Open Access Article
Open Access ArticleModulating acid sites in Y zeolite for valorisation of furfural to get γ-valerolactone†
Malu Thayil Jayakumari and
Cheralathan Kanakkampalayam Krishnan *
*
Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore 632014, India. E-mail: Cheralathan.k@vit.ac.in
First published on 8th July 2024
Abstract
Furfural is a biomass-derived platform molecule that can be converted into a variety of useful products. Catalysts having appropriate balance between Lewis and Brønsted acid sites are suitable for valorisation of furfural. Lewis acidic metal ion incorporated zeolites were studied for this purpose. However, incorporating Lewis acidic metal ions into an alumino-silicate framework of a zeolite is a cumbersome process. Hence, an attempt has been made in this work to modulate the acid sites of Y zeolite via thermal treatment to effect controlled dealumination and use it for valorisation of furfural using isopropyl alcohol, which is a cascade transformation. The thermal treatment of zeolites changed the distribution of acid sites and increased the weak plus moderate to strong acid site ratio. Among the thermally dealuminated Y, beta and mordenite zeolites, with SiO2/Al2O3 ratio 5.2, 25 and 20, only Y zeolite could yield γ-valerolactone, the final product of the aimed cascade transformation. Complete conversion of furfural and 52% γ-valerolactone yield could be achieved under the optimized conditions using NH4Y zeolite thermally dealuminated at 700 °C (TY700). The better catalytic activity of TY700 could be correlated to a combination different factors such as framework structure, suitable weak plus moderate to strong acid site ratio, presence of both penta-coordinated and octahedral Al sites and balance between Brønsted and Lewis acid sites.
1. Introduction
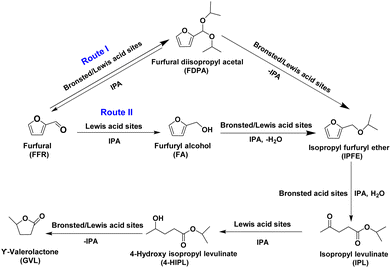
Biomass-derived sustainable fuels and chemicals are desired to decrease the dependence on non-renewable fossil resources. Lignocellulosic biomass is a type of plant-based material made up of lignin, cellulose, and hemicellulose, typically derived from trees, grasses and agricultural wastes. It is an attractive renewable source for producing a wide range of molecules having potential uses to produce industrial chemicals, solvents and fuels.1 Different processes have been employed for breaking down the complex lignocellulosic biomass to get useful products and molecules.2–4 Lignocellulosic biomass upon hydrolysis can yield furanic molecules which can be further employed as precursors for production of a wide range of important chemicals including solvents, monomers, fuels, and fuel additives.5 Furfural (FFR) is one of the thirty platform molecules obtained via hydrolysis of lignocellulosic biomass6–8 and it is mass-produced from hemi cellulosic feedstocks.9,10 Furfural is highly reactive due to the presence of an aldehyde functional group and hence it can be easily functionalized via different chemical routes to get molecules of higher value.11 The different value-added molecules, obtained from FFR are furfuryl alcohol (FA), furfuryl alkyl ethers (FE), levulinic acid (LA), alkyl levulinates (AL), α & β-angelica lactones (AGL) and γ-valerolactone (GVL).10,12–15Conventionally, catalytic reduction of FFR using molecular hydrogen and a noble metal catalyst was used to obtain the value-added molecules. Controlling the reaction pathways and the degree of hydrogenation using a selective catalyst is always a challenge in catalytic hydrogenation of furfural.16 Catalytic transfer hydrogenation (CTH) or Meerwein–Ponndorf–Verley (MPV) reduction of FFR using alcohols as hydrogen sources is considered as an alternative to the conventional catalytic hydrogenation. CTH of FFR is normally carried out using secondary alcohols such as isopropanol (IPA) and secondary butanol as hydrogen donors in the presence of a Lewis acid-base catalyst.17,18 Based on the reaction parameters,19 nature of the catalyst,10 and the kind of solvent used,20 valorisation of FFR may proceed in different pathways. For example, FFR valorisation using isopropanol in the presence of acid catalysts may proceed through different steps and produce a series of products as shown in Scheme 1.12,21–23 If Lewis acid sites suitable for MPV reduction are present, the main pathway followed is CTH of FFR into furfuryl alcohol and its subsequent etherification to form furfuryl ether. If Brønsted acid sites are present along, FFR can undergo acetalization with the alcohol and form diisopropyl furfuryl acetal. Then, by elimination of an alcohol in the presence of acid sites, it can form isopropyl furfuryl ether. In the next step, furanic ring opening of the furfuryl ether gives rise to isopropyl levulinate. Finally, the CTH of isopropyl levulinate and further lactonization may yield GVL (Scheme 1). Lewis acid sites present in zirconium, tin, and titanium containing catalysts including zeolites are known to catalyse MPV reduction.24–28 However, the presence of both Lewis and Brønsted acid sites within the catalyst is important for promoting conversion of the different intermediates shown in Scheme 1 and finally to form GVL. For instance, when Sn- and Zr- loaded beta zeolite is an effective catalyst for the MPV reduction of FFR to furfuryl alcohol,29 Zr supported on dealuminated zeolite Y30 and bimetallic Sn–Zr supported on dealuminated Y zeolite have been shown to yield GVL.31 The importance of Lewis and Brønsted acid sites in converting FFR to GVL has been demonstrated by Bui et al. using a mixture of Zr-beta and Al-MFI zeolite as catalyst.32
The introduction of Lewis acidic metal into the zeolite framework is usually carried out either via direct synthesis33,34 or via post synthetic modifications;35,36 the latter includes dealumination/removal of Al from zeolite and subsequent metal ion incorporation involving cumbersome multiple step procedure.37 In this regard, generating Lewis acid sites stemming from Al intrinsic to zeolites and exploiting the associated Brønsted acid sites of the same zeolite to catalyse FFR valorisation would avoid the use of costly metals and tedious multistep synthesis procedures. For instance, as reported recently, properly tuning the coordination environment of Al in Y zeolite via controlled thermal dealumination can produce a catalyst suitable for MPV reduction of levulinic acid and isopropyl levulinate to yield GVL.38 During dealumination procedure, when zeolite is treated at temperatures >500 °C, in the presence of air (thermal treatment) or air saturated with water vapor (steam treatment), Si–(OH)–Al bonds in the tetrahedral framework are broken completely or partially leading to either extra-framework aluminium (EFAl) or partially detached Al sites.38 Through this dealumination process, different Al sites such as framework-associated, partially detached, distorted-tetrahedral or penta-coordinated Al, and hexa-coordinated extra-framework aluminium could be formed.39 The presence of these multiple Al species detached from the zeolite framework can create Lewis acid sites of different characteristics, and further, along with these Lewis acid sites, Brønsted acid sites originated from tetrahedral Al will coexist within the same zeolite,39 making the dealuminated zeolite suitable for cascade reactions. With this background, in this work, we aim at creating a suitable catalyst via controlled dealumination of zeolite framework for conversion of FFR to GVL. This method has advantage as acid sites could be modulated in a relatively simple procedure without the need of incorporating an external Lewis acidic metal onto the zeolite framework. To fulfil this, ammonium forms of Y, beta and mordenite zeolites were thermally treated at different conditions to effect dealumination. After proper physicochemical characterization, the catalytic performance of the dealuminated zeolites were compared. The nature & distribution of acid sites and different Al species generated during the thermal and steam treatment were correlated with FFR conversion and GVL formation.
2. Experimental
2.1 Materials
Ammonium forms of Y zeolite (NH4Y) (CBV500, SiO2/Al2O3 = 5.2), beta zeolite (CP81E, SiO2/Al2O3 = 25), and mordenite (CBV21A, SiO2/Al2O3 = 20) were obtained from Zeolyst International, USA. Furfural (98%) and levulinic acid (98%) were obtained from Sigma-Aldrich, India. Isopropanol (99%) was procured from SD Fine chemicals, India. Furfuryl alcohol (>98%), γ-valerolactone (>98.0%), α-angelica lactone (>98.0%), mesitylene (>98.0%), were purchased from Tokyo Chemical Industry (India) Private Ltd. Isopropyl levulinate (IPL) was synthesized in the laboratory and confirmed by spectroscopic methods.382.2 Catalyst preparation
The thermally treated zeolite catalysts were prepared as reported earlier.38 Briefly, NH4Y zeolite was heated in the presence of air (thermal treatment) or air saturated with water (steam treatment) at different temperatures ranging from 500–800 °C. The catalysts thus obtained were coded as TYX for thermal treated samples and SYX for steam treated samples, where X denotes the treatment temperatures. In this manner, different catalysts named as TY500, TY600, TY700, TY800, SY500 and SY700 were prepared. NH4 forms of beta and mordenite zeolites were also thermally treated at 500, 700 and 800 °C and named as TB500, TM500, TB700, TM700, TB800 and TM800. In these catalyst codes, Y, B and M denotes Y, beta and mordenite respectively.2.3 Catalysts characterization
Bruker D8 Advance X-ray diffractometer was used to collect the X-ray diffraction (XRD) patterns of the prepared catalysts. Cu Kα X-ray (λ = 1.5406 Å) was used and the catalyst powders were scanned starting from 5 to 90° 2θ. Multipoint Brunauer–Emmett–Teller (BET) surface area of the catalysts was determined using nitrogen adsorption at 77 K using Quantachrome, ASiQwin Autosorb iQ Station. The catalysts were heated at 150 °C for 24 h during outgassing step carried out before N2 adsorption. t-Plot method was used to determine micropore surface area and micropore volume. Microtrac, BELCAT II catalyst analyzer equipped with thermal conductivity detector (TCD) was used to study temperature programmed desorption of ammonia (TPD-NH3). The catalyst was heated and outgassed at 250 °C for 2 h. After the outgassing step, the catalyst was cooled down to 100 °C and ammonia gas (20% in helium) was allowed to flow (flow rate = 15 mL min−1) for 30 min. Then, the catalyst was purged with He for 30 min. After purging, the catalyst was heated from 100 to 700 °C (heating rate = 10 °C min−1) under He flow to facilitate desorption of ammonia. Jeol ECX400 multinuclear FT-NMR spectrometer was used to obtain 27Al MAS NMR spectra of the catalysts. AlCl3 was used as the reference. The catalyst samples were spun at the speed of 10 kHz at radio frequency of 104 MHz. Jasco FT-IR 4100 spectrometer equipped with MCT detector was utilized to obtain pyridine FT-IR spectra of the catalysts. About 40 mg of catalyst was pressed into the form of a disc and then heated at 200 °C for 3 h under vacuum in an IR cell to remove adsorbed water. After this step, pyridine vapour was allowed to adsorb on the catalyst at room temperature. The excess pyridine was removed by applying vacuum and then the spectra were recorded.2.4 Catalytic reaction
The reaction was performed in a 100 mL high-pressure reactor made of stainless-steel and having Teflon lining inside. In a typical experiment, the reactor was charged with 1 mmol FFR dissolved in 20 mL of isopropanol (IPA) followed by addition of predetermined quantity of catalyst and mesitylene as internal standard. The reaction mixture was magnetically stirred at 1000 rpm using a magnetic pellet and heated to different temperatures between 100 to 190 °C. The reaction was carried out for different time durations between 1 to 12 h. After the reaction, the reactor was cooled down to room temperature and the reaction mixture was centrifuged to remove the catalyst and analyzed by using a gas chromatograph (Shimadzu GC 2010 gas chromatograph (GC)), Stabilwax-DA capillary column (30 m, 0.25 mm ID, 0.25 μm) equipped with flame ionization detector. The standards of the expected products (furfuryl alcohol, isopropyl levulinate, α-angelica lactone, levulinic acid and GVL) were used for identification of the products in GC based on their retention time. The products were also confirmed using Gas Chromatography/Mass Spectrometry (GC/MS) (Model: PerkinElmer Clarus 680/PerkinElmer Clarus 600) equipped with Elite-5ms capillary column (30 m × 0.25 mm I.D. × 0.25 μm). The concentrations of the individual products in the reaction mixture were determined by using GC standard calibration plots. As the standards are not available, the quantification of furfural diisopropyl acetal (FDPA) and isopropyl furfuryl ether (IPFE) was done using the calibration plots of FA and IPL respectively. The presence of FDPA and IPFE was also confirmed by the GC-MS analysis (See ESI, Fig. S1 and S2†). The reaction conditions for TOFFFR analysis were optimized to get below 50% conversion of furfural. The TOFFFR of the catalysts were calculated based on FFR conversion and acid sites measured from TPD-NH3 profiles. The following equations were used to find conversion of furfural, product yield, product selectivity and turnover frequency (TOFFFR):3. Result and discussion
3.1 X-ray diffraction and N2 adsorption analysis
Powder X-ray diffraction analysis was carried out to determine crystallinity and structure of the prepared zeolite catalysts and the diffraction patterns are shown in ESI Fig. S3.† In Y zeolites, the peak intensity showed a decreasing trend moving from TY500 to TY800 and SY500 to SY700. In beta zeolites, TB500 & TB700 the XRD patterns were of similar shape and intensities, but in TB800 the peak intensity decreased. In mordenite, the peak intensity decreased moving from TM500 to TM800. The decrease in intensity of the peaks in X-ray diffraction patterns of zeolites, while increasing the treatment temperature greater than 500 °C could be due to breaking and disappearance of Si–(OH)–Al bridges leading to detachment of aluminium from the framework and the resulted structural damage. Nitrogen adsorption isotherms, multipoint BET surface area and pore volume data of the catalysts are given in Fig. S4† and Table 1. As a general trend, increasing thermal and steam-treatment temperature decreases multipoint BET surface area, and micropore surface area of the catalysts. The decrease in X-ray diffraction peak intensity, multipoint point BET surface area and micropore surface area is likely due to structural damage caused to the framework of the zeolites upon heating at higher temperatures.38| Catalyst | BET surface area (m2 g−1) | Micropore surface area (m2 g−1) | External surface area (m2 g−1) | Micropore volume (Vmicro) (cm3 g−1) | Total pore volume (Vtotal) (cm3 g−1) | V(micro)/V(total) (cm3 g−1) |
|---|---|---|---|---|---|---|
| TY500 | 746 | 699 | 47 | 0.259 | 0.379 | 0.683 |
| TY600 | 717 | 671 | 46 | 0.256 | 0.371 | 0.690 |
| TY700 | 713 | 657 | 56 | 0.250 | 0.369 | 0.677 |
| TY800 | 675 | 618 | 57 | 0.237 | 0.380 | 0.623 |
| SY500 | 726 | 667 | 59 | 0.255 | 0.371 | 0.687 |
| SY700 | 664 | 611 | 53 | 0.234 | 0.372 | 0.629 |
| TB500 | 500 | 352 | 148 | 0.142 | 0.809 | 0.175 |
| TB700 | 466 | 314 | 152 | 0.127 | 0.714 | 0.177 |
| TB800 | 413 | 249 | 164 | 0.107 | 0.632 | 0.169 |
| TM500 | 387 | 305 | 82 | 0.120 | 0.285 | 0.421 |
| TM700 | 290 | 163 | 127 | 0.066 | 0.204 | 0.323 |
| TM800 | 224 | 53 | 171 | 0.042 | 0.189 | 0.222 |
3.2 Characterization of acid sites
The nature of acid sites existing in the prepared catalysts were investigated by temperature programmed desorption of NH3. The desorption profiles were deconvoluted using Gaussian function to understand the distribution of weak, moderate, and strong acid site and presented in Fig. S5 in ESI.† In the TPD profiles, the desorption of NH3 between 100 to 250 °C was ascribed to weak acid sites; the desorption from 400 to 600 °C was related to strong acid sites; the desorption feature from 250 to 400 °C was correlated to moderate acid sites.38,40a–c,41a,b All the zeolites studied showed three desorption features corresponding to weak, moderate, and strong acid sites. A comparison of total number of acid sites and contribution from weak, moderate, and strong acid sites is presented in Table 2. Upon thermal treatment of NH4Y zeolite from 500 to 800 °C, a gradual decrease in total number of acid sites could be observed.| Catalyst | Weak (mmol g−1) | Moderate (mmol g−1) | Strong (mmol g−1) | Total (mmol g−1) |
|---|---|---|---|---|
| TY500 | 0.69 | 0.44 | 0.33 | 1.46 |
| TY600 | 0.70 | 0.42 | 0.22 | 1.34 |
| TY700 | 0.70 | 0.40 | 0.15 | 1.25 |
| TY800 | 0.70 | 0.39 | 0.09 | 1.18 |
| SY500 | 0.69 | 0.35 | 0.26 | 1.312 |
| SY700 | 0.59 | 0.35 | 0.13 | 1.087 |
| TB500 | 0.68 | 0.33 | 0.19 | 1.20 |
| TB700 | 0.53 | 0.25 | 0.11 | 0.896 |
| TM500 | 0.68 | 0.23 | 0.39 | 1.30 |
| TM700 | 0.47 | 0.17 | 0.20 | 0.842 |
Further, it can be noticed that the strong acid sites of the Y zeolite are affected the most while increasing thermal treatment temperature. The moderate acid sites of Y zeolite are affected to a lesser extent, while weak acid sites are retained during the thermal treatment. The decrease in the amount of strong acid sites in Y zeolite with increasing temperature can be attributed to breaking and disappearance of Si–(OH)–Al bridges that are responsible for the strong Brønsted acid sites. When this occurs, the resulted detachment of aluminium from the framework may lead to different Al species (Section 3.3). Compared to TY500 and TY700, their steam treated counterparts, SY500 and SY700 showed lower total number of acid sites (TY500 = 1.46 mmol g−1, SY500 = 1.312 mmol g−1, TY700 = 1.25 mmol g−1, SY700 = 1.087 mmol g−1). Further it can be noticed that steam treatment affected all the three, weak, moderate, and strong acid sites without any selectivity. In the case of beta and mordenite zeolites, compared to thermal treatment at 500 °C, thermal treatment at 700 °C drastically decreased the total number of acid sites, and all the three types of acid sites were affected.
The chart of percentage distribution of weak, moderate & strong acid sites in the catalysts is given in Fig. S6 in ESI.† The chart indicates that when the treatment temperature is increased, percentage of strong acid sites in Y zeolites decreases and at the same time percentage of weak acid sites increases. It can be also noted that the distribution of moderate acid sites in Y zeolites is not affected much while varying the thermal treatment temperature. The similar trend was observed upon steam treatment in the case of SY500 & SY700 and thermal treatment in the cases of TB500 & TB700 and TM500 & TM700.
3.3 Coordination environment of aluminium
The 27Al-MAS NMR spectroscopy was utilized to assess the nature of the coordination environment of aluminium in the studied catalysts. Based on the NMR chemical shift values, different aluminium sites, such as tetrahedral Al (AlIV-I, AlIV-II) (chemical shift range = 55–65 ppm), penta-coordinated Al (AlV) (chemical shift range = 30–40 ppm) and octahedral Al (AlVI) (chemical shift range = 0–6 ppm), also known as extra-framework Al could be identified.42,43 Further, based on the area under the resonance peaks in the deconvoluted 27Al-MAS NMR spectra (see ESI Fig. S7†), the distribution of the different aluminium sites was calculated and presented in Fig. S8 in ESI.†In NH4Y zeolite before thermal treatment, AlIV-I, AlV & AlVI sites are present. While increasing the thermal treatment temperature of NH4Y from 500 to 700 °C (TY500, TY600 and TY700), AlIV-II starts to appear and at the same time, AV & AlVI sites tend to increase at the expense of AlIV-I sites. However, this trend is broken in TY800, wherein, AlIV-I and AlVI sites are present, AlIV-II sites are absent and AlV sites decreased. In SY500, like TY500, AlIV-I, AlIV-II, AV & AlVI sites can be seen, but SY700 & TY700 show completely different distribution of the Al sites. In SY700, AlIV-II sites are absent and only a smaller percentage of AlV sites are present compared with TY700.
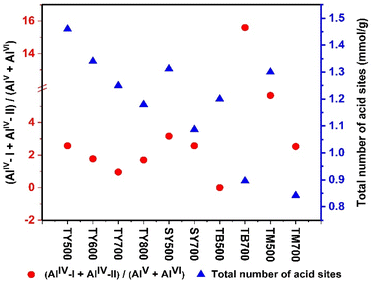
In the case of beta zeolites, TB500 have only AlIV sites, but while increasing the treatment temperature to 700 °C (TB700), AlVI sites appeared. In TM500, AlIV-I, AlIV-II & AlVI sites are observed. While increasing the thermal treatment temperature from 500 to 700 °C, in TM700, AlIV-II & AlVI sites tend to increase at the expense of AlIV-I sites. It can be noted that in beta and mordenite zeolite samples, AlV sites are totally absent. Importantly, compared with all the other zeolites prepared and studied here, in TY700, greater percentage of framework associated penta-coordinated, AV species are present. Table S1 in ESI† compares (AlIV-I + AlIV-II)/(AlV + AlVI) ratio obtained for all the prepared catalysts and the lowest ratio is obtained for TY700, which indicates that it has more Al dislodged from the zeolite framework. Fig. 1 correlates (AlIV-I + AlIV-II)/(AlV + AlVI) ratio with total acid sites, which indicates that when dealumination of the framework occurs and AlV, AlVI sites are generated the total number of acid sites are decreased.
3.4 Catalytic activity studies
The prepared zeolites were studied as catalysts for converting FFR to value-added products in the presence of IPA under same reaction conditions and the results are compared in Table 3. On all the catalysts studied, isopropyl levulinate (IPL) was obtained as the major product. Furfural diisopropyl acetal, isopropyl furfuryl ether and γ-valerolactone were obtained as minor products. Some unidentified products in trace quantities (<1%) and acetone & diisopropyl ether, the products obtained from IPA were also observed in the reaction mixtures. A separate reaction run was also performed in the absence of catalyst under the same reaction conditions, and none of the mentioned products were detected, which confirms the role of catalyst in the reaction. No furfuryl alcohol was detected over any of the zeolites under the studied reaction conditions, but formation of furfural diisopropyl acetal was observed over all the catalysts.| Entry | Catalyst | FFR conversion (%) | FDPA selectivity (%) | IPFE selectivity (%) | IPL selectivity (%) | GVL selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | TY500 | 60 | 18 | 3 | 74 | 4 |
| 2 | TY600 | 64 | 17 | 10 | 67 | 5 |
| 3 | TY700 | 98 | 1 | — | 76 | 21 |
| 4 | TY800 | 55 | 11 | 7 | 73 | 8 |
| 5 | SY500 | 63 | 7 | 2 | 83 | 5 |
| 6 | SY700 | 81 | 5 | 19 | 54 | 3 |
| 7 | TB500 | 78 | 5 | — | 95 | — |
| 8 | TB700 | 88 | — | 100 | — | |
| 9 | TB800 | 62 | 17 | — | 82 | — |
| 10 | TM500 | 30 | 9 | — | 91 | — |
| 11 | TM700 | 52 | 5 | 48 | 47 | — |
| 12 | TM800 | 9 | 18 | 42 | 40 | — |
Further, no furfuryl alcohol formation was observed on Y zeolites even at much lower conversion rates under different reaction conditions as discussed in the later part of this article. These results indicate that over the catalysts, the cascade reaction proceeds through acetalization of furfural (route I in Scheme 1). In fact, acetalization of furfural and reduction of furfural to form furfuryl alcohol are competitive reactions44,45 and the former suppresses the later and requires acid sites.45,46 Different zeolites possessing strong acid sites were found to efficiently catalyse acetalization of furfural.47,48 Hence, it is likely that FFR to FDPA reaction outperformed FFR to FA conversion in this study because all the catalysts possess strong acid sites. As a general trend, increasing the thermal treatment temperature of the Y zeolites from 500 to 700 °C (TY500, TY600 & TY700), increased the conversion of FFR. Similarly, increasing the steam treatment temperature from 500 (SY500) to 700 °C (SY700) increased the conversion. However, compared with TY700, on SY700 and TY800, the conversion of FFR decreased probably due to large decrease in total surface area (SA of TY700 = 713 m2 g−1, SA of SY700 = 664 m2 g−1, SA of TY800 = 675 m2 g−1). The difference in conversion observed among the different framework type zeolites Y, beta and mordenite could be due their structural difference and surface area. While Y and beta zeolites have three-dimensional pore network with the former having super cages, mordenite possesses two-dimensional pore system. Further, mordenite zeolites prepared in this study show relatively lower surface area compared to Y and beta zeolites. So, the relatively lower conversions obtained on TM500, TM700 and TM800 compared to their Y and beta counterparts could be due to these differences.
However, apart from pore structure and surface area, the nature of the acid sites and their distribution might also play a role in the catalytic activity. Since acetalization is found to be the starting point of the cascade reaction (Scheme 1), the acid sites required for the acetalization reaction could be the determining factor for conversion of furfural. Song et al. studied acetalization of furfural with 1,2 propanediol over SAPO-34, SAPO-11, USY, HY and HZSM-5 zeolites and found that SAPO-34 is found to be best performing catalyst due to greater number of moderate and strong acid sites.47 However, when USY and HY zeolites were compared for the same acetalization reaction, USY (11) zeolite with more weak acid sites (2.12 mmol g−1) and moderate plus strong acid sites (0.60 mmol g−1) combination was found to be the better catalyst than HY (weak acid sites = 1.92 mmol g−1, moderate plus strong acid sites = 0.58 mmol g−1).47 Another study reported that H-USY zeolite (Si/Al = 6, acid sites = 835 μmol g−1) is better catalyst than Hβ (Si/Al = 12.5, acid sites = 855 μmol g−1) and H-mordenite (Si/Al = 10, acid sites = 1656 μmol g−1) for acetalization furfural with ethanol.48 A comparative study on acetalization of furfural with ethanol found that weak acid sites and mesoporosity present in hierarchical beta zeolite makes it a better catalyst than conventional beta zeolite.49 Apart from Brønsted acid sites, acetalization of furfural has been reported even on purely Lewis acidic catalysts.29
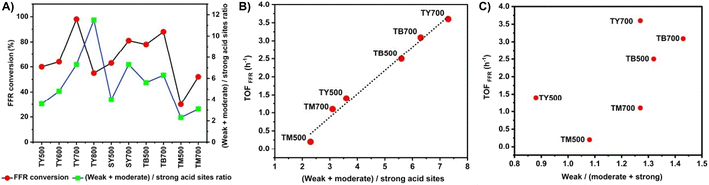
In the present study, the characterization of acid sites of Y zeolites discussed under Section 3.2 indicated that total number of acid sites decrease while increasing thermal and steam treatment temperature. Strong acid sites of the zeolites are affected the most during such treatments, whereas weak and moderate acid sites are not relatively affected much. So, in order to correlate the acid sites distribution and FFR conversion, a correlation graph between, FFR conversion and weak + moderate acid sites to strong acid sites ratio ((W + M)/S ratio) of the catalysts was plotted (Fig. 2(a)). From the graph, it could be seen that within the specific framework type zeolites (Y, beta & mordenite), there is a correlation between FFR conversion and (W + M)/S ratio; when the ratio increases, FFR conversion also increases. Overall, weakening the acid sites by dealumination improves the conversion of furfural, this is in line with previous reports.47–49 However, in TY800, this trend is not followed probably due to its much higher, more than optimum (W + M)/S ratio. Further, to support these conclusions, as shown in Fig. 2(b), a linear relationship was obtained between TOFFFR and (W + M)/S ratio of the thermally dealuminated zeolites. The highest TOFFFR value, 3.6 h−1 obtained for TY700 indicated that it is the best among the catalyst studied. A similar graph of TOFFFR vs. weak acid sites/(moderate acid sites + strong acid sites) ratio was plotted, but no linear relationship could be obtained (Fig. 2(c)). Though IPL was the major product formed over all the different framework zeolites studied, GVL was formed only on Y zeolites. In this aspect, TY700 was found to be the most suitable catalyst for GVL production as it showed 98 ± 2% conversion of FFR and 20 ± 0.4% yield of GVL (Table 3). Incidentally, TY700 is the catalyst having optimum (W + M)/S ratio and higher number of penta-coordinated (AlV) and octahedral aluminium (AlVI) sites (from Section 3.3, 27Al MAS NMR spectroscopy studies). Among the different framework type zeolites studied here, zeolite Y has more Al (SiO2/Al2O3 = 5.2) than beta (SiO2/Al2O3 = 25) and mordenite (SiO2/Al2O3 = 20), hence, probably, it is more susceptible for dealumination and formation penta-coordinated (AlV) and octahedral aluminium (AlVI) sites. In beta and mordenite zeolites though framework eliminated Al species could be generated, they found to be easily reinserted into the framework.50–52 Actually, Lewis acid sites in zeolites can stem from AlIII, AV and extra-framework AlVI species.39,53 However, the tri-coordinated Al sites (AlIII) are in an extremely distorted local environment and normally not visible in the 27Al MAS NMR spectrum because of their strong quadrupolar interactions54,55
Previous study indicated that octahedral extra-framework Al (EFAL) species introduced onto Y and beta zeolites enhance reduction of 4-tert-butylcyclohexanone to its corresponding alcohol, as Lewis acidity of the catalysts is increased.37,56 In another study, Lewis acid sites related to penta-coordinated Al (AV) species in Y zeolite were found to be the potential Lewis acid sites suitable for reduction of levulinic acid and IPL to GVL.38 However, in FFR to GVL conversion via MPV reduction, presence of both Brønsted and Lewis sites are important to get GVL.27,32,57,58 Further, the ratio between Brønsted acid sites/Lewis acid sites (B/L) ratio should be properly tuned for higher GVL yields and selectivity.22,59 In this regard, the B/L ratio of 0.5 to 0.6 is found to be the optimum to maximize the yield/selectivity of GVL.22,59 The pyridine-FTIR spectra of TY500 & TY700 are shown in Fig. S9 (see ESI).† The pyridine-FTIR spectra indicated the presence of both Brønsted and Lewis acid sites which are required for facilitating FFR to GVL transformation. The B/L ratio of the TY500 and TY700 were calculated based on the peak areas under the absorbance bands at 1545 cm−1 and 1450 cm−1 in the spectra. For TY700, the B/L ratio was found to be 0.63 and the same for TY500 was 0.71. The B/L ratio of TY700 (0.63) is close to the B/L ratio requirement reported previously for higher selectivity of GVL.22,59 So, the better catalytic activity of TY700 compared to other catalysts studied here might be due to combination different factors such as framework structure, suitable (W + M)/S acid sites ratio, presence of both AlV and AlVI sites and proper balance between Brønsted and Lewis acid sites.
3.5 Influence of reaction parameters
As TY700 catalyst exhibited the best catalytic activity among the studied catalyst in terms of conversion of FFR and selectivity towards GVL, it was further explored to understand the influence of different reaction parameters. The reaction was carried out at different temperatures such as 100, 150, 175 and 190 °C and the influence of temperature on conversion of FFR and yields of the products are compared in Fig. 3. While increasing the reaction temperature from 100 to 175 °C, conversion increased from 24 ± 0.5% to 98 ± 2% and it became 100% at 190 °C. It could also be observed that at all the studied temperatures, IPL was the major product. No GVL was formed at 100 °C but it started to form (GVL yield = 8 ± 0.2%) when temperature was increased to 150 °C, and its yield reached to 20 ± 0.4% at 175 °C. A drop in GVL yield was observed when the reaction temperature was further increased to 190 °C. The decrease in GVL yield at 190 °C can be attributed to the formation of by-products as suggested by Rao et al.60 At 100 °C the yields of FDPA and IPFE are 3 ± 0.06% and 5 ± 0.1% respectively, and their yields decreased while moving to higher temperatures. From the reaction temperature influence study, it can be concluded that IPL can be produced at relatively lower temperature such as 100 °C on TY700 but higher temperature is required to convert IPL to GVL.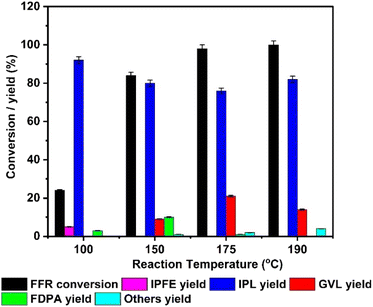 | ||
| Fig. 3 Effect of reaction temperature on furfural conversion and product yield (reaction conditions: FFR = 100 mg (1 mmol), IPA = 20 mL, catalyst wt. = 100 mg, Time = 6 h). | ||
The influence of catalyst loading was studied by varying the weight of TY700 from 50 mg to 500 mg while keeping the other reaction parameters same and the results are depicted in Fig. 4. At the lower catalyst loading of 50 mg, the conversion of furfural was only 49 ± 0.1%, with 26 ± 0.5% yield of IPL and 2 ± 0.1% yield of GVL. Increasing the catalyst loading significantly increased the conversion of FFR and it reached 98 ± 2% at 100 mg and 100% at still higher loadings (200 & 500 mg). As a general trend, increasing the catalyst loading increased the yield of GVL. The maximum yield of GVL, 46 ± 0.1% was obtained when catalyst loading was 200 mg. However, at the same time, the yield of unwanted side products (others) started to increase at 200 and 500 mg loadings. The fall in GVL yield at higher catalyst loading may be due to the conversion of GVL to further products. Formation of IPFE (>2%) was observed only at 50 mg catalyst loading. FDPA yield also decreased from 4 ± 0.08% to 1 ± 0.02% when catalyst loading was changed from 50 to 100 mg.
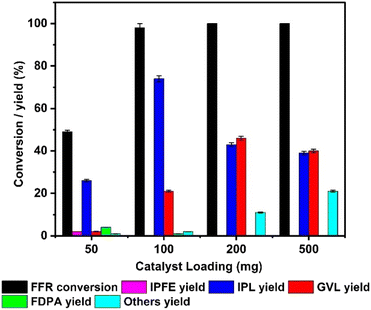 | ||
| Fig. 4 Effect of catalyst loading on furfural conversion and product yield. (Reaction conditions: FFR = 100 mg (1 mmol), IPA = 20 mL, temp. = 175 °C, time = 6 h). | ||
To understand the course of the reaction, the reaction was performed for 12 h under the optimized conditions (FFR = 100 mg (1 mmol), IPA = 20 mL, catalyst wt. = 100 mg, temp. = 175 °C), the conversion of FFR and yields of the products were analysed at regular intervals and the results are plotted in Fig. 5. Initially, after 1 h of reaction, the conversion of FFR was 90 ± 2% and it reached 100% at 8 h reaction time. The yield of IPL and GVL gradually increased while increasing the reaction time from 1 to 6 h. After 6 h, the yield of IPL started to decrease steadily and the same time the GVL yield begun to increase steeply.
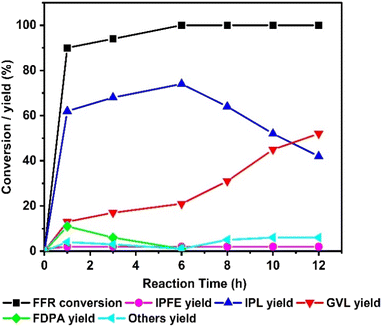 | ||
| Fig. 5 Effect of reaction time on furfural conversion and product yield (reaction conditions: FFR = 100 mg (1 mmol), IPA = 20 mL, catalyst wt. = 100 mg, temp. = 175 °C). | ||
The yields of IPL and GVL crossover after 10 h and the maximum GVL yield of 52 ± 2% was achieved at 12 h. This observation suggests that IPL is the major product formed initially and it is converted into GVL during the course of the reaction. The yield of FDPA decreased from 11 ± 0.5% to <1% while increasing the reaction time from 1 to 6 h indicating its conversion into the next higher product as shown in Scheme 1. The yield of IPFE was found to be low ∼1% thorough out the course of the reaction. It was also observed that the yield of unidentified products (others) is <1% initially and it slightly increases to 2 ± 0.1% after 12 h.
4. Reusability of the catalyst
The reusability of TY700 was investigated and the results are depicted in Fig. 6. The catalyst loading was adjusted to achieve a FFR conversion less than 65%. In the fresh run, TY700 catalyst gave 63 ± 1% FFR conversion and 10 ± 0.2% GVL yield. The catalyst was filtered out of the reaction mixture after the completion of the fresh run, washed with IPA, dried at 100 °C for overnight, and then reused in the following cycle. In this manner, recyclability of the spent catalyst was tested for up to three cycles. The conversion of FFR dropped from 63 ± 1% to 60 ± 1%, 58 ± 1%, 56 ± 1% during the consecutive runs and the yield of GVL dropped from 10 ± 0.2% to 8 ± 0.1%, 6 ± 0.1% and 5 ± 0.1% indicating some loss in catalytic activity. However, when the spent catalyst after three recycles was regenerated by heat treatment in the presence of air at 500 °C (Recycle 4*), it showed an improved conversion FFR (60 ± 1%) and GVL yield (9 ± 0.1%), which is close to that of the fresh catalyst.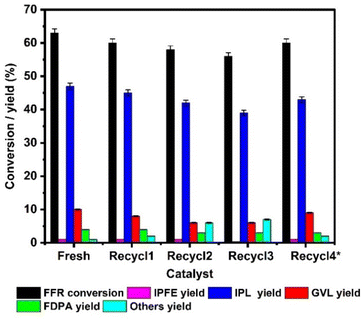 | ||
| Fig. 6 Reusability of the catalyst in MPV reduction of furfural to γ-valerolactone (reaction conditions: FFR = 100 mg (1 mmol), IPA = 20 mL, catalyst wt. = 65 mg, temp. = 175 °C, time = 6 h). | ||
5. Comparison with other reported catalysts
Table 4 summarizes the results obtained in this study on TY700 and some of the previous reports on catalytic transfer hydrogenation of furfural over different catalysts, in which Lewis acidic metal ions, Zr4+and Sn4+, were used as catalytic active sites. The conversion obtained on TY700 is comparable to other reported catalysts. However, direct comparison of the selectivity of GVL on the different catalysts cannot be fair as it is influenced by different reaction parameters such as, temperature, time, and furfural to catalyst ratio. Even though this limitation is considered, still TY700 catalyst's ease of preparation and recyclability outweigh the other catalysts.| Sl. no. | Catalyst | Reaction temp. (°C) | Reaction time (h) | Surface area (m2 g−1) | Conversion of FFR (%) | Selectivity of GVL (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Zr–Al-beta | 170 | 4 | 685 | 100 | 70 | 12 |
| 2 | Zr–Al-beta | 170 | 24 | 539 | 100 | 23 | 57 |
| 3 | ZrO2–SBA-15 | 130 | 7 | 349 | 93 | 3 | 61 |
| 4 | Zr–SBA-15 | 190 | 24 | 605 | 99 | 93 | 62 |
| 5 | Au/ZrO2–ZSM-5 | 120 | 24 | 372 | 100 | 78 | 58 |
| 6 | HZ-ZrP-16 | 180 | 14 | 251 | 100 | 64 | 63 |
| 7 | ZrO2/TPA-beta | 170 | 10 | 392 | 100 | 90 | 60 |
| 8 | ZrO2-[Al]MFI-NS | 170 | 36 | 510 | 100 | 83 | 27 |
| 9 | Sn–Al-beta | 180 | 24 | 486 | 100 | 54 | 23 |
| 10 | TY700 | 175 | 12 | 713 | 100 | 52 | Present work |
6. Conclusions
Ammonium form of Y, beta and mordenite zeolites were thermally dealuminated in a controlled manner to modify their acid sites. Data obtained from TPD of NH3 indicated a gradual decrease in total number of acid sites upon increasing the treatment temperature due to dislodgement of aluminium from the tetrahedral framework. There is a drop in the percentage of strong acid sites and at the same time rise in the percentage of weak acid sites while increasing thermal and steam treatment temperature. The treatment temperature does not influence the distribution of moderate acid sites in all three types of zeolites much. 27Al MAS NMR study showed that in Y zeolites, while increasing the thermal treatment temperature from 500 to 700 °C, more framework associated penta-coordinated Al sites and extra-framework octahedral Al sites are generated. TY700 showed the lowest (AlIV-I + AlIV-II)/(AlV + AlVI) ratio (0.96) compared to all the other prepared catalysts, which indicates that it has more Al dislodged from the tetrahedral zeolite framework.Isopropyl levulinate (IPL) was obtained as the major product whereas furfural diisopropyl acetal, isopropyl furfuryl ether and γ-valerolactone were minor products on all the catalysts studied. No furfuryl alcohol was detected over any of the catalysts under the studied reaction conditions. Hence, cascade reaction starts from acetalization of furfural rather than MPV reduction of furfural to furfuryl alcohol. There is a linear relationship between TOFFFR and weak + moderate acid sites to strong acid sites ratio ((W + M)/S ratio). Overall, weakening the acid sites by dealumination improves the conversion of furfural. However, in TY800, this trend is not followed probably due to its much higher, more than optimum (W + M)/S ratio. Though IPL was the major product formed over all the different framework zeolites studied, GVL was formed only on Y zeolites. In this aspect, TY700 was found to be the most active catalyst. The better catalytic activity of TY700 compared to other catalysts studied could be due to combination different factors such as its framework structure, suitable (W + M)/S acid sites ratio, presence of both AlV and AlVI sites and proper balance between Brønsted and Lewis acid sites. The valorisation of furfural on TY700 catalyst was studied under different reaction conditions to optimize and maximize furfural conversion and GVL yield. 100% furfural conversion and 52% GVL yield was achieved in the following reaction conditions: furfural = 1 mmol, IPA = 20 mL, TY700 catalyst = 100 mg, temperature = 175 °C, time = 12 h. Further, the TY700 catalyst could be recycled for three consecutive runs with small loss in its catalytic activity. Catalytic activity of the spent catalyst could be restored close to that of fresh catalysts, when the spent catalyst after three recycles was regenerated by heat treatment in the presence of air at 500 °C.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
The manuscript was written through contributions of all authors. Malu T. J.: data curation, methodology, investigation, writing – original draft Cheralathan K. K.: conceptualization, resources, supervision, validation, writing – review & editing. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank VIT for providing seed grant (VIT Seed Grant No.: SG20220044) for carrying out this research work. The authors acknowledge Sophisticated Instrumentation Facility (SIF), VIT, Vellore, India, NMR facility in IISC, Bangalore, India, TPD facility in CRF, IIT, Delhi, India, and nitrogen adsorption analysis facility in Material Analysis & Research Centre, Bengaluru, India for their help in characterization. The authors also wish to thank Prof. Masaru Ogura, Institute of Industrial Science, The University of Tokyo, Japan for pyridine-FTIR analysis.References
- Z. Fang and X. Qi, Production of Platform Chemicals from Sustainable Resources, Springer Singapore, 2017 Search PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- G. Dedes, A. Karnaouri and E. Topakas, Catalysts, 2020, 10, 1–25 CrossRef.
- L. Moghaddam, J. Rencoret, V. R. Maliger, D. W. Rackemann, M. D. Harrison, A. Gutiérrez, J. C. Del Río and W. O. S. Doherty, ACS Sustain. Chem. Eng., 2017, 5, 4846–4855 CrossRef CAS.
- W. Yu, K. Xiong, N. Ji, M. D. Porosoff and J. G. Chen, J. Catal., 2014, 317, 253–262 CrossRef CAS.
- Y. Nakagawa, K. Takada, M. Tamura and K. Tomishige, ACS Catal., 2014, 4, 2718–2726 CrossRef CAS.
- R. F. Perez and M. A. Fraga, Green Chem., 2014, 16, 3942–3950 RSC.
- X. Li, P. Jia and T. Wang, ACS Catal., 2016, 6, 7621–7640 CrossRef CAS.
- J. K. Zeitsch, The Chemistry and Technology of Furfural and its Many By-Products, 2000 Search PubMed.
- J. A. Melero, G. Morales, J. Iglesias, M. Paniagua and C. López-Aguado, Ind. Eng. Chem. Res., 2018, 57, 11592–11599 CrossRef CAS.
- Y. Nakagawa, M. Tamura and K. Tomishige, ACS Catal., 2013, 3, 2655–2668 CrossRef CAS.
- W. Cao, W. Luo, H. Ge, Y. Su, A. Wang and T. Zhang, Green Chem., 2017, 19, 2201–2211 RSC.
- Z. Lin, X. Cai, Y. Fu, W. Zhu and F. Zhang, RSC Adv., 2017, 7, 44082–44088 RSC.
- A. O’Driscoll, J. J. Leahy and T. Curtin, Catal. Today, 2017, 279, 194–201 CrossRef.
- M. J. Gilkey and B. Xu, ACS Catal., 2016, 6, 1420–1436 CrossRef CAS.
- J. Song, L. Wu, B. Zhou, H. Zhou, H. Fan, Y. Yang, Q. Meng and B. Han, Green Chem., 2015, 17, 1626–1632 RSC.
- K. Gupta, R. K. Rai and S. K. Singh, ChemCatChem, 2018, 10, 2326–2349 CrossRef CAS.
- G. Giorgianni, S. Abate, G. Centi, S. Perathoner, S. Van Beuzekom, S. H. Soo-Tang and J. C. Van Der Waal, ACS Sustain. Chem. Eng., 2018, 6, 16235–16247 CrossRef CAS.
- W. Tolek, W. Auppahad, P. Weerachawanasak, O. Mekasuwandumrong, P. Praserthdam and J. Panpranot, Catalysts, 2023, 13(3), 498 CrossRef CAS.
- S. Song, L. Di, G. Wu, W. Dai, N. Guan and L. Li, Appl Catal B, 2017, 205, 393–403 CrossRef CAS.
- H. P. Winoto, B. S. Ahn and J. Jae, J. Ind. Eng. Chem., 2016, 40, 62–71 CrossRef CAS.
- A. Saotta, A. Allegri, F. Liuzzi, G. Fornasari, N. Dimitratos and S. Albonetti, ChemEngineering, 2023, 7(2), 23 CrossRef CAS.
- Y. Wang, D. Zhao, D. Rodríguez-Padrón and C. Len, Catalysts, 2019, 9(10), 796 CrossRef.
- J. Hidalgo-Carrillo, A. Parejas, M. J. Cuesta-Rioboo, A. Marinas and F. J. Urbano, Catalysts, 2018, 8(11), 539 CrossRef.
- K. D. Kim, J. Kim, W. Y. Teoh, J. C. Kim, J. Huang and R. Ryoo, RSC Adv., 2020, 10, 35318–35328 RSC.
- R. López-Asensio, J. A. Cecilia, C. P. Jiménez-Gómez, C. García-Sancho, R. Moreno-Tost and P. Maireles-Torres, Appl. Catal., A, 2018, 556, 1–9 CrossRef.
- M. Koehle and R. F. Lobo, Catal. Sci. Technol., 2016, 6, 3018–3026 RSC.
- H. Zhang, W. Yang, I. I. Roslan, S. Jaenicke and G. K. Chuah, J. Catal., 2019, 375, 56–67 CrossRef CAS.
- A. García, R. Sánchez-Tovar, P. J. Miguel, E. Montejano-Nares, F. Ivars-Barceló, J. A. Cecilia, B. Torres-Olea and B. Solsona, Fuel, 2023, 352, 129045 CrossRef.
- L. Bui, H. Luo, W. R. Gunther and Y. Román-Leshkov, Angew. Chem., Int. Ed., 2013, 52, 8022–8025 CrossRef CAS.
- J. M. Guarinos, F. G. Cirujano, A. Rapeyko and F. X. Llabrés i Xamena, Mol. Catal., 2021, 515, 111925 CrossRef CAS.
- M. Popova, I. Trendafilova, M. Oykova, Y. Mitrev, P. Shestakova, M. R. Mihályi and Á. Szegedi, Molecules, 2022, 27(17), 5383 CrossRef CAS PubMed.
- G. Morales, J. A. Melero, J. Iglesias, M. Paniagua and C. López-Aguado, React. Chem. Eng., 2019, 4, 1834–1843 RSC.
- C. López-Aguado, M. Paniagua, J. A. Melero, J. Iglesias, P. Juárez, M. L. Granados and G. Morales, Catalysts, 2020, 10(6), 678 CrossRef.
- S. R. Batool, V. L. Sushkevich and J. A. van Bokhoven, J. Catal., 2022, 408, 24–35 CrossRef CAS.
- M. T. Jayakumari and C. Kanakkampalayam Krishnan, Appl. Catal., A, 2023, 663, 119318 CrossRef CAS.
- M. Ravi, V. L. Sushkevich and J. A. van Bokhoven, Nat. Mater., 2020, 19, 1047–1056 CrossRef CAS PubMed.
- (a) C. Deng, J. Zhang, L. Dong, M. Huang, B. Li, G. Jin, J. Gao, F. Zhang, M. Fan, L. Zhang and Y. Gong, Sci. Rep., 2016, 6(1), 23382 CrossRef CAS PubMed; (b) D. Liu, P. Yuan, H. Liu, J. Cai, D. Tan and T. Chen, Appl. Clay Sci., 2013, 80, 407–412 CrossRef; (c) O. Ojelabi, S. Yousatit, U. Rashid and C. Ngamcharussrivichai, Catalysts, 2023, 13, 982 CrossRef CAS.
- (a) J. B. Condon, Surface Area and Porosity Determinations by Physisorption: Measurements and Theory, the Boulevard, Langford Lane, Kidlington, 2006 Search PubMed; (b) S. Bhogeswararao and D. Srinivas, J. Catal., 2015, 327, 65–77 CrossRef CAS.
- A. Sartbaeva, N. H. Rees, P. P. Edwards, A. J. Ramirez-Cuesta and E. Barney, J. Mater. Chem. A, 2013, 1, 7415–7421 RSC.
- K. Mlekodaj, J. Dedecek, V. Pashkova, E. Tabor, P. Klein, M. Urbanova, R. Karcz, P. Sazama, S. R. Whittleton, H. M. Thomas, A. V. Fishchuk and S. Sklenak, J. Phys. Chem. C, 2019, 123, 7968–7987 CrossRef CAS.
- P. Cao, L. Lin, H. Qi, R. Chen, Z. Wu, N. Li, T. Zhang and W. Luo, ACS Catal., 2021, 11, 10246–10256 CrossRef CAS.
- M. J. Taylor, L. J. Durndell, M. A. Isaacs, C. M. A. Parlett, K. Wilson, A. F. Lee and G. Kyriakou, Appl. Catal., B, 2016, 180, 580–585 CrossRef CAS.
- R. Kosydar, E. Lalik, J. Gurgul, T. Szumełda and A. Drelinkiewicz, Int. J. Hydrogen Energy, 2024, 60, 293–307 CrossRef CAS.
- H. Song, F. Jin, Q. Liu and H. Liu, Mol. Catal., 2021, 513, 111752 CrossRef CAS.
- J. M. Rubio-Caballero, S. Saravanamurugan, P. Maireles-Torres and A. Riisager, Catal. Today, 2014, 234, 233–236 CrossRef CAS.
- I. D. Kopa, R. Y. Barakov, S. O. Sotnik and N. D. Shcherban, Mater. Today Proc., 2022, 62, 7686–7690 CrossRef CAS.
- M. Ravi, V. L. Sushkevich and J. A. Van Bokhoven, J. Phys. Chem. C, 2019, 123, 15139–15144 CrossRef CAS.
- Y. Oumi, S. Nemoto, S. Nawata, T. Fukushima, T. Teranishi and T. Sano, Effect of the framework structure on the dealumination-realumination behavior of zeolite, Mater. Chem. Phys., 2002, 78, 551–557 CrossRef.
- M. Ravi, V. L. Sushkevich and J. A. van Bokhoven, Chem. Sci., 2021, 12, 4094–4103 RSC.
- A. V. Yakimov, M. Ravi, R. Verel, V. L. Sushkevich, J. A. Van Bokhoven and C. Copéret, J. Am. Chem. Soc., 2022, 144, 10377–10385 CrossRef CAS PubMed.
- B. Gil, S. I. Zones, S. J. Hwang, M. Bejblová and J. Čejka, J. Phys. Chem. C, 2008, 112, 2997–3007 CrossRef CAS.
- Y. Kubota, K. Itabashi, S. Inagaki, Y. Nishita, R. Komatsu, Y. Tsuboi, S. Shinoda and T. Okubo, Chem. Mater., 2014, 26, 1250–1259 CrossRef CAS.
- S. R. Batool, V. L. Sushkevich and J. A. van Bokhoven, ACS Catal., 2024, 14, 678–690 CrossRef CAS.
- B. Hernández, J. Iglesias, G. Morales, M. Paniagua, C. López-Aguado, J. L. García Fierro, P. Wolf, I. Hermans and J. A. Melero, Green Chem., 2016, 18, 5777–5781 RSC.
- S. Zhu, Y. Xue, J. Guo, Y. Cen, J. Wang and W. Fan, ACS Catal., 2016, 6, 2035–2042 CrossRef CAS.
- X. Li, X. Yuan, G. Xia, J. Liang, C. Liu, Z. Wang and W. Yang, J. Catal., 2020, 392, 175–185 CrossRef CAS.
- B. Srinivasa Rao, P. Krishna Kumari, P. Koley, J. Tardio and N. Lingaiah, Mol. Catal., 2019, 466, 52–59 CrossRef CAS.
- J. Iglesias, J. A. Melero, G. Morales, M. Paniagua, B. Hernández, A. Osatiashtiani, A. F. Lee and K. Wilson, Catal. Sci. Technol., 2018, 8, 4485–4493 RSC.
- Z. Liu, Z. Zhang, R. Fu, J. Xu, J. Lu, Z. Wen and B. Xue, ACS Appl. Nano Mater., 2023, 6, 13196–13207 CrossRef.
- L. Ye, Y. Han, H. Bai and X. Lu, ACS Sustain. Chem. Eng., 2020, 8, 7403–7413 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray diffraction pattern, N2 adsorption isotherms, TPD of NH3 profiles and 27Al MAS NMR, pyridine adsorption-FTIR and GC-MS spectra. See DOI: https://doi.org/10.1039/d4ra03113j |
| This journal is © The Royal Society of Chemistry 2024 |