocculation experiment of the actual wastewater of the printing and dyeing plant found that the dye wastewater changed obviously from turbidity to clarification, which proved the practical application potential of the flocculant. This work provides a feasible idea for the preparation of bio-based flocculants.
1. Introduction
In view of pollution-carbon reduction and serious water pollution, treatment of wastewater before discharge is warranted.1,2 The residual organic dyes in printing and dyeing wastewater will cause adverse effects on the environment and humans.3–7 Flocculation is a common industrial water treatment process because of its simple operation and wide application range.8–13 However, many of the existing flocculants have safety risks,14–16 so it is meaningful to develop efficient and environmentally suitable flocculants.Chitosan is a natural, non-toxic bio-based material. The rich amino and hydroxyl groups in the structure give it many potential applications in water treatment.17–20 The aerogels with a high absorption rate of copper (Cu2+) were synthesized by chitosan modified with dimethylaminoethyl methacrylate.21 Chitosan was also used to prepare magnetic chitosan particles by the surface coating of Fe3O4.22 Previous studies have mostly focused on the application potential of chitosan in the field of adsorption. The application of chitosan in flocculation faces many challenges due to its poor solubility and viscosity.2,22 On this basis, it is a good measure to modify by a quaternary ammonium salt. At the same time, because the charge carried by the main pollutants in the wastewater is anionic, the cationic modification will have a greater coverage in the treatment of pollutants, and has a good removal effect for dissolved pollutants and suspended solids.23 Yang et al. modified chitosan with a quaternary ammonium salt monomer to obtain quaternary ammonium chitosan, and applied it in the flocculation treatment of Escherichia coli. Cation-modified chitosan makes it possible to apply chitosan in the field of flocculation.24
In the actual wastewater treatment process, inorganic salts cannot be ignored as it is difficult for quaternary aminated chitosan to play a role in the presence of inorganic salts because the inorganic salt ions will cause the long-chain flocculant in the water to be difficult to unfold, thus affecting the removal of subsequent pollutants.18,25,26
In addition, under the folded conformation of the polymer chain, due to the action of polyelectrolytes, salt-induced dehydration reduced the active sites in the polymer chain and enhanced the internal diffusion resistance of contaminants, resulting in an inefficient pollution removal rates.27,28 Most polyelectrolyte polymers have a poor salt tolerance, and under the action of salt ions, they undergo a significant phase transition from water soluble to water insoluble.29,30 This is due to the fact that salt ions with opposite charges weaken the electrostatic repulsion between the main chains of the polyelectrolyte chain through the charge shielding effect and salt-out effect, and transform the originally extended random chain into a compact configuration, resulting in the deterioration of the hydration performance of the polyelectrolyte chain.31 Wang et al. prepared a novel high charge density salt-tolerant chitosan-based flocculant (CTCTA-BGE3) by the etherification of chitosan (CT) with 2,3-epoxy-propyl trimethyl ammonium chloride (CTA) and butyl glycidyl ether (BGE) as the raw materials. It was found that the hydrogen bond hydration between BGE fragments and water molecules weakened the salt-induced polyelectrolyte effect, and the CT-CTA-BGE3 chain maintained an extended conformation in the high saline solution, thus having an excellent salt tolerance.18
As a widespread interaction, hydrogen bonding is an effective strategy to improve the hydration of polyelectrolyte chains. Therefore, increasing the hydrogen bond hydration of the polymer chain can greatly improve the salt tolerance of the polymer, so as to resist the salting out of the polyelectrolyte. Based on this, a polyelectrolyte complex was successfully prepared from anionic poly-(acrylamide-co-acrylic acid-co-2-acrylamido-2-methyl-1-propanesulfonicacid) (PA3) and cationic homo poly((2-methacryloyloxyethyl) trimethyl ammonium chloride) (PDMC). This oppositely charged polyelectrolyte complex may open a new pathway for polymer thickeners in high-salinity environments.27 Therefore, it is necessary to introduce new forces to build the tolerance of the flocculant to the water containing inorganic salts to ensure the tolerance effect of the flocculant. Gallic acid (GA) is a kind of polyphenolic organic compound, and is widely used in food, biology, medicine, chemical industry and other fields.32 As a natural small molecule that exists in nature, its safety is guaranteed. Because GA has three phenolic hydroxyl groups on its molecular structure, the three-functional molecules can provide more possibilities to form hydrogen bonds with water molecules, which is conducive to the stretching of flocculant molecular chains in salt water.33 The carboxyl group on GA provides the possibility for GA to modify chitosan by reacting with the amino group on chitosan.34
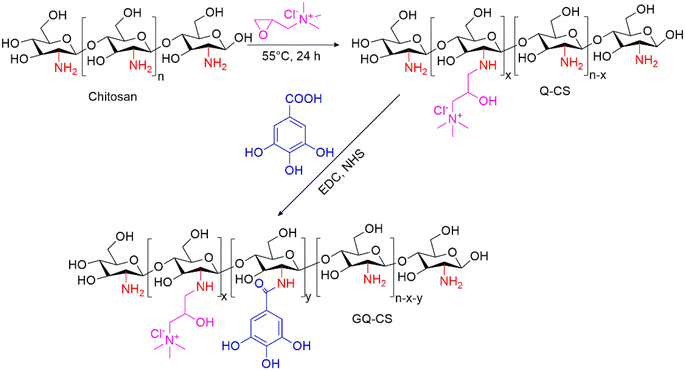
In this work, we grafted quaternary ammonium oxide and gallic acid (GA) onto chitosan (CS). A kind of cationic flocculant GQ-CS with a stable flocculation effect in saline dye wastewater was prepared, and its preparation was proved by means of nuclear magnetic and infrared. The removal effect of GQ-CS was verified by treating simulated wastewater configured with methyl blue (MB) and kaolin. The universality of GQ-CS was also proved by the mixed dye wastewater of Congo red (CR) and MB-CR. The introduction of GA makes GQ-CS have a higher dye removal efficiency and good tolerance to a saline environment.
2. Experiment
2.1 Experiment materials
Chitosan (CS) and glycidyl trimethyl ammonium chloride (GTMAC) were purchased from Adamas Reagent Co., Ltd, gallic acid came from Aladdin, 1-ethyl-3-(3′-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), MB, CR, hydrochloric acid and sodium hydroxide were purchased from the Chengdu Kelon Chemical Reagent Factory.2.2 Experiment method
According to previous work,35 glycidyl trimethyl ammonium chloride (GTMAC) was introduced into the amino group of the chitosan skeleton to obtain quaternized chitosan (Q-CS). Then, 5 g of chitosan was added to 200 mL of deionized water, 1 mL of acetic acid was added to dissolve the CS. Then 8.3 g of GTMAC was added to a mixed solution (GTMAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) –NH2 = 2
–NH2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the reaction was performed at 55 °C for 18 h and centrifugation was performed to remove the insoluble polymer. The product was precipitated with acetone three times and dried at room temperature for three days, and the product was ground to obtain Q-CS.
1), the reaction was performed at 55 °C for 18 h and centrifugation was performed to remove the insoluble polymer. The product was precipitated with acetone three times and dried at room temperature for three days, and the product was ground to obtain Q-CS.
Subsequently, 1 g of Q-CS was dissolved in deionized water with EDC (0.48 g) and NHS (0.29 g), and the pH was adjusted to 4.7. It was stirred for 1 h to activate the carboxyl group. Then 0.43 g of GA was added and stirred overnight. After the reaction, deionized water with pH 5 was used for dialysis for one day, followed by deionized water with an unadjusted pH for 4 h, and the final sample GQ-CS was obtained by cold drying. The synthesis route of the sample is shown in Fig. 1.
2.3 Characterization
The samples were dissolved in heavy water, and the nuclear magnetic hydrogen spectrum (1H NMR) was tested by an Avance III-800 MHz nuclear magnetic resonance instrument (Bruck, Switzerland). The sample powder was pressed with potassium bromide to obtain the sample, and its infrared characteristic peak was represented by a Fourier infrared spectrometer (IRTracer-100) (Shimadzu, Japan). After that, the sample water solution with a mass fraction of 0.5 g L−1 was configured, and the zeta potential of the different samples was tested by the Zetasizer Nano ZSP of the Malvern Company (United Kingdom).2.4 Flocculation experiment
In this experiment, flocculant GQ-CS was configured at a concentration of 2.5 g L−1 for backup. MB and kaolin were used to simulate dissolved pollutants and suspended solids, respectively.MB was stored away from light in a solution with a concentration of 1000 mg L−1 in deionized water, diluted with deionized water at the required concentration, and its pH was adjusted with 1 M of HCl and NaOH. After controlling the conditions, the flocculant was injected into it immediately in accordance with the required dosage and a constant speed shaker was used to start shaking. First, the system was shaken for 10 minutes at a frequency of 240 rpm to ensure that the flocculant solution could be fully mixed with the MB solution, and then the system was continued to be shaken at a frequency of 100 rpm to ensure further mixing of the flocculant and pollutants while avoiding breaking the formed flocs. After standing for 2 hours, the flocculation experiment was completed, and the supernatant was tested by an ultraviolet-visible spectrophotometer at 665 nm. The remaining MB concentration in the supernatant was measured by a concentration–absorbance curve, and the removal effect of the flocculant on pollutants was evaluated by the calculation of formula (1):
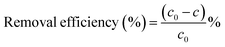 | (1) |
In order to study the universality of GQ-CS to dyes, CR was also selected as the research object in this experiment, and the supernatant was tested by an ultraviolet-visible spectrophotometer at 488 nm.
A kaolin dispersion solution was prepared according to the mass fraction required by the experiment, and stirred to ensure the uniformity of the kaolin dispersion. Kaolin powder needs to be well hydrated and non-agglomerated before use. It contains particles of various sizes, some of which rapidly settle during the turbidity removal test without the need for flocculants. These particles could be discarded before using the suspension. After the pH was adjusted by 1 M of NaOH and HCl, the flocculant aqueous solution was injected into a kaolin dispersion solution for 10 minutes at a speed of 240 rpm and 100 rpm, respectively, and then left for 1 h. The supernatant permeability was measured by a visible-ultraviolet spectrophotometer at 550 nm. In addition, the absence of flocculants was also considered, and blank tests were carried out simultaneously in the kaolin removal experiment. The removal rate of pollutants was evaluated and calculated by formulas (2) and (3) to evaluate the removal effect of the flocculants on pollutants:
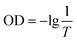 | (2) |
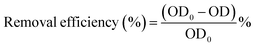 | (3) |
3. Results and discussion
3.1 Chemical structure of GQ-CS
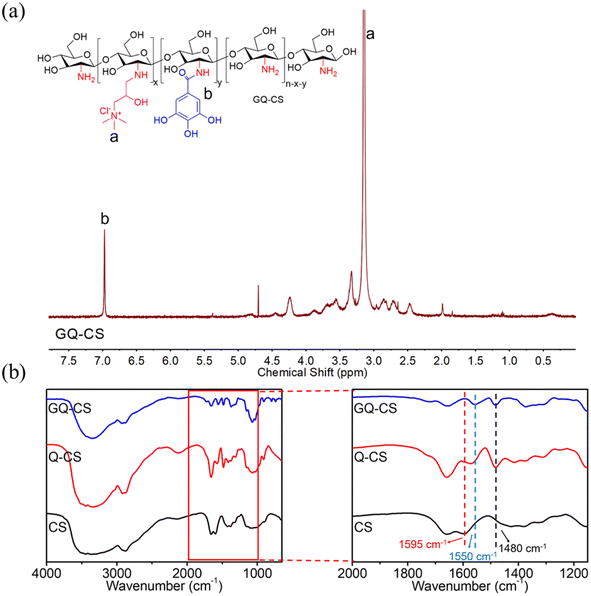
On the basis of the Q-CS synthesis, GQ-CS was successfully prepared by grafting the amino group of chitosan with the carboxyl group of q three-functional gallic acid through an EDC/NHS system. The chemical structure was characterized by 1H NMR and FT-IR.The 1H NMR spectrum diagram of GQ-CS is shown in Fig. 2(a). It can be seen that a large absorption peak appeared at 3.2 ppm (peak a), which can be attributed to the absorption of the H carried on the methyl group of the quaternary ammonium salt. This also proved that the quaternary ammonium salt successfully bonded to the chitosan. There was also a single absorption peak at 7.0 ppm (peak b), which was caused by the H in the benzene ring of gallic acid. Since H in the benzene ring was structurally symmetric, this peak appeared as a single peak. Therefore, the flocculant GQ-CS was successfully prepared.
FT-IR was also used to characterize GQ-CS in this work, and the results can be seen in Fig. 2(b). At 1480 cm−1, both Q-CS and GQ-CS had a larger absorption peak than that chitosan, which can be attributed to the bending vibration of C–H in –N+(CH3)3.35 Differently, GQ-CS had an extra absorption peak to those of chitosan and Q-CS at 1550 cm−1, which can be attributed to the absorption peak caused by the in-plane bending of N–H on the amide bond formed by CS and GA. At 1595 cm−1, CS showed a relatively obvious absorption peak, while the Q-CS peak decreased, and the GQ-CS peak was the least, because –NH2 on chitosan changed from a primary amino to secondary amino.34 The nuclear magnetic spectrum further proved the successful preparation of GQ-CS.
3.2 Flocculation effect of dissolved pollutants
In this experiment, anionic dye MB was selected to simulate dissolved pollutants. The first consideration was the influence of different molecular grafts on the removal effect. Under the condition of the same dye wastewater (cMB = 100 mg L−1, cflocculant = 2.5 g L−1, pH = 5), the relationship between the dosage of different flocculants and the removal rate was explored. As shown in Fig. 3(a), the two modified cationic chitosan-based flocculants can be corrected under an appropriate dosage MB and achieved more than a 95% removal rate (Q-CS/96.2%; GQ-CS/98.7%). But the difference was that the dose required to achieve this removal effect was not the same; in the same MB wastewater conditions, Q-CS needed a 120 mg L−1 dose, and GQ-CS only one of 60 mg L−1. This improvement in flocculation efficiency can be attributed to catechol and catechol groups. Compared to Q-CS, which simply relies on the electrostatic attraction of the quaternary ammonium salt groups, GQ-CS has one benzene ring and three phenolic hydroxyl groups, which enables GQ-CS to strengthen flocculation by other interactions (π–π stacking and hydrogen bonding) with MB on the basis of electrostatic attraction.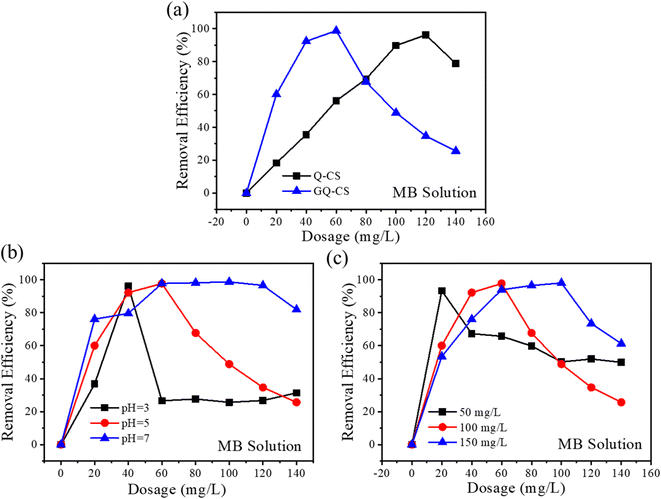 | ||
| Fig. 3 Effect of dosage on the MB removal with different flocculants (a); effects of (b) pH and (c) initial concentration on the removal efficiency of MB. | ||
pH is a key factor affecting the flocculation effect. Maintaining a good removal effect on pollutants is an important indicator to evaluate the performance of flocculants under different pH. As shown in Fig. 3(b), a flocculant of 40 mg L−1 can achieve a 96.1% removal rate of MB at pH 3, and when pH rose to 5 and 7, the optimal removal rates were 97.7% and 98.3%, respectively, indicating that GQ-CS could maintain a good removal effect on pollutants at different pH. However, with the increasing of pH, a higher flocculant dosage was needed to achieve a better removal rate. This can be attributed to changes in the zeta potential. The amount of negative charge carried by pollutant MB itself increased with the increase of pH, while the amount of positive charge carried by GQ-CS decreased with the increase of pH leading to the deprotonation of the unreplaced amino group on its main chain. As a result, the amount of required flocculant increased.
In the actual treatment of wastewater, the concentration of pollutants will not be a fixed value. Therefore, the flocculation effect of GQ-CS was tested by changing the initial concentration of MB solution at pH 5, and the results are shown in Fig. 3(c). When the concentration of MB was 50 mg L−1, a removal rate of 93.4% by GQ-CS at 20 mg L−1 can be achieved. For methyl blue at 100 mg L−1, higher doses (60 mg L−1) can be used to achieve the highest removal rate (97.7%) and when methyl blue was 150 mg L−1, a 100 mg L−1 flocculant dosage was needed to reach the highest removal rate (98.1%). All in all, the prepared flocculants showed a high flocculation effect and stability.
3.3 Flocculation of mixed dyes
In order to study the universality of GQ-CS to dyes, in addition to MB, CR was also selected as a research object in this experiment, and flocculation experiments were carried out under different pH conditions. The removal effect of GQ-CS on CR is shown in Fig. 4(a). At different pH, the highest removal rate of CR by GQ-CS can reach more than 90%, but as the pH of the CR solution increases from 3 to 5 and 7, the dosage required increased from 8 mg L−1 to 16 mg L−1 and 20 mg L−1. This is basically consistent with the trend of GQ-CS to remove MB at different pH values.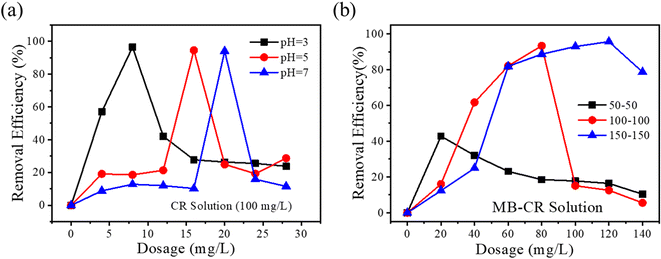 | ||
| Fig. 4 Effects of (a) pH; (b) the initial concentration of mixed dyes on the removal efficiency of the dye pollutants. | ||
The flocculation effect of mixed dyes is of great significance to the performance of flocculants in practical applications. Therefore, MB and CR were also mixed in this study, and different initial concentrations were changed at pH = 5, and then flocculated with GQ-CS and the results are shown in Fig. 4(b). GQ-CS showed a good removal effect under different mixture concentrations of the dyes. When the concentration of the mixed dye was 50 mg L−1–50 mg L−1, GQ-CS at 30 mg L−1 could remove 90.8% of the mixed dye. When the dye concentration rose to 100 mg L−1–100 mg L−1 and 150 mg L−1–150 mg L−1, the dose of GQ-CS required to achieve the optimal removal rate was 80 mg L−1 and 120 mg L−1, respectively. In general, flocculants had a good flocculation effect on mixed dyes with relatively lower concentration.
3.4 Flocculation test of suspended solids
Suspended solids are another typical pollutant in wastewater. Flocculants can be neutralized by static electricity, so as to achieve the removal of suspended solids. In this experiment, kaolin was selected as the simulated sample. A kaolin suspension with a mass fraction of 2 wt% was configured, and it was treated with different flocculants. The specific removal rate is shown in Fig. 5(a). Both flocculants had a good removal effect on kaolin, and the highest removal rate was above 95%, and remained stable. With the dosage of Q-CS and GQ-CS at only 3 mg L−1, the removal rate can reach 95.6% and 97.0%, respectively. This was because the removal of pollutants mainly relies on electrostatic attraction, so the flocculant with a higher positive charge requires less dosage to achieve a better suspended solid removal effect.15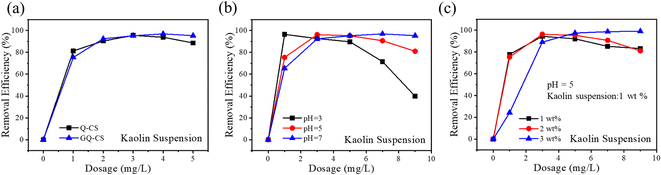 | ||
| Fig. 5 Effect of flocculants (a), pH (b) and initial mass fraction (c) of kaolin on the removal efficiencies of kaolin. | ||
Referring to the test of dissolved pollutants, the performance of the GQ-CS treatment of kaolin suspensions under different conditions was also tested in this experiment. The experimental results are shown in Fig. 5(b); when pH = 3, kaolin has the highest removal rate (96.5%), and the addition amount of GQ-CS was only 1 mg L−1. However, when the dosage of GQ-CS was increased, the removal rate of kaolin further decreased. It can be concluded that the excessive addition of GQ-CS will make the whole kaolin suspension system become stable again due to its own positive charge.35 When pH was 5–7, the removal rate of the pollutants can reach 96.2% and 96.9% at dosages of 3 mg L−1 and 7 mg L−1, respectively. The positive charge of GQ-CS decreased with the increase of pH, but the negative charge of kaolin increased with the increase of pH, so the amount of flocculant required increased with the increase of pH. For Fig. 5(c), the flocculation test was conducted by changing the initial mass fraction of kaolin, and it was found that the flocculation effect of GQ-CS did not change much with the increase of the initial concentration. When the initial mass fraction was 1 wt%, 1 mg L−1 of GQ-CS could remove 96.5% of kaolin, and the removal rate of GQ-CS was close to that of kaolin at 2 wt% mass fraction. When the initial mass fraction of kaolin was increased to 3 wt%, the optimal removal rate of 98.9% could be achieved with the addition amount of 9 mg L−1, which was 6 mg L−1 higher than the original mass fraction of 2 wt%. The exploration of the above conditions indicated that GQ-CS had a high removal rate of suspended solids and good stability.
3.5 Effect of inorganic salts and flocculation time
The flocculation of salt-containing printing and dyeing wastewater is the focus of this work. Therefore, it is a point worth discussing to determine whether the flocculation effect of GQ-CS can remain stable in the presence of inorganic salts in the case that GQ-CS has an excellent effect in MB wastewater configured with deionized water. MB solutions containing different kinds and mass fractions of inorganic salts were configured and flocculated using GQ-CS, as shown in Fig. 6(a). The trend of GQ-CS in different salt solutions was basically the same, the performance was relatively stable, and the dose required to achieve the best removal rate was 60 mg L−1. In Fig. 6(b), comparing the flocculation effect of a MB aqueous solution with deionized water and different inorganic salts (60 mg L−1 GQ-CS addition), it can be seen that the dye removal rate of GQ-CS in the salt solution remained above 94%. This data indicated that GQ-CS was well tolerated in different salt environments.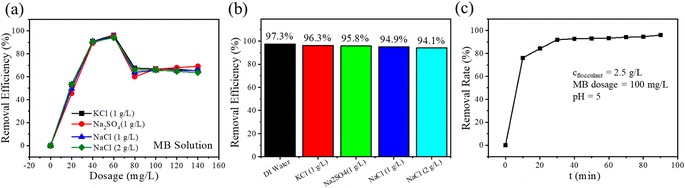 | ||
| Fig. 6 The removal rate of MB (a) and comparison of optimal removal efficiency (b) in different inorganic salt environments; time dependence of the removal rate of MB (c). | ||
Moreover, the time of flocculation is another factor to evaluate the efficiency of flocculation, and the relationship between the removal rate and time after the treatment of MB by GQ-CS was also studied. The timing began when the flocculant and MB solution were fully mixed by shock and left to stand, and a change in the removal rate and time was recorded (Fig. 6(c)). It can be seen that when standing for 10 minutes, there was a relatively good removal effect (76.1%). With the passage of time, when the standing time reached 30 minutes, the removal rate of MB had reached 92.0%, and then it entered a relatively smooth plateau, at which the floc has basically completed precipitation. After 90 minutes of precipitation, the flocculation rate finally reached 96.05%.
3.6 Flocculation mechanism and practical application
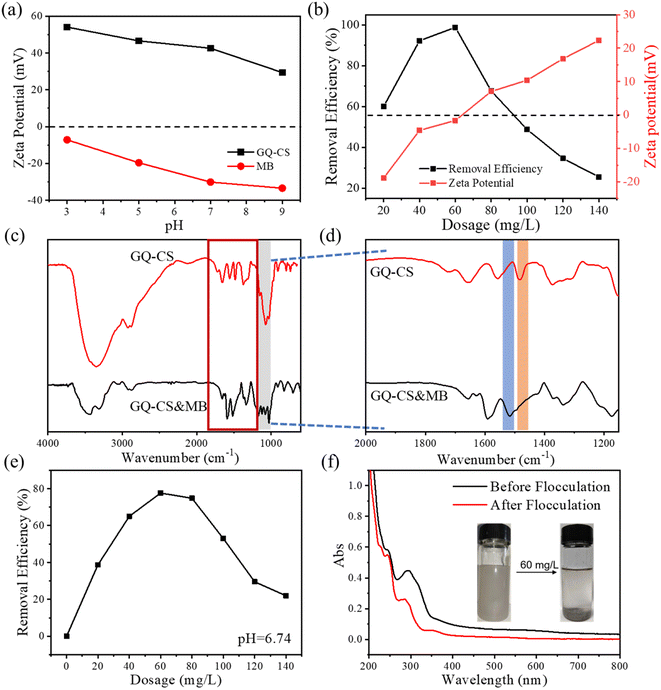
As highlighted above, a crucial function in the flocculation process is the electrostatic attraction between the flocculant and the pollutant. The amount of charge of flocculant and pollutant itself greatly affects the final effect of flocculation. In this work, the Zeta potentials of MB and GQ-CS at different pH were tested and the results are shown in Fig. 7(a). MB carried a negative charge in the range of pH 3–5, and with the increase of pH, the amount of negative charge carried by itself constantly increased. In contrast to MB, GQ-CS always carried a positive charge at pH 3 to 5. The zeta potential decreased with the increase of pH. The opposite charge of the two is the main source of effective flocculation. The change trend of zeta potential was the reason why the higher the pH of GQ-CS was when flocculating MB, the more dosage of flocculant was required. In addition, at pH = 5, the relationship between zeta potential and removal rate of water after different doses of GQ-CS input is shown in Fig. 7(b). As you can see, with the increase of the amount of input of GQ-CS, the zeta potential of the whole system showed a trend from negative to positive. When the zeta potential in the water body was closest to zero, the highest removal rate was 98.8%. Therefore, in the process of flocculation, the zeta potential plays an important role in determining the flocculation effect of the system.In addition to the analysis of zeta potential, the floc produced by GQ-CS flocculation of MB was also analyzed using FT-IR and the results are shown in Fig. 7(c) and (d). As can be seen in Fig. 7(c), GQ-CS&MB had some new absorption peaks compared with those for GQ-CS at 1000–1200 cm−1. According to literature analysis, these absorption peaks belonged to MB. In Fig. 7(d), the absorption peak of the methyl group on the quaternary ammonium salt at 1480 cm−1 in the original GQ-CS also shifted. It was thought that this was due to the absorption caused by the new effect of electrostatic attraction with the pollutants.
To further explore the flocculation effect of GQ-CS, we selected wastewater collected from an actual printing and dyeing plant for flocculation treatment. The obtained water sample was not added with other treatments, GQ-CS was directly used to flocculate, and the removal effect of flocculation was tested with different dosages. As can be seen from Fig. 7(e), GQ-CS can achieve a good removal effect on pollutants at 60 mg L−1, with a removal rate of 75.3%. Compared with MB, the relationship between flocculation efficiency and dose here also presented a peak type. That is, only the appropriate amount of flocculant added can achieve a better removal effect. As can be seen in Fig. 7(f), the absorption peak of GQ-CS decreased significantly after adding flocculant at 60 mg L−1. It is obvious from the illustration that the actual printing and dyeing wastewater changed from cloudy to clarified. In short, the flocculant prepared by this work possessed a good practical application potential.
4. Conclusion
In conclusion, the bio-based flocculant GQ-CS was successfully prepared by grafting gallic acid onto the amino group of the main chain of chitosan using quaternized chitosan as the substrate. The experimental results showed that flocculant GQ-CS had a good removal effect in the face of different concentrations and different kinds of anionic pollutants at different pH. When pH = 5, for 100 mg L−1 MB, GQ-CS can remove 97.9% of the pollutant under 60 mg L−1 and only 3 mg L−1 GQ-CS is needed to achieve a 96.8% removal rate of kaolin. Besides, the flocculant also showed a similar removal effect on a mixed dye MB-CR. The infrared spectra indicated that a new absorption peak belonging to MB appeared in the floc GQ-CS&MB. In the experiment of waste water collected from a printing and dyeing plant, it was found that the removal rate of pollutants reached 75.3% and the wastewater changed obviously from turbid to clear. Compared with quaternized chitosan, GQ-CS has a flocculation stability in the presence of inorganic salts. This provides a new idea for the research and development of chitosan-based flocculants.Author contributions
Jingxuan Liu: writing – original draft, data curation, visualization, formal analysis. Hongyu Zhao: revising & editing, methodology. Mengyue Wang: investigation, data curation, validation, methodology. Wenzheng Ban: conceptualization, investigation, project administration. Xi Lu: writing – review & editing, supervision, project administration, funding support. Bin Yan: writing – review & editing, supervision, project administration, funding support.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The study was supported by the SINOPOEC Science and Technology Research Program, “The Investigation and Application of Chemical Cold Production System of Deep Heavy Oil Reservoir” (P22015) and the National Natural Science Foundation of China (No. 21876119).References
- D. I. M. Al-Risheq, S. M. R. Shaikh, M. S. Nasser, F. Almomani, I. A. Hussein and M. K. Hassan, Enhancing the flocculation of stable bentonite suspension using hybrid system of polyelectrolytes and NADES, Colloids Surf., A, 2022, 638, 128305, DOI:10.1016/j.colsurfa.2022.128305.
- M. Azmana, S. Mahmood, A. R. Hilles, A. Rahman, M. A. B. Arifin and S. Ahmed, A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications, Int. J. Biol. Macromol., 2021, 185, 832–848, DOI:10.1016/j.ijbiomac.2021.07.023.
- A. Ibrahim, A. Z. Yaser and J. Lamaming, Synthesising tannin-based coagulants for water and wastewater application: A review, J. Environ. Chem. Eng., 2021, 9(1), 105007, DOI:10.1016/j.jece.2020.105007.
- M. Lapointe and B. Barbeau, Dual starch–polyacrylamide polymer system for improved flocculation, Water Res., 2017, 124, 202–209, DOI:10.1016/j.watres.2017.07.044.
- B. Liu, H. Lu, S. Wu, Z. Wang, L. Feng and H. Zheng, Octopus tentacle-like molecular chains in magnetic flocculant enhances the removal of Cu(II) and malachite green in water, Sep. Purif. Technol., 2022, 282, 120139, DOI:10.1016/j.seppur.2021.120139.
- G. Wang, J. Xiang, J. Lin, L. Xiang, S. Chen, B. Yan, H. Fan, S. Zhang and X. Shi, Sustainable Advanced Fenton-like Catalysts Based on Mussel-Inspired Magnetic Cellulose Nanocomposites to Effectively Remove Organic Dyes and Antibiotics, ACS Appl. Mater. Interfaces, 2020, 12(46), 51952–51959, DOI:10.1021/acsami.0c14820.
- G. Wang, J. Zhang, S. Lin, H. Xiao, Q. Yang, S. Chen, B. Yan and Y. Gu, Environmentally friendly nanocomposites based on cellulose nanocrystals and polydopamine for rapid removal of organic dyes in aqueous solution, Cellulose, 2019, 27(4), 2085–2097, DOI:10.1007/s10570-019-02944-6.
- J. Ma, K. Fu, X. Fu, Q. Guan, L. Ding, J. Shi, G. Zhu, X. Zhang, S. Zhang and L. Jiang, Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification, Sep. Purif. Technol., 2017, 182, 134–143, DOI:10.1016/j.seppur.2017.03.048.
- H. Maruyama, H. Seki and A. Igi, Flocculation of quartz and kaolin by alginate-protamine complex, Biochem. Eng. J., 2020, 162, 107713, DOI:10.1016/j.bej.2020.107713.
- K. Ren, H. Du, Z. Yang, Z. Tian, X. Zhang, W. Yang and J. Chen, Separation and Sequential Recovery of Tetracycline and Cu(II) from Water Using Reusable Thermoresponsive Chitosan-Based Flocculant, ACS Appl. Mater. Interfaces, 2017, 9(11), 10266–10275, DOI:10.1021/acsami.7b00828.
- I. T. Tomasi, C. A. Machado, R. A. R. Boaventura, C. M. S. Botelho and S. C. R. Santos, Tannin-based coagulants: Current development and prospects on synthesis and uses, Sci. Total Environ., 2022, 822, 153454, DOI:10.1016/j.scitotenv.2022.153454.
- J. Ma, G. Wu, R. Zhang, W. Xia, Y. Nie, Y. Kong, B. Jia and S. Li, Emulsified oil removal from steel rolling oily wastewater by using magnetic chitosan-based flocculants: Flocculation performance, mechanism, and the effect of hydrophobic monomer ratio, Sep. Purif. Technol., 2023, 304, 122329, DOI:10.1016/j.seppur.2022.122329.
- M. S. S. Abujazar, S. U. Karaağaç, S. S. Abu Amr, M. Y. D. Alazaiza and M. J. K. Bashir, Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review, J. Cleaner Prod., 2022, 345, 131133, DOI:10.1016/j.jclepro.2022.131133.
- X. Luo, X. Li, C. Wei, X. Shi, S. Zheng and Z. Deng, Use of carbon dioxide to enhance the brine purification and flocculation performance of PAM flocculants, Sep. Purif. Technol., 2021, 267, 118676, DOI:10.1016/j.seppur.2021.118676.
- Z. Liu, H. Wei, A. Li and H. Yang, Evaluation of structural effects on the flocculation performance of a co-graft starch-based flocculant, Water Res., 2017, 118, 160–166, DOI:10.1016/j.watres.2017.04.032.
- T. Okuda, W. Nishijima, M. Sugimoto, N. Saka, S. Nakai, K. Tanabe, J. Ito, K. Takenaka and M. Okada, Removal of coagulant aluminum from water treatment residuals by acid, Water Res., 2014, 60, 75–81, DOI:10.1016/j.watres.2014.04.041.
- P. S. Bakshi, D. Selvakumar, K. Kadirvelu and N. S. Kumar, Chitosan as an environment friendly biomaterial – a review on recent modifications and applications, Int. J. Biol. Macromol., 2020, 150, 1072–1083, DOI:10.1016/j.ijbiomac.2019.10.113.
- K. Wang, S. Zhang, Q. Xu, T. Lian, Z. Xu, M. Jiang and P. Liu, Fabrication of salt-tolerant chitosan-based polyelectrolyte flocculant through enhancing H-bond hydration effect for treating and recycling of highly saline dyeing wastewater, Sep. Purif. Technol., 2023, 307, 122786, DOI:10.1016/j.seppur.2022.122786.
- S. Wang, L. Zhang, B. Yan, H. Xu, Q. Liu and H. Zeng, Molecular and Surface Interactions between Polymer Flocculant Chitosan-g-polyacrylamide and kaolinite Particles: Impact of Salinity, J. Phys. Chem. C, 2015, 119(13), 7327–7339, DOI:10.1021/acs.jpcc.5b00739.
- W. Wang, C. Xue and X. Mao, Chitosan: Structural modification, biological activity and application, Int. J. Biol. Macromol., 2020, 164, 4532–4546, DOI:10.1016/j.ijbiomac.2020.09.042.
- S. Fan, J. Chen, C. Fan, G. Chen, S. Liu, H. Zhou, R. Liu, Y. Zhang, H. Hu, Z. Huang, Y. Qin and J. Liang, Fabrication of a CO2-responsive chitosan aerogel as an effective adsorbent for the adsorption and desorption of heavy metal ions, J. Hazard. Mater., 2021, 416, 126225, DOI:10.1016/j.jhazmat.2021.126225.
- M. Zhang, Z. Zhang, Y. Peng, L. Feng, X. Li, C. Zhao and K. Sarfaraz, Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range, Int. J. Biol. Macromol., 2020, 156, 289–301, DOI:10.1016/j.ijbiomac.2020.04.020.
- J. Gregory and S. Barany, Adsorption and flocculation by polymers and polymer mixtures, Adv. Colloid Interface Sci., 2011, 169(1), 1–12, DOI:10.1016/j.cis.2011.06.004.
- Z. Yang, J.-R. Degorce-Dumas, H. Yang, E. Guibal, A. Li and R. Cheng, Flocculation of Escherichia coli Using a Quaternary Ammonium Salt Grafted Carboxymethyl Chitosan Flocculant, Environ. Sci. Technol., 2014, 48(12), 6867–6873, DOI:10.1021/es500415v.
- P. Jin, S. Chergaoui, J. Zheng, A. Volodine, X. Zhang, Z. Liu, P. Luis and B. Van der Bruggen, Low-pressure highly permeable polyester loose nanofiltration membranes tailored by natural carbohydrates for effective dye/salt fractionation, J. Hazard. Mater., 2022, 421, 126716, DOI:10.1016/j.jhazmat.2021.126716.
- K. Wang, T. Ran, P. Yu, L. Chen, J. Zhao, A. Ahmad, N. Ramzan, X. Xu, Y. Xu and Y. Shi, Evaluation of renewable pH-responsive starch-based flocculant on treating and recycling of highly saline textile effluents, Environ. Res., 2021, 201, 111489, DOI:10.1016/j.envres.2021.111489.
- Y. Zhang, H. Yin and Y. Feng, Oppositely Charged Polyelectrolyte Complexes for High-Salinity Hydrofracking Fluid, Ind. Eng. Chem. Res., 2019, 58(40), 18488–18497, DOI:10.1021/acs.iecr.9b03170.
- Z. Zhao, Y. Li, K. Muylaert and I. F. J. Vankelecom, Synergy between membrane filtration and flocculation for harvesting microalgae, Sep. Purif. Technol., 2020, 240, 116603, DOI:10.1016/j.seppur.2020.116603.
- S. Xiao, Y. Yang, M. Zhong, H. Chen, Y. Zhang, J. Yang and J. Zheng, Salt-Responsive Bilayer Hydrogels with Pseudo-Double-Network Structure Actuated by Polyelectrolyte and Antipolyelectrolyte Effects, ACS Appl. Mater. Interfaces, 2017, 9(24), 20843–20851, DOI:10.1021/acsami.7b04417.
- Y. Kang, X. Zhao, X. Han, X. Ji, Q. Chen, H. Pasch, A. Lederer and Y. Liu, Conformation and persistence length of chitosan in aqueous solutions of different ionic strengths via asymmetric flow field-flow fractionation, Carbohydr. Polym., 2021, 271, 118402, DOI:10.1016/j.carbpol.2021.118402.
- J. Yang, H. Chen, S. Xiao, M. Shen, F. Chen, P. Fan, M. Zhong and J. Zheng, Salt-Responsive Zwitterionic Polymer Brushes with Tunable Friction and Antifouling Properties, Langmuir, 2015, 31(33), 9125–9133, DOI:10.1021/acs.langmuir.5b02119.
- Y. P. Neo, S. Ray, J. Jin, M. Gizdavic-Nikolaidis, M. K. Nieuwoudt, D. Liu and S. Y. Quek, Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system, Food Chem., 2013, 136(2), 1013–1021, DOI:10.1016/j.foodchem.2012.09.010.
- X. Sun, Z. Wang, H. Kadouh and K. Zhou, The antimicrobial, mechanical, physical and structural properties of chitosan–gallic acid films, LWT–Food Sci. Technol., 2014, 57(1), 83–89, DOI:10.1016/j.lwt.2013.11.037.
- M. Zhu and L. Feng, A sensitive and rapid sensing platform for ochratoxin A detection based on triple-helix molecular switch and CMC-EDC/NHS covalent immobilized paper, Sens. Actuators, B, 2023, 375, 132859, DOI:10.1016/j.snb.2022.132859.
- W. Ban, Q. Yang, W. Huang, X. Li, Z. Wang, S. Chen, L. Xiang and B. Yan, Mussel-Inspired Catechol-Grafted Quaternized Chitosan Flocculant for Efficiently Treating Suspended Particles and Refractory Soluble Organic Pollutants, Ind. Eng. Chem. Res., 2023, 62(2), 1099–1111, DOI:10.1021/acs.iecr.2c03645.
| This journal is © The Royal Society of Chemistry 2024 |