Open Access Article
Open Access ArticleGlycyrrhetinic acid-modified redox-sensitive polymeric mixed micelles for tumor-specific intracellular delivery of cantharidin†
Yu Hua,
Tian Lanb,
Ji Lic,
Lingjun Li‡
*a and
Jizheng Song‡ *a
*a
aSchool of Pharmacy, Shandong University of Traditional Chinese Medicine (TCM), 250355 Jinan, Shandong, China. E-mail: sdzyylilingjun@163.com; songjizheng345@163.com
bInnovative Institute of Chinese Medicine, Shandong University of TCM, 250355 Jinan, Shandong, China
cAffiliated Hospital of Shandong University of TCM, 250011 Jinan, Shandong, China
First published on 10th September 2024
Abstract
Cantharidin (CTD) has been widely used to treat hepatocellular carcinoma (HCC) in clinical practice. However, the current CTD preparations may induce hepatic and renal damage due to their non-specific distribution. Therefore, redox-sensitive polymer Pluronic F127-disulfide bond-poly(D,L-lactide) (F127-SS-PDLA) and active targeting polymer F127-glycyrrhetinic acid (F127-GA) were synthesized to prepare mixed micelles (GA/F127-SS-PDLA/CTD) for effective delivery of CTD. Fourier transform infrared (FTIR) spectroscopy and 1H nuclear magnetic resonance (1H-NMR) spectroscopy were used to verify the successful synthesis of F127-SS-PDLA and F127-GA. During the preparation, this study was the first to screen the density of GA by cellular uptake assay. The results indicated that mixed micelles with 10% and 15% F127-GA (weight fraction) exhibited superior cellular uptake in comparison to micelles with 5% and 20% F127-GA. GA/F127-SS-PDLA/CTD micelles prepared by thin film hydration method demonstrated excellent drug loading capacity for CTD (16.12 ± 0.11%). The particle size and zeta potential of GA/F127-SS-PDLA/CTD micelles were 85.17 ± 1.24 nm and −11.71 ± 0.86 mV, respectively. Hemolysis and stability assay showed that the mixed micelles had good blood compatibility and could remain stable for 30 days at 4 °C. The redox-sensitivity of GA/F127-SS-PDLA/CTD micelles in vitro was verified under reducing conditions through dynamic light scattering (DLS) and an in vitro drug release experiment, which showed obvious particle size variation and rapid drug release ability. In cellular experiments, GA/F127-SS-PDLA/CTD micelles could induce superior cytotoxicity, apoptosis and intracellular reactive oxygen species (ROS) levels compared with free CTD, non-sensitive F127-PDLA/CTD micelles and redox-sensitive F127-SS-PDLA/CTD micelles. The cellular uptake ability of nile red-labeled GA/F127-SS-PDLA micelles, which was evaluated via fluorescent microscope and flow cytometry, indicated that the modification of GA significantly increased micelle uptake in HepG-2 cells. Consequently, GA/F127-SS-PDLA/CTD micelles could be considered as a satisfactory drug administration strategy in the treatment of HCC.
1 Introduction
Hepatocellular carcinoma (HCC) is the sixth commonest form of cancer and the second leading cause of cancer-associated death worldwide.1 Since HCC is asymptomatic in its early stage, most interventions, such as liver transplantation and hepatectomy, are often limited by cancer recurrence and metastasis.2 As a result, chemotherapy is currently a commonly used and effective treatment for HCC in clinical practice.3,4Cantharidin (CTD) is a sesquiterpenoid derivative extracted from the Mylabris.5,6 It is famous for its remarkable and widespread anti-tumor effects.6–8 Modern pharmacological research has shown that the anti-tumor mechanism of CTD primarily involved suppressing tumor cell growth, inducing tumor cell apoptosis and blocking the tumor cell cycle.9,10 Various preparations containing CTD and its derivatives are widely used in treating HCC, such as aidi injection, disodium cantharidinate injection and compound CTD capsule.2,11,12 These preparations can significantly relieve the symptoms of patients and prolong their survival time. However, CTD preparations might cause liver and kidney damage in the long term, which could be attributed to the nonspecific distribution of CTD.13–15 Therefore, it is crucial to establish a novel targeting drug delivery system, which could decrease the side effects and toxicity of the drugs, enhance the anti-tumor effect and expand the scope of CTD.
Nanodrugs have shown great potential in cancer treatment. They can aggregate in tumor tissue through the high permeability and retention effect of solid tumors (EPR effect) by virtue of their small sizes (10–200 nm).16 In aqueous solution, amphiphilic block copolymers are able to self-assemble into polymeric micelles with “core–shell” structure.17 At present, micelles loaded with chemotherapy drugs such as paclitaxel and docetaxel have been approved for clinical use.18,19 These agents have exhibited higher overall response rates and greater safety profiles compared with conventional chemotherapeutic drugs. As reported in the literature, micelles have been used to deliver CTD.5 However, conventional nanoparticles may encounter challenge in incomplete drug release.20,21 The disulfide bond is responsive to the reductive environment. It can cleave rapidly at high concentrations of glutathione (GSH) in tumor cells while remaining stable in normal tissues and blood.22 At present, it has been widely used in the development of anti-tumor drug delivery carriers and exerted superior efficacy.23,24 Nevertheless, the utilization of redox-sensitive formulations for intracellular delivery of CTD has yet to be reported in detail.
An ideal drug delivery carrier should not only trigger the rapid release of drugs under specific stimulation conditions but also exhibit excellent biocompatibility and drug loading capacity.25 Therefore, due to their exceptional physical and biological properties, polymeric materials have become the potential candidates for establishing stimulus-responsive nano-carriers. Pluronic F127 (F127), a triblock copolymer, has been recognized as an excipient by British Pharmacopoeia for intravenous injection.26 Using F127 to prepare micelles could improve the solubility of drugs and target drugs to the tumor site passively.27 Nevertheless, the high critical micelle concentration (CMC) of F127 limited its clinical application.26 Given this circumstance, the hydrophobic poly(D,L-lactide) (PDLA) with good degradability and biocompatibility could be used to modify F127 to increase the hydrophobicity of F127 and subsequently enhance the stability and anti-dilution property of micelles.28
Although passive targeting agents can deliver drugs to the tumor tissue through the EPR effect, they still need more specificity towards tumor cells.29,30 Therefore, it is significant to design nanoparticles with active targeting ability to deliver drugs to tumor cells precisely.31 Glycyrrhetinic acid (GA) receptors are overexpressed on the surface of liver cancer cells, which can specifically recognize and bind with GA.21,32 Therefore, drug delivery carriers modified with GA can minimize non-specific interactions with normal tissues and improve the anti-tumor effect of the drug.21,33 Furthermore, the density of the targeting ligands plays a crucial role in the targeting ability of drug delivery carriers.34 The inappropriate density of ligands may weaken the targeting capacity of nanoparticles.35 However, most reported active targeting nano-preparations have not been screened for the suitable density of targeting ligands particularly GA.
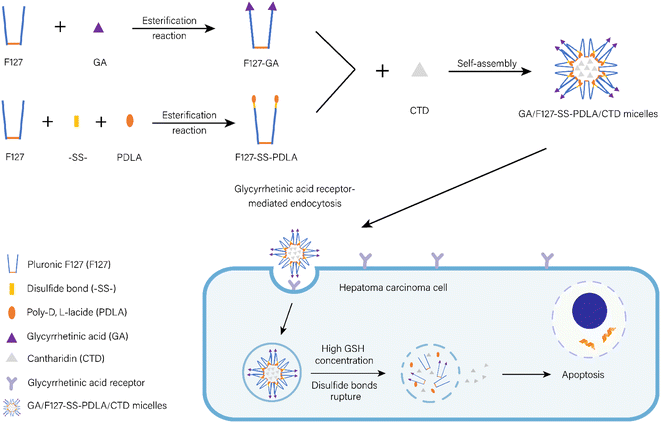
In this study, a CTD-loaded active targeting and reduction-sensitive mixed micelles composed of F127-disulfide bond-PDLA (F127-SS-PDLA) and F127-GA were established for HCC treatment (Fig. 1). Firstly, the disulfide bond was used to connect F127 and PDLA to synthesize F127-SS-PDLA. F127 was esterized with GA to obtain F127-GA. Secondly, in order to further improve the active targeting efficiency of the micelles, the appropriate proportion of F127-GA in the mixed micelle system was explored by cellular uptake assay. Thirdly, CTD-loaded mixed micelles F127-GA/F127-SS-PDLA/CTD (GA/F127-SS-PDLA/CTD) were then formulated by self-assembly of two polymers and characterized by particle size, polydispersity index (PDI), zeta potential, encapsulation efficiency (EE), drug loading (DL) and stability. Meanwhile, to evaluate the redox-sensitive function of the preparation, a non-sensitive polymer material, F127-PDLA, was synthesized and served as the control group of F127-SS-PDLA in the in vitro drug release assay and cell experiments. Finally, the inhibitory effect of GA/F127-SS-PDLA/CTD micelles on human hepatocellular carcinoma cells HepG-2 was investigated by cytotoxicity assay, apoptosis assay, reactive oxygen species (ROS) levels detection and cell uptake assay.
2 Materials and methods
2.1 Materials and chemicals
GA was purchased from J&K Scientific Co., Ltd (Beijing, China). F127 (average molecule weight = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), anhydrous dichloromethane, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), 4-dimethylaminopyridine (DMAP) and CTD were supplied by Aladdin Biotechnology Co., Ltd (Shanghai, China). 3,3′-Dithiodipropionic (SS-COOH) was purchased from Yuanye Biotechnology Co., Ltd (Shanghai, China). P-toluenesulfonic acid (p-TSA) was obtained from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). PDLA (average molecular weight = 4700) was bought from Jinan Daigang Biological Engineering Co., Ltd (Jinan, China). N,N-dimethylformamide (DMF), triethylamine (TEA) and other chemical reagents were from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
600), anhydrous dichloromethane, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), 4-dimethylaminopyridine (DMAP) and CTD were supplied by Aladdin Biotechnology Co., Ltd (Shanghai, China). 3,3′-Dithiodipropionic (SS-COOH) was purchased from Yuanye Biotechnology Co., Ltd (Shanghai, China). P-toluenesulfonic acid (p-TSA) was obtained from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). PDLA (average molecular weight = 4700) was bought from Jinan Daigang Biological Engineering Co., Ltd (Jinan, China). N,N-dimethylformamide (DMF), triethylamine (TEA) and other chemical reagents were from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin streptomycin (P/S), 0.25% trypsin-EDTA were provided by Gibco. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT) was obtained from Beijing Solarbio Technology Co., Ltd (Beijing, China). Annexin V-FITC/propidium iodide (PI) assay kit, ROS assay kit and Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 were supplied by Servicebio Biotechnology Co., Ltd (Wuhan, China). Phosphate buffered saline (PBS) and trypsin solution (free EDTA) were obtained from Biosharp Biotechnology Co., Ltd.
342 were supplied by Servicebio Biotechnology Co., Ltd (Wuhan, China). Phosphate buffered saline (PBS) and trypsin solution (free EDTA) were obtained from Biosharp Biotechnology Co., Ltd.
2.2 Cells culture
HepG-2 cell line was acquired from Shanghai Anwei Biotechnology Co., Ltd (Shanghai, China) and cultured with DMEM containing 10% FBS (v/v) and 1% P/S (v/v) under 37 °C in a humidified atmosphere containing 5% CO2.2.3 Synthesis of polymer materials
2.4 Characterization of polymer materials
The structures of F127-GA, F127-SS-PDLA and F127-PDLA were confirmed by Fourier transform infrared (FTIR) spectroscopy (PerkinElmer Inc., USA). The polymers and potassium bromide powder in a dry state were thoroughly blended together before being compacted into a thin sheet. Then, the composites were analyzed with FTIR in the range of 4000 to 400 cm−1. In addition, the structures were further identified with ADVANCE III HD-600 MHz nuclear magnetic resonance (1H-NMR) spectroscopy (Bruker, Karlsruhe, Germany) with deuterated chloroform or deuterated dimethyl sulfoxide (DMSO) as the solvent.2.5 Studying the proportion of F127-GA in the mixed micelle system
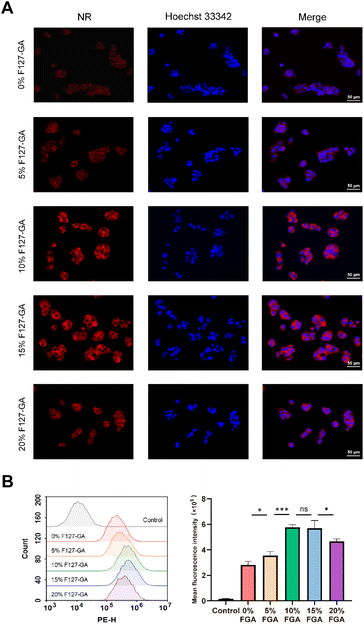
The proportion of targeting ligands in carrier materials had a significant impact on the targeting efficiency of the nanoparticles. For the purpose of screening the appropriate proportion of F127-GA in GA/F127-SS-PDLA/CTD micelles, the uptake ability of HepG-2 cells to various mixed micelles was investigated. Hydrophobic fluorescence probe, nile red (NR), was selected to use in this study. GA/F127-SS-PDLA/NR micelles with F127-GA proportion of 0%, 5%, 10%, 15% and 20% (weight fraction) were prepared for use.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) in a 12-well plate with 1 mL DMEM medium and cultured for 24 h. Then, a novel medium comprising micelles loaded NR (equivalent concentration of 0.3 μg mL−1 NR) was introduced and incubated for 4 h. After washed three times with PBS, the cells were fixed by 4% formalin for 10 min. Subsequently, the formalin was substituted with 1 mL Hoechst 33
000 cells per well) in a 12-well plate with 1 mL DMEM medium and cultured for 24 h. Then, a novel medium comprising micelles loaded NR (equivalent concentration of 0.3 μg mL−1 NR) was introduced and incubated for 4 h. After washed three times with PBS, the cells were fixed by 4% formalin for 10 min. Subsequently, the formalin was substituted with 1 mL Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (1 μg mL−1) and the staining was performed for 5 min. After absorbing the Hoechst 33
342 (1 μg mL−1) and the staining was performed for 5 min. After absorbing the Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 solution, the cells were rinsed twice using PBS, then covered with 0.5 mL of PBS and photographed with the BZ-X810 fluorescent microscope (KEYENCE Ltd, Japan).
342 solution, the cells were rinsed twice using PBS, then covered with 0.5 mL of PBS and photographed with the BZ-X810 fluorescent microscope (KEYENCE Ltd, Japan).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) in 12-well plates and incubated for 24 h. The cells were then incubated for 4 h with the novel medium containing various GA/F127-SS-PDLA/NR micelles (equivalent concentration of 0.3 μg mL−1 NR) instead of the original medium. After eliminating the medium, the cells were cleaned three times with PBS. The cells were collected by digestion and subjected to centrifugation. Subsequently, the precipitations were resuspended in PBS. The intensity of red fluorescence, reflecting the intracellular micelles, was detected by the PE channel of the Novo Ctye Advanteon flow cytometer (Agilent Ltd, USA).
000 cells per well) in 12-well plates and incubated for 24 h. The cells were then incubated for 4 h with the novel medium containing various GA/F127-SS-PDLA/NR micelles (equivalent concentration of 0.3 μg mL−1 NR) instead of the original medium. After eliminating the medium, the cells were cleaned three times with PBS. The cells were collected by digestion and subjected to centrifugation. Subsequently, the precipitations were resuspended in PBS. The intensity of red fluorescence, reflecting the intracellular micelles, was detected by the PE channel of the Novo Ctye Advanteon flow cytometer (Agilent Ltd, USA).2.6 Preparation of CTD-loaded micelles
Non-sensitive micelles F127-PDLA/CTD and reduction-sensitive micelles F127-SS-PDLA/CTD were prepared according to previously reported method.21 Firstly, polymer materials (10 mg) and CTD (2 mg) were completely dissolved in acetone (5 mL). After removing acetone by rotary evaporation at 50 °C, −0.1 Mpa, 100 rpm, a transparent film was formed at the bottom of the eggplant-shaped bottle. Then, 10 mL of normal saline at the same temperature was added and hydrated at 50 °C for 30 min. The solution was cooled to room temperature, and the volume was diluted to a constant volume of 10 mL. The final F127-PDLA/CTD and F127-SS-PDLA/CTD micelles were obtained by filtering through 0.22 μm microporous membrane.A total of 10 mg of F127-GA and F127-SS-PDLA were weighed at a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (w/w), and then GA/F127-SS-PDLA/CTD micelles were prepared as the method described above.
90 (w/w), and then GA/F127-SS-PDLA/CTD micelles were prepared as the method described above.
2.7 Characterization of CTD-loaded micelles
90Plus PALS dynamic light scattering (DLS, Brookhaven, USA) was used to determine the particles size and zeta potential of CTD-loaded micelles. The morphology of CTD-loaded micelles was observed and photographed by JEM 1400 Plus transmission electron microscopy (TEM, JEOL Ltd, Japan). The DL and EE of CTD-loaded micelles were determined by Agilent series 1260 high-performance liquid chromatography (HPLC) (Agilent Technologies, USA) equipped with a UV-vis detector set at 218 nm. The mobile phase was methanol/water (65/35, v/v) and the flow rate was 1 mL min−1. Methanol was added to the micellar solution to destroy the micelle shells. The formulas provided below were utilized to calculate the DL and EE of CTD-loaded micelles:| DL (%) = Ma/(Ma + Mb) × 100% |
| EE (%) = Ma/Mc × 100% |
2.8 The stability of CTD-loaded micelles
F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles were stored at 4 °C for 30 days, and their particle size and PDI were measured by DLS at predetermined time intervals (1, 3, 5, 10, 15, 20 and 30 days).In addition, the particle size changes of CTD-loaded micelles in normal saline with 10% FBS were also investigated to preliminarily evaluate the ability of micelles to maintain their original state when exposed to plasma proteins. Briefly, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles were exposed to 10% FBS and shaken at 100 rpm in 37 °C. At the scheduled time intervals (0, 1, 2, 4, 8, 12 and 24 h), the particle size of micelles was detected using DLS.
2.9 Reduction-responsive behaviour of CTD-loaded micelles
The particle size changes of micelles in 10 mM GSH solution were measured to assess the redox-triggered behaviour of CTD-loaded micelles. In brief, 2 mL of normal saline containing 20 mM GSH was added to 2 mL of F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles, respectively, to obtain the desired concentration of GSH. All of the mixture solutions were shaken at 100 rpm in 37 °C for 24 h. The size distributions of F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles were monitored by DLS at predetermined time points 0, 1, 2, 4, 8, 12 and 24 h.2.10 In vitro drug release
The redox-triggered release of CTD from micelles was estimated through dialysis method. Appropriate amount of F127-PDLA/CTD, F127-SS-PDLA/CTD, GA/F127-SS-PDLA/CTD micelles and free CTD solutions were loaded in dialysis bags (MWCO = 5000), respectively. The dialysis bags were completely immersed in normal saline with different GSH concentrations (0 and 10 mM) and shaken at 100 rpm in 37 °C for 24 h. During this time, 1 mL of sample was collected at specified time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 12 and 24 h) and 1 mL of fresh release medium at the same temperature was added. The amount of released CTD was measured by HPLC. Finally, the in vitro cumulative release curves of free CTD and three CTD-loaded micelles in different release media were plotted with the time and cumulative release rate as the abscissa and ordinate, respectively.2.11 Hemolysis assay
The blood compatibility of blank F127-PDLA, F127-SS-PDLA and GA/F127-SS-PDLA micelles was preliminarily assessed by performing the hemolysis assay using red blood cells (RBCs).21,26 Briefly, the RBCs separated from the whole blood of rat were collected by centrifugation. 2% Erythrocyte suspension (v/v) was obtained by cleaning and diluting RBCs utilizing normal saline. Then, 2 mL of erythrocyte suspension was interacted with 2 mL of blank micelles at varying concentrations. After shaken for 3 h in 37 °C at 100 rpm, the supernatant was collected via centrifugation. A microplate reader (CLARIOstar Plus, BMG Labtech, Germany) was utilized to determine the absorbance of the supernatant at 540 nm. The normal saline and distilled water were served as the negative and positive controls, respectively. The hemolysis rate was calculated based on the following equations:| Hemolysis rate (%) = (A1 − A2) / (A3 − A2) × 100% |
2.12 Cytotoxicity assay
The MTT assay was employed to measure the in vitro cytotoxicity of free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD, GA/F127-SS-PDLA/CTD, blank F127-PDLA, blank F127-SS-PDLA and blank GA/F127-SS-PDLA micelles against HepG-2 cells. These findings are crucial for understanding the potential cytotoxicity of the micelles. HepG-2 cells (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) were seeded in 96-well plates in 100 μL DMEM medium and incubated for 24 h. Then, we investigated the cells treated with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles at different concentrations ranging from 0.5 to 32.0 μg mL−1, respectively. Meanwhile, the cells treated with blank F127-PDLA, blank F127-SS-PDLA and blank GA/F127-SS-PDLA micelles (3.125–200 μg mL−1) were also investigated for the cytotoxicity of polymers. After incubating for 12 or 24 h at 37 °C, the previous solution was replaced with fresh DMEM medium that contained MTT (1 mg mL−1). Then, the medium containing MTT was completely discarded after 4 h and each well received 150 μL of DMSO to dissolve the formazan crystal. The microplate reader measured absorbance values at 570 nm. Cells without treatment were served as control. The cell viability was calculated according to the following equation:
000 cells per well) were seeded in 96-well plates in 100 μL DMEM medium and incubated for 24 h. Then, we investigated the cells treated with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles at different concentrations ranging from 0.5 to 32.0 μg mL−1, respectively. Meanwhile, the cells treated with blank F127-PDLA, blank F127-SS-PDLA and blank GA/F127-SS-PDLA micelles (3.125–200 μg mL−1) were also investigated for the cytotoxicity of polymers. After incubating for 12 or 24 h at 37 °C, the previous solution was replaced with fresh DMEM medium that contained MTT (1 mg mL−1). Then, the medium containing MTT was completely discarded after 4 h and each well received 150 μL of DMSO to dissolve the formazan crystal. The microplate reader measured absorbance values at 570 nm. Cells without treatment were served as control. The cell viability was calculated according to the following equation:| Cell viability (%) = (OD1/OD2) × 100% |
2.13 Apoptosis assay
FITC-labelled Annexin-V was used to identify apoptotic cells that exposed phosphatidylserine (PS) to the outer leaflet. The cells with damaged membranes could also be stained with PI. Thus, flow cytometry was employed to quantify the apoptotic cells induced by various treatments through Annexin V-FITC/PI double staining. HepG-2 cells (250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) were placed in 6-well plates and cultured for 24 h. After treated with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles (equivalent concentration of 2.0 and 4.0 μg mL−1 CTD) for 12 h, the cells were harvested and subjected to centrifugation, then cleaned using PBS. The cell precipitates were resuspended with 0.5 mL of binding buffer (Annexin V-FITC 5.0 μL) and stained the PS for 15 min in darkness. In the end, 5.0 μL of PI was added to stain the nuclei and the mixture were evaluated by the flow cytometer.
000 cells per well) were placed in 6-well plates and cultured for 24 h. After treated with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles (equivalent concentration of 2.0 and 4.0 μg mL−1 CTD) for 12 h, the cells were harvested and subjected to centrifugation, then cleaned using PBS. The cell precipitates were resuspended with 0.5 mL of binding buffer (Annexin V-FITC 5.0 μL) and stained the PS for 15 min in darkness. In the end, 5.0 μL of PI was added to stain the nuclei and the mixture were evaluated by the flow cytometer.
2.14 Studying the effect of CTD-loaded micelles on ROS in HepG-2 cells
We detected the generation of intracellular ROS using a specific staining method with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). This compound is able to penetrate the cell membrane and be intracellularly cleaved by esterase, resulting in the formation of 2′,7′-dichlorodihydrofluorescein (DCFH). ROS further oxidizes DCFH to form dichlorofluorescein (DCF) emitting green fluorescence.21 We seeded HepG-2 cells (130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) in a 12-well plate with DMEM medium and incubated them for 24 h. Then, the cells were treated with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles (equivalent concentration of 2.0 μg mL−1 CTD), respectively. After a 12 hours incubation, the cells were stained with DCFH-DA (10 μM) for 30 min. Finally, cells were collected by digestion as well as washed and resuspended with PBS. The intensity of green fluorescence, reflecting the intracellular ROS, was determined by the FITC channel of the flow cytometer.
000 cells per well) in a 12-well plate with DMEM medium and incubated them for 24 h. Then, the cells were treated with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles (equivalent concentration of 2.0 μg mL−1 CTD), respectively. After a 12 hours incubation, the cells were stained with DCFH-DA (10 μM) for 30 min. Finally, cells were collected by digestion as well as washed and resuspended with PBS. The intensity of green fluorescence, reflecting the intracellular ROS, was determined by the FITC channel of the flow cytometer.
2.15 Cellular uptake assay
For the sake of researching the internalization of mixed micelles within HepG-2 cells, we prepared different micelles labelled with NR using the thin film hydration method described above. Fluorescent microscope and flow cytometry were employed to assay the cellular uptake ability of F127-PDLA/NR, F127-SS-PDLA/NR and GA/F127-SS-PDLA/NR by HepG-2 cells. The experimental methods were referred to the content of 2.4.2.16 Statistical analysis
All the experiments were conducted three times. Results were presented as mean ± standard deviation (SD). The statistical analysis was carried out by GraphPad Prism 8.3.0 and Origin 8.0 software. Statistical comparison was analyzed by t-test.3 Results and discussion
3.1 Characterization of polymer materials
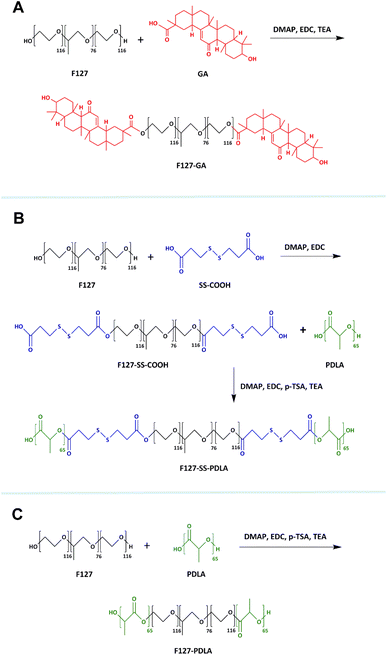
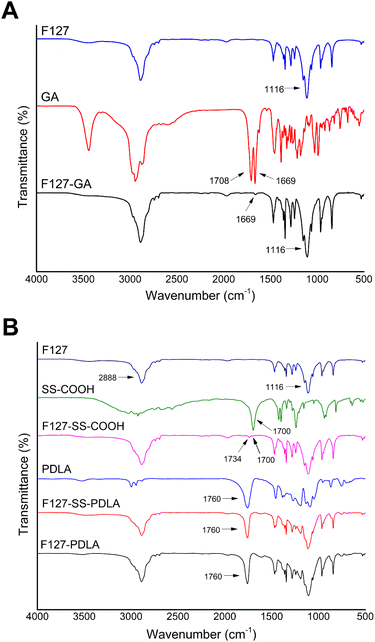
The FTIR spectra of F127, GA, and F127-GA were shown in Fig. 3A. In the spectrum of GA, the stretching vibration absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O forming a p–π conjugate with C
O forming a p–π conjugate with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was at 1669 cm−1. The absorption band of carboxyl group in GA was at 1708 cm−1. Compared with F127 and GA, F127-GA retained the stretching vibration absorption peaks of C–O and C
C was at 1669 cm−1. The absorption band of carboxyl group in GA was at 1708 cm−1. Compared with F127 and GA, F127-GA retained the stretching vibration absorption peaks of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1116 cm−1 and 1669 cm−1, respectively. Meanwhile, the absorption band of carboxyl group in GA (1708 cm−1) disappeared. These changes were attributed to the formation of the ester group between GA and F127.
O at 1116 cm−1 and 1669 cm−1, respectively. Meanwhile, the absorption band of carboxyl group in GA (1708 cm−1) disappeared. These changes were attributed to the formation of the ester group between GA and F127.
Fig. 3B displayed the FTIR spectra of polymer F127-SS-PDLA as well as its raw materials and intermediate. Compared with the spectra of F127, F127-SS-COOH retained the intrinsic absorption peaks of F127 at 2888 cm−1 and 1116 cm−1, and added two stretching vibration absorption bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1700 cm−1 and 1734 cm−1, respectively. The former was assigned to the terminal carboxyl group of F127-SS-COOH, and the latter was attributed to the ester bond. Combined with the spectrum of SS-COOH, the intermediate F127-SS-COOH was confirmed to be synthesized successfully. As shown in the FTIR spectrum of F127-SS-PDLA, a new peak belonging to PDLA arisen at 1760 cm−1, manifesting that F127-SS-PDLA was successfully synthesized.
O at 1700 cm−1 and 1734 cm−1, respectively. The former was assigned to the terminal carboxyl group of F127-SS-COOH, and the latter was attributed to the ester bond. Combined with the spectrum of SS-COOH, the intermediate F127-SS-COOH was confirmed to be synthesized successfully. As shown in the FTIR spectrum of F127-SS-PDLA, a new peak belonging to PDLA arisen at 1760 cm−1, manifesting that F127-SS-PDLA was successfully synthesized.
In the spectrum of F127-PDLA (Fig. 3B), the peaks at 2888, 1116 and 1760 cm−1 were responsible for stretching vibration of C–H, C–O in F127 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in PDLA, inferring that F127-PDLA was synthesized successfully.
O in PDLA, inferring that F127-PDLA was synthesized successfully.
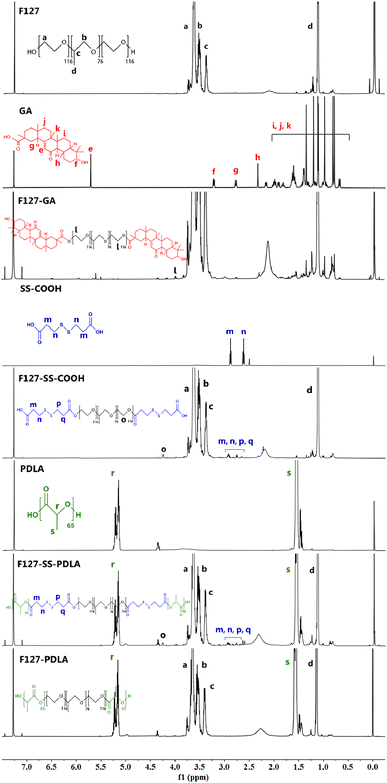
The following characteristic peaks could be observed in the 1H-NMR spectrum of F127. Peaks a (δ = 3.63 ppm) and b (δ = 3.52 ppm) were assigned to CH2 in poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), respectively. Peak c (δ = 3.39 ppm) and d (δ = 1.11 ppm) were the hydrogen signals of CH and CH3 in PPO, respectively. As presented in the spectrum of GA, peak e (δ = 5.71 ppm) was attributed to alkene hydrogen in the steroidal nucleus. Peaks f (δ = 3.25 ppm) was the signal of hydrogen on the carbon linked to hydroxyl group of GA. Peaks g (δ = 2.77 ppm) and h (δ = 2.34 ppm) belonging to CH2 in the steroidal nucleus. The signals between 0.70 and 2.00 ppm, including peaks i, j and k, were assigned to the protons of saturated carbons. In addition to the typical signals of F127 and GA mentioned above, a new peak l (δ = 3.99 ppm) emerged in the spectrum of F127-GA, which possibly due to the change in chemical environment of CH2 in the PEO after the formation of ester bond between F127 and GA. These data indicated that the carboxyl group of GA was successfully connected to the hydroxyl of F127.
In the 1H-NMR spectrum of SS-COOH, peaks m (δ = 2.87 ppm) and n (δ = 2.62 ppm) were assigned to CH2 on either side of the disulfide bond. As shown in the spectrum of F127-SS-COOH, peaks m and n became four peaks, including m, n, p and q (δ = 2.60–3.00 ppm). It was reasonable to refer that the ester group caused the change in the chemical environment of CH2 in SS-COOH. Apart from the characteristic peaks of F127 and SS-COOH, a new peak o (δ = 4.24 ppm) appeared in the spectrum of F127-SS-COOH which may be caused by the changes in chemical environment of CH2 in of PPO after the esterification reaction between F127 and SS-COOH. These results were indicated that the intermediate product F127-SS-COOH was synthesized successfully.
The 1H-NMR spectra of PDLA and F127-SS-PDLA displayed the characteristic peaks of PDLA, including peaks r (δ = 5.18 ppm) and s (δ = 1.55 ppm) which corresponded to the CH and CH3 in the PDLA, respectively. Furthermore, in the spectrum of F127-SS-PDLA, the characteristic peaks of F127-SS-COOH also appeared, implying the successful synthesis.
The specific signals of F127 and PDLA could be found simultaneously from the 1H-NMR spectrum of F127-PDLA, indicating that F127-PDLA was synthesized successfully.
3.2 The appropriate proportion of F127-GA in the mixed micelle system
The results of the fluorescence microscopy imaging system and flow cytometry (Fig. 5) underscore the significance of our work. The red fluorescence intensity of GA/F127-SS-PDLA/NR micelles, with varying proportions of F127-GA, followed a distinct pattern: 10% F127-GA ≈ 15% F127-GA > 20% F127-GA > 5% F127-GA > 0% F127-GA. It was suggested that the introduction of GA could increase the uptake of micelles by HepG-2 cells and the binding affinity of mixed micelles with 10% and 15% F127-GA to HepG-2 cells was significantly higher than that of mixed micelles with 20% and 5% F127-GA.Previous studies have demonstrated that the density of targeting ligands on the surface of drug delivery carriers significantly influenced the nanoparticle's targeting efficacy. The similarity of these results is that higher ligand densities are not always better. Gong et al. reported that polymeric micelles containing 10% folic acid (FA) (molar content) exhibited superior cytotoxicity and cellular uptake on ovarian cancer cell line OVCAR-3 in comparison to micelles with 50% and 91% FA.35 Similarly, Yi et al. found that polymeric vesicles incorporating 2% transferrin (Tf) had higher cellular uptake and more remarkable ability to inhibit multidrug resistance (MDR) in tumor cells than those with 4% and 5% Tf.36 Based on the literature review and the analysis of experimental findings, the uptake capacity of tumor cells for active targeting nanoparticles could be roughly divided into three distinct phases depending on the concentration of targeting ligands. During the initial phase, increasing concentration of targeting ligands resulted in greater uptake of micelles, because the interaction with specific receptors on the surface of cancer cells facilized internalization.37,38 In the second stage, binding saturation was achieved at higher concentration of targeting ligands and there was no further enhancement in targeting ability. In the third phase, when the concentration continued to increase, the targeting ability of the nanoparticles was limited due to steric hindrance effects.35,36,39 Therefore, there should be an optimal range for the concentration of the targeting ligands in nanoparticles to obtain greater targeting activity against tumor cells. Evaluating the density of targeting ligands in future research on active targeting drugs is essential.
It was reported that the redox-sensitivity of polymer mixed micelles tended to decrease when the proportion of reduction-sensitive polymeric materials was reduced.38 Therefore, considering the active targeting function and the reduction sensitivity, we determined the optimal ratio of F127-GA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F127-SS-PDLA to be 10
F127-SS-PDLA to be 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 in the mixed micelle. It could potentially enhance the efficiency and effectiveness of mixed micelles.
90 in the mixed micelle. It could potentially enhance the efficiency and effectiveness of mixed micelles.
3.3 Characterization of CTD-loaded micelles
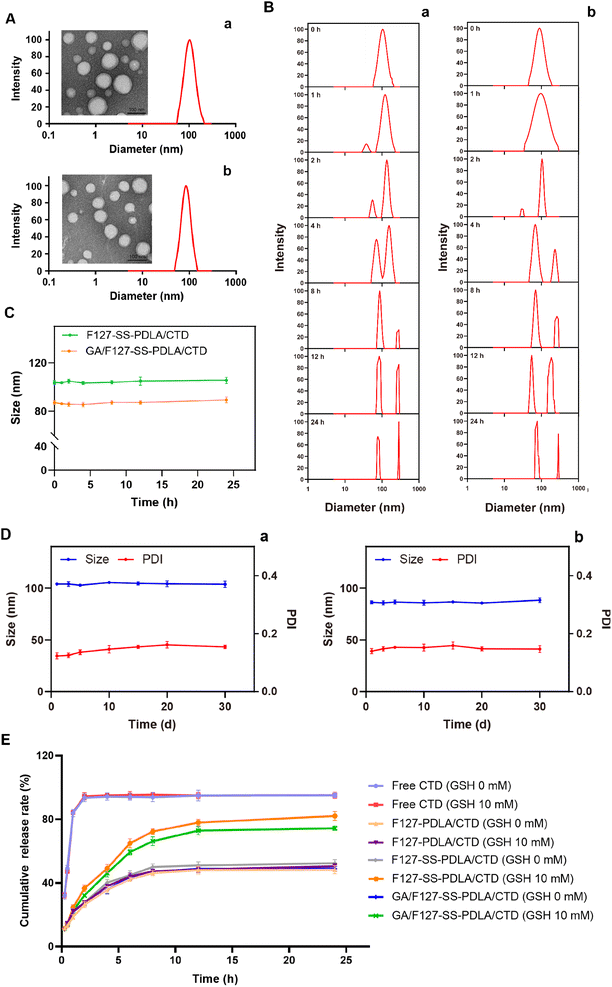
The particle size, PDI, zeta potential, DL and EE of various CTD-loaded micelles were listed in Table 1. The nanoparticles within the size range of 20–200 nm could not only take advantage of EPR effect to enhance the passive accumulation of drugs in tumor tissues, but also evade the phagocytosis by the reticuloendothelial system (RES) and premature elimination through glomerular filtration.40 Consequently, it was implied that CTD-loaded micelles would effectively aggregate in tumor sites. It was noteworthy that polymer mixed micelles GA/F127-SS-PDLA/CTD possessed smaller particle size (85.17 ± 1.24 nm) compared to F127-SS-PDLA/CTD micelles prepared from the single polymer material (102.20 ± 1.98 nm), which was also consistent with previous reports.21 This preponderance of particle size would make polymer mixed micelles more likely to enter the tumor tissue through the EPR effect and be taken up by tumor cells.41 The PDI value of GA/F127-SS-PDLA/CTD micelles was less than 0.200, which facilitating the micelles to cross the biological barrier effectively.42 Moreover, GA/F127-SS-PDLA/CTD micelles had a zeta potential of −11.71 ± 0.86 mV which would contribute to improving hemocompatibility and prolonging the circulation time.40| Micelles | Size (nm) | Polydispersity | Zate potential (mV) | Encapsulation efficiency (%) | Drug loading (%) |
|---|---|---|---|---|---|
| F127-PDLA/CTD | 114.55 ± 2.09 | 0.102 ± 0.015 | −8.47 ± 0.33 | 93.25 ± 1.51 | 15.72 ± 0.22 |
| F127-SS-PDLA/CTD | 102.20 ± 1.98 | 0.141 ± 0.010 | −10.08 ± 0.41 | 95.37 ± 0.93 | 16.02 ± 0.13 |
| GA/F127-SS-PDLA/CTD | 85.17 ± 1.24 | 0.132 ± 0.005 | −11.71 ± 0.86 | 96.09 ± 0.81 | 16.12 ± 0.11 |
Utilizing DLS to determine particle size and distribution has advantages of easy operation and fast analysis, but it cannot reflect the appearance and morphology of particles. Therefore, DLS and TEM are usually combined to comprehensively evaluate the nanoparticle from both quantitative and qualitative perspectives. As illustrated in Fig. 6A, the average particle sizes of GA/F127-SS-PDLA/CTD and F127-SS-PDLA/CTD micelles were about 72 and 89 nm, respectively. They possessed uniform spherical structure with good dispersion and no fragmentation, adhesion or aggregation. The particle sizes observed by TEM were smaller than those measured via DLS, which was attributed to the drying and tightening of micelles during sample preparation.38
3.4 The stability of CTD-loaded micelles
The stability of nanoparticles is not only beneficial for drug transport and storage, but also for the anti-tumor effect of drug.25,43 As displayed in Fig. 6D, when stored at 4 °C for 30 days, negligible variations of particle size and PDI were observed in F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles. It was manifested that CTD-loaded micelles had excellent capacity for long-term storage, which was possibly contributed to the negative charge on the surface of the micelles.41When entering the physiological environment, nanoparticles could be coated with biomacromolecules in the blood such as proteins, which might lead to aggregation and precipitation.44,45 As shown in Fig. 6C, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles were basically stable in 10% FBS within 24 h with little changes in particle size, indicating that the protein adsorbed on the surface of the micelles was less and CTD-loaded micelles possessed the potential to remain stable in blood circulation.
3.5 Reduction-responsive behaviour of CTD-loaded micelles
The disulfide bonds between F127 and PDLA made micelles susceptible to redox environment. The changes in size distribution of F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles in 10 mM GSH were monitored by DLS. As illustrated in Fig. 6B, the particle size of the micelles showed a pronounced bimodal distribution starting from 4 h in 10 mM GSH. Moreover, with the increase of time, the signal intensity of larger particle size increased continuously. It could be inferred that F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles were gradually decomposed to release drugs in the reducing environment. The results were similar to the previous study. Liu et al. reported that significant changes in particle size distribution and morphology of the redox-sensitive micelles were observed under reductive conditions.26 The main reason was that the cleavage of the disulfide bonds connecting F127 and PDLA resulted in the disruption of the micelle structure, the aggregation of hydrophobic chain segments and the formation of larger particles.24,253.6 In vitro drug release
In vitro cumulative release assay is usually used to estimate the drug release rate and the total amount, which is one of the evaluation methods for sustained and controlled-release formulations.46 The drug release behaviors of free CTD and three CTD-loaded micelles were investigated in different release media by the dialysis method. As illustrated in Fig. 6E, free CTD showed a burst release characteristic with approximately 90% CTD were released within 2 h whatever the concentrations of GSH. In contrast, CTD-loaded micelles all exhibited sustained release pattern. The release trend of CTD from F127-PDLA/CTD micelles was roughly the same whatever in normal saline or 10 mM GSH, indicating that the non-sensitive micelles could maintain stability in the reductive environment. The cumulative release rates of F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles in normal saline were 52.35 ± 2.33% and 48.89 ± 1.44% within 24 h, while CTD released from F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles reached 82.10 ± 2.91% and 74.36 ± 1.46% respectively in 10 mM GSH solution. It was related to the hydrolysis and fracture of disulfide bonds, leading to the rapid release of drugs. These results revealed that F127-SS-PDLA conjugate was able to achieve redox-responsive drug release in tumor cells and maintain stability in normal physiological environment, which was of great value for treating cancer and protecting normal cells.3.7 Hemolysis assay
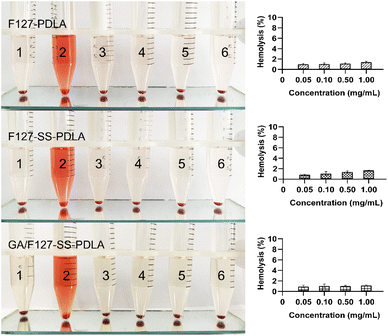
In order to validate the safety of polymer materials, their blood compatibility should be evaluated through a hemolysis assay. As could be seen from Fig. 7, three blank micelles F127-PDLA, F127-SS-PDLA and GA/F127-SS-PDLA exhibited no significant hemolysis even at 1.00 mg mL−1. The American Society for Testing and Materials (ASTM) classified materials into three grades according to their degrees of hemolysis: hemolytic (hemolysis rate greater than 5%), mildly hemolytic (hemolysis rate 2–5%) and non-hemolytic (hemolysis rate less than 2%).26,47 The hemolysis rates of the three materials were all less than 2% in the concentration range of 0.50–1.00 mg mL−1, reflecting that F127-PDLA, F127-SS-PDLA and GA/F127-SS-PDLA micelles possessed great blood compatibility within the experimental concentration range and could meet the requirements of intravenous injection.3.8 Cytotoxicity assay
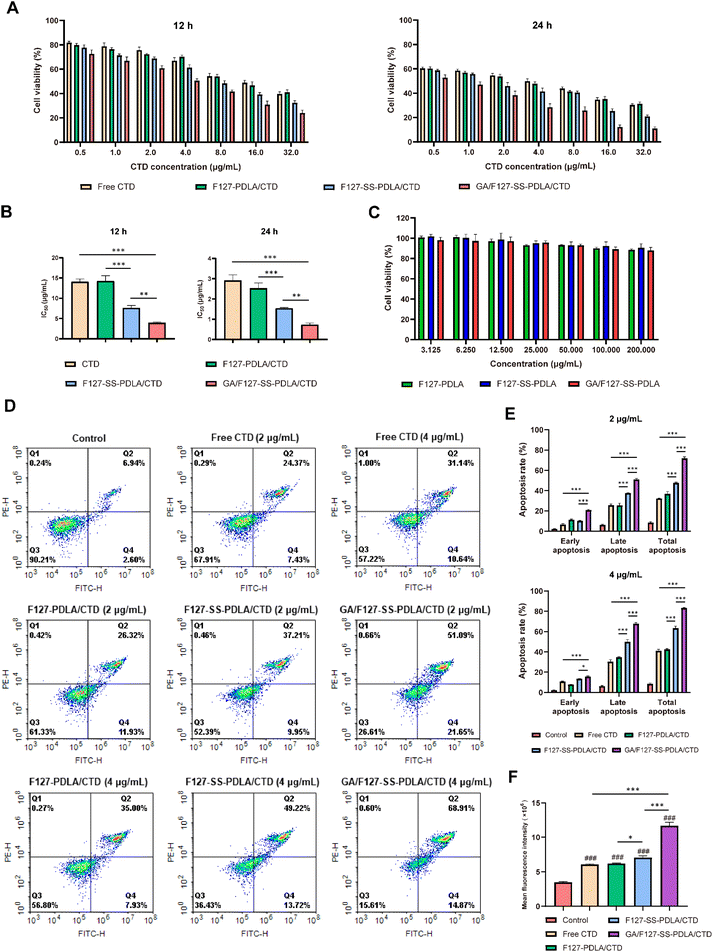
MTT assay were performed on HepG-2 cells to study the cytotoxicity of CTD-loading micelles. In order to further investigate the biocompatibility of carrier materials, the cytotoxicity of blank micelles F127-PDLA, F127-SS-PDLA and GA/F127-SS-PDLA was firstly tested. As illustrated in Fig. 8C, after incubation for 24 h, no significant inhibition of the blank micelles was found on the growth of HepG-2 cells in the concentration range of 3.125–200 μg mL−1 (cellular viability was all above 85%), manifesting that they had great biocompatibility within the range of certain concentration and would exert no influence on the outcomes of subsequent experiments.As showcased in Fig. 8A, when treatment period and concentration of CTD increased, the inhibitory effects of free CTD and CTD-loaded micelles on the growth of tumor cells were enhanced, which exhibited a time and concentration-dependent manner. The half maximal inhibitory concentration (IC50) values of free CTD and CTD-loaded micelles were illustrated in Table 2 and summarized in Fig. 8B. The IC50 value of F127-SS-PDLA/CTD micelles was significantly lower than those of F127-PDLA/CTD micelles and free CTD (P < 0.001). This suggested that redox-sensitive micelles were able to rapidly release drugs in response to the intracellular redox environment, and therefore the equivalent inhibitory effect could be achieved at lower concentration of CTD. Furthermore, GA/F127-SS-PDLA/CTD micelles displayed the strongest inhibition ability, whose IC50 was about 1/2 of that of F127-SS-PDLA/CTD micelles. It was probably resulted from the specific interaction between GA and the GA receptor on the surface of HepG-2 cells, which enhanced the internalization of GA/F127-SS-PDLA/CTD micelles and increased drug accumulation.
| Time (h) | IC50 (μg mL−1) | |||
|---|---|---|---|---|
| Free CTD | F127-PDLA/CTD | F127-SS-PDLA/CTD | GA/F127-SS-PDLA/CTD | |
| 12 | 14.08 ± 0.70 | 14.26 ± 1.32 | 7.631 ± 0.601 | 3.924 ± 0.210 |
| 24 | 2.917 ± 0.274 | 2.527 ± 0.263 | 1.545 ± 0.036 | 0.7315 ± 0.0791 |
3.9 Apoptosis assay
To further confirm whether the inhibitory effect of GA/F127-SS-PDLA/CTD micelles on HepG-2 cell was connected with the induction of apoptosis, we performed apoptosis assay via the mothed of Annexin V/PI double staining. As shown in Fig. 8D and E, compared with the control group, treatment with 2 μg mL−1 and 4 μg mL−1 of free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles for 12 h significantly enhanced the population of apoptotic cells. Furthermore, the concentration of drug had a significant impact on apoptosis rate (P < 0.05). At the concentration of 2 μg mL−1 and 4 μg mL−1, the total apoptosis rates of GA/F127-SS-PDLA/CTD group were 71.86 ± 1.44% and 83.25 ± 0.46%, respectively, which were 2.23 and 2.03 times higher than those of free CTD group, 1.95 and 1.96 times higher than those of F127-PDLA/CTD group, 1.51 and 1.31 times higher than those of F127-SS-PDLA/CTD group. The redox-sensitive F127-SS-PDLA/CTD micelles demonstrated higher late and total apoptotic rates than the non-sensitive F127-PDLA/CTD micelles.However, F127-SS-PDLA/CTD micelles displayed lower early apoptotic rate at 2 μg mL−1. This discrepancy might be attributed to the rapid release of CTD by redox-sensitive micelles in response to intracellular high concentrations of GSH, which directly induce the tumor cells into the late apoptotic state, and therefore the proportion of cells in early apoptosis was reduced. Notably, GA/F127-SS-PDLA/CTD micelles had significantly higher early, late and total apoptosis rates than other groups no matter what the concentration was. In conclusion, GA/F127-SS-PDLA/CTD micelles were more efficient than other preparations in targeting HepG-2 cells and releasing CTD to induce apoptosis of cells.
3.10 Intracellular ROS levels
Intracellular ROS, a by-product of cellular oxygen consumption and metabolism, is closely related to apoptosis and death of tumor cells.48–50 Studies have demonstrated that CTD was able to induce apoptosis in a variety of tumor cells by increasing the levels of intracellular ROS.9,51 Therefore, the generation of ROS in HepG-2 after drug treatment were evaluated via DCFH-DA staining to explore the relationship between ROS and the cytotoxicity. As shown in Fig. 8F, after treatment with free CTD, F127-PDLA/CTD, F127-SS-PDLA/CTD and GA/F127-SS-PDLA/CTD micelles, the generation of ROS in HepG-2 cells was significantly increased. The ROS levels of different groups in HepG-2 cells were in the order of GA/F127-SS-PDLA/CTD micelles > F127-SS-PDLA/CTD micelles > F127-PDLA/CTD micelles ≈ free CTD > control. These results manifested that GA/F127-SS-PDLA/CTD micelles were able to augment intracellular ROS levels through GA receptor-mediated endocytosis and reduction sensitive function. However, the F127-PDLA/CTD micelles group showed no significant disparity compared to the free CTD group, possibly attributed to its slow drug release and lack of active targeting function. Combined with the results above, it was indicated that CTD could upregulate intracellular ROS levels, induce cellular apoptosis, and ultimately cause cell death. Moreover, GA/F127-SS-PDLA/CTD micelles could reinforce this process to some extent.3.11 Cellular uptake assay
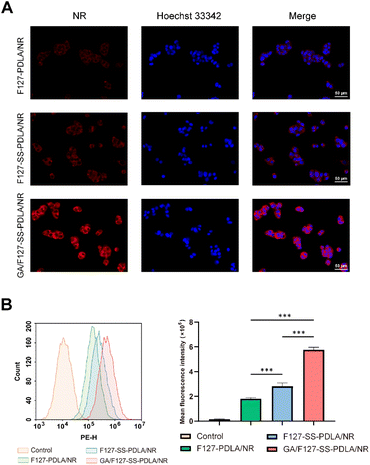
The intracellular uptake ability of F127-PDLA/NR, F127-SS-PDLA/NR and GA/F127-SS-PDLA/NR by HepG-2 cells was observed and imaged by fluorescent microscope. As displayed in Fig. 9A, it could be clearly observed that NR (red fluorescence) and Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (blue fluorescence) were mainly distributed in the cytoplasts and nuclei, respectively. After administration for 4 h, the order of the red fluorescence intensity in HepG-2 cells was as following: GA/F127-SS-PDLA/NR micelles > F127-SS-PDLA/NR micelles > F127-PDLA/NR micelles. Fluorescence intensity of cells was further measured by flow cytometry to quantify the uptake efficiency of various micelles (Fig. 9B). After cultured with F127-SS-PDLA/NR micelles for 4 h, the mean fluorescence intensity in HepG-2 cells was 1.5 times higher than that in F127-PDLA/NR micelles group. Furthermore, the mean fluorescence intensity of GA/F127-SS-PDLA/NR group was 2.0 and 3.2 times higher than those of F127-SS-PDLA/NR and F127-PDLA/NR groups, respectively. This indicated that the release of NR from micelles was promoted by the high GSH concentration in HepG-2 cells, bringing about stronger intracellular fluorescence intensity of reduction-sensitive micelles group compared with non-sensitive micelles group. Similar results were observed in previous.22,52,53 We speculated that those fluorescent substances that had not been released seem to be covered with a “thin film”, so the fluorescence intensity was slightly weaker. Meanwhile, the uptake of GA/F127-SS-PDLA/NR micelles was higher than that of F127-SS-PDLA/NR and F127-PDLA/NR micelles. The results further manifested that the modification of GA could promote the selective uptake of micelles via GA receptor-mediated endocytosis by HepG-2 cells.
342 (blue fluorescence) were mainly distributed in the cytoplasts and nuclei, respectively. After administration for 4 h, the order of the red fluorescence intensity in HepG-2 cells was as following: GA/F127-SS-PDLA/NR micelles > F127-SS-PDLA/NR micelles > F127-PDLA/NR micelles. Fluorescence intensity of cells was further measured by flow cytometry to quantify the uptake efficiency of various micelles (Fig. 9B). After cultured with F127-SS-PDLA/NR micelles for 4 h, the mean fluorescence intensity in HepG-2 cells was 1.5 times higher than that in F127-PDLA/NR micelles group. Furthermore, the mean fluorescence intensity of GA/F127-SS-PDLA/NR group was 2.0 and 3.2 times higher than those of F127-SS-PDLA/NR and F127-PDLA/NR groups, respectively. This indicated that the release of NR from micelles was promoted by the high GSH concentration in HepG-2 cells, bringing about stronger intracellular fluorescence intensity of reduction-sensitive micelles group compared with non-sensitive micelles group. Similar results were observed in previous.22,52,53 We speculated that those fluorescent substances that had not been released seem to be covered with a “thin film”, so the fluorescence intensity was slightly weaker. Meanwhile, the uptake of GA/F127-SS-PDLA/NR micelles was higher than that of F127-SS-PDLA/NR and F127-PDLA/NR micelles. The results further manifested that the modification of GA could promote the selective uptake of micelles via GA receptor-mediated endocytosis by HepG-2 cells.
4 Conclusions
Nano-drug delivery systems can address the problems on poor water solubility, strong toxic and side effects and inadequate targeting ability of anti-tumor ingredients in traditional Chinese medicines. However, due to the intricate tumor microenvironment, drug delivery systems with single-function alone are insufficient to provide effective treatment. In this study, we designed and developed CTD-loaded polymeric mixed micelles GA/F127-SS-PDLA/CTD with active targeting and redox-sensitive functions for HCC therapy. GA/F127-SS-PDLA/CTD micelles exhibited uniform particle size distribution, spherical morphology, excellent stability and biocompatibility, as well as demonstrated exceptional drug-loading ability for CTD and reduction-responsive drug release characteristic. In vitro drug release assay manifested that GA/F127-SS-PDLA/CTD micelles could remain stable under physiological conditions and release rapidly under high GSH conditions. In cytological experiments showed that the anti-tumor ability of reduced sensitive micelles F127-SS-PDLA/CTD was stronger than that of non-sensitive micelles F127-PDLA/CTD, because in response to the intracellular reductive environment, F127-SS-PDLA/CTD micelles were able to release drugs rapidly in tumor cells, and thereby inhibit proliferation and enhance apoptosis. Meanwhile, GA/F127-SS-PDLA/CTD micelles exhibited the most effective cytotoxicity, apoptosis, elevation of ROS level and cellular uptake in HepG-2 cells, since GA/F127-SS-PDLA/CTD micelles had both reduction sensitivity and active targeting function. They could effectively recognize the GA receptor on the surface of tumor cells and promote internalization of micelles. Last but not least, it was found that the targeting ability of GA/F127-SS-PDLA/CTD micelles with 10% and 15% F127-GA was better than those with 5% and 20% F127-GA. Therefore, the density of targeting ligands was not necessarily higher, but should have an optimal range. In conclusion, this novel drug delivery system has the potential to compensate for the insufficient targeting ability of the current CTD preparations, and thereby further enhancing its anti-tumor efficacy.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Strengthening Foundation Plan for Young Teachers of School of Pharmacy, Shandong University of Traditional Chinese Medicine (SDTCM) (No. KYQJ-202314), the project of High-Level Traditional Chinese Medicine Key Disciplines of the State Administration of Traditional Chinese Medicine and the National Science Foundation for Distinguished Young Scholars of China and National Natural Science Foundation of China (Youth Program) (Grant No. 82305051). All authors gratefully acknowledge Dr Jing Sun, Dr Zhenhua Tian and Dr Shuai Man from Chinese Experimental Centre for laboratory equipment of SDTCM for their guidance of HPLC, 1H-NMR and FTIR studies. All the authors thank National Centre for Protein Sciences at Peking University in Beijing, China, for its guidance on TEM.References
- E. Chakraborty and D. Sarkar, Cancers, 2022, 14, 151–172 CrossRef PubMed.
- S. Lu, J. Huang, J. Zhang, C. Wu, Z. Huang, X. Tao, L. You, A. Stalin, M. Chen, J. Li, Y. Tan, Z. Wu, L. Geng, Z. Li, Q. Fan, P. Liu, Y. Lin, C. Zhao and J. Wu, J. Ethnopharmacol., 2024, 319, 117209 CrossRef CAS PubMed.
- M. Zou, Y. Xu, P. Lin, L. Zhou and X. Xia, J. Liposome Res., 2023, 33, 1–17 CrossRef PubMed.
- M. Ibrahim, W. H. Abuwatfa, N. S. Awad, R. Sabouni and G. A. Husseini, Pharmaceutics, 2022, 14, 254 CrossRef CAS PubMed.
- H. Yao, J. Zhao, Z. Wang, J. Lv, G. Du, Y. Jin, Y. Zhang, S. Song and G. Han, Colloids Surf., B, 2020, 196, 111285 CrossRef CAS PubMed.
- F. Naz, Y. Wu, N. Zhang, Z. Yang and C. Yu, Molecules, 2020, 25, 3279 CrossRef CAS PubMed.
- S. Hu, J. Chang, H. Ruan, W. Zhi, X. Wang, F. Zhao, X. Ma, X. Sun, Q. Liang, H. Xu, Y. Wang and Y. Yang, Int. J. Biol. Sci., 2021, 17, 2504–2522 CrossRef CAS PubMed.
- Y. Pan, Q. Zheng, W. Ni, Z. Wei, S. Yu, Q. Jia, M. Wang, A. Wang, W. Chen and Y. Lu, Front. Pharmacol, 2019, 10, 590 CrossRef CAS PubMed.
- W. Huang, S. Ko, H. Tsai, J. Chung, J. Chiang, K. Chen, Y. Chen, H. Chen, Y. Chen and J. Yang, Int. J. Oncol., 2011, 38, 1067–1073 CAS.
- K. Zhu, L. Zhou, M. Zou, S. Ning, S. Liu, Y. Zhou, K. Du, X. Zhang and X. Xia, J. Pharm. Sci., 2020, 109, 2038–2047 CrossRef CAS PubMed.
- B. Zhai, J. Sun, Y. Shi, X. Zhang, J. Zou, J. Cheng, Y. Fan, D. Guo and H. Tian, J. Nanobiotechnol., 2022, 20, 509 CrossRef PubMed.
- P. An, D. Lu, L. Zhang, H. Lan, H. Yang, G. Ge, W. Liu, W. Shen, X. Ding, D. Tang, W. Zhang, X. Luan, H. Cheng and H. Zhang, Phytomedicine, 2022, 103, 154231 CrossRef CAS PubMed.
- X. Liu, L. Zhang, W. Tang, T. Zhang, P. Xiang, Q. Shen, T. Ye and Y. Xiao, Toxicol. Appl. Pharmacol., 2023, 465, 116450 CrossRef CAS PubMed.
- W. Cheng, Y. Wang, J. Liu, X. Li, M. Yu, C. Duan, L. Liu and J. Zhang, J. Appl. Toxicol., 2021, 42, 970–980 CrossRef PubMed.
- D. Jin, N.-N. Huang and J.-X. Wei, Front. Pharmacol, 2023, 14, 1201404 CrossRef CAS PubMed.
- J. Wu, J. Pers. Med., 2021, 11, 771 CrossRef PubMed.
- I. D. Zlotnikov, D. A. Streltsov, A. A. Ezhov and E. V. Kudryashova, Pharmaceutics, 2023, 15, 1135 CrossRef CAS PubMed.
- D. Hwang, J. D. Ramsey and A. V. Kabanov, Adv. Drug Delivery Rev., 2020, 156, 80–118 CrossRef CAS PubMed.
- H. Cabral and K. Kataoka, J. Controlled Release, 2014, 190, 465–476 CrossRef CAS PubMed.
- X.-B. Fang, J.-M. Zhang, X. Xie, D. Liu, C.-W. He, J.-B. Wan and M.-W. Chen, Int. J. Pharm., 2016, 502, 28–37 CrossRef CAS PubMed.
- J. Song, Y. Liu, L. Lin, Y. Zhao, X. Wang, M. Zhong, T. Xie, Y. Luo, S. Li, R. Yang and H. Li, RSC Adv., 2019, 9, 40131–40145 RSC.
- C. Tian, S. Asghar, Z. Hu, Y. Qiu, J. Zhang, F. Shao and Y. Xiao, Int. J. Biol. Macromol., 2019, 136, 143–153 CrossRef CAS PubMed.
- X. Du, S. Yin, F. Zhou, X. Du, J. Xu, X. Gu, G. Wang and J. Li, Int. J. Pharm., 2018, 550, 1–13 CrossRef CAS PubMed.
- L. Zou, X. Liu, J. Li, W. Li, L. Zhang, C. Fu, J. Zhang and Z. Gu, Theranostics, 2021, 11, 4171–4186 CrossRef CAS PubMed.
- Y. Yuan, Z. Wang, S. Su, Y. Mi, Q. Li, F. Dong, W. Tan and Z. Guo, Int. J. Biol. Macromol., 2023, 247, 125849 CrossRef CAS PubMed.
- Y. Liu, S. Fu, L. Lin, Y. Cao, X. Xie, H. Yu, M. Chen and H. Li, Int. J. Nanomed., 2017, 12, 2635–2644 CrossRef CAS PubMed.
- P. Dehghan Kelishady, E. Saadat, F. Ravar, H. Akbari and F. Dorkoosh, Pharm. Dev. Technol., 2014, 20, 1009–1017 CrossRef PubMed.
- P. S. Giram, J. T.-W. Wang, A. A. Walters, P. P. Rade, M. Akhtar, S. Han, F. N. Faruqu, H. M. Abdel-Bar, B. Garnaik and K. T. Al-Jamal, Biomater. Sci., 2021, 9, 795–806 RSC.
- Y. Shi, R. van der Meel, X. Chen and T. Lammers, Theranostics, 2020, 10, 7921–7924 CrossRef PubMed.
- Z. Li, X. Xiong, S. Peng, X. Chen, W. Liu and C. Liu, J. Microencapsulation, 2020, 37, 220–229 CrossRef CAS PubMed.
- A. A. Shitole, N. Sharma, P. Giram, A. Khandwekar, M. Baruah, B. Garnaik and S. Koratkar, Mater. Sci. Eng. C, 2020, 114, 111035 CrossRef CAS PubMed.
- F. Wu, X. Li, B. Jiang, J. Yan, Z. Zhang, J. Qin, W. Yu and Z. Gao, J. Biomed. Nanotechnol., 2018, 14, 1837–1852 CrossRef CAS PubMed.
- C. T. Dinh, H. T. Vu, Q. T. H. Phan, L. P. Nguyen, T. Q. Tran, D. Van Tran, N. N. Quy, D. T. N. Pham and D. T. Nguyen, J. Mater. Sci.: Mater. Med., 2022, 33, 72 CrossRef CAS PubMed.
- Y. Hu, J. Song, A. Feng, J. Li, M. Li, Y. Shi, W. Sun and L. Li, Molecules, 2023, 28, 7767 CrossRef CAS PubMed.
- Y. C. Gong, X. Y. Xiong, X. J. Ge, Z. L. Li and Y. P. Li, Macromol. Biosci., 2019, 19, e1800348 CrossRef PubMed.
- S. L. Yi, Z. L. Li, Y. C. Gong and X. Y. Xiong, Langmuir, 2023, 39, 15920–15931 CrossRef CAS PubMed.
- M. K. Riaz, X. Zhang, K. H. Wong, H. Chen, Q. Liu, X. Chen, G. Zhang, A. Lu and Z. Yang, Int. J. Nanomed., 2019, 14, 2879–2902 CrossRef CAS PubMed.
- Y. Zhong, W. Yang, H. Sun, R. Cheng, F. Meng, C. Deng and Z. Zhong, Biomacromolecules, 2013, 14, 3723–3730 CrossRef CAS PubMed.
- A. Grigoletto, G. Martinez, D. Gabbia, T. Tedeschini, M. Scaffidi, S. Martin and G. Pasut, Pharmaceutics, 2021, 13, 929 CrossRef CAS PubMed.
- J. Liu, N. Liang, S. Li, Y. Han, P. Yan, Y. Kawashima, F. Cui and S. Sun, J. Biomater. Appl., 2020, 34, 1458–1469 CrossRef CAS PubMed.
- N. S. Noor, N. H. M. Kaus, M. R. Szewczuk and S. B. S. Hamid, Int. J. Mol. Sci., 2021, 22, 9420 CrossRef CAS PubMed.
- X. P. Chen, Y. Li, Y. Zhang and G. W. Li, Drug Des., Dev. Ther., 2019, 13, 3569–3578 CrossRef CAS PubMed.
- Y. Liu and X. An, Colloids Surf., B, 2019, 178, 238–244 CrossRef CAS PubMed.
- L. Yu, M. Xu, W. Xu, W. Xiao, X. H. Jiang, L. Wang and H. Gao, Nano Lett., 2020, 20, 8903–8911 CrossRef CAS PubMed.
- L. Rao, Q. F. Meng, L. L. Bu, B. Cai, Q. Huang, Z. J. Sun, W. F. Zhang, A. Li, S. S. Guo, W. Liu, T. H. Wang and X. Z. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 2159–2168 CrossRef CAS PubMed.
- W. Jiang, J. Guo, W. Wen, Y.-G. Jia and S. Liu, Materials, 2019, 12, 1610 CrossRef CAS PubMed.
- F. K. Andrade, J. P. Silva, M. Carvalho, E. M. S. Castanheira, R. Soares and M. Gama, J. Biomed. Mater. Res., Part A, 2011, 98A, 554–566 CrossRef CAS PubMed.
- J. Liu, Y. Xu, H. Lu, R. Wang, Z. Xia, C. Zhao, D. Huang, F. Jiang and W. Chen, Langmuir, 2022, 38, 13955–13962 CrossRef CAS PubMed.
- B. Ren, Z.-C. Cai, X.-J. Zhao, L.-S. Li and M.-X. Zhao, Int. J. Nanomed., 2021, 16, 7023–7033 CrossRef CAS PubMed.
- S. Vafaei, S. A. Sadat Shandiz and Z. Piravar, Biol. Trace Elem. Res., 2020, 198, 109–117 CrossRef CAS PubMed.
- T. C. Hsia, C. C. Yu, S. C. Hsu, N. Y. Tang, H. F. Lu, Y. P. Huang, S.-H. Wu, J.-G. Lin and J.-G. Chung, Int. J. Oncol., 2014, 45, 245–254 CrossRef CAS PubMed.
- L. Han, L. Hu, F. Liu, X. Wang, X. Huang, B. Liu, F. Feng, W. Liu and W. Qu, Asian J. Pharm. Sci., 2019, 14, 531–542 CrossRef PubMed.
- M. Huo, Y. Liu, L. Wang, T. Yin, C. Qin, Y. Xiao, L. Yin, J. Liu and J. Zhou, Mol. Pharm., 2016, 13, 1750–1762 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03171g |
| ‡ These authors contributed equally to this work and should be considered as corresponding author. |
| This journal is © The Royal Society of Chemistry 2024 |