Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spinel cobalt-based binary metal oxides as emerging materials for energy harvesting devices: synthesis, characterization and synchrotron radiation-enabled investigation†
Abdelelah Alshanableha,
Yusuf Selim Ocakab,
Bashar Aljawrneh *c,
Borhan Aldeen Albissa,
Khaled Shawakfehcd,
Latif U. Khane
*c,
Borhan Aldeen Albissa,
Khaled Shawakfehcd,
Latif U. Khane e,
Messaoud Harfoucheee and
Saja Alrousana
e,
Messaoud Harfoucheee and
Saja Alrousana
aNanotechnology Institute, Jordan University of Science & Technology, PO Box 3030, Irbid 22110, Jordan
bDepartment of Physics and Engineering Physics, Morgan State University, Baltimore, Maryland 21234, USA
cDepartment of Physics, Al-Zaytoonah University of Jordan, PO Box 130, Amman 11733, Jordan. E-mail: B.Aljawarneh@zuj.edu.jo
dDepartment of Chemistry, Jordan University of Science & Technology, PO Box 3030, Irbid 22110, Jordan
eSynchrotron-Light for Experimental Science and Applications in the Middle East (SESAME), PO Box 7, Allan 19252, Jordan
First published on 4th July 2024
Abstract
The synthesis and characterization of spinel cobalt-based metal oxides (MCo2O4) with varying 3d-transition metal ions (Ni, Fe, Cu, and Zn) were explored using a hydrothermal process (140 °C for two hours) to be used as alternative counter electrodes for Pt-free dye-sensitized solar cells (DSSCs). Scanning electron microscopy (SEM) and atomic force microscopy (AFM) revealed distinct morphologies for each metal oxide, such as NiCo2O4 nanosheets, Cu Co2O4 nanoleaves, Fe Co2O4 diamond-like, and Zn Co2O4 hexagonal-like structures. The X-ray diffraction analysis confirmed the cubic spinel structure for the prepared MCo2O4 films. The functional groups of MCo2O4 materials were recognized in metal oxides throughout Fourier transform infrared (FTIR) analysis. The local structure analysis using X-ray absorption fine structure (XAFS) at Fe and Co K-edge identified octahedral (Oh) Co3+ and tetrahedral (Td) Co2+ coordination, with Zn2+ and Cu2+ favoring Td sites, while Ni3+ and Fe3+ preferred Oh active sites. Further investigations utilizing the Fourier transformation (FT) analysis showed comparable coordination numbers and interatomic distances ranked as Co–Cu > Co–Fe > Zn–Co > Co–Ni. Furthermore, the utilization of MCo2O4 thin films as counter electrodes in DSSC fabrication showed promising results. Notably, solar cells based on CuCo2O4 and ZnCo2O4 counter electrodes showed 1.9% and 1.13% power conversion efficiency, respectively. These findings indicate the potential of employing these binary metal oxides for efficient and cost-effective photovoltaic device production.
Introduction
Because of the permanent growth in the global population and industrialization, the demand for energy has increased steadily. Limits in fuel-based energy sources make the need for cleaner, sustainable alternative energy sources inevitable. Among all renewable energy sources, solar energy is one of the most impressive ones. Photovoltaic technologies, particularly dye-sensitized solar cells (DSSCs), have emerged as key factors in this transition owing to their low cost and easy production processes. Another important parameter that increases the impression of DSSCs is the capability of harvesting energy from even lower-intensive light.Significant effort has been devoted to increasing the power conversion efficiency and stability of DSSCs while making them cost-effective. To achieve optimal performance in the DSSCs, the counter electrode commonly incorporates platinum (Pt) on a surface of transparent conductive oxide.1,2 Several novel approaches have been formulated to improve the DSSCs' performance including enhancing the light absorption capacity of the sensitizer, charge separation of photo-induced charge at the electrolyte–semiconductor interface, and prompt the migration of charge towards the counter electrode.3–5 The proposed approaches involve the binding of a sensitizer onto wide bandgap semiconductor substrates such as titanium dioxide, which aims to enhance light absorption capabilities.6 Additional efforts have been made to replace the Pt counter electrode in DSSCs. As an alternative to Pt, various kinds of materials, including metal oxides, metal sulfides, carbon, and hybrid electrodes, have been proposed to obtain cost-effective device fabrication.7–11 An alternative candidate with significant potential for DSSCs is spinel cobalt-based metal oxides (MB2O4, M: tetrahedral, B: octahedral sites). Such unique characteristics involve excellent electrical conductivity, efficient charge transfer, and significantly large surface area. Besides that, various oxidation states, and notable electrochemical activity make them highly advantageous to be used in energy and photocatalytic applications.12–15
The spinel cobalt oxide (Co3O4) is one of the most investigated electro-catalyst materials that is widely applied in the counter electrode for DSSCs. The tetrahedral site (Td) is occupied by Co2+ ions, while the octahedral site (Oh) is occupied by two Co3+ ions.16 To date, Co2+ and Co3+ serve as active sites within the spinel cobalt metal oxide Co3O4, and they have been reported to have a crucial role in charge transfer and their catalytic activity.17–19 Recent studies report that replacing the 3rd transition metal ions, such as Ni and Zn ions, with the Co2+ in spinel cobalt oxide (Co3O4) enhances their electrical conductivity.20 For instance, ZnCo2O4 and NiCo2O4 exhibited superior electrical conductivity compared to Co3O4. Zn2+ ions are beneficial for indicating defects in the Td sites.,17–20 as observed in spinel oxides, which in turn enhances the catalytic activity for the oxygen evolution reaction. Moreover, the large surface and electrical conductivity of NiCo2O4 result in better catalytic activity than spinel metal oxide (Co3O4). Also, the Fe3+ in FeCo2O4 occupies the Oh sites, leading to a shift in energy level near the Fermi level, which improves the activity of spinel metal oxide.21,22
Several reports have integrated the spinel-based cobalt structure into DSSC devices. Interestingly, the Co3O4 counter electrode of PCE ∼8.6% in DSSCs exhibits an excellent catalytic performance towards the iodide electrolyte compared to the Pt standard counter electrode.23 It has been reported also that, Co3O4 has a good electro-catalytic activity towards reduction of I3− ions into I− ions.24 Furthermore, zinc substitution for Co in cobalt oxide(II,III) results in the spinel structure of Zn–Co–O with p-type conductivity. The hole transport layer of Zn–Co–O employed in DSSCs results in enhanced diffusion length and transport within a device.25 The DSSCs based spinel NiCo2O4 nanostructures reported high performance and are comparable to the Pt counter electrode.26 To address, the role of the local structure of the spinel-based cobalt materials in DSSCs performance still needs in-depth investigation.
In the present work, three-dimensionally arrays of spinel cobalt-based metal oxide thin films of MCo2O4 (M: Ni, Fe, Cu, Zn) were fabricated through hydrothermal deposition to use them as counter electrodes to obtain cost-effective DSSCs. Surface morphology and roughness of the prepared samples were investigated throughout SEM and AFM analysis. The XAFS technique is employed to examine the atomic coordination and interatomic distances by observing the local structure at Fe and Co K-edge. Also, the XANES spectra indicate the prepared samples have a spinel structure. Then, spinel cobalt-based metal oxide thin films were used in the fabrication of DSSCs as promising counter electrodes, and the photovoltaic performance of DSSCs with MCo2O4 counter electrodes were compared.
Experimental procedures
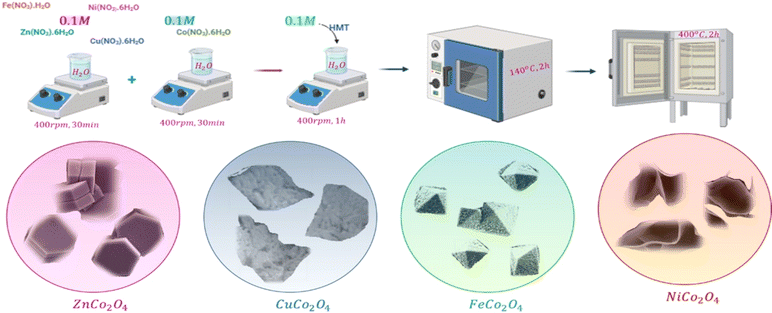
The following materials are used to synthesis the spinel cobalt based oxide films: copper nitrate tetrahydrate (Cu(NO3)2·4H2O), iron monohydrate (Fe(NO3)2·H2O), nickel nitrate hexahydrate (Ni(NO3)2·6H2O), and zinc nitrate hexahydrate (Zn(NO3)2·6H2O), cobalt nitrate hexahydrate (Co(NO3)2·6H2O), hexamethylenetetramine (HMTA, (CH4)6N4), and indium tin oxide (ITO) coated glasses, all are purchased from Sigma Aldrich and used without any further purification.The samples were grown hydrothermally using a solution processing route. The synthesis of spinel cobalt-based metal oxide thin films, MCo2O4, coated over a cleaned ITO substrate is shown in Fig. 1. The ITO substrate was subjected to cleaning in the acetone for 15 min followed by 15 min in the ethanol under sonication. The preparation procedure was performed by mixing 0.1 M of M(NO3)2·xH2O dissolved in 40 mL deionized water, and 0.1 M of Co(NO3)2·6H2O dissolved in 40 mL deionized (DI) water. The solution was then stirred at 400 rpm for 30 min. Then, the 0.1 M of (CH2)6N4 was added to the prepared aqueous solution. The obtained stock solutions were then transferred into a glass-sealed bottle containing a cleaned ITO substrate before being grown in an oven at 140 °C for two hours. Finally, the deposited spinel cobalt-based metal oxides of MCo2O4 films were rinsed with deionized water and annealed at 400 °C in a furnace for another two hours.
Scanning electron microscopy (SEM, Quanta FEG 450) was conducted to investigate the morphology and size distribution of the metal oxide samples. The surface roughness of the MCo2O4 thin films was carried out by using an atomic force microscope (AFM, SmartSPM™ 1000), FTIR (Bruker Vertex 80 and Hyperion 2000 microscope) used to investigate the vibrational modes and chemical bonds of metal oxides. The XAFS data were obtained via the BM-08 XAFS/XRF beamline at the Synchrotron-Light for Experimental Science and Applications in the Middle East (SESAME). The beamline was run in decay condition at an energy level of 2.5 GeV, with 300 mA as a maximum current. The X-ray absorption fine structure (XAFS) data were measured on the BM08-XAFS/XRF beamline of the SESAME operated at 2.5 GeV in “decay” mode with a maximum electron current of 300 mA.27 The XAFS spectra of the materials were acquired in transmission mode in the spectral range of Co K-edge (7709 eV) and Fe K-edge (7112 eV) at room temperature. The X-ray beam intensity was measured by ionization chambers filled with an optimal mixture of noble gases at a total pressure of 1.0 bar, and the XAFS data of the samples were acquired from the signals measured at ion chambers subsequently amplified by Stanford picoammeters and digitalized by a voltage to frequency converter, using a double-crystal Si (111) monochromator. The energy was calibrated at the Co K-edge (7709 eV) and Fe K-edge (7112 eV) of the Co and Fe standard metal foils, respectively. The sample was prepared in pallet form (13 mm diameter) by pressing a homogeneous mixture of calculated quantity of finely ground material and polyvinylpyrrolidone (PVP) powder. The amount of material in the pellet was calculated using XAFS mass software to give an absorption μt ∼1.5, just above the Co K-edge and Fe–K edge absorptions.
The DSSCs were prepared using TiO2 nanoparticle decorated ITO coated photoanodes. The electrodes were sintered at 450 °C in air ambient for 30 min to remove contaminations from the TiO2 nanoparticle surfaces. And then was immersed in N719 dye for a night. The photoanodes were washed with ethanol and dried using a heat gun. MCo2O4 coated ITO counter electrodes and dye-coated TiO2 photoanodes were sealed together and an electrolyte (iodide/tri-iodide in a nitrile solvent) was used to fill the space between two electrodes. The performances of DSSCs were measured under a class A solar simulator (ABET Technology Sun2000) with an AM 1.5 G filter and 100 mW cm−2 light intensity using a Keithley 2425 source meter.
Results and discussion
The DSSCs device performance is directly influenced by the morphology of the counter electrode in particular redox/oxidation electrolyte at the interface. SEM images can give insight into crystal arrays and defective surfaces of spinel cobalt oxides. The surface morphologies of as-prepared MCo2O4 thin films are illustrated in Fig. 2. Interestingly, the morphology of the prepared samples can be correlated to the variations in source metal ions. Fig. 2(a) and (b) manifested uniform arrays of nano-leave structures that coincide with the CuCo2O4 thin film. Whereas Fig. 2(c) and (d) displays uniform arrays of octahedral-shaped structures observed in the FeCo2O4 thin film. The FeCo2O4 thin film is comprised of nano-blocks with average size of about 500 nm. The observed octahedron structure demonstrates high-pressure conditions controlled by the (111) plane, devoid of any preferential axis for selective growth.28 Fig. 2(e) and (f) displays a hierarchical structure of NiCo2O4 nano-sheets observed with hollow arrays. Fig. 2(g) and (h) depicts a twin hexagonal configuration of nano-block ZnCo2O4 arrays observed with non-uniform distribution due to anisotropic growth along [100] and [001] directions.29 It is impressive to find that different Zn2+, Fe2+, Ni2+, and Cu2+ reacted species tend to form spinel structures in a cubic crystal phase. The intricate reaction pathways of these species during the hydrothermal process drive the formation of distinct morphologies of spinel metal oxides. Initially, the decomposed reacted species undergo a recrystallization process to form MCo2O4 with preferential growth on selective planes, depending on the nature of the ions. The synthesis of MCo2O4 is driven by the formation of M(OH)2 and Co(OH)2 before transforming into MCo2(OH)6 complex compound, as demonstrated by the NR Chodankar group.30 Alternatively, another reaction route suggests that the formation pathway is governed by the formation of Co3O4 and MO, which are then reduced to the MCo2O4 structure.31 The annealing process reduces the OH− ions in the complex compound to the oxide form, resulting in the obtaining of bimetal oxides.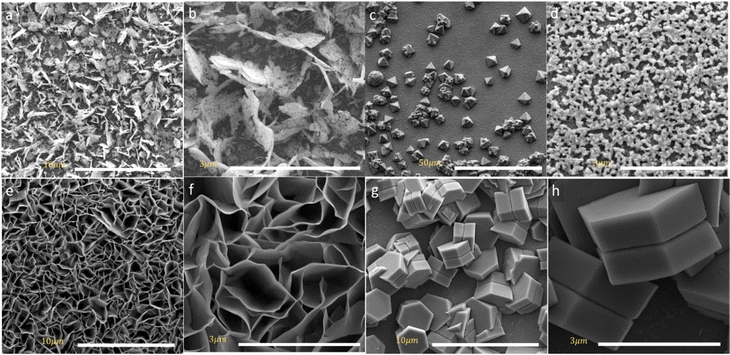 | ||
| Fig. 2 SEM images of the obtained (a and b) CuCo2O4 thin film (c and d) FeCo2O4 thin film (e and f) NiCo2O4 thin film and (g and h) ZnCo2O4 thin film. | ||
The HMT acts as an alkaline source that assists in the formation of M–Co precursor. During the hydrolysis of HMT, NH3 is released, making the solution alkaline. This process also produces OH− ions in the hydrothermal synthesis, which react with Zn2+, Fe2+, Ni2+, Cu2+, and Co2+ ions to form spinel metal oxide. Furthermore, studies have found HMT useful for obtaining a crystalline spinel metal oxide structure. The alkaline environment facilitated by HMT is preferable for precipitating M2+ and Co2+ ions during hydrothermal deposition, accelerating the formation of the crystalline MCo2O4 structure.32,33
The surface area and roughness of the electrodes can provide significant insight into understanding the charge transfer mechanism and overall device performance. The AFM imaging was performed for the MCo2O4 thin films and illustrated in Fig. 3. The AFM images of the prepared CuCo2O4 thin film exhibit a high root mean square (RMS) roughness of 0.16 μm and a large surface area of 38.74 μm2. While the FeCo2O4 thin films show a polygon-shaped structure with an overall RMS of 0.03 μm and a surface area of 29.9 μm2. The phase image demonstrates a variation between – 50° and 20° indicating low phase separation at the grain boundaries. The NiCo2O4 nanosheets thin films depict a large surface area of 58.87 μm2 and RMS of 0.26 μm with an average thickness of nearly 20 nm, while a high roughness was observed for the hexagonal structure of ZnCo2O4 with an RMS of 0.35 μm and surface area of 34.9 μm2. The hexagonal plates of ZnCo2O4 are stacked in a non-uniform structural array.
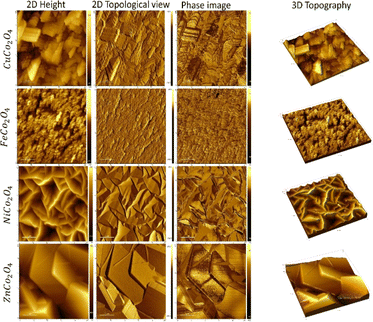 | ||
| Fig. 3 AFM images of as-deposited metal oxides of NiCo2O4, CuCo2O4, ZnCo2O4, NiCo2O4, and FeCo2O4 in 2D and 3D profile. | ||
In DSSCs, the device performance is highly dependent on the crystal quality of the counter electrode based a binary metal oxide films. The cubic spinel structure was confirmed for various metal oxide films from crystallographic X-ray diffraction plots in Fig. S1.† As seen, the diffracted XRD peaks of FeCo2O4 film match the (220), (311), (400), (422), and (440) crystal planes indexed to Fd3m space group (JCPDS card No. 04-0850). The crystal profile of NiCo2O4 nano-sheets assigned to (111), (220), (311), (222), (400), (331), and (511) crystal planes suggested Fd3m space group (JCPDS card No. 01-073-1702). For CuCo2O4 nano-leaves, the observed XRD profile in agreement with (111), (220), (311), (400), (422), and (511) crystallographic planes analogue to Fd3m space group (JCPDS card No. 23-1390). The ZnCo2O4 tends to form a crystal structure throughout hydrothermal synthesis similar to the above metal oxide films. The crystal diffraction profile of ZnCo2O4 resembles (220), (311), (222), (511), and (620) crystal planes for Fd3m (227) space group (JCDP card No. 00-001-1149).
The UV-vis-NIR spectral of the spinel metal oxide films plots in Fig. S2† and the corresponding extrapolated absorption band gap energies (Eg) were determined according to Tauc plot. The NiCo2O4 nanosheets exhibit higher absorbance compared to the other spinel materials oxides followed by crystal CuCo2O4 nanoleaves. The straight-line interception yields two band gap energies of 1.98 and 2.50 eV for ZnCo2O4, while the obtained band gap energies of 2.00 and 3.35 eV for NiCo2O4 in agreement with the reported values of 2.00 and 3.30 eV.34 The valence band in NiCo2O4 was constructed from O 2p orbital and the conduction band from 3d orbitals of Ni and Co. And has an electron configuration of tetrahedral high spin Co2+ (eg4 t2g3), octahedral low spin Co3+ (t2g6), and Ni3+ (t2g6eg1). Therefore, the electron can be excited from Co-3d-t2g to the partially filled Co 3d-eg orbital. Consequently, the presence of two band gap energies can be assigned to high spin and low spin of Co3+ in the spinel structure.34 The spinel CuCo2O4 band gap energies was 1.88 eV and 3.0 eV and FeCo2O4 yields 2.18 eV and 2.82 eV. The determined values are close to those reported in the literature.35,36
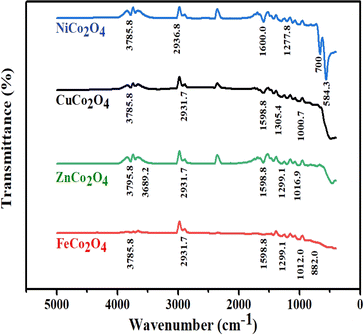
The solution processed DSSCs device via hydrothermal usually contains –OH functional group. To verify the presence of the –OH group and other functional groups that could influence the device performance, the FTIR profile illustrated in Fig. 4 corresponds to the prepared MCo2O4 films. The vibration modes that match the M–O group can be seen at 584.3 cm−1 in various sample profiles. The M–O vibration modes at 584.3 cm−1 and 700 cm−1 may be associated with octahedral and tetrahedral sites.37 Furthermore, stretching modes located at approximately 882 cm−1 and 1000 cm−1 are assigned to the C–O group. Furthermore, the weak vibrational mode at 1277.8 cm−1 and 1299.1 cm−1 is linked to the NO3− group.38 Additional stretching characteristics identified at 1598.8 cm−1 and 1600 cm−1 were assigned to COO and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O molecules.39 The stretching modes at 2931.7 cm−1, 2936.8 cm−1, 3785.8 cm−1, and 3795.8 cm−1 are attributed to the O–H functional group.40,41 A higher content of OH– molecules was noticed in the case of ZnCo2O4 associated with a prominent shift in the OH vibration mode.
O molecules.39 The stretching modes at 2931.7 cm−1, 2936.8 cm−1, 3785.8 cm−1, and 3795.8 cm−1 are attributed to the O–H functional group.40,41 A higher content of OH– molecules was noticed in the case of ZnCo2O4 associated with a prominent shift in the OH vibration mode.
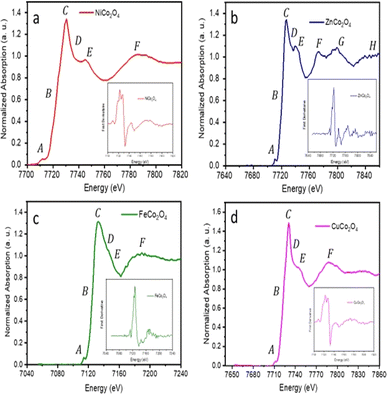
The local structure of the MCo2O4 thin films could play an effective role in the DSSC device performance. The applied XAFS integrates to resolve the local structure of spinel cobalt oxide devices. The XANES spectra at Co absorption K-edge are presented in Fig. 5 of the prepared samples. Resolving the FeCo2O4 structure at the Co absorption K-edge is elusive and therefore XANES is applied at the Fe absorption K-edge. According to the XANES data, the Co absorption K-edge was (7722.53 eV) for NiCo2O4, (7724.02 eV) for CuCo2O4 and (7722.18 eV) for ZnCo2O4. The observed Co absorption K-edge aligns with the value reported in the literature at 7721.4 eV, while the Fe absorption K-edge was 7125.53 eV for FeCo2O4. The oxidation state of the cobalt cation is Co3+ and the observed XANES spectrum reveals the spinel structure of the prepared samples.42,43
The observed XANES spectrum reveals a low-intensity pre-edge peak at A, which can be assigned to 1s → 3d quadrupole transition in spinel cobalt oxide (Co3O4).44 A transition edge (shoulder region) was observed at B, along with the prominent white-line peak of high intensity at C. These transition edges at B and C can be attributed to resonance peaks.45,46 The white-line region can be ascribed to the 1s → np transition.46
Following that, minor peaks of low intensity were observed at D and E. The broadening of peak D, in particular, in FeCo2O4 can be attributed to the coordination atoms in the vicinity of the Co absorber atom. Moreover, two peaks at E and F indicate the presence of multiple scattering associated with the coordination of the medium-range structure surrounding the Co absorber atom.47,48 Furthermore, two peaks at G and H were observed in the XANES spectrum coinciding with the ZnCo2O4 sample, which can be attributed to the existence of multi-scattering effects.
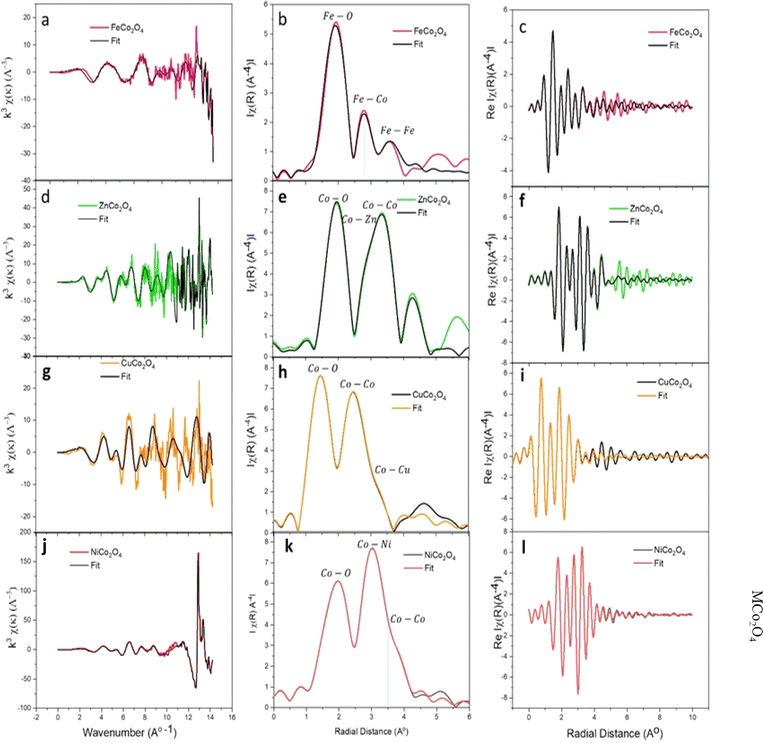
To facilitate a comprehensive quantitative analysis of the local atomic structure of the MCo2O4 nanomaterials, the EXAFS fitting in R-space was conducted.49 The experimental EXAFS data in R-space in the range of 1.0 to 5.0 (Å) with Hanning window and k range of 3.0 Å−1 were analyzed to the best fit. The initial four high-ranking single scattering paths of Co–O and M–Co bond pairs were included in the fit. The passive electrons reduction factor S02 and energy shift E0 were set similarly for all the paths in the fit, and the mean-square relative displacement σ2 and interatomic distance R were refined relatively to get the best fit result.
Fig. 6 plots k3-weighted EXAFS with the best fit, and the FT, respectively. The fit parameters with details are listed in Table 1. The FT analysis of spinel FeCo2O4 structure shows a strong peak around 1.500 Å assigned to the Fe–O1 (2.239 Å) coincides with Td Fe2+ and Fe–O2 (1.963 Å) assigned to Oh Fe3+. The Fe–Co1 and Fe–Fe1 bonds are observed near the 3 Å which reveals the Co2+ cations are substituted by Fe2+ cations in the spinel Co3O4 structure (FeCo2O4). The Fe–Co1 can be resolved to Oh Fe3+ and Td Co2+ sites. According to the reported data, the Fe, Cu, and Ni cations exhibit a tendency to occupy the octahedral sites (Oh) and leave tetrahedral (Td) sites in spinel Co3O4.50 On the other side, the Co2+ ions in the Co3O4 compound are substituted by Zn2+ ions, which exhibit tetrahedral symmetry (Td), leading to the formation of ZnCo2O4.46 Interestingly, the ZnCo2O4 tends to form a spinel structure as the Zn2+ occupy the tetrahedral sites (Td) and leaving the Co3+ at octahedral sites (Oh).51 The crystal ZnCo2O4 has a spinel structure (A2+Co23+O4, where, A is tetrahedral and B is octahedral sites) closely similar to the Co3O4 structure.51 Indeed, the FT shows Co–O1 (1.966 Å) interaction, which is characteristic of Oh Co3+. Also, the Co–Zn1 bond suggested Td Zn2+ and Oh Co3+ in the spinel structure of ZnCo2O4. The Co–O1 and Co–Co1 own octahedral symmetry. This finding shows a good agreement with the previous reports that confirmed the CoO6 structure has octahedral symmetry, whereas the CoO4 exhibits tetrahedral symmetry.52 Furthermore, the results imply that the Zn2+ cations have a tendency to occupy active sites characterized by a low oxidation state, whereas Co3+ cations are left in octahedral locations. It is clear that Td Zn2+ and Oh Co3+ ions were presented in the crystalline structure of the ZnCo2O4 spinel structure.
| Sample | Bond type | N | R (Å) | σ2 (Å2) | So2 | Eo (eV) | Rfactor |
|---|---|---|---|---|---|---|---|
| CuCo2O4 | Co–O1 | 2 | 1.917 ± 0.010 | 0.0023 ± 0.0044 | 1 | 0.530 | 0.1057 |
| Co–O2 | 2 | 2.058 ± 0.091 | 0.0147 ± 0.0423 | 1 | 0.530 | 0.1057 | |
| Co–Co1 | 2 | 3.237 ± 0.150 | 0.0094 ± 0.0057 | 1 | 0.530 | 0.1057 | |
| Co–Cu1 | 4 | 3.462 ± 0.067 | 0.0066 ± 0.0087 | 1 | 0.530 | 0.1057 | |
| NiCo2O4 | Co–O1 | 2 | 1.912 ± 0.017 | 0.0009 ± 0.0020 | 1 | 0.679 | 0.0103 |
| Co–O2 | 4 | 1.940 ± 0.174 | 0.0238 ± 0.0072 | 1 | 0.679 | 0.0103 | |
| Co–Ni1 | 4 | 2.835 ± 0.042 | 0.0037 ± 0.0088 | 1 | 0.679 | 0.0103 | |
| Co–Co1 | 2 | 2.988 ± 0.078 | −0.0015 ± 0.0099 | 1 | 0.679 | 0.0103 | |
| ZnCo2O4 | Co–O1 | 4 | 1.966 ± 0.036 | 0.0045 ± 0.0023 | 1 | 0.250 | 0.0571 |
| Co–O2 | 6 | 3.808 ± 0.166 | 0.0045 ± 0.0268 | 1 | 0.250 | 0.0571 | |
| Co–Zn1 | 4 | 3.197 ± 0.058 | 0.0057 ± 0.0038 | 1 | 0.250 | 0.0571 | |
| Co–Co1 | 6 | 3.439 ± 0.234 | 0.0001 ± 0.0303 | 1 | 0.250 | 0.0571 | |
| FeCo2O4 | Fe–O1 | 2 | 2.239 ± 0.173 | 0.0056 ± 0.027 | 1 | 0.527 | 0.0320 |
| Fe–O2 | 4 | 1.963 ± 0.105 | 0.0045 ± 0.0054 | 1 | 0.527 | 0.0320 | |
| Fe–Co1 | 4 | 3.224 ± 0.252 | 0.0006 ± 0.0123 | 1 | 0.527 | 0.0320 | |
| Fe–Fe1 | 2 | 3.409 ± 0.266 | −0.0015 ± 0.012 | 1 | 0.527 | 0.0320 |
Moreover, the FT first peak at 1.900 Å interatomic distance in spinel CuCo2O4 structure corresponds to the Co–O1 (1.917 Å) of Oh Co3+ and Co–O2 (2.058 Å) of Td Co2+. The results in accordance with the previous reports.51,53 The FT peak at 3.200 Å is correlated to Co–Co1 (3.237 Å) and Co–Cu1 (3.462 Å) bond interactions. It has been reported that the Co–Co interactions can be found in Oh and Td as Co3+–Co3+ (2.85 Å), Co3+–Co2+ (3.346 Å), and Co2+–Co2+(3.495 Å).54 Therefore, the Co–Co1 interactions in spinel CuCo2O4 assigned to Oh Co3+ and Td Co2+. Moreover, the Co–Cu1 bridges the Td Cu2+ and Td Co2+.
The above-reported XANES (Fig. 5b) pre-edge of NiCo2O4 suggested the presence of Td Co2+ symmetry.55 It can be preliminary concluded that, the NiCo2O4 is close to the spinel Co3O4 structure and the oxidation state for both is 8/3 as oxidation state corresponding to the presence of Oh Ni3+.56 The FT analysis of NiCo2O4 reveals a peak at the lowest interatomic distance of 1.94 Å assigned to Co–O1 of Oh (Co3+O6) and Co–O2 of Td (Co2+O4).57 The next FT peak at 2.9 Å corresponds to Co–Co1 (2.988 Å) and 4Co–Ni1 (2.835 Å) interactions. It's evident the Co–Co1 is characteristic of Oh Co3+ and the Co–Ni1 coincides characteristic of Oh Co3+ and the Co–Ni1 coincides with Oh Co3+ and Oh Ni3+. In general, the Co–O interatomic distances at Td or Oh sites are shorter than M–O distances which is correlated with a small atomic radius of Co3+ in comparison with M2+. It is obvious that the Co–O1 and Co–O2 in ZnCo2O4 are longer compared to other spinel structures. It is worth noting that, the interatomic distances of M–Co introduced in Table 1 are observed as Co–Cu > Co–Fe > Zn–Co > Co–Ni.
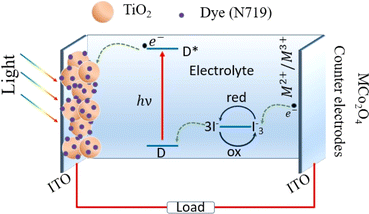
The working mechanism of DSSCs in this study, as illustrated in Fig. 7, involves several key components and processes. The TiO2 nanoparticle-decorated ITO-coated photoanode serves as the light-harvesting layer. When light is absorbed by the sensitizer dye (here N719), it excites electrons from the dye into the conduction band of the TiO2. These photo-generated electrons then move through the TiO2 nanoparticles and are collected at the ITO substrate, flowing into the external circuit to generate electric current. The oxidized dye molecules are regenerated by electrons from the iodide (I−) in the electrolyte, which is subsequently oxidized to tri-iodide (I3−). The tri-iodide ions diffuse towards the spinel cobalt-based metal oxide MCo2O4 thin film counter electrodes, where they are reduced back to iodide ions, completing the circuit. This process is facilitated by the high electrocatalytic activity of the MCo2O4 counter electrodes, which efficiently catalyzes the reduction of tri-iodide. The high surface area and conductivity of the MCo2O4 thin films can play an important role in efficient electron transfer and low charge transfer resistance, contributing to the high performance of the DSSCs. Additionally, the chemical and thermal stability of MCo2O4 provides durability and long-term performance stability, making it an excellent choice for counter electrodes in high-performance DSSCs.
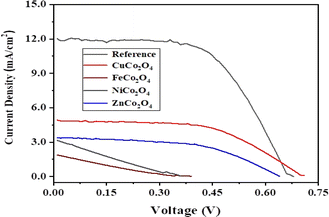
Fig. 8 shows current density–voltage (J–V) plots of the DSSCs fabricated using sensitized MCo2O4 based counter electrodes in comparison with Pt-based one as reference solar cell under illumination of 100 mW cm−2. Table 2 summarizes the obtained results and highlights significant differences among the examined thin films. As presented in Table 2, the DSSC with a counter electrode based on CuCo2O4 nano-leave gives the best photovoltaic response with a Jmax of 4.23 mA cm−2 and a Vmax of 550 mV which results in 1.9% power conversion efficiency. It is also seen that the open circuit voltage (VOC) value of this device (705.8 mV) superior to the one for conventional DSSC with Pt electrode (664.1 mV). The second-best solar cell efficiency is reported as 1.13% for the DSSC obtained using ZnCo2O4 hexagonal-like structures. The VOC value of this device with 642.0 mV is very close to the cell with Pt electrode. The other two solar cells obtained from NiCo2O4 nanosheets and FeCo2O4 diamond-like structures have lower PCE values of 0.27 and 0.15%, respectively. The lower short-circuit current density (JSC) values observed in our study for DSSCs utilizing alternative counter electrodes, such as CuCo2O4, FeCo2O4, NiCo2O4, and ZnCo2O4, compared to conventional Pt-based DSSCs. It suggests potential limitations in transport mechanisms associated with these materials. Factors such as differences in catalytic activity, electronic structure, and surface morphology may contribute to the reduced efficiency of photogenerated charge carriers and subsequent lower JSC values. Similar studies have been performed for various metal oxides including binary metal oxides.58–60 For instance, Kaya et al. hydrothermally sensitized CuCrO2 delofossite oxide nanoparticles to use them as photocathode in the fabrication of high efficient tandem p–n photoelectrochemical cells using CuCrO2 delafossite semiconductors as photocathodes coupling with traditional n-type TiO2 based photoanodes.59 They reported the efficiency of solar cells between 1.67 and 2.33% for various annealing temperatures.
| DSSCs | Jmax mA cm−2 | Vmax mV | Jsc mA cm−2 | Voc mV | FF | PCE % |
|---|---|---|---|---|---|---|
| CuCo2O4 | 4.23 | 550 | 4.80 | 705.8 | 56.1 | 1.90 |
| FeCo2O4 | 0.99 | 150 | 1.96 | 401.8 | 18.8 | 0.15 |
| NiCo2O4 | 1.67 | 160 | 3.25 | 364.9 | 22.6 | 0.27 |
| ZnCo2O4 | 2.83 | 400 | 3.41 | 642.0 | 51.7 | 1.13 |
| Pt | 10.81 | 440 | 12.1 | 664.1 | 59.2 | 4.76 |
Considering the structural properties examined in this study across various spinel metal oxides, the samples consistently demonstrated high crystallinity, primarily in the cubic phase. Notably, the identification of active structural planes in these samples indicates a correlation between electrochemical activity and charge injection with these specific planes. For instance, the (311) plane was prominent in the ZnCo2O4 structure, the (220) plane in CuCo2O4, both (311) and (220) planes in NiCo2O4, and the (220) plane in FeCo2O4. Furthermore, distinctive characteristics and morphologies were observed in the hexagonal structure of ZnCo2O4 and the nanosheet morphology of CuCo2O4, which are thought to enhance the photocurrent and efficiency of DSSCs.
Conclusions
The spinel cobalt-based metal oxides of MCo2O4 (M: Ni, Cu, Fe, or Zn), were synthesized through the hydrothermal growth technique on ITO substrate to use them as counter electrodes in the fabrication of Pt free DSSCs. The oxides displayed a uniform and homogeneous surface morphology. Interestingly, NiCo2O4 exhibits nanosheets, Fe Co2O4 displays diamond shapes, CuCo2O4 shows nanoleaves, and ZnCo2O4 presents a hexagonal shape. These morphologies exhibit unique structural features characterized by a significantly large surface area and the existence of surface defect sites to enhance chemical reactivity. To probe the local structure of the prepared samples, EXAFS data was carried out at Fe and Co K-edge. The absorption Co K-edge was close to the 7721.4 eV value reported in the literature. The Fe Kedge was 7125.53 eV for FeCo2O4. The pre-edge peak in XANES of the prepared samples suggested Td Co2+. The shape of the XANES spectrum reveals the spinel structure of the obtained samples. The FT reveals a Co–O1 coincides with Oh Co3+ and Co–O2 is characteristic for Td Co2+. On the contrary, Fe–O1 reveals Td Fe2+ and Fe–O2 associated with Oh Fe3+. The interatomic distances evaluated from FT analysis of M–Co were observed as Co–Cu > Co–Fe > Zn–Co > Co–Ni. In addition, it is reported that these spinels cobalt-based binary metal oxides, especially CuCo2O4 nanoleaves and ZnCo2O4 hexagonal-like structures are promising candidates for the low cost and high efficient DSSCs with 1.9 and 1.13% power conversion efficiency values, respectively.Data availability
The data that support the findings of this study are available from the corresponding author, Bashar Aljawrneh, upon reasonable request. Researchers interested in accessing the data should contact Bashar Aljawrneh at E-mail: b.aljawarneh@zuj.edu.jo.Author contributions
Abdelelah Alshanableh: conceptualization, investigation, methodology, data curation, writing – original draft, writing – review & editing. Yusuf Selim Ocak: conceptualization, investigation, methodology, data curation, writing – original draft, writing – review & editing. Bashar Aljawrneh: conceptualization, investigation, methodology, data curation, writing – original draft, writing – review & editing. Borhan Aldeen Albiss: supervision, investigation, review & editing, funding acquisition. Khaled Shawakfeh: supervision, investigation, review & editing, funding acquisition. Latif U. Khane: investigation, methodology, data curation, writing – original draft. Messaoud Harfouchee: investigation, methodology, data curation, writing – original draft. Saja Alrousan: investigation, methodology, data curation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work supported by Jordan University of Science and Technology (JUST) under grants No. 135/2023 and 148/2022.References
- M. Gurulakshmi, A. Meenakshamma, K. Susmitha, N. Charanadhar, V. Srikanth, S. N. Babu, Y. V. Subbaiah, K. Venkateswarlu and M. Raghavender, Sol. Energy, 2019, 193, 568–575 CrossRef CAS.
- Y. L. Lee, C. L. Chen, L. W. Chong, C. H. Chen, Y. F. Liu and C. F. Chi, Electrochem. Commun., 2010, 12, 1662–1665 CrossRef CAS.
- G. Boschloo and A. Hagfeldt, Acc. Chem. Res., 2009, 42, 1819–1826 CrossRef CAS PubMed.
- R. Güzel, Y. S. Ocak, Ş. N. Karuk, A. Ersöz and R. Say, J. Power Sources, 2019, 440, 227119 CrossRef.
- R. Güzel, F. Yediyıldız, Y. S. Ocak, F. Yılmaz, A. Ersöz and R. Say, J. Photochem. Photobiol., A, 2020, 401, 112743 CrossRef.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- A. Pérez-Tomás, A. Mingorance, D. Tanenbaum and M. Lira-Cantú, in The Future of Semiconductor Oxides in Next-Generation Solar Cells, Elsevier, 2018, pp. 267–356 Search PubMed.
- A. Kunzmann, M. Stanzel, W. Peukert, R. D. Costa and D. M. Guldi, Adv. Energy Mater., 2016, 6, 1501075 CrossRef.
- J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan, G. Luo, Y. Lin, Y. Xie and Y. Wei, Chem. Soc. Rev., 2017, 46, 5975–6023 RSC.
- S. Rashidi, S. Rashidi, R. K. Heydari, S. Esmaeili, N. Tran, D. Thangi and W. Wei, Prog. Photovolt.: Res. Appl., 2021, 29, 238–261 CrossRef CAS.
- M. Wu, M. Sun, H. Zhou, J. Y. Ma and T. Ma, Adv. Funct. Mater., 2020, 30, 1906451 CrossRef CAS.
- I. H. Inoue, S. Yasuda, H. Akinaga and H. Takagi, Phys. Rev. B, 2008, 77, 035105 CrossRef.
- K. Galatsis, Y. Li, W. Wlodarski, E. Comini, G. Sberveglieri, C. Cantalini, S. Santucci and M. Passacantando, Sens. Actuators, B, 2002, 83, 276–280 CrossRef CAS.
- A. M. A. Ali, N. M. Ahmed, N. A. Kabir, A. M. Al-Diabat, N. A. Algadri, A. Alsadig, O. A. Aldaghri and K. H. Ibnaouf, Materials, 2023, 16, 1868 CrossRef CAS PubMed.
- N. A. Algadri, A. M. AL-Diabat and N. M. Ahmed, Instrum. Sci. Technol., 2023, 51, 144–161 CrossRef CAS.
- H. Tüysüz, Y. J. Hwang, S. B. Khan, A. M. Asiri and P. J. N. R. Yang, J. Nano Res., 2013, 6, 47–54 CrossRef.
- M. Risch, F. Ringleb, M. Kohlhoff, P. Bogdanoff, P. Chernev, I. Zaharieva and H. Dau, J. Energy Environ. Sci., 2015, 8, 661–674 RSC.
- W. Liu, J. Han, I. Yamada and S. Yagi, J. Catal., 2021, 394, 50–57 CrossRef CAS.
- M. Harada, F. Kotegawa and M. Kuwa, J. ACS Appl. Energy Mater., 2022, 5, 278–294 CrossRef CAS.
- W. Deeloed, T. Priamushko, J. Čížek, S. Suramitr and F. Kleitz, J. ACS Appl. Mater. Interfaces, 2022, 14, 23307–23321 CrossRef CAS PubMed.
- Z. Zhang, J. Li, J. Qian, Z. Li, L. Jia, D. Gao and D. Xue, J. Small, 2022, 18, 2104248 CrossRef CAS PubMed.
- L. An, Y. Hu, J. Li, J. Zhu, M. Sun, B. Huang, P. Xi and C. H. Yan, J. Adv. Mater., 2022, 34, 2202874 CrossRef CAS PubMed.
- S. Lu, Y. Wang, F. Li, G. Yang, H. Yang, X. Zhang and Y. Liu, J. Phys. Chem. C, 2017, 121, 12524–12530 CrossRef CAS.
- J. Sharma, P. Srivastava, G. Singh, M. S. Akhtar and S. Ameen, Mater. Sci. Eng., B, 2015, 193, 181–188 CrossRef CAS.
- C. C. Mercado, A. Zakutayev, K. Zhu, C. J. Flynn, J. F. Cahoon and A. J. Nozik, J. Phys. Chem. C, 2014, 118, 25340–25349 CrossRef CAS.
- F. Yang, X. Tian, Y. Gu, K. Zhang and L. Liu, RSC Adv., 2019, 9, 24880–24887 RSC.
- L. U. Khan, Z. U. Khan and M. A. Umar, The Nucleus, 2023, 60, 78–85 CAS.
- L. Zhang, Q. Tang, X. Chen, B. Fan, K. Xiao, S. Zhang, W. Deng and A. Hu, J. Alloys Compd., 2017, 722, 387–393 CrossRef CAS.
- L. Xie, Y. Liu, H. Bai, C. Li, B. Mao, L. Sun and W. Shi, J. Colloid Interface Sci., 2018, 531, 64–73 CrossRef CAS PubMed.
- N. R. Chodankar, D. P. Dubal, Y. Kwon and D.-H. Kim, NPG Asia Mater., 2017, 9, 419 CrossRef.
- X. Zhang, F. Yang, H. Chen, K. Wang, J. Chen, Y. Wang and S. Song, Small, 2020, 16, 2004188 CrossRef CAS PubMed.
- G. Gao, H. B. Wu and X. W. Lou, Adv. Energy Mater., 2014, 4, 1400422 CrossRef.
- D. P. Dubal and R. Holze, RSC Adv., 2012, 2, 12096–12100 RSC.
- L. Hu, L. Wu, M. Liao, X. Hu and X. Fang, Adv. Funct. Mater., 2012, 22, 998–1004 CrossRef CAS.
- B. Palanivel, M. S. Hossain, R. R. Macadangdang Jr., C. Ayappan, V. Krishnan, R. Marnadu, T. Kalaivani, F. A. Alharthi and G. Sreedevi, ACS Omega, 2021, 6, 34563–34571 CrossRef CAS PubMed.
- H. Guo, J. Chen, W. Weng, Q. Wang and S. Li, Chem. Eng. J., 2014, 239, 192–199 CrossRef CAS.
- A. N. Naveen and S. Selladurai, Phys. B, 2015, 457, 251–262 CrossRef CAS.
- A. J. C. Mary and A. C. Bose, Appl. Surf. Sci., 2017, 425, 201–211 CrossRef CAS.
- V. Crocella, F. Cavani, G. Cerrato, S. Cocchi, M. Comito, G. Magnacca and C. Morterra, J. Phys. Chem. C, 2012, 116, 14998–15009 CrossRef CAS.
- S. Verma, H. M. Joshi, T. Jagadale, A. Chawla, R. Chandra and S. Ogale, J. Phys. Chem. C, 2008, 112, 15106–15112 CrossRef CAS.
- O. C. Pore, A. V. Fulari, C. D. Chavare, D. S. Sawant, S. S. Patil, R. V. Shejwal, V. J. Fularimm and G. H. Lohar, Chem. Phys. Lett., 2023, 824, 140551 CrossRef CAS.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- M. Risch, K. Klingan, F. Ringleb, P. Chernev, I. Zaharieva, A. Fischer and H. Dau, J. ChemSusChem, 2012, 5, 542–549 CrossRef CAS PubMed.
- G. P. Huffman, N. Shah, J. Zhao, F. E. Huggins, T. E. Hoost, S. Halvorsen and J. G. Goodwin, J. Catal., 1995, 151, 17–25 CrossRef CAS.
- X. Wang, Y. Liu, T. Zhang, Y. Luo, Z. Lan, K. Zhang, J. Zuo, L. Jiang and R. J. A. C. Wang, J. ACS Catal., 2017, 7, 1626–1636 CrossRef CAS.
- S. Zhang, L. Zhang, H. Li, J. Li, Z. Jiang, W. Chu, Y. Huang, J. Wang and Z. Wu, J. Synchrotron Radiat., 2010, 17, 600–605 CrossRef CAS PubMed.
- Y. Wang, W.-B. Chen, F.-Y. Liu, D.-W. Yang, Y. Tian, C.-G. Ma, M. Dramićanin and M. G. Brik, J. Results Phys., 2019, 13, 102180 CrossRef.
- Q. Zhang, W. Tian, R. Nepal, A. Huq, S. Nagler, J. DiTusa and R. Jin, J. Chem. Mater., 2023, 35, 2330–2341 CrossRef CAS PubMed.
- L. U. Khan, Z. U. Khan, L. Blois, L. Tabassam, H. F. Brito and S. J. Figueroa, J. Inorg. Chem., 2023, 62, 2738–2750 CrossRef CAS PubMed.
- H. Y. Wang, S. F. Hung, Y. Y. Hsu, L. Zhang, J. Miao, T. S. Chan, Q. Xiong and B. Liu, J. Phys. Chem. Lett., 2016, 7, 4847–4853 CrossRef CAS PubMed.
- Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier and H. Dai, J. Am. Chem. Soc., 2012, 134, 3517–3523 CrossRef CAS PubMed.
- Y. Lou, J. Ma, X. Cao, L. Wang, Q. Dai, Z. Zhao, Y. Cai, W. Zhan, Y. Guo and P. J. A. C. Hu, J. ACS Catal., 2014, 4, 4143–4152 CrossRef CAS.
- A. Y. Khodakov, J. Lynch, D. Bazin, B. Rebours, N. Zanier, B. Moisson and P. Chaumette, J. Catal., 1997, 168, 16–25 CrossRef CAS.
- J. Wang, P. A. Chernavskii, A. Y. Khodakov and Y. Wang, J. Catal., 2012, 286, 51–61 CrossRef CAS.
- O. Knop, K. I. Reid and Y. N. Sutarno, J. Can. J. Chem., 1968, 46, 3463–3476 CrossRef CAS.
- A. V. Chadwick, S. L. Savin, S. Fiddy, R. Alcantara, D. Fernández Lisbona, P. Lavela, G. F. Ortiz and J. L. Tirado, J. Phys. Chem. C, 2007, 111, 4636–4642 CrossRef CAS.
- J. Zheng, X. Peng, Z. Xu, J. Gong and Z. Wang, J. ACS Catal., 2022, 12, 10245–10254 CrossRef CAS.
- A. A. Qureshi, S. Javed, H. M. A. Javed, A. Akram, M. Jamshaid and A. Shaheen, Opt. Mater., 2020, 109, 110267 CrossRef CAS.
- I. C. Kaya, S. Akin, H. Akyildiz and S. Sonmezoglu, Sol. Energy, 2018, 169, 196–205 CrossRef CAS.
- A. Agrawal, S. A. Siddiqui, A. Soni and G. D. Sharma, Sol. Energy, 2022, 233, 378–407 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03462g |
| This journal is © The Royal Society of Chemistry 2024 |