Open Access Article
Open Access ArticleTsOH-catalyzed dehydroxylative cross-coupling of alcohols with phenols: rapid access to propofol derivatives†
Yuqiu Lianga,
Chengxiu Liua,
Youchun Li*b and
Lu Ouyang *a
*a
aSchool of Pharmacy, Gannan Medical University, Ganzhou 341000, Jiangxi Province, P. R. China. E-mail: oyl3074@163.com
bThe Affiliated Ganzhou Hospital, Jiangxi Medical College, Nanchang University, Ganzhou 341000, Jiangxi Province, P. R. China. E-mail: liyouchun2007@163.com
First published on 23rd August 2024
Abstract
Modification of the parent structure of molecules often alters their physicochemical properties and biological activities. Herein, a practical, efficient, and highly regioselective C–H alkylation of phenols with alcohols via dehydroxylative cross-coupling was developed to produce para-alkylated phenols with excellent regioselectivities and yields, using which propofol derivatives were rapidly synthesized. This process is performed under mild and simple conditions and is well-compatible with a variety of alcohols (secondary and tertiary benzylic alcohols as well as allyl alcohols) as alkylated agents. In addition, high aryl ether derivatives were also obtained using this catalytic system.
Phenols and their derivatives are vital compounds that not only serve as intermediates in organic synthesis but also act as core scaffolds in numerous natural products, pharmaceuticals, agrochemicals, and dyes.1 For instance, propofol is a typical example of phenols serving as an intravenous anesthetic, which is widely applied in anesthesia induction, anesthesia maintenance, ICU patient sedation, and epidural anesthesia.2 Propofol shows the advantages of rapid onset, high plasma clearance, rapid reduction of blood concentration and suitability for continuous infusion; however, the injection pain limits its clinical application. Modification of the parent structure of drugs often alters their physicochemical properties and biological activities. Therefore, structural modification of phenols and their derivatives for the rapid construction of complicated molecules could provide wide applications in organic synthesis, pharmaceutical chemistry, as well as new drug development.
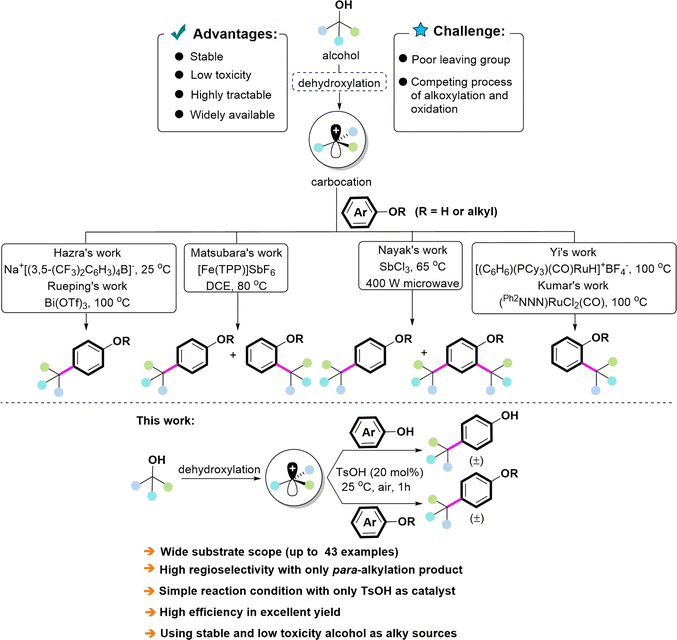
Dehydrative coupling of phenols and their derivatives is of great importance in the synthesis of organic molecules.3 Obviously, Friedel–Crafts alkylation of phenols and their derivatives is the most studied and classical procedure.4 In this case, alkyl halides were typically employed as electrophiles, which is in contrast with the principles of “green chemistry” (producing large amounts of waste and salt contaminants and requiring harsh reaction conditions). In addition, the formation of ortho-, para-, and poly-alkylated products hinders its application. Therefore, the development of a straightforward method for the selective alkylation of phenols and their derivatives that is highly efficient, economical, and environmentally friendly is highly desirable.
Carbon-based pro-electrophiles have been employed as alkylated sources due to the drawbacks of alkyl halides.5 In this content, alcohols, which are characterized by stability, wide availability, high tractability, and low toxicity, have been identified as excellent electrophile partners in coupling reactions.6 In this process, water is the only byproduct of the dehydration of alcohol, which is consistent with the requirements of green chemistry. A variety of catalytic systems for the activation of alcohols have been established for the alkylation of phenols and their derivatives.7 For instance, differential catalytic systems including SbCl3-microwave,8 Fe(TPP)SbF6,9 Bi(OTf)3,10 (Ph2NNN)RuCl2(CO),11 [(C6H6)(PCy3)(CO)RuH]+BF4−,12 and Na+[(3,5-(CF3)2C6H3)4B]− 13 have been developed for this purpose. Despite these advancements, the following challenges remain: (1) the hydroxyl group is a poor leaving group, which makes the activation of alcohols difficult. (2) The competing process of alkoxylation and oxidation hinders the application of alcohols in catalytic C–H coupling reactions. Therefore, the development of a concise and efficient catalytic system for the selective alkylation of phenols and aryl ethers using alcohols as the alkylated sources is still in demand.
With our research interest in alkylation, we developed alkylation reactions with carboxylic acid, alkynes, or ketones as alkylated sources and Cp*Ir complexes as catalysts.14 In continuation of our work on alkylation, we hereby disclose a TsOH-catalyzed dehydroxylative cross-coupling of alcohols with phenols by which only para-alkylated products were afforded in excellent yields (Scheme 1). This process performs under mild and simple conditions, and is well compatible with a variety of alcohols (secondary and tertiary benzylic alcohols, allyl alcohols) as alkylated agents. In addition, high-value aryl ether derivatives were also achieved via this catalytic system (Scheme 1).
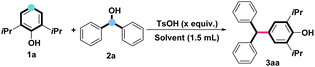
In pursuit of the optimal conditions for the dehydroxylative cross-coupling of alcohols with phenols, we started our research by using propofol (1a) and diphenylmethanol (2a) as a model reaction (Table 1). It is known that Lewis acid could activate the dehydroxylation of alcohols to generate carbon cation intermediates (See the ESI, Scheme S1†). According to our previous work,14 TsOH was chosen as the catalyst to realize the dehydroxylative cross-coupling of alcohols with phenols and their derivatives. Unsurprisingly, the alkylated product (3aa) of propofol was generated in the mixture solvent (p-xylene/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in 34% yield at 80 °C after 12 h (Table 1, entry 1). Screening of other solvents, such as 1,4-dioxane, TFEA, DMF, MeCN, p-xylene, and H2O (Table 1, entries 2–8), evidenced that TFEA was the optimal media to afford the corresponding product 3aa in 98% yield (Table 1, entry 3). Treatment of 1a and 2a using differential loadings of TsOH delivered similar yields of the desired product (Table 1, entries 9–12). The control experiment indicated the TsOH was essential for this transformation (Table 1, entry 13). Further loading of alcohol screening revealed that 1a could be fully converted into the desired alkylated product 3aa even if 1.0 equivalent of 2a was employed (Table 1, entry 18). It is interesting to note that high yields of 3aa could also be achieved by lowering the reaction temperature or shortening the reaction time (Table 1, entries 19–25).
4) in 34% yield at 80 °C after 12 h (Table 1, entry 1). Screening of other solvents, such as 1,4-dioxane, TFEA, DMF, MeCN, p-xylene, and H2O (Table 1, entries 2–8), evidenced that TFEA was the optimal media to afford the corresponding product 3aa in 98% yield (Table 1, entry 3). Treatment of 1a and 2a using differential loadings of TsOH delivered similar yields of the desired product (Table 1, entries 9–12). The control experiment indicated the TsOH was essential for this transformation (Table 1, entry 13). Further loading of alcohol screening revealed that 1a could be fully converted into the desired alkylated product 3aa even if 1.0 equivalent of 2a was employed (Table 1, entry 18). It is interesting to note that high yields of 3aa could also be achieved by lowering the reaction temperature or shortening the reaction time (Table 1, entries 19–25).
| Entry | TsOH (equiv.) | 2a (equiv.) | Solvent | Reaction time | Yield of 3aa b (%) |
|---|---|---|---|---|---|
a Reaction condition: 1a (0.5 mmol), and solvent (1.5 mL) under air condition at 80 °C for 12 h.b Yield was determined by NMR with dimethyl terephthalate as internal standard.c The ratio of the mixed solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v).d 25 °C.e 40 °C.f 60 °C. 4 (v/v).d 25 °C.e 40 °C.f 60 °C. |
|||||
| 1c | 1.0 | 2.0 | p-Xylene/H2O | 12 h | 34 |
| 2 | 1.0 | 2.0 | 1,4-Dioxane | 12 h | 92 |
| 3 | 1.0 | 2.0 | TFEA | 12 h | 98 |
| 4 | 1.0 | 2.0 | DMF | 12 h | n.r |
| 5 | 1.0 | 2.0 | MeCN | 12 h | 92 |
| 6c | 1.0 | 2.0 | TFEA/H2O | 12 h | 68 |
| 7 | 1.0 | 2.0 | p-Xylene | 12 h | 96 |
| 8 | 1.0 | 2.0 | H2O | 12 h | 22 |
| 9 | 0.8 | 2.0 | TFEA | 12 h | 97 |
| 10 | 0.6 | 2.0 | TFEA | 12 h | 97 |
| 11 | 0.4 | 2.0 | TFEA | 12 h | 98 |
| 12 | 0.2 | 2.0 | TFEA | 12 h | 98 |
| 13 | — | 2.0 | TFEA | 12 h | n.r. |
| 14 | 0.2 | 1.8 | TFEA | 12 h | 96 |
| 15 | 0.2 | 1.6 | TFEA | 12 h | 99 |
| 16 | 0.2 | 1.4 | TFEA | 12 h | 98 |
| 17 | 0.2 | 1.2 | TFEA | 12 h | 97 |
| 18 | 0.2 | 1.0 | TFEA | 12 h | 98 |
| 19d | 0.2 | 1.0 | TFEA | 12 h | 98 |
| 20e | 0.2 | 1.0 | TFEA | 12 h | 97 |
| 21f | 0.2 | 1.0 | TFEA | 12 h | 99 |
| 22d | 0.2 | 1.0 | TFEA | 10 min | 90 |
| 23d | 0.2 | 1.0 | TFEA | 20 min | 92 |
| 24d | 0.2 | 1.0 | TFEA | 30 min | 95 |
| 25d | 0.2 | 1.0 | TFEA | 1 h | 99 |
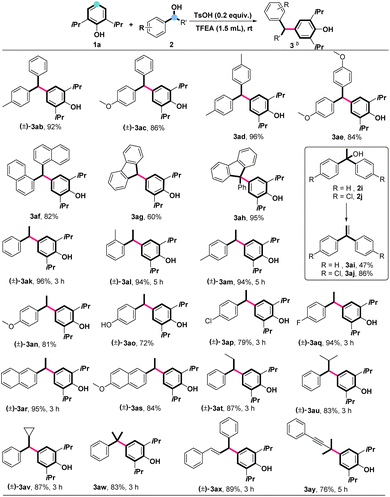
With the optimal conditions in hand, we investigated the alcohol scope in conjunction with propofol (Scheme 2). As shown in Scheme 2, a wide range of substituted secondary diaryl alcohols, including electron-donating groups of methyl and methoxy, proved to be suitable as alkylated sources, delivering the desired products (3ab–3ae) in excellent yields. Notably, larger steric hindrance of 1-naphthyl alcohol could also undergo this dehydroxylative cross-coupling to afford product 3af in 82% yield. Interestingly, using 9H-fluoren-9-ols as alkylated sources could also provide the corresponding products 3ag and 3ah in yields of 60% and 95%, respectively. However, no desirable alkylated products but 1,1-diarylethenes were formed in moderate to good yields (3ai and 3aj) when diaryl tertiary alcohols (2i, 2j) were loaded as alkylated sources under standard conditions. Importantly, alkylated agents of 1-arylethanol were also surveyed. Regardless of the positions and substituents of the alcohols, a wide range of 1-arylethanols was compatible with this catalytic system, affording the corresponding products 3ak–3as in excellent yields. Similarly, other substrates of 1-phenyl alkyl alcohols were also amenable to the catalytic system, furnishing 3at–3aw in 83–87% yields. Again, substrates of enol and alkynyl alcohol were compatible with the standard conditions to give the desirable products 3ax and 3ay in excellent yields.
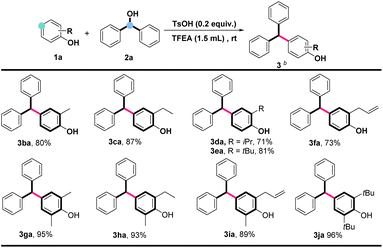
The promising results showcased above motivated us to further explore the dehydroxylative cross-coupling of diphenylmethanol (2a) with general phenols. As indicated in Scheme 3, a range of substituted phenols, including o-methyl (2b, 2g), o-ethyl (2c, 2h), o-isopropyl (2d), o-tertiary (2e) substituted phenols, were subjected under aforementioned optimal conditions to deliver desired products (3ba–3ea, 3ga and 3ha) in excellent yields. Apparently, the sensitive functional group of allyls on phenol has no influence on the yield of the products, affording 3fa and 3ia in 73% and 89%, respectively. Moreover, a substrate with a larger hindrance of 2,6-di-tert-butylphenol could also be compatible with this system to afford the desired product 3ja in excellent yield, indicating good substrate versatility.
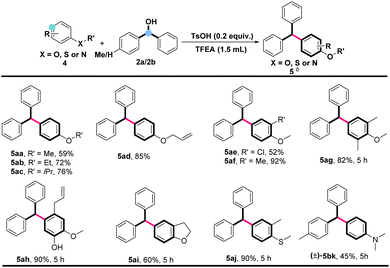
The substrate scope of this established methodology was also investigated with various aryl ethers. As shown in Scheme 4, a variety of substituted anisoles were alkylated with diphenylmethanol (2a) under standard conditions to produce the corresponding products (5aa–5ac, 5ae–5ah) in excellent yields. Allyl phenyl ether (4d), which contains an unsaturated double bond, was employed under standard conditions and delivered 5d in 85% yield. Additionally, alkylation of benzo-tetrahydrofuran could also take place to give product 5ai in 60% yield. Interestingly, using thioether as a substrate exhibited similar excellent results to furnish product 5aj in 90% yield. In addition, Electron-rich arylamine of N,N-dimethylaniline was also compatible with this catalytic system, giving the desired product in moderate yield (5bk).
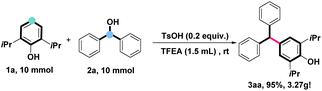
To demonstrate the synthetic utility of this dehydroxylative cross-coupling protocol, the gram-scale experiment was performed (Scheme 5). The model reaction of propofol (1a) and diphenylmethanol (2a) could be conducted on a 10.0 mmol scale, giving 3.27 grams of 3aa in the yield of 95%, showing promising prospects in industrial production.
Conclusions
In conclusion, a practical and efficient dehydroxylative cross-coupling of alcohols with phenols and their derivatives was developed to produce para-alkylated phenols/aryl ethers in excellent regioselectivities and yields and propofol derivatives could be rapidly accessed. This process was performed under mild and with only TsOH as a catalyst, is well compatible with a variety of alcohols (secondary and tertiary benzylic alcohols, allyl alcohols) as alkylated agents. In addition, high-value aryl ether derivatives were also furnished using this catalytic system. Studies on the physicochemical properties and biological activities of propofol derivatives are in progress and will be reported soon.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Yuqiu Liang, and Chengxiu Liu: investigation, data curation, validation, visualization, manuscript, and supplementary information writing and editing. Youchun Li and Lu Ouyang: conceptualization, funding acquisition, project administration, resources, supervision, visualization, revising the manuscript and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ganzhou Bureau of Science and Technology (2022-YB1402), the Fundamental Research Funds for Gannan Medical University (TD2021YX05) for financial support.Notes and references
- (a) K. A. Scott, P. B. Cox and J. T. Njardarson, Phenols in Pharmaceuticals: Analysis of a Recurring Motif, J. Med. Chem., 2022, 65, 7044–7072 CAS; (b) W. Zhao, B. Wang, Y. Liu, L. Fu, L. Sheng, H. Zhao, Y. Lu and D. Zhang, Design, synthesis, and biological evaluation of novel 4Hchromen-4-one derivatives as antituberculosis agents against multidrug-resistant tuberculosis, Eur. J. Med. Chem., 2020, 189, 112075 Search PubMed; (c) E. E. A. Osman, N. S. Hanafy, R. F. George and S. M. El-Moghazy, Design and synthesis of some barbituric and 1,3-dimethylbarbituric acid derivatives: A non-classical scaffold for potential PARP1 inhibitors, Bioorg. Chem., 2020, 104, 104198 Search PubMed.
- S. H. Lee, S. E. Lee, S. Chung, H. J. Lee and S. Jeong, Impact of time interval between remifentanil and propofol on propofol injection pain, J. Clin. Anesth., 2016, 34, 510–515 CrossRef CAS PubMed.
- (a) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Transition-Metal-Catalyzed C-H Alkylation Using Alkenes, Chem. Rev., 2017, 117, 9333–9403 CAS; (b) Z.-Y. Tian, Y. Ma and C.-P. Zhang, Alkylation Reactions with Alkylsulfonium Salts, Synthesis, 2022, 54, 1478–1502 CrossRef CAS; (c) S. B. Ankade, A. B. Shabade, V. Soni and B. Punji, Unactivated Alkyl Halides in Transition-Metal-Catalyzed C-H Bond Alkylation, ACS Catal., 2021, 11, 3268–3292 CrossRef CAS.
- (a) C. Friedel and J. M. Crafts, A new general synthetical method of producing hydrocarbons, J. Chem. Soc., 1877, 32, 725–791 Search PubMed; (b) R. M. Roberts and A. A. Khalaf, Friedel-Crafts Alkylation Chemistry. A Discovery, Marcel Dekker, New York, 1984 Search PubMed; (c) M. Rueping and B. J. Nachtsheim, A Review of New Developments in the Friedel-Crafts Alkylation-From green chemistry to asymmetric catalysis, Beilstein J. Org. Chem., 2010, 6, 6 CrossRef PubMed.
- (a) X.-H. Chen, T. Lhazom and H.-L. Cui, NH4OAc/DMSO-Promoted Benzylation of Pyrrolo[2,1-a]isoquinolines, J. Org. Chem., 2023, 88, 13598–13609 CrossRef CAS PubMed; (b) R.-J. Tang, T. Milcent and B. Crousse, Bisulfate Salt-Catalyzed Friedel–Crafts Benzylation of Arenes with Benzylic Alcohols, J. Org. Chem., 2018, 83, 14001–14009 CrossRef CAS PubMed; (c) R. Liu, Y. Wei and M. Shi, Rhodium(III)-Catalyzed C-H Benzylation of Indole's C3 Position with Aza-o-Quinone Methides, Adv. Synth. Catal., 2020, 362, 3649–3654 CrossRef CAS; (d) X. Li, Y. Mao, P. Fan and C. Wang, Nickel/Photo-Cocatalyzed Acyl C-H Benzylation of Aldehydes with Benzyl Chlorides, Eur. J. Org Chem., 2022, 2022, e202200214 CrossRef CAS; (e) G. Schäfer and J. W. Bode, Friedel-Crafts benzylation of activated and deactivated arenes, Angew. Chem., Int. Ed., 2011, 50, 10913–10916 CrossRef PubMed; (f) K. J. Schwarz, C. Yang, J. W. Fyfe and T. N. Snaddon, Enantioselective α-Benzylation of Acyclic Esters Using π-Extended Electrophiles, Angew. Chem., Int. Ed., 2018, 57, 12102–12105 CrossRef CAS PubMed.
- (a) M. Dryzhakov, E. Richmond and J. Moran, Recent Advances in Direct Catalytic Dehydrative Substitution of Alcohols, Synthesis, 2016, 48, 935–959 CrossRef CAS; (b) P. H. Huy, Lewis Base Catalysis Promoted Nucleophilic Substitutions-Recent Advances and Future Directions, Eur. J. Org Chem., 2020, 2020, 10–27 CrossRef CAS; (c) S. Estopiná-Durán and J. E. Taylor, Brønsted Acid-Catalysed Dehydrative Substitution Reactions of Alcohols, Chem. Eur. J., 2021, 27, 106–120 CrossRef PubMed; (d) H. Li, A. Riisager, S. Saravanamurugan, A. Pandey, R. S. Sangwan, S. Yang and R. Luque, Carbon-Increasing Catalytic Strategies for Upgrading Biomass into Energy-Intensive Fuels and Chemicals, ACS Catal., 2018, 8, 148 CrossRef CAS; (e) J. Huang, J. Chen, Z. Huang, T. Liu, Y. Ding, H. Li and C. He, Solventless autothermic production of energy-intensive furanic biofuels expedited by photothermal effect, Fuel, 2024, 359, 130458 CrossRef CAS; (f) H. Li, S. Saravanamurugan, S. Yang and A. Riisager, Catalytic Alkylation of 2-Methylfuran with Formalin Using Supported Acidic Ionic Liquids, ACS Sustainable Chem. Eng., 2015, 3, 3274 CrossRef CAS.
- (a) M. Noji, T. Ohno, K. Fuji, N. Futaba, H. Tajima and K. Ishii, Secondary Benzylation Using Benzyl Alcohols Catalyzed by Lanthanoid, Scandium, and Hafnium Triflate, J. Org. Chem., 2003, 68, 9340–9347 CrossRef CAS PubMed; (b) K. Mertins, I. Lovel, J. Kischel, A. Zapf and M. Beller, Gold-Catalyzed Benzylation of Arenes and Heteroarenes, Adv. Synth. Catal., 2006, 348, 691–695 CrossRef CAS; (c) J. Liu, E. Muth, U. Florke, G. Henkel, K. Merz, J. Sauvageau, E. Schwake and G. Dyker, Alkylation of Arenes with Benzylic and Propargylic Alcohols-Classical versus Fancy Catalysts, Adv. Synth. Catal., 2006, 348, 456–462 CrossRef CAS; (d) S.-S. Meng, X. Tang, X. Luo, R. Wu, J.-L. Zhao and A. S. C. Chan, Borane Catalyzed Chemoselectivity-Controllable N-alkylation and ortho C-alkylation of Unprotected Arylamines Using Benzylic Alcohols, ACS Catal., 2019, 9, 8397–8403 CrossRef CAS; (e) S. Estopina-Duran, E. B. Mclean, L. J. Donnelly, B. M. Hockin and J. E. Taylor, Arylboronic Acid Catalyzed C-Alkylation and Allylation Reactions Using Benzylic Alcohols, Org. Lett., 2020, 22, 7547–7551 CrossRef CAS PubMed.
- P. Shukla, M. K. Choudhary and S. K. Nayak, Microwave-Accelerated Alkylation of Arenes/Heteroarenes with Benzylic Alcohols Using Antimony(III) Chloride as Catalyst: Synthesis of O-Heterocycles, Synlett, 2011, 11, 1585–1591 Search PubMed.
- S. Teranishi, T. Kurahashi and S. Matsubara, Cationic Iron(III) Porphyrin Catalyzed Dehydrative Friedel-Crafts Reaction of Alcohols with Arenes Dehydrative Friedel–Crafts Reaction, Synlett, 2013, 24, 2148–2152 CrossRef CAS.
- M. Rueping, B. J. Nachtsheim and W. Ieawsuwan, An Effective Bismuth-Catalyzed Benzylation of Arenes and Heteroarenes, Adv. Synth. Catal., 2006, 348, 1033–1037 CrossRef CAS.
- K. Das, E. Yasmin and A. Kumar, Pincer-Ruthenium Catalyzed Oxygen Mediated Dehydrative Etherification of Alcohols and Ortho-Alkylation of Phenols, Adv. Synth. Catal., 2022, 364, 3895–3909 CrossRef CAS.
- N. Yadav, J. Khan, A. Tyagi, S. Singh and C. K. Hazra, Rapid Access to Arylated and Allylated Cyclopropanes via Brønsted Acid-Catalyzed Dehydrative Coupling of Cyclopropylcarbinols, J. Org. Chem., 2022, 87, 6886–6901 CrossRef CAS PubMed.
- D.-H. Lee, K.-H. Kwon and C.-S. Yi, Dehydrative C-H Alkylation and Alkenylation of Phenols with Alcohols: Expedient Synthesis for Substituted Phenols and Benzofurans, J. Am. Chem. Soc., 2012, 134, 7325–7328 CrossRef CAS PubMed.
- (a) L. Ouyang, R. Miao, Z. Yang and R. Luo, Iridium-catalyzed Reductive Amination of Carboxylic Acids, J. Catal., 2023, 418, 283–289 CrossRef CAS; (b) L. Ouyang, Y. Liang, S. Wang, R. Miao, J. Liao, Z. Yang and R. Luo, Iridium-Catalyzed Reductive Hydroamination of Terminal Alkynes in Water, J. Catal., 2023, 427, 115096 CrossRef CAS; (c) L. Ouyang, Y. Liang, S. Wang, J. Liao and R. Luo, Access of arylmethanes via iridium-catalyzed deoxygenative cross-coupling of aryl ketones with anilines/phenols, J. Catal., 2024, 433, 115492 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04674a |
| This journal is © The Royal Society of Chemistry 2024 |