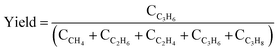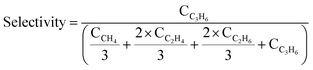Open Access Article
Open Access ArticlePropane dehydrogenation catalysis of group IIIB and IVB metal hydrides†
Xiaoming Hua,
Mengwen Huanga,
Tetsuya Kinjyob,
Shinya Mine c,
Takashi Toyao
c,
Takashi Toyao a,
Yoyo Hinuma
a,
Yoyo Hinuma d,
Masaaki Kitano
d,
Masaaki Kitano ef,
Toyoto Satog,
Norikazu Namiki
ef,
Toyoto Satog,
Norikazu Namiki b,
Ken-ichi Shimizu
b,
Ken-ichi Shimizu a and
Zen Maeno
a and
Zen Maeno *b
*b
aInstitute for Catalysis, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan
bSchool of Advanced Engineering, Kogakuin University, 2665-1, Nakano-cho, Hachioji, 192-0015, Japan. E-mail: zmaeno@cc.kogakuin.ac.jp
cNational Institute of Advanced Industrial Science and Technology (AIST), Research Institute for Chemical Process Technology, 4-2-1 Nigatake, Miyagino, Sendai 983-8551, Japan
dDepartment of Energy and Environment, National Institute of Advanced Industrial Science and Technology, 1-8-31, Midorigaoka, Ikeda 563-8577, Japan
eMDX Research Center for Element Strategy, International Research Frontiers Initiative, Tokyo Institute of Technology, Midori, Yokohama 226-8503, Japan
fAdvanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan
gDepartment of Engineering Science and Mechanics, College of Engineering, Shibaura Institute of Technology, Tokyo 135-8548, Japan
First published on 25th July 2024
Abstract
Catalytic propane dehydrogenation (PDH) has mainly been studied using metal- and metal oxide-based catalysts. Studies on dehydrogenation catalysis by metal hydrides, however, have rarely been reported. In this study, PDH reactions using group IIIB and IVB metal hydride catalysts were investigated under relatively low-temperature conditions of 450 °C. Lanthanum hydride exhibited the lowest activation energy for dehydrogenation and the highest propylene yield. Based on kinetics studies, a comparison between the reported calculation results and isotope experiments, the hydrogen vacancies of metal hydrides were involved in low-temperature PDH reactions.
1. Introduction
Propane dehydrogenation (PDH) is a simple method used to synthesize propylene, which is an important bulk chemical for the production of polymers and other fine chemicals. Owing to the increasing demand for propylene brought about by the shift in feedstock from naphtha to shale gas, the development of PDH catalysts has recently attracted significant attention from academia and industry.1–5 Commercially, PtSn alloys and CrOX catalyst systems have been used for PDH.6–9 Owing to the reaction's exothermic nature, the operating temperature was approximately 600 °C.2 Significant efforts have been made toward developing metal- (mainly Pt and its alloy nanoparticles) and metal oxide-based (Ga2O3, Al2O3, TiO2, ZrO2, etc.) catalyst systems for efficient PDH reactions.10–20 To develop new catalyst systems, the exploration of PDH catalysis by materials other than metals and metal oxides is of considerable interest.Catalytic applications of hydrogen-containing compounds have been a topic of interest in catalysis research.21–25 Several metal hydrides and hydrogen-containing mixed-anion compounds are effective catalysts and/or supports for the hydrogenation of CO2 and N2, with lattice hydrogen atoms and/or hydrogen vacancies playing an important role.26–34 However, their effectiveness in dehydrogenation has not been reported since early studies were conducted in the 1920s–1950s.35,36 Recently, our group reported that titanium hydrides exhibit a superior propylene formation rate compared to that achieved using titanium oxides at 450 °C.37 As a continuation of that study, we investigated the PDH reaction over group IIIB (Sc, Y, and La) and IVB (Ti, Zr, and Hf) metal hydrides, all of which showed higher activity compared to that exhibited by the corresponding metal oxides at 450 °C. Among the tested metal hydrides, lanthanum hydride afforded the highest propylene yield. Notably, the activation barrier for PDH using lanthanum hydride was 34.6 kJ mol−1, which is much lower than those using PtSn and CrOX catalysts. The involvement of hydrogen vacancies in metal hydrides in low-temperature PDH reactions is also discussed.
2. Experimental
2.1. Catalyst preparation
The group IIIB metal hydrides (ScH2, YH3, and LaH3) were prepared by reacting metal chips (purity: >99.9%, RARE METALLIC Co.,Ltd) in an H2 atmosphere (1.5 MPa) at various temperatures (400 °C for ScH2 and YH3, room temperature for LaH3) for 4 h. Other group IVB metal hydrides, ZrH2 (powder, Mitsuwa Chemical Co.,Ltd) and HfH2 (powder, <75 μm, purity: >99.5%, Goodfellow), were commercially obtained. The ball-milled metal hydrides were prepared using a Fritsch P-6 planetary ball mill. For example, LaH3 (1 g) was milled together in an ZrO2 pot (80 mL) with zirconia balls (ø5 mm, 100 g). The milling conditions were 200 rpm with a 1 min interval after every 6 min of milling for 1 h. In order to minimize the effect of oxidation on the experiment, the metal hydrides were handled in the glove box under an argon atmosphere. PtSn and CrOX catalysts were prepared according to impregnation methods (The details are described in ESI†).2.2. Catalytic reactions
Under an Ar atmosphere in a glove box, 100 mg of metal hydride was loaded into a U-shaped quartz reactor equipped with a 4-way valve. The reactor was removed, taking care to ensure no exposure to air, and placed in an electric furnace to conduct the PDH reaction (Fig. S1†). The propylene yield (YC3H6) was determined using a gas chromatograph equipped with a flame-ionizing detector (details are provided in ESI†). The compositions (Cn: n denotes the kind of gas) of methane, ethane, ethylene, propane, and propylene were determined on the basis of effective carbon numbers and GC areas. Note that other products with higher carbon number were not detected. The yield and selectivity of propylene were calculated from the gas compositions by the following equations.To investigate the temperature-dependency of formation rate, the PDH reaction was carried out under the following conditions: 100 mg of catalysts, 10 mL min−1 of 10% C3H8/He, 430–460 °C for metal hydrides, 410–440 °C for PtSn/SiO2 and CrOX–Al2O3. The average propylene formation rate at each temperature was used for data analysis. For the comparison among a series of metal hydrides, the propylene formation rate is calculated based on each mole of catalyst, while for the comparison of LaH3 with PtSn and CrOX catalysts, the propylene formation rate is calculated based on each gram of catalyst. For the effect of partial propane pressure, the reaction was performed under the following conditions: 100 mg of metal hydrides, 50 mL min−1 of 2–8% C3H8/He, 450 °C. For the effect of partial H2 pressure, the reaction was performed under the following conditions: 100 mg of metal hydrides, 50 mL min−1 of 2–8% H2 + 10% C3H8/He, 450 °C.
2.3. Temperature programmed desorption (TPD)
The TPD experiment was performed using 10 mg of as-prepared LaH3 under He flow. The signal of hydrogen (m/z = 2) was observed by a quadrupole mass spectrometer (BELMASS, MicrotracBEL). After the signal for m/z = 2 was stable, the temperature was increased from 50 °C to 800 °C with ramping rate of 5 °C min−1, then be kept for an hour.2.4. Isotope experiment
100 mg of as-prepared LaH3 or LaD3 were used for PDH with monitoring the generated gases including H2, HD (m/z = 3), and D2 (m/z = 4), by BELMASS. LaD3 was prepared from the La chip and D2 in the similar way described above. To monitor the generated D2, Ar was used as a carrier gas instead of He. The temperature was increased from room temperature to 450 °C in 20 minutes under Ar flow. After the signals for m/z = 2, 3, and 4 were stable, 10% C3H8/Ar was introduced at 450 °C to investigate PDH reactions.3. Results and discussion
The PDH reactions using metal hydrides were carried out at 450 °C using a flow-type reactor under atmospheric pressure. The initial catalytic activities at 20 min were compared in Table 1. The group IIIB metal hydrides, such as ScH2, YH3, and LaH3, exhibited higher YC3H6 values (0.3–0.5%) than group IVB metal hydrides such as TiH2, ZrH2, and HfH2 (0.1–0.2%). To increase YC3H6, a series of metal hydrides were ball milled (BM) to increase surface area. The specific surface area values, as determined by N2 adsorption, increased from <1.0 to 4.2–12.5 m2 g−1, resulting in improved YC3H6 values from 0.1–0.5% to 1.2–11.0%, with LaH3_BM presenting the highest YC3H6 (Fig. 1). The propylene formation rate normalized specific surface area of ball-milled LaH3 was also highest. The ball-milled LaH3, TiH2, ZrH2, and HfH2 showed relatively good propylene selectivity (SC3H6) (81.8–92.7%), whereas the ball-milled ScH2 and YH3 showed moderate to low SC3H6 of 36.6% and 53.2%, respectively. The main byproduct was methane regardless of different metal hydrides. The possible reason for decrease of SC3H6 is the cracking reaction of propane with hydrides derived from metal hydride catalysts. To discuss this possibility, we investigated H2 TPD experiments for ScH2 (as-prepared) and ScH2_BM because the biggest decrease of SC3H6 from 99.9% to 36.6% was observed. The H2 desorption occurred above 500 °C for as-prepared ScH2 (Fig. S2†). In contrast, the main desorption peaks shifted to lower temperature region (below 500 °C), supporting the above hypothesis. The favorable SC3H6 of LaH3_BM was maintained by extending the reaction time from 20 to 120 min, although the YC3H6 unfortunately decreased (Fig. S3 and S4†). PDH catalysis using group IIIB and IVB metal oxides was investigated under similar reaction conditions because some of them, such as TiO2 and ZrO2, have been reported to exhibit considerable activity for alkane dehydrogenation.15,16 Each metal oxide demonstrated a lower YC3H6 and propylene formation rate (normalized based on the specific surface area value) than those of the corresponding metal hydrides, proving that group IIIB and IVB metal hydrides have the potential to be effective catalysts for low-temperature PDH reactions.| Catalyst | Conv.a (%) | SC3H6a (%) | YC3H6a (%) |
|---|---|---|---|
| a Reaction conditions: 100 mg of catalyst, 10 mL min−1 of 10% C3H8/He, and 450 °C. Details of determination are described in Experimental section. | |||
| ScH2 | 0.5 | >99.9 | 0.5 |
| ScH2_BM | 8.3 | 36.6 | 3.1 |
| Sc2O3 | 0.1 | 17.6 | <0.1 |
| YH3 | 0.5 | 60.4 | 0.3 |
| YH3_BM | 4.5 | 53.2 | 2.4 |
| Y2O3 | <0.1 | 58.7 | <0.1 |
| LaH3 | 0.6 | 88.8 | 0.5 |
| LaH3_BM | 11.8 | 92.7 | 11.0 |
| La2O3 | 0.4 | 34.8 | 0.1 |
| TiH2 | 0.4 | 52.6 | 0.2 |
| TiH2_BM | 1.5 | 82.6 | 1.2 |
| TiO2_Anatase | 0.5 | 73.0 | 0.4 |
| TiO2_Rutile | 0.7 | 96.1 | 0.7 |
| ZrH2 | 0.2 | 90.5 | 0.2 |
| ZrH2_BM | 1.9 | 86.6 | 1.7 |
| ZrO2 | 0.4 | 47.7 | 0.2 |
| HfH2 | 0.2 | 82.9 | 0.2 |
| HfH2_BM | 2.5 | 81.8 | 2.0 |
| HfO2 | 0.0 | 56.2 | 0.0 |
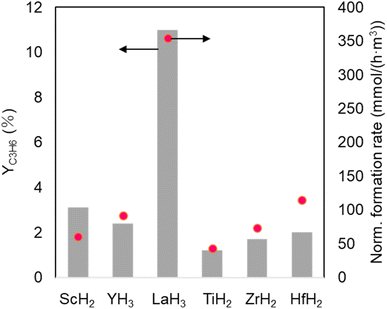 | ||
| Fig. 1 YC3H6 and normalized formation rate based on surface area in PDH using group IIIB and IVB metal hydrides (ball-milled for 1 h) at 450 °C. | ||
Our previous study on PDH over TiH2 revealed that partially dehydrogenated titanium hydrides are more active than Ti metal.37 In this study, LaH3 was pre-treated under He flow at different temperatures to promote the desorption of lattice hydrogen and was then applied to the PDH reaction at 450 °C. The He treatments at 450 and 600 °C did not significantly affect the reaction, and thus similar YC3H6 values were obtained (0.5%, Fig. 2a). When the pre-treatment temperature was increased to 700 and 800 °C, YC3H6 decreased to 0.3% and <0.1%, respectively. In the TPD of LaH3, a small desorption peak at 300–500 °C and a larger peak at 700–800 °C were observed (Fig. 2b). The loss of activity by high temperature pretreatment is possibly deep dehydrogenation of LaH3 to La metal. To discuss this consideration, we conducted H2 TPD of LaH3 up to 800 °C to completely release hydrides and then successively investigated H2 temperature programmed reduction (TPR) to study the H2 absorption property. The main H2 absorption peak was observed from 300–400 °C (Fig. S5†). The H2-treated sample showed the intermediate YC3H6 value (0.3%), which was lower than that of fresh LaH3 (0.5%) and higher than Ar-treated one (<0.1%), supporting the above consideration.
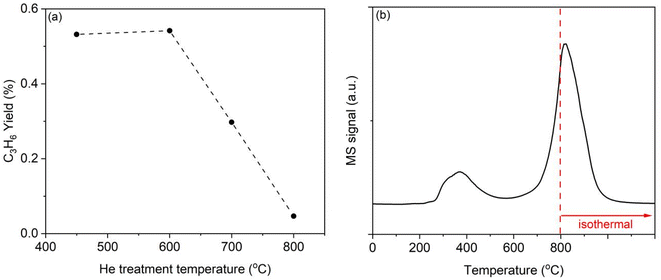
The XRD measurements of LaH3 before and after PDH at 450 °C showed that the diffraction peaks shifted toward lower angles after the reaction (Fig. S6†). A decrease in hydrogen content induces lattice expansion in the lanthanum hydride system.38 To study the change of lattice constant in detail, the synthesized LaH3 was treated under argon or propane (1 atm) atmosphere at 450 °C in a batch reactor, and then the treated samples were characterized by XRD with using Si powder as an internal standard (Fig. 3a and b). Using indexing program TREOR97 and least-squares refinement of lattice constant program PIRUM (see ESI†), observed Bragg peaks of as-prepared sample were indexed by a pseudo-cubic unit cell with a = 5.6119(11) Å, which corresponds to H/La = 2.77 as estimated by comparison with calculated lattice constant of LaH3 and LaH2 (Fig. 3c), showing the formation of relatively highly-hydrogenated lanthanum hydrides. The treatment under argon (as an inert gas) induced the increase of lattice constant to 5.6188(8) Å (the pseudo-cubic unit cell), and the propane treatment resulted in the further slightly increase to 5.6246(4) Å (the pseudo-cubic unit cell). The estimated H/La values were 2.61 and 2.49, respectively. These results show that the formation of hydrogen vacancy mainly occurred by thermal treatment and indicate that the incorporation of hydrogen derived from propane into the generated vacancy is unlikely to occur during PDH.
We also investigated the PDH using LaH3 under higher space velocity conditions (100 mL min−1 of 10% C3H8/Ar). The detailed results including the carbon balance value were shown in Table S1.† Although the initial conversion was relatively high (15.4%), the SC3H6 value was quite low owing to cracking reaction and the carbon balance was moderate (60%), resulting in a low YC3H6. The conversion decreased to 2–3% whereas was good SC3H6 and carbon balance values was maintained. The used catalyst was treated with H2 at 300 °C to try to regenerate the initial activity. Unfortunately, the initial activity was not recovered (Table S2†), indicating that deep dehydrogenation is unlikely to cause the deactivation. The specific surface area of the used ball-milled LaH3 was 6.5 m2 g−1, which was similar to the original one (7.5 m2 g−1), indicating that the sintering was unlikely to occur. Because the carbon balance value at initial stage was relatively low in PDH using fresh LaH3, the coke formation is more plausible reason for deactivation.
The temperature dependence of the propylene formation rate using different metal hydrides was plotted, and the apparent activation energies (Ea) were compared. TiH2 was excluded because the Ea value is too dependent on H2 co-feeding due to the interconversion between titanium hydride and Ti metal.37 The Ea values for the group IIB metal hydrides were lower than those for the group IVB metal hydrides (Fig. 4a). LaH3 exhibited the lowest Ea (34.6 kJ mol−1), whereas the highest Ea (147.5 kJ mol−1) was observed for HfH2. Notably, under low-temperature conditions, the Ea for LaH3 is lower than those for PtSn- and CrOX-based catalysts (74.0 and 94.0 kJ mol−1, respectively), as shown in Fig. 4b. In addition, industrially used K–PtSn/Al2O3 was prepared according to the previous report and investigated kinetic study. The Ea value (117 kJ mol−1) was higher than that for LaH3 (Fig. S7†), indicating the high catalytic potential of LaH3.
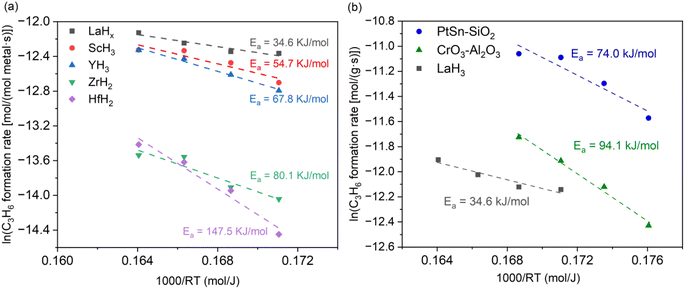 | ||
| Fig. 4 Temperature dependence of propylene formation rate in PDH using (a) a series of metal hydrides and (b) PtSn- and CrOX-based catalysts. | ||
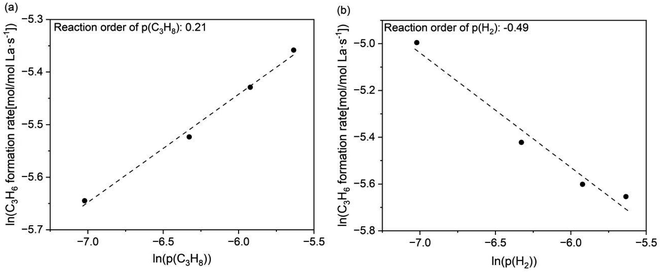
In previous studies on PDH over group IVB metal oxides, such as TiO2 and ZrO2, oxygen vacancies generated by H2 or CO pre-treatment were involved in C–H bond cleavage, promoting PDH.15,16 In this study, we discuss the possible involvement of hydrogen vacancies during PDH based on partial pressure changes and the relationship between activity and the formation energy of surface hydrogen vacancies. The effects of the partial pressures of propane and H2 (p(C3H8) and p(H2)) on PDH using LaH3 were investigated. The reaction order of p(H2) was determined to be approximately −0.5, whereas the reaction order of p(C3H8) was relatively low and positive (approx. 0.2) (Fig. 5). Similar partial pressure effects were observed in the PDH using another metal hydride (Fig. S8†). Interestingly, the PtSn-based catalyst system exhibited a zero-order dependency on p(H2).39,40 It can be considered that an increase in p(H2) suppresses the formation of hydrogen vacancies, resulting in a decreased propylene formation rate. The initial YC3H6 values are plotted as a function of theoretical formation energies of the surface hydrogen vacancies (EHvac).41 As EHvac decreased, the initial YC3H6 increased, and the lanthanum hydrides possessing the lowest EHvac exhibited the highest YC3H6 (Fig. 6). These results imply that hydrogen vacancies in metal hydrides are involved in the PDH reaction.
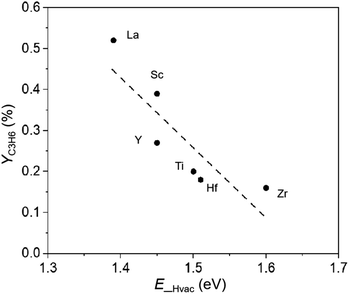 | ||
| Fig. 6 Correlation of initial YC3H6 with theoretical formation energy of surface hydrogen vacancies (E_Hvac) for group IIIB and IVB metal hydrides.41 | ||
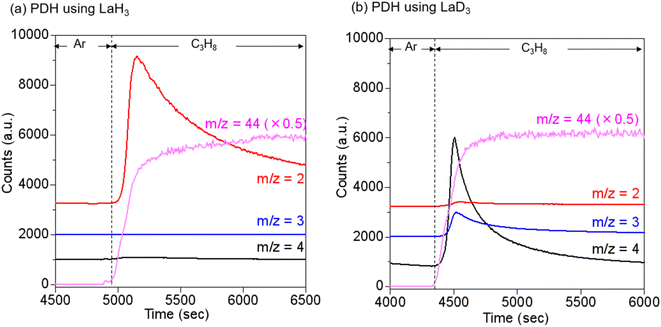
To further investigate the involvement of hydrogen vacancies, LaD3 was prepared from a lanthanum chip and D2 gas for testing in the PDH reaction. The generated gas products, including H2, HD, and D2, were monitored using mass spectrometry (m/z = 2, 3, and 4, respectively) and then compared with the PDH reactions using LaH3. During the pre-treatment of LaH3 under Ar flow, H2 desorption occurred as the temperature was increased from room temperature to 450 °C (Fig. S9†), indicating the generation of hydrogen vacancies under PDH reaction conditions. After the introduction of propane at 450 °C, H2 was formed, and D2 and HD were barely detected (Fig. 7a). When LaD3 was used instead of LaH3, D2 desorption was observed during a similar pre-treatment under Ar flow (Fig. S9†). In the PDH reaction, D2 and HD were simultaneously formed, and the signal intensity for H2 was much lower than that observed when using LaH3 (Fig. 7b). In a separate experiment, LaD3 exhibited PDH activity, in which the time course of YC3H6 was similar to that obtained when using LaH3 (Fig. S10†). These results indicate that the H atoms of C3H8 are incorporated into the hydrogen vacancies of LaD3 and then exchanged/combined with lattice D atoms, resulting in the formation of D2/HD as co-products. To determine whether an exchange reaction occurred between LaD3 and the gas-phase H2, the H–D exchange reaction of LaD3 with H2 was also conducted at 450 °C. The peak for D2 immediately appeared after Ar pre-treatment and the introduction of H2, while the signals for HD and H2 were hardly detected directly after the introduction of H2. With a decreasing signal for D2, a peak for HD formation was observed, and the signal intensity for H2 increased (Fig. S11†). This observation was different from that of the PDH reaction, suggesting that the exchange reaction of LaD3 with H2 was not a plausible formation pathway for D2 or HD. These isotope experiments provide further indicate for the involvement of the hydrogen vacancies in LaH3 in PDH reactions.
4. Conclusions
In conclusion, the low-temperature activities of group IIIB and IVB metal hydrides in PDH reactions were investigated. All the metal hydrides exhibited higher activity than the corresponding metal oxides. Among the tested metal hydrides, LaH3 was the most active and reacted through the lowest activation barrier, which was significantly lower than those observed for PtSn- and CrOX-based catalyst systems. Kinetics studies and isotope experiments indicated that hydrogen vacancies are possibly involved in low-temperature PDH using metal hydrides. Although the durability and reusability of La hydrides are relatively low as PDH catalysts, our findings demonstrate the potential of bulk metal hydrides to activate light alkanes under non-oxidative conditions.Data availability
The data that support the findings of this study are available from the corresponding author, Z. M., upon reasonable request.Author contributions
Z. M. conceived the idea of study and supervised the conduct of this study. X. H., M. H., and S. M. performed the experiments for catalyst preparation and catalytic reactions. Y. H. analyzed the experimental data based on computational study. M. K. synthesized a part of metal hydrides. T. K. and T. S. conducted the XRD measurements and details analysis. X. M. also wrote the draft, and T. T., N. N. K. S., and Z. M. critically reviewed it. All authors approved the final version of the manuscript to be published.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This study was financially supported by KAKENHI (Grant No. JP20H02518, JP20H02775, JP20KK0111, and JP21H04626) from the Japan Society for the Promotion of Science (JSPS) and KAKENHI on Innovative Areas “Hydrogenomics” (No. JP21H00019 and JP21H00012). This study was also supported by the JST-CREST project JPMJCR17J3, JST-SPRING project JPMJSP2119, and Joint Usage/Research Center for Catalysis.References
- S. Chen, X. Chang, G. Sun, T. Zhang, Y. Xu, Y. Wang, C. Pei and J. Gong, Chem. Soc. Rev., 2021, 50, 3315–3354 RSC.
- J. J. H. B. Sattler, J. Ruiz-Martinez, E. Santillan-Jimenez and B. M. Weckhuysen, Chem. Rev., 2014, 114, 10613–10653 CrossRef CAS PubMed.
- O. O. James, S. Mandal, N. Alele, B. Chowdhury and S. Maity, Fuel Process. Technol., 2016, 149, 239–255 CrossRef CAS.
- M. Artetxe, G. Lopez, M. Amutio, G. Elordi, J. Bilbao and M. Olazar, Chem. Eng. J., 2012, 207–208, 27–34 CrossRef CAS.
- A. Corma, F. V. Melo, L. Sauvanaud and F. Ortega, Catal. Today, 2005, 107–108, 699–706 CrossRef CAS.
- J. Wang, Y. H. Song, Z. T. Liu and Z. W. Liu, Appl. Catal., B, 2021, 297, 120400 CrossRef CAS.
- L. Liu, M. Lopez-Haro, C. W. Lopes, S. Rojas-Buzo, P. Concepcion, R. Manzorro, L. Simonelli, A. Sattler, P. Serna, J. J. Calvino and A. Corma, Nat. Catal., 2020, 3, 628–638 CrossRef CAS.
- Z. P. Hu, Y. Wang, D. Yang and Z. Y. Yuan, J. Energy Chem., 2020, 47, 225–233 CrossRef.
- Y. Shan, Z. Sui, Y. Zhu, D. Chen and X. Zhou, Chem. Eng. J., 2015, 278, 240–248 CrossRef CAS.
- B. Zheng, W. Hua, Y. Yue and Z. Gao, J. Catal., 2005, 232, 143–151 CrossRef CAS.
- Y. Xie, W. Hua, Y. Yue and Z. Gao, Chin. J. Chem., 2010, 28, 1559–1564 CrossRef CAS.
- T. P. Otroshchenko, V. A. Kondratenko, U. Rodemerck, D. Linke and E. V. Kondratenko, Catal. Sci. Technol., 2017, 7, 4499–4510 RSC.
- D. Shee and A. Sayari, Appl. Catal., A, 2010, 389, 155–164 CrossRef CAS.
- E. V. Kondratenko, J. Catal., 2017, 348, 282–290 CrossRef.
- Y. Zhang, Y. Zhao, T. Otroshchenko, H. Lund, M. M. Pohl, U. Rodemerck, D. Linke, H. Jiao, G. Jiang and E. V. Kondratenko, Nat. Commun., 2018, 9, 3794 CrossRef PubMed.
- C. F. Li, X. Guo, Q. H. Shang, X. Yan, C. Ren, W. Z. Lang and Y. J. Guo, Ind. Eng. Chem. Res., 2020, 59, 4377–4387 CrossRef CAS.
- Y. Nakaya and S. Furukawa, ChemPlusChem, 2022, 87, e202100560 CrossRef CAS PubMed.
- L. Rochlitz, Q. Pessemesse, J. W. A. Fischer, D. Klose, A. H. Clark, M. Plodinec, G. Jeschke, P. A. Payard and C. Copéret, J. Am. Chem. Soc., 2022, 144, 13384–13393 CrossRef CAS PubMed.
- M. D. Marcinkowski, M. T. Darby, J. Liu, J. M. Wimble, F. R. Lucci, S. Lee, A. Michaelides, M. Flytzani-Stephanopoulos, M. Stamatakis and E. C. H. Sykes, Nat. Chem., 2018, 10, 325–332 CrossRef CAS PubMed.
- H. Zhang, Y. Jiang, G. Wang, N. Tang, X. Zhu, C. Li and H. Shan, Mol. Catal., 2022, 519, 112143 CrossRef CAS.
- H. Hosono and M. Kitano, Chem. Rev., 2021, 121, 3121–3185 CrossRef CAS PubMed.
- H. Nishino, T. Fujita, N. T. Cuong, S. Tominaka, M. Miyauchi, S. Iimura, A. Hirata, N. Umezawa, S. Okada, E. Nishibori, A. Fujino, T. Fujimori, S. I. Ito, J. Nakamura, H. Hosono and T. Kondo, J. Am. Chem. Soc., 2017, 139, 13761–13769 CrossRef CAS PubMed.
- K. Hayashi, P. V. Sushko, Y. Hashimoto, A. L. Shluger and H. Hosono, Nat. Commun., 2014, 5, 3515 CrossRef PubMed.
- K. Fukutani, J. Yoshinobu, M. Yamauchi, T. Shima and S. Orimo, Catal. Lett., 2022, 152, 1583–1597 CrossRef CAS.
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Nat. Commun., 2018, 9, 772 CrossRef PubMed.
- Y. Tsuji, K. Okazawa, Y. Kobayashi, H. Kageyama and K. Yoshizawa, J. Phys. Chem. C, 2021, 125, 3948–3960 CrossRef CAS.
- S. Kato, S. K. Matam, P. Kerger, L. Bernard, C. Battaglia, D. Vogel, M. Rohwerder and A. Züttel, Angew. Chem., Int. Ed., 2016, 55, 6028–6032 CrossRef CAS PubMed.
- Y. Cao, A. Saito, Y. Kobayashi, H. Ubukata, Y. Tang and H. Kageyama, ChemCatChem, 2021, 13, 191–195 CrossRef CAS.
- M. Miyazaki, K. Ogasawara, T. Nakao, M. Sasase, M. Kitano and H. Hosono, J. Am. Chem. Soc., 2022, 144, 6453–6464 CrossRef CAS PubMed.
- Y. Kobayashi, Y. Tang, T. Kageyama, H. Yamashita, N. Masuda, S. Hosokawa and H. Kageyama, J. Am. Chem. Soc., 2017, 139, 18240–18246 CrossRef CAS PubMed.
- M. Kitano, J. Kujirai, K. Ogasawara, S. Matsuishi, T. Tada, H. Abe, Y. Niwa and H. Hosono, J. Am. Chem. Soc., 2019, 141, 20344–20353 CrossRef CAS PubMed.
- P. Wang, F. Chang, W. Gao, J. Guo, G. Wu, T. He and P. Chen, Nat. Chem., 2017, 9, 64–70 CrossRef CAS PubMed.
- M. Hattori, S. Iijima, T. Nakao, H. Hosono and M. Hara, Nat. Commun., 2020, 11, 2001 CrossRef CAS PubMed.
- C. Färber, P. Stegner, U. Zenneck, C. Knüpfer, G. Bendt, S. Schulz and S. Harder, Nat. Commun., 2022, 13, 3210 CrossRef PubMed.
- L. Wright and S. Weller, J. Am. Chem. Soc., 1954, 76, 5305–5308 CrossRef CAS.
- V. V. Lunin, G. V. Lisichkin, Y. V. Vlasenko and A. E. Agronomov, Zh. Fiz. Khim., 1974, 48, 2465 CAS.
- S. Yasumura, Y. Wen, T. Toyao, Y. Kanda, K. Shimizu and Z. Maeno, Chem. Lett., 2022, 51, 88–90 CrossRef CAS.
- P. Klavins, R. N. Shelton, R. G. Barnes and B. J. Beaudry, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 29, 5349 CrossRef CAS.
- S. Saerens, M. K. Sabbe, V. V. Galvita, E. A. Redekop, M. F. Reyniers and G. B. Marin, ACS Catal., 2017, 7, 7495–7508 CrossRef CAS.
- G. di Wang, J. W. Jiang, Z. J. Sui, Y. A. Zhu and X. G. Zhou, ACS Omega, 2022, 7, 30773–30781 CrossRef PubMed.
- Y. Hinuma, S. Mine, T. Toyao, Z. Maeno and K. Shimizu, Phys. Chem. Chem. Phys., 2021, 23, 16577–16593 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02473g |
| This journal is © The Royal Society of Chemistry 2024 |