Open Access Article
Open Access ArticleBiomass as an alternative feedstock to oleochemicals
Zeni Rahmawati *a,
Liangga Santosoa,
Wan Nazwanie Wan Abdullahb,
Abdul Hamidc,
Nor Laili Azua Jamari
*a,
Liangga Santosoa,
Wan Nazwanie Wan Abdullahb,
Abdul Hamidc,
Nor Laili Azua Jamari d,
Djarot Sugiarsoa,
Yatim Lailun Ni'maha and
Alfa Akustia Widati*e
d,
Djarot Sugiarsoa,
Yatim Lailun Ni'maha and
Alfa Akustia Widati*e
aChemistry Department, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember, Keputih, Sukolilo, Surabaya 60111, Indonesia. E-mail: zeni.rahmawati@its.ac.id
bSchool of Chemical Science, Universiti Sains Malaysia, 11800 Penang, Malaysia
cDepartment of Heavy Equipment Mechanical Engineering, Politeknik Negeri Madura, Indonesia
dDepartmen of Chemistry & Biology, Centre of Defence Studies, National Defence University of Malaysia, Kem Sungai Besi, Kuala Lumpur 57000, Malaysia
eDepartment of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia. E-mail: alfaakustia@fst.unair.ac.id
First published on 10th September 2024
Abstract
The huge demands for petrochemicals have led to a rapid increase in the production of these fossil-based derivatives. Biomass represents a promising feedstock for addressing the challenges related to petrochemicals in terms of the necessity to apply renewable sources and the need to decrease carbon emissions. Among the natural biomass products, most studies have attempted to upgrade natural oils owing to their promising advantages of worldwide availability, low-cost processing, and built-in functionality. This paper discusses the upgradation of natural oils to the most beneficial oleochemicals, including fatty acids, fatty alcohols, and fatty acid methyl esters. This review also covers the utility, physico-chemical properties, and the production processes for such materials. The interconnected reaction routes to produce oleochemicals and the affecting parameters (catalyst design, temperature, and pressure) are also elucidated. Furthermore, this article discusses the future perspective of oleochemicals based on their development in recent years.
1. Introduction
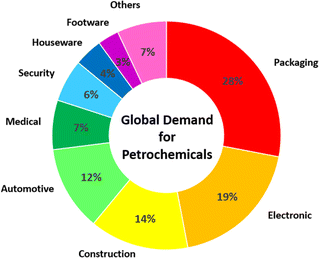
The dependence of modern society on petrochemicals has been rapidly growing day by day. The huge demands for petrochemicals in numerous sectors, including packaging, electronics, construction, automotive, medicine, security, and houseware, have driven an increase in the production of these oil and gas derivatives (Fig. 1). Subsequently, petrochemicals have emerged as a rapid growth driver of global oil consumption. In 2023, petrochemicals contributed to about a third of global oil demand, and their contribution is predicted to increase to about 50% by 2050.1Nevertheless, the production of petrochemicals is facing several challenges, including depletion of the finite source of oil and gas as well as the rise in carbon dioxide emissions. In addition, many environmental problems, such as climate change and air and water pollution are associated with the production, utilization, and disposal of petrochemical products. Consequently, alternative sustainable sources are needed to replace petrochemical feedstocks.2–4
Biomass has emerged as a promising feedstock for addressing the drawbacks of petrochemicals in regard to the necessity of renewable sources and reduced carbon emissions. Biomass has been attracting increasing attention from researchers since many important chemicals can be derived from biomass conversion. History records the employment of biomass as raw materials for medicinal drugs, flavourings, and fragrances for centuries. For instance, industrial-scale biomass conversion commenced in the second half of the 19th century. Unfortunately, biomass-based chemicals were undesirable back then from an economic point of view since petroleum-based chemicals were cheaper, and the production had been stable for more than 100 years.4,5
Basically, biomass comprises four main products: sugar and starch bioproducts, oil- and fat-based products, gum and wood, and cellulose derivatives.5,6 Biomass is also classified based on the energy content of components such as terpenes, vegetable oils, lignin, and sugars.7–9 Amongst all the products, natural oils are the most common focus of research associated with biomass conversion. A large number of studies have attempted to upgrade natural oil as this biomass exhibits superior advantages, such as worldwide availability, low-cost processing, and built-in functionality.8,10 As can be seen in Fig. 2, the global production of natural oil has been increasing each year.11
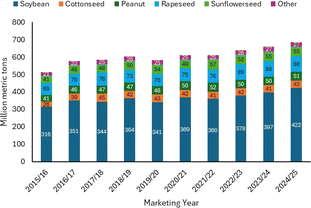 | ||
| Fig. 2 Global vegetable oil production from 2015 to 2024.11 | ||
Most importantly, natural oils possess a chemical structure similar to long-chain hydrocarbons derived from petroleum. Natural oil is a potential renewable resource to replace petroleum despite showing several differences, which arise since natural oils are generally oxidized and unsaturated. Nevertheless, the structural differences can be addressed by some chemical processes with particular parameters.
In this paper, the upgrading of natural oil into oleochemicals and green diesel is described with particular emphasis on the scope of utility, physico-chemical properties, and the production aspects. The interconnected reaction pathways to produce oleochemicals as well as the affecting parameters, such as catalyst design, temperature, and pressure, are also thoroughly discussed. This review also provides a future perspective for oleochemicals based on its development in recent years. To the best of our knowledge, an updated review in this area is still unavailable, so this work will fill an important gap.
2. Natural oil refining process
Apart from triglycerides, natural oil comprises several chemicals that can generate adverse effects on the quality of the oil and its derivative products.12,13 These substances include natural components, oxidation products, and chemical pollutants. The most common natural components observed are phospholipids, glycolipids, unsaponifiable matter, waxes, tocopherols, phytosterols, squalene, and terpenoids.12,14,15 The oxidation products consist of peroxides, oxidized fatty acids, and aldehydes. Undesirable chemical pollutants may also be present in the form of pesticides, heavy toxic metals, mineral oil, organic solvent, aflatoxins, PAH, etc.13 Despite their minor proportions (1–2%), these chemicals can influence the properties of the products, such as colour, smoke and foam formation, odours, precipitation, flavour, toxicity, and oxidative stability.16 Subsequently, the refining process must be able to remove unwanted and toxic chemicals. This process embodies two main types: physical and chemical refining.12,13Physical refining eliminates the unwanted substances by physical separation processes, like atmospheric distillation, steam distillation, and high vacuum distillation.13,16 Physical refining involves certain main principles: degumming for phosphide removal, bleaching-filtration for off-colour elimination, and deodorization for addressing the discharge of volatile substances.12,15 Another report also included deacidification to remove free fatty acids in this category.13 The biggest advantage of physical refining lies in the environmental aspects, energy requirement, and economic point of view.17 Nevertheless, the application of physical refining is limited to certain types of oil, especially crude ones with high acidity. Physical refining also requires high temperature and a vacuum and carries a risk of unwanted products.12
Chemical refining utilizes chemical substances to remove minor unwanted compounds. The principal techniques are similar to the physical one, including degumming, neutralization, washing–drying, bleaching, and deodorization.18 In these processes, several chemicals may be applied, such as acids, enzymes, caustic soda, adsorbents, and membranes.14,17 Chemical refining is considered a highly efficient method with high practicality. However, chemical refining also has some drawbacks, such as excessive oil loss, the formation of undesired side products, and high cost.12,13,17
3. Natural oil upgrade to basic oleochemicals
Natural oil contains a large proportion of triglycerides, with a percentage of 96–98%, while the remaining compounds include phospholipids, free fatty acids, diglycerides, monoglycerides, and minor amounts of sterol, sterol esters, tocopherols, tocotrienols, and trace metals.19,20 The structure of triglycerides comprise three fatty acids attached to one glycerol.21–25 These fatty acids can be saturated or unsaturated with 12–22 carbon atoms in the carbon chain, as shown in Fig. 3. This structure allows distinguishing natural oils from each other by their properties, such as density, viscosity, boiling point, and degree of saturation.21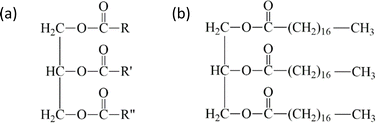 | ||
| Fig. 3 Structure of triglyceride (a) and tri-acyl glyceride of stearic acid as an example (b).21 | ||
The wide-scale application of natural oil has gone through an extensive history, especially a century ago when natural oil was first utilised to fuel an engine by Rudolph Diesel.26 This history emphasizes the feasibility of using natural oil despite its limitations, such as high viscosity, low volatility, and high triglycerides content with a large molecular weight.27,28 From this point, numerous research studies have focused on upgrading natural oil as a biofuel, with many successful examples, such as rapeseed, soybean, sunflower, palm, jatropha, and corn oil. It has also been claimed that biofuel from natural oil is a clean resource and offers some important benefits, such as a stable supply, reduction in CO emissions, and opportunities for the agricultural economy.29–31
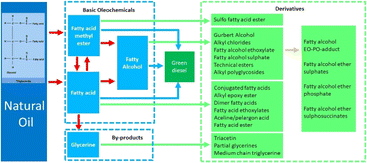
Apart from biofuel, natural oil has been extensively investigated as a promising feedstock for many important oleochemicals. Fig. 4 illustrates the products from the upgrading of natural oil, consisting of basic oleochemicals and derivatives. Triglycerides in natural oil can be split up into the two highly important basic oleochemicals, namely fatty acids and fatty acid methyl esters. These two compounds are generated from different reactions with the same by-product of glycerine. Another basic oleochemical type is fatty alcohols, which can be obtained from the hydrogenation of fatty acids or fatty acid methyl esters. All basic oleochemicals are the intermediates of some essential derivatives processed through several different reactions. Detailed discussions of the basic oleochemicals and green diesel are given in the following sub chapters.
3.1. Fatty acids
The structure of fatty acids consists of aliphatic groups and carboxyl groups at the end point (Fig. 5). Normally, the aliphatic group exhibits an even number of carbon atoms with the total number differing from one fatty acid to another. Based on the presence of double bonds, fatty acids can be categorised as either saturated or unsaturated.32–35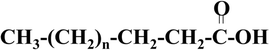 | ||
| Fig. 5 Structure of saturated fatty acids.33 | ||
The absence of a double bond indicates a saturated fatty acid with variation of the number of carbon atoms between 4 to 24. Most commonly, saturated fatty acids consist of 12 to 22 carbon atoms. Unsaturated fatty acids are identified by the existence of double bonds as well as the number, the position, and the geometry of the double bonds.32 Table 1 contains some examples of saturated and saturated fatty acids. The presence of double bonds influences the properties of fatty acids, in which unsaturated fatty acids have a lower boiling melting point and a higher reactivity towards oxidation. Nevertheless, all fatty acids have a very low solubility in water and high dissolution in nonpolar solvents.19,32
| Common name | Carbon atoms | IUPAC name |
|---|---|---|
| Lauric | 12 | Dodecanoic |
| Myristic | 14 | Tetradecanoic |
| Palmitic | 16 | Hexadecanoic |
| Stearic | 18 | Octadecanoic |
| Arachidic | 20 | Eicosanoic |
| Behenic | 22 | Docosanoic |
| Lauroleic | 12 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 decenoic 10 decenoic |
| 12 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 decenoic 5 decenoic |
|
| Myristoleic | 14 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 tetradecenoic 10 tetradecenoic |
| 14 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 tetradecenoic 5 tetradecenoic |
|
| Palmitoleic | 16 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 hexadecenoic 10 hexadecenoic |
| Oleic | 18 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 octadecenoic 10 octadecenoic |
| Linoleic | 18 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 11 7, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 octadecanoic 12 octadecanoic |
Fatty acids can be derived from natural oils by a process of fat splitting through a hydrolysis reaction.6,36 Chemically, the process is carried out by the addition of water to convert triglycerides to glycerol and fatty acids (Fig. 6).8,37 There are three steps involved in the transformation to split three fatty acid groups attached in triglycerides that result in diglycerides, monoglycerides, and glycerol as by-products for each step, respectively. Therefore, three moles of water are required to transform one mole of triglycerides.
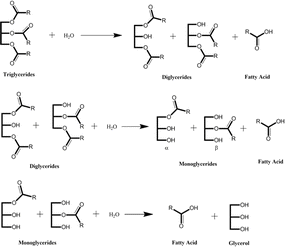 | ||
| Fig. 6 Hydrolysis of triglycerides to produce fatty acids.8 | ||
Commercially, there are three methods for the hydrolysis of natural oil: the Twitchell, autoclave, and continuous method.6,32,38 The Twitchell process is the simplest fat splitting process carried out by heating up natural oil with boiling water in the presence of a catalyst. Sulfuric acid is the common catalyst applied with a low concentration of 1–2%. Nevertheless, this process requires a very long time to accomplish, and gives a maximum yield of 95%.8,38
The autoclave method exhibits a higher efficiency of 95–96% within a shorter reaction time. A high purity of fatty acid is obtained over basic oxide catalysts, such as magnesium and calcium oxides.8,32,38 In addition, this method relies on heat energy to accelerate the reaction, leading to some considerations in terms of the higher investment in energy, maintenance, and cost. Finally, the continuous method is the most viable and preferable technique at the industrial scale.8,32,38 In this latter process, fatty acids are produced by passing water and natural oil at high temperature and pressure, with the continuous removal of glycerol. A highest efficiency of 96–98% and low cost are the main benefits of this method.
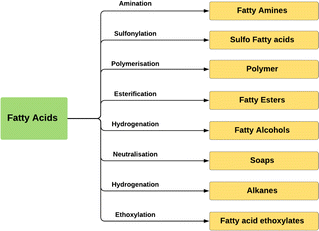
Many applications of fatty acids have been mentioned in industry, including lubricants and greases, emulsifiers, textiles, soaps, cosmetics, candles, pharmaceuticals, waxes, and adhesives. Fatty acids are also the first derivative of natural oil as an intermediate to many other oleochemicals, such as such as fatty alcohols, fatty amines, fatty acid methyl esters, fatty acid ethoxylates, and green diesel. The derivatization of fatty acids to these oleochemicals can involves several reactions; for instance, esterification, ethoxylation, neutralisation, amination, and hydrogenation (Fig. 7).
3.2. Fatty acid methyl ester (FAME)
Fatty acid methyl ester is derived from natural oil directly through a transesterification reaction in the presence of an alcohol and catalyst. Methanol is the most common alcohol applied in this process due to its reactivity and availability.39–42Scheme 1 depicts the transesterification of triglycerides to produce fatty acid methyl ester, in which R1, R2, and R3 are long-chain fatty acids. There are five types of fatty acids ordinarily found in natural oil or animal fat, namely palmitate, stearate, oleate, linoleate, and linolenate. Initially, triglyceride is transformed into diglyceride and subsequently monoglyceride. The final product of transesterification is glycerol and methyl esters depending on the fatty acid chains. Therefore, three moles of alcohol are required to convert one mole of triglycerides during transesterification.6
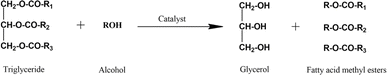 | ||
| Scheme 1 Transesterification of triglycerides.39 | ||
FAME production can be performed by three main pathways: transesterification over acid catalysts, transesterification over base catalysts, and non-catalytic transesterification under supercritical alcohol. Commercially, the main synthesis method applied is the transesterification of triglycerides with strong base catalysts, such as NaOH and KOH.39,43–45 The drawbacks of homogeneous catalysts are their saponification susceptibility and separation issues. Heterogeneous catalysts are also commonly applied for biodiesel production. This type of catalyst has attracted enormous attention due to its excellent activity, high reusability, corrosive resistance, and low-cost.
FAME production through the transesterification reaction is influenced by the methanol oil ratio, reaction time, temperature, and catalyst concentration.46,47 The transesterification can be conducted at a relatively low temperature, whereby the optimum temperature was reported to be around 50–60 °C.48,49 However, higher temperatures are sometimes applied due to the viscosity of the feedstock; for instance, for jatropha oil, soybean oil, and olive oil.27,50–54 The reaction time applied in the transesterification is about 0.25 to 6 h and most studies have mentioned that an effective reaction time is 0.5 to 2 h.46,51,55–60 Excess methanol is required to shift the equilibrium to the product side since transesterification is a reversible reaction. The ratio of methanol/oil mentioned in the literature is in the range of 1–60. However, the range of 10–30 has mainly been reported,61–67 while the catalyst loading varies depending on the catalyst and the feedstock.
FAME is also well known as a biodiesel, primarily as an alternative fuel to replace fossil fuels.68 This compound has some properties similar to the characteristics of fossil fuels (Table 2). Other minor applications include as surfactants or thickening and plastifying agents. Despite being considered a promising alternative fuel, it is worth noting that biodiesel has not yet really achieves the quality of fossil fuels. Hence so far, biodiesel is applied by mixing with fossil fuels. Also, biodiesel has a high oxygen content, which can cause incomplete combustion and lead to the accumulation of carbon in the engine, filter, and nozzles. Furthermore, biodiesel can have degradation issues, with a gum-like formation due to oxidation and polymerization during storage. Moreover, it has a low energy content and thermal stability, which are further matters that require solutions.22,69,70
| Fuel properties | Petro-diesel | Biodiesel | |
|---|---|---|---|
| Density | kg m−3 | 796–841 | 880 |
| Viscosity at 40 °C | mm2 s−1 | 1.9–4.1 | 2.9–11 |
| Flash point | °C | 54–148 | 100–180 |
| Net calorific value | MJ kg−1 | 42.34–43.1 | 37.2–38 |
| Cetane number | Min | 40–67 | 45–65 |
| Oxygen | — | 11.2 | |
| Water content | ppm | — | — |
| Sulfur | ppm | <10 | <1 |
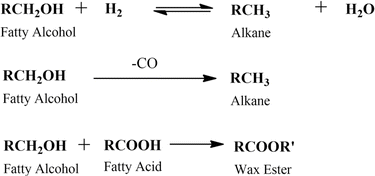
FAME can be upgraded to green diesel by oxygen removal via a deoxygenation reaction. This reaction will produce green diesel with the same structure as alkanes. In addition, FAME can also be transformed into another valuable oleochemical known as fatty alcohol. The selective hydrogenation of FAME has been applied for years to produce fatty alcohols.71 These reactions are interconnected as described in Fig. 8.
3.3. Fatty alcohols
Fatty alcohols have a structure of aliphatic hydrocarbons with a hydroxyl group in the primary position (Fig. 9).73,74 The aliphatic group provides hydrophobic properties while the hydroxyl group is responsible for hydrophilicity. The aliphatic group commonly consists of chain lengths with 8 to 22 carbon (maximum 38 carbon atoms), sometimes with one or more double bonds. Therefore, fatty alcohols are also categorised as saturated and unsaturated. Other categories include linear, branched, primary, and secondary fatty alcohols.73–76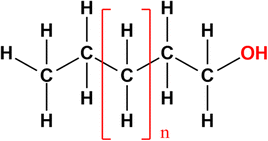 | ||
| Fig. 9 Structure of fatty alcohols.72 | ||
The physical and chemical properties of fatty alcohols are influenced by the number of aliphatic chains and the presence of double bonds. Unsaturated fatty alcohols exhibit a lower melting point, and longer aliphatic chain is insoluble in water. Some examples of fatty alcohols together with their physical and chemical properties are illustrated in Table 3. Fatty alcohols are oleochemicals with a broad range of applications in industry, such as fragrances, detergents, cosmetics, plasticizers, emulsifiers, lubricants, health supplements, and pharmaceuticals.69,77–79 The chemical structure of fatty alcohols having both hydrophobic and hydrophilic properties is the key to these extensive applicable functions.73,74
| Synthetic name | Trivial name | Carbon number | Density (g cm−3) | Melting point (°C) | Boiling point (°C) |
|---|---|---|---|---|---|
| Butanol | Butyl | 4 | 0.81 | −90 | 117 |
| Pentanol | Amyl | 5 | 0.815 | −79 | 137.5 |
| Hexanol | Caproyl | 6 | 0.815 | −51.6 | 157 |
| Heptanol | Onantyl | 7 | 0.819 | −34.6 | 175.8 |
| Octanol | Caprylic | 8 | 0.827 | −16 | 194 |
| Nonanol | Pelorgonyl | 9 | 0.828 | −5 | 215 |
| Decanol | Capryl | 10 | 0.83 | 6.4 | 232.9 |
| Dodecanol | Lauryl | 12 | 0.831 | 24 | 259 |
| Tetradecanol | Myristyl | 14 | 0.824 | 38 | 289 |
| Hexadecanol | Cetyl | 16 | 0.811 | 49 | 344 |
| Octadecanol | Stearyl | 18 | 0.811 | 59 | 360 |
Fig. 10 describes the main applications of fatty alcohols based on the chain length. Cosmetics, foods, and plasticisers require shorter chain fatty alcohols, while middle chain fatty alcohols with 12–14 carbons chain lengths are predominantly utilized in detergent applications, while the longer chain fatty alcohols are used in pharmaceuticals and personal care products. Larger chain fatty alcohols are also commonly applied as biofuel, non-ionic surfactants, and emulsifiers.8,79,80 Additionally, fatty alcohols are intermediates for many important chemicals, such as fatty alcohol ethoxylates, metal alkoxides, and alkyl halides (Fig. 11).
 | ||
| Fig. 10 Main applications of fatty alcohols.80 | ||
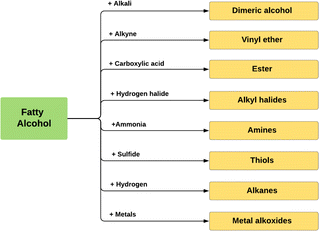 | ||
| Fig. 11 Fatty alcohols transformation into derivative compounds.81 | ||
Aside from their extensive range of applications, the importance of fatty alcohols can be also observed from their global demand, which has risen each year, amounting to 3.9 million tonnes in 2022.82 The largest consumers are found in Asia-Pacific countries, which account for 45% of global demand. The huge demand is associated with the rapid use of sanitizers, detergents, and soaps during the Covid-19 pandemic. This huge demand is predicted to continuously rise to up to 5.5 million tons by 2030. As with consumption, Asia-Pacific also dominates fatty alcohol production. While individually, Indonesia is one of the largest fatty alcohol suppliers worldwide, with a total capacity of the oleochemical industry of 1.99 million tons per year. Fatty alcohol production alone is estimated to increase 85% each year. The high production is attributed to the abundance of raw materials in the country. Indonesia has many kinds of natural oils as raw materials for oleochemicals and derivative compounds. So far, palm oil is still the main resource for the oleochemical industries.
Fatty alcohols can be produced from petroleum and natural based resources. The synthesis of fatty alcohols from petroleum resources is carried out via the Ziegler process or the oxo process. In the Ziegler process, ethylene is polymerised with tri-ethyl aluminium followed by oxidation or hydrolysis, while the oxo process is performed by the hydroformylation of propylene followed by hydrogenation of the aldehyde.79,83,84
Natural based fatty alcohols can be derived from natural oil via direct hydrogenation. However, the process is undesirable due to cost considerations since excessive hydrogen pressure is required. Moreover, the harsh reaction conditions may possibly degrade the catalyst. These challenges have driven the use of natural derivatives as fatty alcohol and fatty acid methyl ester feedstocks.85,86
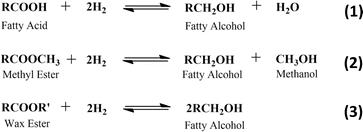
Natural based fatty alcohols can be produced by the hydrogenation of several feedstocks, such as fatty acid, fatty acid methyl ester, and wax ester (Scheme 2).23,77,79,87 Commercially, fatty alcohol production is carried out at elevated temperature (200–300 °C) and pressure (100–300 bar) over Cu–Cr-based catalysts.23,79,81,88,89 Table 4 shows some examples of fatty alcohol production on an industrial scale.
| Process condition | Slurry reactor | Fixed bed | |
|---|---|---|---|
| Liquid phase | Gas phase | ||
| Pressure (bar) | 250–300 | 250–300 | 40 |
| Temperature (°C) | 280 | 180–220 | 200–240 |
| Feed | Methyl or wax ester | Methyl or wax ester | Methyl or wax ester |
| Catalyst shape | Powder | Tablets or extrudates | Tablets or extrudates |
| H2/feed (molar ratio) | 25–50 | 40–100 | 250 and above |
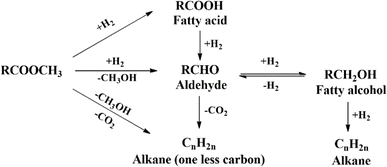
The main challenge for fatty alcohol production is to maintain the selectivity while enhancing the conversion. Excessive hydrogenation, decarbonylation, and esterification often occur simultaneously, resulting in alkanes and wax ester as by-products (Scheme 3). Fortunately, wax ester is generally recycled to produce fatty alcohols through hydrogenation.71,90 There are several factors that influence fatty alcohol production, including the catalyst and reaction conditions, which are covered in detail below.
The history of heterogeneous catalysts in natural based fatty alcohol production dates back to 1931. It was initiated by Adkin et al. (1999), who successfully conducted the hydrogenation of esters to fatty alcohols over Cu–Cr catalysts at 250 °C and 250 bar.92 Afterwards, numerous research attempts to optimise the catalytic activity of the Cu–Cr catalyst were performed through some modifications, such as the catalyst preparation method.93 A further optimisation focused on the reaction conditions with the same Cu–Cr catalyst, such as the application of a supercritical solvent to reduce the hydrogen.94,95
Many regulations have been formed against the application of Cr-based catalyst due to its adverse effects on the environment, such as US Environmental Protection Agency, the European Union, and REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals).79 Following such restrictions, many efforts have been dedicated to replacing chromium with other catalyst promoters to obtain excellent activity and high acid resistance. The composition thus shifted to CuZn, CuAl, and CuFe. The application of CuZn-supported catalysts was assessed with some methyl esters and acids, such as methyl acetate, methyl laurate, and lauric acid.91,96–100 Nevertheless, CuZn exhibited a low alcohol selectivity, high selectivity towards heavy esters, and suffered from leaching. Similarly, CuFe showed a slow activity although it provided higher selectivity for alcohol.96 Furthermore, harsh reaction conditions were required during the hydrogenation of ester over a CuFe-supported catalyst.101 On the other hand, supported Cu–Al–O generated a lower conversion and alcohol selectivity even at elevated temperature and high pressure.100,102 Afterwards, the focus of catalyst development then deviated towards noble metal and transition metal catalysts as Cu replacements.
Platinum is one of the most employed hydrogenation catalysts owing to its high activity and hydrogen dissociation capability. For instance, Manyar et al. examined the role of Pt/TiO2 on the hydrogenation of stearic acid under the following reaction conditions: stearic acid 1.4 g, dodecane as a solvent 40 ml, 4% Pt/TiO2 0.3 g, 130 °C, and H2 pressure 20 bar.103 Complete conversion was achieved after 20 h with 93% fatty alcohol selectivity and 7% alkanes. The role of rhenium as a promoter and several supports, such as SiO2, Al2O3, ZrO2, and CreO4, were also investigated. Nevertheless, the addition of rhenium only enhanced the activity yet lowered the selectivity of stearyl alcohol to 70%. Additionally, the effect of the support on the reaction rate was trivial.
Another noble catalyst is palladium. Predominantly, palladium supported catalysts were applied in the hydrogenation of fatty acids and methyl esters in the form of bimetallics or combined with promoters. Silica-supported Pd–Re was tested by Takeda et al. in the hydrogenation of stearic acid.89 The optimum conditions were found to be Re/Pd = 8, H2 initial pressure 80 bar, 140 °C, and a reaction time of 4 h. A selectivity to stearyl alcohol of 96.6% was obtained at a stearic acid conversion of 45.3%.
Some other noble metals, such as rhenium and ruthenium, were also tested in previous studies and are commonly used in bimetallic forms. Pt–Re/TiO2 showed a high activity in stearic acid hydrogenation, with a selectivity to stearyl alcohol reaching 89%. The rhenium metal in this catalyst increased the oxophilicity, leading to a higher reaction rate.104 On the other hand, the catalytic activity of monometallic Ru was very low and only increased with the addition of other metals. The addition of Sn in RuSn/N–C improved the yields of stearyl alcohol up to 38%.105 Other noble metal catalysts are summarised in Table 5. However, the low reaction rates and high cost of catalysts must be considered for industrial application.
| Catalysts | Reactant | Reaction conditions | Result | Ref. |
|---|---|---|---|---|
| Pt/TiO2 | Stearic acid | 50 bar, 130 °C, reactant 1.4 g, catalyst 0.3 g, 20 h | Conv. = 100% | 103 |
| Sel. = 93% | ||||
| Pt–Re/TiO2 | Stearic acid | 20 bar, 130 °C, reactant 1.42 g, catalyst 0.5 g, 5 h | Conv. = 86% | 104 |
| Sel. = 89% | ||||
| Pt–Re/TiO2 | Methyl hexanoate | 50 bar, 180 °C, reactant 0.4 mmol, catalyst 3 mg, 8 h | Conv. = 11% | 106 |
| Sel. = 98% | ||||
| Pt–ReO2/TiO2 | Hexanoic acid | 50 bar, 130 °C, reactant 0.4 mol, catalyst 2 g, 5 h | Conv. = 95% | 107 |
| Yield = 92% | ||||
| Re–Pd/SiO2 | Stearic acid | 80 bar, 130 °C, reactant 1 g, catalyst 0.15 g, 9 h | Conv. = 7.9% | 89 |
| Yield = 97% | ||||
| ReOx/TiO2 | Stearic acid | 40 bar, 200 °C, reactant 1 g, catalyst 0.1 g | Conv. = 80% | 78 |
| Sel. = 93% | ||||
| Re/Nb/Al2O3 | Palm methyl ester | 70 bar, 280 °C, 10 h | Conv. = 90% | 108 |
| Sel. = 79% | ||||
| Ni–Re/SBA-15 | Stearic acid | 40 bar, 150 °C, reactant 0.1 g, catalyst 0.02 g, 5 h | Conv. = 100% | 109 |
| Sel. = 94.5 | ||||
| Ru–Sn/Al2O3 | Methyl laurate | 60 bar, 300 °C, reactant 40 g, catalyst 0.8 g, 4 h | Conv. = 56.9% | 110 |
| Sel. = 18.3% | ||||
| Ru–Sn/Al2O3 | Methyl oleate | 80 bar, 270 °C, reactant 100 ml, catalyst 2.2 g | Conv. = 70% | 111 |
| Sel. = 73% | ||||
| Ru–Sn–B/Al2O3 | Oleic acid | 53 bar, 300 °C, 1 h | Conv. = 50% | 112 |
| Sel. = 15% | ||||
| Ru/NH2-rGO | Palmitic acid | 100 bar, 210 °C, reactant 0.04 g, catalyst 0.025 g, 22 h | Conv. = 99% | 88 |
| Sel. = 93% | ||||
| RuSn/N–C | Stearic acid | 50 bar, 140 °C, reactant 0.4 g, catalyst 0.1 g, 6 h | Conv. = 100% | 105 |
| Sel. = 96% | ||||
| RuSn/ZnO | Octanoic acid | 20 bar, 300 °C, catalyst 0.5 g, WHSV 2 h−1 | Conv. = 99.4% | 113 |
| Sel. = 93% | ||||
| Ru–MoOx/TiO2 | Lauric acid | 40 bar, 170 °C, reactant 0.065 g, catalyst 0.05 g, 7 h | Conv. = 99% | 114 |
| Sel. = 82% | ||||
| Rh–Sn/Al2O3 | Methyl laurate | 60 bar, 300 °C, reactant 40 g, catalyst 0.8 g, 4 h | Conv. = 71.7% | 110 |
| Sel. = 43.2% | ||||
| Rh–Sn–B/γ-Al2O3 | Oleic acid | 50.7 bar, 270 °C, reactant 3.5 g, catalyst 1 g, 2.5 h | Conv. = 100% | 115 |
| Sel. = 94.4% | ||||
| Pd/CuZnAl | Methyl decanoate | 20 bar, 200 °C, reactant 0.05 g, catalyst 0.05 g, 8 h | Conv. = 96.6% | 116 |
| Sel. = 95.9% |
In the quest for lower-priced catalysts, transition metals have emerged as potential alternatives with higher abundance. In the production of petroleum-based fatty alcohols, homogeneous cobalt carbonyl was proposed for the hydroformylation reaction. A heterogeneous cobalt catalyst was also applied in the production of natural based fatty alcohols.
The cobalt-based catalyst showed high activity in FAMEs hydrogenation. Co/ZrO2 showed 96% selectivity in methyl laurate hydrogenation at 180 °C and 20 bar.117 Other reports showed high selectivity in ethyl palmitate hydrogenation, with 1-hexadecanol selectivity reaching 85.7% at 200 °C and 20 bar with a Co/ZrO2 catalyst.118 In some reports, cobalt was combined with tin in order to prevent excess hydrogenation and to selectively produce unsaturated alcohols. For instance, the application of CoSn/Al2O3 in the hydrogenation of methyl oleate at 270 °C and 80 bar was reported, and the selectivity of the heavy ester oleyl oleate was higher than oleyl alcohol.119 The preference for heavy ester was also observed in the hydrogenation of methyl oleate over CoSn/ZnO,120 and CoSnB/Al2O3.121
Aside from cobalt, nickel has also been mentioned for fatty alcohol production. The activity of a nickel catalyst (Ni-VOx/TiO2) in the hydrogenation of methyl palmitate was investigated under the following reaction conditions: methyl palmitate 0.1 g, catalyst 0.05 g at 220 °C. A maximum acetyl alcohol selectivity of 90% was attained after 7.5 h.122
Other than metal active sites, the selection of the catalyst also requires consideration of other aspects, such as the catalyst support, catalyst loading, and catalyst stability. Basically, the role of a catalyst support is to reduce the metal amount and produce active metal species. In heterogeneous catalysis, the support allows the metal particles to have mechanical and thermal stability. Furthermore, supports influence the overall catalytic properties, such as metal dispersion, particle size, and acidity.76,123 For the purpose of comparison, some studies have been attempted to investigate the nature of support in fatty alcohol formation.
Al2O3 is the most popular support, with excellent performance reported by many studies. The application of Al2O3 as a catalyst support provides some benefits for metal dispersion, thermal stability, and moderate acidity.121 Al2O3 displayed excellent activity compared to ZrO2 and MgO in the hydrogenation of lauric acid over copper catalysts.124 The conversion rates of lauric acid were determined as 53.9%, 96.9%, and 98.9% over Cu/ZrO2, Cu/MgO, and Cu/Al2O3 respectively. Aside from conversion, Al2O3 also surpassed ZrO2 and MgO in terms of the lauryl alcohol yield, with a yield of 99.2%. The authors argued that the excellent performance of the alumina support was on account of the higher dispersion, smaller particle size, and lower acidity. In addition, the higher acidity of ZrO2 was reported to induce the formation of the ester.
The superior activity of Al2O3 above several other supports was also mentioned in the study as being related to the support effect in the hydrogenation of methyl hexadecanoate over supported ruthenium tin catalysts.125 Al2O3 exhibited a superior activity of 86.4% conversion followed by SiO2 and TiO2. The change in conversion was related to ruthenium dispersion generated by the ruthenium-support interaction. However, the alcohol selectivity remained unaffected by the catalyst support alteration within the range of 83–86%.
Many other reports also presented the use of Al2O3, such as the hydrogenation of methyl laurate over Ru–Sn/Al2O3,110 hydrogenation of oleic acid over Ru–Sn–B/Al2O3,112 hydrogenation of lauric acid over Cu/Al2O3,124 stearic acid over Ni/Al2O3,126 methyl oleate over CoSn/Al2O3,121 octanoic acid over CuIn/Al2O3,127 and palmitic acid over Pt/Al2O3,128 hydrogenation of oleic acid over Rh–Sn–B/Al2O3,115 hydrogenation of palm methyl ester over Re/Nb/Al2O3,108 and palmitic acid over Ni/Al2O3.129
SiO2 is also a common catalyst support applied in some works related to fatty alcohol production. This catalyst support was reported to have similar activity in the hydrogenation of stearic acid over nickel-supported catalysts. Both SiO2 and Al2O3 exhibited 41% conversion after 360 min reaction. However, SiO2 produced stearyl alcohol with three times selectivity for Al2O3. On the other hand, a similar trend for the selectivity of stearyl alcohol was generated from the hydrogenation of methyl palmitate over Ru–Sn/Al2O3 and Ru–Sn/SiO2.110 The exquisite performance of SiO2 as a catalyst support was mentioned in the hydrogenation of stearic acid over Re–Pd/SiO2 (ref. 89) and methyl acetate over Cu/SiO2,71 methyl octanoate over NiIn/SiO2,77 and oleic acid over NiFe/SiO2–ZrO2.130
Another promising support is TiO2 with medium acidity–basicity that could be expected to inhibit the formation of heavy ester. The role of the support TiO2 in Pt–Re activity was investigated in methyl hexanoate hydrogenation with varied catalyst supports, including TiO2, Al2O3, CeO2, ZrO2, CeZrO2, and carbon.106 The highest conversion was generated from Pt–Re supported on TiO2 followed by Al2O3. Another TiO2 application was mentioned in the hydrogenation of stearic acid over Pt/TiO2,103 methyl palmitate over Ni–VOx/TiO2,122 stearic acid over Ni3Fe/R–TiO2,87 stearic acid over Pt–Re/TiO2,104 and lauric acid over Ru–MoOx/TiO2.114
Furthermore, other mentioned catalyst supports include H-ZSM-5, ZrO2, Al-incorporated silica molecular sieve AlSBA-15, silicoaluminophosphate SAPO-11, and carbon.105,109,126,131–133
Apart from the metal active site and support, the influence of the catalyst mass is the least reported. The observed impact of the catalyst mass in the conversion and product distribution varies from one report to the next. Predominantly, the conversion increases with the catalyst mass. The influence of the mass of Pt–Re/TiO2 was investigated in the hydrogenation of hexanoic acid at 180 °C, 50 bar, and 125 mg hexanoic acid.106 Less than 10% hexanoic acid was converted over 3 mg catalyst, but it was fully converted when the catalyst addition was 18 mg. A significant impact of the catalyst mass was also observed in the hydrogenation of lauric acid over Cu/Al2O3.124 The reaction was performed at 330 °C for 3 h with 50 mg of lauric acid and catalyst mass was varied from 0–15 mg. Hexanoic acid was continuously converted from 15.6% to 98.9%. The same impact was observed in the hydrogenation of stearic acid over Ni/Al2O3,126 sunflower oil over CuZn,85 oleic acid over Co–CoOxAl2O3,81 and with Co catalyst.134
Nevertheless, the opposite effect was reported for methyl hexanoate hydrogenation over Ru–Sn–B/Al2O3.125 The impact of the catalyst amount was negligible even when the catalyst mass was increased fourfold. Notably, a remarkable decrease in hexanol selectivity from around 85% to 30% occurred when the catalyst mass was double. A decline in fatty alcohol selectivity was also found in the hydrogenation of stearic acid over Ni/Al2O3.126 The effect of the catalyst mass on stearyl alcohol selectivity was studied by altering the catalyst concentration from 0.1 to 0.75 (w/v%) under the following reaction conditions: concentration of stearic acid 0.018 mol, n-dodecane 100 ml, 270 °C, and 8 bar. Interestingly, heptadecane was the only product found with the 0.1 (w/v%) catalyst loading. The selectivity reached a maximum of 34.9% when the catalyst loading was doubled, but constantly decreased afterwards.
Fatty alcohol production involves many compounds, from the reactant and products, that can potentially deactivate the catalyst through poisoning, reoxidation, coking, and sintering. Deactivation of the catalyst may disturb the stability and the reusability, especially irreversible deactivation, which would permanently reduce the activity.
Some investigations of the catalyst stability have been reported by some studies. Takeda et al. assessed the stability of Re–Pd/SiO2 in the hydrogenation of stearic acid under the following conditions: reaction temperature 140 °C, pressure 80 bar, reaction time 1 h, catalyst amount 78.2 mg, stearic acid 0.7–1 g.135 The stability tests were performed with two different methods. First, the catalyst was dried in air for 12 h after separation from the reaction products by filtration and applied to the second reaction, followed by the third reaction afterwards. The result showed a significant decrease in catalytic activity with a conversion loss from 15% to 6%. The conversion rate also declined from 5.3 to 2.1 mmol gcat−1 h−1 while the stearyl alcohol selectivity remained stable at around 96–97%.
Second, the catalyst was separated by decantation after the reaction under nitrogen and applied to the second reaction straight away. The same procedure was repeated for the third reaction. Surprisingly, an insignificant loss was observed in the conversion, with a conversion of 18% for the first reaction and 16% for both the second and third reuse. The conversion rate was also slightly slower from 6 to 4.7 mmol gcat−1 h−1. Additionally, stearyl alcohol selectivity was also stable at 96–97%. These result imply that exposure of the catalyst to air induces the oxidation of Re active sites due to the high oxophicility of Re.
Excellent stability was also observed in NiFe catalysts applied in the hydrogenation of stearic acid at 250 °C and 50 bar for 2 h. For the sake of the reusability tests, the catalyst was separated by a permanent magnet and applied in several reaction cycles (Fig. 12). Obviously, it showed a slight loss in performance in the conversion of stearic acid and stearic alcohol selectivity. The XRD and XPS analyses indicated there were barely any changes in the spent catalyst compared to the catalyst in its original state.136 Similar results have been reported in other studies.88,107
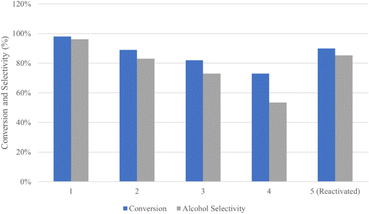 | ||
| Fig. 12 Reusability of the NiFe catalyst in several reaction cycles at 250 °C and under 50 bar for 2 h.136 | ||
Other catalyst have also exhibited excellent reusability for stearic acid hydrogenation at 235 °C and 30 bar for 2 h. The catalytic activity and selectivity to stearyl alcohol of Ni–MoOx/CeO2 decreased by 25% and 42%, respectively, after three recycling tests. ICP-OES-MS technique revealed the decreases in Ni and Mo species by 1.2 wt% and 1.9 wt% indicating the leaching of the metal species. XPS characterization confirmed that the metal species were oxidized after three cycles with lower amounts of Ni0 and higher amounts of Mo6+. Reactivating the spent catalyst improved the conversion and selectivity towards the alcohol product by 17% and 32%.109,137 Similar results were reported in another study.87
On the other hand, a significant loss of catalytic activity was observed in the reaction cycles for lauric acid hydrogenation over Cu/Al2O3.124 The yield of lauric acid remarkably dropped from 80% to 20% after the third reaction cycle. This trend was mirrored by the lauryl alcohol yield with a significant decline from 72.45% to 3% (Fig. 13). Unfortunately, insufficient data or further analysis was presented in the report to explain this phenomenon further.
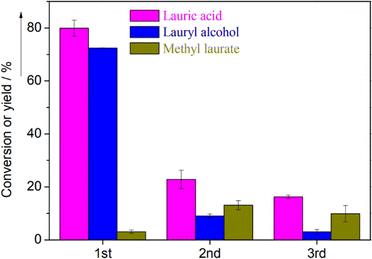 | ||
| Fig. 13 Conversion and product yields of lauric acid over Cu/Al2O3 after the first, second, and third catalyst use.124 | ||
Apart from the catalyst design, the reaction conditions significantly determine the conversion and fatty alcohol selectivity. It is well known that increasing the reaction temperature will enhance the reaction rate, leading to a higher conversion. On the other hand, thermodynamic studies have implied that the equilibrium constant of fatty alcohol formation decreases with the rise in temperature. Excess hydrogenation is an endothermic reaction, whereby higher temperature would accelerate the formation of by-products and lower the selectivity of fatty alcohols. Consequently, a compromise between the overall reaction rate and the selectivity of alcohols should be considered.
In terms of conversion, the temperature has been predominantly reported to have a positive impact. Kumar et al. reported that the reaction time significantly affected the hydrogenation of stearic acid over Ni/Al2O3 15 wt%.126 The reaction was conducted under the following reaction conditions: concentration of stearic acid 0.18 kmol m−3, n-dodecane 100 ml, hydrogen pressure 8 bar, catalyst loading 0.5 (w/v%), in the range of temperature from 260 °C to 290 °C. The smallest conversion of 66% was achieved after 6 h at 260 °C, and complete conversion was obtained with a shorter reaction time of 4 h at 290 °C. Similar results were mentioned for the preparation of oleyl alcohol from methyl oleate over CoSn/Al2O3.121 At temperatures of 240 °C, 270 °C, and 300 °C, it was observed that the conversion increased as a function of the reaction temperature. The increase in temperature from 240 °C to 270 °C caused a rise in methyl oleate conversion from 50% to almost 100%. Raising the temperature up to 300 °C made the time required to achieve complete conversion even shorter from 12 h to 8 h. The dependence of stearic acid conversion on the reaction temperature was also reported in the hydrogenation of oleic acid over Ru–Sn–B/Al2O3 at various temperature from 250 °C to 400 °C.112 Furthermore, the relationship between the conversion and reaction temperature was mentioned in stearic acid hydrogenation over Re/TiO2 (ref. 78) and Pt/TiO2,103 methyl laurate over Co/ZrO2,117 and palm ester over Re/Nb2O5 (ref. 138) and Re/Nb/Al2O3.108
Generally, the reaction temperature improves the reaction rate. Increasing the temperature from 180 °C to 220 °C enhanced the reaction rate from 0.07 to 0.39 mol g−1 h−1 in the hydrogenation of stearic acid over Re/TiO2, as observed in the first 30 min of reaction.78 A rate enhancement was also observed in the hydrogenation of stearic acid over 4% Pt/TiO2 at various temperature of 110 °C to 180 °C. Raising the temperature from 110 °C to 180 °C elevated the reaction rate from 0.0005 to 0.0025 mol g−1 h−1.103 A higher reaction rate, constant as a function of reaction time, was also found in the hydrogenation of stearic acid over Ni/Al2O3 in the range of temperature between 260–290 °C. About ten times an enhancement of the reaction rate constant was mentioned when the reaction temperature was raised from 260 °C to 290 °C.126
In contrast, the opposite behaviour was observed with the selectivity of fatty alcohol in the hydrogenation of oleic acid over Ru–Sn–B/Al2O3. For the purpose of comparison, the reaction temperature was altered from 250 °C to 400 °C under the same reaction conditions. The maximum alcohol selectivity of 45% was generated at 300 °C and constantly decreased until it was completely diminished at 400 °C.112 Notably, other reports also reported that the selectivity of alcohol started to decrease at temperatures above 300 °C. Above this point, the selectivity of alkane and wax ester became more favourable.114,122
The maximum temperature for the highest alcohol selectivity varied in some studies. A 90% stearyl alcohol selectivity was achieved at 200 °C with 80% conversion in the hydrogenation of stearic acid over Re/TiO2.78 A lower reaction temperature of 180 °C showed the maximum stearyl alcohol selectivity of 93%, yet at much smaller conversion of 30%. However, a lower reaction temperature has the consequence of a slow reaction rate and/or low conversion.
Hydrogen pressure crucially influences the product distribution as well as the reaction pathways. A high reaction pressure is required to drive the reaction in a hydrodeoxygenation route; whereas a lower hydrogen pressure initiates the formation of alkanes, as decarboxylation and/or decarbonylation become more favourable. High hydrogen coverage on the catalyst surface permits the hydride to insert into carboxylic acid group followed by water release. However, an excessively higher hydrogen pressure leads to excess hydrogenation.
In accordance with the reaction temperature, the hydrogen pressure was also taken into account for the conversion and reaction rate. Increasing the hydrogen pressure from 20 bar to 40 bar resulted in a rise in conversion from 40% to 70% in the hydrogenation of stearic acid over Re/TiO2, after only 30 min reaction.78 Additionally, the conversion of oleic acid over Ru–Sn–B/Al2O3 continuously increased as a function of hydrogen pressure. The variation of pressure from 17.3 to 70 bar raised oleic acid conversion from 30% to 75%.112 The dependence of the reaction rate and conversion on the hydrogen pressure was also reported in the hydrogenation of stearic acid over Re–Pd/SiO2 (ref. 89) and RuSn/N–C,105 oleic acid over CuIn/Al2O3 (with co catalyst In2O3)127 and NiFe/SiO2–ZrO2.130 On the other hand, the increase in methyl oleate conversion over CoSn/Al2O3 only occurred from 40 to 60 bar, and only a negligible change was observed when the pressure was increased to 100 bar.119
Hydrogen pressure plays a crucial role on fatty alcohol selectivity since an appropriate hydrogen amount should be available to drive the reaction to favour fatty alcohol. The hydrogen pressure was reported to maintain a high selectivity of stearyl alcohol in the hydrodeoxygenation of stearyl alcohol over ReO/TiO2.78 The highest stearyl alcohol selectivity of 93% was achieved at 20 bar with 80% conversion. The increase in pressure to 40 bar led to the complete conversion with the same stearyl alcohol selectivity. An insignificant addition of oleyl alcohol was noticed when the pressure was increased from 17.3 to 53 bar in the hydrogenation of methyl oleate.119 However, the oleyl alcohol started to decline beyond that point. The dependence of alcohol selectivity on the hydrogen pressure can be found in several studies.77,127,129
Furthermore, the hydrogen pressure also suppresses the formation of wax ester. The selectivity of heavy ester in the hydrogenation of oleic acid over Ru–Sn–B/Al2O3 was found to be constant at 25% in the range of hydrogen pressure of 17.3–53 bar.112 An increase in pressure from 53 to 70 bar inhibited the heavy ester formation for up to 15% selectivity. Therefore, the authors argued that the optimal hydrogen pressure was 35–50 bar in the respect of the fatty alcohol and wax ester formation. The inhibition of wax ester formation was also reported in the hydrogenation of stearic acid over Re/TiO2.78 The selectivity of stearyl stearate decreased as the pressure was increased from 20 to 40 bar. The same phenomenon was also found in the hydrogenation of methyl oleate.119 The selectivity of oleyl oleate declined when the pressure was constantly increased above 40 bar. Another study revealed a similar result.113
However, it has to be pointed out that the low formation of wax ester due to the increased pressure was often accompanied by the formation of alkanes. At higher pressure, the excess hydrogenation of alcohol was preferable compared to the esterification of alcohol with acids. The second explanation was that heavy ester can be hydrogenated to form alkane and alcohol, whereby higher pressure enhances the rate of these pathways compared to the wax ester formation.119
In summary, the catalyst and reaction conditions, including temperature and pressure, determine the reaction pathway and subsequent product distribution. An appropriate selection of the catalyst and the reaction conditions must be considered thoroughly in regards to the desired product, since the fatty alcohols are also the intermediates of green diesel formation, as described in more detail in the next sub section.
4. Life cycle assessment (LCA) and techno-economic analysis (TEA)
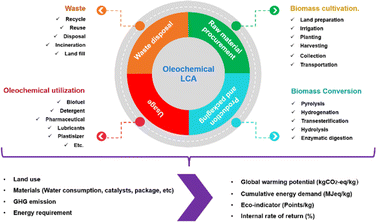
Life cycle assessment analyses the environmental impacts of a product in every stage (from cradle to grave), such as raw material procurement – processing, manufacturing – packaging, usage/application, and disposal. This assessment aims at excellent decision-making for the overall production process with ensuring effectives cost and meeting all regulatory/mandated requirements. The LCA method is standardized by the International Organization for Standardization (ISO) under code 14044-14073. This method comprises four steps: goal and scope definition, inventory analysis, impact analysis, and interpretation.139,140In general, the oleochemical life cycle consists of four stages with interconnected impacts on the environment and society (Fig. 14).141 The cycle starts with the raw material procurement through biomass cultivation. This stage involves land preparation, irrigation, planting, harvesting, and collection. The second stage is the production of oleochemicals through several reactions, such as pyrolysis, hydrogenation, transesterification, and hydrolysis. Afterward, the products pass through the packaging process and enter the usage phase (stage 3). Mostly, oleochemical product are widely applied in several industries as detergents, pharmaceuticals, cosmetics, lubricants, biofuel, etc. The waste produced throughout the lifecycle is treated in the last stage with several options possible depending on the type of the waste, including recycling or disposal in a regulated manner.
The LCA of oleochemicals determines the use of materials, land usage, energy consumption, waste production throughout the process and over the overall lifetime of the products.141 The environmental impact is rationalized using several metrics, including global warming potentials (kg CO2), cumulative energy demand, eco indicators, and internal rate of return (IRR).142,143 Fig. 15 describes the LCA metrics of oleochemicals for Kao (Japan) in 2023.144 The highest energy was required for the manufacturing process, and GHG emissions resulted from the material procurement, equating to 4103.9 thousand tons CO2.
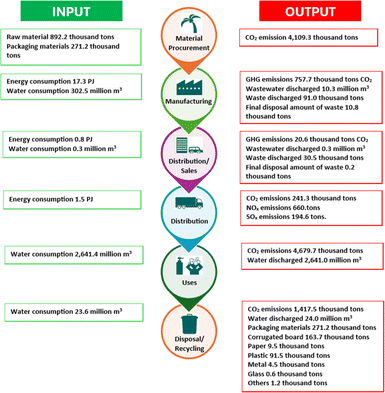 | ||
| Fig. 15 LCA metrics of oleochemicals by Kao.144 | ||
Several other LCA metrics by the oleochemical industries are mentioned in Table 6. As the highest oleochemical supplier in the world, Wilmar international consumes the highest energy and produces the most waste. Even though BASF came as the runner up, their GHG emissions were much higher than for Wilmar International. Meanwhile, Green Oleo exhibited a much lower scores in every metric due to their smaller production scale.
Apart from life cycle assessment, techno-economic analysis is also used to defines the feasibility of oleochemical production. This analysis plays a significant role in determining the opportunity to develop up to the industrial scale based up lab-scale or pilot-scale data.148 TEA is applied to bridge the technical perspective and economic feasibility involving the cash flow analysis, market analysis, environmental cost assessment, technology assessment, and sensitivity analysis.141 In detail, capital cashflow includes the scale, initial investment, operational investments, operational profit, and annual profit.142 TEA also considers several aspects of feedstock supply and logistics, the conversion process, use stage, market impacts, and relevant policies/regulations.148
The TEA evaluates the capital cost, operating cost, mass balance, and energy balance.141 The result focuses on the process optimization with certain concerns about the total cost of investment, annual operating cost, and minimum product selling price.148 The metrics of the TEA are based around the net present value (NPV), internal rate return (IRR), dynamic payback period, revenue, gross margin, return of investment, etc.142,143,148
Fig. 16 exhibits the TEA of the volatile fatty acid (VFA) production from food waste and grass through anaerobic digestion.149 This result represents the economic feasibility according a consideration of the NPV, revenue, capital expenditure (CapEx), operational expenditure (OpEx), and energy costs. In terms of profitability, it can be seen that the pilot scale was unable to provide a profitable system, as inferred by the NPV of less than zero, even though the theoretical yield confirmed otherwise. It was also claimed that the revenue and OpEx varied due to the raw material procurement, in which grass incurred a higher cost. Further analysis from this study implied that a 30% increase in the selling price and pilot-scale improvement would be beneficial to attain economic feasibility.
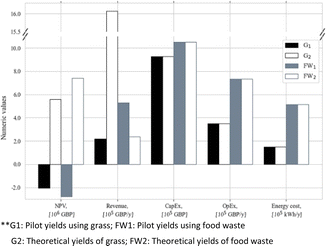 | ||
| Fig. 16 TEA result for volatile fatty acid production from food waste and grass through anaerobic digestion.149 | ||
5. Future outlook
Oleochemicals are among the most promising substitutes for petrochemicals on account of their functional efficiency, high performance, and sustainability. Since petrochemicals represent the third largest contributor to oil and gas demand, with 3.7 mb/d, the rapid development of oleochemicals by other means could be expected to reduce the global dependence on petrochemicals significantly.1The current development of oleochemicals has been studied in many outlook documents. The global markets of oleochemicals are spread out all over the world, as depicted in Fig. 17.150 This vast growing market of oleochemicals is supported by political, economic, social, technological, legal, and environment factors. The awareness of climate change and the demand for environmentally friendly products remain as the roots of this support. In 2023, the global market for oleochemicals reached USD 24.4 billion. This market was dominated by Asia-Pacific with a 41.5% revenue share due to the existence of the largest oleochemical producers in this region, i.e. Indonesia and Malaysia. The key players in the oleochemicals markets include Eastman Chemical Company, Kao Corporation, BASF SE, Wilmar International, Emery Oleochemicals, Oleon NV, KLK Oleo, Musim Mas Group, Croda, International, IOI Group, and Procter & Gamble Co.150–152
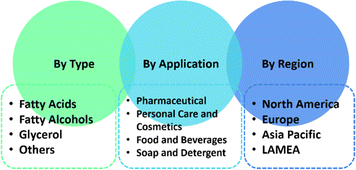
Fig. 18 elaborates the growing demand for oleochemicals by type, application, and region. In terms of the oleochemical types, fatty acids still dominate the market with over fatty alcohols, glycerol, fatty acid methyl ester, and others.151 Fatty acids contributed the highest share of 49% in 2023.152 Oleochemicals are mostly applied in pharmaceuticals, followed by personal care and cosmetics, food and beverages, as well as soaps and detergents. It has been claimed that North America is greatest consumer of oleochemicals, but other report mentioned that Asia-Pacific has both the highest production and highest consumption at the same time.150,151
Individually, several reports have stated there is a continuous increasing market demand for all oleochemicals. The fatty acids market size increased from USD 23.2 billion dollars in 2023 to 24.5 billion in 2024. This number is forecast to reach 34.2 billion dollars in 2032 with a compound annual growth rate (CAGR) of 5.73%. This increase is encouraged by the increasing demand for fatty acids for several applications, such as cosmetics, pharmaceuticals, as well as cleaning supplies, including soaps, detergents, bleaches, and cleaners.153 On the other hand, the global market for FAME was valued at USD 23.3 billion in 2024. Net zero emission policies have accelerated the demand for fatty acid methyl ester for biodiesel application. The promising properties of FAME also result in a wide range of uses in lubricants, coatings, food, and agriculture.154 The fatty alcohol market demand is the lowest one, amounting to USD 5.46 billion dollars in 2023 and estimated to reach 5.71 billion in 2024. This increase is especially driven by escalating demands from the detergents and cosmetics industries.155
On the forecast trajectory, overall the oleochemicals market is projected to reach 52.27 billion in 2031, with a predicted CAGR in the range of 7.5%. This value is supported by the swift growth in oleochemicals, especially fatty alcohol with a CAGR of 8.6%. The application of oleochemicals will be dominated by the personal care and cosmetics markets. In terms of producers, the same companies will maintain their position as the key players.1,150–152
Nevertheless, the success story of oleochemicals is still facing some challenges to address in both the short and long term. The main issue is oleochemical production still heavily depends on the feedstock price and availability. Palm oil is still the top feedstock, despite the environmental issues associated with palm plantation. The shift of feedstock to non-edible oil will ultimately need to be addressed. Another environmental concern highlights the inevitable demand to conduct more environmentally friendly processing. For instance, fatty alcohol production still relies on Cr-based catalysts, which can have adverse effects on the environment. To date, some studies have reported alternative catalysts to replace the current CuCr-based catalyst with excellent activity. However, the ideal catalyst has not been achieved yet, since the most developed catalyst is centred on the application of noble metals, which is undesirable from an economic point of view.
6. Conclusions
Biomass, especially natural oil, is a promising feedstock for petrochemical on account of its similar chemical structure with long-chain hydrocarbons, thus allowing creating a substitute product termed oleochemicals. The triglycerides in the natural oil can be transformed into three basic oleochemicals, namely fatty acids, fatty acid methyl ester (FAME), and fatty alcohols, through hydrogenation and transesterification, respectively. These basic oleochemicals are the feedstock of many important chemicals, such as fatty alcohol and green diesel. Fatty alcohol can be obtained from fatty acids, fatty acid methyl ester, and direct natural oil via deoxygenation involving several pathways, including hydrodeoxygenation, decarboxylation, and decarbonylation. Overall, the production of oleochemicals is significantly influenced by the catalyst design (active site, support, and properties) and reaction conditions (pressure, temperature, reaction time, etc.).Data availability
The authors confirm that the data supporting the findings of this study are available within the article. No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Author contributions
ZR (conceptualization, writing – original draft, writing – review & editing, funding acquisition); LS (methodology, validation, formal analysis, investigation, writing – original draft); WNWA (resources, visualization, writing – review & editing); YLN (investigation, visualization, writing – review & editing) AH (investigation, writing – original draft, writing – review & editing); NLAJ (investigation, writing – review & editing, resources), DS (project administration, investigation, writing – review & editing) AAW (supervision, conceptualization).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the Institut Teknologi Sepuluh Nopember for this work, under project scheme of the Publication Writing and IPR Incentive Program (PPHKI) 2024.References
- Y. Akizuki, A. Bressers, J. Couse, C. Healy, P. Mackey, D. Martin, J. Messing, J. Thomson, J. Canu, L. Fernando Rosa, T. Bosoni, K. Sadamori, D. Mooneesawmy, Y. Arsalane, A. Blasi, L. Cozzi, P. Frankl, T. Gould, P. Grimal, J. Hilaire, C. McGlade, J. Moorhouse, A. Petropoulos and G. Tonolo, Oil 2023: Analysis and Forecast to 2028, 2023 Search PubMed.
- A. T. Hoang, M. Tabatabaei, M. Aghbashlo, A. P. Carlucci, A. I. Ölçer, A. T. Le and A. Ghassemi, Renewable Sustainable Energy Rev., 2021, 135, 110204 CrossRef CAS.
- A. S. Carlsson, Biochimie, 2009, 91, 665–670 CrossRef CAS PubMed.
- M. Alkazimi, J. Spitzy, M. Benmerabet, R. Salo, N. Guerer, I. Etiobhio, J. Ban, E. Vafaie Fard, H. Aliefendic, M. Sugungun, J. Walker, M. Tallett, A. Bock-Butler, E. Kaditi, D. McKirdy, B. Baikalizadeh, B. AlSeiari, M. Zarie Zare, M. Mekerba, H. Hassani, H. Balfakeih, M. A. Danesh, Y. Sariahmed, A. Yahyai, P. Christodoulides, H. Eichner, R. Kammerer, K. Stoeger, M. Sattar, Z. Arifin, M. Mihnev and J. Pelenis, 2020 World Oil Outlook 2045, Vienna, Autria, 2020 Search PubMed.
- J. G. de Vries, Curr. Opin. Green Sustainable Chem., 2023, 39, 100715 CrossRef CAS.
- K. D. Maher and D. C. Bressler, Bioresour. Technol., 2007, 98, 2351–2368 CrossRef CAS PubMed.
- B. Liu and Z. Zhang, ACS Catal., 2016, 6, 326–338 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- S. Gundekari, J. Mitra and M. Varkolu, in Advanced Functional Solid Catalysts for Biomass Valorization, ed. C. Mustansar Hussain and P. Sudarsanam, Elsevier, 2020, pp. 89–120 Search PubMed.
- W. Deng, Y. Feng, J. Fu, H. Guo, Y. Guo, B. Han, Z. Jiang, L. Kong, C. Li, H. Liu, P. T. T. Nguyen, P. Ren, F. Wang, S. Wang, Y. Wang, Y. Wang, S. S. Wong, K. Yan, N. Yan, X. Yang, Y. Zhang, Z. Zhang, X. Zeng and H. Zhou, Green Energy Environ., 2023, 8, 10–114 CrossRef CAS.
- M. Bukowski, B. Swearingen and K. Vaiknoras, Oil Crops Outlook: May 2024, 2024 Search PubMed.
- S. Gharby, Sci. World J., 2022, 2022, 6627013 Search PubMed.
- C. Wen, M. Shen, G. Liu, X. Liu, L. Liang, Y. Li, J. Zhang and X. Xu, Process Biochem., 2023, 124, 168–179 CrossRef CAS.
- S. Manjula and R. Subramanian, Sep. Purif. Technol., 2009, 66, 223–228 CrossRef CAS.
- X. Liu, W. Wang, L. Xu, Z. Li, Q. Xu, B. Yang, D. Lan and Y. Wang, LWT-Ed., 2023, 185, 115126 CrossRef CAS.
- S. M. Ghazani and A. G. Marangoni, J. Am. Oil Chem. Soc., 2013, 90, 923–932 CrossRef CAS.
- W. Wang, B. Yang, F. Huang, C. Zheng, W. Li, T. Liu and C. Liu, LWT-Ed., 2022, 168, 113939 CrossRef CAS.
- S. Gharby, A. Hajib, M. Ibourki, E. H. Sakar, I. Nounah, H. EL Moudden, M. Elibrahimi and H. Harhar, Chem. Data Collect., 2021, 33, 100702 CrossRef CAS.
- M. K. Gupta, in Practical Guide to Vegetable Oil Processing, ed. M. K. Gupta, AOCS Press, 2nd edn, 2017, pp. 7–25 Search PubMed.
- M. J. A. Romero, D. Duca and G. Toscano, Processes, 2022, 47, 62–68 Search PubMed.
- B. Smith, H. C. Greenwell and A. Whiting, Energy Environ. Sci., 2009, 2, 262–271 RSC.
- L. Giraldo, G. Camargo, J. Tirano and J. C. Moreno-Piraján, J. Chem., 2010, 7, 439801 Search PubMed.
- M. A. Sánchez, G. C. Torres, V. A. Mazzieri and C. L. Pieck, J. Chem. Technol. Biotechnol., 2017, 92, 27–42 CrossRef.
- M. Ramos, A. P. S. Dias, J. F. Puna, J. Gomes and J. C. Bordado, Energies, 2019, 12, 4408 CrossRef CAS.
- F. Esmi, V. B. Borugadda and A. K. Dalai, Catal. Today, 2022, 404, 19–34 CrossRef CAS.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- K.-S. Chen, Y.-C. Lin, K.-H. Hsu and H.-K. Wang, Energy, 2012, 38, 151–156 CrossRef CAS.
- S. Janampelli and S. Darbha, Catal. Surv. Asia, 2019, 23, 90–101 CrossRef CAS.
- S. Z. Naji, C. T. Tye and A. A. Abd, Process Biochem., 2021, 109, 148–168 CrossRef CAS.
- A. V Yate, P. C. Narváez, A. Orjuela, A. Hernández and H. Acevedo, Food Bioprod. Process., 2020, 122, 72–81 CrossRef.
- W. Song, R. Apointe and M. Guo, in Practices and Perspectives in Sustainable Bioenergy: A Systems Thinking Approach, ed. M. Mitra and A. Nagchaudhuri, Springer India, New Delhi, 2020, pp. 57–73 Search PubMed.
- H. C. Black, W. S. Baldwin, H. Wittcoff, M. R. McCorkle, P. L. Du Brow, G. Zinzalian, R. D. Aylesworth, R. H. Dhonau, L. A. Stegemeyer and W. J. Shibe, Fatty Acids for Chemical Specialties, New York, 1955 Search PubMed.
- M. S. Gamal, N. Asikin-Mijan, W. N. A. W. Khalit, M. Arumugam, S. M. Izham and Y. H. Taufiq-Yap, Fuel Process. Technol., 2020, 208, 106519 CrossRef CAS.
- A. C. Rustan and C. A. Drevon, Encyclopedia of Life Sciences, John Wiley & Sons, Oslo, 2005, pp. 1–7 Search PubMed.
- E. Stavila, F. Yuliati, A. Adharis, J. A. Laksmono and M. Iqbal, RSC Adv., 2023, 13, 14747–14775 RSC.
- F. O. Nitbani, P. J. P. Tjitda, B. A. Nurohmah and H. E. Wogo, J. Oleo Sci., 2020, 69, 277–295 CrossRef CAS PubMed.
- N. Hongloi, P. Prapainainar and C. Prapainainar, Mol. Catal., 2022, 523, 111696 CrossRef CAS.
- F. O. Nitbani, P. J. P. Tjitda, H. E. Wogo and A. I. R. Detha, J. Oleo Sci., 2022, 71, 781–793 CrossRef CAS PubMed.
- D. Y. C. Leung, X. Wu and M. K. H. Leung, Appl. Energy, 2010, 87, 1083–1095 CrossRef CAS.
- C. V McNeff, L. C. McNeff, B. Yan, D. T. Nowlan, M. Rasmussen, A. E. Gyberg, B. J. Krohn, R. L. Fedie and T. R. Hoye, Appl. Catal., A, 2008, 343, 39–48 CrossRef.
- C. C. Cardoso, A. S. Cavalcanti, R. O. Silva, S. A. Junior, F. P. de Sousa, V. M. D. Pasa, S. Arias and J. G. A. Pacheco, J. Braz. Chem. Soc., 2020, 31, 756–767 CAS.
- J.-M. Jung, J.-I. Oh, D. Kwon, Y.-K. Park, M. Zhang, J. Lee and E. E. Kwon, Energy Convers. Manage., 2019, 195, 1–6 CrossRef CAS.
- S. Nisar, M. A. Hanif, U. Rashid, A. Hanif, M. N. Akhtar and C. Ngamcharussrivichai, Catalysts, 2021, 11, 1085 CrossRef CAS.
- A. Martínez, G. E. Mijangos, I. C. Romero-Ibarra, R. Hernández-Altamirano and V. Y. Mena-Cervantes, Fuel, 2019, 235, 277–287 CrossRef.
- F. Guo, Z.-G. Peng, J.-Y. Dai and Z.-L. Xiu, Fuel Process. Technol., 2010, 91, 322–328 CrossRef CAS.
- D. Y. C. Leung and Y. Guo, Fuel Process. Technol., 2006, 87, 883–890 CrossRef CAS.
- Y. Zhang, S. Niu, K. Han, Y. Li and C. Lu, Renewable Energy, 2021, 168, 981–990 CrossRef CAS.
- T. Eevera, K. Rajendran and S. Saradha, Renewable Energy, 2009, 34, 762–765 CrossRef CAS.
- T. Qu, S. Niu, Z. Gong, K. Han, Y. Wang and C. Lu, Renewable Energy, 2020, 159, 873–884 CrossRef CAS.
- Y. Xiao, Y. Liu, X. Zhang, J. Hou, X. Liu, Y. Yuan and X. Liao, Catal. Commun., 2022, 165, 106448 CrossRef CAS.
- Y. H. Taufiq-Yap, H. V Lee, M. Z. Hussein and R. Yunus, Biomass Bioenergy, 2011, 35, 827–834 CrossRef CAS.
- C. Ben-Youssef, A. Chávez-Yam, A. Zepeda, J. M. Rivera and S. Rincón, Int. J. Environ. Sci. Technol., 2021, 18, 3313–3326 CrossRef CAS.
- M. Kouzu, T. Kasuno, M. Tajika, Y. Sugimoto, S. Yamanaka and J. Hidaka, Fuel, 2008, 87, 2798–2806 CrossRef CAS.
- S. Shrivastava, P. Prajapati, Virendra, P. Srivastava, A. P. S. Lodhi, D. Kumar, V. Sharma, S. K. Srivastava and D. D. Agarwal, Ind. Crops Prod., 2023, 192, 116002 CrossRef CAS.
- J. Tantirungrotechai, S. Thepwatee and B. Yoosuk, Fuel, 2013, 106, 279–284 CrossRef CAS.
- C. Ngamcharussrivichai, P. Totarat and K. Bunyakiat, Appl. Catal., A, 2008, 341, 77–85 CrossRef CAS.
- C. C. C. M. Silva, N. F. P. Ribeiro, M. M. V. M. Souza and D. A. G. Aranda, Fuel Process. Technol., 2010, 91, 205–210 CrossRef CAS.
- A. C. Alba-Rubio, J. Santamaría-González, J. M. Mérida-Robles, R. Moreno-Tost, D. Martín-Alonso, A. Jiménez-López and P. Maireles-Torres, Catal. Today, 2010, 149, 281–287 CrossRef CAS.
- M. Kouzu, S. Yamanaka, J. Hidaka and M. Tsunomori, Appl. Catal., A, 2009, 355, 94–99 CrossRef CAS.
- Z. Kesica, I. Lukic, M. Zdujic, H. Liu and D. Skala, Procedia Eng., 2012, 42, 1169–1178 CrossRef.
- A. K. Endalew, Y. Kiros and R. Zanzi, Energy, 2011, 36, 2693–2700 CrossRef CAS.
- A. Patel, V. Brahmkhatri and N. Singh, Renewable Energy, 2013, 51, 227–233 CrossRef CAS.
- H. Wu, J. Zhang, Q. Wei, J. Zheng and J. Zhang, Fuel Process. Technol., 2013, 109, 13–18 CrossRef CAS.
- Y. Tang, J. Xu, J. Zhang and Y. Lu, J. Cleaner Prod., 2013, 42, 198–203 CrossRef CAS.
- A. P. S. Dias, J. Bernardo, P. Felizardo and M. J. N. Correia, Fuel Process. Technol., 2012, 102, 146–155 CrossRef CAS.
- H. Joshi, B. R. Moser, J. Toler and T. Walker, Biomass Bioenergy, 2010, 34, 14–20 CrossRef CAS.
- W. Xie and T. Wang, Fuel Process. Technol., 2013, 109, 150–155 CrossRef CAS.
- H. I. Mahdi, A. Bazargan, G. McKay, N. I. W. Azelee and L. Meili, Chem. Eng. Res. Des., 2021, 174, 158–187 CrossRef CAS.
- J. C. Serrano-Ruiz and J. A. Dumesic, Energy Environ. Sci., 2011, 4, 83–99 RSC.
- G. C. Li, P. Anastas and P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- D. S. Brands, E. K. Poels and A. Bliek, Appl. Catal., A, 1999, 184, 279–289 CrossRef CAS.
- S. M. Mudge, S. E. Belanger and A. M. Nielsen, Fatty Alcohols: Anthropogenic and Natural Occurrence in the Environment, The Royal Society of Chemistry, 2008 Search PubMed.
- F. Valoppi, S. Calligaris and A. G. Marangoni, in Edible Oleogels, ed. A. G. Marangoni and N. Garti, AOCS Press, 2nd edn, 2018, pp. 219–234 Search PubMed.
- E. F. Hill, G. R. Wilson and E. C. Steinle, Ind. Eng. Chem., 1954, 46, 1917–1921 CrossRef CAS.
- R. Sree, N. Seshu Babu, P. S. Sai Prasad and N. Lingaiah, Fuel Process. Technol., 2009, 90, 152–157 CrossRef CAS.
- Y. Zhou, J. Remón, Z. Jiang, A. S. Matharu and C. Hu, Green Energy Environ., 2023, 8, 722–743 CrossRef CAS.
- L. Wang, X. Niu and J. Chen, Appl. Catal., B, 2020, 278, 119293 CrossRef CAS.
- B. Rozmysłowicz, A. Kirilin, A. Aho, H. Manyar, C. Hardacre, J. Wärnå, T. Salmi and D. Yu. Murzin, J. Catal., 2015, 328, 197–207 CrossRef.
- D. S. Thakur and A. Kundu, J. Am. Oil Chem. Soc., 2016, 93, 1575–1593 CrossRef CAS.
- S. Yue, X. Ding, X. Liu, Y. Guo and Y. Wang, Catal. Today, 2022, 405–406, 221–226 CrossRef CAS.
- Y. Chen, Y. Zhang, W. Lin, X. Cheng, J. Wang, X. Liu, C. Charles Xu and R. Nie, Fuel, 2023, 345, 128136 CrossRef CAS.
- ChemAnalyst, Decode the Future of Fatty Alcohol, https://www.chemanalyst.com/industry-report/fatty-alcohol-market-634, accessed 5 August 2024.
- P. Munkajohnpong, C. Kesornpun, S. Buttranon, J. Jaroensuk, N. Weeranoppanant and P. Chaiyen, Biofuels, Bioprod. Biorefin., 2020, 14, 986–1009 CrossRef CAS.
- A. Zarli, in Studies in Surface Science and Catalysis, ed. A. Basile, G. Centi, M. De Falco and G. Iaquaniello, Elsevier, 2020, vol. 179, pp. 77–95 Search PubMed.
- A. Smirnov, W. Wang, O. Kikhtyanin, L. Xiao, W. Wu and D. Kubička, Catal. Today, 2023, 424, 113841 CrossRef CAS.
- P. Foley, A. Kermanshahi pour, E. S. Beach and J. B. Zimmerman, Chem. Soc. Rev., 2012, 41, 1499–1518 RSC.
- F. Long, S. Wu, Y. Chen, X. Cao, J. Zhao, P. Liu, J. Jiang, X. Zhang and J. Xu, Chem. Eng. J., 2023, 464, 142773 CrossRef CAS.
- L. M. Martínez-Prieto, M. Puche, C. Cerezo-Navarrete and B. Chaudret, J. Catal., 2019, 377, 429–437 CrossRef.
- Y. Takeda, M. Tamura, Y. Nakagawa, K. Okumura and K. Tomishige, ACS Catal., 2015, 5, 7034–7047 CrossRef CAS.
- L. C. T. Andrade, G. J. Muchave, S. T. A. Maciel, I. P. da Silva, G. F. da Silva, J. M. A. R. Almeida and D. A. G. Aranda, Int. J. Chem. Eng., 2022, 2022, 6402004 Search PubMed.
- O. Kikhtyanin, J. Aubrecht, V. Pospelova and D. Kubička, Catalysts, 2021, 11, 1417 CrossRef CAS.
- H. Adkins, K. Folkers, R. Connor and Madison, US Pat., 2091800, 1937, pp. 1–4 Search PubMed.
- R. D. Rieke, D. S. Thakur, B. D. Roberts and G. T. White, J. Am. Oil Chem. Soc., 1997, 74, 341–345 CrossRef CAS.
- S. van den Hark and M. Härröd, Appl. Catal., A, 2001, 210, 207–215 CrossRef CAS.
- S. van den Hark, M. Härröd and P. Møller, J. Am. Oil Chem. Soc., 1999, 76, 1363–1370 CrossRef CAS.
- K. Kandel, U. Chaudhary, N. C. Nelson and I. I. Slowing, ACS Catal., 2015, 5, 6719–6723 CrossRef CAS.
- K. G. Kanade, B. B. Kale, R. C. Aiyer and B. K. Das, Mater. Res. Bull., 2006, 41, 590–600 CrossRef CAS.
- F. T. Van De Scheur and L. H. Staala, Appl. Catal., A, 1994, 108, 63–83 CrossRef CAS.
- 5345005, 1994.
- X. Gou, F. Okejiri, Z. Zhang, M. Liu, J. Liu, H. Chen, K. Chen, X. Lu, P. Ouyang and J. Fu, Fuel Process. Technol., 2020, 205, 106426 CrossRef CAS.
- Y. Hattori, K. Yamamoto, J. Kaita, M. Matsuda and S. Yamada, J. Am. Oil Chem. Soc., 2000, 77, 1283–1288 CrossRef CAS.
- 6916457 B2, 2005.
- H. G. Manyar, C. Paun, R. Pilus, D. W. Rooney, J. M. Thompson and C. Hardacre, Chem. Commun., 2010, 46, 6279–6281 RSC.
- K. Ralphs, G. Collins, H. Manyar, S. L. James and C. Hardacre, ACS Sustain. Chem. Eng., 2022, 10, 6934–6941 CrossRef CAS.
- A. Ali, B. Li, Y. Lu and C. Zhao, Green Chem., 2019, 21, 3059–3064 RSC.
- K. Liu, J. Pritchard, L. Lu, R. van Putten, M. W. G. M. (Tiny) Verhoeven, M. Schmitkamp, X. Huang, L. Lefort, C. J. Kiely, E. J. M. Hensen and E. A. Pidko, Chem. Commun., 2017, 53, 9761–9764 RSC.
- A. Suknev, V. Zaikovskii, V. Kaichev, E. Paukshtis, E. Sadovskaya and B. Bal'Zhinimaev, J. Energy Chem., 2015, 24, 646–654 CrossRef.
- G. J. Muchave, J. M. A. R. Almeida and D. A. G. Aranda, Int. J. Sci. Res, 2022, 11, 516–531 Search PubMed.
- X. Cao, J. Zhao, F. Long, P. Liu, X. Jiang, X. Zhang, J. Xu and J. Jiang, Appl. Catal., B, 2022, 312, 121437 CrossRef CAS.
- T. Miyake, T. Makino, S. Taniguchi, H. Watanuki, T. Niki, S. Shimizu, Y. Kojima and M. Sano, Appl. Catal., A, 2009, 364, 108–112 CrossRef CAS.
- Y. Pouilloux, F. Autin, C. Guimon and J. Barrault, J. Catal., 1998, 176, 215–224 CrossRef CAS.
- V. O. Rodina, D. Yu. Ermakov, A. A. Saraev, S. I. Reshetnikov and V. A. Yakovlev, Appl. Catal., B, 2017, 209, 611–620 CrossRef CAS.
- M. J. Hidajat, G.-N. Yun and D.-W. Hwang, Mol. Catal., 2021, 512, 111770 CrossRef CAS.
- Rodiansono, H. P. Dewi, K. Mustikasari, M. D. Astuti, S. Husain and Sutomo, RSC Adv., 2022, 12, 13319–13329 RSC.
- C. A. Fonseca Benítez, V. A. Mazzieri, C. R. Vera, V. M. Benitez and C. L. Pieck, React. Chem. Eng., 2021, 6, 726–746 RSC.
- Z. Guo, F. Zhou, H. Wang, X. Liu, G. Xu, Y. Zhang and Y. Fu, Green Chem., 2019, 21, 5046–5052 RSC.
- Y. Zhou, L. Liu, G. Li and C. Hu, ACS Catal., 2021, 11, 7099–7113 CrossRef CAS.
- Y. Zhou, X. Liu, P. Yu and C. Hu, Fuel, 2020, 278, 118295 CrossRef CAS.
- Y. Pouilloux, F. Autin, A. Piccirilli, C. Guimon and J. Barrault, Appl. Catal., A, 1998, 169, 65–75 CrossRef CAS.
- K. De Oliveira Vigier, Y. Pouilloux and J. Barrault, Catal. Today, 2012, 195, 71–75 CrossRef CAS.
- Y. Pouilloux, F. Autin and J. Barrault, Catal. Today, 2000, 63, 87–100 CrossRef CAS.
- X. Cao, F. Long, G. Zhang, J. Xu and J. Jiang, ACS Sustain. Chem. Eng., 2021, 9, 9789–9801 CrossRef CAS.
- G. Busca, in Heterogeneous Catalytic Materials, ed. G. Busca, Elsevier, Amsterdam, 2014, pp. 297–343 Search PubMed.
- Z. Zhang, F. Zhou, K. Chen, J. Fu, X. Lu and P. Ouyang, Energy Fuels, 2017, 31, 12624–12632 CrossRef CAS.
- V. M. Deshpande, W. R. Patterson and C. S. Narasimhan, J. Catal., 1990, 121, 165–173 CrossRef CAS.
- P. Kumar, S. R. Yenumala, S. K. Maity and D. Shee, Appl. Catal., A, 2014, 471, 28–38 CrossRef CAS.
- G. Onyestyák, S. Harnos and D. Kalló, Catal. Commun., 2012, 26, 19–24 CrossRef.
- L. Zhou and A. Lawal, Appl. Catal., A, 2017, 532, 40–49 CrossRef CAS.
- J. Chen, D. Wang, F. Luo, X. Yang, X. Li, S. Li, Y. Ye, D. Wang and Z. Zheng, Fuel, 2022, 314, 122780 CrossRef CAS.
- F. Wang, S. Yu, H. Xu, J. Feng, F. Guo, X. Jiang and J. Jiang, Fuel, 2023, 345, 128170 CrossRef CAS.
- J. Ni, W. Leng, J. Mao, J. Wang, J. Lin, D. Jiang and X. Li, Appl. Catal., B, 2019, 253, 170–178 CrossRef CAS.
- B. Rozmysłowicz, P. Mäki-Arvela, A. Tokarev, A.-R. Leino, K. Eränen and D. Yu. Murzin, Ind. Eng. Chem. Res., 2012, 51, 8922–8927 CrossRef.
- E. W. Qian, N. Chen and S. Gong, J. Mol. Catal. A: Chem., 2014, 387, 76–85 CrossRef CAS.
- J. Wang, R. Nie, L. Xu, X. Lyu and X. Lu, Green Chem., 2019, 21, 314–320 RSC.
- Y. Takeda, Y. Nakagawa and K. Tomishige, Catal. Sci. Technol., 2012, 2, 2221–2223 RSC.
- X. Kong, Z. Fang, X. Bao, Z. Wang, S. Mao and Y. Wang, J. Catal., 2018, 367, 139–149 CrossRef CAS.
- X. Cao, F. Long, Q. Zhai, J. Zhao, J. Xu and J. Jiang, Fuel, 2021, 298, 120829 CrossRef CAS.
- G. J. Muchave, J. M. A. R. Almeida and D. A. G. Aranda, Int. J. Drug Dev. Res., 2022, 12, 56082–56089 Search PubMed.
- C. Watkins, P. G. Boakye, K. C. Jones, N. Latona, C. Liu, S. A. Besong, S. E. Lumor, V. T. Wyatt, K. E. Joseph, C. Krumm, P. Jimenez-penalver, X. Font, T. Gea, B. Dolman and J. Winterburn, IFORM: International News on Fats, Oils, and Related Materials, AOCS Press, 5th edn, 2017, vol. 28 Search PubMed.
- European Commission Joint Research Center, International Reference Life Cycle Data System (ILCD) Handbook: General Guide for Life Cycle Assessment - Provision and Action Steps, Publication Office of the European Union, Luxembourg, 1st edn, 2010 Search PubMed.
- A. I. Osman, B. Fang, Y. Zhang, Y. Liu, J. Yu, M. Farghali, A. K. Rashwan, Z. Chen, L. Chen, I. Ihara, D. W. Rooney and P. S. Yap, Environ. Chem. Lett., 2024, 22, 1115–1154 CrossRef CAS.
- S. Chen, H. Feng, J. Zheng, J. Ye, Y. Song, H. Yang and M. Zhou, Processes, 2020, 8, 122780 Search PubMed.
- J. L. Zheng, Y. H. Zhu, M. Q. Zhu, G. T. Sun and R. C. Sun, Green Chem., 2018, 20, 3287–3301 RSC.
- Sustainability Report 2023, Kao Group, Tokyo, 2023.
- Sustainability Report, Green Oleo S.p.A., Cremona, 2024.
- Annual Sustainability Report 2023, Wilmar International Limited, Singapore, 2024.
- G. U. dos Santos, R. Leenknecht and Z. M. Yang, BASF Sustainability Report, 2023, Ludwigshafen, 2024.
- C. D. Scown, N. R. Baral, M. Yang, N. Vora and T. Huntington, Curr. Opin. Biotechnol., 2021, 67, 58–64 CrossRef CAS PubMed.
- A. S. S. Pinto, L. J. McDonald, R. J. Jones, J. Massanet-Nicolau, A. Guwy and M. McManus, Bioresour. Technol., 2023, 388, 129726 CrossRef CAS PubMed.
- A. Analytica, Global Oleochemicals Market: Industry Dynamics, Market Size, and Opportunity Forecast 2023-2031, https://www.astuteanalytica.com/industry-report/oleochemicals-market, accessed 29 June 2023.
- S. Moon, A. Sayyad and E. Prasad, Oleochemical Market: Global Opportunity Analysis and Industry Forecast 2021-2030, Portland, 2023 Search PubMed.
- Oleochemicals Market Size, Share and Industry Analysis, by Type, Application, and Regional Forecast 2023-2030, San Fransisco, 2023 Search PubMed.
- P. Nandi, Global Fatty Acid Market Overview, New York, 2024 Search PubMed.
- Global Fatty Acid Methyl Ester Market 2024–2033, https://www.custommarketinsights.com/report/fatty-acid-methyl-ester-market/, accessed July, 2024.
- Fatty Alcohols Market by Source (Natural, synthetic), Type (Higher Chain, Long Chain, Pure, and Mid Cut), End-Use-Global Forecast 2024-2030, Tokyo, 2024.
| This journal is © The Royal Society of Chemistry 2024 |