Open Access Article
Open Access ArticleQuantitative and equipment-free paper-based agglutination assay of bacterial cells
Mohammad Al-Tamimi *a,
Shahed Altarawneha,
Minas A. Mustafa
*a,
Shahed Altarawneha,
Minas A. Mustafa b,
Mariam El-sallaqa and
Penelope Shihabc
b,
Mariam El-sallaqa and
Penelope Shihabc
aDepartment of Microbiology, Pathology and Forensic Medicine, Faculty of Medicine, The Hashemite University, Zarqa, Jordan. E-mail: mohammad.altamimi@hu.edu.jo; Fax: +962 (5) 3826613; Tel: +962 (5) 3903333
bDepartment of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, The Hashemite University, Zarqa, Jordan
cWyoming Center for Entrepreneurship and Innovation, University of Wyoming, WY, USA
First published on 27th June 2024
Abstract
Background: point-of-care (POC) tests are useful for bedside/home applications, emergencies, frequent follow-ups, and resource-limited areas. Limited quantitative and equipment-free POC assays have been reported. This study aims to develop, validate, and apply a simple, quantitative, paper-based POC assay. Methods: wax-channeled paper treated with specific anti-Brucella and anti-Salmonella antibodies was used for distance-based chromatographic elution of stained bacterial cell agglutinations. Results: a qualitative paper-based agglutination POC test was developed using color intensity, tail appearance, and “+/−” signs that clearly distinguish the positive and negative results. The optimization of the test for paper type, microfluidic channel design, antibody and bacterial cell concentrations, and elution methods was carried out. Quantitative assay transformation was successfully developed using the color intensity of the original reaction zone, intensity of elution tail, and distance-based migration that correspond to bacterial agglutination size. The migration distance of eluted bacterial agglutination bands corresponds to the target concentration with good linearity and minimal variability. Reporting of colored band migration with numbers using microfluidic patterns was used to enhance non-technical end-user applications. A distance-based POC assay prototype was then successfully used for the accurate detection of known and unknown samples in comparison with standard assays. Conclusions: the migration distance of an eluted stained bacterial agglutination correlated with anti-bacterial antibody concentrations. A simple, cheap, quantitative, and equipment-free paper-based POC assay of bacterial cell agglutination was developed. This test can be used for simple “+/−” results, thermometer-like quantification, or text reporting with numbers corresponding to target concentrations. The assay has extended applications to different human disease biomarkers.
Introduction
Point-of-care (POC) medical testing attracts huge attention due to its wide real-life applications at home, health care sites, and emergency and low-resource situations.1 Traditional diagnostic laboratory testing is equipment-dependent, labor-intensive, time-consuming, and expensive.1,2 POC testing is affordable, user-friendly, rapid, equipment-free, and deliverable to users.1,2 Microfluidic technologies are interesting for POC testing as they require less time and small volumes of samples yet provide high accuracy.2 Paper-based microfluidic devices have placed themselves on the front line for POC development.2–4Most POCs are qualitative and provide a “yes/no” answer with relatively limited sensitivity, which limits their wide applications.1 POC devices that provide quantitative results are very expensive.5,6 Other less expensive devices including scanners, smartphone applications, portable pressure meters, and other devices were proposed.1,5,6 Quantitative and equipment-free POCs are needed and would be of huge advantage.1,4 Many attempts for the development of quantitative and equipment-free POCs were reported using intensity-based methods, ladder-bar detection, time-based detection, distance-based detection, counting-based strategy, and text-based detection.1,4–7
Quantitative, equipment-free, distance-based paper POCs that rely on reading a visual signal length that corresponds to target concentration in a thermometer-like approach have been developed.8–17 Mechanisms involved enzyme, metal complexion, enzyme-linked immunosorbent assay (ELISA), immunochromatography enzyme channeling, coffee ring effect, magnetic beads, nanoparticles aggregation, aptamer/invertase-functionalized sepharose beads, interfacial interactions, liquid crystalline visualization, and hydrogel-based to generate color band height or distance.8–20 Similarly, an internal clock reaction using enzyme immunochromatography assay9 and length of flow of amphiphilic biosamples in paper microchannels21 was used to generate distance-based signals. Some of these POC devices require the presence of a smart phone for accurate detection.14,17
Distance-based paper POCs were used for the measurement of cholesterol, high-density lipoprotein, glucose, bilirubin, nickel, glutathione, immunoglobulin, aerosol oxidative activity, cocaine, lactoferrin, theophylline, and metals.8–20 Infectious diseases are common in developing countries with limited resources; accordingly, the development of reliable and equipment-free POC devices for the diagnosis of different infections is required.22 Bacterial infections including Brucella and Salmonella are endemic in many countries in Asia, South America, the Middle East and Africa. Serological tests for the detection of anti-Brucella or anti-Salmonella antibodies are commonly used for establishing the diagnosis using slide agglutination test or ELISA methods.23–25 To the best of our knowledge, paper-based bacterial agglutination POC tests that report results using the migration distance for the quantification of anti-bacterial antibodies have not been reported before. Furthermore, converting distance to text numbers is a new and simple approach that shows results directly with no need for further interpretation.
Materials and methods
Bacterial cells agglutination tests
The Rose Bengal Brucella test is a slide agglutination test for the qualitative and semi-quantitative specific detection of anti-Brucella antibodies in human and animal serum. The test principal relies on the abilities of anti-Brucella IgG or IgM antibodies (agglutinin) in patients' serum to induce the visible agglutination of stained Brucella abortus bacterial suspension (Rose Bengal Brucella reagent).23 The test procedure was performed according to manufacturer instructions (Arcomex, Jordan) by mixing 40 μL of serum or controls with one drop of Rose Bengal reagent on a slide or white card at room temperature, placed on a rotator for proper mixing for 2–4 minutes and then observed for visible macroscopic agglutination. Serial two-fold dilutions of the sample in 9 g L−1 saline solution followed by applying the same steps can be used for the semi-quantitative determination of Brucella antibodies. Positive control (Serum with anti-B. abortus concentration 50 IU mL−1) and negative control are provided in the kit.The Salmonella slide test applies a similar principle and procedure for the qualitative and semi-quantitative detection of anti-Salmonella antibodies using visible agglutination. The test was performed according to manufacturer instructions (Arcomex, Jordan). Semi-quantitative measurements require the dilution of the serum sample followed by the determination of the degree of agglutination from +4 (strong agglutination with 100% clear supernatant) to +1 (weak agglutination about 25%).26
Determination of Brucella and Salmonella antibodies by standard laboratory tests
The quantitative determination of anti-Brucella antibodies for positive control and plasma samples was carried out using a specific ELISA kit by a reference diagnostic laboratory according to standard procedures.Filter paper and hydrophobic wax channels creation
Whatman qualitative filter paper grades 598, 595, and quantitative filter paper 589/2 (Whatman, USA), fiberglass MGC paper (sartorius, Germany), and chromatography paper (sartorius, Germany), and fine Sterilized Facial Tissues (Kleenex) (Fine, Jordan) were used for experiments. Numbers, channels, reaction zones, text, and symbols like “+/−” were prepared using Microsoft Word with different shapes and sizes and then printed automatically on filter or chromatography papers using Xerox 8580 wax solid ink printer to create the hydrophobic surfaces, followed by heating with a laminator device.27Paper-based bacterial agglutination assay
The same reagents provided with the Rose Bengal test and the Salmonella test including stained bacterial suspension and controls were used to develop the paper-based agglutination test. The procedure involves adding the positive control to the paper (4 μL), then the reagent was added directly to the same zone (4 μL). The paper was incubated for 5 minutes at room temperature. In the case of ascending wash, the paper was then placed into a chromatography tank containing the washing buffer, which allowed the reaction zone to be washed ascendingly until the paper was completely soaked. The direct washing of the paper was performed by directly pipetting the washing buffer on the reaction zone after placing the paper on a tissue paper (50 μL for up to 10 times). Positive reaction was indicated by the fixation of stained reagent cells in paper while negative reaction was indicated by the elution of cells.For the semi-quantitative testing of the paper-based assay, the negative control was used as 0%, the positive control was either used as supplied (100%) or diluted with normal saline (Polifarma, Turkey) into 75%, 50%, 25% or was concentrated on the reaction zone by adding it twice or three times to the same location with a separating time frame of 3–5 minutes. Different types of washing buffers were used, including normal saline, normal saline + 0.1 tween 20 (Scharlau, Spain), and normal saline + 0.2 tween 20. Rose Bengal reagent was concentrated using a centrifuge (Sigma, USA), followed by discarding the required volume of the supernatant.
Pictures of the reactions were taken by a Canon EOS 250D digital camera (Canon, Japan) and the washed tail length was measured using a ruler. Pictures were then added to the ImageJ program (National Institutes of Health and the Laboratory for Optical and Computational Instrumentation, University of Wisconsin, USA) for absorbance (OD) measurement of the reaction zones, and the ODs were analyzed using Microsoft Excel.
Results
Qualitative and semi-quantitate bacterial slide agglutination
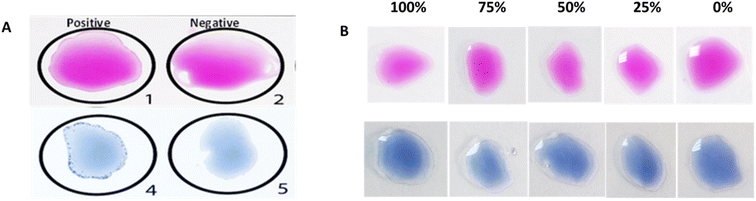
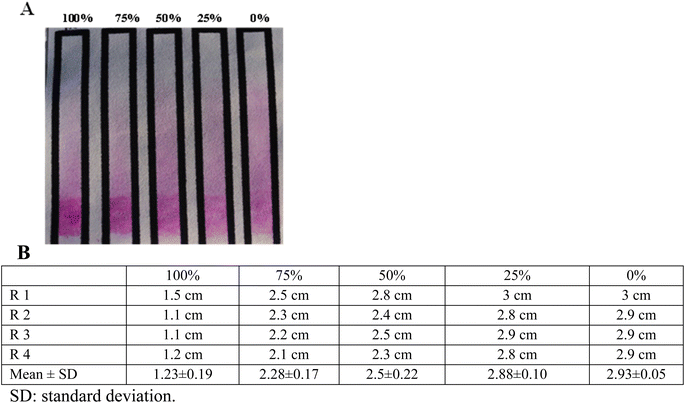
Fig. 1A shows a positive slide/card agglutination obtained when doing both the Rose Bengal test (pink) and the Salmonella test (blue). Fig. 1B shows serial dilutions of positive controls for the Rose Bengal test (pink) and Salmonella test (blue), where agglutination can be observed for the first two dilutions (100% and 75%) with difficulties.Qualitative paper-based bacterial agglutination assay
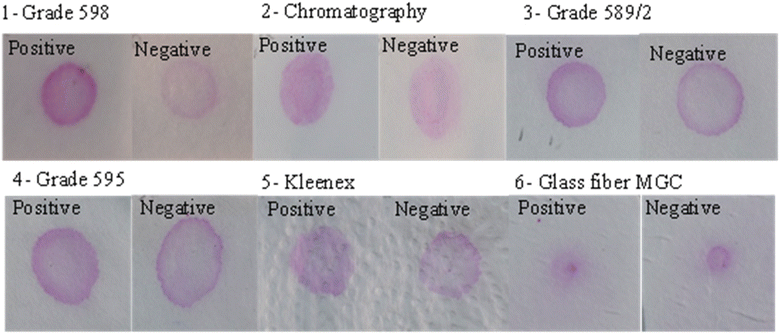
Positive and negative paper-based agglutination assay results were compared according to the wash method (direct addition of washing buffer or ascending wash of buffer) for Rose Bengal test (pink) and Salmonella test (blue color) using Whatman filter paper grade 598 (Fig. 2). The negative test results were washed for the Rose Bengal and Salmonella test when using direct wash (Fig. 2A), ascending wash (Fig. 2B), and when using a positive and negative sign as a reaction zone shape (Fig. 2C).Optimization of paper-based agglutination assay
The optimization of the Rose Bengal agglutination reaction on filter paper was performed relying on testing factors such as paper type, washing buffer type, positive control concentrations, and Rose Bengal reagent concentrations. Three parameters were observed to determine the optimal conditions; the visual appearance of the original reaction zone after washing (fixed or eluted), the visual appearance of the elution tail with ascending washing with the migration distance of the tail measured in cm, and the color intensity of the reaction zone or elution tail measured by the ImageJ program.The absorbances of each filter paper agglutination (concentrations 100% and 0%) were measured using the ImageJ program after using saline as a wash. Kleenex had the highest differences between the two concentrations absorbances but failed to leave a washing tail, which could be possibly used as a concentration measurement tool. Grade 598 had the next highest OD difference, followed by grade 589/2. Glass fiber MGC paper failed to have a notable OD difference between the two concentrations (Fig. 4).
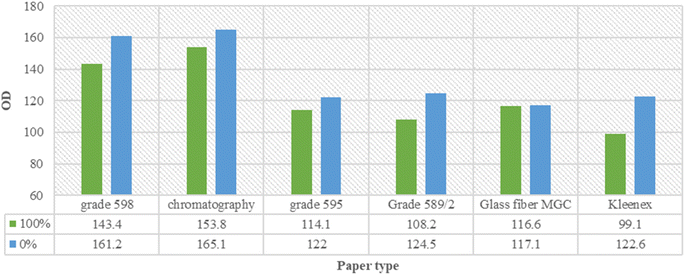 | ||
| Fig. 4 OD readings and variations among the used filter papers for concentrations 100% and 0% washed with ascending normal saline. | ||
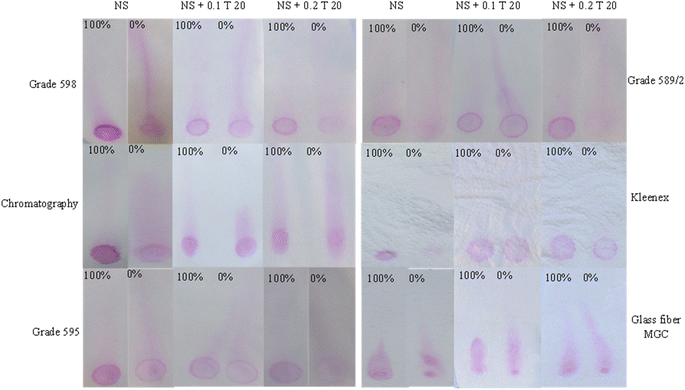
Filter paper grade 598 and chromatography paper were tested for the possibility of using direct washing of positive and negative agglutination reactions. Filter paper grade 598 had a nearly completely washed negative reaction zone and a non-washed positive agglutination. However, chromatography paper and Kleenex filter paper grades 595 and 589/2 positive and negative reaction zones had close color intensities for positive and negative results with a slightly more washed negative zone (Fig. 5).
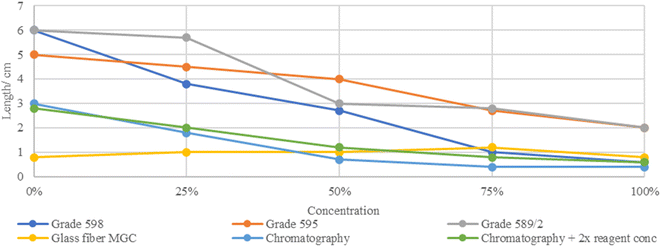
The washed tails lengths were measured for each concentration on each paper type too. Grades 598 and 589/2 had 0% tail washed through the whole paper length, then reduced the tail length according to the concentrations. The chromatography paper resulted in a short tail for 0%, which could be useful for future tail level quantification but had a fluctuated tail length for the rest of the concentrations, which became more linear and concentration-related when the concentration of the reagent was doubled (2×). Grade 595 showed tail lengths positively correlated with the expected wash level according to each concentration. However, glass fiber MGC paper showed no tail lengths according to the concentrations (Fig. 6).
Quantitative paper-based agglutination assay development
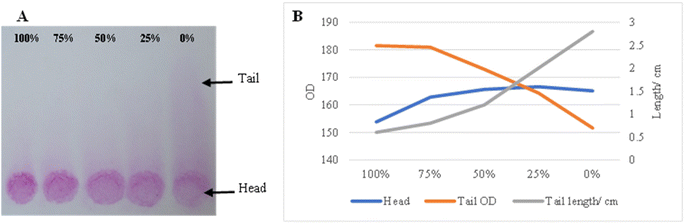
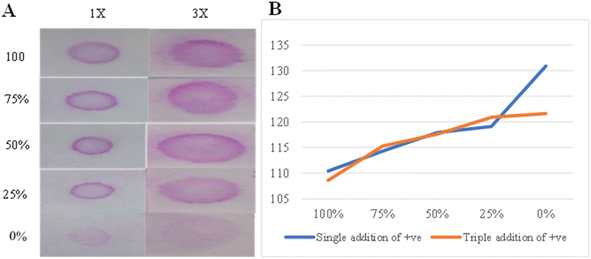
Chromatography paper was used for semi-quantification paper-based agglutination development. The absorbance of the reaction zones and washed tails resulting from the ascending washing of agglutination on chromatography paper was measured and correlated with positive control concentrations 100%, 75%, 50%, 25%, and 0% as well as the washed tails length in centimeters. The pink color intensity of the head zones observed by the naked eye is positively correlated with the concentrations. The tail lengths had opposite results, forming a ladder of increased length when the concentrations were reduced (Fig. 8A). As expected, the head absorbances and tail lengths were adversely correlated with the positive control concentrations, but the head readings did not vary significantly from concentrations 50% to 0%. The tail absorbances decreased significantly when the concentrations decreased (Fig. 8B).Filter paper grade 598 was used for a semi-quantitative agglutination reaction using a direct wash of normal saline. The positive control was used in 1× and 3× additions, and 100%, 75%, 50%, 25%, and 0% concentrations were used in the reactions. A reduction in the pink color intensity was observed according to the reduced concentrations in both 1× and 3× reactions with an almost vanished pink color for 0%. However, visualizing the variations in color intensities according to concentrations was slightly easier using the 3× reaction compared to the 1× one (Fig. 9A). Adversely, ImageJ showed more significant variations in the absorbance results for the 1× reaction compared to the 3× one (Fig. 9B).
Assay linearity and variability and determination of unknown samples
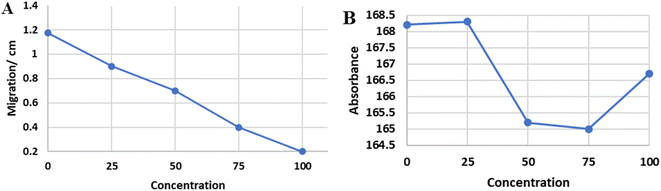
The Rose Bengal reaction was performed for five concentrations (100%, 75%, 50%, 25%, 0%) with four trials on chromatography paper. After the ascending wash, the migration of the reaction was measured in centimeters by a ruler, and the average of the four readings was calculated for each concentration (Fig. 10A). A nice linear concentration-dependent tail migration distance can be observed with minimal variability between different trails. Furthermore, the reaction zone absorbances for the same trails were measured using ImageJ, and the average was calculated (Fig. 10B). Poor concentration-dependent linearity can be noted. The concentration of 5 unknown samples was determined accurately for all the samples using tail migration distance plotted in Fig. 10A, while only the concentration of 2/5 unknown samples was determined using optical density plotted in Fig. 10B.Assay prototyping using different text (numbers) reporting methods
Different designs of quantitative number reporting methods were tested (Fig. 11). A simple ruler can be used to correspond to analyte concentration (Fig. 11A). Hydrophobic white color wax-printed numbers that appear as the colored cells migrate within the hydrophilic space around it (Fig. 11B). Hydrophilic connected number design surrounded by wax-filled hydrophobic space and numbers appear as colored cells migrate inside numbers (Fig. 11C). Using pink color wax printed numbers that disappear as bacterial cells of equal color intensity migrate (Fig. 11D).Discussion
The development of quantitative and equipment-free POCs that provide an onsite sample-to-answer approach has gained wide interest in recent years due to their wide applications, cheapness, portability, accessibility, convenience and quick read-out.5,6 Different strategies have been tested including color intensity, ladder-bar, time, distance, and text. However, challenges including user variability, environmental conditions effects, complexity, multiple steps, the requirement for fixed attention, low accuracy, insufficient sensitivity, and poor reproducibility limit their clinical applicability.1,4,5,7 The different techniques, applications, pros and cons of distance-based paper microfluidic assays have been recently reviewed.5,6,28 Equipment-free, distance-based paper microfluidic devices have been summarized in Table 1.8–21 As described in Table 1, the techniques and principles applied for generating color band height that corresponds to targets concentrations include enzymatic reaction, immune chromatography, ELISA, coffee-ring effect, nanoparticles aggregation, fluorescence, sweet hydrogel, and liquid crystalline or combination of more than one technique. Distance-based elution of agglutinated bacterial cells has not been used before (Table 1).8–21 In this study, color generation was mediated directly using stained bacterial cells, which decrease the number of steps needed to generate color by other techniques (enzyme, fluorescence, etc.) and can be expanded to any naturally colored or stained cells, beads, or particles (Table 1).8–21 Furthermore, these distance-based techniques were used for measuring drugs, metals, cholesterol, total human IgG, invasive fungi, bilirubin, and glucose (Table 1).8–21 However, none of these techniques were applied for the measurement of infections-related antibodies like Brucella or Salmonella applied in this study. Distance-based assays have a higher ability for quantitative transformation,1 text reporting is regarded as the most user-friendly,5 while paper is considered the most applicable substrate.2,3| Technique | Application | Principal | Quantitation | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Enzyme immunochromatography | Quantifying drugs in biological fluids (theophylline) | Immobilized specific antibody, enzyme reagent glucose oxidase and horseradish peroxidase and a color-developer solution containing substrates for both enzymes | Height of a color front | Simple, rapid, stable, reliable, with no need for external calibration, or blood separation | Complex with three steps and two incubations | 8 |
| Other applications were not discussed | ||||||
| Internal clock enzyme immunochromatography | Quantifying theophylline | Immobilization of glucose oxidase and monoclonal antibody to theophylline on chromatographic paper followed by the addition of enzyme reagents glucose, dicarboxidine, ascorbate, and theophylline-labeled horseradish peroxidase (HRP) | Color band height | One step, simple, and accurate | Require 20 minutes with multiple reagents and antibodies and applications limited to therapeutic drug monitoring | 9 |
| The ascorbate acts as an internal clock | ||||||
| Enzyme chromatography | Measurement of cholesterol in whole blood | Enzymatic reaction with cholesterol to produce hydrogen peroxide and quantification using redox-coupled indicator | Height of color band | One step, separate plasma from whole blood, accurate, and stable | Complex design | 10 |
| Enzyme chromatography | Measurement of high-density lipoprotein in whole blood | Serum separation and erythrocyte agglutination followed by lipoprotein separation, the first reaction chamber contains chromatography paper with immobilized HRP, the second chamber contains immobilized cholesterol esterase, the last chamber contains immobilized cholesterol oxidase | Color band | Simple, accurate, stable, combine all steps in a single device | Complex design | 11 |
| Coffee-ring effect and ELISA | Human IgG | Paper-based color ELISA combined with coffee-ring effect | Ruler readout of color band width | Simple, rapid, and not affected by ambient temperature | Accuracy and precision compared to standard tests were not performed | 12 |
| Fluorescent detection on surface-modified cellulosic filter papers | Tear lactoferrin | TbCl3 and NaHCO3 solutions were used for fluorescent detection of lactoferrin on sulfated anionic polysaccharide ι-carrageenan-modified cellulosic filter paper | Distance-based readout of green fluorescence band | Simple, cheap, fast with no need for calibration | Require UV illumination | 13 |
| Accuracy and precision are less than other assays | ||||||
| Clinical applications are not clear | ||||||
| Sweet hydrogel integrated paper-based device | Cocaine and adenosine in urine | Aptamer cross-linked hydrogel for target recognition, cascade enzymatic reactions for signal amplification, within microfluidic paper-based device | Distance-based color readout | Cost-effective, disposable, easy to fabricate, sensitive, selective, detection of a variety of targets | Detection by a cellular phone camera after 30 minutes, requires controlled conditions and multiple washing steps | 14 |
| Aptamer and enzyme-based origami paper device | Cocaine and adenosine | Aptamer and invertase-DNA conjugate functionalized sepharose beads, and cascade enzymatic reactions, within a 3D microfluidic paper-based device | Brown bar chart reading | One step, low-cost, small volume, disposable, applicable to many targets, and simple | Accuracy and stability were not discussed | 15 |
| CRISPR Cas12a-hydrogel-integrated paper-based devices | Invasive fungi (Candida and Aspergillus) | CRISPR Cas12a for target recognition, a DNA hydrogel coupled with a cascade of enzymatic reactions for signal amplification and transduction, within a paper-based microfluidic chips | Brown color ruler-readout | Sensitivity, selectivity, low-cost, and ease of operation | Stability was not tested | 16 |
| Liquid crystalline visualization | Bilirubin in biological samples | Immobilizing 4-cyano-40-pentylbiphenyl (5CB) (liquid crystalline) followed by ultrasound-assisted decomposition by hydroxyl radical generated from the oxidase enzymatic reaction of the analyte | Length of color | Simple, accurate with low detection limit, applicable to many biomarkers and reproducible | Require ultrasound systems controlled by a smartphone | 17 |
| Enzymatic, metal complexation, and nanoparticle aggregation device | Glucose, nickel, and glutathione | Enzymatic reactions, metal complexation, and nanoparticle aggregation | Distance-based of color band | Simple, fast, and cost-effective | Sample needs to be solubilized, can be affected by environmental conditions | 18 |
| Silver nanoparticle aggregation on paper-based devices | Aerosol oxidative activity | Elman's reagent with chemical oxidation of dithiothreitol on silver nanoparticle aggregation coupled to glutathione oxidation in a paper-based device | Length of color | Sensitive, rapid, simple, portable, low-cost | Environmental applications only | 19 |
| Paper/poly(methyl methacrylate) (PMMA) integrated microfluidic ELISA-Chip | Immunoglobulin G | Utilizes the movement of immunoassay complexes with magnetic beads using a permanent magnet in PMMA | Distance-based of color | Accurate, cost-effective, disposable, and easy to fabricate | Stability was not tested | 20 |
| Passive flow of amphiphilic biosample on a microfluidic device | Amphiphilic biosample (surfactants, DNA, bovine serum albumin, human albumin, nitrite, glucose, and low-density lipoprotein) | The flow length decreased with increasing concentration of an amphiphilic sample because of adsorption of the sample on the hydrophobic barrier | Distance-based of color band | Simple, no reagents, immediate, portable, small volume, and cheap | Semi-quantitative, not specific, affected by environmental conditions | 21 |
In this study, we combined color intensity, chromatographic migration distance, and text reporting by ruler or numbers using patterned paper to generate a new quantitative and equipment-free prototype for antibody detection using stained bacterial agents. The assay prototype was optimized, validated and successfully detected anti-Brucella and anti-Salmonella antibodies in samples. The assay can have extended applications to measure different analytes/antibodies/antigens in the serum or plasma of targeted patients. The color can be provided using naturally colored cells (e.g., red blood cells),29 stained cells (e.g., bacterial cells),23 or antigens/antibodies absorbed to colored beads/particles (e.g., latex beads or nanoparticles).26 Concentration-dependent migration can be achieved by agglutination, aggregation, competitive inhibition, flow-dependent saturation, and other methods.12–20 Quantification can be achieved by printing numbers as a ruler,12,16 or as described in this study, by printing numbers as hydrophilic or hydrophobic patterns (Fig. 11), or using match color intensity of numbers and cells.
Limited assays have reported equipment-free, text-based signal readout of paper-based POCs.5,6 Paper-based assay that reports ABO/RhD blood grouping “in writing” was initially reported by Li et al.,29 followed by reporting other secondary blood groups as “+/−”.30 Yamada et al. reported human albumin in text using “–, Tr., 1+, 2+, 3+, 4+” displayed on a barrier-involved patterned paper-based analytical device.31 A barrier-free paper-based assay was developed that reports DNA aptamers text as “0, Lo, Med, Hi”32 and metal ions as “Fe” “Ni” or “Cu” with color intensity increased with increased ion concentration.33 All these assays are qualitative or semi-quantitative.29–33 In this study, printed numbers were used to report the exact quantity of serum antibodies using a new approach and microfluidic design. This approach significantly enhances the applicability of the device for user-friendly unambiguous quantitative detection by showing the target concentration as a “number”.
The agglutination test depends on the ability of antibodies to react with antigens on cells or particles to form clumps. Agglutination can be visualized by the naked eye using slide, card, and tube methods or through automated machines. The test has a wide range of applications including blood grouping, detection of infections, and autoimmunity.34,35 Among infections, agglutination tests are widely used for Brucella and Salmonella diagnosis.24,25 The assessment of slide/card agglutination test results by the naked eye is influenced by observers' subjectivity, affecting its accuracy,35 requires an experienced operator, which limits its applicability to POC devices,34 and requires immediate analysis and proper storage of antibodies and reagents.36 The semi-quantitative assessment of slide/card agglutination test using a 4+ to 0 scale is furthermore difficult and subjective.34,35 In this study, we developed, validated, and applied a quantitative, equipment-free, paper-based POC assay of bacterial cell agglutination. This test can be used for simple “+/−” results or the quantitative analysis of a visual signal length that corresponds to the target concentration in a thermometer-like approach. The assay is simple, fast, reliable, uses cheap materials in an innovative design, and can have extended applications of agglutination assays.
The advantage of using the paper-based agglutination assay developed in this study includes open reading time as the results are stable for days in the paper while standard slide agglutination had a very short reading window of a few minutes before the reaction fades due to dryness and disabled agglutination.36 The clarity of positive versus negative results using color intensity and the elution tail of paper-based agglutination assay compared to standard slide/card agglutination assays in which the visual observation of positive agglutination reaction is subjective.35 Clarity and simplicity can be further enhanced in paper-based agglutination assay using “yes/no” or “+/−” design, which increases its applicability to POCs test by the lay personnel. Importantly, quantitative assessment of slide/card agglutination assays is compromised by the difficulty in observation of the agglutination strength,34,35 while distance-based quantification in paper-based agglutination assay is more accurate and reliable.
The paper-based assay used the chromatographic elution of colored agglutination clumps based on size within a porous paper matrix to provide a distance-based migration that corresponds to the target concentration. The mechanism has been described previously for the qualitative detection of blood groups or plasma and serum separation.8,27,37,38 Quantitative and equipment-free POCs were used for the measurement of enzymes, proteins, nucleic acids, and metal ions with limited reports on infectious agents or cells.1,4,5,7,12,16,20 The validation of bacterial cell agglutination, quantitative transformation based on migration distance, and clinical applications on Brucella and Salmonella infections were not reported before. Implications of POC tests for infectious diseases diagnosis including Brucella and Salmonella are huge, especially in endemic low resources countries.22–26 Other approaches for simple, quantitative, distance-based paper POC assays were developed.8–17 Color band height or distance were used for cholesterol, high-density lipoprotein, glucose, bilirubin, nickel, glutathione, immunoglobulin, aerosol oxidative activity, cocaine, lactoferrin, theophylline, and metals measurements.8–20 Semi-quantitative flow distance measurements of DNA, bovine serum albumin, human albumin, and low-density lipoprotein were also reported.21
Conclusions
Colored agglutinated bacterial cells by corresponding antibodies migrate within the porous paper structure according to the size/strength of agglutination after chromatographic elution with a buffer. The distance of migration can be used as a reading point in a thermometer-like approach to quantify antibody concentrations. This principle was optimized and validated for the quantitative detection of anti-Brucella and anti-Salmonella antibodies. The same approach can be used for the detection of other anti-bacterial antibodies or other agglutination assays. Furthermore, the principle can be applied to human cells including red blood cells agglutination, latex beads agglutination, and other types of beads to detect and quantify the corresponding antibodies in different diseases. A simple, quantitative, and equipment-free paper-based POC prototype that detects serum antibodies using bacterial cell agglutination elution was successfully developed. This test can be used for simple “+/−” results or distance-based quantitative analysis of corresponding target concentrations. Different text reporting with numbers using microfluidic patterns has been developed to enhance the non-technical end-user applications.Ethical approval
The study protocol was approved by the IRB at The Hashemite University. Blood samples were collected from volunteers or patients after obtaining informed consent.Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
All authors declare no conflicts of interest.Acknowledgements
This work was funded by Al-Quds Academy for Scientific Research (QASR), ARIF 2019. Amman, Jordan. We would like to thank Al-Quds Academy for Scientific Research.References
- T. Tian, J. Li, Y. Song, L. Zhou, Z. Zhu and C. J. Yang, Distance-based microfluidic quantitative detection methods for point-of-care testing, Lab Chip, 2016, 16(7), 1139–1151, 10.1039/c5lc01562f.
- Y. Hou, C. C. Lv, Y. L. Guo, X. H. Ma, W. Liu, Y. Jin, B. X. Li, M. Yang and S. Y. Yao, Recent Advances and Applications in Paper-Based Devices for Point-of-Care Testing, J. Anal. Test., 2022, 6(3), 247–273, DOI:10.1007/s41664-021-00204-w.
- R. Pelton, Bioactive paper provides a low-cost platform for diagnostics, Trends Anal. Chem., 2009, 28(8), 925–942, DOI:10.1016/j.trac.2009.05.005.
- S. T. Phillips and G. G. Lewis, The expanding role of paper in point-of-care diagnostics, Expert Rev. Mol. Diagn., 2014, 14(2), 123–125, DOI:10.1586/14737159.2014.887445.
- Z. Li, M. You, Y. Bai, Y. Gong and F. Xu, Equipment-Free Quantitative Readout in Paper-Based Point-of-Care Testing, Small Methods, 2020, 4, 1900459, DOI:10.1002/smtd.201900459.
- D. Yang, C. Hu, H. Zhang and S. Geng, Recent Developments in Paper-Based Sensors with Instrument-Free Signal Readout Technologies (2020-2023), Biosensors, 2024, 14(1), 36, DOI:10.3390/bios14010036.
- E. Fu, Enabling robust quantitative readout in an equipment-free model of device development, Analyst, 2014, 139(19), 4750–4757, 10.1039/c4an01003e.
- R. F. Zuk, V. K. Ginsberg, T. Houts, J. Rabbie, H. Merrick, E. F. Ullman, M. M. Fischer, C. C. Sizto, S. N. Stiso and D. J. Litman, Enzyme immunochromatography--a quantitative immunoassay requiring no instrumentation, Clin. Chem., 1985, 31(7), 1144–1150 CrossRef CAS.
- R. Chen, T. M. Li, H. Merrick, R. F. Parrish, V. Bruno, A. Kwong, C. Stiso and D. J. Litman, An internal clock reaction used in a one-step enzyme immunochromatographic assay of theophylline in whole blood, Clin. Chem., 1987, 33(9), 1521–1525 CrossRef CAS.
- M. P. Allen, A. DeLizza, U. Ramel, H. Jeong and P. Singh, A noninstrumented quantitative test system and its application for determining cholesterol concentration in whole blood, Clin. Chem., 1990, 36(9), 1591–1597 CrossRef CAS.
- V. Y. Liu, T. Y. Lin, W. Schrier, M. Allen and P. Singh, AccuMeter noninstrumented quantitative assay of high-density lipoprotein in whole blood, Clin. Chem., 1993, 39(9), 1948–1952 CrossRef CAS.
- D. Zhang, B. Gao, Y. Chen and H. Liu, Converting colour to length based on the coffee-ring effect for quantitative immunoassays using a ruler as readout, Lab Chip, 2018, 18(2), 271–275, 10.1039/c7lc01127j.
- K. Yamada, T. G. Henares, K. Suzuki and D. Citterio, Distance-Based Tear Lactoferrin Assay on Microfluidic Paper Device Using Interfacial Interactions on Surface-Modified Cellulose, ACS Appl. Mater. Interfaces, 2015, 7(44), 24864–24875, DOI:10.1021/acsami.5b08124.
- X. Wei, T. Tian, S. Jia, Z. Zhu, Y. Ma, J. Sun, Z. Lin and C. J. Yang, Microfluidic Distance Readout Sweet Hydrogel Integrated Paper-Based Analytical Device (μDiSH-PAD) for Visual Quantitative Point-of-Care Testing, Anal. Chem., 2016, 88(4), 2345–2352, DOI:10.1021/acs.analchem.5b04294.
- T. Tian, Y. An, Y. Wu, Y. Song, Z. Zhu and C. Yang, Integrated Distance-Based Origami Paper Analytical Device for One-Step Visualized Analysis, ACS Appl. Mater. Interfaces, 2017, 9(36), 30480–30487, DOI:10.1021/acsami.7b09717.
- D. Huang, D. Ni, M. Fang, Z. Shi and Z. Xu, Microfluidic Ruler-Readout and CRISPR Cas12a-Responded Hydrogel-Integrated Paper-Based Analytical Devices (μReaCH-PAD) for Visible Quantitative Point-of-Care Testing of Invasive Fungi, Anal. Chem., 2021, 93(50), 16965–16973, DOI:10.1021/acs.analchem.1c04649.
- K. Khachornsakkul, J. J. Chang, P. H. Lin, Y. H. Lin, W. Dungchai and C. H. Chen, Highly sensitive distance-based liquid crystalline visualization for paper-based analytical devices, Anal. Chim. Acta, 2021, 1154, 338328, DOI:10.1016/j.aca.2021.338328.
- D. M. Cate, W. Dungchai, J. C. Cunningham, J. Volckens and C. S. Henry, Simple, distance-based measurement for paper analytical devices, Lab Chip, 2013, 13(12), 2397–2404, 10.1039/c3lc50072a.
- W. Dungchai, Y. Semeion, O. Chailapakul, J. Volckens and C. S. Henry, Determination of aerosol oxidative activity using silver nanoparticle aggregation on paper-based analytical devices, Analyst, 2013, 138(22), 6766–6773, 10.1039/c3an01235b.
- M. F. Abate, M. G. Ahmed, X. Li, C. Yang and Z. Zhu, Distance-based paper/PMMA integrated ELISA-chip for quantitative detection of immunoglobulin G, Lab Chip, 2020, 20(19), 3625–3632, 10.1039/d0lc00505c.
- Y. T. Chen and J. T. Yang, Detection of an amphiphilic biosample in a paper microchannel based on length, Biomed. Microdevices, 2015, 17(3), 9954, DOI:10.1007/s10544-015-9954-9.
- O. Clerc and G. Greub, Routine use of point-of-care tests: usefulness and application in clinical microbiology, Clin. Microbiol. Infect., 2010, 16(8), 1054–1061, DOI:10.1111/j.1469-0691.2010.03281.x.
- R. Díaz, A. Casanova, J. Ariza and I. Moriyón, The Rose Bengal Test in human brucellosis: a neglected test for the diagnosis of a neglected disease, PLoS Neglected Trop. Dis., 2011, 5(4), e950, DOI:10.1371/journal.pntd.0000950.
- G. Di Bonaventura, S. Angeletti, A. Ianni, T. Petitti and G. Gherardi, Microbiological Laboratory Diagnosis of Human Brucellosis: An Overview, Pathogens, 2021, 10(12), 1623, DOI:10.3390/pathogens10121623.
- D. P. Neupane, H. P. Dulal and J. Song, Enteric Fever Diagnosis: Current Challenges and Future Directions, Pathogens, 2021, 10(4), 410, DOI:10.3390/pathogens10040410.
- L. Wijedoru, S. Mallett and C. M. Parry, Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever, Cochrane Database Syst. Rev., 2017, 5(5), CD008892, DOI:10.1002/14651858.CD008892.
- M. Al-Tamimi, S. Altarawneh, M. Alsallaq and M. Ayoub, Efficient and Simple Paper-Based Assay for Plasma Separation Using Universal Anti-H Agglutinating Antibody, ACS Omega, 2022, 7(44), 40109–40115, DOI:10.1021/acsomega.2c04908.
- B. Alsaeed and F. R. Mansour, Distance-based paper microfluidics; principle, technical aspects and applications, Microchem. J., 2020, 155, 104664, DOI:10.1016/j.microc.2020.104664.
- M. Li, J. Tian, M. Al-Tamimi and W. Shen, Paper-based blood typing device that reports patient's blood type "in writing", Angew Chem. Int. Ed. Engl., 2012, 51(22), 5497–5501, DOI:10.1002/anie.201201822.
- M. Li, W. L. Then, L. Li and W. Shen, Paper-based device for rapid typing of secondary human blood groups, Anal. Bioanal. Chem., 2014, 406(3), 669–677, DOI:10.1007/s00216-013-7494-9.
- K. Yamada, K. Suzuki and D. Citterio, Text-Displaying Colorimetric Paper-Based Analytical Device, ACS Sens., 2017, 2(8), 1247–1254, DOI:10.1021/acssensors.7b00464.
- C. Carrasquilla, J. R. Little, Y. Li and J. D. Brennan, Patterned paper sensors printed with long-chain DNA aptamers, Chemistry, 2015, 21(20), 7369–7373, DOI:10.1002/chem.201500949.
- L. Zhang, L. Guan and Z. Lu, et al., Barrier-free patterned paper sensors for multiplexed heavy metal detection, Talanta, 2019, 196, 408–414, DOI:10.1016/j.talanta.2018.12.096.
- M. Huet, M. Cubizolles and A. Buhot, Red Blood Cell Agglutination for Blood Typing Within Passive Microfluidic Biochips, High-Throughput, 2018, 7(2), 10, DOI:10.3390/ht7020010.
- N. Sheng, L. Liu and H. Liu, Quantitative determination of agglutination based on the automatic hematology analyzer and the clinical significance of the erythrocyte-specific antibody, Clin. Chim. Acta, 2020, 510, 21–25, DOI:10.1016/j.cca.2020.06.042.
- E. J. Mann, S. J. Rogerson and J. G. Beeson, An alternative agglutination assay to measure antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes, Trans. R. Soc. Trop. Med. Hyg., 2003, 97(6), 717–719, DOI:10.1016/s0035-9203(03)80111-6.
- F. S. Ebrahimi, M. Paknejad and M. Aminian, Paper-based analytical devices for blood grouping: a comprehensive review, Biomed. Microdevices, 2021, 23(3), 34, DOI:10.1007/s10544-021-00569.
- M. Al-Tamimi, M. El-Sallaq, S. Altarawneh, A. Qaqish and M. Ayoub, Development of Novel Paper-Based Assay for Direct Serum Separation, ACS Omega, 2023, 8(23), 20370–20378, DOI:10.1021/acsomega.3c00215.
| This journal is © The Royal Society of Chemistry 2024 |