Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silver nanoparticles alter the dimerization of Aβ42 studied by REMD simulations†
Quynh Mai Thai ab,
Phuong-Thao Tran
ab,
Phuong-Thao Tran c,
Huong T. T. Phung
c,
Huong T. T. Phung d,
Minh Quan Pham
d,
Minh Quan Pham ef and
Son Tung Ngo
ef and
Son Tung Ngo *ab
*ab
aLaboratory of Biophysics, Institute of Advanced Study in Technology, Ton Duc Thang University, Ho Chi Minh City, Vietnam. E-mail: ngosontung@tdtu.edu.vn
bFaculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, Vietnam
cHanoi University of Pharmacy, 13-15 Le Thanh Tong, Hanoi, Vietnam
dNTT Hi-Tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam
eInstitute of Natural Products Chemistry, Vietnam Academy of Science and Technology, Hanoi, Vietnam
fGraduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi, Vietnam
First published on 8th May 2024
Abstract
The aggregation of amyloid beta (Aβ) peptides is associated with the development of Alzheimer's disease (AD). However, there has been a growing belief that the oligomerization of Aβ species in different environments has a neurotoxic effect on the patient's brain, causing damage. It is necessary to comprehend the compositions of Aβ oligomers in order to develop medications that may effectively inhibit these neurotoxic forms that affect the nervous system of AD patients. Thus, dissociation or inhibition of Aβ aggregation may be able to prevent AD. To date, the search for traditional agents and biomolecules has largely been unsuccessful. In this context, nanoparticles have emerged as potential candidates to directly inhibit the formation of Aβ oligomers. The oligomerization of the dimeric Aβ peptides with or without the influence of a silver nanoparticle was thus investigated using temperature replica-exchange molecular dynamics (REMD) simulations. The physical insights into the dimeric Aβ oligomerization were clarified by analyzing intermolecular contact maps, the free energy landscape of the dimeric oligomer, secondary structure terms, etc. The difference in obtained metrics between Aβ with or without a silver nanoparticle provides a picture of the influence of silver nanoparticles on the oligomerization process. The underlying mechanisms that are involved in altering Aβ oligomerization will be discussed. The obtained results may play an important role in searching for Aβ inhibitor pathways.
Introduction
The self-assembly of Aβ peptides into oligomers1,2 is associated with AD,3 which is one of the most common dementias.4,5 In 2023, more than 11 million Americans provided 18.4 billion working hours in order to take care of about 6.9 million elders who are living with AD.6 $346.6 billion is the cost of health, long-term care, and hospice services for these elders. Despite several previous research studies,7–12 the mechanism of AD pathogenesis is unidentified, resulting in the failure of treatment.12–14 The pathogenic mechanism of AD has not been determined despite intensive and extensive studies,15 which hinders the prevention and treatment of the disease. The patient's brain is slowly destroyed, which reduces cognition and life skills.16 Many works have indicated a wide variety of probable reasons for AD, which can be grouped into three categories, including molecular, cellular, and genetic imbalances. For example, Ca2+ homeostasis falls under the category of cellular imbalances, while genetic imbalance is associated with DNA damage. Here we focus on molecular imbalances, including tau,17 amyloid,18 and cholinergic19,20 hypotheses. Among these, numerous pieces of evidence indicate that the oligomerization of Aβ peptides into extracellular transient oligomers plays a role as neurotoxicity agents causing AD.18,21,22 The main types of Aβ peptides are Aβ40 and Aβ42 with 40 and 42 residues,23 respectively. It should be noted that the structural Aβ peptides are very flexible in solution, resulting in a lack of stable conformations. Aβ peptide is a molecule having low hydrophobicity, a high net charge, and a few aggregation-prone regions.24 The Aβ oligomerization is highly sensitive to the sequences25,26 and is associated with AD hallmarks, including the amyloid hypothesis.27–30The amyloid hypothesis states that the aggregation of Aβ is causally linked to the development of AD. Recently, there has been a growing belief that the oligomerization of Aβ species in different environments has a neurotoxic effect on the patient's brain, causing damage.31,32 Characterizing the shapes of Aβ oligomers is thus required to develop agents to prevent the neurotoxic forms.33–36 Several investigations are thus targeted on clarifying the formation of Aβ in solution via both MD and REMD simulations.37–39 In particular, the structural properties of the Aβ16–35 fragment were studied as a model for the Aβ peptide via REMD simulations.40 The influence of mutations on the folding process of dimeric and monomeric forms of Aβ peptides was clarified.41 The probability of the solvated Aβ40/42 peptide forming tetrameric β-barrel structures was determined.42 Therefore, computational approaches were extensively and intensively used for studying the influence of various inhibitors on the conformations of Aβ peptides.43 In particular, the stronger inhibitors normally form larger binding affinity Aβ peptides. The good inhibitors also prevent the formation of β-structure effectively. Numerous compounds were suggested to be able to inhibit the Aβ oligomerization, such as β-sheet breaker peptides,37 curcumin,44 epigallocatechin gallate,45 astaxanthin,34 resveratrol,46 etc. Unfortunately, there is no cure for AD based on small compounds targeting Aβ peptides.14 However, recent AD therapeutic developments are associated with antibody drugs against the aggregates.47,48
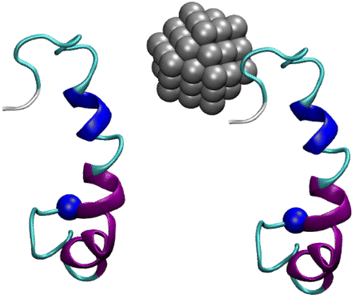
Nanoparticles are particles with physical, chemical, or biological effects whose sizes are within the nanoscale range (1–100 nm).49 Nanoparticles can be used for a variety of pharmaceutical and medical applications, such as drug delivery, imaging, and cancer therapy.50 In this context, nanoparticles emerge as highly potent substrates that may be used to treat AD by preventing the oligomerization of Aβ peptides (cf. Fig. 1). The 11-mercapto-1-undecanesulfonate-coated gold nanoparticles have been informed to be able to spot the generation of amyloid fibrils derived from various amyloidogenic proteins.51 These nanoparticles are powerful tools for investigating amyloid morphologies, using cryogenic transmission electron microscopy (cryo-EM).51 Moreover, silver nanoparticles are also small particles of silver that have been reduced to a size of less than 100 nanometers. They have a number of unique properties, including the ability to kill bacteria and viruses. This makes them useful for a variety of applications, including wound dressings, food preservation, and water purification.52 Similar to gold nanoparticles, silver nanoparticles can induce fast disintegration of the fibrils. Triangular silver nanoplates stabilized with poly(vinyl)pyrrolidone and carrying a negative charge were more efficient compared to silver nanospheres stabilized with poly(vinyl)pyrrolidone.53,54 In particular, it was demonstrated that when Aβ fibrils were exposed to triangular silver nanoplates and subjected to near infrared illumination, the fibrils were dissolved in just 1 hour. In contrast, it took approximately 70 hours for the nanospheres to achieve the same result. Silver nanoparticles were used to monitor the dynamical behavior of Aβ25–35 peptides.55 The silver nanoparticles were thus suggested that they are able to a good therapy solution to prevent amyloidosis diseases such as AD.55
In this context, studying the influence of nanoparticles on the oligomerization of Aβ peptides is of great interest. Therefore, in the project, we propose to use atomistic simulations to assess the folding process of Aβ peptides wild-type under the effects of silver nanoparticle. In particular, the structural change, dynamic behavior, and kinetics of the Aβ oligomerization in the presence and absence of nanoparticles will be clarified.
Materials and methods
The Aβ42 dimer and Aβ42 dimer + silver nanoparticle in solution
The initial conformation of the monomeric Aβ42 peptide can be downloaded from the Protein Data Bank (PDB) with PDB ID 1Z0Q.56 The aqueous solution structure of Aβ42 was often used to probe the interaction between Aβ and different molecules in solution.57 The Aβ peptides are randomly inserted into a dodecahedron box with the minimum distance between various monomers larger than 12.0 Å. The silver nanoparticle with 55 atoms is generated via the nanomaterial modeler tools via CHARMM GUI58 according to the stable structures of nanoparticles, referring to the previous work.59 In particular, the interface force field (IFF)60 was employed to present the silver nanoparticle. It should be noted that IFF facilitates using computational methods to probe biomaterials and advanced materials. Using IFF, the interaction between nanomaterials and proteins was successfully characterized, including silver nanomaterials and SARS-CoV-2 receptor-binding protein.61 The Aβ peptides are parameterized via CHARMM36m force fields.62 The TIP3P water model is employed to describe the water molecule.63 The interface force field60 will then be used to parameterize the silver nanoparticle. An example of Aβ42 dimer and Aβ42 + silver nanoparticle is shown in Fig. 1. In particular, both complexes were inserted into the dodecahedron box with a volume of 584 nm3, respectively, and the systems consist of ca. 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 atoms totally.
300 atoms totally.
MD simulations
The solvated Aβ peptides in the presence and absence of nanoparticles were simulated using GROMACS version 2019.6.64 In particular, the MD simulations utilize the leap-frog stochastic dynamics integrator with a time step of 2 fs. The pressure of simulation was selected as 1 bar. A relaxation time of 0.1 picoseconds is selected. The V-rescale thermostat and Parrinello–Rahman barostat were used for temperature and pressure simulations.65,66 The LINCS67 approach restricts all covalent bonds to a fourth order. The non-bonded interaction pair list is updated using a 0.9 nm cut-off every 10 femtoseconds. The rapid and efficient particle-mesh Ewald electrostatics method is used to calculate the interactions between charged particles, with a cutoff distance equal to the range of non-bonded interactions. The van der Waals (vdW) interactions are calculated using a cut-off distance of 0.9 nm. In the first step, energy minimization using the steepest descent protocol was performed. The minimized systems were formerly relaxed in NVT and NPT ensembles with the positionally restrained condition using a weak harmonic potential (100 ps each). The last snapshots of NPT simulations were then used as the initial structures for REMD simulations at different temperatures.REMD simulations
Temperature REMD simulation68 has been widely used to investigate the structural change of disordered proteins, including Aβ systems.69–71 The last snapshots of NPT simulations (mentioned above) were used as initial conformations of REMD simulations. The temperature REMD simulations with a length of 500 ns will be carried out as previously described72 using 44 temperatures ranging from 308.50 to 384.33 K (cf. the ESI†). In particular, the temperatures were generated via a web-server generator.73 Atomic coordinates and other data (energy and velocity, etc.) will be recorded every 10 ps during REMD simulations to analyze the structural changes of the systems.Structural analysis
The free energy landscapes (FEL) of the Aβ systems over the equilibrium region of REMD simulations will be obtained using the GROMACS tool “gmx sham”,74,75 in which the radius of gyration (Rg) and root mean square deviation (RMSD) will be used as the reaction coordinates. Moreover, the FEL will be possibility constructed via the principal component analysis (PCA) method, with the first and second principal components being reaction coordinates.76 In combination with the FEL results, the clustering method will be used to obtain the representative conformation of the considered systems.77 IMPACT tools were used to calculate the collision cross-section (CCS) of Aβ peptides.78 The intermolecular non-bonded contacts were counted when the minimum distance between non-hydrogen atoms of different Aβ residues to Aβ residues or to nanoparticles was smaller than 4.5 Å. The intermolecular hydrogen bond (HB) between the Aβ residues and the Aβ residues/nanoparticles was endorsed when the angle ∠ acceptor (A)–hydrogen (H)–donor (D) is larger than 3π/4 and the pair A–D is smaller than 3.5 Å. The secondary structure of Aβ peptides can be predicted via the Dictionary of Protein Secondary Structure (DSSP).79Results and discussion
The conformational change of Aβ peptides was popularly investigated via REMD simulations, which is one of the most effective enhanced sampling approaches.80 In particular, the large difference in temperature range can increase the efficiency of the approach compared with conventional MD simulations.81,82 The temperatures were selected in the range from 308.50 to 384.33 K by using a web-server generator.73 The exchange rates diffused in the range from 21 to 24% implying the efficiency of the REMD simulations. The superposition of computed values over the various intervals mentions the simulating convergence (Fig. S1 and S2 of the ESI file†). The REMD simulations were performed over 500 ns of MD simulations, which resulted in ca. 44 μs of MD simulations totally. The systemic coordinates at 310 K were recorded every 10.0 ps.The structural change of dimeric Aβ peptides in the presence and absence of silver nanoparticle was monitored and analyzed (cf. Fig. 2) over equilibrium domains, which ranged from 260 to 500 ns in the simulations. The radius of gyration, Rg, of dimeric Aβ42 peptides without the presence of silver nanoparticle diffuses in the range from 1.26 to 3.56 nm with a mean value of 1.70 ± 0.37 nm. The value is slightly larger than that interacting with silver nanoparticle, which varies in the range from 1.30 to 3.14 nm with an average value of 1.60 ± 0.23 nm. The RMSD value of the isolated Aβ dimer ranges from 1.15 to 2.90 nm with a mean value of 1.87 ± 0.18 nm. While, the dimeric Aβ peptides in the presence of Ag55 forms RMSD in the range from 1.30 to 2.70 nm, with an average value of 1.89 ± 0.25 nm. The presence of silver nanoparticle alters the CCS curve of the dimeric Aβ42 (cf. Fig. 2). The mean CCS values of the dimers are changed, among these, the dimers with and without silver nanoparticle form an average CCS of 12.77 ± 1.50 and 12.32 ± 0.96 nm2, respectively. The total number of sidechain (SC) contacts between different residues of different chains is also changed. The silver nanoparticle significantly alters the SC contact between two monomers. The mean of SC contacts is 75.03 ± 30.41 and 81.76 ± 36.70 nm2 corresponding to the dimeric Aβ42 in the absence and presence of Ag55, respectively.
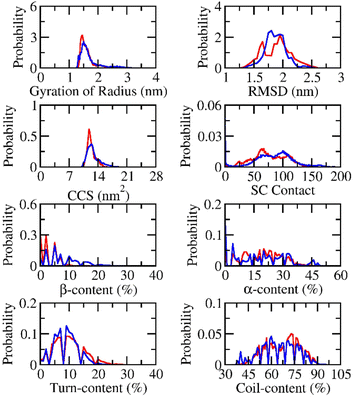 | ||
| Fig. 2 Computed metrics of dimeric Aβ42 in the presence (red curve) and absence (blue curve) of the silver nanoparticle. | ||
The secondary structure terms were also investigated via the Define Secondary Structure of Proteins (DSSP) protocol.79,83 The obtained results are presented in Fig. 2. In details, the β-content changes from 0 to 33% with an average of 8.2 ± 5.7% and from 0 to 26% with a mean value of 3.5 ± 3.8% in the absence and presence of Ag55. The free Aβ42 dimer in the present work adopts more and less β-content compared with the previous works by Barz et al.,84 which occupied 5.6 ± 0.7% and Maryam et al.,85 which formed 13%. The α-content of the free Aβ42 dimer varies in the range from 0 to 51% with a mean value of 18.2 ± 13.3%. The appearance of Ag55 turns the metric to 19.0 ± 10.3% and ranges from 0 to 48%. In similar, the Ag55 increases the turn-content of Aβ42 dimer from 8.9 ± 4.1 to 9.9 ± 5.2%. The coil-content of Aβ42 dimer increases from 64.7 ± 11.7 to 67.6 ± 10.9% via the influence of Ag55. Besides, secondary structure terms fall in a large range mentioning a broad structural change observed over the REMD simulations.
The secondary structure terms over individual residues were also reported (cf. Fig. 3). The outcomes are in good consistent with the previous investigation via MD simulation using the same force field.85 In good agreement with the total metrics above, almost dimeric residues adopt a less β-content when silver nanoparticle was induced. Moreover, the N-terminal of the dimer significantly increases α-content with the presence of Ag55, whose residues range from 10–20. Correspondingly, the coil-content of the sequence 10–20 was significantly decreased when Ag55 was induced. Turn-content population density per residues was also altered as shown in Fig. 3. The outcomes are similar results to the secondary structure of the Aβ40 monomer in binding mode with gold nanoparticle.86 In addition, the local secondary structure term of a residue along sequence of the dimeric Aβ was thus reported, which is in good consistent with the previous work.87
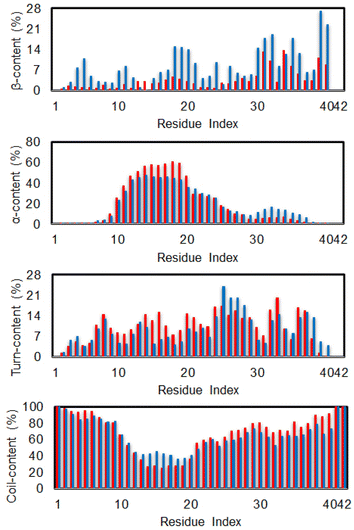 | ||
| Fig. 3 Secondary structure per residues of the dimeric Aβ42 in the presence (red bar) and absence (blue bar) of Ag55. | ||
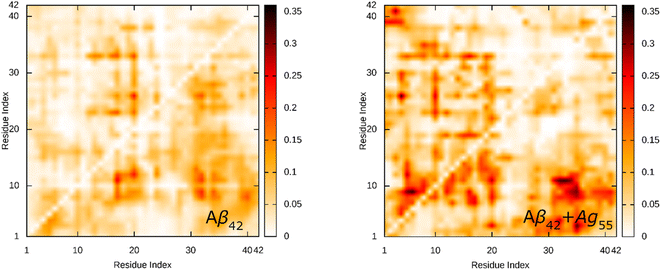
The SC contact maps between individual chains of the dimeric Aβ42 and Aβ42 + Ag55 were generated and shown in Fig. 4. Interestingly, the appearance of Ag55 enhanced the binding between different residues of various chains of the dimeric Aβ42 peptide. The obtained results are in good consistent with the analysis of the total number of SC contact between different chains. It may be argued that the Ag55 nanoparticle force the dimer to stick together.
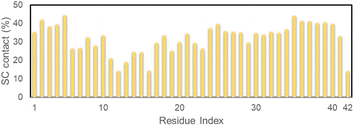
The referential binding site of Ag55 to the dimeric Aβ42 can be probed by computing the distribution of SC contact between Ag55 and the dimeric Aβ42. In particular, the SC contact was counted when the spacing between non-hydrogen atoms of Aβ42-specific residues and Ag55 was smaller than 0.45 nm. The obtained results are shown in Fig. 5. Ag55 forms SC contacts to two domains, including sequences 2–5 and 35–40, over more than 38% of considered snapshots. It may be argued that the silver nanoparticle prefers to bind to these domains.
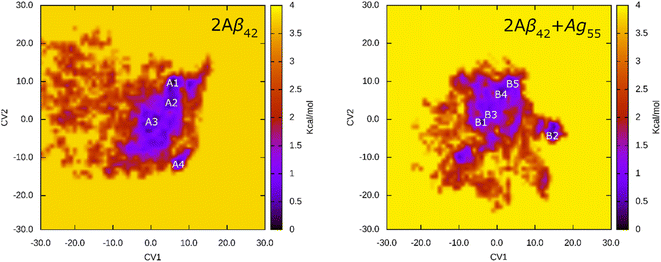
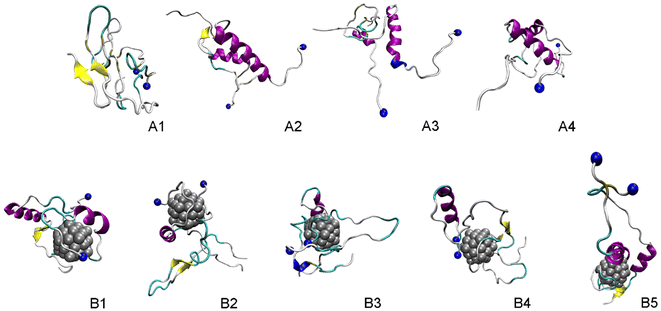
The FEL of dimeric Aβ42 with and without Ag55 was produced over the interval 260–500 ns of REMD simulations at 310 K. 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 snapshots of each dimeric Aβ42 system were used for PCA analysis. The representative structure of dimeric Aβ42 with and without the presence of Ag55 nanoparticle was then obtained via the clustering method.77,88 The FEL was obtained and shown in Fig. 6. Absolutely, the appearance of silver nanoparticle modifies the FEL of the dimeric Aβ42 peptide. The number of minima was also altered. Among these, the isolated Aβ42 dimer forms 4 minima denoted as A1, A2, A3, and A4, while the Aβ42 dimer + Ag55 adopts 5 minima denoted as B1, B2, B3, B4, and B5. Every dimeric Aβ42 structures located in the minima was collected to evaluate the representative conformation via the clustering approach on the backbone with a cutoff of 0.03 nm.
000 snapshots of each dimeric Aβ42 system were used for PCA analysis. The representative structure of dimeric Aβ42 with and without the presence of Ag55 nanoparticle was then obtained via the clustering method.77,88 The FEL was obtained and shown in Fig. 6. Absolutely, the appearance of silver nanoparticle modifies the FEL of the dimeric Aβ42 peptide. The number of minima was also altered. Among these, the isolated Aβ42 dimer forms 4 minima denoted as A1, A2, A3, and A4, while the Aβ42 dimer + Ag55 adopts 5 minima denoted as B1, B2, B3, B4, and B5. Every dimeric Aβ42 structures located in the minima was collected to evaluate the representative conformation via the clustering approach on the backbone with a cutoff of 0.03 nm.
The representative structures of the dimeric Aβ42 and Aβ42 + Ag55 were obtained and shown in Fig. 7. Among these, A1–A4 correspond to 4 minima of the isolated dimeric Aβ42 system, which coordinates (CV1, CV2) at (7.0, 9.0), (5.0, 4.0), (0.5, −3.5), and (8.5, −12.5), respectively. The population of these minima is 12, 10, 12, and 4%, respectively. Besides, the conformations B1–B5 correspond to 5 minima of the dimeric Aβ42 + Ag55, which coordinates (CV1, CV2) at (−4.5, −1.5), (13.5, −4.5), (−3.5, 0.5), (0.5, 6.0), and (3.5, 9.5), respectively. The population of these conformation is of 8, 10, 7, 10, and 7%, respectively. In good agreement with the whole trajectory analysis above, the dimeric Aβ42 adopts more coil-structure when Ag55 was induced. It may be argued that the silver nanoparticle forms strong effects on the conformation of the dimeric Aβ42.
Conclusions
In this work, the folding process of the dimeric Aβ42 with and without the presence of Aβ42 was investigated via extensive REMD simulations. Several conformations of the dimers were produced and recorded.The structural metrics including RMSD, Rg, CCS, and secondary structure terms vary in a large range implying the reality of the simulations. In particular, the appearance of silver nanoparticle rigidly alters the structure of the dimeric Aβ42. The β-content was significantly reduced when Ag55 was induced. Inversely, the α-, turn-, coil-content of the dimer was increased. Moreover, the size of the dimer is slightly increased due to the influence of silver nanoparticle. However, the number of SC contacts between different chains of the dimer is significantly increase when the Ag55 was induced. Absolutely, the dimeric Aβ42 peptide in binding mode with Ag55 forms much more of structural change than the isolated dimeric Aβ42. The silver nanoparticle prefers to bind to two domains, including sequences 2–5 and 35–40. The FEL of the dimer is thus altered under the influence of the nanoparticle. Among these, the number of minima has increased. The obtained results may play an important role in the searching for Aβ inhibitor pathway.
Conflicts of interest
There are no conflicts to decare.Acknowledgements
This research was funded by Vingroup Innovation Foundation (VINIF) under project code VINIF.2022.DA00061 and Ho Chi Minh City Department of Science and Technology under project code 115/QD-SKHCN.References
- P. H. Nguyen, A. Ramamoorthy, B. R. Sahoo, J. Zheng, P. Faller, J. E. Straub, L. Dominguez, J.-E. Shea, N. V. Dokholyan, A. De Simone, B. Ma, R. Nussinov, S. Najafi, S. T. Ngo, A. Loquet, M. Chiricotto, P. Ganguly, J. McCarty, M. S. Li, C. Hall, Y. Wang, Y. Miller, S. Melchionna, B. Habenstein, S. Timr, J. Chen, B. Hnath, B. Strodel, R. Kayed, S. Lesné, G. Wei, F. Sterpone, A. J. Doig and P. Derreumaux, Chem. Rev., 2021, 121, 2545–2647 CrossRef CAS
.
- U. Sengupta, A. N. Nilson and R. Kayed, EBioMedicine, 2016, 6, 42–49 CrossRef
.
- A. Z. Alzheimer, J. Psychiatry Psych-Forensic Med., 1907, 64, 146–148 Search PubMed
.
- H. W. Querfurth and F. M. LaFerla, N. Engl. J. Med., 2010, 362, 329–344 CrossRef CAS PubMed
.
- M. G. Iadanza, M. P. Jackson, E. W. Hewitt, N. A. Ranson and S. E. Radford, Nat. Rev. Mol. Cell Biol., 2018, 19, 755–773 CrossRef CAS PubMed
.
- Alzheimer’s Association, 2024 Alzheimer’s disease facts and figures, 2024, vol. 20, pp. 1–145 Search PubMed
.
- R. Ahmed, M. Akcan, A. Khondker, M. C. Rheinstädter, J. C. Bozelli, R. M. Epand, V. Huynh, R. G. Wylie, S. Boulton, J. Huang, C. P. Verschoor and G. Melacini, Chem. Sci., 2019, 10, 6072–6082 RSC
.
- S. Eketjäll, J. Janson, K. Kaspersson, A. Bogstedt, F. Jeppsson, J. Fälting, S. B. Haeberlein, A. R. Kugler, R. C. Alexander and G. Cebers, J. Alzheimer's Dis., 2016, 50, 1109–1123 Search PubMed
.
- J. Hu, Y.-D. Huang, T. Pan, T. Zhang, T. Su, X. Li, H.-B. Luo and L. Huang, ACS Chem. Neurosci., 2019, 10, 537–551 CrossRef CAS PubMed
.
- S. Ghosh, K. Jana and B. Ganguly, Phys. Chem. Chem. Phys., 2019, 21, 13578–13589 RSC
.
- V. Armiento, A. Spanopoulou and A. Kapurniotu, Angew. Chem., Int. Ed., 2020, 59, 3372–3384 CrossRef CAS
.
- J. Cummings, G. Lee, A. Ritter, M. Sabbagh and K. Zhong, Alzheimer’s Dement. Transl. Res. Clin. Interv., 2019, 5, 272–293 CrossRef PubMed
.
- M. Tolar, S. Abushakra and M. Sabbagh, Alzheimer’s Dementia, 2019, 16, 1553–1560 CrossRef
.
- D. J. Selkoe and J. Hardy, EMBO Mol. Med., 2016, 8, 595–608 CrossRef CAS
.
- J. Nasica-Labouze, P. H. Nguyen, F. Sterpone, O. Berthoumieu, N.-V. Buchete, S. Coté, A. De Simone, A. J. Doig, P. Faller, A. Garcia, A. Laio, M. S. Li, S. Melchionna, N. Mousseau, Y. Mu, A. Paravastu, S. Pasquali, D. J. Rosenman, B. Strodel, B. Tarus, J. H. Viles, T. Zhang, C. Wang and P. Derreumaux, Chem. Rev., 2015, 115, 3518–3563 CrossRef CAS PubMed
.
- J. L. Cummings, N. Engl. J. Med., 2004, 351, 56–67 CrossRef CAS PubMed
.
- K. S. Kosik, C. L. Joachim and D. J. Selkoe, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 4044–4048 CrossRef CAS PubMed
.
- G. G. Glenner and C. W. Wong, Biochem. Biophys. Res. Commun., 1984, 122, 1131–1135 CrossRef CAS PubMed
.
- D. A. Drachman and J. Leavitt, Arch. Neurol., 1974, 30, 113–121 CrossRef CAS
.
- P. Davies and A. J. F. Maloney, Lancet, 1976, 308, 1403 CrossRef
.
- M. D. Benson, J. N. Buxbaum, D. S. Eisenberg, G. Merlini, M. J. M. Saraiva, Y. Sekijima, J. D. Sipe and P. Westermark, Amyloid, 2018, 25, 215–219 CrossRef CAS PubMed
.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed
.
- J. Kang, H.-G. Lemaire, A. Unterbeck, J. M. Salbaum, C. L. Masters, K.-H. Grzeschik, G. Multhaup, K. Beyreuther and B. Muller-Hill, Nature, 1987, 325, 733–736 CrossRef CAS PubMed
.
- A. K. Dunker, M. M. Babu, E. Barbar, M. Blackledge, S. E. Bondos, Z. Dosztányi, H. J. Dyson, J. Forman-Kay, M. Fuxreiter, J. Gsponer, K.-H. Han, D. T. Jones, S. Longhi, S. J. Metallo, K. Nishikawa, R. Nussinov, Z. Obradovic, R. V. Pappu, B. Rost, P. Selenko, V. Subramaniam, J. L. Sussman, P. Tompa and V. N. Uversky, Intrinsically Disord. Proteins, 2013, 1, e24157 CrossRef PubMed
.
- M. C. Owen, D. Gnutt, M. Gao, S. K. T. S. Wärmländer, J. Jarvet, A. Gräslund, R. Winter, S. Ebbinghaus and B. Strodel, Chem. Soc. Rev., 2019, 48, 3946–3996 RSC
.
- J. Dobson, A. Kumar, L. F. Willis, R. Tuma, D. R. Higazi, R. Turner, D. C. Lowe, A. E. Ashcroft, S. E. Radford, N. Kapur and D. J. Brockwell, Proc. Natl. Acad. Sci. U.S.A., 2017, 114, 4673–4678 CrossRef CAS
.
- K. Beyreuther and C. L. Masters, Brain Pathol., 1991, 1, 241–251 CrossRef CAS PubMed
.
- J. Hardy and D. Allsop, Trends Pharmacol. Sci., 1991, 12, 383–388 CrossRef CAS PubMed
.
- D. J. Selkoe, Neuron, 1991, 6, 487–498 CrossRef CAS PubMed
.
- J. A. Hardy and G. A. Higgins, Science, 1992, 256, 184–185 CrossRef CAS PubMed
.
- F. Panza, M. Lozupone, G. Logroscino and B. P. Imbimbo, Nat. Rev. Neurol., 2019, 15, 73–88 CrossRef PubMed
.
- S. R. Chowdhury, F. Xie, J. Gu and L. Fu, Pharmaceutical Fronts, 2019, 01, e22–e32 CrossRef
.
- G. T. Heller, F. A. Aprile, T. C. T. Michaels, R. Limbocker, M. Perni, F. S. Ruggeri, B. Mannini, T. Löhr, M. Bonomi, C. Camilloni, A. De Simone, I. C. Felli, R. Pierattelli, T. P. J. Knowles, C. M. Dobson and M. Vendruscolo, Sci. Adv., 2020, 6, eabb5924 CrossRef CAS
.
- H. Minh Hung, M. T. Nguyen, P.-T. Tran, V. K. Truong, J. Chapman, L. H. Quynh Anh, P. Derreumaux, V. V. Vu and S. T. Ngo, J. Chem. Inf. Model., 2020, 1399–1408, DOI:10.1021/acs.jcim.9b01074
.
- T. C. T. Michaels, A. Šarić, J. Habchi, S. Chia, G. Meisl, M. Vendruscolo, C. M. Dobson and T. P. J. Knowles, Annu. Rev. Phys. Chem., 2018, 69, 273–298 CrossRef CAS PubMed
.
- S. L. Bernstein, N. F. Dupuis, N. D. Lazo, T. Wyttenbach, M. M. Condron, G. Bitan, D. B. Teplow, J.-E. Shea, B. T. Ruotolo, C. V. Robinson and M. T. Bowers, Nat. Chem., 2009, 1, 326–331 CrossRef CAS PubMed
.
- M. H. Viet, S. T. Ngo, N. S. Lam and M. S. Li, J. Phys. Chem. B, 2011, 115, 7433–7446 CrossRef CAS
.
- M. H. Viet and M. S. Li, J. Chem. Phys., 2012, 136, 245105 CrossRef
.
- S. T. Ngo, H. T. T. Phung, K. B. Vu and V. V. Vu, RSC Adv., 2018, 8, 41705–41712 RSC
.
- N. A. Alves and R. B. Frigori, J. Phys. Chem. B, 2018, 122, 1869–1875 CrossRef CAS PubMed
.
- Y. Lu, G. Wei and P. Derreumaux, J. Phys. Chem. B, 2011, 115, 1282–1288 CrossRef CAS PubMed
.
- P. H. Nguyen, J. M. Campanera, S. T. Ngo, A. Loquet and P. Derreumaux, J. Phys. Chem. B, 2019, 123, 6750–6756 CrossRef CAS PubMed
.
- A. J. Doig and P. Derreumaux, Curr. Opin. Struct. Biol., 2015, 30, 50–56 CrossRef CAS PubMed
.
- S. T. Ngo and M. S. Li, J. Phys. Chem. B, 2012, 116, 10165–10175 CrossRef CAS
.
- S. T. Ngo, D. T. Truong, N. M. Tam and M. T. Nguyen, J. Mol. Graphics Modell., 2017, 76, 1–10 CrossRef CAS PubMed
.
- C. Regitz, E. Fitzenberger, F. L. Mahn, L. M. Dussling and U. Wenzel, Eur. J. Nutr., 2016, 55, 741–747 CrossRef CAS PubMed
.
- A. Mullard, Nat. Rev. Drug Discovery, 2023, 22, 89 CrossRef CAS PubMed
.
- J. Couzin-Frankel, Science, 2023 DOI:10.1126/science.adj8110
.
- FDA_USA, Administration, Considering whether an FDA-Regulated Product Involves the Application of Nanotechnology FDA, https://clinicaltrials.gov/show/NCT01354444 Search PubMed.
- T. M. Joseph, D. K. Mahapatra, A. Esmaeili, Ł. Piszczyk, M. S. Hasanin, M. Kattali, J. Haponiuk and S. Thomas, Nanomaterials, 2023, 13, 574 CrossRef CAS PubMed
.
- U. Cendrowska, P. J. Silva, N. Ait-Bouziad, M. Müller, Z. P. Guven, S. Vieweg, A. Chiki, L. Radamaker, S. T. Kumar, M. Fändrich, F. Tavanti, M. C. Menziani, A. Alexander-Katz, F. Stellacci and H. A. Lashuel, Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 6866–6874 CrossRef CAS PubMed
.
- X.-F. Zhang, Z.-G. Liu, W. Shen and S. Gurunathan, Int. J. Mol. Sci., 2016, 17, 1534 CrossRef
.
- S. Sudhakar and E. Mani, Langmuir, 2019, 35, 6962–6970 CrossRef CAS
.
- A. Chakraborty, S. S. Mohapatra, S. Barik, I. Roy, B. Gupta and A. Biswas, Biosci. Rep., 2023, 43, BSR20220324 CrossRef CAS PubMed
.
- A. Garcia-Leis and S. Sanchez-Cortes, ACS Appl. Nano Mater., 2021, 4, 3565–3575 CrossRef CAS
.
- S. Tomaselli, V. Esposito, P. Vangone, N. A. van Nuland, A. M. Bonvin, R. Guerrini, T. Tancredi, P. A. Temussi and D. Picone, ChemBioChem, 2006, 7, 257–267 CrossRef CAS PubMed
.
- S. Cao, Z. Song, J. Rong, N. Andrikopoulos, X. Liang, Y. Wang, G. Peng, F. Ding and P. C. Ke, ACS Appl. Mater. Interfaces, 2023, 15, 40317–40329 CrossRef CAS
.
- S. Jo, T. Kim, V. G. Iyer and W. Im, J. Comput. Chem., 2008, 29, 1859–1865 CrossRef CAS PubMed
.
- M. Itoh, V. Kumar, T. Adschiri and Y. Kawazoe, J. Chem. Phys., 2009, 131, 174510 CrossRef PubMed
.
- H. Heinz, T.-J. Lin, R. K. Mishra and F. S. Emami, Langmuir, 2013, 29, 1754–1765 CrossRef CAS PubMed
.
- M. Khavani, A. Mehranfar and M. R. K. Mofrad, J. Chem. Inf. Model., 2023, 63, 1276–1292 CrossRef CAS PubMed
.
- J. Huang, S. Rauscher, G. Nawrocki, T. Ran, M. Feig, B. L. de Groot, H. Grubmüller and A. D. MacKerell, Nat. Methods, 2017, 14, 71–73 CrossRef CAS PubMed
.
- W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey and M. L. Klein, J. Chem. Phys., 1983, 79, 926–935 CrossRef CAS
.
- M. J. Abraham, T. Murtola, R. Schulz, S. Páll, J. C. Smith, B. Hess and E. Lindahl, SoftwareX, 2015, 1–2, 19–25 CrossRef
.
- G. Bussi, D. Donadio and M. Parrinello, J. Chem. Phys., 2007, 126, 014101 CrossRef PubMed
.
- M. Parrinello and A. Rahman, J. Appl. Phys., 1981, 52, 7182–7190 CrossRef CAS
.
- B. Hess, H. Bekker, H. J. C. Berendsen and J. G. E. M. Fraaije, J. Comput. Chem., 1997, 18, 1463–1472 CrossRef CAS
.
- Y. Sugita and Y. Okamoto, Chem. Phys. Lett., 1999, 314, 141–151 CrossRef CAS
.
- B. Strodel, J. W. L. Lee, C. S. Whittleston and D. J. Wales, J. Am. Chem. Soc., 2010, 132, 13300–13312 CrossRef CAS PubMed
.
- N. Miyashita, J. E. Straub and D. Thirumalai, J. Am. Chem. Soc., 2009, 131, 17843–17852 CrossRef CAS PubMed
.
- S. T. Ngo, P. H. Nguyen and P. Derreumaux, J. Phys. Chem. B, 2021, 125, 3105–3113 CrossRef CAS PubMed
.
- S. T. Ngo, M. T. Nguyen, N. T. Nguyen and V. V. Vu, J. Phys. Chem. B, 2017, 121, 8467–8474 CrossRef CAS PubMed
.
- A. Patriksson and D. van der Spoel, Phys. Chem. Chem. Phys., 2008, 10, 2073–2077 RSC
.
- Y. Mu, P. H. Nguyen and G. Stock, Proteins: Struct., Funct., Bioinf., 2005, 58, 45–52 CrossRef CAS PubMed
.
- E. Papaleo, P. Mereghetti, P. Fantucci, R. Grandori and L. De Gioia, J. Mol. Graphics Modell., 2009, 27, 889–899 CrossRef CAS PubMed
.
- A. Amadei, A. B. M. Linssen and H. J. C. Berendsen, Proteins: Struct., Funct., Genet., 1993, 17, 412–425 CrossRef CAS
.
- E. Papaleo, P. Mereghetti, P. Fantucci, R. Grandori and L. De Gioia, J. Mol. Graphics Modell., 2009, 27, 889–899 CrossRef CAS PubMed
.
- E. G. Marklund, M. T. Degiacomi, C. V. Robinson, A. J. Baldwin and J. L. P. Benesch, Structure, 2015, 23, 791–799 CrossRef CAS PubMed
.
- W. G. Touw, C. Baakman, J. Black, T. A. H. te Beek, E. Krieger, R. P. Joosten and G. Vriend, Nucleic Acids Res., 2015, 43, D364–D368 CrossRef CAS PubMed
.
- R. C. Bernardi, M. C. R. Melo and K. Schulten, Biochim. Biophys. Acta, Gen. Subj., 2015, 1850, 872–877 CrossRef CAS PubMed
.
- H. Nymeyer, J. Chem. Theory Comput., 2008, 4, 626–636 CrossRef CAS PubMed
.
- A. Liwo, C. Czaplewski, S. Ołdziej and H. A. Scheraga, Curr. Opin. Struct. Biol., 2008, 18, 134–139 CrossRef CAS PubMed
.
- R. P. Joosten, T. A. H. te Beek, E. Krieger, M. L. Hekkelman, R. W. W. Hooft, R. Schneider, C. Sander and G. Vriend, Nucleic Acids Res., 2011, 39, D411–D419 CrossRef CAS PubMed
.
- B. Barz and B. Urbanc, PLoS One, 2012, 7, e34345 CrossRef CAS PubMed
.
- M. H. Dehabadi and R. Firouzi, J. Mol. Graph. Model., 2022, 115, 108207 CrossRef CAS PubMed
.
- F. Tavanti, A. Pedone and M. C. Menziani, Int. J. Mol. Sci., 2021, 22, 26 CrossRef CAS PubMed
.
- T. Škrbić, A. Maritan, A. Giacometti and J. R. Banavar, Protein Sci., 2021, 30, 818–829 CrossRef PubMed
.
- S. Vivekanandan, J. R. Brender, S. Y. Lee and A. Ramamoorthy, Biochem. Biophys. Res. Commun., 2011, 411, 312–316 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2. Fig. S1 shows the superposition of computed metrics of the dimeric Aβ42 + Ag55 over the different intervals 260–500 and 380–500 ns of REMD simulations. Fig. S2 describes the superposition of computed metrics of the dimeric Aβ42 over the different intervals 260–500 and 380–500 ns of REMD simulations. See DOI: https://doi.org/10.1039/d4ra02197e |
| This journal is © The Royal Society of Chemistry 2024 |