Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advancements in biohydrogen production – a comprehensive review of technologies, lifecycle analysis, and future scope
Aarnav Hetan Sanghvia,
Amarjith Manjoo b,
Prachi Rajputb,
Navya Mahajanb,
Natarajan Rajamohan*c and
Iyman Abrar
b,
Prachi Rajputb,
Navya Mahajanb,
Natarajan Rajamohan*c and
Iyman Abrar *b
*b
aDepartment of Electrical & Electronics Engineering, Birla Institute of Technology and Science, Pilani – Hyderabad Campus, Shameerpet, Hyderabad, Telangana-500078, India
bDepartment of Chemical Engineering, Birla Institute of Technology and Science, Pilani – Hyderabad Campus, Shameerpet, Hyderabad, Telangana-500078, India. E-mail: iyman@hyderabad.bits-pilani.ac.in
cChemical Engineering Section, Faculty of Engineering, Sohar University, Sohar, P C-311, Oman. E-mail: rnatarajan@su.edu.om
First published on 18th November 2024
Abstract
The global shift towards sustainable energy sources, necessitated by climate change concerns, has led to a critical review of biohydrogen production (BHP) processes and their potential as a solution to environmental challenges. This review evaluates the efficiency of various reactors used in BHP, focusing on operational parameters such as substrate type, pH, temperature, hydraulic retention time (HRT), and organic loading rate (OLR). The highest yield reported in batch, continuous, and membrane reactors was in the range of 29–40 L H2/L per day at an OLR of 22–120 g/L per day, HRT of 2–3 h and acidic range of 4–6, with the temperature maintained at 37 °C. The highest yield achieved was 208.3 L H2/L per day when sugar beet molasses was used as a substrate with Clostridium at an OLR of 850 g COD/L per day, pH of 4.4, and at 8 h HRT. The integration of artificial intelligence (AI) tools, such as artificial neural networks and support vector machines has emerged as a novel approach for optimizing reactor performance and predicting outcomes. These AI models help in identifying key operational parameters and their optimal ranges, thus enhancing the efficiency and reliability of BHP processes. The review also draws attention to the importance of life cycle and techno-economic analyses in assessing the environmental impact and economic viability of BHP, addressing potential challenges like high operating costs and energy demands during scale-up. Future research should focus on developing more efficient and cost-effective BHP systems, integrating advanced AI techniques for real-time optimization, and conducting comprehensive LCA and TEA to ensure sustainable and economically viable biohydrogen production. By addressing these areas, BHP can become a key component of the transition to sustainable energy sources, contributing to the reduction of greenhouse gas emissions and the mitigation of environmental impacts associated with fossil fuel use.
1. Introduction
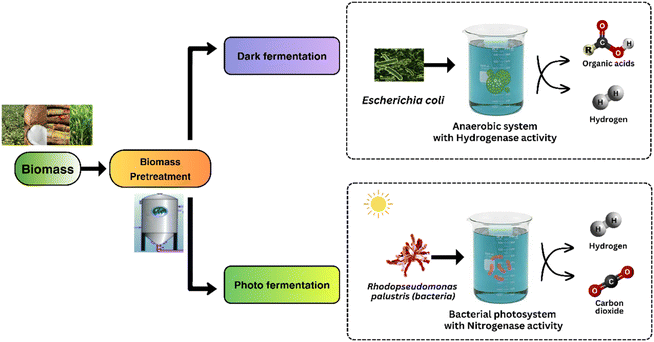
Demand for fossil fuels has increased multifold, creating an imbalance between production and consumption. It has become increasingly important to rely on environmentally clean fuels like hydrogen. Hydrogen, a very efficient fuel with one of the highest calorific values (120–142 MJ kg−1), is considered as a clean fuel because it releases only water vapour as a byproduct during combustion.1 Out of the several methods available to generate hydrogen, biological processes have long been preferred due to favourable operating parameters like low temperature and pressure and low costs of operation.2 Among the different renewable energy resources available, waste biomass has proven to be one of the most potent choices for producing green fuels.3 Biohydrogen Production (BHP) is a propitious domain for sustainable energy generation, with its viability depending on substrate costs and pretreatment technologies. Fermentation of carbohydrates from organic substrates like agricultural biomass holds potential, yet their complex structure poses efficiency challenges.4Two primary BHP technologies utilizing biomass as fuel are thermochemical and biological processes. Dark fermentation, carried out by fermentative bacteria, utilizes a variety of organic biomasses to produce biohydrogen and organic acids.5 Photo fermentation, which involves photosynthetic purple non-sulfur (PNS) bacteria, produces biohydrogen from organic acids under anaerobic, light-driven conditions.6 Biohydrogen production offers significant environmental and operational benefits over conventional chemical hydrogen generation methods. Traditional chemical processes, like steam methane reforming and coal gasification, are energy-intensive and heavily reliant on fossil fuels, resulting in high greenhouse gas emissions.7 In contrast, BHP uses renewable feedstocks, such as agricultural waste and wastewater, creating a sustainable, low-emission hydrogen source. BHP systems typically operate under milder conditions, which not only reduces energy costs but also enables simpler process setups.8 Understanding the kinetics of these processes and optimizing conditions through pretreatment methods are crucial for enhancing efficiency and economic viability in BHP.
Different types of reactors play an important role in BHP and their design and affecting parameters influences the yield of production. The batch reactor provides BHP with advantages such as flexibility, robust control strategy implementation, and reduced cost. However, challenges exist, including the need for substrate pretreatment, low hydrogen yields from complex organic materials, and the difficulty in achieving high hydrogen yields due to biochemical and thermodynamic constraints.9 Research efforts to enhance the efficiency of BHP processes include modelling, optimization, metabolic engineering, using mixed cultures, and changing reactor layouts.10 Furthermore, the comparison between continuous and batch reactors indicates that batch reactors may yield higher hydrogen rates due to varying substrate concentrations but only in the early stages of the batch, else continuous reactors are more efficient.11 Continuous stirred tank reactors (CSTRs), packed bed reactors (PBRs) and membrane bioreactors (MBRs) play a vital role in the continuous production of biohydrogen. A short hydraulic retention time (HRT) and washout of biomass have been identified as constraints in CSTR performance.12 Additionally, the integration of CSTRs with other bioreactors, such as upflow anaerobic sludge blanket (UASB) reactors, has been explored for simultaneous hydrogen and methane production. PBRs are known for their simple design with minimal moving parts, which makes them cost-effective and easy to operate. PBRs offer a wide range of operational times and high biomass concentrations, as the microorganisms attach to the solid support material, leading to long operational periods.13 A highest BHP rate was achieved at 2 h HRT for PBRs operated at various HRTs varying from 1.2 to 24 h, and using paper mill effluent as substrate.14 However, PBRs struggle with clogging from complex substrates, uneven flow distribution, and optimal temperature maintenance. MBRs have emerged as a more efficient BHP alternative, when particularly combined with biological processes. MBRs show higher hydrogen yields when compared to PBRs15 and they also provide better control over microbial diversity and process stability, leading to more consistent BHP rates.16 The addition of biofilm support was observed to result in BHP rate of 44.22 L/L per day, when polyester screen mesh was used compared to BHP rate of 51.64 L/L per day with stainless-steel.17 MBRs, though they offer higher yields and better control, they come with their own drawbacks such as membrane fouling. Both PBRs and MBRs have limitations in their microbial communities, requiring further research to understand their full potential.
In recent years, several machine learning (ML) models have been implemented to boost the efficiency of BHP. Artificial intelligence (AI) tools help in predicting and alleviating problems that might be faced during the process. Some important and frequently-used AI tools such as artificial neural network (ANN) models used to estimate BHP yield and support vector machines (SVM) models used to classify errors have been discussed in the paper. By integrating AI tools such as ANNs and SVMs into the biohydrogen production process, it is possible to achieve significant improvements in efficiency, reliability, and yield. These tools enable precise predictive modeling, process optimization, anomaly detection, and automated control, ultimately leading to a more sustainable and economically viable BHP system. Before commercializing biohydrogen produced through any methods, doing a thorough life cycle assessment (LCA) and technoeconomic analyses (TEA) is very important. TEA gives an idea about the financial viability of a biological procedure by examining economic aspects involved during the production process.16,17 LCA analyses the effect the procedure would have on the environment throughout the life cycle.
The existing literature on BHP reveals critical gaps, particularly in reactor design and the integration of AI-driven optimization methods. The low yields of dark and photofermentation processes demand advanced reactor designs, often complex and resource-intensive. While scaling up reactor systems for diverse feedstocks has potential to enhance efficiency, it remains a formidable challenge. AI models, including ANN and Adaptive Neuro-Fuzzy Inference Systems (ANFIS), are underutilized in this field despite their proven effectiveness in other biotechnological applications for process parameter optimization and yield prediction. The lack of real-time AI-driven monitoring and control also restricts the ability to dynamically adjust reactor conditions, thereby limiting efficiency, adaptability, and scalability improvements across various operating conditions.18 Another notable gap includes sustainable management of post-fermentation broth, which is critical for reducing environmental impact but often overlooked in biohydrogen processes. Current strategies lack comprehensive waste management approaches that incorporate reuse, recycling, or safe disposal practices, which are essential for a sustainable BHP lifecycle. Addressing these gaps with integrated waste management solutions could facilitate the broader environmental acceptance and practical applicability of biohydrogen.19
This review offers an in-depth analysis of light and dark fermentation, exploring their operational mechanisms and contextual applications, and provides insights into biohydrogen yield optimization for various reactor types, while detailing their respective advantages and constraints. Furthermore, it introduces AI-driven prediction tools to predict reactor performance represents a novel approach to enhance process optimization and gain deeper insights into the dynamic behaviour of BHP processes. The review also considers the broader sustainability and economic implications of BHP, with comprehensive assessments via LCA and TEA to align with global sustainability and energy transition objectives. Future research should prioritize integrated cultivation and waste management systems to address both efficiency and environmental impact. Expanding the role of real-time AI-based monitoring and process control in BHP, alongside adaptable reactor designs, could enhance operational precision and pave the way for sustainable scale-up and industrial application.
2. Technology overview and mechanism
The technological and kinetic viability of BHP is majorly influenced by the costs associated with the substrate and also the technology used for pretreatment process. In accordance with fermentation principles, the conversion of biohydrogen from carbohydrates, particularly those found in organic substrates like agricultural biomass, holds considerable promise. Nevertheless, the intricate and heterogeneous structure of matrices poses challenges to the efficient fermentation of carbohydrates.20 To overcome this obstacle, substrates necessitate pretreatment to liberate more accessible carbohydrates which eventually enhances fermentation efficiency.21 Selecting the most suitable pretreatment method depends on the substrate type and composition of the substrate.22 The pre-existing pretreatment methods, pyrolysis and ultrasound performs better in the case of cell destruction and needs lesser treatment time. Although, these techniques require high energy conditions which can lead to further breakdown of sugars, hence becoming unsuitable.23 While pyrolysis, combustion, liquefaction and gasification are key thermo-chemical processes that use biomass for hydrogen generation, direct and indirect bio-photolysis, dark, photo, and integrated fermentation (a combination of both dark and photo fermentation) are the main microbial processes.4 The different pathways of dark and photo fermentation for BHP has been depicted in Fig. 1.2.1. Dark fermentation for BHP
Several fermentative bacteria (either obligatory or facultative) produce biohydrogen through dark fermentation and may use a larger variety of organic biomass or wastes as substrate. The capacity of dark-fermentative bacteria to degrade anaerobic or anaerobic biomass distinguishes them as obligatory or facultative anaerobes. The fermentative bacteria convert organic substrates into hydrogen and other by-products under anaerobic conditions. This process is carried out in the absence of light, distinguishing it from photofermentation. The primary reactions involved in dark fermentation include the breakdown of carbohydrates into simpler compounds, resulting in the production of H2, CO2, and volatile fatty acids (VFAs) such as acetic acid and butyric acid.24 The accumulation of these acids can affect the overall yield and efficiency of hydrogen production, making their monitoring and control essential for optimizing the process. Dark fermentation commonly produces hydrogen yields of 1–2 moles per mole of carbohydrates consumed, depending on the microbial strain and operating conditions. The process efficiency is influenced optimal pH, correct temperature and the extent of byproducts produced. Many studies report optimal conditions at a pH of 5.5–6.0 and temperatures around 35–37 °C for hydrogen-producing Clostridium strains, maximizing yields close to 2.0 moles H2 per mole of glucose. Organic acids like acetate and butyrate are byproducts, with yields of up to 2–3 g L−1 depending on the carbon source and fermentation conditions.25The kinetic parameters of biohydrogen generation, which rely on the kind of substrates being fermented and the inoculum microbial population, characterize the activities of the dark fermentation process and span a large range. The formation of dark fermentative biohydrogen has been extensively described using kinetic models, such as the Modified Gompertz Model given by eqn (1) which discusses the regular changes in the growth rate of biohydrogen producing bacteria substrate concentrations, and the Modified Logistic Model given by eqn (2) which aid in defining the precise functions of factors that affect H2 yield and aid in process design.5
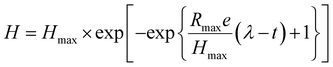 | (1) |
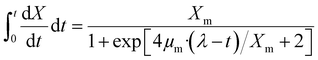 | (2) |
In dark fermentation, microbial contamination impacts hydrogen production by introducing non-hydrogen-producing organisms that consume essential substrates or even the hydrogen itself, reducing yield. Contaminants like methanogens can outcompete hydrogen-producing bacteria such as Clostridium and Enterobacter by using substrates like acetate and butyrate, diverting the process toward methane production instead of hydrogen. To address this, pretreatment techniques—such as heat, chemical, or pH treatments—help deactivate unwanted microbes, creating an environment where hydrogen producers can thrive. Additionally, metabolic engineering of hydrogen-producing microbes is explored to improve their substrate utilization and resistance to contamination, potentially increasing hydrogen output.26
2.2. Photo fermentation for BHP
Under anoxygenic conditions using light as their energy source, photosynthetic PNS bacteria may produce hydrogen from organic acids (butyrate, acetate, succinate, malate, etc.) and CO2.27 Consequently, photo-fermentation offers the possibility of generating hydrogen from a variety of substrates, such as wastewater and organic acid-rich waste.28 Depending on the carbon source, the literature indicates a maximum light conversion efficiency of 9.3% and a maximum hydrogen production of 80%. The chemical oxygen demand (COD) removal efficiency and hydrogen output of photo-fermentation are both high in theory, but their economic viability is constrained by the activity of the hydrogen-producing enzyme (nitrogenase) and light intensity.29 Several PNS bacteria strains, including Rhodobacter sulfidophilus, Rhodopseudomonas palustris, Rhodobacter sphaeroides O.U001, Rhodobacter capsulitis, R. sphaeroides RV, and others, have been examined for photo fermentative hydrogen generation. In this fermentation, photosynthetic bacteria like Rhodobacter species can achieve theoretical yields of up to 3.5–4 moles H2 per mole of organic acid under optimal conditions, though practical yields often range around 2.5–3.0 moles. Light intensity plays a key role; low light can limit yield, while high light can induce photoinhibition. Typical light intensities of 4000–6000 lux are reported to support optimal conversion rates, though reactor design and light penetration are significant limiting factors.30 Eqn (3) illustrates the hydrogen production from acetate via photo fermentative method.| 2CH3COOH + 2H2O → 4H2 + 2CO2, ΔG0 = +104 kJ | (3) |
The logistic equation for the fermentation process explains and discusses the growth kinetics of Rhodobacter sphaeroides O.U001. The logistic model, a sigmoidal-shaped model, has recently become the most popular due to its ‘goodness of fit’ and has been frequently utilized to describe the proliferation of microorganisms. The logistic model has the advantage of portraying the whole growth curve, including the lag phase (if present), exponential growth, and stationary periods. Eqn (4) is based on logistic model which accounts for growth-associated hydrogen generation in photo fermentation.6
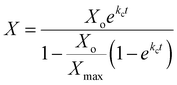 | (4) |
Eqn (4) depicts the corelation between biomass production concentration (X) and fermentation time (t) where X max and k, kinetic parameters are calculated using logistic curve.
Microbial contamination poses a challenge in photo-fermentation for hydrogen production as unwanted microbial growth competes for nutrients, reducing hydrogen yield. Contaminants can also disrupt the delicate balance of environmental conditions essential for photo-fermentative bacteria, such as Rhodobacter and Rhodopseudomonas species, thereby lowering efficiency. To address contamination, pre-treatment methods like pasteurization and pH adjustments are used to inhibit unwanted microbes without affecting target bacteria. Combining photo-fermentation with dark fermentation (two-stage fermentation) is another strategy that boosts yields and stabilizes pH, minimizing contamination issues.31
3. Reactor types
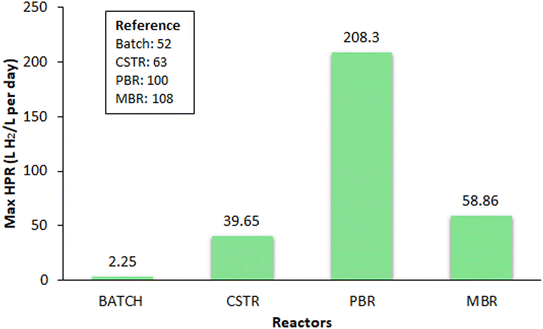
The core of BHP technology depends upon the efficiency of bioreactors, specially engineered to optimize the microbial BHP processes. This section provides an in-depth exploration of different reactor configurations used in BHP, including batch reactor, CSTR, PBR, and MBR, with the maximum BHP rate represented in Fig. 2. Furthermore, a comprehensive understanding of the various parameters and several challenges encountered in the pursuit of efficient BHP have also been discussed. Parameters such as pH, temperature, substrate concentration, HRT must be optimized to maximize BHP while minimizing the operational costs and environmental impacts, and also taking other challenges such as system integration, and development of cost-effective reactor materials into consideration.3.1. Application of batch reactor for BHP
Studies have reported that batch operation generally yields higher amounts of hydrogen compared to continuous operation, because bacteria in a batch reactor experience a distinct environment with varying substrate concentrations during the early stages of the batch compared to low-substrate microenvironment in continuous operation.32 A viable substitute for BHP using organic solid waste is the sequential batch reactor (SBR), which offers the advantages of flexible operation, comprehensive instrumentation possibilities, robust control strategy implementation, and reduced cost.11 An efficient feedstock-retaining reactor that runs on self-immobilized biomass is called an anaerobic sequencing batch reactor (ASBR). Therefore, if enhanced hydrogen-generating bacterial populations are attained, operational systems that uses ASBR have a higher chance of producing hydrogen for a longer period and at a higher yield. In addition to these benefits, the ASBR is superior to other systems because of its versatility, ease of use, and ability to settle within the same reacting vessel.33| Substrate | Micro-organism | OLR | pH | Temperature | HRT | BHP rate | Reference |
|---|---|---|---|---|---|---|---|
| Vinicultural biomass (grape residues) | Clostridia species | 14 to 28 g COD/L | 5.7 ± 0.5 | 37 ± 1 °C | 24 h | 2.2 × 10−4 mol H2 per min | 34 |
| Winery wastewater | Lactobacillus, Pectinatus, and Clostridium species | 108.6 g COD/L per day | 5.0–5.6 | 37 ± 1 °C | 5.5 h | 0.05 L H2/L per day | 35 |
| Palm oil mill discharge | Clostridiaceae | 45.4 g COD/L per day | 4.5 ± 2 | 60 ± 1 °C | 8 h | 6.1 ± 0.03 L H2/L per day | 36 |
| Tequila vinasses | Clostridium and Bacillus species | 20 g COD/L per day | 7.5 | 54.7 °C | 29 h | 0.15 L H2/L per h | 37 |
| Cheese whey | Rhodobacter sphaeroides | 70.4 kg COD/m3 per day | 4.25 | 28 ± 2 °C | 8 h | 3.78 ± 3.33 × 10−3 mol H2 per day | 38 |
| Food waste | Enterococcus and Veillonella | 22 g COD/L per day | 5.5–7.0 | 37 °C | 3 days | 0.73 ± 0.28 L H2/L per day | 39 |
| Apple pulp waste | Sporolactobacillus (39%), Clostridium (15%), and Coprothermobacter (10%) | 100 g COD/L per day | 7.2 | 37 ± 1 °C | 12 h | 0.07 L per g volatile solids | 40 |
| Cacao pod husk | Ethanoligenes | 35 g COD/L per day | 4.0–4.5 | 25.24–26.16 °C | 78–103 h | 0.25 ± 0.007 L/L per day | 41 |
| Potato waste | Enterobacter aerogenes MTCC2822 | 20 g COD/L per day | 7.0 | 50 °C | 48 h | 1.58 L H2/L per day | 42 |
| Brewery wastewater | Clostridia species | 38.44 g COD/L per day | 4.13–4.72 | 55 °C | 20 days | 0.60 to 1.50 g/L per day | 43 |
| Cassava wastewater | Eubacterium | 14 g COD/L per day | 6.0 | 30 ± 1 °C | 4 h | 0.7 L H2/L per day | 44 |
| Citrus peel waste | Escherichia and Clostridium | 30 g COD/L per day | 5.5–8.5 | 30–44 °C | 20.9 h | 0.84 L H2/L per day | 45 |
| Crude glycerol | Clostridiales | 20 g COD/L per day | 5.5 | 37 °C | 28 h | 3.94 L H2/L per day | 46 |
One of the most efficient ways for BHP in a batch reactor is using starch because of its of abundance, fermentability by various bacteria, high hydrogen yield, and cost-effectiveness. The hydrogen yield is capable of achieving 0.199–0.240 LH2 g−1 in a batch reactor.52 It was found that the initial concentration given to the reactor had an impact on the amount of biogas and hydrogen generated, and the temperature and HRT had an impact on the extent to which this effect was produced.10 Using an ASBR without pH control, the batch biohydrogen yield of rice wastes (rice bran and rice straw) was assessed by combined assimilation with heat-shocked slurry under ambient and high temperatures. Due to its higher lignin concentration of 19.34% compared to rice straw, rice husk exhibited the lowest experimental yield under ambient conditions.53 The process of dark fermentation of palm oil mill discharge involves the evaluation of the two-stage process by employing enriched mixed culture in an ASBR system. It was observed that the two-stage ASBR system with thermophilic and mesophilic BHP was effective for both total suspended solids degradation and combined sugar utilization in palm oil mill discharge at the ideal HRT of 12 h.54
The impact of several parameters, including pH and temperature, on hydrogen output was assessed for BHP from food waste through dark fermentation. It was observed that at 5.5 pH, temperature of 37 °C, and 0.80 L of working volume, the hydrogen yield was 0.064 L g−1 the energy yield was 0.7 kJ g−1. Under other conditions, involving a 0.15 L working volume, 37 °C temperature, and pH of 5.5, the energy yield was 1.2 kJ g−1 and BHP was 0.106 L g−1. These results illustrate the manner in which operating conditions impact energy recovery and hydrogen-generating yield.55 In utilizing chemical wastewater as a substrate, a sequencing batch reactor configured with a biofilm was employed to generate hydrogen via fermentative processes. The results revealed a discernible correlation between the variation in OLR and the corresponding fluctuation in the rate of hydrogen production, indicating a decrease in hydrogen generation with higher OLRs.51
3.2. Applications of CSTR
Hydrogen-producing microbes are thoroughly combined and suspended from the mixing pattern in the reactor liquid in a CSTR for continuous hydrogen production. Mass transport and a good substrate–microbe interaction can be achieved under such hydrodynamics. Short HRTs may result in biomass washout, which significantly limits the rates at which hydrogen can be produced.59 Microorganisms leveraging photosynthesis degrade organic compounds through photolysis to contribute to hydrogen production.60 Photosynthetic hydrogen generation can be achieved by microalgae and their different metabolic pathways using photosystems and light-independent fermentation. As a result of this process, a variety of small organic compounds are produced, including formate, acetate, and ethanol, in combination with hydrogen.61 Dark fermentation also produces low-weight organic compounds such as VFA and alcohols. This process of assimilation can be carried out by the genus of bacteria belonging to the genera Prevotella, Lactobacillus, Clostridium, Selenomonas, Megasphaera, and Enterobacter, with the most representative members belonging to these genera.62 CSTRs, being the most versatile reactor type, have been utilized by continuously introducing authentic wastewater to achieve high-rate BHP.63| Substrate | Micro-organism | OLR | pH | Temperature | HRT | BHP rate | Reference |
|---|---|---|---|---|---|---|---|
| Poplar leaves | Enterobacter aerogenes ATCC 13048 | 200 g COD/L per day | 5–8 | 30–40 °C | 14 h | 0.6 mol H2/mol per h | 64 |
| Date waste | Thermotoga maritima | 204 g COD/L per day | 7.0 | 80 °C | 4 h | 0.002 to 0.017 mol H2/L per h | 65 |
| Peanut shell | Firmicutes, Bacteroidetes and Proteobacteria | 12 g COD/L per day | 4.85–5 | 60 °C | 6 h | 0.58 L H2/L per day | 66 |
| Acidic cheese whey | Clostridium and Lactobacillus species | 309 g COD/L per day | 5 | 55 °C | 4.5 h | 3.2 L H2/L per day | 67 |
| Sugarcane biorefineries | Clostridium and Thermoanaerobacterium | 30–120 g COD/L per day | 5.0–6.0 | 55 °C | 8–16 h | 3.63 L H2/L per day | 2 |
| Pig manure | Enterobacter aerogenes | 6.5 g COD/L per day | 5.5 | 35 °C | 3 days | 15.8 L H2/L per day | 68 |
| Waste molasses | Propionibacterium, Desulfobulbus, Methylobacterium, Clostridium | 70 g COD/L per day | 5.7 | 60 °C | 6 h | 0.417 L H2/L per day | 69 |
| Sewage sludge and wine vinasse | Eubacteria | 51.4 g COD/L per day | 5.0–6.0 | 55 °C | 12 h | 35.19 L H2/L per day | 70 |
| Catering waste | Methanosaeta concilii | 91.5 g COD/L per day | 6.5 | 27 °C | 24 h | 3.84 L H2/L per day | 71 |
| Xylose | Lactobacillus and Sporolactobacillus species | 21.61 g COD/L per day | 7.5 | 30 °C | 3 h | 30.26 ± 1.19 L H2/L per day | 72 |
| Rice straw | Ruminiclostridium species | 20 g COD/L per day | 6.5 | 55 °C | 3 days | 24.8 L H2/L per day | 73 |
In an effort to increase the volumetric hydrogen generation rate and the hydrogen yields, the impact of various HRT and OLRs with cheese lactoserum powder solution was examined. It exhibited the highest hydrogen molar yield of 2.8 mol H2 per mol lactose at an OLR of 138.6 g lactose/L per day and a HRT of 6 h. This combination resulted in the highest volumetric hydrogen production rate of 0.046 molH2 L−1 h−1. The microbial communities enriched at each culture condition were investigated using polymerase chain reaction – denaturing gradient gel electrophoresis, and the major metabolites produced were also observed. A clear dominance of Clostridium species, specifically Clostridium butyricum CM-C86 and Clostridium butyricum CM-C97 was detected.78 The microbial ecology of hydrogen-producing and lactate/acetate-utilizing bacteria that were enriched in the CSTR was examined using molecular techniques. The cloning and sequencing indicated that Clostridium tyrobutyricum was considered the major hydrogen-producing bacteria in the CSTR fed with lactate and acetate, which resulted in OLR of 55.64 g COD/L per day, for fermentation along with 6.60 mol H2 per mol lactose.79 A laboratory-scale CSTR employing hybrid immobilization with a substrate concentration of 60 g L−1 of galactose was operated at an OLR of 120 g/L per day and a HRT of 3 h. The reactor exhibited a peak BHP rate of 25.9 L H2/L per day, while a 6 h HRT yielded the maximum hydrogen yield of 2.21 mol H2 per mol galactose. Briefly, reducing the HRT to 2 h resulted in a recorded BHP rate of 39.65 L H2/L per day; however, the process failed to sustain due to the rapid washout of the hydrogen production consortium.63 The integration of a CSTR with an UASB reactor was explored in a two-stage process aimed at biohydrogen and methane production. Under optimal HRT of 3 h, the CSTR yielded methane at a rate of 2.25 L/L per day and achieved a maximum BHP rate of 17.5 L/L per day.80 Additionally, a separate investigation utilized a thermophilic mixed microbial culture as the inoculum, evaluating the fermentative hydrogen generation potential of waste and sludge obtained from a tofu-processing anaerobic digester, both in batch and continuous operation modes. At 4 h HRT, higher yields of 2.3 mol H2 per mol glucose equivalent and BHP rate of 12 L H2/L per day were attained.81
3.3. Applications of PBR for BHP
PBRs are widely used in BHP due to their versatile nature and efficiency. For continuous BHP, PBRs are suitable for certain biohydrogen-producing species such as Clostridium.87 To allow for the fast transfer of biohydrogen and to lessen the strong effect of partial pressure and supersaturation of biohydrogen, a horizontal fixed bed reactor was designed with pH stabilized at 5.9. At 6 h HRT and OLR of 0.88 g L−1, maximum affordable conditions for BHP by Clostridium butyricum in mixed culture was found. PBRs also have high biomass concentration as the immobilized microorganisms attached to the solid support material, provides a large surface area for the microorganisms to grow and multiply. These immobilized microorganisms are more stable and less prone to washout, therefore improving their ability to operate for longer periods of time.13 PBRs only need minimum amount of additional equipment and can be used in small commercial units. Other advantages of these reactors include easy extraction of catalyst from reactor effluent stream and the wide variety of times at which these reactors can be operated.The pH is another critical factor that impacts the performance of PBRs for continuous BHP. During continuous BHP, the pH inside the reactor may drop due to organic acid accumulation, inhibiting metabolic activity and reducing the BHP rate.88 It was observed that increasing pH, led to increase of production ability in hydrogen producing bacteria. Optimal pH for fermentative BHP in a continuous PBR was reported by researchers to be 4.2 and 7 respectively.93,94 In another study, researchers investigated the BHP from traditional Chinese medicine wastewater as substrate in AnPBR by varying the pH range from 5.6 to 6.4 and also by trying different HRTs from 24 h to 6 h.95 HRT is also another factor that influences the BHP rate by affecting the residence time of microorganisms. The performance of an anaerobic packed bed reactor under different HRTs was studied from 6 h to 2 h to generate biohydrogen gas, with glucose as the substrate.96 HRTs varied between 2 h and 3 h, concluded that volumes ranged from 4.5 L per day and 5 L per day. The volumetric BHP rate saw an increase from 3.7 L per day to 4.73 L per day when HRT was changed from 2 h to 6 h, and at 6 h of HRT, the maximum yield of 0.89 mol was obtained for BHP. Interestingly, the hydrogen percentage remained stable at around 60% for all the HRTs examined. The BHP rate in a PBR by using paper mill effluent as a source of hydrogen was studied at HRT range of 2 to 24 h, and found that the highest BHP rate 6.21 L H2/L per day at the HRT of 2 h and the highest biohydrogen concentration of 41.5% in produced gas to be at HRT of 4 h.14 A longer HRT can also lead to the accumulation of organic acids, thereby reducing the BHP rate, whereas a shorter HRT reduces reactor volume and increases the BHP rate, resulting in a more compact and efficient system.88 An overview of the influence of various parameters on BHP is given in Table 3.
| Substrate | Micro-organism | OLR | pH | Temperature | HRT | BHP rate | Reference |
|---|---|---|---|---|---|---|---|
| Pre-settled paper mill effluent | Anaerobic sludge | 27.35 g COD/L per day | 7 | 37 °C | 2 h | 6.21 L H2/L per day | 14 |
| Traditional Chinese medicine wastewater | Clostridium butyricum | 328.2 g COD/L per day | 6.6 | 37 °C | 6 h | 7.92 × 10−3 mol H2/L per h | 95 |
| Glucose | Clostridium, Ethanoligenens, Lactobacillus | 40 g COD/L per day | 5.7 | 35 °C | 24 h | 5.3 L H2/L per day | 97 |
| Glucose | Clostridium, Ethanoligenens, Lactobacillus | 80 g COD/L per day | 5.7 | 35 °C | 24 h | 4 L H2/L per day | 97 |
| Rice straw | Anaerobic seed sludge | — | 5.3 | 37 °C | 2 h | 0.252 L H2/L per h | 98 |
| Winery wastewater | Lactobacillus, pectinatus, Clostridium | 523 g COD/L per day | 5.5 | 37 °C | 5.5 h | 1.44 L H2/L per day | 35 |
| Glycerol | Rhodopseudomonas Palustris | — | 7 | 28 °C | — | 0.012 L H2/g per h | 99 |
| Sugar beet molasses | Clostridium pasteurianum, Clostridium tyrobutyricum | 850 g COD/L per day | 4.4 | — | 8 h | 208.3 L H2/L per day | 100 |
| Sugar beet molasses | Clostridium pasteurianum, Clostridium tyrobutyricum | 790 g COD/L per day | 4.6 | — | 9 h | 100.1 L H2/L per day | 100 |
| Glucose | Anaerobic sludge | 64 g glucose/L per day | 5 | 48 °C | 6 h | 4.73 L H2/L per day | 96 |
3.4. Application of MBR for BHP
Membrane bioreactors (MBRs) are now emerging as one of the most efficient systems. The usage of MBRs has been concentrated on the BHP, mainly when combined with biological processes. MBRs can be classified mainly on their microbial structure and their performance. MBRs can be classified mainly on their microbial structure and their performance. Based on their microbial structure, MBRs are classified as aerobic (AeMBRs) and anaerobic MBRs (AnMBRs). AnMBRs are designed by adding together the membrane technology with traditional anaerobic fermenters. The main difference between these two types of MBRs is the lack of aeration system and low biomass production rate in AnMBRs. But in comparison to AeMBRs, AnMBRs only require lesser energy because of the lack of aeration system.104| Substrate | Micro-organism | OLR | pH | Temperature | HRT | BHP rate | Reference |
|---|---|---|---|---|---|---|---|
| Diluted grape deposits | Dark fermentation of biomass | 2 g COD/L per day | 5 | 37 °C | 8 h | 4.5 L H2/L per day | 105 |
| Mixed sugar | Clostridium pasteurianum | 2.54 g COD/L per day | 5.5–6 | — | 3 h | 28.52 L H2/L per day | 106 |
| Glucose (MBR with polyester mesh) | Clostridium pasteurianum | 2.63 g COD/L per day | 5.2 | — | 2 h | 44.22 L H2/L per day | 17 |
| Glucose (MBR with stainless steel mesh) | Clostridium puniceum | 2.99 g COD/L per day | 5.2 | — | 2 h | 51.64 L H2/L per day | 17 |
| Glucose | Clostridium butyricum | 21.66 g COD/L per day | 5.5–6 | 37 °C | 2 h | 50.37 L H2/L per day | 107 |
| Glucose | Clostridium butyricum, sludge originated biohydrogen producers | 18.88 g COD/L per day | 5.5–6 | 37 °C | 2 h | 58.57 L H2/L per day | 107 |
| Glucose | Clostridium butyricum | 21.3 g COD/L per day | 5.5–6 | 37 °C | 2 h | 58.86 L H2/L per day | 108 |
| Glucose | Clostridium beijerinckii, Clostridium pasteurianum, Enterobactor species | 0.9 g glucose/L per day | 4.8 | 37 °C | 12.5 h | 0.135 L H2/L per h | 109 |
| Food waste | Clostridium species | 9.42 g COD/L per day | 5.5–6 | 37 °C | 8 h | 7.09 L H2/L per day | 110 |
| Xylose | Clostridium species | 17.37 g COD/L per day | — | 37 °C | 3 h | 30.26 L H2/L per day | 72 |
| Algal biomass | Clostridium species, Anaerostipes species, Caproiciproducens species | 21.4 g COD/L per day | 5.5 | — | 3 h | 21.58 L H2/L per day | 111 |
| Food waste | Clostridium thermopalmarium, Clostridium butyricum | 18.61 g COD/L per day | 7 | 55 °C | 120 h | 0.146 L H2 per g volatile solid added | 112 |
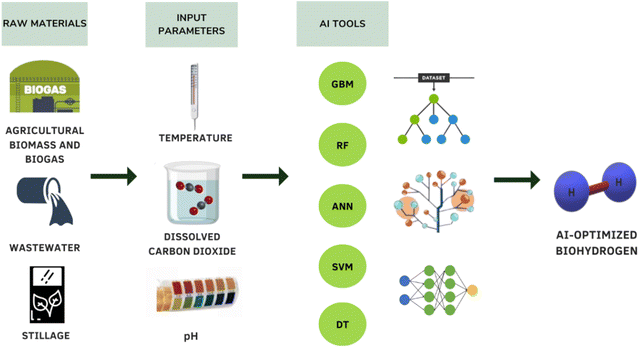
4. AI integration
The BHP from dark fermentation is a highly complex procedure, but this process can be made efficient and economical by employing various AI techniques. Recent research has shown that producing biohydrogen from agricultural biomass is a comparatively green technique because it requires lower temperatures and pressures during the synthesis process. Due to its economic and environmental potential, BHP from agricultural waste has attracted attention on a global scale. Design and optimization techniques can prove to be useful to scale-up bioprocess technology to the industrial level. Innovation is supported by ML in a number of domains, such as waste-to-energy network design and online process control. The manufacturing processes can be improved and modelled to reach a maximum yield and BHP rate, as shown in Fig. 3. The main objective of modelling is to improve yields by optimizing the processes involved in producing these biofuels.125,126ML tools are increasingly integrated into BHP processes, offering notable benefits in efficiency, reliability, and cost reduction. By utilizing ML algorithms to analyze data patterns in BHP, these tools allow for the identification of critical trends, aiding in performance optimization. ML, as a reliable predictive model, assists in estimating process efficiency and predicting yield outcomes across BHP stages, making it a valuable tool for enhancing overall productivity while lowering operational expenses.127 Since ML models rely heavily on pre-existing data, they provide rapid response capabilities by approximating complex, non-linear behaviors of input parameters in BHP, thereby allowing for real-time monitoring and control.128 This adaptability is especially advantageous in BHP due to the variable nature of input conditions, enabling quick, data-driven adjustments to optimize yield and maintain process stability. By offering insights into parameter interactions and predicting outcomes, AI-driven models play a critical role in efficiently managing BHP processes with minimal trial-and-error experimentation.
4.1. Artificial neural networks for BHP
The production of biohydrogen involves several complex steps, and the application of ANN to different steps can result in enhanced outputs and effective production. Three ANN models used to predict parameters such as BHP rate, accumulated hydrogen production and hydrogen yield, by determining the profiles of volatile fatty acids generated during manufacturing using a developed ANN model reported highly accurate results (R2 > 0.987).129 ANN has been used to estimate BHP rate in a CSTR, with a minimum training error of 0.0438 and maximum R2 value of 0.92, by using input parameters such as HRT, biomass concentrations and pH to model a separate neural network.130 An ANN model with a feed-forward algorithm, four input and two hidden layers was used to model BHP from a continuous anaerobic sludge blanket filter using 109 data points for training. The ANN model correlated the inputs and outputs using optimum weights with R2 value of 0.9981.131 ANN modelling carried out on the efficiency of BHP on sugar cane molasses revealed a R2 value of 0.91, revealing that ANN has great accuracy in modelling relationships between input and output parameters for fermentative BHP. In this study, back propagation algorithm was used to train the ANN model.132 The estimated BHP in fermentative processes and the quantity of biohydrogen released from genetically-altered E coli bacteria was predicted using BPNN with twelve nodes and one hidden layer. The study reported a correlation coefficient (R2) of 0.955.126 Similarly, back propagation algorithm modelled to train and estimate error, in a process meant to predict hydrogen yield during the dark fermentation of coffee mucilage wastes give an error less than 0.002.133 Another back propagation model of BHP in a hybrid up-flow anaerobic sludge blanket reactor made use of four inputs and one output reported training regression coefficient R2 of 0.929 in the operation phase of BHP.134ANN have been effectively integrated into BHP, especially in optimizing complex bioprocesses with multiple interdependent steps. By modeling intricate interactions between input and output parameters, ANN enables a better understanding of how different inputs affect the biohydrogen yield without requiring a comprehensive mechanistic understanding of the underlying processes.135 In scenarios where governing frameworks are either undefined or complex, ANN provides a robust solution by accurately representing these non-linear relationships, facilitating real-time decision-making.136,137 ANN proves particularly useful in tasks such as prediction, monitoring, and process control in BHP, which are critical to optimizing production costs and time efficiency. It enhances the process by forecasting performance trends, identifying optimal operating conditions, and adjusting parameters for consistent yield maximization.56 By reducing reliance on extensive experimental setups and time-consuming manual adjustments, ANN contributes to cost-effective, reliable BHP operations, underscoring its value as a primary AI tool for improving BHP productivity.
4.2. Support vector machine for BHP
SVM is another very important algorithm used majorly in classification and troubleshooting. BHP from wastewater using the dark fermentation procedure was predicted using different ML models including SVMs. The SVM model resulted in a high determination coefficient (R2) of 0.885, which was dependent on a variety of parameters such as environmental temperature, pH of solution, materials used and HRT.138 Another investigation reported that SVM had an improved performance in terms of classification and regression analysis when compared with other traditional statistical algorithms.128 SVM used to model the process involving hydrogen generation from organic wastes reported a high R2 of 0.988 of the hydrogen yield.139 Different ML models were used to check the yield of hydrogen produced from supercritical water gasification of biomass, which resulted in an R2 value of 0.9768 and a root mean square error of 0.8740 for the SVM model, which performed better than their ANN model.140 SVM has been reported to be highly affected by noisy inputs, has high computation cost and is a slower, more expensive method than ANN.128 However, it is a huge asset when users need to control the output error, and in cases where input data is highly disorganized.141 One major advantage of using SVM algorithm is that it can allow users to specify or set the threshold levels for the amount of error that should be endured by the model.142 This provides greater control over the error and leads to better, more accurate results.4.3. Other algorithms for BHP
Decision Tree (DT) is a widely-used ML algorithm used for creating classification systems relying on several variables. Random Forest (RF) is an algorithm that prevents data points on different decision trees from converging by using bootstrap aggregation.143 In a study of microalgal hydrogen production, the researchers noted that RF was resilient to over-fitting, a situation where the learning fits completely over the data used to train it. This affects the ability of model to foresee future outcomes.141 Gradient boosting machine (GBM) is an improvement model used along with regression trees to generate strong, highly reliable, and comprehensible algorithms. In comparison to other ML and regression models, GBM is more flexible, has superior precision and involves less usage of processing power.144 Researchers reported an R2 value of 0.976 in the validation phase and 0.805 in the testing phase with RF. Furthermore, the mean squared error was only 0.004 in the validation phase and 0.023 in the testing phase.138 In case of GBM, the R2 and mean squared error value of were 0.985 and 0.002, respectively in the training or validation phase and 0.802 and 0.023, respectively in the testing phase. All models showcased similar strengths (R2 value), and almost identical mean squared error values. However, while comparing mean absolute error, SVM to show least error, followed by GBM and RF. Another gradient boosting DT to ascertain properties of cellulose-rich materials produced from lignocellulosic biomass reported a low R2 value of approximately 0.90.145 In situations where only limited or partial inputs are present, RF holds a clear advantage over other ML algorithms like ANN.5. Life cycle and technoeconomic analysis
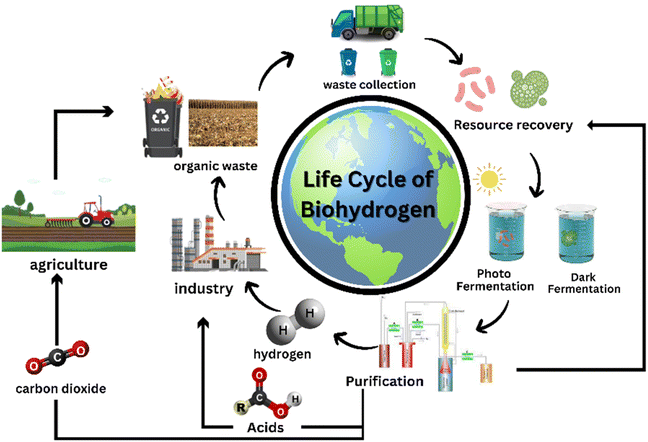
LCA is considered a crucial requirement to prove the sustainability of the process or technology.146 Though not sustainable, fossil fuels are widely used to produce hydrogen as the process involved is inexpensive as compared to greener alternatives.113 BHP from organic sources helps in pollution control as well as gives us an alternative source of energy, as shown in Fig. 4. The researchers observed that around 75% of the total greenhouse gas emissions could be attributed to the supply of heat and more than 15% was due to electricity used in the entire process of biohydrogen produced through dark photosynthesis.147 Another LCA study revealed that among various BHP processes like gasification of softwood and steam reforming, production of biohydrogen through eucalyptus leaves and their gasification is responsible for the least carbon footprint of approximately 1.6 kg of CO2 equivalent per kg hydrogen.148 The two processes extensively used to produce hydrogen from ethanol, i.e., dehydrogenation and steam reforming showed that using the steam reforming method released carbon even though it was cheaper, whereas dehydrogenation had zero carbon emission coupled with the release of industry-relevant chemicals like ethyl acetate and acetaldehyde.149 Three approaches have been listed from a life-cycle viewpoint for evaluating the methods to valorize stillage produced during bioethanol production via composting, one-stage anaerobic digestion and dark fermentation followed by anaerobic digestion.150 Although anaerobic digestion was a more favourable approach, there was not much difference between the LCA of dark fermentation and anaerobic digestion for anyone to make a reasonable distinction between them.Technoeconomic analysis are conducted to verify the economic feasibility and establish the optimisation pathways. Though costs to produce biohydrogen may differ depending on the procedure used or method followed, it takes an estimated $3.2–$48.96 to produce one kg biohydrogen through dark fermentation. Pretreatment costs vary from procedure to procedure and can sometimes contribute to more than 50% of the total operational cost.151 Raw materials used to maintain the BHP plants may contribute up to 80% of production costs. An investigation carried out to compare the cost of hydrogen production from conventional sources and fermentation concluded that producing hydrogen through the former using methods such as electrolysis, natural gas reforming and coal gasification is less expensive.113 A comprehensive breakdown of expenditure related to BHP through dark photosynthesis found the plant costs contributed to 28% while direct and indirect costs contributed to 27% of the total capital expenditure. Operational expenditures, on the other hand, are expenses made to keep the production plant up and running. These include resources like heat, water and electricity.147 Another study on TEA listed the minimum costs required to produce biohydrogen through several methods and claimed that at $1.20 per kg hydrogen, gasification is the most feasible process, followed by anaerobic digestion at $1.25 per kg. However, hydrogen generated from domestic biomass was less expensive ($2.40 per kg hydrogen) than that produced from agricultural residue ($4.55 per kg hydrogen).148 An all-inclusive analysis of the costs associated with different thermochemical technologies to produce hydrogen were found to be economically feasible with an approximate payback time of 5.10 to 7.18 years.152 Some improved technologies like photo-bioreactors and solar photovoltaic-based production plants can produce biohydrogen at much cheaper rates of $2 per kg and $14 per kg, respectively.153
6. Future directions
Research efforts are increasingly focused on biohydrogen production as a viable and efficient alternative energy source, driven by the escalating decline of fossil fuels and its consequential effect on the natural ecosystem. Currently, steam reforming of methane is the most cost-effective technique of hydrogen synthesis, contributing more than 50% of world's hydrogen generation.154 The focused problem is costing, which is higher because of reduced hydrogen yields and the process instability. Fermentation process provide lower yields with low conversion efficacy, which may be related to the biomass structure, necessitating sophisticated processing techniques during the process.155 In addition, the hunt for cheaper biomass is critical. Operating time, capital investment, electricity produced, and conversion efficiency are all factors that influence biohydrogen production rate during the electrolysis process.156 A rising strategy for dealing with the main economic concern is the shift toward integrated hydrogen production systems capable of increasing hydrogen yields. In addition, combined dark and photo fermentative procedures (two-stage integration) are being used to assist hydrogen generation techniques.157 The incorporation of hydrogen with the biodiesel manufacturing process can boost energy recovery by 90.3% over single stage hydrogen synthesis, which has to be thoroughly investigated.158 A hydrogen molar production of 77% of the theoretical maximum of burned feedstock was achieved during 15 days of operation using integrated dark-photo-fermentative technique.159 Furthermore, the use of synthetic enzymatic pathways based on a set of recombinant enzymes allows for virtually full conversion of various types of carbohydrates into ‘green’ hydrogen with stoichiometric conversion yields close to theoretical values. Although conversion rates and total prices need to be reduced, cost projections predict that in the future, this sustainable and ecologically friendly strategy will be comparable with thermochemically generated hydrogen.160 The extent of hydrogen production in the next years will be determined not only by scientific breakthroughs (improvements in efficiency through genetically modified microbes, bioreactor development, efficient manufacture of thermostable enzymes, among other things), but also by economic factors.7. Conclusion
In light of the urgent need for sustainable energy solutions to address depleting fossil fuels and environmental concerns, biohydrogen production offers significant potential as a renewable energy source. This review highlights that optimizing reactor configurations and operational parameters, such as substrate concentration, microbial strain selection, temperature, pH, and HRT, is crucial for maximizing efficiency. However, the reactors face challenges like substrate inhibition, operational stability, and membrane fouling, which necessitate ongoing research and innovation to overcome these limitations. Key findings indicate that while each BHP method and reactor type presents unique advantages and challenges, optimizing operational parameters such as substrate selection, temperature control, and pH balance is critical for maximizing hydrogen yield and process efficiency. The integration of AI-driven tools into BHP processes represents a promising development, enabling predictive modeling, process optimization, and improved real-time control that could drive significant gains in efficiency and scalability. Furthermore, it is important for manufacturers to conduct LCA and TEA, which allows to decrease greenhouse emissions by using alternative production processes and minimizing costs by reducing direct and indirect plant costs.Moving forward, future research should focus on overcoming these limitations through innovative reactor designs, AI integration, and sustainable waste management practices. As BHP technology advances, ongoing policy support, combined with rigorous research and development, will be essential to realizing the potential of biohydrogen as a foundation of the global renewable energy transition.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.References
- X. Zhang, M. Schwarze, R. Schomäcker, R. van de Krol and F. F. Abdi, Nat. Commun., 2023, 14, 991 CrossRef CAS.
- L. T. Fuess, Int. J. Hydrogen Energy, 2024, 49, 532–553 CrossRef CAS.
- H. C. Puchakayala, A. Viswanathan, I. Abrar and N. Rajamohan, Sustain. Energy Technol. Assessments, 2023, 60, 103539 CrossRef.
- D. Levin, Int. J. Hydrogen Energy, 2004, 29, 173–185 CrossRef CAS.
- P. Mishra, S. Krishnan, S. Rana, L. Singh, M. Sakinah and Z. Ab Wahid, Energy Strat. Rev., 2019, 24, 27–37 CrossRef.
- H. Koku, Int. J. Hydrogen Energy, 2003, 28, 381–388 CrossRef CAS.
- S. Moscow, V. Kavinkumar, M. Sriramkumar, K. Jothivenkatachalam, P. Saravanan, N. Rajamohan, Y. Vasseghian and M. Rajasimman, Chemosphere, 2022, 299, 134343 CrossRef CAS PubMed.
- A. V. Samrot, D. Rajalakshmi, M. Sathiyasree, S. Saigeetha, K. Kasipandian, N. Valli, N. Jayshree, P. Prakash and N. Shobana, Sustainability, 2023, 15, 12641 CrossRef CAS.
- V. Lalit Babu, S. Venkata Mohan and P. N. Sarma, Int. J. Hydrogen Energy, 2009, 34, 3305–3312 CrossRef CAS.
- G. Buitrón and C. Carvajal, Bioresour. Technol., 2010, 101, 9071–9077 CrossRef PubMed.
- V. Piemonte, L. Di Paola, S. Chakraborty and A. Basile, Int. J. Hydrogen Energy, 2014, 39, 4863–4869 CrossRef CAS.
- K. Y. Show, Z. P. Zhang, J. H. Tay, D. T. Liang, D. J. Lee, N. Ren and A. Wang, Int. J. Hydrogen Energy, 2010, 35, 13350–13355 CrossRef CAS.
- S. Roy, M. Vishnuvardhan and D. Das, Appl. Energy, 2014, 136, 51–58 CrossRef CAS.
- M. Namdarimonfared, H. Zilouei and H. Tondro, Fuel, 2023, 338, 127231 CrossRef CAS.
- K. Y. Show, D. J. Lee, J. H. Tay, C. Y. Lin and J. S. Chang, Int. J. Hydrogen Energy, 2012, 37, 15616–15631 CrossRef CAS.
- M. Aslam, R. Ahmad, M. Yasin, A. L. Khan, M. K. Shahid, S. Hossain, Z. Khan, F. Jamil, S. Rafiq, M. R. Bilad, J. Kim and G. Kumar, Bioresour. Technol., 2018, 269, 452–464 CrossRef CAS.
- J. Yang, Y.-B. Sim, S. Moon Kim, H.-H. Joo, J.-H. Jung and S.-H. Kim, Bioresour. Technol., 2023, 377, 128900 CrossRef CAS PubMed.
- A. I. Osman, T. J. Deka and D. C. Baruah, Biomass Convers. Biorefin., 2023, 13, 8383–8401, DOI:10.1007/s13399-020-00965-x.
- A. Tiwari and K. Nakamura, Int. J. Hydrogen Energy, 2024, 81, 595–614 CrossRef CAS.
- Z.-T. Zhao, J. Ding, B.-Y. Wang, M.-Y. Bao, B.-F. Liu, J.-W. Pang, N.-Q. Ren and S.-S. Yang, Chem. Eng. J., 2024, 481, 148444 CrossRef CAS.
- E. Yang, K. Chon, K.-Y. Kim, G. T. H. Le, H. Y. Nguyen, T. T. Q. Le, H. T. T. Nguyen, M.-R. Jae, I. Ahmad, S.-E. Oh and K.-J. Chae, Bioresour. Technol., 2023, 369, 128380 CrossRef CAS.
- I. Abrar, T. Arora and R. Khandelwal, Fuel Process. Technol., 2023, 242, 107646 CrossRef CAS.
- J. Cheng, C. Huang, Y. Zhan, S. Han, J. Wang, X. Meng, C. G. Yoo, G. Fang and A. J. Ragauskas, Chem. Eng. J., 2022, 443, 136395 CrossRef CAS.
- I. Valdez-Vazquez and H. M. Poggi-Varaldo, Renew. Sustain. Energy Rev., 2009, 13, 1000–1013 CrossRef CAS.
- J. Arun, T. Sasipraba, K. P. Gopinath, P. Priyadharsini, S. Nachiappan, N. Nirmala, S. S. Dawn, N. Thuy Lan Chi and A. Pugazhendhi, Fuel, 2022, 327, 125112 CrossRef CAS.
- Y. Cao, H. Liu, W. Liu, J. Guo and M. Xian, Microb. Cell Factories, 2022, 21, 166 CrossRef CAS PubMed.
- H. Argun and F. Kargi, Int. J. Hydrogen Energy, 2011, 36, 7443–7459 CrossRef CAS.
- M. Ni, D. Y. C. Leung, M. K. H. Leung and K. Sumathy, Fuel Process. Technol., 2006, 87, 461–472 CrossRef CAS.
- H.-S. Lee, W. F. J. Vermaas and B. E. Rittmann, Trends Biotechnol., 2010, 28, 262–271 CrossRef CAS.
- M. N. I. Siddique, M. Momeen Ul Islam, N. Shila Rasit, N. Binti Ali and W. Sani Wan Nik, Int. J. Environ. Monit. Anal., 2021, 9, 190 CAS.
- S. Gupta, A. Fernandes, A. Lopes, L. Grasa and J. Salafranca, Appl. Sci., 2024, 14, 1191 CrossRef CAS.
- I. Ntaikou, G. Antonopoulou and G. Lyberatos, Waste Biomass Valorization, 2010, 1, 21–39 CrossRef CAS.
- D. Cheong, C. L. Hansen and D. K. Stevens, Biotechnol. Bioeng., 2007, 96, 421–432 CrossRef CAS PubMed.
- Z. Guellout, E. Francois-Lopez, Y. Benguerba, C. Dumas, K. K. Yadav, A. M. Fallatah, A. Pugazhendhi and B. Ernst, J. Environ. Manage., 2022, 305, 114393 CrossRef CAS PubMed.
- C. Mejía-Saucedo, G. Buitrón, M. F. León-Galván and J. Carrillo-Reyes, Int. J. Hydrogen Energy, 2022, 47, 5891–5900 CrossRef.
- A. Akhbari, O. C. Chuen and S. Ibrahim, Int. J. Hydrogen Energy, 2021, 46, 10191–10204 CrossRef CAS.
- A. Toledo-Cervantes, F. Villafán-Carranza, J. Arreola-Vargas, E. Razo-Flores and H. O. Méndez-Acosta, Int. J. Hydrogen Energy, 2020, 45, 11000–11010 CrossRef CAS.
- R. Rao and N. Basak, Appl. Biochem. Biotechnol., 2021, 193, 2297–2330 CrossRef CAS PubMed.
- I. Moreno-Andrade, M. J. Berrocal-Bravo and I. Valdez-Vazquez, J. Chem. Technol. Biotechnol., 2023, 98, 230–237 CrossRef CAS.
- Ö. B. Gökçek, F. Baş, H. Muratçobanoğlu and S. Demirel, Fuel, 2023, 339, 127475 CrossRef.
- J. A. Kriswantoro and C.-Y. Chu, J. Clean. Prod., 2024, 434, 140407 CrossRef CAS.
- S. R. Das and N. Basak, Biomass Convers. Biorefin., 2024, 14, 4791–4811 CrossRef CAS.
- W. G. Sganzerla, L. Sillero, T. Forster-Carneiro, R. Solera and M. Perez, Bioenergy Res., 2023, 16, 1073–1083 CrossRef CAS.
- A. G. Mari, C. L. Andreani and T. Uana, Int. J. Hydrogen Energy, 2020, 45, 5165–5174 CrossRef CAS.
- F. P. Camargo, I. K. Sakamoto, A. Bize, I. C. S. Duarte, E. L. Silva and M. B. A. Varesche, Int. J. Hydrogen Energy, 2021, 46, 7794–7809 CrossRef CAS.
- C. Varella, F. Abraham, R. Alcaraz, M. Gustavo, A. Varella, F. Motteran, M. Angela, T. Adorno, M. Bernadete, A. Varesche and S. Imaculada, Renewable Energy, 2020, 162, 701–711 CrossRef.
- P. Kirankumar, S. V. Krishna, N. Chaitanya, D. Bhagawan, V. Himabindu and M. L. Narasu, Biofuels, 2017, 8, 693–699 CrossRef CAS.
- F. Masihi, F. Rezaeitavabe, A. Karimi-Jashni and G. Riefler, Int. J. Hydrogen Energy, 2024, 52, 295–305 CrossRef CAS.
- S. G. Santiago, J. M. Morgan-Sagastume, O. Monroy and I. Moreno-Andrade, Int. J. Hydrogen Energy, 2020, 45, 25681–25688 CrossRef CAS.
- J. Ortigueira, T. Pinto, L. Gouveia and P. Moura, Energy, 2015, 88, 528–536 CrossRef CAS.
- S. V. Mohan, G. Mohanakrishna, S. Veer Raghavulu and P. N. Sarma, Int. J. Hydrogen Energy, 2007, 32, 3284–3292 CrossRef CAS.
- L. Chatellard and A. Marone, in Biohydrogen Production: Sustainability of Current Technology and Future Perspective, 2016, pp. 69–95 Search PubMed.
- A. Sattar, C. Arslan, C. Ji, S. Sattar, M. Umair, S. Sattar and M. Z. Bakht, Int. J. Hydrogen Energy, 2016, 41, 11050–11061 CrossRef CAS.
- R. M. Maaroff, J. Md Jahim, A. M. Azahar, P. M. Abdul, M. S. Masdar, D. Nordin and M. A. Abd Nasir, Int. J. Hydrogen Energy, 2019, 44, 3395–3406 CrossRef CAS.
- T. Jarunglumlert, C. Prommuak, N. Putmai and P. Pavasant, Int. J. Hydrogen Energy, 2018, 43, 634–648 CrossRef CAS.
- M. A. Mohd Asrul, M. F. Atan, H. Abdul Halim Yun and J. C. H. Lai, Int. J. Hydrogen Energy, 2022, 47, 16785–16804 CrossRef CAS.
- E. Taheri, M. Mehdi, A. Fatehizadeh and M. Rezakazemi, J. Environ. Manage., 2021, 292, 112759 CrossRef CAS PubMed.
- Y. Wang, G. Yang, V. Sage, J. Xu, G. Sun, J. He and Y. Sun, Environ. Prog. Sustainable Energy, 2021, 40, 13485 CrossRef.
- K.-Y. Show, D.-J. Lee and J.-S. Chang, Bioresour. Technol., 2011, 102, 8524–8533 CrossRef CAS.
- D. Das and T. N. Veziroǧlu, Int. J. Hydrogen Energy, 2001, 26, 13–28 CrossRef CAS.
- E. Eroglu and A. Melis, Int. J. Hydrogen Energy, 2016, 41, 12772–12798 CrossRef CAS.
- R. Palomo-Briones, E. Razo-Flores, N. Bernet and E. Trably, Appl. Energy, 2017, 198, 77–87 CrossRef CAS.
- G. Kumar, P. Sivagurunathan, J.-H. Park, J.-H. Park, H. Park, J. Yoon and S. Kim, Bioresour. Technol., 2016, 206, 188–194 CrossRef CAS.
- A. Y. Goren, M. Kenez, I. Dincer and A. Khalvati, J. Clean. Prod., 2024, 434, 140080 CrossRef.
- R. Saidi, M. Hamdi and H. Bouallagui, Waste Biomass Valorization, 2023, 14, 1859–1868 CrossRef CAS.
- N. Qi, X. Zhao, X. Hu, J. Wang and C. Yang, Renewable Energy, 2023, 219, 119492 CrossRef CAS.
- E. J. Ordoñez-Frías, K. M. Muñoz-Páez and G. Buitrón, Int. J. Hydrogen Energy, 2024, 52, 389–403 CrossRef.
- T. Weide, R. E. Hernández Regalado, E. Brügging, M. Wichern and C. Wetter, Chem. Eng. Technol., 2020, 43, 1578–1587 CrossRef CAS.
- K.-S. Lee, S.-L. Chen, C.-Y. Lin and J.-S. Chang, Int. J. Hydrogen Energy, 2021, 46, 16546–16554 CrossRef CAS.
- M. Tena, M. Perez and R. Solera, Int. J. Hydrogen Energy, 2021, 46, 7810–7820 CrossRef CAS.
- K. Z. Elwakeel, A. M. Elgarahy, H. M. Alghamdi and M. El-Qelish, J. Environ. Chem. Eng., 2023, 11, 110955 CrossRef CAS.
- J.-H. Baik, J.-H. Jung, Y.-B. Sim, J.-H. Park, S. M. Kim, J. Yang and S.-H. Kim, Bioresour. Technol., 2022, 344, 126205 CrossRef CAS PubMed.
- H. Chen, J. Wu, R. Huang, W. Zhang, W. He, Z. Deng, Y. Han, B. Xiao, H. Luo and W. Qu, Chemosphere, 2022, 286, 131655 CrossRef CAS PubMed.
- M. F. Arooj, S. K. Han, S. H. Kim, D. H. Kim and H. S. Shin, Int. J. Hydrogen Energy, 2008, 33, 3289–3294 CrossRef CAS.
- T. A. Kotsopoulos, I. A. Fotidis, N. Tsolakis and G. G. Martzopoulos, Biomass Bioenergy, 2009, 33, 1168–1174 CrossRef CAS.
- A. Castillo-Hernández, I. Mar-Alvarez and I. Moreno-Andrade, Int. J. Hydrogen Energy, 2015, 40, 17239–17245 CrossRef.
- W. Han, Y. Y. Hu, S. Y. Li, F. F. Li and J. H. Tang, Bioresour. Technol., 2016, 221, 318–323 CrossRef CAS.
- G. Davila-Vazquez, C. B. Cota-Navarro, L. M. Rosales-Colunga, A. de León-Rodríguez and E. Razo-Flores, Int. J. Hydrogen Energy, 2009, 34, 4296–4304 CrossRef CAS.
- C.-W. Wu, L.-M. Whang, H.-H. Cheng and K.-C. Chan, Bioresour. Technol., 2012, 113, 30–36 CrossRef CAS.
- C. Nualsri, P. Kongjan and A. Reungsang, Int. J. Hydrogen Energy, 2016, 41, 17884–17895 CrossRef CAS.
- M. Kim and D. Lee, Bioresour. Technol., 2010, 101, S48–S52 CrossRef CAS.
- E. Yahaya, S. W. Lim, W. S. Yeo and J. Nandong, Int. J. Hydrogen Energy, 2022, 47, 30404–30427 CrossRef CAS.
- A. K. Saini, T. Radu, K. Paritosh, V. Kumar, D. Tripathi and V. Vivekanand, Bioengineered, 2021, 12, 6418–6433 CrossRef CAS PubMed.
- M. A. Abdelkareem, B. Soudan, M. S. Mahmoud, E. T. Sayed, M. N. Almallahi, A. Inayat, M. Al Radi and A. G. Olabi, Chem. Eng. Res. Des., 2022, 182, 66–86 CrossRef CAS.
- M. Abdul, I. Ihsanullah, R. Ahmad, S. Ismail, A. Khan and A. Nizami, Int. J. Hydrogen Energy, 2022, 47, 37154–37170 CrossRef.
- R. Banu J, M. Usman T M, K. S, Y. Kannah R, Y. K N, S. P, A. Bhatnagar and G. Kumar, Int. J. Hydrogen Energy, 2021, 46, 16565–16590 CrossRef CAS.
- S. Hiligsmann, L. Beckers, J. Masset, C. Hamilton and P. Thonart, Int. J. Hydrogen Energy, 2014, 39, 6899–6911 CrossRef CAS.
- E. L. Cavalcante de Amorim, A. R. Barros, M. H. Rissato Zamariolli Damianovic and E. L. Silva, Int. J. Hydrogen Energy, 2009, 34, 783–790 CrossRef CAS.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2009, 34, 799–811 CrossRef CAS.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2008, 33, 5392–5397 CrossRef CAS.
- C. Li and H. H. P. Fang, Crit. Rev. Environ. Sci. Technol., 2007, 37, 1–39 CrossRef CAS.
- C. Lin, C. Wu and C. Hung, Int. J. Hydrogen Energy, 2008, 33, 43–50 CrossRef CAS.
- Y. Mu, H.-Q. Yu and Y. Wang, Chemosphere, 2006, 64, 350–358 CrossRef CAS.
- Q.-B. Zhao and H.-Q. Yu, Bioresour. Technol., 2008, 99, 1353–1358 CrossRef CAS.
- C. Sun, T. Sheng, L. Li and L. Yang, RSC Adv., 2021, 11, 5601–5608 RSC.
- I. Karapinar, P. Gokfiliz Yildiz, R. T. Pamuk and F. Karaosmanoglu Gorgec, Int. J. Hydrogen Energy, 2020, 45, 3524–3531 CrossRef CAS.
- Z. Zhang, K. Ding, X. Ma, S. Tang, Z. Wang, H. Lu, W. Jiang and B. Si, Energy, 2023, 271, 127059 CrossRef CAS.
- M. Safari, H. Tondro and H. Zilouei, Int. J. Hydrogen Energy, 2022, 47, 5879–5890 CrossRef CAS.
- B. S. Ross and R. W. M. Pott, Int. J. Hydrogen Energy, 2021, 46, 1715–1727 CrossRef CAS.
- A. Detman, D. Laubitz, A. Chojnacka, E. Wiktorowska-Sowa, J. Piotrowski, A. Salamon, W. Kaźmierczak, M. K. Błaszczyk, A. Barberan, Y. Chen, E. Łupikasza, F. Yang and A. Sikora, Front. Microbiol., 2021, 11, 612344 CrossRef PubMed.
- M. Nazarpour, A. Taghizadeh-Alisaraei, A. Asghari, A. Abbaszadeh-Mayvan and A. Tatari, Energy, 2022, 253, 124059 CrossRef CAS.
- B. Senthil Rathi, P. Senthil Kumar, G. Rangasamy and S. Rajendran, Int. J. Hydrogen Energy, 2024, 52, 115–138 CrossRef CAS.
- P. Sinha and A. Pandey, Int. J. Hydrogen Energy, 2011, 36, 7460–7478 CrossRef CAS.
- B. Jabbari, E. Jalilnejad, K. Ghasemzadeh and A. Iulianelli, Membranes, 2019, 9, 100 CrossRef CAS.
- M. Renaudie, C. Dumas, S. Vuilleumier and B. Ernst, J. Clean. Prod., 2024, 437, 140759 CrossRef CAS.
- S. M. Kim, Y.-B. Sim, J.-H. Baik, J. Yang, A. K. Pandey, H.-H. Joo, J.-H. Jung and S.-H. Kim, Chem. Eng. J., 2023, 452, 139384 CrossRef CAS.
- Y.-B. Sim, J. Yang, S. M. Kim, H.-H. Joo, J.-H. Jung, D.-H. Kim and S.-H. Kim, Bioresour. Technol., 2022, 366, 128181 CrossRef CAS PubMed.
- J.-H. Jung, Y.-B. Sim, J.-H. Park, A. Pandey and S.-H. Kim, Chem. Eng. J., 2021, 420, 127685 CrossRef CAS.
- M. Renaudie, C. Dumas, S. Vuilleumier and B. Ernst, Bioresour. Technol., 2021, 319, 124203 CrossRef CAS PubMed.
- J.-H. Jung, Y.-B. Sim, J. Ko, S. Y. Park, G.-B. Kim and S.-H. Kim, Bioresour. Technol., 2022, 352, 127094 CrossRef CAS PubMed.
- Y.-B. Sim, J.-H. Jung, J.-H. Baik, J.-H. Park, G. Kumar, J. Rajesh Banu and S.-H. Kim, Bioresour. Technol., 2021, 340, 125562 CrossRef CAS.
- M. S. Akca, O. Bostancı, A. K. Aydin, I. Koyuncu and M. Altinbas, Int. J. Hydrogen Energy, 2021, 46, 27941–27955 CrossRef CAS.
- G. Mohanakrishna, N. P. Sneha, S. M. Rafi and O. Sarkar, Sci. Total Environ., 2023, 888, 163801 CrossRef CAS PubMed.
- Y. Ueno, T. Kawai, S. Sato, S. Otsuka, M. Morimoto and J. Ferment, Bioengineered, 1995, 79, 395–397 CAS.
- E. Vanniel, Int. J. Hydrogen Energy, 2002, 27, 1391–1398 CrossRef CAS.
- T. Kanai, H. Imanaka, A. Nakajima, K. Uwamori, Y. Omori, T. Fukui, H. Atomi and T. Imanaka, J. Biotechnol., 2005, 116, 271–282 CrossRef CAS PubMed.
- C. Y. Chang and F. Lin, Int. J. Hydrogen Energy, 2004, 29, 33–39 CrossRef.
- Z. Zhang, S. S. Adav, K. Show, J. Tay, D. T. Liang, D. Lee and A. Su, Biotechnol. Bioeng., 2008, 101, 926–936 CrossRef CAS PubMed.
- M. El-Qelish, G. K. Hassan, S. Leaper, P. Dessì and A. Abdel-Karim, J. Environ. Manage., 2022, 316, 115239 CrossRef CAS PubMed.
- I. Hussy, F. R. Hawkes, R. Dinsdale and D. L. Hawkes, Biotechnol. Bioeng., 2003, 84, 619–626 CrossRef CAS PubMed.
- P. Dange, S. Pandit, D. Jadhav, P. Shanmugam, P. K. Gupta, S. Kumar, M. Kumar, Y.-H. Yang and S. K. Bhatia, Sustainability, 2021, 13, 8796 CrossRef CAS.
- Z. Wang, J. Ma, C. Y. Tang, K. Kimura, Q. Wang and X. Han, J. Membr. Sci., 2014, 468, 276–307 CrossRef CAS.
- B. C. Crone, J. L. Garland, G. A. Sorial and L. M. Vane, Water Res., 2016, 104, 520–531 CrossRef CAS PubMed.
- D. J. Batstone, T. Hülsen, C. M. Mehta and J. Keller, Chemosphere, 2015, 140, 2–11 CrossRef CAS PubMed.
- A. Kumar Sharma, P. Kumar Ghodke, N. Goyal, S. Nethaji and W.-H. Chen, Bioresour. Technol., 2022, 364, 128076 CrossRef CAS PubMed.
- L. M. Rosales-Colunga, R. G. García and A. De León Rodríguez, Int. J. Hydrogen Energy, 2010, 35, 13186–13192 CrossRef CAS.
- A. Alagumalai, B. Devarajan, H. Song, S. Wongwises, R. Ledesma-Amaro, O. Mahian, M. Sheremet and E. Lichtfouse, Biofuel Res. J., 2023, 10, 1844–1858 CrossRef CAS.
- A. K. Pandey, J. Park, J. Ko, H.-H. Joo, T. Raj, L. K. Singh, N. Singh and S.-H. Kim, Bioresour. Technol., 2023, 370, 128502 CrossRef CAS PubMed.
- E. B. Sydney, E. R. Duarte, W. J. Martinez Burgos, J. C. de Carvalho, C. Larroche and C. R. Soccol, Int. J. Hydrogen Energy, 2020, 45, 5175–5181 CrossRef CAS.
- B. O. Nikhil, A. Visa, C.-Y. Lin, J. A. Puhakka and O. Yli-Harja, Int. J. Math. Phys. Eng. Sci., 2008, 2, 80–85 CAS.
- P. Mullai, M. K. Yogeswari, K. Sridevi and P. R. Ross, Singaporean J. Sci. Res., 2013, 5, 1–7 Search PubMed.
- J. K. Whiteman and E. B. Gueguim Kana, Bioenergy Res., 2014, 7, 295–305 CrossRef CAS.
- E. L. M. Cárdenas, A. D. Zapata-Zapata and D. Kim, Energies, 2020, 13, 1663 CrossRef.
- K. Sridevi, E. Sivaraman and P. Mullai, Bioresour. Technol., 2014, 165, 233–240 CrossRef CAS PubMed.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2009, 34, 1253–1259 CrossRef CAS.
- N. Nasr, H. Hafez, M. H. El Naggar and G. Nakhla, Int. J. Hydrogen Energy, 2013, 38, 3189–3195 CrossRef CAS.
- Y. Shi, G. S. Gai, X. T. Zhao, J. J. Zhu and P. Zhang, 2010 4th Int. Conf. Bioinforma. Biomed. Eng. iCBBE 2010, 2010, pp. 1–4 Search PubMed.
- A. Hosseinzadeh, J. L. Zhou, A. Altaee and D. Li, Bioresour. Technol., 2022, 343, 126111 CrossRef CAS.
- C. Mahata, S. Ray and D. Das, Energy Convers. Manag., 2020, 219, 113047 CrossRef CAS.
- S. Zhao, J. Li, C. Chen, B. Yan, J. Tao and G. Chen, J. Clean. Prod., 2021, 316, 128244 CrossRef CAS.
- M. Z. Ahmad Sobri, A. Redhwan, F. Ameen, J. W. Lim, C. S. Liew, G. R. Mong, H. Daud, R. Sokkalingam, C.-D. Ho, A. Usman, D. H. Nagaraju and P. V. Rao, Fermentation, 2023, 9, 243 CrossRef CAS.
- S. S. Hossain, B. V. Ayodele, S. S. Ali, C. K. Cheng and S. I. Mustapa, Sustainability, 2022, 14, 7245 CrossRef CAS.
- F. Bagherzadeh, M.-J. Mehrani, M. Basirifard and J. Roostaei, J. Water Process Eng., 2021, 41, 102033 CrossRef.
- J. Cai, K. Xu, Y. Zhu, F. Hu and L. Li, Appl. Energy, 2020, 262, 114566 CrossRef.
- Y.-Q. Gu, T. Shu, B. Ge, P. Wang, C. Gao and H. Heydari, Int. J. Chem. Chem. Eng., 2021, 2021, 1–9 CrossRef.
- İ. T. Özen Daş, S. Özmıhçı and N. Büyükkamacı, Int. J. Hydrogen Energy, 2024, 56, 1446–1463 CrossRef.
- J. Full, M. Geller, S. Ziehn, T. Schließ, R. Miehe and A. Sauer, Int. J. Hydrogen Energy, 2024, 52, 594–609 CrossRef CAS.
- P. Ganeshan, V. S. Vigneswaran, S. C. Gowd, D. Kondusamy, C. Sanjay kumar, N. Krishnamoorthy, D. Kumar, A. Juneja, B. Paramasivan, N. N. Raju, K. Rajendran and A. Pugazhendhi, Fuel, 2023, 341, 127601 CrossRef CAS.
- P. Khamhaeng, N. Laosiripojana, S. Assabumrungrat and P. Kim-Lohsoontorn, Int. J. Hydrogen Energy, 2021, 46, 30891–30902 CrossRef CAS.
- S. Estévez, R. Rebolledo-Leiva, D. Hernández, S. González-García, G. Feijoo and M. T. Moreira, Energy, 2023, 274, 127319 CrossRef.
- B. Basak, B.-H. Jeon, T. H. Kim, J.-C. Lee, P. K. Chatterjee and H. Lim, Renew. Sustain. Energy Rev., 2020, 133, 110338 CrossRef CAS.
- D. J. Nouwe Edou and J. A. Onwudili, Renewable Energy, 2022, 189, 704–716 CrossRef CAS.
- R. Pachaiappan, L. Cornejo-Ponce, A. A. Sagade, M. Mani, V. Aroulmoji, V. Femilaa Rajan and K. Manavalan, Sustain. Energy Technol. Assessments, 2024, 62, 103606 CrossRef.
- C. M. Kalamaras and A. M. Efstathiou, Conference Paper in Energy, 2013, vol. 2013, pp. 1–9 Search PubMed.
- R. K. Mahato, D. Kumar and G. Rajagopalan, Renewable Energy, 2020, 153, 1368–1377 CrossRef CAS.
- S. Li, F. Li, X. Zhu, Q. Liao, J.-S. Chang and S.-H. Ho, Chemosphere, 2022, 291, 132717 CrossRef CAS.
- N. Ren, W. Guo, B. Liu, G. Cao and J. Ding, Curr. Opin. Biotechnol., 2011, 22, 365–370 CrossRef CAS PubMed.
- S. Ghosh and S. Roy, Bioresour. Technol., 2019, 288, 121603 CrossRef CAS.
- E. Özkan, B. Uyar, E. Özgür, M. Yücel, I. Eroglu and U. Gündüz, Int. J. Hydrogen Energy, 2012, 37, 2044–2049 CrossRef.
- J.-E. Kim, E.-J. Kim, H. Chen, C.-H. Wu, M. W. W. Adams and Y.-H. P. Zhang, Metab. Eng., 2017, 44, 246–252 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |