Open Access Article
Open Access ArticleEffect of diammonium hydrogen phosphate coated with silica on flame retardancy of epoxy resin
Chao Liu ab,
Lixin Wei*b,
Boyu Mac,
Yuanping Zhang
ab,
Lixin Wei*b,
Boyu Mac,
Yuanping Zhang c and
Xinlei Jiad
c and
Xinlei Jiad
aHeilongjiang Provincial Key Laboratory of Oilfield Applied Chemistry and Technology, Daqing Normal University, Daqing, 163712, China
bKey Laboratory of Enhanced Oil Recovery, Northeast Petroleum University, Ministry of Education, Daqing, 163318, China. E-mail: weilixin73@163.com
cSchool of Coal Engineering, Shanxi Datong University, Datong, 037003, China
dCollege of Chemical Engineering and Safety, Shandong University of Aeronautics, Binzhou, 256603, China
First published on 12th September 2024
Abstract
Epoxy resin has become one of the most widely used polymers owing to their excellent comprehensive properties. However, the inherent inflammability of epoxy resin (EP) has seriously limited its application in a range of fields with high fire safety requirements. Herein, a novel shell–core hierarchy architecture (DAP@SiO2) was prepared, composed of diammonium hydrogen phosphate (DAP) and in situ grown silica, and its structure and morphology were characterized by electron microscopy and infrared spectroscopy. The results indicated that silica particles were uniformly coated onto the surface of DAP. The modified DAP was used to reinforce the epoxy resin. The thermal stability of the EP blends was studied with the use of thermogravimetric analysis. The diammonium phosphate in DAP@SiO2 flame retardant produces pyrolysis gases such as NH3 and N2 during decomposition, diluting the concentration of oxygen and flammable volatile products around the flame. Secondly, silica migrates to the surface of epoxy resin to form a shielding layer, forming compounds containing Si–O–Si and O–Si–C structures, which can be cross-linked with other condensed phase products, greatly improving the thermal stability of the carbon layer. Fire behavior was evaluated using the limiting oxygen index (LOI), vertical burning test, and the cone calorimetry, and the flame retardancy mode of action was explained. With 12% of DAP@SiO2 involved, the EP blend passes UL-94 V-0 level, and its limiting oxygen index (LOI) reaches 33.2%. The incorporation of DAP@SiO2 in an EP matrix showed a slight reduction in the heat release and smoke production. The flame retardant mode of the EP polymer shows that its flame retardant and smoke suppression characteristics are related to the interaction of flame retardants in the gas phase and condensed phase. The mechanical properties test results illustrated that the tensile strength, elastic modulus and impact strength of EP/3%DAP@SiO2 are improved compared with pure EP. This is due to the crosslinking reaction between a large number of amino groups on the surface of DAP@SiO2 and the epoxy group on the epoxy resin, which significantly enhances the interfacial compatibility between DAP@SiO2 and epoxy resin, making the combination of DAP@SiO2 and epoxy resin closer.
1. Introduction
As a commonly used thermosetting polymer, epoxy resin (EP) has been widely used in various fields due to its high temperature resistance, low shrinkage on curing, strong corrosion resistance, enhanced mechanical properties, and excellent electrical insulation.1–3 However, EP has the characteristics of high flammability and releases a lot of smoke and toxic gaseous substances during combustion, which restricts its application in the fields of electronic devices, building materials and aviation.4 Many studies have reported improvements through different strategies, but in the process of improvement, flame retardants may have a negative impact on the mechanical properties and other properties of the material while improving the flame retardancy. First, the introduction of nanoparticles or flakes into the polymer matrix is a widely used strategy to improve the flame retardancy of materials. Mira Park et al.5 explored the method of preparing a fly ash/polystyrene composite nanofiber membrane by using waste polystyrene (PS) and fly ash (FA) particles, and evaluated the potential of the material as a flame retardant material. Secondly, adding traditional halogen-based flame retardants to epoxy resin is an effective way for improving their flame retardancy. Nevertheless, the damage to humans and environment caused by the halogen-containing flame retardants have gradually aroused attention, and the research of halogen-free flame retardants in epoxy resins has become a future development trend.6 In recent years, phosphorus-containing compounds have gradually attracted extensive attention of researchers because they meet the environmental protection requirements of flame retardants. Diammonium hydrogen phosphate (DAP) is a phosphorus–nitrogen compound that can be used as both acid source and foaming agent in polymer flame retardancy. Moreover, DAP has the characteristics of small smoke density, low toxicity, and high flame retardancy efficiency. Son et al.7 introduced diammonium hydrogen phosphate flame retardant into polyurethane/rice husk (PUF–RH) composites. Up to now, there is no report about the use of diammonium hydrogen phosphate and its modified products to improve the flame retardancy of epoxy resins. However, enhancing the flame retardant properties of polymers solely through the addition of specific flame retardant elements often yields subtle improvements. The integration of different flame retardant elements to create a novel flame retardant system can effectively meet the stringent demands for enhanced flame resistance. The performance of DAP flame retardants can be better enhanced by the cooperative effect of other elements.The silicon-based additives have attracted much attention since they exhibit good fire performance for various polymer materials. Silica, as an inorganic and environmental friendly fillers, have the advantages of non-toxic, chemically inert, excellent thermal stability and strong hardness, and show obvious flame retardant effect in the condensed phase.8,9 The silicon-containing components migrate and accumulate on the surface of the combustion polymer, and the silicon protective layer formed on the surface prevents further degradation of the sample. At present, silica has absorbed an increasing attention as an attractive and promising material to prepare the efficient flame retardants. Importantly, related studies have demonstrated that there is a good cooperative flame retardant effect between nitrogen, phosphorus and silicon elements. Xu et al.10 studied the flame retardance of polyvinyl alcohol (PVA) modified with silicon dioxide-coated h-BN (SiO2@BN) hybrid and guanidine phosphate (GP), and proved that char residue of PVA composites increased significantly by the incorporation of SiO2@BN and GP. When the contents of SiO2@BN and GP were 2 wt% and 8 wt%, respectively, the LOI reached 28.4%. The pHRR and THR values of modified PVA were decreased by 65.2% and 44.4%. Zhou et al.11 synthesized an amino-silica functionalized triazine-rich polyphosphazene tube (MTP@NH2–SiO2), and introduced them into the EP system. The experimental results showed that the addition of 1.5 wt% MTP@NH2–SiO2 could effectively improve the flame retardancy and smoke suppression of the EP composites. Micro/nano particles with core/shell architecture offer a wide range of applications in healthcare, food, construction, environment and other fields.12 Recently, micro/nano particle technology has also been applied in the resistance of the composite to flame growth, and the addition of encapsulated flame retardants to flammable polymer materials significantly reduces the fire risk.13 Flame retardants coated with inorganic shell materials can better isolate heat transfer and inhibit smoke release compared to conventional flame retardants.14 Based on the research mentioned above, it is reasonable and imperative to design a phosphorus–nitrogen–silicon cooperative flame retardant system through the introduction of silicon to diammonium hydrogen phosphate system.
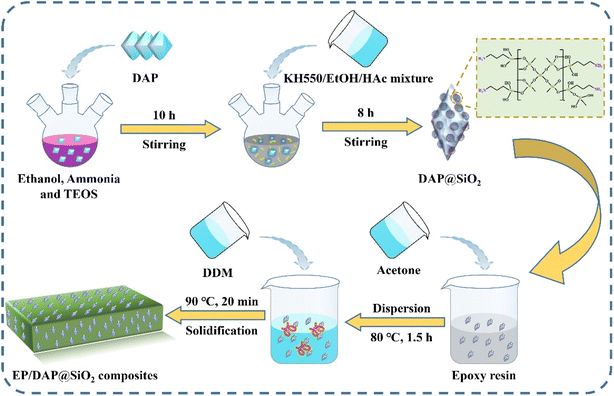
Have synthesized silica microcapsule structures centered on diammonium hydrogen phosphate by sol–gel route. The synthesized silica microcapsule (DAP@SiO2) were subsequently added to the epoxy resin as excellent flame-retardant additives to improve the flame-retardant properties of epoxy resin. The apparent morphology of DAP@SiO2 was observed using scanning electron microscope (SEM), and its chemical structure was characterized by Fourier transform infrared (FTIR). The thermal stability of the EP blends was assessed through Thermogravimetric analysis (TGA). The influence of the addition of DAP@SiO2 on the flame retardant behavior of EP blends was investigated via the limiting oxygen index (LOI), the vertical burning (UL-94), cone calorimetery (CONE). In addition, the mechanical properties of EP samples were measured by electronic universal testing machine and pendulum impact tester. The strategy can effectively improve the flame retardant efficiency of epoxy resin, and provide a reference for the synthesis of high efficiency flame retardants for epoxy resin. The use of DAP@SiO2 is expected to bring about a double improvement in flame retardancy and mechanical properties compared with the existing literature. In terms of flame retardancy, DAP@SiO2 can promote the formation of carbon layer, improve the thermal stability of the material, and reduce the heat release rate and smoke production during combustion. In terms of mechanical properties, the addition of DAP@SiO2 can enhance the toughness and strength of the material, and maintain structural integrity even at high temperatures. Finally, the Thermo Gravimetric Analysis was carried out to measure the mass change of the material in the controlled atmosphere. The LOI test was carried out to measure the oxygen concentration required for the combustion of the material. Successfully improving the flame retardancy of EP may have an important impact on future material design.
2. Experimental
2.1 Materials
Diammonium hydrogen phosphate (DAP) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Epoxy resin (E-51) was obtained from Shandong Usolf Chemical Technology Co., Ltd. Anhydrous ethanol was purchased through Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd. Tetraethyl orthosilicate (TEOS) was provided by Tianjin Tiantai Fine Chemical Co., Ltd. Aqueous ammonia and acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd. 4,4′- Diaminodiphenylmethane (DDM) was supplied by Shanghai Macklin Biochemical Technology Co., Ltd. 3-Aminopropyltriethoxysilane (KH550) was purchased from Changsha Zhongyi Chemical Co., Ltd. Deionized water is made by us in the laboratory.2.2 Synthesis of DAP@SiO2
The core–shell structure of DAP@SiO2 was obtained by sol–gel method through the hydrolysis of TEOS on the surface of diammonium phosphate particles. Firstly, DAP (2 g) were uniformly dispersed in a mixed solution of ammonia (5 mL), ethanol (160 mL) and deionized water (32 mL). During ultrasound treatment, TEOS (5 mL) was slowly added into the mixture. After 1 h of ultrasound, the mixture was stirred magnetically at room temperature for 10 h. Then, 2 mL ethanol/acetic acid mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dissolved with 0.045 g KH550 was added to the above mixture, and then magnetically stirred for 8 h. The product was filtered and placed in a vacuum oven at 60 °C for 8 h to obtain DAP@SiO2 powder.
1) dissolved with 0.045 g KH550 was added to the above mixture, and then magnetically stirred for 8 h. The product was filtered and placed in a vacuum oven at 60 °C for 8 h to obtain DAP@SiO2 powder.
2.3 Preparation of EP blends
Different mass fractions of DAP@SiO2 and epoxy resin were added to acetone, followed by ultrasonic dispersion, for 30 min. The mixtures were subjected to oven at 80 °C for 1.5 h to make sure that the acetone was completely removed. After that, DDM was added to the above mixture and stirred at 90 °C for 20 min. The mixture was transferred to a vacuum drying oven to remove air bubbles, after which it was poured into polytetrafluoroethylene mold, and cured at 120 °C for 2 h and 160 °C for 3 h. EP/DAP@SiO2 blends were obtained by demolding after cooling. The pure EP samples containing only curing agents were prepared by similar method. The synthetic route of EP/DAP@SiO2 is shown in Fig. 1.2.4 Characterization
The morphology of the char residues and DAP@SiO2 was observed with TM 4000 plus (Hitachi, Japan) scanning electron microscope at an acceleration voltage of 15 kV.The FTIR spectra of samples were investigated by Nicolet 380 (Thermo Fisher Scientific, America) Fourier transform infrared spectroscopy in the range of 4000–500 cm−1.
The thermal analysis was conducted on TGA 55 (TA Instruments, America) with a heating rate of 10 °C min−1 under a nitrogen atmosphere from 40 to 700 °C.
The limiting oxygen index (LOI) was carried out by JF-3 (Jiangning Analytical Instrument Co., Ltd., China) according to ASTM D2863-97, and the dimension of samples was 130 mm × 6.5 mm × 3 mm.
Vertical combustion test (UL-94) was measured by using a CZF-3 (Jiangning Analytical Instrument Co., Ltd., China) based on ASTM D3801, and the surface of the material should be flat without obvious defects and bubbles. The sample was placed vertically, and the sample size was 120 mm × 13 mm × 3.2 mm. The Bunsen lamp was placed vertically, and the lamp mouth was kept at a distance of 10 mm from the sample.
The combustion behavior of epoxy resin and its blend materials was characterized by a iCone Plus (FTT, UK) cone calorimeter (CONE) according to ISO 5660 in the atmosphere of 50 kW m−2 heat flux, and the material should have no defects such as bubbles and cracks, and the surface should be clean. The ignition time is 30 seconds, the ignition power is set to 50 kW m−2, and the duration of the test is 15 minutes.
The tensile properties of epoxy resin samples were surveyed by a WDW-300M universal testing machine (Jinan Liling Testing Machine Co., Ltd., China) according to ASTM D638-14. The samples were standard dumbbell-shaped specimens. The tensile speed was 5 mm min−1.
The impact properties of epoxy resin samples were carried out according to ISO 179-1 using a HT-843-5.5D pendulum impact tester (Guangdong Hongtuo Instrument Technology Co., Ltd., China). The sample size was 80 mm × 10 mm × 4 mm.
3. Results and discussion
3.1 Morphology and structure characterization of DAP@SiO2
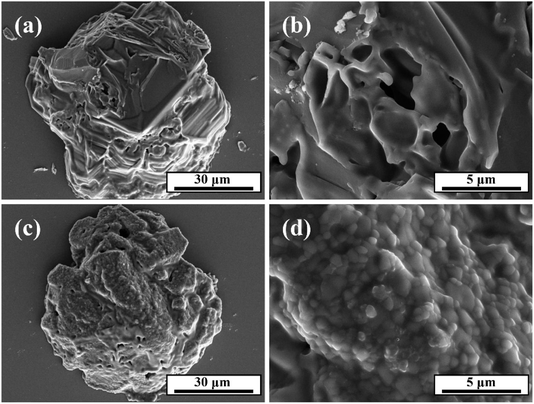
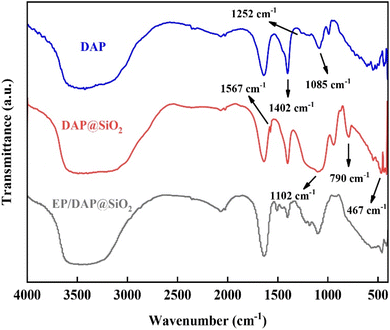
The morphologies of DAP and DAP@SiO2 are presented in Fig. 2. Fig. 2a shows that DAP have irregular particles with rough and uneven surface. Compared with DAP, the diameter of DAP@SiO2 in Fig. 2b is maintained at 80–90 μm, the surface becomes smooth, the clear and sharp edges are obviously weakened, and the surface is covered with dense and uniform silica particles. The smooth and uniform surface maximizes the effective surface area that DAP@SiO2 can interact with epoxy resin. Uniformity ensures that the active sites on the surface can be equally accessed, promoting a consistent reaction rate and process within the material.Fig. 3 illustrates the FTIR spectra of DAP and DAP@SiO2. In the infrared spectrum of DAP, the characteristic peaks at 1085 cm−1 and 1252 cm−1 are attributed to the P–O and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and 1402 cm−1 appears the characteristic peak of NH4+. In addition, the characteristic peak at 1102 cm−1 in DAP@SiO2 becomes wider and stronger, which is due to the coincidence of P–O, P
O stretching vibration, and 1402 cm−1 appears the characteristic peak of NH4+. In addition, the characteristic peak at 1102 cm−1 in DAP@SiO2 becomes wider and stronger, which is due to the coincidence of P–O, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Si–O–Si antisymmetric stretching vibration of DAP. Moreover, other characteristic peaks of DAP@SiO2 also appeared in the infrared spectrum of modified DAP. The absorption peak at 1567 cm−1 is ascribed to the N–H bond in amino group. The peaks at 790 cm−1 and 467 cm−1 are assigned to the symmetric stretching vibration and bending vibration of Si–O, respectively. It is indicated that SiO2 particles have been successfully grafted on the surface of DAP and the attached SiO2 is amino-functionalized.
O and Si–O–Si antisymmetric stretching vibration of DAP. Moreover, other characteristic peaks of DAP@SiO2 also appeared in the infrared spectrum of modified DAP. The absorption peak at 1567 cm−1 is ascribed to the N–H bond in amino group. The peaks at 790 cm−1 and 467 cm−1 are assigned to the symmetric stretching vibration and bending vibration of Si–O, respectively. It is indicated that SiO2 particles have been successfully grafted on the surface of DAP and the attached SiO2 is amino-functionalized.
3.2 Thermal stability of EP/DAP@SiO2 blends
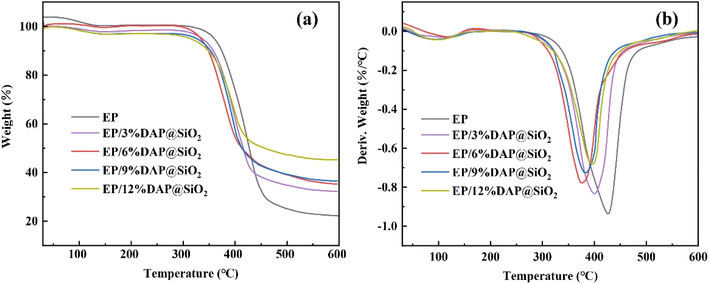
The TGA and DTG curves of EP and EP/DAP@SiO2 blends are shown in Fig. 4, and the detailed data from above curves are summarized in Table 1. It can be seen from the table that the highest DAP@SiO2 content (12%) reduced T5% to a minimum of 313.7 °C, and the Tmax of pure EP was 426.8 °C. With the increase of DAP@SiO2 content, Rmax gradually decreased. The thermal weight loss curves of epoxy resin and its blends showed roughly the same downward trend. Both EP and EP/DAP@SiO2 samples showed one-step decomposition, and the main thermal decomposition range was mainly concentrated in the range of 300 °C to 460 °C. The T5% of pure EP is 359.8 °C, but the T5% of all EP blends with DAP@SiO2 was lower than 340 °C, the T5% of EP blends gradually decreases with the increase of DAP@SiO2. This may be due to the initial decomposition temperature of flame retardant is lower than that of epoxy resin. Secondly, DAP can release ammonia during heating, which may further catalyze the degradation of epoxy matrix. So the addition of DAP@SiO2 leads to a decrease in the temperature of epoxy resin system at 5% weight loss. With the increasing content of DAP@SiO2, the Tmax of all flame retardant EPs decreased to below 400 °C. The Tmax value of EP blends decreased from 426.8 °C of pure EP to 397.3 °C with the addition amount of 12% DAP@SiO2. This may be due to the fact that more interfaces may be formed inside the material when the content of DAP@SiO2 nanocomposites increases. These interfaces may introduce additional thermal resistance, thereby reducing the overall heat transfer efficiency of the material, resulting in a decrease in Tmax. The Rmax of EP/DAP@SiO2 decreased from 0.94 of EP to 0.68 indicating that DAP@SiO2 effectively delayed the decomposition of epoxy resin matrix. The char residue of EP blends was significantly improved compared with EP resin. The residual mass of EP/12%DAP@SiO2 at 600 °C reached 45.1%, which was 23.4% higher than that of pure EP, because decomposition of diammonium hydrogen phosphate produces phosphoric acid and other phosphorus-containing substances, which will slightly increase the char residue after combustion of the polymer. In addition, the increase in residual carbon is related to the loading of silica particles on the surface of DAP, which has excellent heat resistance under high temperature conditions compared to epoxy resin polymer. The final residue generated after combustion of EP blends may be a mixture of silica and phosphorus-containing char residue. Consequently, the flame retardant DAP@SiO2 is helpful to improve the thermal stability of EP matrix in high temperature.| Sample | T5% (°C) | Tmax (°C) | Rmax (% °C−1) | Char residue at 600 °C (%) |
|---|---|---|---|---|
| EP | 359.8 | 426.8 | 0.94 | 21.7 |
| EP/3%DAP@SiO2 | 338.0 | 399.7 | 0.83 | 32.3 |
| EP/6%DAP@SiO2 | 333.4 | 376.3 | 0.78 | 35.1 |
| EP/9%DAP@SiO2 | 325.3 | 383.5 | 0.72 | 36.6 |
| EP/12%DAP@SiO2 | 313.7 | 397.3 | 0.68 | 45.1 |
3.3 Combustion behavior of EP/DAP@SiO2 blends
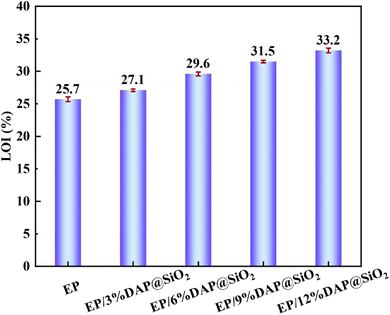
Flame retardant performance is an important index to measure the fire risk of materials.15 The flame retardant properties of EP blends were evaluated by limiting oxygen index and horizontal vertical combustion test. Fig. 5 and Table 2 are the LOI and UL-94 test results of EP/DAP@SiO2 system. The LOI value of pure EP was only 25.7% and it could not pass the UL-94 test, which indicates that the epoxy resin is extremely flammable. It is worth noting that the LOI value of epoxy resin blends increases with the increase of DAP@SiO2. When the addition amount of DAP@SiO2 was 3 wt%, 6 wt% and 9 wt%, the UL-94 grade of the epoxy resin blends increased from no grade of pure EP to V-1 grade, and the EP blends did not show melt dripping during the combustion process. This is because the carbonized material formed on the surface of EP blends can play a role in preventing the dripping of liquid molten material.16 The LOI value of EP/12%DAP@SiO2 material was 33.2% and the UL-94 reached V-0 grade. After adding DAP@SiO2, the LOI of EP matrix increased significantly, which indicated that DAP@SiO2 could effectively improve the fire safety of epoxy resin.| Samples | LOI (%) | UL-94 | |||
|---|---|---|---|---|---|
| t1 (s) | t2 (s) | Dripping | Rating | ||
| EP | 25.7 | None | None | Yes | NR |
| EP/3%DAP@SiO2 | 27.1 | 19 | 15 | Yes | V-1 |
| EP/6%DAP@SiO2 | 29.6 | 17 | 12 | No | V-1 |
| EP/9%DAP@SiO2 | 31.5 | 15 | 11 | No | V-1 |
| EP/12%DAP@SiO2 | 33.2 | 12 | 7 | No | V-0 |
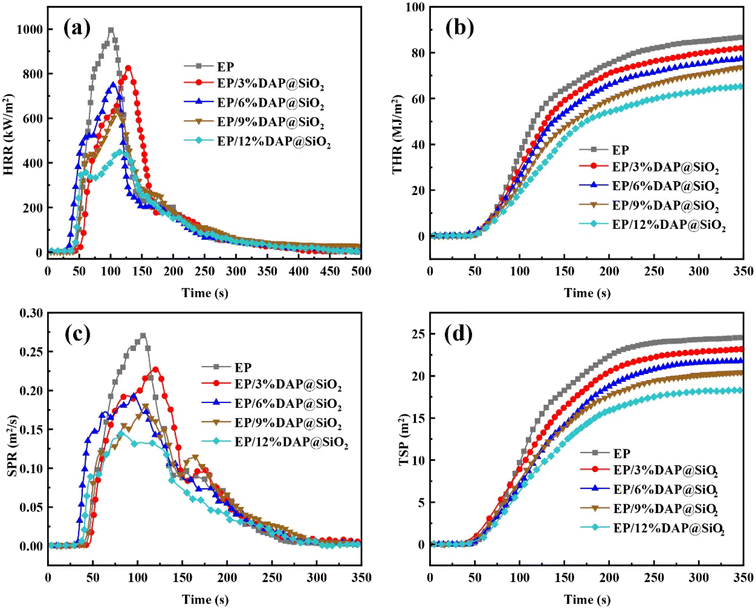
The cone calorimeter test can simulate the combustion of polymers under real fire conditions, and it is an important means to study the flame retardancy of polymers.17 The flame retardant properties of EP blends were further analyzed through cone calorimetry test. Fig. 6 shows the HRR (Heat Release Rate), THR (Total Heat Release), SPR (Smoke Production Rate) and TSP (Total Smoke Production) curves of pure EP and EP/DAP@SiO2, and the test data are shown in Table 3. As can be seen from Table 3, the TTI value of the EP/DAP@SiO2 samples decreased to different degrees compared with that of pure EP. The TTI of pure EP was 47 s, while that of EP blends containing 6% DAP@SiO2 was shortened to 44 s. When 12% DAP@SiO2 was added, the TTI of EP samples decreased to 41 s. In the initial stage of combustion of EP blends, the decomposition of DAP promoted the thermal decomposition process of epoxy resin, resulting in the shortening of ignition time of EP blends, which was consistent with the decrease of initial decomposition temperature in thermal stability analysis. As shown in Fig. 6, it can be observed that the heat release rate rised sharply, indicating that pure EP burns violently after being ignited. The PHRR (Peak Heat Release Rate) and THR of pure EP were 1001.8 kW m−2 and 86.3 MJ m−2, respectively. Compared with pure EP, the PHRR of the EP blends reinforced by DAP@SiO2 decreased significantly. When the loading of DAP@SiO2 was 12%, the PHRR value of the EP blends was decreased to 443.9 kW m−2, which was decreased by 55.7% compared with pure EP. It is worth noting that the HRR curve of pure EP reaches the HRR peak after the HRR rises rapidly during the combustion process. However, the addition of the flame retardant also resulted in a distinct small peak before reaching the HRR peak, and the peak became more obvious as the flame retardant content increased.
| Samples | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | TSR (m2) | PSPR (m2 s−1) | av-COY (kg kg−1) | av-EHC (MJ kg−1) | Residue (wt%) |
|---|---|---|---|---|---|---|---|---|
| EP | 47 | 1001.8 | 86.3 | 24.4 | 0.27 | 0.050 | 22.6 | 9.1 |
| EP/3%DAP@SiO2 | 45 | 827.9 | 82.0 | 22.9 | 0.23 | 0.063 | 21.3 | 10.7 |
| EP/6%DAP@SiO2 | 44 | 757.2 | 76.7 | 21.8 | 0.19 | 0.076 | 19.1 | 11.3 |
| EP/9%DAP@SiO2 | 42 | 641.5 | 73.2 | 20.2 | 0.18 | 0.082 | 17.5 | 12.1 |
| EP/12%DAP@SiO2 | 41 | 443.9 | 65.3 | 18.3 | 0.14 | 0.107 | 16.2 | 13.7 |
This is because the EP blend material forms a protective char layer during combustion, which acts as a barrier layer to slow down the continuous growth of heat release rate, thereby protecting the internal polymer from continued degradation due to heat radiation.18 In addition, the THR value of EP blend system showed a downward trend with the increase of DAP@SiO2. The THR of EP/12%DAP@SiO2 sample decreased to 65.3 MJ m−2, with a decrease of 24.3%. The addition of DAP@SiO2 forms a stable carbon layer during the combustion process. This carbon layer can isolate heat and oxygen, which can effectively reduce the HRR and THR values of the epoxy resin matrix during the combustion process, thereby delaying flame propagation.
Smoke release rate (SPR) and total smoke release (TSR) can be used as important evaluation parameters for smoke release during polymer combustion. The SPR and TSR curves of EP/DAP@SiO2 blend system are presented in Fig. 6(c) and (d). The PSPR and TSR of pure EP were 0.27 m2 s−1 and 24.4 m2, respectively. Compared with pure EP, the PSPR and TSR values of all EP blends with DAP@SiO2 decreased significantly. When the loading of DAP@SiO2 was 12%, the PSPR and TSR values of the blends were decreased to 0.14 m2 s−1 and 18.3 m2, which decreased by 48.1% and 25%, respectively. The decrease of PSPR and TSR indicates that DAP@SiO2 has a significant effect on the smoke suppression of reinforced EP blends, which creates conditions for the escape of people in fire accidents.
As shown in Table 3, the av-COY (Average Carbon Monoxide Yield) of pure EP was 0.05 kg kg−1. With the increase of DAP@SiO2, the av-COY value of EP blends presented an upward trend. When the loading of DAP@SiO2 was 3%, the av-COY value of EP blend system was increased to 0.063 kg kg−1. When the content of DAP@SiO2 was increased to 12%, the av-COY of flame retardant system reaches the maximum value of 0.107 kg kg−1. This is due to the incomplete combustion of EP blends induced by DAP@SiO2. The dense char layer formed during the combustion of material prevents the inner resin from obtaining sufficient oxygen, which ultimately leads to an increase in av-COY.19 The av-EHC (Average Effective Heat of Combustion) of EP was 22.6 MJ kg−1, and the av-EHC of EP/12%DAP@SiO2 was decreased to 16.2 MJ kg−1, with a decrease of 28.3%. This may be due to the dilution of the concentration of volatile cracking products by the nonflammable gases, such as NH3 and H2O, generated by the thermal decomposition of DAP@SiO2, which reduces the combustion rate of volatile gases in the gas phase.20 The char residue rate of EP blends increased from 10.2% of pure EP to 17.2%, which indicated that EP blends had stronger char layer formation ability than pure EP materials in high temperature environment, and the heat resistance of EP matrix was greatly improved. It is necessary to balance the effects of av-COY and av-EHC when designing and selecting materials. For specific applications, it may be necessary to select materials with lower carbon monoxide production and appropriate heat of combustion to ensure that performance requirements are met while minimizing fire risk.
3.4 Morphology of the char residue
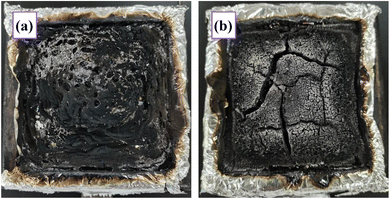
In order to further explore the flame retardant mechanism about EP blend flame retardant materials, the residual char after cone calorimeter test was analyzed. Fig. 7 shows the digital photographs of char residue of epoxy resin and its blends after cone calorimeter test under the same thermal radiation condition. In Fig. 7a, there is less char residue left after the combustion of pure EP, and the residual char layer is thin and unevenly distributed. It can be observed that there is a relatively sparse area of char residue distribution. This shows that the epoxy resin almost burnt out, thus reducing the remaining char layer and exposing the underlying aluminum foil to thermal radiation. With the increase of DAP@SiO2 content, the carbon residue of EP/12% DAP@SiO2 system increased significantly, which indicated that the addition of DAP@SiO2 promoted the formation of higher quality carbon layer. This carbon layer is more complete, dense and continuous, effectively blocking heat transfer and external air inflow, thereby maximizing the prevention of continuous combustion of the epoxy resin matrix. The presence of this carbon layer not only slows down the transfer of heat and oxygen to the interior of the material, but also improves the flame retardancy of the material and reduces the heat release and smoke generation during fire. The quality of the carbon layer has been greatly improved, which provides a good flame retardant effect in the condensed phase. DAP@SiO2 has a significant promoting effect on the carbonization of epoxy resin, which may be due to the interaction between DAP@SiO2 and the carbon layer generated during the combustion of epoxy resin, forming a more stable and continuous protective layer. This carbon layer can not only isolate heat, but also slow down the diffusion of oxygen and combustible gas, thus effectively inhibiting the combustion rate of the material.The microstructure of char residue of EP blends with different flame retardant content was observed by SEM after cone calorimeter test. The char residue photos of EP samples are shown in Fig. 8. It can be found that the amount of DAP@SiO2 added has a significant effect on the char residue of EP blends. When no flame retardant is added, a large number of pores with different sizes were distributed on the surface of char residue. The porous char layer cannot prevent the release of pyrolysis products from the interior of the EP matrix to the gas phase, thus accelerating the further degradation of the resin material at the bottom after ignition. Compared with EP, the pore structure of the char layer surface of the EP blend loaded with 6% DAP@SiO2 was significantly improved, and the thickness of the residual char was increased, but the cracks of the residual carbon were still large, showing loose and discontinuous. The char layer cannot effectively inhibit the heat transfer and the diffusion of flammable volatile small molecules. Although the decomposition rate of epoxy resin decreases, the flame retardant ability of EP matrix in the condensed phase is limited by the residual char cracks.21 The continuity of the char residue structure of EP blends is significantly improved with the more addition of DAP@SiO2. The char layer of EP/12%DAP@SiO2 system is dense and complete, and the surface of char layer is covered with a lot of silica particles. This is due to the migration of silica to the surface of epoxy resin during the combustion process. Most of the heat and air transmission can be effectively blocked by the physical barrier layer, which helps to improve the barrier effect of EP polymer in the condensed phase.22 Moreover, the char layer of the bottom layer shows an expansive shape, which is because the diammonium phosphate in the DAP@SiO2 flame retardant is decomposed into pyrolysis gases such as ammonia gas, which are blocked by the internal char layer, thereby increasing the thickness of char layer. The char layer can greatly improve the flame retardant efficiency of epoxy resin.
 | ||
| Fig. 8 SEM images of char residues for EP (a and b), EP/6%DAP@SiO2 (c and d) and EP/12%DAP@SiO2 (e and f). | ||
3.5 Flame retardant mechanism
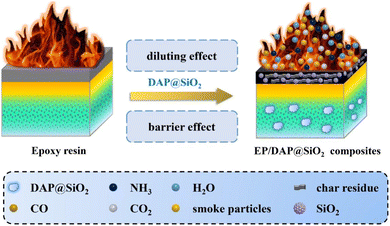
Based on the above analysis, DAP@SiO2 flame retardant has a significant effect on the flame retardant properties of EP blends, and the possible flame retardant mechanism is shown in Fig. 9. After the EP blend was ignited, the SiO2 on the DAP@SiO2 fell off, and the DAP core began to decompose. As a compound rich in N element, NH3 and N2 are released during combustion. These non-combustible gases and other gases such as CO and CO2 produced by EP material degradation can dilute the concentration of oxygen and flammable volatile products around the flame, so that the temperature is reduced to below the ignition point, thereby achieving the purpose of inhibiting flame growth.23,24 In addition, diammonium hydrogen phosphate decomposes to produce a large amount of substances such as ammonium dihydrogen phosphate and ammonium metaphosphate, which are further decomposed into phosphoric acid. After the degradation of phosphoric acid, the phosphorus-containing components are produced. In the condensed phase, the phosphorus-containing pyrolysis products generated by DAP decomposition react with the EP matrix to form chemical structures such as P–O–C, which improves the density of char layer. These continuous and complete char layers can effectively isolate the heat exchange between the outside and inside of epoxy resin.25 In addition, SiO2 migrates to the surface of epoxy resin to form a shielding layer, forming a compound containing Si–O–Si and O–Si–C structures, which can be cross-linked by other condensed phase products, greatly improving the thermal stability of the carbon layer, thus effectively inhibiting the further pyrolysis of the EP material inside the char layer.263.6 Mechanical performance of EP/DAP@SiO2 blends
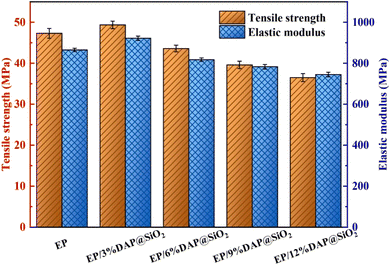
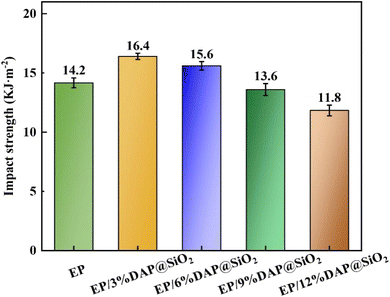
Tensile strength and elastic modulus of pure EP and flame retardant epoxy resin are shown in Fig. 10. The results showed that the tensile strength and elastic modulus of pure EP are 47.3 MPa and 865 MPa, respectively. The mechanical properties of EP blends were improved after the small amount addition of DAP@SiO2. For the EP sample containing 3% of DAP@SiO2 only, the tensile strength and elastic modulus were increased to 49.4 MPa and 922 MPa, respectively, which were 4.4% and 6.6% higher than those of pure EP. This might be due to the cross-linking reaction between a large number of amino groups on the surface of DAP@SiO2 and the epoxy groups on the epoxy resin, so that the interfacial compatibility between DAP@SiO2 and epoxy resin is significantly enhanced, and the combination of DAP@SiO2 and the epoxy resin is closer. However, when 12% of DAP@SiO2 was added, the tensile strength and elastic modulus of EP blends were decreased to 36.5 MPa and 745 MPa, respectively. With the continuous addition of DAP@SiO2, the tensile strength and elastic modulus of EP/DAP@SiO2 exhibited a declining trend. This can be attributed to the fact that a large amount of DAP@SiO2 is prone to agglomerate, resulting in poor compatibility between DAP@SiO2 and EP matrix, and the phenomenon will deteriorate the tensile strength and elastic modulus of EP blends.The impact strength test results of EP blends are presented in Fig. 11. The impact strength of EP is 14.2 kJ m−2. When 3% DAP@SiO2 was introduced into EP, the impact strength of EP blends was 16.4 kJ m−2, which was increased by 15.5% compared with pure EP. This can be attributed to the uniform dispersion of DAP@SiO2 in EP, which plays the role of uniform dispersion of stress. When the loading of DAP@SiO2 exceed 3%, the impact strength of EP blends decrease. With the addition of 6% DAP@SiO2, the impact strength of EP blends is 15.6 kJ m−2. The impact strength of EP/12%DAP@SiO2 decreased to 11.8 kJ m−2. The dispersion effect of DAP@SiO2 in epoxy resin is poor, and the stress concentration is caused by the agglomeration and uneven distribution, thus reducing the impact strength of EP samples.
4 Conclusions
We have synthesized DAP@SiO2 via the hydrolysis of TEOS on the surface of diammonium phosphate. Subsequently, DAP@SiO2 were introduced into epoxy resin to prepare EP blends with desired thermal stability and flame retardance. TG results revealed that the addition of DAP@SiO2 improved the char residue of epoxy resin blends. When 12% DAP@SiO2 was introduced, the LOI value of the EP mixture reached 33.2%, compared with Son et al.7 The PUF–RH system with 25% DAP reached UL-94 V-0 level, and the limiting oxygen index (LOI) was 26.3%, which meant that the EP/12% DAP@SiO2 composite had excellent flame retardancy and reached the V-0 level in the UL-94 test. The V-0 rating indicates that the material exhibits the highest flame retardant grade in the UL-94 vertical combustion test. The peak of heat release rate (PHRR) and peak of smoke production rate (PSPR) decreased by 55.7% and 48.1% comparing to the relative values of pure EP. Reducing PHRR and PSPR can help slow the spread of fires and reduce the toxic smoke generated by fires. DAP@SiO2 enhanced the fire safety of EP blends and played a flame retardant role in both gas phase and condensed phase. The flame retardant mode of EP polymer shows that after adding DAP@SiO2, NH3 and N2 are released during the combustion process, which can dilute the concentration of oxygen and flammable volatile products around the flame. In addition, the phosphorus-containing components produced by the decomposition of diammonium phosphate react with the EP matrix in the condensed phase to form chemical structures such as P–O–C, which increases the density of the carbon layer. Therefore, its flame retardant and smoke suppression characteristics are related to the interaction of flame retardants in the gas phase and the condensed phase. After adding DAP@SiO2, DAP is thermally decomposed to produce a large amount of ammonium dihydrogen phosphate and ammonium phosphate, which are further decomposed into phosphoric acid. The phosphorus-containing components produced after the degradation of phosphoric acid react with the EP matrix in the condensed phase to form chemical structures such as P–O–C, which increases the density of the carbon layer. In addition, silica migrates to the surface of epoxy resin to form a shielding layer, forming compounds containing Si–O–Si and O–Si–C structures, which can be cross-linked with other condensed phase products, greatly improving the thermal stability of the carbon layer. A small amount of DAP@SiO2 may improve the mechanical properties of the material by forming a good interface with the EP matrix, and the tensile strength and impact strength of 3% DAP@SiO2 were 49.4 MPa and 16.4 kJ m−2, respectively, which were 4.4% and 15.5% higher than that of pure EP. However, with the increase of DAP@SiO2 content, aggregation may be caused, resulting in stress concentration and defects, thus reducing the mechanical strength of the material. The improvement of mechanical properties of flame retardant materials can reduce the damage of fire to buildings and objects, protect property safety and reduce the repair cost after fire.Data availability
All relevant data are within the manuscript and its additional files.Conflicts of interest
There are no conflicts to declare.References
- J. Y. Li, H. C. Zhao and G. X. Sui, Renewable green reactive diluent for bisphenol a epoxy resin system: curing kinetics and properties, RSC Adv., 2022, 12(49), 31699–31710 RSC.
- C. H. Zhang, Z. C. Xu and W. B. Sui, Synergistic effect of novel ionic liquid/graphene complex on the flame retardancy of epoxy nanocomposites, Carbon Lett., 2022, 33(2), 501–516 CrossRef.
- G. M. Raja, A. Vasanthanathan and K. Jeyasubramanian, Novel ternary epoxy resin composites obtained by blending graphene oxide and polypropylene fillers: An avenue for the enhancement of mechanical characteristics, J. Inorg. Organomet. Polym. Mater., 2022, 33(2), 383–397 CrossRef.
- Y. C. Han, H. Zhao, J. C. Chen, X. L. Wang, L. X. Chen, D. Ran, Z. Y. Wang and P. J. Zeng, A new phosphorus flame-retard curing agent for epoxy resin/anhydride system, Polym. Adv. Technol., 2021, 33(3), 927–936 CrossRef.
- M. Park, Y.-S. Kuk, O. H. Kwon, J. Acharya, G. P. Ojha, J.-K. Ko, H.-S. Kong and B. Pant, Fly Ash-Incorporated Polystyrene Nanofiber Membrane as a Fire-Retardant Material: Valorization of Discarded Materials, Nanomaterials, 2022, 12(21), 3811 CrossRef CAS PubMed.
- G. Zhang, Y. L. Dong, M. Yao, Y. M. Gui, W. H. Meng, S. S. Wang, H. Q. Qu and J. Z. Xu, Preparation of a MOF flame retardant containing phosphazene ring and its effect on the flame retardant of epoxy resin, React. Funct. Polym., 2023, 191, 105670 CrossRef CAS.
- T. Son, T. T. H. Nguyen, Q. T. H. Phan, T. C. Pham, T. B. Nguyen, H. L. Pham, V. V. T. Do, H. Vothi, J. Kim and Q. D. Hoang, Phosphorus/phosphorus-nitrogen flame retardants applied to polyurethane/rice husk eco-composites: thermal behavior, flame retardancy, and physico-mechanical properties, Polym. Bull., 2020, 78(5), 1–17 Search PubMed.
- S. Lai, G. Chen, W. Hu, B. J. Liu, X. Yang and K. Gao, Preparation and performance of DOPO-nano-SiO2 modified polyacrylic acid-based flame retardant dust suppressant for coal, New J. Chem., 2021, 45(37), 17461–17474 RSC.
- W. Z. Xu, L. J. Fan, Z. Q. Qin, Y. C. Liu and M. Li, Silica-coated metal-organic framework-β-FeOOH hybrid for improving the flame retardant and smoke suppressive properties of epoxy resin, Plast., Rubber Compos., 2021, 50(8), 396–405 CrossRef CAS.
- W. Z. Xu, C. W. Xu, J. L. Liu, D. Ding, Y. Zhang and Y. C. Zhou, A guanidine phosphate-assisted boron nitride network enabled simultaneous improvements in flame resistance and thermal conductivity of polyvinyl alcohol (PVA), Polym. Degrad. Stab., 2022, 206, 110178 CrossRef CAS.
- X. Zhou, J. Wang, L. X. He, S. L. Qiu and R. M. Pen, Preparation of an integrated P–N–Si tube containing phosphazene-triazine-silica structure: An efficient flame retardant agent for epoxy resin, Compos. Commun., 2023, 38, 101504 CrossRef.
- S. B. Kang, Y. Jo, N. H. Lam, N. T. N. Truong, J. H. Jung and C. Kim, Nitrogen-Doped Nickel Graphene Core Shell Synthesis: Structural, Morphological, and Chemical Composition for Planar Hybrid Solar Cells Application, Photonics, 2022, 10(1), 18–27 CrossRef.
- X. S. Wang, L. Lu, Y. J. Tong and W. D. Chen, Synthesis of Core/Shell Structured Zinc Borate/Silica and Its Surface Charring for Enhanced Flame Retardant Properties, Polym. Degrad. Stab., 2020, 183, 109432 CrossRef.
- H. X. Sun, Y. L. Qi and J. Zhang, Effect of magnesium hydroxide as a multifunctional additive on high solar reflectance, thermal emissivity, and flame retardancy properties of PP/SEBS/oil composites, Polym. Compos., 2020, 41(10), 4010–4019 CrossRef CAS.
- Y. C. Xiao, Y. R. Yang, Q. L. Luo, B. L. Tang, J. P. Guan and Q. Tian, Construction of carbon-based flame retardant composite with reinforced and toughened property and its application in polylactic acid, RSC Adv., 2022, 12(34), 22236–22243 RSC.
- W. D. Wang and Z. Z. Wang, Facile synthesis of carbon microspheres/tin ethylenediamine tetramethylene phosphonate hybrid for improving the mechanical, flame-retardant, and thermal properties of epoxy resin, Polym. Adv. Technol., 2021, 32(8), 2953–2968 CrossRef CAS.
- M. J. Niu, Z. Z. Zhang, Z. Z. Wei and W. J. Wang, Effect of a Novel Flame Retardant on the Mechanical, Thermal and Combustion Properties of Poly (Lactic Acid), Polymers, 2020, 12(10), 2407 CrossRef CAS PubMed.
- Y. D. Yang, H. F. Shen, Y. Z. Luo, R. H. Zhang, J. J. Sun, X. Y. Liu, Z. F. Zong and G. Tang, Rigid polyurethane foam composites based on iron tailing: Thermal stability, flame retardancy and fire toxicity, Cell. Polym., 2022, 41(5), 189–207 CrossRef CAS.
- W. Xie, B. W. Wang, Q. Wang and Z. Q. Yang, Flame retardancy of a novel high transparent poly(methyl methacrylate) modified with phosphorus-containing compound, React. Funct. Polym., 2020, 153, 104631 CrossRef CAS.
- W. Yan, M. Q. Zhang, J. Yu, S. Q. Nie, D. Q. Zhang and S. H. Qin, Synergistic Flame-retardant Effect of Epoxy Resin Combined with Phenethyl-bridged DOPO Derivative and Graphene Nanosheets, Chin. J. Polym. Sci., 2019, 37(01), 79–88 CrossRef CAS.
- D. Y. Liu, W. H. Zhao, Y. H. Cui, T. L. Zhang and P. F. Ji, Influence of the Chemical Structure on the Flame Retardant Mechanism and Mechanical Properties of Flame-Retardant Epoxy Resin Thermosets, Macromol. Mater. Eng., 2022, 307(9), 2200169 CrossRef CAS.
- Y. K. Chen, Q. X. Lu, G. Zhong, H. G. Zhang, M. F. Chen and G. P. Liu, DOPO-based curing flame retardant of epoxy composite material for char formation and intumescent flame retardance, J. Appl. Polym. Sci., 2020, 138(9), 49918 CrossRef.
- P. Qi, Y. C. Li, J. Sun, X. G. Wang, K. H. Wang, D. Meng, X. Y. Gu, H. F. Li and S. Zhang, Flame retardant and anti-dripping surface treatment through a co-deposition of polydopamine/polyphosphonamide for fabric and foam materials, Composites, Part B, 2022, 247, 110262 CrossRef CAS.
- S. P. Liu, H. Wei, Y. Xiong, Y. G. Ding and L. L. Xu, Synthesis of a highly efficient flame retardant containing triazine and pentaerythritol phosphate groups and its intumescent flame retardancy on epoxy resin, High Perform. Polym., 2022, 34(8), 889–903 CrossRef CAS.
- Y. Wei, S. Y. Zhu, Q. W. Qian, Q. R. Jiang, L. Y. Zhang, K. J. Jin, W. S. Liu and Y. P. Qiu, Hexachlorocyclotriphosphazene functionalized lignin as a sustainable and effective flame retardant for epoxy resins, Ind. Crops Prod., 2022, 187, 115543 CrossRef CAS.
- H. Z. Fan, J. M. Zhao, J. F. Zhang, H. F. Li, S. Zhang, J. Sun, F. Xin, F. Liu, Z. D. Qin and W. F. Tang, TiO2/SiO2/kaolinite hybrid filler to improve the flame retardancy, smoke suppression and anti-aging characteristics of epoxy resin, Mater. Chem. Phys., 2022, 277, 125576 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |