Open Access Article
Open Access ArticleAntiproliferative effects of resorcylic acid lactones from the Beibu Gulf coral-derived fungus Curvularia lunata GXIMD 02512 on prostate cancer cells†
Jiaxi Wang‡
a,
Humu Lu‡a,
Wenxuan Fangab,
Miaoping Lina,
Yuyao Fenga,
Xin Qia,
Chenghai Gaoa,
Yonghong Liua,
Xueni Wang*b and
Xiaowei Luo *a
*a
aGuangxi Key Laboratory of Marine Drugs, University Engineering Research Center of High-efficient Utilization of Marine Traditional Chinese Medicine Resources, Guangxi Institute of Marine Drugs, Guangxi University of Chinese Medicine, Nanning 530200, China. E-mail: luoxiaowei1991@126.com
bGuangxi Zhuang Yao Medicine Center of Engineering and Technology, Guangxi University of Chinese Medicine, Nanning 530200, China. E-mail: wangxueni@gxtcmu.edu.cn
First published on 9th December 2024
Abstract
Four novel resorcylic acid lactones (RALs), curvulomycins A–D (1–4), and six known congeners were isolated from the Beibu Gulf coral-derived fungus Curvularia lunata GXIMD 02512. Their structures including absolute configurations were established by extensive spectroscopic analyses along with experimental and calculated ECD spectra. Structurally, compound 3 harbors a unique γ-pyrone moiety rarely found in the natural RAL family. Notably, curvulomycin D (4) represents the first reported compound of the rare 8-membered RAL derivatives. Compounds 1 and 5 exhibited significant antiproliferative potency against two prostate cancer cell lines, with IC50 values of 9.70 ± 0.77 μM and 7.64 ± 0.46 μM for PC-3 cells and 5.96 ± 0.43 μM and 3.15 ± 0.27 μM for 22Rv1 cells, respectively. Moreover, compound 1 inhibited clonal cell colonies in a dose-dependent manner, blocked the S and G2 phases in the PC-3 cell cycle and the G1 phase in the 22Rv1 cell cycle, which further induced apoptosis in both PC-3 and 22Rv1 cells, indicating its potential as a promising lead compound for anti-prostate cancer therapy.
1. Introduction
Global cancer statistics for 2022 revealed nearly 20 million new cancer cases and close to 10 million cancer-related deaths. Projections suggest that the number of new cases will rise to 35 million by 2050, posing huge economic as well as societal challenges worldwide in the coming decades.1 Prostate cancer (PC) is the most commonly diagnosed cancer in men, predominantly in regions with a high human development index. It remains the second most common malignancy and was the fifth leading cause of cancer-related deaths among men in 2022.1,2 Patients with localized PC may progress to an advanced and lethal stage known as metastatic castration-resistant PC (CRPC) after undergoing standard therapeutic regimens. These treatments often result in poor outcomes owing to the lack of effective therapies.3 Although current first-line therapies, such as AR antagonist enzalutamide and the androgen synthesis inhibitor abiraterone have shown initial improvement in the disease outcomes in some patients, de novo and acquired resistance continue to hinder the curable medications of CRPC.4 Therefore, there is an urgent need for innovative molecular drugs with novel structures and more efficient and/or less toxic properties.Natural products have consistently played a pivotal role in pharmaceutical research, exerting profound impacts on the treatment of human diseases, especially cancers.5,6 Structurally complex compounds from traditional Chinese medicine as well as marine organisms have been recently evidenced as potential sources of new drugs against PC.7,8 Notably, DD1, a daphnane diterpenoid isolated from Daphne genkwa, was identified as a highly potent importin-β1 inhibitor for the treatment of CRPC,4 which was further optimized to an improved analog DD1-Br with better tolerability and oral bioavailability for overcoming drug resistance in advanced CRPC.9 Elaiophylin, an antibiotic derived from marine Streptomyces, was found as a novel RORγ antagonist targeting CRPC, which strongly suppressed tumor growth in both cell line-based and patient-derived PC xenograft models.10
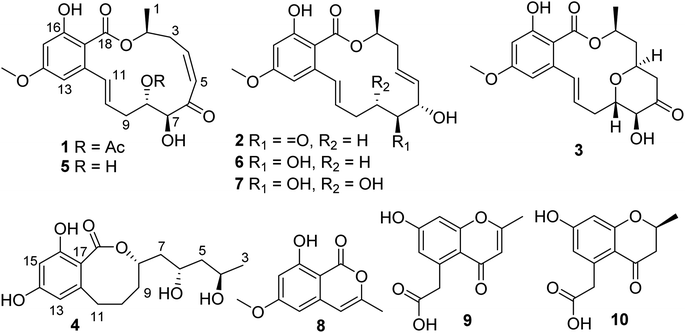
Resorcylic acid lactones (RALs) are fungal polyketides that consist of a β-resorcylic acid residue (2,4-dihydroxybenzoic acid) embedded in a macrolactone ring. The structural diversity of RALs is mainly due to the size as well as extensive modification of the macrolactone ring. Over 200 RALs have been reported from different genera of fungi hitherto, including Aigialus, Curvularia, Lasiodiplodia, Penicillium, Pochonia, and Monocillium, which were found to have diverse biological activities, including anticancer, antimicrobial, antimalarial, heat shock protein 90 (Hsp90) inhibitory, and protein tyrosine kinase inhibitory activities, eliciting considerable interest from communities of chemistry and pharmacology.11,12 In the course of our continuous search for novel bioactive natural products from marine microorganisms, a series of anticancer compounds has been recently obtained, including ascochlorins,13–16 piericidins,17–19 epipolythiodioxopiperazines,20 and azaphilones.21 In this study, four new RALs and six known related congeners were obtained from the Beibu Gulf coral-derived fungus Curvularia lunata GXIMD 02512 by HPLC-DAD-guided isolation (Fig. 1). Herein, the isolation, structural elucidation, and anti-PC activities of these RALs along with their congeners are described in detail.
2. Results and discussion
Compound 1 was isolated as a white powder. The molecular formula was determined as C21H24O8 by the HR-ESIMS peak at m/z of 427.1367 [M + Na]+ (calcd for C21H24O8Na+, 427.1363), indicating 10 degrees of unsaturation (DOU). Its UV spectrum displayed UV absorption bands at 231, 270, and 309 nm, suggesting the presence of benzoic acid functionality in the structure. The 1H NMR data (Table 1) in conjunction with HSQC data showed the presence of two methyls, H3-1 (δH 1.38, d, J = 6.5 Hz) and H3-2′ (δH 2.06, s), one methoxy group, H3-19 (δH 3.75, s), two methylenes, H2-3 (δH 2.64, m; 3.27, m) and H2-9 (δH 2.19, m), three methines, H-2 (δH 5.22, m), H-7 (δH 4.52, dd, J = 5.0, 1.5 Hz), and H-8 (δH 5.03, m), and six aromatic/olefinic protons, H-4 (δH 6.32, td, J = 11.5, 3.0 Hz), H-5 (δH 6.49, dd, J = 11.5, 2.0 Hz), H-10 (δH 6.02, ddd, J = 16.0, 9.0, 5.5 Hz), H-11 (δH 6.76, d, J = 16.0 Hz), H-13 (δH 6.39, d, J = 2.5 Hz), and H-15 (6.40, d, J = 2.5 Hz). Apart from the above-mentioned proton-containing groups, there were seven quaternary carbons (δC 104.9, 141.8, 163.3, 163.4, 170.2, 170.4, and 199.2) in the 13C NMR data of 1. The spectroscopic features illustrated that 1 was a RAL derivative, which closely resembled those of co-isolated LL-Z1640-2 (5).22 The main difference was the presence of an additional acetyl group (δH/C 2.06/21.1, CH3-2′; δC 170.4, C-1′) at C-8 in 1 instead of a hydroxy group in 5, which was confirmed by the HMBC correlations (Fig. 2) from H-8 and H3-2′ to C-1′, along with the molecular formula. Further analysis of the HSQC, COSY, and HMBC data allowed the explicit assignment of all NMR resonances of 1.| Pos | 1b | 2c | 3c | 4c | ||||
|---|---|---|---|---|---|---|---|---|
| δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |
| a For 1, δH 5.41, d (5.0), 7-OH; 11.71, s, 16-OH.b In DMSO-d6.c In CD3OD. | ||||||||
| 1 | 20.1, CH3 | 1.38, d (6.5) | 18.9, CH3 | 1.42, d (6.5) | 21.9, CH3 | 1.40, d (6.5) | ||
| 2 | 73.7, CH | 5.22, m | 72.7, CH | 5.52, m | 73.7, CH | 5.20, m | ||
| 3 | 36.3, CH2 | 2.64, m; 3.27, m | 38.3, CH2 | 2.50, m | 38.2, CH2 | 1.66, dq (16.0, 1.5) | 24.2, CH3 | 1.17, d (6.0) |
| 2.15, ddd (16.0, 11.0, 7.5) | ||||||||
| 4 | 145.4, CH | 6.32, td (11.5, 3.0) | 131.6, CH | 6.28, ddd (15.5, 9.0, 6.0) | 74.4, CH | 4.60, m | 65.5, CH | 3.94, m |
| 5 | 126.0, CH | 6.49, dd (11.5, 2.0) | 133.3, CH | 5.43, dd, (15.5, 8.0) | 47.5, CH2 | 2.86, dd (15.0, 6.0) | 46.3, CH2 | 1.45, t (6.0) |
| 2.41, dd (15.0, 5.0) | ||||||||
| 6 | 199.2, C | 80.1, CH | 4.81, d (8.0) | 209.1, C | 66.2, CH | 3.92, m | ||
| 7 | 78.3, CH | 4.52, dd (5.0, 1.5) | 210.2, C | 77.1, CH | 4.18, d (9.5) | 45.7, CH2 | 1.93, dt (14.0, 7.0) | |
| 1.63, dt (14.0, 6.0) | ||||||||
| 8 | 74.8, CH | 5.03, m | 35.7, CH2 | 2.61, ddd (18.0, 6.5, 3.0) | 78.8, CH | 3.91, td (9.5, 3.0) | 80.4, CH | 4.52, dd (16.0, 7.0) |
| 3.25, ddd (18.0, 11.0, 3.0) | ||||||||
| 9 | 33.0, CH2 | 2.19, m | 25.3, CH2 | 2.39, m; 2.72, m | 34.4, CH2 | 2.40, overlapped | 35.3, CH2 | 1.91, m; 1.79, m |
| 2.64, ddd (14.5, 9.0, 3.5) | ||||||||
| 10 | 130.9, CH | 6.02, ddd (16.0, 9.0, 5.5) | 132.2, CH | 6.16, dt (16.0, 4.5) | 127.4, CH | 5.83, dt (16.0, 6.5) | 28.0, CH2 | 2.08, m; 1.56, m |
| 11 | 131.5, CH | 6.76, d (16.0) | 129.7, CH | 6.70, dt (16.0, 1.5) | 136.5, CH | 7.09, d (16.0) | 34.2, CH2 | 2.75, dd (13.5, 7.5) |
| 2.58, t (13.5) | ||||||||
| 12 | 141.8, C | 143.0, C | 144.9, C | 145.5, C | ||||
| 13 | 106.4, CH | 6.39, d (2.5) | 106.9, CH | 6.49, d (2.5) | 108.0, CH | 6.33, d (2.5) | 109.4, CH | 6.20, d (2.5) |
| 14 | 163.4, C | 165.0, C | 165.3, C | 162.8, C | ||||
| 15 | 100.3, CH | 6.40, d (2.5) | 100.8, CH | 6.28, d (2.5) | 100.5, CH | 6.35, d (2.5) | 102.1, CH | 6.23, d (2.5) |
| 16 | 163.3, C | 164.7, C | 164.7, C | 159.3, C | ||||
| 17 | 104.9, C | 106.8, C | 106.9, C | 110.7, C | ||||
| 18 | 170.2, C | 171.9, C | 172.1, C | 173.5, C | ||||
| 19 | 55.5, CH3 | 3.77, s | 55.9, CH3 | 3.79, s | 55.9, CH3 | 3.80, s | ||
| 1′ | 170.4, C | |||||||
| 2′ | 21.1, CH3 | 2.06, s | ||||||
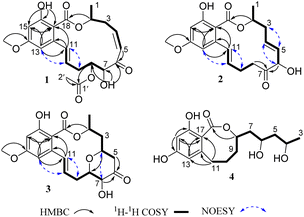
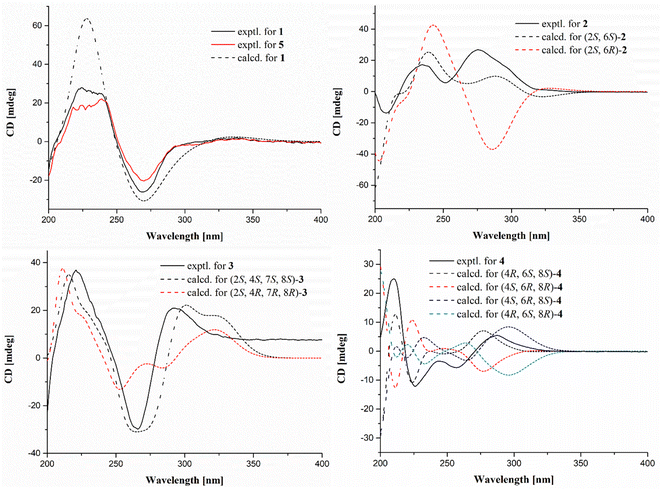
The relative configurations of 1 were determined by proton coupling constants and NOESY correlations. The large coupling constant between H-10 and H-11 (J = 15.5 Hz) along with the NOESY correlations (Fig. 2) of H-11/H2-9 and H-10/H-13 revealed the E configuration of the Δ10 double bond. However, the Z configuration of the Δ4 double bond in 1 was assigned by the small coupling constant (JH-4/H-5 = 11.5 Hz), combined with the absent NOESY correlation of H-5/H2-3. From a biosynthetic point of view, the absolute configurations of (2S, 7S, 8S) in 1 should be the same as those of 5. This deduction was further ascertained by the shared nearly identical experimental ECD curves (Fig. 3) as well as the well matched calculated and experimental ECD curves of 1, which showed shared positive Cotton Effect at about 230 nm and negative Cotton Effect at about 270 nm. Thus, the gross structure of 1 was resolved as shown in Fig. 1 and was given the trivial name curvulomycin A.
Curvulomycin B (2) was obtained as a white powder with the molecular formula C19H22O6, as assigned by the HR-ESIMS data (9 DOU). Interpretation of the spectroscopic characteristics (Table 1) of 2 was also indicative of an RAL-like structure, which showed great similarity to that of co-isolated deoxyaigialomycin C (6).22 The major difference was the presence of a carbonyl group (δC 210.2, C-7) in 2 instead of a hydroxy-methine one in 6, which was confirmed by the HMBC correlations (Fig. 2) of C-7/H-6 (δH 4.81) and H2-8 (δH 3.25, 2.61). Both Δ4 and Δ10 double bonds were determined as E configuration based on the NOESY correlations and large proton coupling constants of JH-4/H-5 (15.5 Hz) and JH-10/H-11 (16.0 Hz).
As in the same case of 1, the stereogenic carbon at C-2 in these obtained 14-membered RALs (1–3 and 5–7) was assigned as S-configuration on the basis of the shared biosynthetic origin. Thus, two possible diastereoisomers of 2 were further subjected to ECD calculations at the B3LYP/6-31+G (d, p) level by time-dependent density functional theory (TDDFT).13 The calculated ECD curve (Fig. 3) of (2S, 6S)-2 coincided well with the experimental one, suggesting (2S, 6S)-configuration of 2.
Curvulomycin C (3) was isolated as a white powder with the molecular formula C17H20O4 (6 DOU), as determined by the HR-ESIMS ion peak. The 1H NMR data (Table 1) underscored the presence of one methyl, H3-1 (δH 1.40, d, J = 6.5 Hz), one methoxy group, H3-19 (δH 3.80, s), three methylenes, H2-3 (δH 1.66, dq, J = 16.0, 1.5 Hz; 2.15, ddd, J = 16.0, 11.0, 1.5 Hz), H2-5 (δH 2.41, dd, J = 15.0, 5.0 Hz; 2.86, dd, J = 15.0, 6.0 Hz), and H2-9 (δH 2.40, overlapped; 2.64, ddd, J = 14.5, 9.0, 3.5 Hz), four oxygenated methines, H-2 (δH 5.20, m), H-4 (δH 4.60, m), H-7 (δH 4.18, d, J = 9.5 Hz), and H-8 (δH 3.91, td, J = 9.5, 3.0 Hz), and four aromatic/olefinic protons, H-10 (δH 5.83, dt, J = 16.0, 6.5 Hz), H-11 (δH 7.09, d, J = 16.0 Hz), H-13 (δH 6.33, d, J = 2.5 Hz), and H-15 (6.35, d, J = 2.5 Hz). Aside from the above 13 corresponding hydrogen-bearing carbons, six remaining quaternary carbons in the 13C NMR data were attributed to two carbonyls (δC 172.1, 209.1) and four olefinics (δC 106.9, 144.9; two oxygenated ones, 164.7, 165.3). The above spectral data of 3 also revealed a 14-membered RAL analogue structurally related to (7′E)-6′-oxozeaenol,22 except for the observation of oxygenated methine (CH-4) in 3. Meanwhile, the aforementioned functionalities accounted for 8 out of 9 DOU, indicative of an additional ring in 3. The key HMBC correlation of H-4/C-8 allowed the establishment of a γ-pyrone ring by an ether bridge. Further analysis of the HSQC, COSY, and HMBC data confidently supported the NMR resonances of 3. However, compound 3 was found as a synthetic RAL derivative from (5Z)-7-oxozeaenol,23 which is relatively uncommon in the natural RAL family.
Similar to the case of 1 and 2, the Δ10 double bond in 3 was also assigned the E configuration based on the NOESY correlations (Fig. 2) in association with a large proton coupling constant of 16.0 Hz. Moreover, the NOESY correlation of H-4/H-7 suggested that H-4 and H-7 were located on the same face of the γ-pyrone ring. Meanwhile, the absent NOESY correlation of H-4/H-8 tentatively revealed H-4 and H-8 were in the opposite side. This deduction was further confirmed by the anti-facial relationship of H-7/H-8, as deduced by the large proton coupling constant of 9.5 Hz in 3 (synthetic one: 9.6 Hz).23 Further comparison between the experimental and calculated ECD spectra of 3 permitted the (2S,4S,7S,8S)-configuration of 3.
Curvulomycin D (4) was obtained as a colorless oil with the molecular formula C16H22O6 (6 DOU), as determined by HR-ESIMS data. The 1H NMR data (Table 1) combined with HSQC data showed the signals indicative of one methyl, H3-3 (δH 1.17, d, J = 6.0 Hz), five methylenes, H2-5 (δH 1.45, d, J = 6.0 Hz), H2-7 (δH 1.93, dt, J = 14.0, 7.0 Hz; 1.63, dt, J = 14.0, 6.0 Hz), H2-9 (δH 1.91, m; 1.79, m), H2-10 (δH 2.08, m; 1.56, m), and H2-11 (δH 2.75, dd, J = 13.5, 7.5 Hz; 2.58, t, J = 13.5 Hz), three oxygenated methines, H-4 (δH 3.94, m), H-6 (δH 3.92, m), and H-8 (δH 4.52, dd, J = 16.0, 7.0 Hz), two aromatic protons, H-13 (δH 6.20, d, J = 2.5 Hz) and H-15 (δH 6.23, d, J = 2.5 Hz). Besides the 11 corresponding hydrogen-bearing carbons, five carbons remained in the 13C NMR data, including one carbonyl (δC 173.5) and four aromatics (δC 110.7, 145.5; two oxygenated ones, 159.3, 162.8). The aforementioned NMR characteristics of 4 suggested the presence of a β-resorcylic acid ring (5 DOU) and a branched C9 aliphatic chain with a 4,6,8-triol unit, which were confirmed by the HMBC correlations of H-13/C-15, C-17 and H-15/C-14, C-16, C-17 as well as sequential 1H–1H COSY correlations of H3-3/H-4/H2-5/H-6/H2-7/H-8/H2-9/H2-10/H2-11. Another ring was required to fulfill the remaining one DOU. Notably, the HMBC correlation of H-8/C-18 and H2-11/C-12, C-13, C-17 allowed the establishment of a rare 8-membered lactone ring tethered to the β-resorcylic acid core. The relative configuration of the 4-OH/6-OH cluster was deduced as an anti-relationship by well-known Kishi's Universal NMR Database for the 1,3-diol moiety (Fig. 4).24
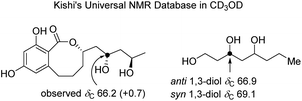 | ||
| Fig. 4 Kishi's NMR data set for the elucidation of the relative configuration of the 1,3-diol moiety in compound 4. ΔδC value between 4 and the model system is shown in brackets. | ||
Many initial attempts exemplified by the modified Mosher's method applied to establish the absolute configurations of 4 turned failed, meanwhile more exhaustive efforts were hampered by the limited obtained quantity. With this information in mind, four remaining possible stereoisomers of 4 were also alternatively subjected to ECD calculations. The calculated ECD spectrum of (11S,13S,15R)-4 showed good agreement with the experimental one, indicating (11S,13S,15R)-configuration in 4 (Fig. 3). To our knowledge, curvulomycin D (4) is obtained as the first case of 8-membered RAL derivatives.
Meanwhile, the known compounds were identified by comparing their physicochemical and spectroscopic data with the literature values, which were LL-Z1640-2 (5),22 deoxyaigialomycin C (6),22 zeaenol (7),22 8-hydroxy-6-methoxy-3-methylisocoumarin (8),25 2-methyl-5-carboxymethyl-7-hydroxychromone (9),26 and 2-methyl-5-carboxymethyl-7-hydroxychromanone (10).26
The cytotoxicity of compounds 1–10 toward two human PC cell lines, namely, 22Rv1 and PC-3, was tested using the MTT assay.13,16,21 Amongst them, compounds 1 and 5 exhibited significant inhibitory activity with IC50 values of 9.70 ± 0.77 μM and 7.64 ± 0.46 μM for PC-3, and 5.96 ± 0.43 μM and 3.15 ± 0.27 for 22Rv1, respectively, which revealed that the acetyl group at C-8 in 1 would slightly decrease the inhibitory activity against the two PC cell lines. Moreover, compounds 1 and 5 were also tested in two normal PC cell lines, namely, WPMY-1 and RWPE-1, which also displayed cytotoxicities with IC50 values of 6.91 ± 0.12 μM and 5.74 ± 0.52 μM for WPMY-1, and 3.01 ± 0.09 μM and 3.18 ± 0.11 μM for RWPE-1, respectively.
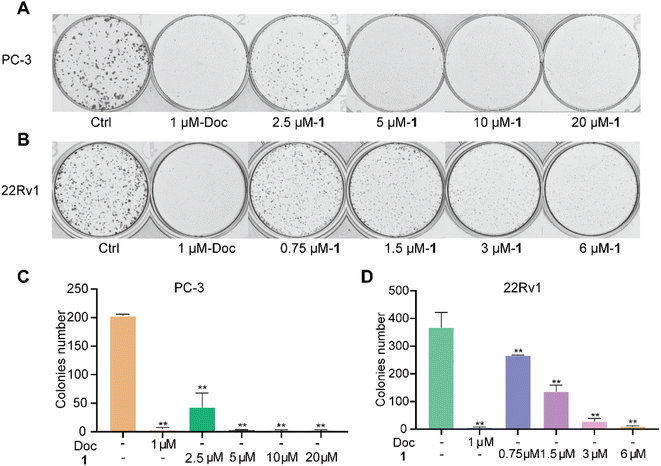
We further examined the effects of the new compound 1 on the proliferation of two PC cell lines using a plate cloning assay. The experimental results showed that PC cells in the control group proliferated normally and formed dense clonal cell colonies. However, all PC cells in the docetaxel group were induced to undergo apoptosis and could not form obvious clonal cell colonies. Meanwhile, compound 1 showed significant inhibition of the PC clonal cell colonies in a dose-dependent manner (Fig. 5).
Confirming that compound 1 inhibited the proliferation of PC cells, we further examined its effect on the cell cycle and cell apoptosis by flow cytometry. PC-3 and 22Rv1 cells were treated with different concentrations of 1 (5 μM, 10 μM, and 20 μM) and docetaxel (1 μM) for 48 h. The results showed that the proportion of docetaxel-treated PC-3 and 22Rv1 cells in the G2/M phase was significantly higher than those of the control group, which verified that docetaxel blocked the cell cycle of both PC-3 and 22Rv1 cells at the G2/M phase. When PC-3 cells were treated with different concentrations of 1, the percentage of cells in the G1 phase gradually decreased with increasing concentration, while the percentage of cells in the S and G2 phases increased (Fig. 6A and C). Meanwhile, the treatment of 22Rv1 cells with different concentrations of 1 resulted in a gradual increase in the percentage of cells in the G1 phase and a decrease in the percentage of cells in the S and G2 phases with increasing concentration (Fig. 6B and D). The above data suggested that 1 affected the cycle distribution of the two prostate cancer cell lines by blocking the cell cycle of PC-3 cells in the S and G2 phases, while it blocked the cell cycle of 22Rv1 cells in the G1 phase.
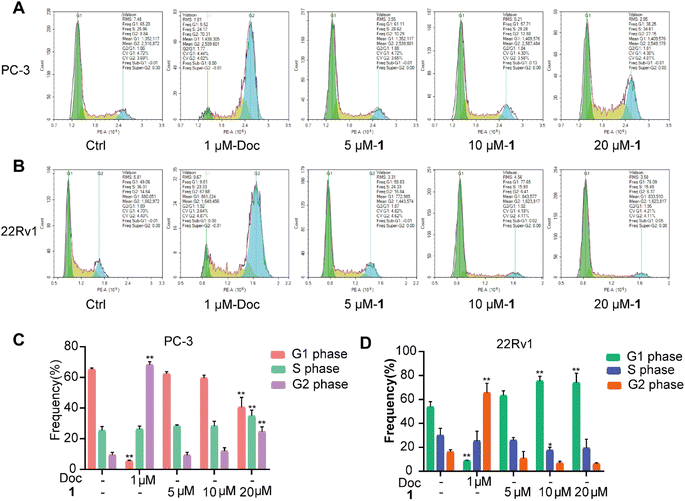 | ||
| Fig. 6 Effect of 1 on the cell cycle of PC-3 (A and C) and 22Rv1 (B and D) cells in vitro. *P < 0.05, **P < 0.01 vs. Ctrl. | ||
Meanwhile, PC-3 and 22Rv1 cells were also treated with different concentrations of 1 (5 μM, 10 μM, 20 μM, and/or 40 μM) and docetaxel (1 μM) for the evaluation of cell apoptosis. The total apoptotic cells (early and late apoptotic cells) induced by docetaxel rose by 11.6% for PC-3 cells and 35.3% for 22Rv1 cells at 1 μM (Fig. 7), respectively. Meanwhile, the total apoptotic cells induced by 1 rose by 15.0% for PC-3 cells (Fig. 7A and C) and 34.2% for 22Rv1 cells (Fig. 7B and D) at 20 μM, suggesting a promising anti-PC lead compound.
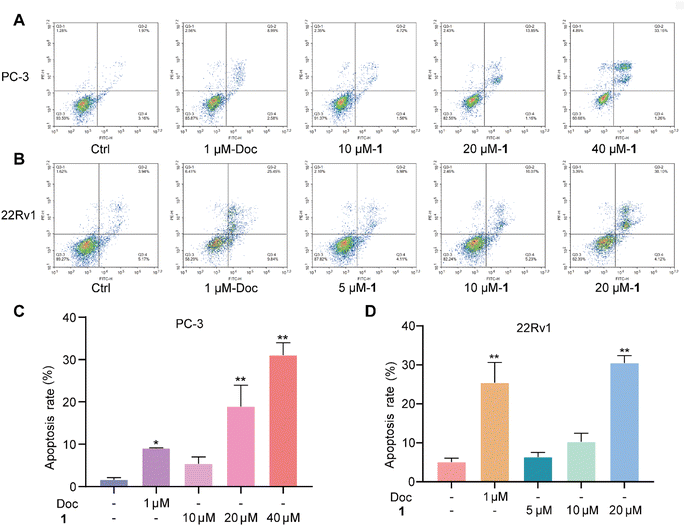 | ||
| Fig. 7 Effect of 1 on the cell apoptosis of PC-3 (A and C) and 22Rv1 (B and D) cells in vitro. *P < 0.05, **P < 0.01 vs. Ctrl. | ||
3. Experimental
3.1. General experimental procedures
The ECD spectra were measured on a JASCO J-1500 polarimeter (JASCO Corporation, Tokyo, Japan). The NMR spectra were obtained on a Bruker Avance spectrometer (Bruker BioSpin, Fällanden, Switzerland) operating at 500 MHz for 1H NMR and 125 MHz for 13C NMR, using TMS as an internal standard. HR-ESIMS spectra were collected on a Waters Xevo G2-S TOF mass spectrometer (Waters Corporation, Milford, MA, USA). TLC and column chromatography (CC) were performed on plates precoated with silica gel GF254 (10–40 μm) and over silica gel (200–300 mesh) (Qingdao Marine Chemical Factory, Qingdao, China), respectively. All solvents employed were of analytical grade (Shanghai Titan Scientific Co. Ltd, Shanghai, China). Semi-preparative high-performance liquid chromatography (Semi-prep HPLC) was performed on a Shimadzu SCL-10VAP (Shimadzu, Tokyo, Japan), equipped with an ODS column (YMC-pack ODS-A, YMC Co. Ltd, Kyoto, Japan, 10 × 250 mm, 5 μm, 2 mL min−1). The artificial sea salt was a commercial product (Guangzhou Haili Aquarium Technology Company, Guangzhou, China).3.2. Fungal material
The fungus GXIMD 02512 was isolated from the coral Pocillopora damicornis collected from the Weizhou Islands in Guangxi, China. It was taxonomically identified as Curvularia lunata GXIMD 02512 by sequence analysis of the internal spacer (ITS) region of the rDNA (GenBank accession no. PP535558). The voucher specimen was deposited at the Institute of Marine Drugs, Guangxi University of Chinese Medicine.3.3. Fermentation, extraction, and isolation
The large-scale fermentation of C. lunata GXIMD 02512 was carried out in rice solid medium (120 g rice, 3.0 g artificial sea salt, 0.3 g corn steep liquor, and 150 mL H2O) employing 1 L × 60 Erlenmeyer flasks at room temperature for 36 days. The rice fermentation products were extracted with ethyl acetate (EtOAc) and evaporated in vacuo to obtain the crude extract (73 g). The crude extract was divided by medium-pressure liquid chromatography using step gradient elution with petroleum ether/CH2Cl2/CH3OH (petroleum ether/CH2Cl2, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100; CH2Cl2/methanol, 100
100; CH2Cl2/methanol, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–50
0–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v). Nine fractions (Frs.1–9) were further combined and obtained according to TLC observations. Fr.8 was separated by reverse phase silica gel column chromatography, and 10 sub-fractions (Frs.8-1–8-10) were obtained by gradient elution (CH3OH/H2O, 10
50, v/v). Nine fractions (Frs.1–9) were further combined and obtained according to TLC observations. Fr.8 was separated by reverse phase silica gel column chromatography, and 10 sub-fractions (Frs.8-1–8-10) were obtained by gradient elution (CH3OH/H2O, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90–100
90–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, v/v). Fr.8-5 was isolated by semi-prep HPLC (CH3CN/H2O, 50
0, v/v). Fr.8-5 was isolated by semi-prep HPLC (CH3CN/H2O, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 2 mL min−1) to yield compounds 2 (tR = 41 min, 9.1 mg) and 3 (tR = 32 min, 4.5 mg). Fr.8-6 was purified by semi-prep HPLC (CH3CN/H2O, 58
50, 2 mL min−1) to yield compounds 2 (tR = 41 min, 9.1 mg) and 3 (tR = 32 min, 4.5 mg). Fr.8-6 was purified by semi-prep HPLC (CH3CN/H2O, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42, 2 mL min−1) to obtain compound 1 (tR = 32 min, 2.8 mg).
42, 2 mL min−1) to obtain compound 1 (tR = 32 min, 2.8 mg).
Fr.9 was separated by reversed-phase silica gel column chromatography with gradient elution (CH3OH/H2O, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90–100
90–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, v/v) to provide 23 fractions. Fr.9-5 was isolated by semi-prep HPLC (CH3CN/H2O, 20
0, v/v) to provide 23 fractions. Fr.9-5 was isolated by semi-prep HPLC (CH3CN/H2O, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 2 mL min−1) to afford compound 8 (tR = 47 min, 11.7 mg) and three sub-fractions, namely, Frs.9-5-1–9-5-3 and Fr.9-5-1, were further purified by semi-prep HPLC (CH3CN/H2O, 20
80, 2 mL min−1) to afford compound 8 (tR = 47 min, 11.7 mg) and three sub-fractions, namely, Frs.9-5-1–9-5-3 and Fr.9-5-1, were further purified by semi-prep HPLC (CH3CN/H2O, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 2 mL min−1) to yield compounds 4 (tR = 25 min, 4.1 mg) and 9 (tR = 27.5 min, 5.2 mg). Fr.9-5-3 was purified using semi-prep HPLC (CH3CN/H2O, 30
80, 2 mL min−1) to yield compounds 4 (tR = 25 min, 4.1 mg) and 9 (tR = 27.5 min, 5.2 mg). Fr.9-5-3 was purified using semi-prep HPLC (CH3CN/H2O, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 2 mL min−1) to obtain compound 10 (tR = 16 min 2.8 mg). Fr.9-12 was isolated by semi-prep HPLC (CH3CN/H2O, 48
70, 2 mL min−1) to obtain compound 10 (tR = 16 min 2.8 mg). Fr.9-12 was isolated by semi-prep HPLC (CH3CN/H2O, 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52, 2 mL min−1) to produce compounds 7 (tR = 12.5 min, 28.8 mg), 5 (tR = 21 min, 3.3 mg), and 6 (tR = 23 min, 6.8 mg).
52, 2 mL min−1) to produce compounds 7 (tR = 12.5 min, 28.8 mg), 5 (tR = 21 min, 3.3 mg), and 6 (tR = 23 min, 6.8 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 231 (4.06), 270 (3.57), 309 (3.32) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 229 (+26.5), 269 (−26.1) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 427.1367 [M + Na]+(calcd for C21H24O8Na+ 427.1363).
ε) 231 (4.06), 270 (3.57), 309 (3.32) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 229 (+26.5), 269 (−26.1) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 427.1367 [M + Na]+(calcd for C21H24O8Na+ 427.1363).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 235 (4.21), 270 (3.83), 311 (3.50) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 208 (−13.8), 235 (+17.1), 252 (+5.6), 276 (+26.7) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 369.1315 [M + Na]+(calcd for C19H23O6+ 369.1309).
ε) 235 (4.21), 270 (3.83), 311 (3.50) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 208 (−13.8), 235 (+17.1), 252 (+5.6), 276 (+26.7) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 369.1315 [M + Na]+(calcd for C19H23O6+ 369.1309).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 229 (4.12), 265 (3.72), 303 (3.47) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 221 (+11.7), 266 (−15.7), 293 (+5.1) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 361.1289 [M − H]−(calcd for C19H21O7− 361.1293).
ε) 229 (4.12), 265 (3.72), 303 (3.47) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 221 (+11.7), 266 (−15.7), 293 (+5.1) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 361.1289 [M − H]−(calcd for C19H21O7− 361.1293).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 200 (4.1205), 262 (3.38) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 210 (+32.2), 226 (−15.6), 244 (−4.37), 287 (+7.07) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 333.1315 [M + Na]+(calcd for C16H22O6Na+ 333.1309).
ε) 200 (4.1205), 262 (3.38) nm; ECD (0.25 mg mL−1, CH3OH) λmax (Δε) 210 (+32.2), 226 (−15.6), 244 (−4.37), 287 (+7.07) nm; 1H NMR and 13C NMR data, see Table 1; HR-ESIMS m/z 333.1315 [M + Na]+(calcd for C16H22O6Na+ 333.1309).3.4. ECD calculations
Conformational analysis was subjected to Spartan'14 software (Wavefunction Inc., Irvine, CA, USA) using the Merck Molecular Force Field (MMFF), as previously described.13,21 Low-energy conformers with a Boltzmann distribution over 1% were chosen for DFT/TD-DFT calculations by Gaussian 16 software (Gaussian, Wallingford, CT, USA) at the B3LYP/6-311+G (d, p)//B3LYP/6-31+G (d) level in methanol by adopting 50 excited states. The ECD spectra were generated by SpecDis 1.7 (University of Wurzburg, Wurzburg, Germany) using a half bandwidth of 0.2–0.3 eV, according to the Boltzmann-calculated contribution of each conformer after UV correction.3.5. Cytotoxicity bioassay
Human prostate cancer PC-3 and 22Rv1 cells, along with human normal prostate RWPE-1 and WPMY-1 cells, were obtained from the Cell Bank/stem Cell Bank of the Chinese Academy of Sciences. All cell lines were also cultured in the medium according to the recommendations of the Cell Bank/stem Cell Bank of the Chinese Academy of Sciences. Cells in logarithmic growth phase were inoculated into 96-well cell culture dishes with 5000 cells per well. After all cells were attached to the bottom of the plate, the cells were primarily treated with these obtained compounds with a screening concentration of 10 μM. Compounds 1 and 5 with inhibitions over 50% were further subjected to the gradient test by the MTT method,16 which were 0.3125 μM, 0.625 μM, 1.25 μM, 2.5 μM, 5 μM, 10 μM, 20 μM, and 40 μM, while the control group was treated with dimethyl sulfoxide (DMSO, 0.1%, D8371, Solarbio). After the cells were treated by the compounds for 72 h, MTT solution (M8180, Solarbio) was added to the reaction with the cells for 4 h. Then, the OD values were detected by a multiwell plate reader (Synergy™ H1, Bio-Tek), and the IC50 values were fitted with GraphPad Prism 8.0. Docetaxel was used as the positive control with IC50 values of 0.12 μM and 0.030 μM for PC-3 and 22Rv1 cells, respectively.3.6. Clone formation assay
PC-3 cells and 22Rv1 cells were inoculated into 6-well plates, and compound 1 was added to treat the cells after they were completely attached to the bottom of the wells. In PC-3 cells, the concentration gradient of 1 was 2.5 μM, 5 μM, 10 μM, and 20 μM. In 22Rv1 cells, the concentration gradient of 1 was 0.75 μM, 1.5 μM, 3 μM, and 6 μM. Docetaxel (S1148, Selleck, China) was added to the positive drug control at a concentration of 1 μM, and DMSO was added to the control at a concentration of 0.1% (v/v). After one week of compound treatment, the cells were given fresh medium and compound treatment again. Two weeks later, obvious clonal colonies of the cells were formed, then the cells were fixed with 4% cell tissue fixative (P1110, Solarbio), stained with crystal violet (G1063, Solarbio), and finally, the photographs of the clonal colonies of the cells were taken with a colony counter (GelCount™, Oxford-Optronix).3.7. Cell cycle and apoptosis assays
Cells were inoculated into 6-well plates at a density of 2.0 × 105 per well for 48 h and then treated with either DMSO (0.1%, v/v), docetaxel (1 μM), or compound 1 (5, 10, and 20 μM) for 48 h, respectively. Cells were digested and collected with 0.25% trypsin solution and fixed with 75% ice ethanol overnight. Cells were then stained with FxCycle™ PI/RNase solution (F10797, Invitrogen) for 30 min and the cell cycle distribution was examined by flow cytometry (NovoCyte 2060R, ACEA Biosciences).The cells were inoculated with 2.0 × 105 cells per well and cultured in 6-well plates for 48 h, which were then treated with 1. PC-3 cells were treated with 1 at concentrations of 10, 20, and 40 μM, and 22Rv1 cells were treated with 1 at concentrations of 5, 10, and 20 μM. Docetaxel functioned as the positive control with a concentration of 1 μM. DMSO was added to the control group at a concentration of 0.1% (v/v). Then, the cells were cultured for 48 h and collected with 0.25% trypsin solution without EDTA (T1350, Solarbio), which were stained with propidium iodide and Annexin-V/FITC (BMS500FI-300, Invitrogen) according to the manufacturer's instructions. Cell apoptosis was detected and analyzed by flow cytometry (NovoCyte 2060R, ACEA Biosciences).
4. Conclusions
In summary, four new RALs and six known congeners were identified from the Beibu Gulf coral-derived fungus Curvularia lunata GXIMD 02512. To our knowledge, curvulomycin C (3) harboring a unique γ-pyrone ring is relatively uncommon in the natural RAL family. Notably, curvulomycin D (4) is obtained as the first case of rare 8-membered RAL derivatives. Curvulomycin A (1) and LL-Z1640-2 (5) exhibited significant antiproliferative activity against two prostate cancer cell lines with IC50 values of 9.70 ± 0.77 μM and 7.64 ± 0.46 μM for PC-3 cells, 5.96 ± 0.43 μM and 3.15 ± 0.27 μM for 22Rv1 cells, respectively. The acetyl group at C-8 in 1 would slightly decrease the inhibitory activity. Moreover, compound 1 inhibited clonal cell colonies in a dose-dependent manner, blocked S and G2 phases in the PC-3 cell cycle and the G1 phase in the 22Rv1 cell cycle, which further induced apoptosis in PC-3 and 22Rv1 cells. Collectively, our findings could expand the chemical and biological diversity of natural RALs and provide a promising anti-PC lead compound.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Jiaxi Wang: data curation, investigation, methodology, writing – original draft. Humu Lu: data curation, methodology, funding acquisition. Wenxuan Fang: data curation, investigation. Miaoping Lin: data curation, formal analysis. Yuyao Feng: investigation, validation. Xin Qi: investigation, validation. Chenghai Gao: methodology, validation. Yonghong Liu: project administration. Xueni Wang: conceptualization, writing – review & editing. Xiaowei Luo: conceptualization, funding acquisition, project administration, resources, supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by Natural Science Foundation of Guangxi (2024GXNSFFA010004, 2024GXNSFBA010145), National Natural Science Foundation of China (U20A20101, 82260692), Guangxi Young and Middle-aged University Teachers' Scientific Research Ability Enhancement Project (2023KY0296), High-Level Talent Training Project Foundation of Guangxi University of Chinese Medicine (GUCM) (No. 202407, 2022C038), Foundation of GUCM (2023QN004).References
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263, DOI:10.3322/caac.21834.
- R. J. Rebello, C. Oing, K. E. Knudsen, S. Loeb, D. C. Johnson, R. E. Reiter, S. Gillessen, T. V. D. Kwast and R. G. Bristow, Nat. Rev. Dis. Primers., 2021, 7, 9, DOI:10.1038/s41572-020-00243-0.
- J. J. Xie, H. He, W. N. Kong, Z. W. Li, Z. T. Gao, D. Q. Xie, L. Sun, X. F. Fan, X. Q. Jiang, Q. G. Zheng, G. Li, J. D. Zhu and G. Y. Zhu, Nat. Chem. Biol., 2022, 18, 1341–1350, DOI:10.1038/s41589-022-01151-y.
- J. L. Huang, X. L. Yan, W. Li, R. Z. Fan, S. Li, J. H. Chen, Z. H. Zhang, J. Sang, L. Gan, G. H. Tang, H. W. Chen, J. J. Wang and S. Yin, J. Am. Chem. Soc., 2022, 144, 17522–17532, DOI:10.1021/jacs.2c06449.
- Z. W. Luo, F. C. Yin, X. B. Wang and L. Y. Kong, Chin. J. Nat. Med., 2024, 22, 195–211, DOI:10.1016/S1875-5364(24)60582-0.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803, DOI:10.1021/acs.jnatprod.9b01285.
- F. M. Kong, C. R. Wang, J. Zhang, X. Q. Wang, B. X. Sun, X. Xiao, H. J. Zhang, Y. Q. Song and Y. J. Jia, Chin. Herb. Med., 2023, 15, 485–495, DOI:10.1016/j.chmed.2023.05.003.
- E. Montuori, C. A. C. Hyde, F. Crea, J. Golding and C. Lauritano, Int. J. Mol. Sci., 2023, 24, 1435, DOI:10.3390/ijms24021435.
- J. L. Huang, X. L. Yan, D. Huang, L. Gan, H. H. Gao, R. Z. Fan, S. Li, F. Y. Yuan, X. Y. Zhu, G. H. Tang, H. W. Chen and J. J. Wan, Acta Pharm. Sin. B, 2023, 13, 4934–4944, DOI:10.1016/j.apsb.2023.07.017.
- J. W. Zheng, J. F. Wang, Q. Wang, H. Y. Zou, H. Wang, Z. H. Zhang, J. H. Chen, Q. Q. Wang, P. X. Wang, Y. S. Zhao, J. Lu, X. L. Zhang, S. T. Xiang, H. B. Wang, J. P. Lei, H. W. Chen, P. Q. Liu, Y. H. Liu, F. H. Han and J. J. Wang, Acta Pharm. Sin. B, 2020, 10, 2313–2322, DOI:10.1016/j.apsb.2020.07.001.
- S. Kuttikrishnan, K. S. Prabhu, A. H. Al Sharie, Y. O. Al Zu'bi, F. Q. Alali, N. H. Oberlies, A. Ahmad, T. El-Elimat and S. Uddin, Drug Discovery Today, 2022, 27, 547–557, DOI:10.1016/j.drudis.2021.10.001.
- W. F. Xu, N. N. Wu, Y. W. Wu, Y. X. Qi, M. Y. Wei, L. M. Pineda, M. G. Ng, C. Spadafora, J. Y. Zheng, L. Lu, C. Y. Wang, Y. C. Gu and C. L. Shao, Mar. Life Sci. & Technol., 2022, 4, 88–97, DOI:10.1007/s42995-021-00103-0.
- X. W. Luo, G. D. Cai, Y. F. Guo, C. H. Gao, W. F. Huang, Z. H. Zhang, H. M. Lu, K. Liu, J. H. Chen, X. F. Xiong, J. P. Lei, X. F. Zhou, J. J. Wang and Y. H. Liu, J. Med. Chem., 2021, 64, 13918–13932, DOI:10.1021/acs.jmedchem.1c01402.
- Z. H. Zhang, Y. D. Zhang and C. J. Yang, Cell Biol. Int., 2021, 45, 2380–2390, DOI:10.1002/cbin.11674.
- L. Guo, X. W. Luo, P. Yang, Y. T. Zhang, J. L. Huang, H. Wang, Y. F. Guo, W. F. Huang, Z. Q. Chen, S. S. Wang, J. J. Wang, J. P. Lei, S. T. Xiang and Y. H. Liu, Front. Pharmacol., 2021, 12, 723729, DOI:10.3389/fphar.2021.723729.
- X. Gan, X. W. Luo, J. Q. Chen, W. X. Fang, M. Y. Nie, H. M. Lu, Y. H. Liu and X. N. Wang, Mol. Cell. Biochem., 2024 DOI:10.1007/s11010-024-05026-9.
- P. Yu, T. W. Gu, Y. Y. Rao, W. M. Liang, X. Zhang, H. G. Jiang, J. D. Lu, J. L. She, J. M. Guo, W. Yang, Y. H. Liu, Y. F. Tu, L. Tang and X. F. Zhou, Acta Pharm. Sin. B, 2024, 14, 3232–3250, DOI:10.1016/j.apsb.2024.03.005.
- Z. Liang, Y. L. Chen, T. W. Gu, J. L. She, F. H. Dai, H. G. Jiang, Z. K. Zhan, K. L. Li, Y. H. Liu, X. F. Zhou and L. Tang, J. Med. Chem., 2021, 64, 9943–9959, DOI:10.1021/acs.jmedchem.1c00175.
- X. F. Zhou, Z. Liang, K. L. Li, W. Fang, Y. X. Tian, Y. X. Tian, X. W. Luo, Y. L. Chen, Z. K. Zhan, T. Zhang, S. R. Liao, S. W. Liu, Y. H. Liu, W. Fenical and L. Tang, J. Med. Chem., 2019, 62, 7058–7069, DOI:10.1021/acs.jmedchem.9b00598.
- M. P. Lin, Z. Z. Tang, J. X. Wang, H. M. Lu, C. W. Wang, Y. T. Zhang, X. M. Liu, Y. H. Liu and X. W. Luo, J. Zhejiang Univ., Sci., B, 2023, 24, 275–280, DOI:10.1631/jzus.B2200622.
- X. W. Luo, X. P. Lin, H. M. Tao, J. F. Wang, J. Y. Li, B. Yang, X. F. Zhou and Y. H. Liu, J. Nat. Prod., 2018, 81, 934–941, DOI:10.1021/acs.jnatprod.7b01053.
- W. F. Xu, X. J. Xue, Y. X. Qi, N. N. Wu, C. Y. Wang and C. L. Shao, Nat. Prod. Res., 2021, 35, 490–493, DOI:10.1080/14786419.2019.1633646.
- L. Fakhouri, T. El-Elimat, D. P. Hurst, P. H. Reggio, C. J. Pearce, N. H. Oberlies and M. P. Croatt, Bioorgan. Med. Chem., 2015, 23, 6993–6999, DOI:10.1016/j.bmc.2015.09.037.
- Y. Kobayashi, C. H. Tan and Y. Kishi, Helv. Chim. Acta, 2000, 83, 2562–2571, DOI:10.1002/1522-2675(20000906)83:9<2562::AID-HLCA2562>3.0.CO;2-Z.
- L. X. Feng, B. Y. Zhang, H. J. Zhu, L. Pan and F. Cao, Chem. Nat. Compd., 2020, 56, 974–976, DOI:10.1007/s10600-020-03206-9.
- Y. Kashiwada, G. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1984, 32, 3493–3500, DOI:10.1248/cpb.32.3493.
Footnotes |
| † Electronic supplementary information (ESI) available: ECD calculations, HRESIMS, 1D and 2D NMR, and UV spectra of compounds 1–4. See DOI: https://doi.org/10.1039/d4ra06292b |
| ‡ J. Wang and H. Lu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |