Open Access Article
Open Access ArticleA ternary system of meloxicam with matching hydrophilic polymer and cyclodextrin for improved stability in liquid preprations
Si Lia,
Longfa Kouac,
Yimeng Qina,
Yimeng Wangb,
Yinghua Sun *b,
Zhonggui Heb and
Xiaohong Liu*a
*b,
Zhonggui Heb and
Xiaohong Liu*a
aSchool of Pharmacy, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang 110016, China
bWuya College of Innovation, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang 110016, China. E-mail: sunyinghua77@aliyun.com; Fax: +86-24-23986321; Tel: +86-24-23986321
cDepartment of Pharmacy, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325035, China
First published on 5th July 2024
Abstract
The purpose of the study is to investigate the effect of ternary systems consisting of meloxicam with cyclodextrins (HP-β-CD or SBE-β-CD) and different polymers (HA, HPMC and PVP) on the stability of meloxicam. The t0.9 values of meloxicam were determined within all the aforementioned systems and the influence of various polymers on the alteration in meloxicam's stability was evaluated. All three polymers altered the stability of meloxicam to varying degrees, with the extent of the effect being related to hydrophilicity, concentration of components, and the interaction of the newly formed ternary system. Among them, meloxicam demonstrated its highest degree of stabilization within the ternary system formed by SBE-β-CD&HPMC and HP-β-CD&HA. We characterized the ternary system of meloxicam using differential scanning calorimetry (DSC), X-ray diffraction, and scanning electron microscopy analysis, which determined the presence of ternary system inclusions. In addition, we investigated the optimized prescription of eye drops of meloxicam using the ternary system and further determined that the ternary system improved the stability of the drug in liquid formulations.
1. Introduction
Meloxicam (MLX) has been mainly used to treat rheumatoid arthritis and ankylosing spondylitis,1 in recent years, and ophthalmic formulations of meloxicam have also been reported. For example, Zhang et al.2 designed bovine serum protein–meloxicam aggregates to treat post-cataract endophthalmitis; Hebatallah et al.3 investigated the therapeutic effects of chitosan nanoparticles of meloxicam in a rabbit eye model. Meloxicam is almost insoluble in water and has poor stability in liquid formulations,4 therefore, in previous studies, researchers conducted different methods to prepare the formulations. For example, Zhang2 prepared bovine serum protein–meloxicam aggregates using an acid–base neutralization method, and Hebatallah3 prepared chitosan nanoparticles loaded with meloxicam (MLX-CS-NPs) by electrostatic interactions between cationic chitosan and anionic MLX using polyethylene glycol 400 or trimeric phosphate solutions as solvents.Complexation solubility methods involve specific host–guest interactions, and complexation can result in faster and higher drug solubilization than traditional co-solvent, pH adjustment, and emulsion solubilization methods.5 Pillar[n]arenes can be linked to guest molecules through the special structure of methylene bridges, and functionalize with different functional hydrophilic groups, thereby delivering anticancer drugs in a targeted manner.6 Cucurbit[n]urils have a pumpkin-shaped structure and can be used to complex methylene groups with guest molecules to delivery of chemotherapy drugs.7 Calixarenes are cyclic oligomers, and the flexibility of the ring and the further modification of their basic core and edges make them ideal for use as a drug and drug carrier.8 Cyclodextrins (CDs) are cyclic oligosaccharides composed of D-glucopyranoside units linked by glycosidic bonds. Their main property is the ability to modify the physicochemical and biological characteristics of low-soluble drugs through the formation of drug-CD inclusion complexes.9 In addition, complexation solubility methods can have the advantage of reducing irritation and masking undesirable odors. It has been reported10 that drug solubility in aqueous media can be improved by complexation with cyclodextrins (CD), because of hydrophobic cavities of CD. The drug, as a guest molecule, can enter the cavities of the main CD molecule through non-covalent interactions, thus achieving new physicochemical and biological properties of the drug without changing its properties. And because of the different toxic and hemolytic properties of cyclodextrin derivatives, the use of hydroxypropyl-β-CD (HP-β-CD) and sulfobutyl ether-β-CD (SBE-β-CD) can effectively reduce the formation of intermolecular hydrogen bonds, improve solubility and stability, and eliminate systemic toxicity.11 If CD has a low complexation efficiency to the drug, the use of low doses of CD may not result in the required increase in solubility, so this is when the introduction of ternary reagents such as polymers is needed to increase the efficiency of CD and increase the solubility and stability of the drug in water-soluble media by first forming polymeric materials between water-soluble polymers and CD, and then encapsulating the drug.12
In previous studies, the solubilizing ability of the ternary system was reported,13 while the assessment of its stabilizing ability was neglected. In this study, we employed aqueous stirring method to prepare eye drop formulations by establishing a ternary system involving MLX, cyclodextrin, and water-soluble polymers, and investigated the stability of MLX in the presence or absence of three prevalent hydrophilic polymers: polyvinyl pyrrolidone (PVP), hydroxypropyl methyl cellulose (HPMC) and hyaluronic acid (HA). This study extensively examined the potential impact of the hydrophilic polymer, serving as the third component within the ternary system, on the drug stability.
2. Materials and methods
2.1 Materials
MLX was purchased from Ningxia Kangya Pharmaceutical Co. Ltd China. HP-β-CD and SBE-β-CD were kindly provided by Shandong Xinda Chemical Co. Ltd China. PVP k30, HPMC E15 and HA were purchased from Tianjin Bodi Chemical Co. Ltd China, Huzhou Zhanwang Co. Ltd China, Shandong Furuida Biological Chemical Co. Ltd China, respectively. All other reagents and solvents were of analytical-grade quality.2.2 Instrumentation
High Performance Liquid Chromatography (HPLC) measurements were carried out using an Hitachi chromaster at 365 nm, equipped with 5410 UV Detector, a 5110 pump, an auto-sampler 5210, a column oven 5310.2.3 Estimation of MLX
A content determination method of MLX was established on a HPLC (Hitachi, pump-1-2130, UV-VIS Detector L-2420, D-2000 Elite, Japan). The content of meloxicam in different cases was determined to characterize its stability. A C18 column (250 mm × 4.6 mm, 5 μm) was adopted. The mobile phase was 42 volume of a mixture of 87 volume of methanol and 13 volume of isopropyl alcohol, and 58 volume of 0.2% w/v solution of diammonium phosphate adjust to pH 7.5. The flow rate was 1.0 mL min−1. The column temperature was maintained at 40 °C. The wavelength was set at 360 nm. This determination method was validated for linearity, accuracy, precision and interference.2.4 The degradation kinetics of MLX in aqueous solutions
In order to determine the rate constant, the reaction order must be determined at first. The prescribed amount of MLX was added into 100 mL 90 °C water, stirred until it was dissolved completely, filtered through 0.22 μm filter, dispensed into ampoules. It was placed in 100 °C constant temperature water path (DZKW, Beijing Yongguangming Medical instrument factory). Sampling and immediate testing were performed at special time points of 0, 0.5, 1, 1.5, 2, 3, 4, 5 days. The results were calculated by linear regression to determine the order of reaction.2.5 Inclusion complex preparation
Binary and ternary complexes were obtained by aqueous stirring mixing method. Different weight ratios of MLX and HP-β-CD were dispersed in pre-heated PBS solution (50 °C), then they were stirred until fully dissolved. Subsequently, the binary inclusion complex solution was obtained through filtration using a 0.22 μm filter. As for the ternary system, hydrophilic polymer solution was mixed with the binary inclusion complex and incubated at 50 °C for 2 h to get uniform liquid system. Physical mixtures (PM) of binary and ternary systems were prepared in the same weight ratio.2.6 Differential scanning calorimeter (DSC)
The DSC curves were obtained through conventional differential scanning calorimetry (DSD-60100VAC, Shimadzu Corporation Japan). Test conditions were as follows: detection cell: A1; reference material: Al2O3; ambient gas: nitrogen; initial temperature: 30 °C; heating rate: 10.00 (°C min−1); control temperature: 310 °C.2.7 X-ray diffraction (XRD)
The complexes crystal structures were obtained through X-ray Diffraction (Smart Lab 3 kW, Rigaku, Japan). Test conditions were scanning area of 5–50° and scanning rate of 10° min−1.2.8 Scanning electron microscope analysis (SEM)
The crystal structures were obtained through Scanning electron microscope analysis (JSM-7800F, JEOL, Japan). Test conditions were magnification 1000 times.2.9 Constant temperature accelerated tests
Stability difficulties arise more frequently in liquid pharmaceutical preparations than in solid dosage forms. Currently, there are a lot of for predicting drug stability, but the basic approach continues to rely on constant temperature accelerated tests.14 For most of the reactions, an elevation in temperature is concomitant with an increase in the reaction rate.The Arrhenius equation gives “the dependence of the rate constant k of chemical reactions on the temperature T (in Kelvin) and activation energy Ea”, as shown below:
| k = Ae−Ea/RT |
According to the corresponding formula, then we calculated the time of decomposition drug by 10% (t0.9).15
The impact of the binary and ternary systems on the stability of MLX solution was studied by using constant temperature accelerated tests. All samples, including MLX solution, MLX binary system and MLX ternary system were sealed into ampoule bottles and placed in water baths with constant temperature set at 90 °C, 80 °C, and 70 °C. Samples in 90 °C water bath were measured on day 0, day 1, day 2, day 3, day 4, while at temperatures of 80 °C and 70 °C, the samples were measured at intervals of 0, 1, 2, 3, 4, 6, 8 days. An Arrhenius plot was adopted to analyze the influence of temperature on the rate of degradation. Then the t0.9 at room temperature was calculated according to the obtained slope under various temperature rate constant (k).
2.10 Preparation and optimization of the MLX eye drops
The prescribed amount of NaH2PO4, Na2HPO4 and cyclodextrins were added to 50 mL distilled water and heated to 50 °C until completely dissolved, and then the MLX was added with continues stirring. Following this, the other additives (ethylparapen, sodium chloride) were dissolved in the solution. Finally, the solution was mixed with 50 mL HPMC, filtrated through 0.22 μm microporous membrane and embedded in the eye drops bottles. An orthogonal experimental design was introduced to optimize the formulation of the eye drops formulation.2.11 Short-term stability tests for MLX eye drops
The stability stress testing for MLX eye drops was carried out at the environment of light (4500 ± 500 Lx) and high temperature (40 °C and 60 °C) for 10 days. The samples were packaged as commercial products and placed in the stress testing conditions. Samples were collected at the time points of 0, 5, and 10 days, and an evaluation was conducted to assess changes in the appearance, content, purity, and pH value of the MLX eye drop formulation.2.12 Statistical analysis
Data were expressed as mean ± standard deviation. Student's t-test and two-way ANOVA were used for statistical analysis using the software GraphPad 8.0. (San Diego, CA, USA).3. Results and discussions
3.1 Kinetics of MLX degradation
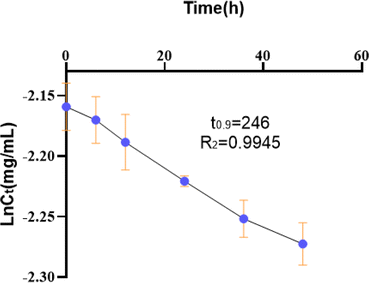
The kinetics of degradation was studied using a set of experimental conditions. The initial concentration of 10.00 mg mL−1 MLX was prepared in buffer of pH 7.4 and then heated in a water bath at 100 °C for 5 days. Upon plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct versus time, a straight line was obtained, conforming to a pseudo-1st-order reaction (r > 0.99) (Langmuir–Hinshelwood model) with the value t0.9 as 246 h (Fig. 1). This model has been widely considered as the basis for degradation of organic compounds. The results showed that the degradation of MLX could be modeled using first-order kinetics. With this premise, we could empoly the constant temperature accelerated tests to evaluate the stability of MLX solution or MLX system.
Ct versus time, a straight line was obtained, conforming to a pseudo-1st-order reaction (r > 0.99) (Langmuir–Hinshelwood model) with the value t0.9 as 246 h (Fig. 1). This model has been widely considered as the basis for degradation of organic compounds. The results showed that the degradation of MLX could be modeled using first-order kinetics. With this premise, we could empoly the constant temperature accelerated tests to evaluate the stability of MLX solution or MLX system.
3.2 The effects of HP-β-CD and SBE-β-CD on the stability of MLX
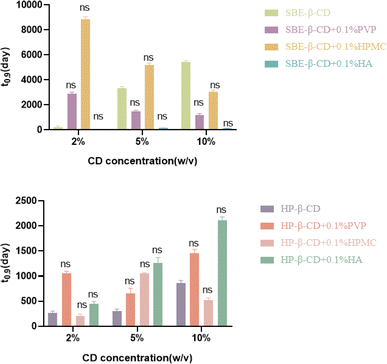
The hydrophilic CD derivatives can increase the aqueous stability of hydrophobic drug via the formation of inclusion complex. The formed complexes were usually more stable than the free active ingredients in the solution form. As shown in the Fig. 2, it is clear that at three different concentrations (2%, 5% and 10%, w/v), both HP-β-CD and SBE-β-CD could dramatically increase the stability of the MLX solution. Unlike HP-β-CD, SBE-β-CD showed a concentration-dependent trend. With the concentration ranging from 2% to 10%, the value of t0.9 increased from 199 days to 5445 days, indicating a dramatic enhanced stability attributed to SBE-β-CD. However, HP-β-CD showed no extra stabilization effect while increasing the concentration from 2% to 10%. This difference might be related to the surface properties of the two cyclodetrins. Knowing that, as a non-ionic CD compound, HP-β-CD could easily unite the hydrophobic drug to form the inclusion complexes. However, while the concentration exceeded the required amount to form the inclusion complexes, their capacity to augment stability exhibited limitations upon further increases in concentration. Typically, HP-β-CD could establish a conventional host–guest complex with MLX, wherein guest molecules can readily disassociate from the complex. Without the protection of CDs, MLX can be easily degraded. On the contrary, SBE-β-CD, as negative charged cyclodetrins, could increase the stability of drug by introducing the charge repulsion effect, which is a highly common stabilization mechanism in the liquid system. As the charged substitute groups exercise mutual electrostatic repulsion, SBE-β-CD has a peculiar structure which can provide a favorable position for drug. As a result, there was an increase in its hydrophobic properties and complexation capacity. Otherwise, the charged groups of SBE-β-CD were far away from the hydrophobic cavity, thus intensifying the solubilizing capacity. These reasons might be responsible for the fantastic enhancement of MLX stability from SBE-β-CD.3.3 The effects of hydrophilic polymers on the stability of MLX-CD system
When a water-soluble polymer, a CD and a drug are mixed together in a solution to obtain the so-called ternary complexes, it is possible to further increase drug stability when compared to the binary system, which is a result of the synergistic effect from hydrophilic polymer and CD. The interaction of water-soluble polymers with drug molecules may occur by means of ion–ion, ion–dipole and dipole–dipole electrostatic bonds, van der Waals force, or 3-center, 2-electron bonds. Similarly, the interaction between polymers and CDs and inclusion complexes begin to occur on the external surface of the CD molecule. CDs, polymers and inclusion complexes form aggregates are capable of stabilizing drugs and other hydrophobic molecules.Several types of interactions between polymers and drugs might be established as a result of the structural difference and polarity of CD molecules, which could give rise to various complexation efficiencies. As for the different matches in Fig. 3, the best complexation efficiency results were obtained from HPMC together with SBE-β-CD and HA together with HP-β-CD. Usually, it was recognized that, in aqueous solutions, hydrophilic polymers could stabilize micelles and other types of aggregates, reduce CD mobility and increase the solubility and the stability of complexes by changing the hydration properties of CD molecules. Moreover, this interaction between the polymer and CD compound could be enhanced and accelerated by heating the ternary system.16 Thus, it was possible to activate the bonds between system components during the preparation of complexes by heating them. For example, the heating method was employed in the preparation process of MLX ternary system.
According to our preliminary experiments results, hydrophilic polymers could somehow increase the stability of MLX. The potential mechanism was that the molecular chain of the hydrophilic polymer containing a lot of hydroxyl and carbonyl groups could entangle with the drug in the solution to form a helical coil or mesh structure, which played a stabilizing role for MLX. Here, the effect of hydrophilic polymer on the stability of the ternary system was investigated to better understand the stability mechanism with or without the third hydrophilic polymer added.
The effects of hydrophilic polymers on the stability of MLX-CD solution are shown in the Fig. 3. From the results, new added hydrophilic polymers did produce different influence on the stability of MLX ternary system. However, we could still find some basic trends. In most of the cases, HP-β-CD could be competitive to form a ternary system. In the ternary system, HP-β-CD always achieved a lower t0.9 value at all the concentrations compared with SBE-β-CD. These might be due to the nice properties of SBE-β-CD which have been explained above. As for HP-β-CD, even though the polymer was added to form a ternary system, the stabilization efficiency was still not enough. Here, what really matter was that how the hydrophilic polymer could affect the binary system.
For the MLX&SBE-β-CD ternary system, PVP showed a positive effect in the low SBE-β-CD concentration with the t0.9 up to almost 15 fold. However, this improvement became less pronounced while the concentration went higher to 5% and 10%. It seemed that PVP displayed a negative effect rather than positive. As for the HP-β-CD, the third component PVP could increase the value of t0.9, but the extent really depended on the concentration. The t0.9 reached a lowest level with 5.0% HP-β-CD in the ternary system.
As like PVP, HPMC, a non-ionic polymer, exhibited a similar effect on the MLX&SBE-β-CD system. Overall, HPMC decreased the stability of the system while SBE-β-CD went from 2% to the higher concentration. Smith et al. obtained a similar result that the synergistic effect occurred from the addition of HPMC to the complex formed by SBE-β-CD and carbamazepine, but this positive effect disappeared with increasing HPMC concentration.17 For the MLX&HP-β-CD system, HPMC showed a totally different result. An optimized stability was got at 5.0% HP-β-CD, and either a lower or higher HP-β-CD concentration resulted in a decreased stability.
Other than PVP and HPMC, HA is a negatively charged polymer, with a long chain containing repeating disaccharide units of Na-glucuronate-N-acetylglucosamine. With HA added, the MLX& SBE-β-CD&HA system achieved a remarkably decreased t0.9 compared with the original binary system. Interestingly, HA exhibited a totally contrary effect on the MLX&HP-β-CD system, and the HA-containing complexation system got a desired stability with t0.9 doubled or tripled at all the concentrations.
The above results shows that PVP, HPMC and HA could affect the stability of the inclusion complexes. However, this effect varied by the component and the concentration of the CD derivative. The stability of MLX-CD solution changed s after adding hydrophilic polymers, the change is not significant, but indicating that the polymers could interact with inclusion complex. Faticci had reported that polymers could interacted with drug inclusions via various mechanisms, including van der Waals dispersion forces, hydrophobic bonds, ionic bonds and molecular hydrogen bonds.18 Ribeiro also pointed out that the vinpocetine CD inclusion could be formed as trimer with hydrophilic polymers (PVP and HPMC), and then improved the stability of drug.19 Here, the polymers we used contained lots of carbonyl groups that were easy to form hydrogen bonds with the hydroxyl groups of CD. HPMC and PVP were non-ionic polymers, and sodium hyaluronate was anionic. In addition, HP-β-CD was electrically neutral molecule, and SBE-β-CD was anionic.
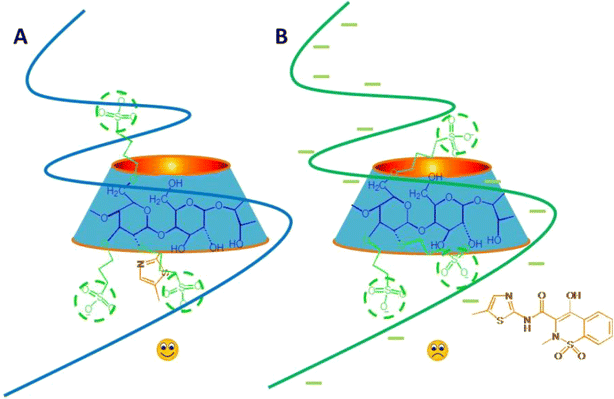
The presence of anionic polymer sodium hyaluronate significantly reduced the stabilization of anionic SBE-β-CD on MLX, but increased the stability of neutral HP-β-CD &MLX complex. It was indicated that the ion repulsion effect existed between anionic polymer and anionic CD derivative, which inhibited the extension of sulfobutyl ether groups of SBE-β-CD, and then compressed the size of CD molecular cavity, limited the degree of drug inserted and the protection from CD, thereby reduced the stabilization effect of SBE-β-CD on MLX (Fig. 4). Moreover, the hydrophilic side chains of HP-β-CD were stretched more through the formation of intermolecular hydrogen bonds with sodium hyaluronate, which increased the inclusion of drug and enhanced the stability of MLX. Furthermore, sodium hyaluronate could also form hydrogen bonds with SBE-β-CD, but reduced the stability of MLX-CD. It suggested that the ion repulsion interactions were dominant.
PVP and HPMC are nonionic hydrophilic polymers. When the concentration of CD was low, these two kinds of polymers exerted the synergistic effect with CD and promoted the stability, indicating that the intermolecular hydrogen bonds would form between CD and polymer, so as to expand the size of molecular cavity.16,20 Then the increased inclusion effect resulted in an enhanced stability of MLX. With the high concentration of CD, the stability of MLX-CD was reduced in the presence of polymers, suggesting that the addition of polymer would compress the extension space of CD hydrophilic side due to the limited space of the whole system. So the weakened inclusion effect decreased the stability of MLX.
Furthermore, from our finding, it was indicated that, in the ternary system, the match of a charged component and a hydrophilic component could provide a better stabilization for the unstable drug. As for MLX, the SBE-β-CD&HPMC and HP-β-CD&HA always showed better results than other matches. If two neutral components were employed, the stabilization effect would be not enough. If two same charged components were employed, the stability of the system will be decreased significantly because of the ion repulsion effect. Certainly, the stabilization capacity of the ternary system seriously varied depending on the hydrophilic properties, the concentration of the component, as well as the potential interaction in the new formed ternary system.
3.4 Characterization of the MLX ternary system
 | ||
| Fig. 5 DSC curves of meloxicam (a), MLX-HP-β-CD-PVP inclusion complex (b), MLX-HP-β-CD-HPMC inclusion complex (c), and MLX-HP-β-CD-HA inclusion complex (d). | ||
 | ||
| Fig. 6 DSC curves of meloxicam (a), MLX-SEB-β-CD-PVP inclusion complex (b), MLX-SEB-β-CD-HPMC inclusion complex (c), and MLX-SEB-β-CD-HA inclusion complex (d). | ||
The results showed that the anhydrous crystalline substance meloxicam had an obvious absorption peak at 256.8 °C, and the MLX-HP-β-CD inclusion complex and MLX-SEB-β-CD inclusion complex did not show any obvious heat absorption peak at 256.8 °C, which indicated that meloxicam was encapsulated and the inclusion complexes were successfully formed.
 | ||
| Fig. 7 XRD patterns of meloxicam (a), MLX-HP-β-CD-HPMC inclusion complex (b), MLX-HP-β-CD-HA inclusion complex (c), and MLX-HP-β-CD-PVP inclusion complex (d). | ||
 | ||
| Fig. 8 XRD patterns of meloxicam (a), MLX-SEB-β-CD-HPMC inclusion complex (b), MLX-SEB-β-CD-HA inclusion complex (c), and MLX-SEB-β-CD-PVP inclusion complex (d). | ||
The test results showed that meloxicam had a crystalline structure, and the obvious characteristic peak appeared at 25.86°; the peak shapes and intensities of MLX-HP-β-CD-HPMC inclusion, MLX-HP-β-CD-HA inclusion, MLX-HP-β-CD-PVP inclusion, and MLX-SEB-β-CD-HPMC inclusion, MLX-SEB-β-CD-HA inclusion, and MLX-SEB-β-CD-PVP inclusion complexes were obviously different from those of the above samples, and were amorphous, and the characteristic peak at 25.86° disappeared, and the crystalline shape of the diffraction peaks of the API changed significantly before and after the encapsulation, which indicated the successful formation of the inclusion complexes.
The results showed that the MLX was in the form of massive crystals, and HP-β-CD and SEB-β-CD were in the form of spherical structure; the morphology of the MLX, HP-β-CD and SEB-β-CD disappeared in the MLX-HP-β-CD inclusion and the MLX-HP-β-CD inclusion, indicating that the original lattice arrangement of the host–guest molecules changed after the formation of the two inclusions and the formation of a new physical phase.
3.5 MLX liquid formulation: eye drops-preparation and stability text
The drug-CD-polymer complexes could be administered in any dosage form for the treatment of a variety of ailments, depending on the biological activity of the complexed drug. Researchers on ternary complexes have gained prominence in recent decades, and it is possible to find a considerable number of studies in which drug-CD-polymer obtained to get a better drug ability.Here, considering the properties of the eye drops, we adopted two commonly used materials in the eye drop formulation, HPMC and SBE-β-CD. Three factors of eye drops formulation were arranged according to an L9(34) orthogonal experimental table (Table 1).
| Factor | Level | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Concentration of HPMC (%, w/v) | 0.1 | 0 | 1 |
| pH value | 6.98 | 7.38 | 7.73 |
| Concentration of SBE-β-CD (%, w/v) | 2.0 | 3.5 | 5.0 |
According to Table 2, A3B1C3 was the optimized formulation, consisting of 1% HPMC and 5% SBE-β-CD at pH 6.98. Other than that, we could conclude from Table 3 that HPMC not only stabilize the formulation but also had a big influence on the viscosity of the MLX eye drops. Compared with the concentration of the hydrophilic HPMC, the pH and the concentration of the cyclodetrins showed limited effect on the viscosity of the formulation. In sum, the comparison of the effect of all three factors was as follows: A > B > C.
| No. | A | B | C | D (error) | Viscosity |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1.090 |
| 2 | 1 | 2 | 2 | 2 | 1.260 |
| 3 | 1 | 3 | 3 | 3 | 1.214 |
| 4 | 2 | 1 | 2 | 3 | 1.723 |
| 5 | 2 | 2 | 3 | 1 | 1.709 |
| 6 | 2 | 3 | 1 | 2 | 1.571 |
| 7 | 3 | 1 | 3 | 2 | 2.690 |
| 8 | 3 | 2 | 1 | 3 | 2.373 |
| 9 | 3 | 3 | 2 | 1 | 2.013 |
| K1/2 | 1.188 | 1.834 | 1.678 | ||
| K2/3 | 1.668 | 1.781 | 1.665 | ||
| K3/3 | 2.359 | 1.599 | 1.871 | ||
| R | 1.171 | 0.235 | 0.206 |
| Source of variation | SS | df | MS | F |
|---|---|---|---|---|
| A | 2.078 | 2 | 1.039 | 23.519 |
| B | 0.091 | 2 | 0.045 | 1.03 |
| C | 0.08 | 2 | 0.04 | 0.902 |
| Between groups | 0.088 | 2 | 0.044 | |
| Within groups | 3.673 |
The results of stress testing showed that the appearance, content and pH values of MLX eye drops were not changed under the conditions of high temperature (40 °C and 60 °C) and light (4500 ± 500 Lx) in 10 days (shown in Fig. 10). The results proved that there was no statistical difference, which proved that the stability of MLX eye drops could meet the requirements for medical use.
4. Conclusions
This study mainly investigated the influence of hydrophilic polymers (PVP, HPMC and HA) and cycle dextrins (SBE-β-CD and HP-β-CD) on the stability of MLX. From our findings, it could be concluded that hydrophilic polymers showed a considerably effect on the stability for two different type MLX inclusion complexes, but this effect seriously varied depending on the hydrophilic properties, the concentration of cyclodetrins as well as the potential interaction in the new formed ternary system. Based on the results, an optimized model formulation, eyedrops, was prepared using the ternary system (5% SBE-β-CD and 1% HPMC). Short-term stability results showed that combination of HPMC and SBE-β-CD achieved a nice stabilizing effect, which met the requirements on the stability of MLX eyedrops. Hydrophilic polymers and cycle dextrins were matched together to form a stable ternary system. For MLX, the third component hydrophilic polymer could interact with MLX binary system, and then form a trimer, thus play a role in the stabilization of MLX. The main purpose of our study is to increase the water solubility of meloxicam and its stability in liquid preparations through the ternary system, so as to lay a good foundation for the development of new dosage forms. At the same time, we also proposed the prescription of meloxicam eye drops, which is also of great significance in practical application. The study has a great guiding significance on improving the stability of liquid formulations of poorly water-soluble and unstable drugs via introducing a ternary complex system.Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.Author contributions
This work was financially supported by National Natural Science Foundation of China (No. 82173766). The authors acknowledge the work was supported by a research group from Shenyang Pharmaceutical UniversityConflicts of interest
The authors have no relevant financial or non-financial interests to disclose.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 82173766).The authors acknowledge the work was supported by a research group from Shenyang Pharmaceutical University, including the financial support. And get the vigorous help and encouragement from all the teachers and students of Municipal Key Laboratory of Biopharmaceutics.References
- G. Engelhardt, D. Homma and K. Schlegel, et al., Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance, Inflammation Res., 1995, 44, 423–433 CrossRef CAS PubMed
.
- W. Zhang and D. Zu, Bovine serum albumin–meloxicam nanoaggregates laden contact lenses for ophthalmic drug delivery in treatment of postcataract endophthalmitis, Int. J. Pharm., 2014, 475, 25–34 CrossRef CAS PubMed
.
- H. B. Mohamed, M. A. Attia Shafie and A. I. Mekkawy, Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model, Pharmaceutics, 2022, 14(5), 893 CrossRef CAS PubMed
.
- T. Irie and K. Uekama, Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation, J. Pharm. Sci., 1997, 86, 147–162 CrossRef CAS PubMed
.
- V. Suvarna and P. Gujar, Supramolecular ternary inclusion complexes of Irbesartan with hydroxypropyl-beta-cyclodextrin, J. Drug Delivery Sci. Technol., 2022, 67, 1773–2247 Search PubMed
.
- S. A. Fahmy, A. Ramzy, B. M. Saleh and H. M. El-Said Azzazy, Stimuli-Responsive Amphiphilic Pillar[n]arene Nanovesicles for Targeted Delivery of Cancer Drugs, ACS Omega, 2021, 6(40), 25876–25883 CrossRef CAS PubMed
.
- O. A. A. Alabrahim, et al., Stimuli-Responsive Cucurbit[n]uril-Based Supramolecular Nanocarriers for Delivery of Chemotherapeutics, ACS Appl. Nano Mater., 2023, 6(5), 3139–3158 CrossRef CAS
.
- A. Yousaf, S. A. Hamid, N. M. Bunnori and A. A. Ishola, Applications of calixarenes in cancer chemotherapy: facts and perspectives, Drug Des., Dev. Ther., 2015, 9, 2831–2838 CAS
.
- R. Challa, A. Ahuja and J. Ali, et al., Cyclodextrins in drug delivery: an updated review, AAPS PharmSciTech, 2005, 6, E329–E357 CrossRef PubMed
.
- S. Ketan, G. Anuradha and S. Jignasa, Drug solubility: importance and enhancement techniques, ISRN Pharm., 2012, 1–10 Search PubMed
.
- S. Gould and R. C. Scott, 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review, Food Chem. Toxicol., 2005, 43, 1451–1459 CrossRef CAS PubMed
.
- J. D. Heidel and T. Schluep, Cyclodextrin-containing polymers: versatile platforms of drug delivery materials, J. Drug Delivery, 2012, 17, 2012 Search PubMed
.
- R. Han, T. Huang and X. Liu, et al., Insight into the Dissolution Molecular Mechanism of Ternary Solid Dispersions by Combined Experiments and Molecular Simulations, AAPS PharmSciTech, 2019, 20, 274 CrossRef PubMed
.
- M. I. Santoro, E. R. Hackmann and V. M. Borges, Stability of phenobarbital sodium in liquid pharmaceutical preparations, Boll. Chim. Farm., 1992, 131(6), 226–229 CAS
.
- F. S. Bandarkar and P. R. Vavia, Physico-chemical characterization and in vivo pharmacodynamic evaluation of lyophilized meloxicam: β-cyclodextrin inclusion complexes, Int. J. Pharm. Pharm. Sci., 2013, 5, 159–165 CAS
.
- T. Loftsson, D. Hreinsdóttir and M. Masson, Evaluation of cyclodextrin solubilization of drugs, Int. J. Pharm., 2005, 302, 18–28 CrossRef CAS PubMed
.
- J. Smith, R. MacRae and M. Snowden, Effect of SBE7-β-cyclodextrin complexation on carbamazepine release from sustained release beads, Eur. J. Pharm. Biopharm., 2005, 60, 73–80 CrossRef CAS PubMed
.
- S. Gould and R. C. Scott, 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review, Food Chem. Toxicol., 2005, 43, 1451–1459 CrossRef CAS PubMed
.
- L. Ribeiro and F. Veiga, Complexation of vinpocetine with cyclodextrins in the presence or absence of polymers. Binary and ternary complexes preparation and characterization, J. Inclusion Phenom. Macrocyclic Chem., 2002, 44, 251–256 CrossRef CAS
.
- Z. Sun, H. Zhang, H. He, L. Sun, X. Zhang, Q. Wang, K. Li and Z. He, Cooperative effect of polyvinylpyrrolidone and HPMC E5 on dissolution and bioavailability of nimodipine solid dispersions and tablets, Asian J. Pharm. Sci., 2019, 14(6), 668–676 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |