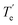Open Access Article
Open Access ArticleResearch on TNTON/DNTF eutectic characteristics: an exploration of low eutectic mixture based melt-cast explosive carriers†
Xinyu Feng a,
Qi Xuea,
Junlin Zhangab,
Kaidi Yanga,
Kunkai Wanga,
Bozhou Wang
a,
Qi Xuea,
Junlin Zhangab,
Kaidi Yanga,
Kunkai Wanga,
Bozhou Wang *ab and
Fuqiang Bi*ab
*ab and
Fuqiang Bi*ab
aXi'an Modern Chemistry Research Institute, Xi'an, 710065, China. E-mail: bifuqiang@msn.com; wbz600@163.com
bState Key Laboratory of Fluorine & Nitrogen Chemicals, Xi'an, 710065, China
First published on 20th November 2024
Abstract
To modify the sensitivity and melting point of the casting of DNTF, a eutectic system of insensitive explosive 3,5,5-trinitro-1,3-oxazinane (TNTON) and DNTF was prepared through a new method. The melting and liquefaction processes of TNTON/DNTF at different ratios were investigated, and a T–x phase diagram was established. The melting and decomposition processes of TNTON, DNTF, and TNTON/DNTF eutectic at different heating rates were compared, while the sensitivity tests were conducted to study the desensitizing effect of TNTON on DNTF. Using EXPLO-5 software, the detonation performance of the TNTON/DNTF eutectic was calculated. The experimental results show that the stoichiometric composition of the TNTON/DNTF eutectic is 58.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.74, with an average melting point of 69 °C. With the increase of heating rate, both the melting and decomposition reactions are delayed. According to the activation energy (Ea) curve, the thermal decomposition is through an autocatalytic process. The impact and friction sensitivity of the TNTON/DNTF eutectic are 38 J and 252 N, respectively. Theoretical density of the TNTON/DNTF eutectic is 1.906 g cm−3, and the calculated detonation velocity is 8921 m s−1. The TNTON/DNTF eutectic exhibits good thermal stability and can significantly reduce the sensitivity of DNTF while maintaining its high energy level. The detonation performance of TNTON/DNTF low eutectic cast explosive is better than that of TNT based cast explosive.
41.74, with an average melting point of 69 °C. With the increase of heating rate, both the melting and decomposition reactions are delayed. According to the activation energy (Ea) curve, the thermal decomposition is through an autocatalytic process. The impact and friction sensitivity of the TNTON/DNTF eutectic are 38 J and 252 N, respectively. Theoretical density of the TNTON/DNTF eutectic is 1.906 g cm−3, and the calculated detonation velocity is 8921 m s−1. The TNTON/DNTF eutectic exhibits good thermal stability and can significantly reduce the sensitivity of DNTF while maintaining its high energy level. The detonation performance of TNTON/DNTF low eutectic cast explosive is better than that of TNT based cast explosive.
1 Introduction
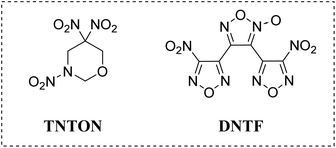
Melt-cast explosives are currently one of the main charging methods for warheads,1–3 but the existing formula of melt-cast explosives based on a single explosive molecule (such as TNT4) has obvious shortcomings in terms of energy, safety, charging quality, and mechanical properties5–7 as a carrier. The melt-cast process normally includes the addition of solid high-energy components into liquid molten carrier explosives and could effectively improve the final detonation and molding performances. Despite the low energy, poor mechanical properties, poor safety, and high toxicity, TNT remains the most important and widely used carrier for molten explosives to date. During the last few decades, continuous efforts in the exploration of new casting carriers to replace TNT were made,8 among which, DNTF9 has attracted widespread attention worldwide.Energetic materials based on tandem furazan–furoxan framework often exhibit superior energetic performance due to their high enthalpy of formation,10,11 and DNTF is the most representative and efficient material with the advantages of prominent energetic properties (density of 1.937 g cm−3, detonation velocity of 9250 m s−1, detonation pressure of 41.1 GPa, heat decomposition temperature of 253.6 °C under atmospheric pressure, and 5 s explosion point of 308 °C), outstanding enthalpy of formation, as well as high nitrogen content.9 With a melting point of 110 °C, DNTF has been regarded as one of the most important casting carriers for future melt-cast explosives. However, in current stage, the high sensitivity characteristics of DNTF still seriously hinder its large-scale application in casting explosives and propellants. Therefore, some research groups, including us, have proposed that the exploration of low eutectic mixture based on DNTF and other energetic components could be a promising solution to this problem.12
Energetic eutectics refer to energetic mixtures formed by mixing two or more energetic compounds, where one or more components are in a molten state, and other components dissolve in it, the energetic eutectics formed has a melting point lower than each of its single component.13 Energetic eutectics formed through the addition of a second component will compensate for the shortcomings of single-component explosives in terms of melting point and sensitivity, which has become a hot topic of research in recent years.14–16 In 2020 and 2022, we reported the preparation of two eutectic systems by mixing BOM3 and (3,5-dinitro-1,3-oxazinan-5-yl) methyl nitrate (TNOP)17 with DNTF and achieved a eutectic temperature of 75.5 °C and 95.4 °C respectively. But the sensitivities and energetic performances of the eutectic mixtures have not yet met our expectations.
TNTON18 is an excellent nitrogen-containing cyclic energetic compound with a density of 1.78 g cm−3, a melting point of 89 °C, and a heat decomposition temperature of 231 °C, exhibiting good stability19 and low sensitivity (38 J, 360 N). Compared to most widely applied explosives as casting carriers, TNTON demonstrates a better overall performance, which ensures a more ideal energy level for the TNTON/DNTF eutectic. This article utilizes the high melting point and low sensitivity characteristics of TNTON to prepare a binary mixture system with DNTF, trying to reduce the final casting process temperature and sensitivity, meanwhile maintain a high energetic level to meet the requirements for advanced warheads and propellants. It is noteworthy that a new way to prepare the binary mixture has been reported in this article, which is different from the traditional strategies. By avoiding heating/melting operations and abandoning the direct grinding of solid powders, this new method significantly improved the safeties. This research aims to establish the T–x phase diagram of the TNTON/DNTF binary system, determine the composition and melting temperature of the eutectic, and analyze the influence of different heating rates on the melting and decomposition processes of the eutectic through DSC,20 as well as carry out the sensitivity tests and detonation performance calculations of the eutectic, in order to achieve the purpose of reducing the sensitivity of DNTF, adjusting the melting point of DNTF, and maintaining its high energy level (Fig. 1).
2 Experimental section
2.1 Reagents and instruments
2.2 Compatibility test of TNTON/DNTF and preparation of eutectic
Eleven groups of mixtures of TNTON and DNTF with mass ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 5
6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7
4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 8
3, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 9
2, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
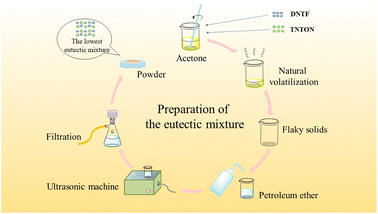
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 were weighed in small beakers, with a total mass of 1 g for each group, named from 1# to 11#. As shown in Fig. 2, each group of mixtures was completely dissolved in acetone to form a saturated solution, stirred for 5 min, and then allowed to slowly solidify at room temperature as the solvent evaporated. After complete solidification, petroleum ether was added to thoroughly wet the solid, followed by grinding the solid into a powder form dispersed in petroleum ether. After grinding, the mixture was filtered, air-dried at room temperature, and the resulting powders were collected for related tests. Based on the T–x phase diagram and other analysis results, the composition of the lowest eutectic was determined, and TNTON/DNTF eutectic was prepared using the same method.
0 were weighed in small beakers, with a total mass of 1 g for each group, named from 1# to 11#. As shown in Fig. 2, each group of mixtures was completely dissolved in acetone to form a saturated solution, stirred for 5 min, and then allowed to slowly solidify at room temperature as the solvent evaporated. After complete solidification, petroleum ether was added to thoroughly wet the solid, followed by grinding the solid into a powder form dispersed in petroleum ether. After grinding, the mixture was filtered, air-dried at room temperature, and the resulting powders were collected for related tests. Based on the T–x phase diagram and other analysis results, the composition of the lowest eutectic was determined, and TNTON/DNTF eutectic was prepared using the same method.
A sample with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of TNTON and DNTF was subjected to DSC testing. The compatibility was evaluated according to the thermal analysis evaluation compatibility standards proposed by Honeywell Company in the United States,21,22 using the maximum exothermic peak temperature change Δtp for evaluation.
1 of TNTON and DNTF was subjected to DSC testing. The compatibility was evaluated according to the thermal analysis evaluation compatibility standards proposed by Honeywell Company in the United States,21,22 using the maximum exothermic peak temperature change Δtp for evaluation.
| Δtp = tp1 − tp2 | (1) |
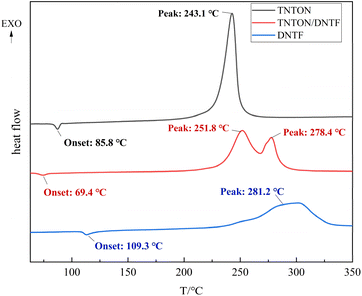
The compatibility evaluation standard is shown in Table 1,23,24 and the DSC curves of TNTON and DNTF and their mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture are shown in Fig. 3. As shown in Fig. 3, the thermal decomposition peak of TNTON/DNTF mixed system is 2.8 °C earlier than that of DNTF. According to the compatibility evaluation criteria in Table 1, TNTON and DNTF are compatible.
1 mixture are shown in Fig. 3. As shown in Fig. 3, the thermal decomposition peak of TNTON/DNTF mixed system is 2.8 °C earlier than that of DNTF. According to the compatibility evaluation criteria in Table 1, TNTON and DNTF are compatible.
| Δtp/°C | Rating | Note |
|---|---|---|
| 0–3 | A | Mixed system compatibility |
| 3–5 | B | The hybrid system is slightly sensitive and can be used for a short time |
| 6–15 | C | The hybrid system is sensitive. It's best not to use it |
| >15 | D | Mixed system dangerous, strictly prohibited use |
2.3 Performance testing
3 Results and discussion
3.1 Binary phase diagram of TNTON/DNTF system
DSC tests were conducted on TNTON/DNTF mixtures with mass ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 5
6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7
4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 8
3, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 9
2, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, represented by 1#, 2#, 3#, 4#, 5#, 6#, 7#, 8#, 9#, 10#, and 11# (Fig. 4).
0, represented by 1#, 2#, 3#, 4#, 5#, 6#, 7#, 8#, 9#, 10#, and 11# (Fig. 4).
Pure TNTON and DNTF each produced a single endothermic peak, indicating that the melting points of TNTON and DNTF are 89 °C and 110 °C, respectively. The DSC curves of the TNTON/DNTF mixtures showed two endothermic peaks, the first of which is the melting peak of the eutectic, and the second is the liquefaction peak of the remaining components. As the content of TNTON increased, the liquefaction temperature of the binary system first decreased and then increased. As shown in Fig. 4, the low eutectic temperature of different systems remained approximately constant, ranging from 67.9 to 70.6 °C, with an average low eutectic temperature of 69.0 °C. This is due to the lowering of the freezing point phenomenon caused by the chemical potential of any component in the mixed solution system being lower than that of the pure substance under the same temperature and pressure conditions, according to the colligative properties of dilute solutions.24 The absorption peaks gradually merged into a single absorption peak at a component mass ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, indicating that this mixture system is very close to the composition of the TNTON/DNTF eutectic.
5, indicating that this mixture system is very close to the composition of the TNTON/DNTF eutectic.
According to the definition of DSC determination of melting point, the phase change temperature (melting point) of pure substance (or low eutectic) melting should be the initial temperature t0 of the tangent extrapolation of the front edge of the melting heat absorption peak, and the temperature difference between the temperature of complete melting (or liquefaction) Te and T0 is the so-called “melting range”, the melting range on the DSC curve is related to the rate of heating. To obtain the accurate liquefaction temperature of the mixed system, it is necessary to correct the obtained DSC temperatures (Fig. 5).
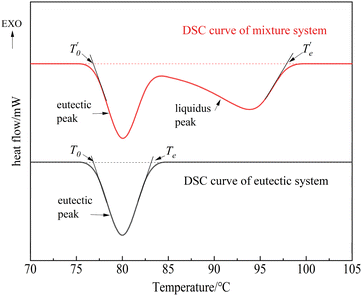 | ||
| Fig. 5 Schematic diagram of liquefaction temperature obtained from DSC curves of the binary system.16,24,25 | ||
The temperature gap between the end temperature Te and the starting temperature T0 of the melting peak of the eutectic mixture was taken as the correction amount to determine the complete liquefaction temperature TL of the mixture in Fig. 5. The correction formula for the liquefaction temperature is shown in eqn (2).
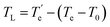 | (2) |
 is the end temperature of the liquefaction peak of the mixed system DSC curve; T0 is the starting temperature of the eutectic peak; Te is the end temperature of the eutectic peak.
is the end temperature of the liquefaction peak of the mixed system DSC curve; T0 is the starting temperature of the eutectic peak; Te is the end temperature of the eutectic peak.
The liquefaction temperature and component content of the binary system have the following relationship:24
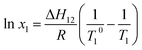 | (3) |
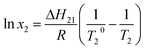 | (4) |
According to eqn (2), the liquefaction temperature of the mixed components is obtained, and combined with equation eqn (3) or eqn (4), a plot of the liquefaction temperature TL versus component content xi (the T–x phase diagram of the binary eutectic)is made.26
The DSC curve characteristic data of different molar ratio TNTON/DNTF mixtures are shown in Table 2. Teu is the starting temperature of the low eutectic peak, that is, the low eutectic temperature; 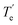 is the end temperature of the liquefaction peak on the DSC curve of the mixed system; TL is the corrected temperature of the mixture after complete liquefaction. According to Fig. 4, the 6# group with a molar ratio of nTNTON
is the end temperature of the liquefaction peak on the DSC curve of the mixed system; TL is the corrected temperature of the mixture after complete liquefaction. According to Fig. 4, the 6# group with a molar ratio of nTNTON![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nDNTF = 58.43
nDNTF = 58.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.57 has only one melting peak in its DSC curve, indicating that the mixture system forms a complete eutectic at this ratio. Therefore, when calculating the liquefaction temperature of the mixed system, the melting range of 14 °C of this mixture system peak is used for calculation.
41.57 has only one melting peak in its DSC curve, indicating that the mixture system forms a complete eutectic at this ratio. Therefore, when calculating the liquefaction temperature of the mixed system, the melting range of 14 °C of this mixture system peak is used for calculation.
| Samples | TNTON/DNTF | Teu/°C | TL/°C | /°C | |
|---|---|---|---|---|---|
| Mass ratio | Molar ratio | ||||
| 1 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.00 100.00 |
109.3 | 109.3 | — |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
13.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86.49 86.49 |
68.4 | 104.3 | 118.3 |
| 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
26.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74.00 74.00 |
68.8 | 97.3 | 111.3 |
| 4 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
37.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62.41 62.41 |
68.4 | 86.6 | 100.6 |
| 5 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
48.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.63 51.63 |
70.6 | 77.8 | 91.8 |
| 6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
58.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.57 41.57 |
69.4 | 69.4 | 83.4 |
| 7 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
67.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.17 32.17 |
70.1 | 73.6 | 87.6 |
| 8 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
76.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.37 23.37 |
68.7 | 77.5 | 91.5 |
| 9 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
84.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.10 15.10 |
67.9 | 80.9 | 94.9 |
| 10 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.33 7.33 |
68.4 | 84 | 98 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
85.8 | 85.8 | — |
According to the data in Table 2, a plot of the liquification temperature tL versus component content x gives the T–x phase diagram of the binary eutectic. Fig. 6 shows the phase diagram of TNTON content versus temperature for the TNTON/DNTF binary mixture. The points in the diagram are the measured values, and the solid line is the fitted curve. It can be observed that the two fitted liquification temperature curves intersect with the fitted melting temperature curve at one point, with the coordinates x(TNTON) = 0.584, indicating that the composition of the TNTON/DNTF eutectic is xTNTON![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xDNTF = 58.4
xDNTF = 58.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.6.
41.6.
By performing linear regression of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x, 1/T where x is the molar fraction of TNTON or DNTF in the mixed system, and T is the liquification temperature of TNTON or DNTF in the mixed system, the relationships for DNTF and TNTON regression are obtained as shown in eqn (5) and (6):
x, 1/T where x is the molar fraction of TNTON or DNTF in the mixed system, and T is the liquification temperature of TNTON or DNTF in the mixed system, the relationships for DNTF and TNTON regression are obtained as shown in eqn (5) and (6):
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xDNTF = 7.1586 − 2750/T, R = 0.997 xDNTF = 7.1586 − 2750/T, R = 0.997
| (5) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xTNTON = 10.953 − 3934.9/T, R = 0.998 xTNTON = 10.953 − 3934.9/T, R = 0.998
| (6) |
The low eutectic point of TNTON/DNTF is the intersection point of eqn (5) and (6). Therefore, the molar ratio of TNTON to DNTF in the low eutectic of TNTON/DNTF system is 58.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.74, and the low eutectic temperature is 342.37 K (69.2 °C).The average Teu of the mixed system in Table 2 is 69 °C (342.15 K) as the low eutectic temperature of the TNTON/DNTF system, and the component content of DNTF or TNTON in the low eutectic can be calculated by taking the T of eqn (5) or (6) as 342.15 K. The content of TNTON in the low eutectic could be obtained by 1 − xDNTF while the content of DNTF in eutectic be determined by 1 − xTNTON.The molar ratio of TNTON to DNTF in the eutectic of TNTON/DNTF system is calculated to be 58.5
41.74, and the low eutectic temperature is 342.37 K (69.2 °C).The average Teu of the mixed system in Table 2 is 69 °C (342.15 K) as the low eutectic temperature of the TNTON/DNTF system, and the component content of DNTF or TNTON in the low eutectic can be calculated by taking the T of eqn (5) or (6) as 342.15 K. The content of TNTON in the low eutectic could be obtained by 1 − xDNTF while the content of DNTF in eutectic be determined by 1 − xTNTON.The molar ratio of TNTON to DNTF in the eutectic of TNTON/DNTF system is calculated to be 58.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.5 and 57.8
41.5 and 57.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.2 according to these two methods respectively. It can be seen from the above results that the composition and temperature of the low eutectic calculated by the three methods are very close, which indicates that the regression calculation method is reliable to calculate the composition and temperature of the low eutectic.
42.2 according to these two methods respectively. It can be seen from the above results that the composition and temperature of the low eutectic calculated by the three methods are very close, which indicates that the regression calculation method is reliable to calculate the composition and temperature of the low eutectic.
The eutectic composition calculated by the intersection of eqn (5) and (6) is close to the average of the individual component ratios calculated by eqn (5) or (6) alone. Therefore, the molar ratio of TNTON to DNTF in the TNTON/DNTF eutectic is determined to be 58.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.74, the mass ratio is 49.8
41.74, the mass ratio is 49.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.2, and the eutectic temperature is 69 °C.
50.2, and the eutectic temperature is 69 °C.
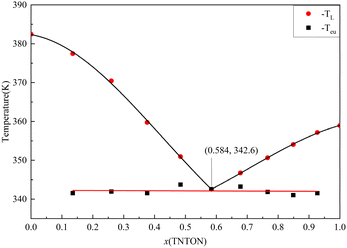
Infrared (IR) spectral analysis of the TNTON/DNTF eutectic, as shown in Fig. 7 and Table 3, reveals all major vibrational bands of pure TNTON and DNTF in the eutectic. The peaks of 2968 cm−1, 3020 cm−1 and 3079 cm−1 are the stretching vibrations of C–H bonds in TNTON, in which the C–O–C fragment in the TNTON ring skeleton has a stretching vibration peak at 1090 cm−1. The peak of vibration at 1000 cm−1 corresponds to the stretching vibrations of O–N in the furoxan ring. The bands 1639 cm−1, 1515 cm−1 and 1447 cm−1 are the skeleton characteristic peaks of furaxan ring, and 1585 cm−1, 1563 cm−1, 1447 cm−1, 1409 cm−1 are the skeleton characteristic peaks of furaxan ring. Similarly, NO2 symmetric and asymmetric stretching vibrations were observed at 1320 ± 40 cm−1 and 1550 ± 50 cm−1. According to these results, the functional groups in the eutectic complex were determined to be the same as those in the raw materials, indicating that during the preparation of the TNTON/DNTF mixture, no new chemical bond was formed between the original TNTON and the original DNTF, indicating good material compatibility. In addition, the peak 1308 cm−1 is related to TNTON's C–NO2 symmetric stretching vibration, and this peak moves to 1318 cm−1 in the eutectic, which is likely due to the interaction between the two molecules.
| TOTON | DNTF | TNTON/DNTF | |
|---|---|---|---|
| C–H stretching | 3079, 3020, 2968 | 3079, 3020, 2968 | |
| NO2(N–NO2) asymmetric stretching vibration | 1581 | 1585 | |
| NO2(C–NO2) asymmetric stretching vibration | 1560 | 1515 | 1563, 1516 |
| NO2(C–NO2) symmetric stretching vibration | 1308 | 1355 | 1318, 1354 |
| NO2(N–NO2) symmetric stretching vibration | 1288 | 1287 | |
| C–O–C asymmetric stretching vibration | 1090 | 1090 | |
| C–NO2 structural bond C–N stretching | 890 | 890 | |
| N–O stretching | 1000 | 1000 | |
| Skeletal stretching (furoxan ring) | 1639, 1515, 1447 | 1639, 1516, 1447 | |
| Skeletal stretching (furaxan ring) | 1585, 1563, 1447, 1409 | 1585, 1563, 1447, 1409 |
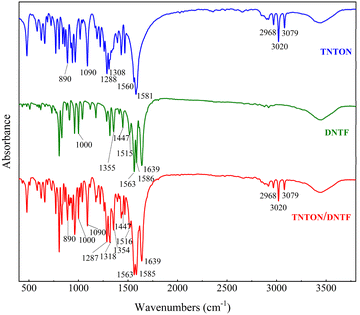
Based on Fig. 8, it can be observed that the melting point of TNTON and DNTF is significantly reduced after forming the eutectic mixture, and the onset temperature of thermal decomposition is delayed by nearly 10 °C compared to pure TNTON. This may be due to the intermolecular forces between the two molecules, which make the overall structure more stable, leading to an increased decomposition temperature. Moreover, the decomposition temperature of the DNTF component within the eutectic mixture occurs earlier than that of pure DNTF, which might be due to some decomposition products of TNTON exerting a catalytic effect on the thermal decomposition process of DNTF.
3.2 The effect of heating rate on the melting and decomposition process of TNTON/DNTF eutectic
In order to study the effect of heating rate on the melting of low eutectic, different heating rates of 5, 10, 15, 20 K °C min−1 were used to carry out experiments, and the composition ratio of low eutectic was prepared according to the calculation results of T–x phase diagram (nTNTON![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nDNTF = 58.26
nDNTF = 58.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
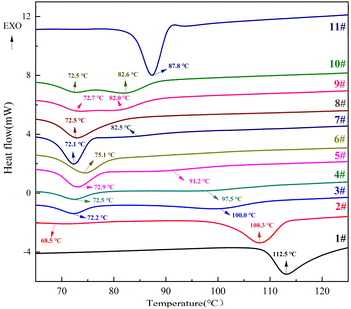
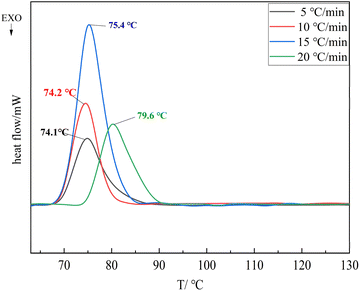
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.74). Fig. 9 shows the melting change curve of TNTON/DNTF eutectic at different heating rates, and Fig. 10 shows the DSC curve of the decomposition process. As can be seen from Fig. 9 and 10, with the increase of heating rate, both the start time and peak value of melting reaction and decomposition reaction are delayed. The peak melting temperature of low eutectic is delayed from 74.1 °C at 5 °C min−1 to 79.6 °C at 20 °C min−1, and the average peak melting temperature is 75.8 °C. The delay in peak temperature of low eutectic point is mainly caused by the overheating phenomenon of low eutectic melting caused by high heating rate. Under constant pressure conditions, the temperature of solid–liquid equilibrium is called the equilibrium melting point, and when the solid phase reaches the equilibrium melting point, the phenomenon of non-melting is called overheating. When the melting process of the eutectic is studied by DSC, the heating furnace and the sample are close to the heat equilibrium state at the low heating rate, but the increase of the heating rate will cause the internal temperature release of the sample to be uneven and overheating. The faster the heating rate, the more obvious the overheating phenomenon.
41.74). Fig. 9 shows the melting change curve of TNTON/DNTF eutectic at different heating rates, and Fig. 10 shows the DSC curve of the decomposition process. As can be seen from Fig. 9 and 10, with the increase of heating rate, both the start time and peak value of melting reaction and decomposition reaction are delayed. The peak melting temperature of low eutectic is delayed from 74.1 °C at 5 °C min−1 to 79.6 °C at 20 °C min−1, and the average peak melting temperature is 75.8 °C. The delay in peak temperature of low eutectic point is mainly caused by the overheating phenomenon of low eutectic melting caused by high heating rate. Under constant pressure conditions, the temperature of solid–liquid equilibrium is called the equilibrium melting point, and when the solid phase reaches the equilibrium melting point, the phenomenon of non-melting is called overheating. When the melting process of the eutectic is studied by DSC, the heating furnace and the sample are close to the heat equilibrium state at the low heating rate, but the increase of the heating rate will cause the internal temperature release of the sample to be uneven and overheating. The faster the heating rate, the more obvious the overheating phenomenon.
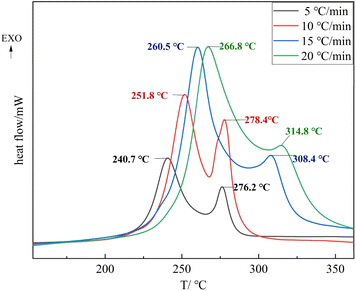
From Fig. 9, it can be seen that the two stages of the decomposition process of TNTON/DNTF eutectic are delayed at different heating rates. The first stage of the decomposition peak temperature ranges from 240.7 to 266.8 °C, with an average decomposition peak temperature of 255.0 °C, indicating good thermal stability. The difference between the thermal decomposition peak temperature and the average melting peak temperature is 179.2 °C, which is significant, indicating that TNTON/DNTF eutectic has good process applicability as a casting explosive carrier.8
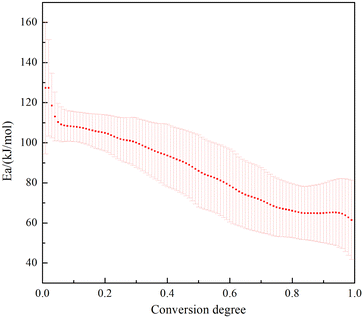
Resulting DSC curves were applicated to calculate the kinetic parameters such as apparent Ea. The software NETZSCH Kinetics NEO was used to explore the optimum simulation method, which obtains many kinds and different methods has a huge impact on the parameter results. DSC curves at different heating rates were employed at four different heating rates of 5, 10, 15 and 20 K min−1. The decomposition temperatures increased with the increase of the heating rates. We tried several thermal decomposition equations such as Kissinger, Ozawa and Friedman. The last method is most suitable for the molecular decomposition model and the correlation factor is R2 = 0.984. As can be seen from the Fig. 11, with the progress of thermal decomposition reaction, the Ea has a trend of decreasing, indicating that the thermal decomposition product catalyzes its further decomposition, which is an “autocatalytic decomposition process”. The above-mentioned values have been showed in ESI Table S1† for respectively.
3.3 Performance of TNTON/DNTF eutectic
The impact sensitivity and friction sensitivity test results of TNTON, DNTF, and TNTON/DNTF eutectic are shown in Table 4. In addition, the detonation performance of TNTON/DNTF eutectic was calculated using EXPLO-5 software.34 The calculation results were compared with the detonation performance parameters of common casting explosive carriers. As can be seen from Table 4, the friction sensitivity of TNTON/DNTF eutectic is 252 N, and the impact sensitivity is 38 J, showing a significant improvement in safety performance compared to DNTF. The density of TNTON/DNTF eutectic is 1.906 g cm−3, higher than most of single-component casting explosives; the thermal decomposition starting temperature is 234.6 °C, close to TNT; the detonation velocity is 8921 m s−1, and the detonation pressure is 36.6 GPa. The energy level is higher than that of current common explosive carriers such as DNAN, DNP, 5,5′-ethanitrat-3,3′-bi-1,2,4-oxadiazole (BOM), and the detonation velocity is comparable to that of 1-methyl-2,4,5-trinitroimidazole (MTNI), indicating that compared with single-component DNTF, the density and detonation performance of TNTON/DNTF eutectic have not significantly decreased, in the meantime, the presence of TNTON greatly reduces the sensitivity of DNTF, indicating its broad application prospects.| Substrate | ρ/(g cm−3) | ΔHf0/(kJ mol−1) | Tm/°C | Tdec/°C | Vdet/(m s−1) | Pcj/GPa | FS/N | IS/J |
|---|---|---|---|---|---|---|---|---|
| TNTON18 | 1.78 | −249 | 89 | 231 | 8322 | 31.0 | 360 | 38 |
| DNTF9 | 1.937 | 570.0 | 110 | 253.6 | 9250 | 41.1 | 82 | 5 |
MTNTON![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MDNTF MDNTF |
1.906 | 84.7 | 69 | 234.6 | 8921 | 36.6 | 252 | 38 |
49.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.2 50.2 |
||||||||
| TNT27,28 | 1.64 | −59.4 | 80.8 | 266.5 | 6940 | 19.0 | 353 | 15 |
| RDX28 | 1.86 | 92.6 | 204 | 235 | 9014 | 33.91 | 120 | 7.4 |
| DNAN29–31 | 1.54 | −184.0 | 94.6 | 351.7 | 5974 | 9.5 | 179 | — |
| DNP15 | 1.87 | 115.2 | 85 | 347 | 8100 | 29.4 | — | — |
| MTNI32 | 1.8 | 64.7 | 94.7 | 314.9 | 8800 | 35.6 | — | — |
| BOM33 | 1.832 | −140.3 | 84.5 | 183.4 | 8180 | 29.4 | 282 | 8.6 |
3.4 Theoretical performance of melt cast explosives based on TNTON/DNTF eutectic
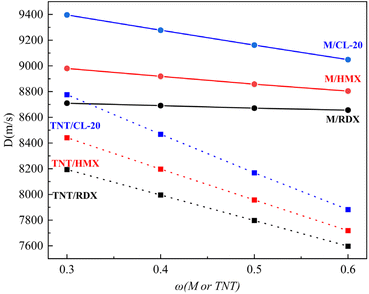
Melt cast explosives have a simple filling process, are suitable for filling irregularly shaped warheads, can adjust various properties through formulation, have strong batch production capabilities, and high automation levels. They still have significant advantages among different types of explosives in the foreseeable future. Melt cast explosives with TNT as the carrier accounted for more than 90% of military mixed explosives in the past, but traditional TNT-based melt cast explosives can no longer meet the requirements of new-era weapon equipment. Improving energy, safety performance, mechanical properties, and reducing harm to personnel and the environment are the long-term development goals of melt cast explosives.35To further verify the application prospects of TNTON/DNTF eutectic in the field of melt cast explosives, we also calculated the detonation velocity, detonation pressure, and density of mixed explosives with TNTON/DNTF eutectic replacing TNT as the melt cast carrier, and CL-20, RDX, HMX as solid high-energy components. Considering the rheology,36,37 process requirements,5,38 energy39 and mechanical performance of melt cast explosives, the proportion of solid high-energy components in the mixed explosive system is generally between 40% and 70%,35,40 TNTON/DNTF eutectic-based mixed explosives with solid high-energy components CL-20, RDX, HMX at carrier proportions of 30%, 40%, 50%, and 60% were studied. The performance of TNT-based mixed explosives with the same formulation was also compared (Fig. 12). For convenience, “M” represents “TNTON/DNTF eutectic”. In Fig. 12, the detonation velocity of TNTON/DNTF eutectic-based melt cast explosives is very high, all higher than 8600 m s−1, with a significant improvement compared to TNT-based melt cast explosives. In addition, with the same solid high-energy components, the increase of TNTON/DNTF eutectic content has a small effect on the detonation velocity, and the performance is relatively stable under different formulations. In practical applications, small adjustments in the mixed explosive formulation can also ensure its excellent detonation performance. This indicates that TNTON/DNTF eutectic-based melt cast explosives have better performance than TNT-based melt cast explosives, and TNTON/DNTF eutectic has the potential to become a new type of melt cast explosive carrier for industrial applications (Fig. 12).
As shown in Table 5, The data outside the brackets are the theoretical properties of M-base fusion-cast explosives, and the data inside the brackets are the theoretical properties of TNT based fusion-cast explosives. TNTON/DNTF eutectic cast explosive has high detonation pressure and density. The detonation pressure of M-base fusible cast explosive is all higher than 33 GPa under different formulations, while that of TNT-based fusible cast explosive is in most cases lower than 30 GPa. The detonation density of M-base fusing cast explosives is above 1.85 g cm−3, while the detonation pressure of TNT base fusing cast explosives is below 1.8 g cm−3. Therefore, the comprehensive properties of TNTON/DNTF-based cast explosives are obviously better than those of TNT based cast explosives.
| Mass ratio | ρ/(g cm−3) | Vdet/(m s−1) | Pcj/GPa | |
|---|---|---|---|---|
M(TNT)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RDX RDX |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1.816(1.752) | 8710(8194) | 33.7(28.6) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
1.822(1.737) | 8691(7996) | 33.6(27.0) | |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.827(1.722) | 8671(7797) | 33.3(25.5) | |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.833(1.707) | 8656(7597) | 33.4(24.1) | |
M(TNT)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HMX HMX |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1.893(1.824) | 9198(8441) | 36.2(30.8) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
1.888(1.797) | 8980(8197) | 35.8(28.8) | |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.882(1.771) | 8858(7957) | 35.2(26.9) | |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.877(1.745) | 8804(7718) | 34.8(25.1) | |
M(TNT)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL-20 CL-20 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1.981(1.905) | 9697(8776) | 41.0(34.8) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
1.962(1.864) | 9397(8468) | 39.5(31.9) | |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.943(1.824) | 9278(8168) | 38.5(29.0) | |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.925(1.787) | 9162(7881) | 37.3(26.6) |
4 Conclusions
In conclusion, a TNTON/DNTF eutectic was successfully synthesized, which was characterized by a low eutectic temperature of 69 °C and molar ration of 58.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.74 and mass ratios of 49.8
41.74 and mass ratios of 49.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.2. The average temperature differences between the melting and decomposition peaks under various heating rates is as high as 179.2 °C, which implies the eutectic's favorable processing characteristics for use in casting explosives. The thermal decomposition of TNTON/DNTF eutectic is an autocatalytic process. This eutectic effectively decreased the melting point and sensitivity of DNTF, while preserving its high energy potential. It holds a competitive edge over current explosive carriers, offering enhanced detonation velocity, pressure, and density that surpass those of TNT-based explosives. The similar performance across diverse formulations makes the TNTON/DNTF eutectic as a prospective alternative to TNT in the realm of melt cast explosives.
50.2. The average temperature differences between the melting and decomposition peaks under various heating rates is as high as 179.2 °C, which implies the eutectic's favorable processing characteristics for use in casting explosives. The thermal decomposition of TNTON/DNTF eutectic is an autocatalytic process. This eutectic effectively decreased the melting point and sensitivity of DNTF, while preserving its high energy potential. It holds a competitive edge over current explosive carriers, offering enhanced detonation velocity, pressure, and density that surpass those of TNT-based explosives. The similar performance across diverse formulations makes the TNTON/DNTF eutectic as a prospective alternative to TNT in the realm of melt cast explosives.
Data availability
Data supporting the findings of this study are publicly available in the manuscript's references. The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22205176) and Natural Science Foundation of Shaanxi Province (No. 2024JC-ZDXM-07).Notes and references
- B. Zheng, G. Luo, Y. Shu and P. Wang, Chem. Ind. Eng. Prog., 2013, 32, 1341–1346 CAS.
- N. Liu, S. Zeman, Y.-j. Shu, Z.-k. Wu, B.-z. Wang and S.-w. Yin, RSC Adv., 2016, 6, 59141–59149 RSC.
- X. Yang, J. Zhou, X. Xing, Y. Huang, Z. Yan, Q. Xue, X. Wang and B. Wang, RSC Adv., 2020, 10, 26425–26432 RSC.
- Q. H. Wang, Energetic Mater., 2004, 12, 46–55 CAS.
- Y. Wang, J. H. Rui and S. S. Feng, J. Beijing Inst. Technol., 2011, 31, 757–760 CAS.
- O. H. Johansen, J. D. Kristiansen and R. Gjersoe, Propellants, Explos., Pyrotech., 2008, 33(1), 20–24 CrossRef CAS.
- Q. L. Jiang, H. Wang, Y. M. Luo, W. Wang, H. X. Wang, J. Gao and K. Zhao, Initiators Pyrotech., 2014, 3, 42–45 Search PubMed.
- F. Chen, Y. C. Liu, Y. Wang and Q. H. Zhang, Energetic Mater., 2020, 28, 1109–1119 CAS.
- D. Chavez, L. Hill, M. Hiskey and S. Kinkead, J. Energ. Mater., 2000, 18, 219–236 CrossRef CAS.
- V. Pepekin, B. Korsunskii and Y. N. Matyushin, Combust. Explos. Shock Waves, 2008, 44, 110–114 CrossRef.
- Y. Li, J. M. Yuan, W. Zhao, Y. Qu, X. W. Xing, J. W. Meng and Y. C. Liu, Russ. J. Gen. Chem., 2021, 91, 445–455 CrossRef CAS.
- G. Luo, H. Huang, S. Zhang, P. S. Wang, Z. Z. Cai and Y. Zhang, Energetic Mater., 2012, 20, 437–440 CAS.
- L. Tang, H. Li, Y. Tan, T. Liu and Z. Yang, CrystEngComm, 2020, 22, 2173–2182 RSC.
- L. Chen, Y. J. Shu, R. J. Xu, T. Xu and X. F. Wang, Energetic Mater., 2013, 21, 108–115 CAS.
- B. B. Li, Y. M. Luo, W. Lei, M. M. Zhang and W. Wang, Energetic Mater., 2021, 29, 308–314 CAS.
- J. Gao, H. Wang, Y. M. Luo, H. X. Wang and W. Wang, Chin. J. Explos. Propellants, 2020, 43, 213–218+224 Search PubMed.
- K. Yang, F. Bi, J. Zhang, Q. Xue, J. Zhang, K. Wang and B. Wang, Arab. J. Chem., 2022, 15, 103947 CrossRef CAS.
- Q. Xue, F. Bi, L. Zhai, T. Guo, J. Zhang, S. Zhang, B. Wang and J. Zhang, ChemPlusChem, 2019, 84, 913–918 CrossRef CAS PubMed.
- G. R. Desiraju, Acc. Chem. Res., 1996, 29, 441–449 CrossRef CAS PubMed.
- D. Trache, K. Khimeche, R. Benelmir and A. Dahmani, Thermochim. Acta, 2013, 565, 8–16 CrossRef CAS.
- N. E. Beach and V. K. Canfield, 1971.
- J.-F. Pei, F.-Q. Zhao, H.-L. Lu, X.-D. Song, R. Zhou, Z.-F. Yuan, J. Zhang and J.-B. Chen, J. Therm. Anal. Calorim., 2016, 124, 1301–1307 CrossRef CAS.
- Q. L. Yan, X. J. Li, L. Q. Liao, X. H. Zhang and Z. R. Liu, Energetic Mater., 2008, 309–314 CAS.
- Z. R. Liu, Thermal Analyses of Energetic materials[M], Beijing, National Defense Industry, 2009, pp. 388–394 Search PubMed.
- X. N. Ren, S. Y. Heng, Y. H. Shao, Z. R. Liu, G. Zhang, X. H. Wang and F. Han, Energetic Mater., 2009, 17, 455–458 CAS.
- S. J. Chen, Phase Diagram Analysis and Application, 2007 Search PubMed.
- C. B. Storm, J. R. Stine and J. F. Kramer, in Chemistry and Physics of Energetic Materials, ed. S. N. Bulusu, Springer Netherlands, Dordrecht, 1990, pp. 605–639, DOI:10.1007/978-94-009-2035-4_27.
- M. Sućeska, EXPLO5, Brodarski Institute, Zagreb, Croatia, 2013, p. 6 Search PubMed.
- K. Zhao, H. Wang, W. Wang, F. Yang, R. P. Liu and Y. J. Zhu, Chin. J. Explos. Propellants, 2016, 39, 68–72 Search PubMed.
- H. X. Wang, F. F. Jiang, H. Wang, Y. M. Luo and J. Gao, Energetic Mater., 2012, 20, 423–426 CAS.
- H. X. Wang, X. F. Wang, Y. M. Luo and F. F. Jiang, Energetic Mater., 2009, 17, 183–186 CAS.
- M. Anniyappan, K. Vijay Varma, R. S. Amit and J. K. Nair, J. Energ. Mater., 2020, 38, 111–125 CrossRef CAS.
- X. Yang, J. Zhou, X. Xing, Y. Huang, Z. Yan, Q. Xue, X. Wang and B. Wang, RSC Adv., 2020, 10(44), 26425–26432 RSC.
- Q. L. Yan, Z. W. Song, T. An, X. H. Zhang and F. Q. Zhao, Chin. J. Explos. Propellants, 2016, 39, 1–12 Search PubMed.
- B. H. Zhen, G. Luo, Y. J. Shu and P. S. Wang, Chem. Ind. Eng. Prog., 2013, 32, 1341–1346 Search PubMed.
- M. A. Parry and H. H. Billon, The viscosity of (molten) rdx/tnt suspensions, Proceedings of the 18th International Annual Conference of ICT, 1987, pp. 1–3 Search PubMed.
- W. F. Larsen, A. A. R. DEVELOPMENT and E. C. P. A. N. P. A. DIRECTORATE, The Use of Low Viscosity 70/30 Octol in Dragon Warheads M224, AD-A053473, 1977 Search PubMed.
- R. L. Hatch, Method for processing explosives containing 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazatetracyclo [5.5. 0.05, 903, 11]-dodecan (CL-20) with naphthenic and paraffinic oils, US Pat., 6736913, 2004 Search PubMed.
- Y. X. Ou, Z. Meng and J. Q. Liu, Chem. Ind. Eng. Prog., 2007, 1690–1694 CAS.
- H. X. Wang, X. F. Wang and Y. M. Luo, Explos. Mater., 2021, 50, 1–9 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03904a |
| This journal is © The Royal Society of Chemistry 2024 |