Open Access Article
Open Access ArticleControl and surveillance of redox potential for 233Pa dissolution in 2LiF–BeF2 molten salt
Zhongqi Zhao abc,
Junxia Geng
abc,
Junxia Geng ab,
Zhiqiang Chengab,
Wenxin Liab,
Qiang Douab,
Lan Zhangab and
Qingnuan Li*ab
ab,
Zhiqiang Chengab,
Wenxin Liab,
Qiang Douab,
Lan Zhangab and
Qingnuan Li*ab
aShanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
bCentre of Excellence TMSR Energy System, Chinese Academy of Sciences, Shanghai 201800, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 17th May 2024
Abstract
233Pa, the precursor nuclide of 233U in the thorium–uranium conversion is prone to reductive deposition in 2LiF–BeF2 (66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 mol%, FLiBe) molten salt. We explored the adjustment and control of the redox potential of the FLiBe melt to avoid the 233Pa reduction deposition. The experimental data indicated that the deposited 233Pa can be completely dissolved and reentered into the molten salt with the addition of oxidant NiF2, and the distribution and behaviour of uranium, thorium, neptunium, and most fission products did not have any significant change in the NiF2-oxidised FLiBe molten salt, showing the feasibility of this manner to make 233Pa exist stably in the melt. The effects of NiF2-addition on the behaviour of the fission products 95Nb and 131I in the FLiBe molten salt were also investigated. It was found that 131I could be used as a redox indicator to monitor the redox potential of the oxidation-enhanced FLiBe molten salt. All the information drawn from this study could provide significant support for the control and surveillance of the redox potential of the FLiBe molten salt in the upcoming thorium molten salt reactor (TMSR).
34 mol%, FLiBe) molten salt. We explored the adjustment and control of the redox potential of the FLiBe melt to avoid the 233Pa reduction deposition. The experimental data indicated that the deposited 233Pa can be completely dissolved and reentered into the molten salt with the addition of oxidant NiF2, and the distribution and behaviour of uranium, thorium, neptunium, and most fission products did not have any significant change in the NiF2-oxidised FLiBe molten salt, showing the feasibility of this manner to make 233Pa exist stably in the melt. The effects of NiF2-addition on the behaviour of the fission products 95Nb and 131I in the FLiBe molten salt were also investigated. It was found that 131I could be used as a redox indicator to monitor the redox potential of the oxidation-enhanced FLiBe molten salt. All the information drawn from this study could provide significant support for the control and surveillance of the redox potential of the FLiBe molten salt in the upcoming thorium molten salt reactor (TMSR).
1 Introduction
The molten salt reactor (MSR), a liquid-fuelled Generation IV reactor,1 has come to the forefront of the international nuclear community because of its intrinsic characteristics suitable for the thorium–uranium fuel cycle.2–6 In a thorium molten salt reactor (TMSR), 232Th can be converted to the fissile nuclide 233U by absorbing one neutron followed by two consecutive β− decays. As the key intermediate nuclide in the thorium–uranium conversion, 233Pa has a relatively long half-life of 27 days, leading to its large accumulation in the TMSR. The high accumulation of 233Pa in the reactor, together with its relatively high neutron capture cross section, would cause an undesirable influence on the neutron economy of the reactor and the breeding rate of 233U.7 Accordingly, it is necessary to remove 233Pa on time from the TMSR and then return it to TMSR after its conversion to 233U.3,4,7We found that most of the 233Pa was deposited in the FLiBe melt and that the 233Pa activity decreased by 1 to 2 orders of magnitude after Hastelloy specimen or metallic Li were introduced.8 Because of the similarity in the chemical properties of Pa and the fission product Nb, the decrease in 233Pa activity could be explained by reduction deposition, as mentioned in the studies on the behaviour of fission products in the molten salt reactor experiment (MSRE) reported by Oak Ridge National Laboratory (ORNL). The recovery technique of 233Pa recommended by ORNL was based on the reduction extraction process of its metallic state. The separation factors of Pa against Th and RE could reach as high as 1200.9 These results indicate the reductive nature of Pa.
Once the deposition of 233Pa occurs in the primary circuit of TMSR, the on-line removal of 233Pa from the fuel salt will not be achieved. More seriously, the accumulation of the fissile nuclide 233U in the 233Pa sediment would possibly cause overheating induced by the fission of 233U under neutron irradiation.7,8,10 To operate the TMSR efficiently and safely, it is necessary to understand the behaviour of 233Pa in the FLiBe molten salt to prevent 233Pa deposition. Unfortunately, no information on the behaviour of 233Pa related with TMSR has been reported in ORNL's work, because the fertile material 232Th was not added into fuel salt during the development of their MSRE.
This paper reports the behaviour of 233Pa produced by irradiating thorium fluorides with photon neutron and how to make the deposited 233Pa dissolve and exist stably in the FLiBe molten salt by adjusting the redox potential of the salt with the oxidant NiF2. The distribution and behaviour of several actinides and the fission products were examined in the oxidation-enhanced FLiBe molten salt. To monitor the redox potential of the oxidation-enhanced FLiBe molten salt, a surveillance protocol based on iodine fission product as redox indicators is proposed.
2 Experimental
The materials, apparatus, and methods described in our previous work were employed after minor modifications.8 Briefly, ThF4 powder (>99.5%, China National Nuclear Corporation) was mixed with LiF (99.85%, Sinopharm Chemical Reagent Co., Ltd.) and melted at 600 °C, followed by purification with H2/HF for 6 h to remove the possible oxides from this ThF4–LiF (FLiTh, mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture. The LiF–BeF2 eutectic salt (molar ratio of 66
20) mixture. The LiF–BeF2 eutectic salt (molar ratio of 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34) was prepared by a similar method. The oxide content of both salt mixtures was determined to be approximately 100 ppm.
34) was prepared by a similar method. The oxide content of both salt mixtures was determined to be approximately 100 ppm.
The FLiTh and UF4 powders were sealed separately in two PMMA boxes and then irradiated on the photon neutron source driven by a 15 MeV electron linear accelerator for about three days. After cooling for about 24 h, the well mixed FLiTh/UF4 powder of about 0.2 g was weighed accurately and measured directly with a calibrated HPGe detector (GMX30P4-70, ORTEC) to obtain the original inventory of radionuclides in the irradiated sample. 25.5 g of LiF–BeF2 eutectic salt, 2.5 g of irradiated FLiTh, and 2.0 g of irradiated UF4 were well mixed in a nickel crucible and then heated in a furnace mounted in an argon atmosphere glove box. The molten salt was maintained at 650 °C for at least 24 h and was ready for subsequent experiments. To examine the distribution and behaviour of 233Pa in the oxidation-enhanced molten salt, NiF2 powder (99.95%, Sinopharm Chemical Reagent Co., Ltd.) was added to the molten salt. The amounts of NiF2 used for all the four experiments involved in this study are listed in Table 1. After each addition of NiF2, the salt was allowed to react completely for 8 h before sampling. Sampling was performed using the 5 mm inner diameter quartz tubes at intervals of 8 to 12 h. After cooling and weighing, the samples were subjected to measure the γ-spectra with the calibrated HPGe detectors.
| Experiment | Times of NiF2 added | Mass of NiF2 in each addition, mg |
|---|---|---|
| Run 21-3 | 2 | 34.0, 71.0 |
| Run 21-4 | 2 | 25.0, 57.0 |
| Run 22-2 | 7 | 6.6, 8.8, 11.1, 9.1, 8.2, 11.5, 6.2 |
| Run 22-3 | 5 | 1.8, 3.0, 4.9, 7.4, 13.0 |
The counting time of the γ-spectrum measurements varied from 0.5 h to 12 h, depending on the activity of the nuclides of interest. The nuclear data used for the nuclide identification and the activity calculation were provided by ENDF/B VIII.0,11 and are listed in Table 2. The uncertainties in the activities mainly include the statistical error in the γ-ray counts, a 6% error in the detector efficiency, and an approximately 8% error in the sample geometry. The typical uncertainty for 233Pa was about 12%. All the activity data were decay corrected to the start time point of each experiment.
| Nuclide | Half-life | Energy, keV | Intensity, % | Energy, keV | Intensity, % |
|---|---|---|---|---|---|
| 233Pa | 27.0 d | 311.9 | 38.50 | 300.1 | 6.63 |
| 235U | 7.04 × 108 a | 185.7 | 57.20 | 143.8 | 10.96 |
| 237U | 6.75 d | 208.0 | 21.20 | 164.6 | 1.86 |
| 239Np | 2.36 d | 277.6 | 14.44 | 228.2 | 11.14 |
| 228Ac | 6.15 h | 911.2 | 26.20 | 969.0 | 15.90 |
| 234mPa | 1.16 min | 1001.9 | 0.84 | 766.4 | 0.32 |
| 141Ce | 32.5 d | 145.4 | 48.20 | — | — |
| 143Ce | 1.38 d | 293.3 | 42.80 | 664.6 | 5.69 |
| 140Ba | 12.8 d | 537.3 | 24.39 | 162.7 | 6.22 |
| 95Nb | 35.0 d | 765.8 | 99.81 | — | — |
| 95Zr | 64.0 d | 756.7 | 54.38 | 724.2 | 44.27 |
| 99Mo | 2.75 d | 739.5 | 12.26 | 181.1 | 6.14 |
| 99mTc | 6.01 h | 140.5 | 89.00 | — | — |
| 103Ru | 39.2 d | 497.1 | 91.00 | 610.3 | 5.76 |
| 132Te | 3.20 d | 228.2 | 88.00 | 49.7 | 14.96 |
| 132I | 2.30 h | 667.7 | 98.70 | 772.6 | 75.60 |
| 131I | 8.03 d | 364.5 | 81.50 | 284.3 | 6.12 |
3 Results and discussion
The redox condition of molten salts can be described in a variety of ways. D. Olander has suggested that the redox condition of molten fluoride salts should be defined quantitatively as the chemical potential of fluorine,12 whereas it is common to express the redox condition in terms of redox potential. Redox potential is defined as the tendency of a chemical species to lose electrons and become oxidised (or gain electrons and become reduced), and it can be obtained directly from experimental measurements, or it can be calculated from the Nernst equation using the standard electrode potential and the activities of the oxidised and reduced species. This study does not include any electrochemical measurements, but only a simple estimation of the redox potential from reference data.For the fuel salt containing UF4, where the U4+/U3+ couple is dominant, the redox potential can be conveniently measured and controlled by the U4+/U3+ mole ratio. According to ORNL reports, the electrode potentials of U4+/U3+, NbF5(g)/Nb, I(g)/I− and Ni2+/Ni are −1.106 V, −0.311 V, −0.051 V and 0.409 V (vs. HF(g)/H2, F− in FLiBe) at 650 °C, respectively.13 Therefore, NiF2 can be used as an oxidant, and the redox condition of the molten salt can also be roughly described by the amount of NiF2 added.
3.1 Oxidative dissolution of 233Pa
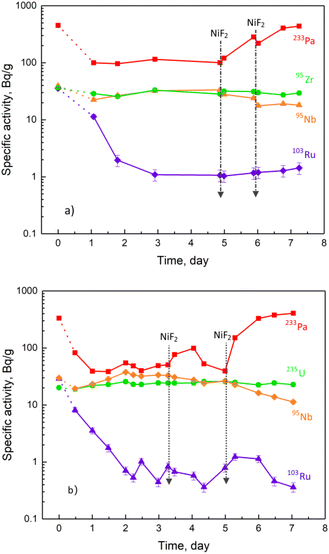
Two parallel experiments were carried out, in each of which NiF2 was added to the molten salt twice, 34 mg and 71 mg in one experiment (run 21-3) and 25 mg and 57 mg in the other (run 21-4). Each experiment lasted for about 8 days, and the specific activities of some selected nuclides in the melt were determined. After correction for the decay of the radionuclides, the nuclide specific activities with the addition of NiF2 from two experiments were obtained, and depicted in Fig. 1(a) and (b).The specific activity of 233Pa in the FLiBe melt decreased obviously when compared to the expected value based on the experimentally determined inventory. The large decrease in 233Pa activity confirms the observation in our previous work.8 Furthermore, the behaviour of the steep decrease in 233Pa activity is similar to that of the typical noble fission product 103Ru. The decrease in 233Pa activity should result from reduction followed by deposition in the form of metal granules, similar to the noble metal fission products.14
In addition, the increase in the redox potential resulting from the addition of NiF2 to the melt could be an approach to eliminating the reductive deposition of 233Pa. As seen also from Fig. 1, the specific activity of 233Pa increased significantly with the addition of the oxidant NiF2. When sufficient NiF2 was added, the specific activity of 233Pa could match the value calculated from the inventory, exhibiting the ability of the oxidant NiF2 to oxidise the deposited Pa to a soluble compound, most likely, PaF4.
| Pa + 2NiF2 = PaF4 + 2Ni | (1) |
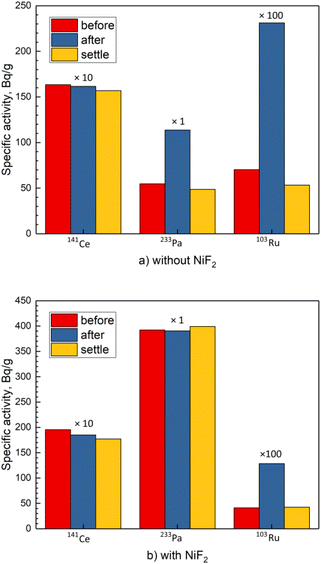
To further demonstrate the deposition of 233Pa in the melt and its dissolution after the addition of NiF2, the test molten salt containing 233Pa and the fission products was stirred thoroughly with a graphite rod and then allowed to settle for several hours. Three samples were taken from the molten salt at different moments, i.e., before stirring, immediately after stirring, and 4 hours later after stirring, respectively. The same procedure was repeated for the other test molten salt, where NiF2 was added beforehand. The measurements of the specific activity obtained from the experiments are shown in Fig. 2 for the nuclides 141Ce, 233Pa and 103Ru.
 | ||
| Fig. 2 Specific activities of 233Pa, 103Ru and 141Ce in molten salt before and after stirring. (a) Without NiF2; (b) with NiF2. | ||
As indicated in our previous work,8 the deposited 233Pa and the noble metal fission products were distributed mainly in the bottom of the molten salt. When the molten salt was stirred thoroughly, the precipitates were dispersed throughout the entire melt in the form of small granules, resulting in an obvious increase in the activities of these nuclides. After settling for several hours, these small granules sink, and their activities in the melt decreased to the original level, as shown in Fig. 2(a) for 233Pa and 103Ru. In contrast, the specific activity of the salt-seeking fission product, represented by 141Ce, did not change no matter whether stirred or settled, due to its good solubility in the molten salt.
In the experiment where the oxidant NiF2 was added into the FLiBe molten salt beforehand, the pattern of 103Ru's specific activities was the same as before, implying that 103Ru behaved as a typical noble metal fission product even in oxidation-enhanced melt. However, a significant variation in the specific activities of 233Pa occurred after the oxidant NiF2 was added. At this time, 233Pa behaved more like 141Ce rather than 103Ru and its specific activity remained unchanged no matter whether stirred or settled (Fig. 2(b)), implying a complete dissolution of 233Pa in the oxidation-enhanced melt resulting from the addition of NiF2. It can be concluded that the increase in the redox potential of the FLiBe molten salt was able to eliminate the deposition of 233Pa or to promote the re-dissolution of the deposited 233Pa. Consequently, maintaining the redox potential at a suitably high level is essential to the oxidative dissolution of 233Pa.
3.2 Behaviour of actinides in the NiF2-oxidised FLiBe molten salt
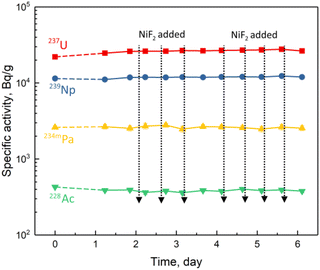
ORNL has made great efforts to study the behaviour and distribution of radioactive nuclides in the MSRE14–17 because of their importance to the performance and safety of the MSRE operation. As mentioned above, the redox potential of the FLiBe molten salt in the TMSR should be maintained at a higher level than that in the MSRE to eliminate the possible deposition of 233Pa in the TMSR. Therefore, it is necessary to clarify whether the distribution and behaviour of the actinides and fission products in the oxidation-enhanced FLiBe molten salt have any negative impact on the operation of the TMSR.As shown in Fig. 1(b), the specific activity of the long-lived nuclide 235U remained constant after sufficient NiF2 was added, indicating an independent behaviour of uranium on the redox potential in the FLiBe molten salt. In another experiment (run 22-2), a total amount of 61.5 mg of NiF2 was added to molten salt, and the specific activities of four actinides, 237U, 239Np, 234mPa and 228Ac, are shown in Fig. 3. 237U is the product of the 238U (n, 2n) reaction, 239Np is the decay daughter of 239U produced by the 238U (n, γ) reaction, 234mPa is the daughter of 234Th produced by the α-decay of 238U, and 228Ac is the daughter of 228Ra produced by the α-decay of 232Th. When the oxidant NiF2 was gradually added to the molten salt, their specific activities remained constant, indicating the stable existence of their precursors, i.e., U, Np, and Th, in the melt, which were not affected by the addition of NiF2. Therefore, the increase in redox potential would not cause an undesirable change in the distribution and behaviour of these actinides in the FLiBe molten salt.
3.3 Behaviour of fission products in the NiF2-oxidised FLiBe molten salt
According to the behaviour in the FLiBe molten salt of MSRE, ORNL classified the fission products into three categories, i.e., the gas fission products, the salt-seeking fission products, and the noble metal fission products.14–17 Among them, the salt-seeking fission products could dissolve well in the FLiBe molten salt and remain stable. In the experiment mentioned above, the specific activities of the typical salt-seeking fission products, 95Zr, 141Ce, 143Ce and 140Ba, were determined and shown in Fig. 4. No significant variation in their specific activities was observed, indicating the stable presence of these salt-seeking fission products in the NiF2-oxidised FLiBe melt.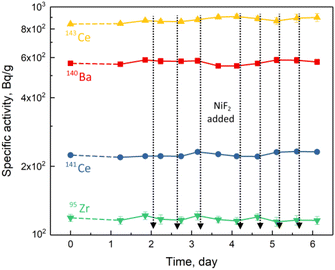 | ||
| Fig. 4 Specific activities for the salt-seeking fission products in FLiBe molten salt with the addition of NiF2. | ||
Compared to the salt-seeking fission products, the variation of the specific activities of the noble metal fission products, such as 99mTc, 99Mo, 103Ru and 132Te, showed a quite different pattern. As shown in Fig. 5, their specific activities decreased rapidly by more than one order of magnitude before the addition of NiF2, which is very similar to that of 233Pa shown in Fig. 1. On the other hand, the variation in specific activity during the over period of NiF2 addition was different from 233Pa. No increase in the specific activity of the noble metal fission products was found with the continuous addition of NiF2. As a result, their specific activities were found to be widely scattered and fluctuated at a low activity level due to the large statistical errors of the γ-counts. The different effects of the specific activities on NiF2 addition between the 233Pa and the noble metal fission products implicates that the latter were more difficult to oxidise by NiF2 and redissolved in the melt. Thus, the behaviour and distribution of noble metal fission products in the NiF2-oxidised FLiBe molten salt were not significantly different from those observed by ORNL in the MSRE.14,16 After sufficient NiF2 was added, the noble metal fission products remained in the form of sediment, while 233Pa was already oxidised and dissolved in the molten salt.
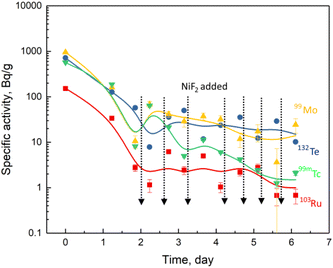 | ||
| Fig. 5 Specific activities for the noble metal fission products in FLiBe molten salt with the addition of NiF2. | ||
3.4 Control and monitoring of the redox potential in NiF2-oxidised FLiBe molten salt
As mentioned above, the deposition of 233Pa in the FLiBe melt could be eliminated by increasing the redox potential of the melt, and the increase in redox potential did not essentially change the behaviour and distribution of U, Np, Th, and most fission products. It is therefore not only necessary but also feasible to increase the redox potential of the FLiBe melt. Thus, the issues are how to control the desired range of the redox potential and how to monitor the redox potential in the FLiBe melt of TMSR.Electrochemical measurements of redox potential are time consuming, and the optical spectroscopy techniques for determining the U4+/U3+ mole ratio may be limited due to the corrosive nature of fluoride salts.18 A strategy worth exploring is to use the behaviour of specific fission products to detect or assess the redox potential of the molten salt, i.e., to use the fission products as redox indicators.
As recommended by ORNL, appropriate values of the U4+/U3+ mole ratio were set in the range of 10–100 to prevent the formation of uranium carbide and to mitigate the corrosion of the structural materials of the MSRE.15 However, in the present work it was shown that the redox potential of the melt had to be adjusted to a higher level to avoid the deposition of 233Pa. In such a situation, the question is whether 95Nb could still be used as a redox indicator in the NiF2-oxidised FLiBe molten salt.
A positive conclusion would be drawn from the experimental results shown in Fig. 1. Contrary to the increase of the 233Pa activity in the molten salt, the 95Nb activity decreased with the addition of the oxidant NiF2. The addition of 105 mg NiF2 increased the activity of 233Pa by a factor of 5, while the activity of 95Nb decreased by about 50%. The decrease in 95Nb activity may be attributed to the oxidation of dissolved NbF4 to volatile NbF5 by the addition of NiF2, followed by the evaporation of NbF5 at high temperature.20 ORNL calculations indicated that Nb could be oxidised and volatilised from the molten salt at a redox potential corresponding to a U4+/U3+ mole ratio greater than 104.13 Therefore, if the activity of 95Nb in the molten salt decreases while 95Nb can be detected in the gas phase, it suggests that the molten salt is under oxidising condition. In other words, 95Nb could still be able to be used as a redox indicator in oxidation-enhanced FLiBe melt. However, its low sensitivity to the redox potential of the melt might more or less limit its practical application compared to iodine fission products as seen below.
The behaviour of iodine fission products and their correlation with the redox potential in the FLiBe molten salt have been studied in our previous work.21,22 The investigations showed that as the redox potential of the molten salt increases, the iodine is oxidised into the iodine molecule I2 and released from the salt, leading to a decrease in the iodine activity remaining in the melt. In the present work, two parallel experiments were carried out, where the specific activities of 131I and 233Pa in the FLiBe and their correlation with the redox potential were examined simultaneously (Fig. 6). Similar to Fig. 1, the specific activity of 233Pa increased to the inventory value with the addition of NiF2 due to its oxidative dissolution in the molten salt. In contrast, the specific activity of 131I decreased continuously with the addition of NiF2 due to its oxidation and subsequent volatilisation. It should be noted that when 233Pa was completely dissolved and returned to the salt, almost no 131I was present in the molten salt any longer. Furthermore, the variation in the specific activities of 131I was much larger than that of 95Nb when NiF2 was added, suggesting that the sensitivity of 131I, as a redox indicator, was better than that of 95Nb. Consequently, 131I could be used as an indicator to monitor the deposition of 233Pa in the FLiBe melt.
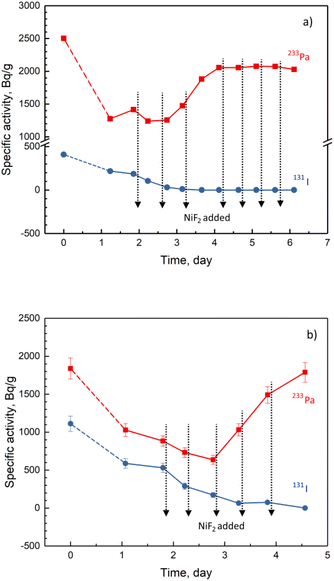 | ||
| Fig. 6 Specific activities of 131I and 233Pa in FLiBe molten salt with the addition of NiF2. The amount of NiF2 in each addition: (a) run 22-2; (b) run 22-3. | ||
The chemical principle that allows the fission product iodine to be used as a redox indicator is based on its volatility caused by oxidation. ORNL evaluated the correlation between the redox potential of the FLiBe melt and the oxidation of iodine and indicated that when massive I− ions were oxidised and volatilised, the redox potential of the melt corresponded to a U4+/U3+ mole ratio of 108,13 even higher than the 104 mentioned above for 95Nb. Although large differences exist for the U4+/U3+ mole ratios estimated from different indicators of iodine and niobium, it can be inferred that the U4+/U3+ mole ratio in the FLiBe melt should be controlled to be in the range of 104 to 108, significantly higher than the U4+/U3+ mole ratio of 10 to 102 proposed by ORNL for their MSRE. To monitor the redox potential of the melt, the electrochemical method or the spectral method might suffer from the difficulty related to the huge difference in the concentration between U4+/U3+.18 Consequently, it would be preferable to use fission product 131I as a redox indicator to adjust and monitor the redox potential of the FLiBe melt for 233Pa dissolution.
As indicated above, increasing redox potential of the FLiBe melt may not change the behaviour of Th, U, Np, and most of the fission products present in TMSR, but some of the possible influences on the operation of TMSR should be further evaluated. 135Xe, the most important neutron poison, is the daughter of 135I. The ORNL report indicated that the residual 135Xe in the reactor was strongly dependent on the removal rate of 135I from the molten salt, i.e., the more iodine was removed, the less 135Xe remained in the molten salt.14,15,23 Thus, increasing the redox potential of the FLiBe melt would remarkably raise the neutron economy of the reactor.
On the other hand, however, the escape of iodine fission products caused by the higher oxidation potential would also lead to the loss of some of the delayed neutron precursors of the iodine isotopes, such as 137–140I with very short half-lives, which will bring about a kind of issues in the control of reactor to some extent. In addition, the increase in the redox potential of the melt might exacerbate the corrosion of the Hastelloy structural materials of the reactor. Therefore, it is necessary to further investigate and evaluate the influence of increasing the redox potential of the FLiBe molten salt on the efficiency and safety of TMSR operation.
4 Conclusions
To eliminate the reductive deposition of the key nuclide 233Pa in the thorium–uranium conversion, a method of increasing the redox potential of the FLiBe molten salt was proposed for the first time. The experimental results obtained from these studies again showed a great deposition of 233Pa in the FLiBe molten salt, and the addition of NiF2 could promote the complete dissolution of the deposited 233Pa. No adverse changes in the distribution and behaviour of Th, U, Np, and most of the fission products were found in the NiF2-oxidised FLiBe molten salt. In the NiF2-oxidised FLiBe molten salt, the fission products 95Nb and 131I, especially the latter, could be used as suitable redox indicators. The results obtained from these studies would have significant reference value for the development and operation of TMSR. However further studies are required using the actual fuel salt from the upcoming TMSR.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their sincere thanks to the Free Electron Group for their encouragement and help. This work was supported by the “Strategic Priority Research Program” (XDA02030000), “Frontier Science Key Program” (QYZDYSSW-JSC016),“the Young Potential Program of Shanghai Institute of Applied Physics, Chinese Academy of Sciences” (E0552901), “National Natural Science Foundation of China” (No. 12175303 and U2267226) and “Xinjiang Uygur Autonomous Region Key R&D Task Special Project” (2022B01039).Notes and references
- U.S. Department of Energy, Phil. Rev., 2002, 66, 239–241 Search PubMed.
- L. Mathieu, D. Heuer, R. Brissot, C. Garzenne, C. Le Brun, D. Lecarpentier, E. Liatard, J. M. Loiseaux, O. Méplan, E. Merle-Lucotte, A. Nuttin, E. Walle and J. Wilson, Prog. Nucl. Energy, 2006, 48, 664–679 CrossRef CAS.
- R. C. Robertson, MSRE Design & Operations Report Part 1 Description of Reactor Design, ORNL-TM-728, 1965 Search PubMed.
- R. C. Robertson, Conceptual Design Study of a Single-Fluid Molten-Salt Breeder Reactor, ORNL-4541, 1971 Search PubMed.
- Advanced Reactor Concepts, Technical Review Panel Report: Evaluation and Identification of Future R&D on Eight Advanced Reactor Concepts, Conducted April–September 2012, United States, 2012 Search PubMed.
- Department for Business Innovation & Skills, Nuclear Industrial Strategy, https://www.gov.uk/government/collections/nuclear-industrial-strategy Search PubMed.
- W. Li and Q. Li, J. Nucl. Radiochem., 2016, 38, 327–336 CAS.
- Z. Zhao, J. Hu, Z. Cheng, J. Geng, W. Li, Q. Dou, J. Chen, Q. Li and X. Cai, RSC Adv., 2021, 11, 7436–7441 RSC.
- L. M. Ferris, J. C. Mailen, J. J. Lawrence, F. J. Smith and E. D. Nogueira, J. Inorg. Nucl. Chem., 1970, 32, 2019–2035 CrossRef CAS.
- A. M. Weinberg, The First Nuclear Era: The Life and Times of a Technological Fixer, American Institute of Physics, New York, NY, United States, 1994 Search PubMed.
- M. B. Chadwick, D. A. Brown and R. Capote, et al., Nucl. Data Sheets, 2018, 148, 1–142 CrossRef.
- D. Olander, J. Nucl. Mater., 2002, 300, 270–272 CrossRef CAS.
- C. F. Baes Jr, Nucl. Metall., 1969, 15, 617–644 Search PubMed.
- E. L. Compere, S. S. Kirslis, E. G. Bohlmann, F. F. Blankenship and W. R. Grimes, Fission Product Behavior in the Molten Salt Reactor Experiment, ORNL-4865, 1975 Search PubMed.
- R. E. Thoma, Chemical Aspects of MSRE Operations, ORNL-4658, 1971 Search PubMed.
- R. J. Kedl, Migration of a Class of Fission Products (Noble Metals) in the Molten-Salt Reactor Experiment, ORNL-TM-3884, 1972 Search PubMed.
- W. R. Grimes, Nucl. Appl. Technol., 1970, 8, 137–155 CrossRef CAS.
- J. Zhang, C. W. Forsberg, M. F. Simpson, S. Guo, S. T. Lam, R. O. Scarlat, F. Carotti, K. J. Chan, P. M. Singh, W. Doniger, K. Sridharan and J. R. Keiser, Corros. Sci., 2018, 144, 44–53 CrossRef CAS.
- Z. Cheng, Z. Zhao, J. Geng, W. Li, Q. Dou and Q. Li, J. Nucl. Mater., 2022, 566, 153807 CrossRef CAS.
- J. H. Junkins, R. L. Farrar Jr, E. J. Barber and H. A. Bernhardt, J. Am. Chem. Soc., 1952, 74, 3464–3466 CrossRef CAS.
- J. Geng, Z. Zhao, Z. Cheng, W. Li, Q. Dou, H. Fu, J. Hu, X. Cai, J. Chen and Q. Li, RSC Adv., 2021, 11, 22611–22617 RSC.
- J. Geng, Z. Zhao, Z. Cheng, W. Li, Q. Dou and Q. Li, Inorg. Chem., 2022, 61, 7406–7413 CrossRef CAS PubMed.
- M. W. Rosenthal, P. N. Haubenreich and R. B. Briggs, Development Status of Molten-Salt Breeder Reactors, ORNL-4812, 1972 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |