Open Access Article
Open Access ArticlePhysicochemical characterization and antibacterial activities of silver nanoparticles prepared by amidated low-methoxyl pectin†
Pei-Jun Li *ab,
Run-Sheng Xieab,
Jiang-Juan Panb,
Yu-Qiu Jiangab and
Xing Liuc
*ab,
Run-Sheng Xieab,
Jiang-Juan Panb,
Yu-Qiu Jiangab and
Xing Liuc
aGuangdong Provincial Key Laboratory of Utilization and Conservation of Food and Medicinal Resources in Northern Region, College of Food Science & Technology, Shaoguan University, Shaoguan 512005, China
bCollege of Chemistry and Bioengineering, Guilin University of Technology, Guilin 541004, China
cInstitute for Agro-food Standards and Testing Technology, Shanghai Academy of Agricultural Sciences, Shanghai, 201403, China
First published on 6th December 2024
Abstract
Pectin-based silver nanoparticles (AgNPs) have been used in the field of antibacterials for food due to their excellent antibacterial properties. Herein, in order to achieve higher antibacterial performance, AgNPs were synthesized using high-methoxyl pectin (HMP) and amidated low-methoxyl pectin (ALMP) as precursors. Initially, ALMP-1, -2, and -4 were obtained by pectin amidation with increasing concentrations of NH4OH. Later, HMP and ALMPs were used to prepare AgNPs, and their physicochemical property and antibacterial activities were studied. Transmission electron microscopy (TEM) showed that the mean diameters of HMP-Ag and ALMP-4-Ag were 11.9 ± 3.8 and 13.0 ± 5.4 nm, respectively. EDS analysis revealed that ALMP-4-Ag combined with more Ag element than HMP-Ag. X-ray photoelectron spectroscopy (XPS) indicated that ALMP-4-Ag led to a lower ratio of Ag0 to Ag+ on the surface of AgNPs. Interestingly, ALMP-4-Ag had the strongest antimicrobial effect against Escherichia coli and Staphylococcus aureus, with the lowest inhibitory concentrations (MICs) of up to 33 μg mL−1, which was 16-fold enhanced compared with HMP-Ag (MICs = 533 μg mL−1). Finally, ALMP-4-Ag-treated cells revealed higher levels of protein and sugar leakage as well as increased levels of reactive oxygen species (ROS) and malondialdehyde (MDA) than HMP-Ag.
1. Introduction
The World Health Organization (WHO) has reported that antibiotic-resistant bacteria kill ≈700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people every year globally, and this number is predicted to increase to 10 million deaths by 2050 with an estimated economic cost of 100 trillion USD.1 Among the various antimicrobial products, silver nanoparticles (AgNPs) have been demonstrated to be active in treating several multidrug-resistant bacterial infections.2,3 In general, AgNPs are synthesized using surfactants, reducing agents, and hazardous chemicals, which have toxic and harmful effects on the environment and human health.4,5
000 people every year globally, and this number is predicted to increase to 10 million deaths by 2050 with an estimated economic cost of 100 trillion USD.1 Among the various antimicrobial products, silver nanoparticles (AgNPs) have been demonstrated to be active in treating several multidrug-resistant bacterial infections.2,3 In general, AgNPs are synthesized using surfactants, reducing agents, and hazardous chemicals, which have toxic and harmful effects on the environment and human health.4,5
In order to solve the above problems and develop alternatives, more environmentally friendly methods are used for synthesizing AgNPs. Renewable plant polysaccharides are highlighted as attractive biopolymers for the synthesis of AgNPs, due to their ability to chelate different metal ions.6 These polysaccharides can act as both stabilizing agents, helping to prevent AgNPs from aggregating, and as reducing agents, facilitating the reduction of silver ions to form AgNPs.7 Pectin is a natural polysaccharide obtained from fruit extracts and mainly consists of chains of D-galacturonic acid units joined by glycosidic linkages, which form the structural backbone of pectin molecules. The alcoholic functions of the galacturonic acid units are efficient Ag+ reductants, as has been shown in the synthesis of AgNPs.8–10
Pectin-based AgNPs are suitable for various applications, especially in the food industry, due to their low toxicity, excellent antibacterial properties, and favorable mechanical properties. Shankar et al.11 synthesized pectin/AgNPs nanocomposite films, which exhibited strong antibacterial activity against food-borne pathogenic bacteria. Ardjoum et al.12 reported the in situ synthesis of AgNPs in a pectin matrix using γ-irradiation and the preparation of antibacterial pectin-based nanocomposite films. Our research group prepared pectin-based AgNPs through a reaction involving silver nitrate and alkali hydrolyzed pectin under microwave irradiation, resulting in the formation of AgNPs with significant antibacterial and antifungal activities.13 In another study, uniform and stable hesperidin–pectin AgNPs were prepared, and the combination of hesperidin and pectin resulted in a synergistic effect, significantly enhancing the antibacterial properties of AgNPs.4 Our recent study showed that the synthesis of pectin nanosilver sponges (PE-S/Ag), through an in situ freeze–drying process resulted in sponge materials with superior sponge properties, good antibacterial properties, and coagulation properties.14
Although pectin-based AgNPs exhibit some level of antibacterial activity, their antibacterial efficiency can be further improved. Currently, pectin is mostly modified in order to obtain some specific properties, including by substitution (alkylation, amidation, quaternization, thiolation, sulfation, oxidation, etc.), chain elongation (cross-linking and grafting), and depolymerization (chemical, physical, and enzymatic degradation).15 However, there have been relatively few reports on the biosynthesis of AgNPs by using modified pectin. In one though, Hileuskaya et al.10 prepared AgNPs by using different types of pectin, including high-methoxyl pectin (HMP-Ag), low-methoxyl pectin (LMP-Ag), and amidated low-methoxyl pectin (ALMP-Ag), and ALMP-Ag displayed the lowest antibacterial activity compared to the others. Interestingly, however, the present work showed different results, with ALMP-Ag exhibiting significantly higher antibacterial activity than HMP-Ag, and the antibacterial activities of ALMP-Ag increased with the acylation degree of pectin. We speculated that the acyl and –NH2 groups of ALMP played an important role in the reduction of Ag+, which led to alterations in the physicochemical properties of the resulting AgNPs and had implications for their antibacterial activities.
On this basis, the main objective of the present study was to investigate how specific characteristics of ALMP, such as the degree of esterification, type of functional groups (including acyl and –NH2 groups), and molecular weight, influence the properties of the AgNPs, as well as to assess the antibacterial activities of these pectin-based AgNPs. Based on this, we synthesized AgNPs using HMP and ALMP, both of which served as reducers and stabilizers. First, we investigated various properties of the modified pectin, including the galacturonic acid content, degree of esterification, degree of amidation, and molecular weight. Second, the AgNPs were characterized by UV-vis spectroscopy, zeta potential analysis, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), XPS, etc. Finally, the antibacterial properties of the synthesized AgNPs were evaluated against both Gram-positive and Gram-negative bacteria, and the antibacterial mechanism was revealed through investigating the generation of reactive oxygen species (ROS), the levels of malondialdehyde (MDA), and the leakage of proteins and sugars within bacterial cells.
2. Experimental section
2.1. Materials
High-methoxyl pectin (HMP, from citrus peel, P9135, galacturonic acid ≥ 74.0%) and polygalacturonic acid were supplied by Sigma-Aldrich; silver nitrate (AgNO3, AR, purity ≥ 99.8%) was purchased from Xilong Scientific Co., Ltd (Shantou, China); the nutrient broths used for the antibacterial activities tests were purchased from Qingdao Hope Bio-Technology Co., Ltd (Qingdao, China); DCFH-DA kit was purchased from Shanghai Qiaoxing Trading Co., Ltd (Shanghai, China); the protein detection kit was purchased from Xian Hete Biotechnology Co., Ltd (Xian, China). All other reagents used in the study were analytically pure. E. coli (strain number: 133264) and S. aureus (strain number: 186335) were purchased from Beijing Beina Chuanglian Biotechnology Research Institute (Beijing, China).2.2. Preparation of amidated low-methoxyl pectin (ALMP) and AgNPs
ALMP was prepared following a modified version of the method previously described in the literature.16 Briefly, a 3.0 g sample of HMP was dissolved in 50 mL of distilled water and stored at 5 °C. At the same time, 40 mL of NH4OH (1, 2, and 4 M) and isopropanol mixtures (60%, v/v) were prepared and stored at 5 °C, respectively. NH4OH demethylation was started by mixing the samples and solutions in Erlenmeyer flasks and continuously stirring the reaction mixtures at 5 °C for 40 min. These samples were immediately filtered, and rapidly washed with 60% isopropanol, followed by a wash with 1 M HCl in 60% isopropanol. The filter cake was then stirred with acid alcohol for 20 min to remove ammonia present in the salt form. The samples were washed with isopropanol, dried at 60 °C, ground, and analyzed. The ALMPs prepared with different concentrations of NH4OH (1, 2, and 4 M) were named as ALMP-1, ALMP-2, and ALMP-4, respectively.The biosynthesis of the pectin-based AgNPs was conducted with some modifications based on our previous methods.13 Briefly, 0.2 g of pectin powder was dissolved in 100 mL of NaOH aqueous solution (1 g L−1, w/v), and the mixtures were prepared by adding 2 mL of 0.5 mol L−1 AgNO3 (final concentration of 2 g L−1 pectin and 10 mmol L−1 AgNO3). The prepared solutions were then exposed to microwave radiation (Galanz, 100 Hz) at 400 W for 2 min. The slurry of AgNPs obtained was washed three times, with distilled water and dried in a hot air oven to obtain the powder. In this study, the AgNPs prepared using high-methoxyl pectin were referred to as HMP-Ag, and the AgNPs prepared using different ALMPs were named as ALMP-1-Ag, ALMP-2-Ag, and ALMP-4-Ag, respectively.
2.3. Characterization of HMP, ALMP, and AgNPs
The solution was transferred to a distillation flask, and an extension tube of the condenser was inserted into a receiving bottle containing 150 mL of carbon dioxide-free water and 20 mL of 0.1 M HCl. Next, 20 mL of 0.5 M NaOH solution was added, and the solution was heated and distilled until 80–120 mL of distillate was collected. Then methyl red indicator was added, and the solution was titrated with a 0.1 M NaOH standard solution until the red color disappeared (S, mL). Another 20 mL of 0.1 M HCl solution used as a blank test (B, mL) was titrated with the 0.1 M NaOH standard solution. The amido titration was calculated as (B–S) and recorded as “V3”. The formulas for calculating the DE and DA contents were as follows:
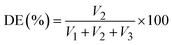 | (1) |
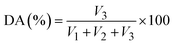 | (2) |
2.4. Antibacterial activities
The minimum inhibitory concentration (MIC) was determined as the lowest concentration of the test compound that inhibited the visible growth of bacteria, as confirmed by measuring the OD600 for all the samples.16 Here, 3.2 mg AgNPs powder was ultrasonically dispersed in LB broth (1 mL), and gradient dilution was performed to obtain AgNPs suspensions at concentrations of 0, 0.2, 0.4, 0.8, 1.6, and 3.2 mg mL−1. In the experiment, the LB broth (200 μL), AgNPs suspension (50 μL), and bacterial suspension (50 μL) were thoroughly mixed and placed into well plates, with AgNPs concentrations of 0, 33, 67, 133, 267, and 533 μg mL−1 in each well, respectively. The cultures were incubated at 37 °C for 24 h, and the absorbance of the cultures at 600 nm was measured every 3 h using a microplate reader.2.5. Antibacterial mechanism
| MDA [mmol g−1] = [6.452 × (A532 − A600) − 0.559 × A600] × Vt/(Vs × W) | (3) |
3. Results and discussion
3.1. Preparation and physicochemical properties of ALMP
In this work, ALMP-1, ALMP-2, and ALMP-4 were obtained by subjecting pectin to amidation with increasing concentrations of NH4OH. As shown in Table 1, with the increase in ammonia concentration, the GA content in pectin increased from 70.5% ± 1.4% to 89.1% ± 0.8%, suggesting that the alkaline hydrolysis process led to the reduction of heteropolysaccharides in the pectin hair zone. Meanwhile, the DE value of amidated pectin decreased significantly with the higher ammonia concentrations, which was consistent with previous research.24 After 40 min, the DE value of pectin decreased from an initial value of 85.7% ± 0.8% to 38.6% ± 0.9%, and higher concentrations of ammonia led to lower DE values. In addition, the degrees of amidation (DA) of ALMP-1, ALMP-2, and ALMP-4 were 3.2% ± 0.1%, 9.6% ± 0.6%, and 18.6% ± 0.9%, respectively (Table 1). This data indicated that more amide groups were formed by treatment with higher concentrations of NH4OH.| Materials | Properties | ||||
|---|---|---|---|---|---|
| PY (%) | GA (%) | DE (%) | DA (%) | Mw (kDa) | |
| a Note: PY – pectin yield, GA – galacturonic acid, DE – degree of esterification, DA – degree of amidation. Means within different letters (a, b, c, and d) are significantly different (p < 0.05); n = 3. | |||||
| HMP | — | 70.5 ± 1.4a | 85.7 ± 0.8a | 0.0 ± 0.1a | 308.5 ± 9.2a |
| ALMP-1 | 87.8 ± 0.8 | 78.4 ± 2.8b | 66.2 ± 1.1b | 3.2 ± 0.1b | 261.5 ± 5.9b |
| ALMP-2 | 86.8 ± 1.0 | 81.8 ± 2.2b | 45.7 ± 0.5c | 9.6 ± 0.6c | 215.1 ± 4.7c |
| ALMP-4 | 86.7 ± 1.3 | 89.1 ± 0.8c | 38.6 ± 0.9d | 18.6 ± 0.9d | 196.5 ± 6.1d |
Molecular weight is an important indicator of the physical, chemical, and structural properties of polysaccharides. The size and distribution of the molecular weight directly affect the digestion, absorption, and biological activity of polysaccharides.19 The molecular weight distributions of pectin and amidated pectin are shown in Fig. S1.† Table 1 also shows that the Mw values of HMP, ALMP-1, ALMP-2, and ALMP-4 were 308.5 ± 9.2, 261.5 ± 5.9, 215.1 ± 4.7, and 196.5 ± 6.1 kDa, respectively. This suggests that β-degradation resulted in the chain breakage of the pectin chain with the amidated modification, which led to a decrease in the molecular weight of pectin. These findings illustrate that the concentration of ammonia during the amidation process affected the chemical composition and molecular characteristics of the modified pectin samples. In a previous report, the extent of ammonolysis and hydrolysis of pectin in aqueous solution were reported to be highly dependent on ammonia concentration and temperature.24
3.2. Characterization of AgNPs
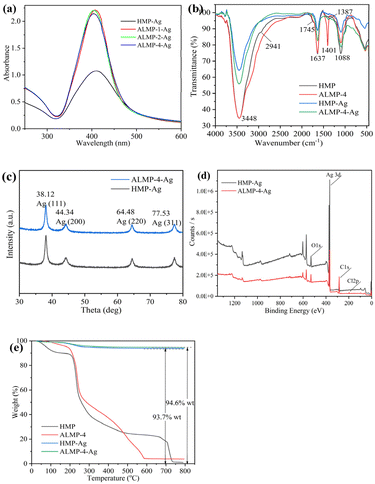 | ||
| Fig. 1 UV-vis absorption spectroscopy (a), FTIR spectroscopy (b), XRD (c), XPS full spectrum (d), and TGA (e) analyses of HMP and ALMP-Ag. | ||
| AgNPs | Characteristics of AgNPs | MIC values (μg mL−1) | |||||
|---|---|---|---|---|---|---|---|
| λmax, nm | FWHM, nm | dH, nm | dTEM, nm | ξ-Potential, mV | E. coli | S. aureus | |
| a dH – hydrodynamic diameter of NPs measured with DLS, dTEM – diameter of NPs measured with TEM, MIC – minimum inhibitory concentration, mean within different letters (a, b, c, and d) are significantly different (p < 0.05); n = 3. | |||||||
| HMP-Ag | 410 | 111 | 202.4 ± 2a | 11.9 ± 3.8 | −20.4 ± 0.3a | 533 | 533 |
| ALMP-1-Ag | 407 | 83 | 174.1 ± 3b | 12.1 ± 2.3 | −19.2 ± 1.6a | 267 | 267 |
| ALMP-2-Ag | 403 | 83 | 157.2 ± 1c | 13.5 ± 0.7 | −16.1 ± 1.8b | 133 | 133 |
| ALMP-4-Ag | 404 | 85 | 119.1 ± 2d | 13.0 ± 5.4 | −14.8 ± 0.8b | 33 | 33 |
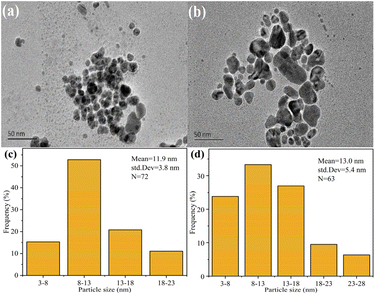
Fig. S2 and S3† show the hydrodynamic diameters and zeta potential values of the AgNPs, respectively. According to Table 2, the dH values (hydrodynamic diameter of NPs measured with DLS) of ALMP-Ag were lower than those of HMP-Ag, and ALMP-4-Ag showed the minimum particle size value. Contrary to this finding, Hileuskaya et al.10 reported that the mean diameter of HMP-Ag was lower than that of ALMP-Ag. This inconsistency could be attributed to the different synthesis process and physicochemical properties of ALMP-Ag. In addition, HMP-Ag showed more negative surface charges than ALMP-Ag, which indicated that the acylation reaction reduced the negative charges of pectin macromolecules.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of nonionic carboxy groups), 1637 cm−1 (–C
O of nonionic carboxy groups), 1637 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1088 cm−1 (C–OH) were various characteristic bands of free pectin.20 In the IR spectrum of the ALMP-4 sample, the band at 2941 cm−1 had disappeared, and a new band at 1401 cm−1 was observed. The results imply that the amidation reaction reduced the –OH group in HMP, and a new amide bond was generated. For the HMP-Ag and ALMP-4-Ag samples, the vibrational band located at 3448 cm−1 was weakened, indicating that O–H exhibited reducing properties and participated in the synthesis process of the AgNPs. The disappearance of the band located at 1745 cm−1 indicated that all the ester groups (-COOCH3) of HMP and ALMP were hydrolyzed to form –COOH under alkaline conditions. After the reduction reaction with Ag+, the IR spectrum of ALMP-4 showed a significant decrease in the amide bond, with the peak shifted from 1401 to 1387 cm−1, indicating that the amide bond was involved in the reduction reaction of Ag+. In conclusion, alkaline conditions caused the –COOCH3 of ALMP to be hydrolyzed to form –COOH, which combined with Ag+ to form a carboxylate. Ag+ was then reduced by amide bonding and O–H, resulting in the synthesis of ALMP-Ag.
O), and 1088 cm−1 (C–OH) were various characteristic bands of free pectin.20 In the IR spectrum of the ALMP-4 sample, the band at 2941 cm−1 had disappeared, and a new band at 1401 cm−1 was observed. The results imply that the amidation reaction reduced the –OH group in HMP, and a new amide bond was generated. For the HMP-Ag and ALMP-4-Ag samples, the vibrational band located at 3448 cm−1 was weakened, indicating that O–H exhibited reducing properties and participated in the synthesis process of the AgNPs. The disappearance of the band located at 1745 cm−1 indicated that all the ester groups (-COOCH3) of HMP and ALMP were hydrolyzed to form –COOH under alkaline conditions. After the reduction reaction with Ag+, the IR spectrum of ALMP-4 showed a significant decrease in the amide bond, with the peak shifted from 1401 to 1387 cm−1, indicating that the amide bond was involved in the reduction reaction of Ag+. In conclusion, alkaline conditions caused the –COOCH3 of ALMP to be hydrolyzed to form –COOH, which combined with Ag+ to form a carboxylate. Ag+ was then reduced by amide bonding and O–H, resulting in the synthesis of ALMP-Ag.As shown in Fig. 1c–e, HMP-Ag and ALMP-4-Ag were characterized by XRD, XPS, and TGA, respectively. Fig. 1c shows that XRD peaks could be observed at 38.12°, 44.34°, 64.48°, and 77.53°, which were consistent with metallic silver, and in line with previous reports.25 The complete XPS spectra showed the presence of the elements C, Ag, and O in both HMP-Ag and ALMP-4-Ag (Fig. 1d). Li et al.25 believed that the C and O elements of AgNPs came from precursor pectin macromolecules. The appearance of the Cl element in ALMP-4-Ag was noteworthy, and could be explained by the use of HCl during the amidation reaction. The combustion residues from HMP-Ag and ALMP-4-Ag were 93.7% and 94.6% (w/w), respectively (Fig. 1e), indicating that ALMP-4 combined more Ag than HMP during the synthesis process of the AgNPs.
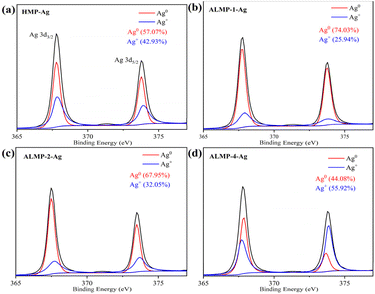
To identify the metallic components and their reduction degree, XPS spectra were recorded and compared. Deconvolution of the high-resolution XPS spectrum of the Ag 3d revealed the chemical character of Ag in the synthesized AgNPs (Fig. 2). According to the standard spectrum in NIST, the binding energy of Ag+ is higher than that of Ag0.26 In the XPS spectra (Fig. 2b–d), the higher degree of amidation of pectin, the lower the ratio of Ag0 to Ag+ on the surface of the synthesized ALMP-Ag. This suggests that the surface of AgNPs prepared with higher amidated pectin is more prone to oxidation, forming Ag2O, and subsequently Ag+ is formed in acidic environments. This higher Ag+ content likely contributed to their enhanced antibacterial properties. Zheng et al.27 believed that the generation and release of Ag+ ions were often initiated with the oxidation of Ag0, which could trigger the production of ROS and lead to bacterial cell damage, ultimately resulting in an antibacterial effect.
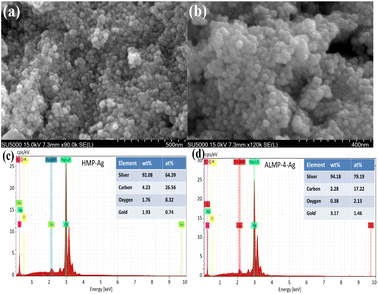
Fig. 4a and b show that both AgNPs prepared by HMP and ALMP-4-Ag were uniform spherical particles, while the particle size of the former was smaller than that of the latter. EDS analysis revealed that the main component of both HMP-Ag and ALMP-4-Ag was metallic silver, and the normalized percentages of silver in HMP-Ag and ALMP-4-Ag were 92.08% and 94.18%, respectively (Fig. 4c and d). The results for EDS analysis of the silver elements were highly consistent with the TGA analysis data (Fig. 1e), while the C and O elements were derived from the precursor pectin.
3.3. Antibacterial activities of the AgNPs
The antibacterial activities of AgNPs determined by the MIC tests are shown in Table 2 and Fig. 5. After 24 h of incubation, the MIC values for HMP-Ag, ALMP-1-Ag, ALMP-2-Ag, and ALMP-4-Ag against both E. coli and S. aureus were found to be 533, 267, 133, and 33 μg mL−1, respectively. ALMP-4-Ag showed an impressive 16-fold increase in bacterial inhibition against E. coli and S. aureus compared to HMP-Ag. The pectin-based AgNPs previously prepared under microwave irradiation by our research group showed MIC values of 160 mg mL−1 against E. coli and S. aureus. Similarly, Ibraheem et al.33 synthesized AgNPs employing Parkia biglobosa pectin, demonstrating antibacterial activity at a concentration of 100 μg mL−1. Furthermore, Wang et al.34 reported the synthesis of AgNPs from the polysaccharides of walnut green husk, which showed inhibitory effects on E. coli and S. aureus at a concentration of 50 μg mL−1. In this work, the results indicated that the antimicrobial activity of ALMP-4-Ag was superior to that reported in previous studies.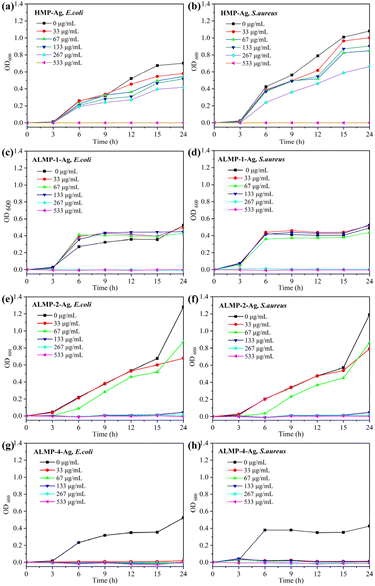 | ||
| Fig. 5 E. coli and S. aureus growth curves at HMP-Ag (a, b), ALMP-1-Ag (c, d), ALMP-2-Ag (e, f), and ALMP-4-Ag (g, h), respectively. | ||
Obviously, the antibacterial activities of ALMP-Ag tended to increase with an increasing DA value and decreasing Mw of pectin. The results could be explained by the following factors. First, the special structural characteristics of ALMP are one of the important factors, with a higher content of GA, lower DE values, higher DA, and lower Mw (Table 1), which are different from those of HMP. The higher GA content and lower DE in ALMP resulted in the presence of more diol moieties in the galacturonic acid units, while the higher DA indicated the presence of more amide groups, and the lower Mw might provide better accessibility to the functional groups, which is particularly conducive to reducing Ag+ and facilitating the formation of AgNPs with superior antibacterial properties.8 However, in a previous report, compared with PectLM-Ag and PectHM-Ag, ALMP-Ag displayed lower antibacterial activities against strains of Bacillus bacteria compared to PectLM-Ag and PectHM-Ag, and it displayed no growth suppression against E. coli.10 Although the structural characteristics of ALMP in this work were very similar to those reported in the study of Hileuskaya et al. (DE = 32%, DA = 18%, Mw = 120 kDa), there were still significant differences in the synthesis process of AgNPs, which led to the difference in antibacterial efficiency. Second, the physicochemical properties of AgNPs are another inevitable factor, including the particle size distribution, zeta potential, and the proportion and valence state of Ag elements. Last but not least, the amount of released Ag+ is also an important factor affecting the antibacterial efficiency of AgNPs.32 A profound investigation is currently in progress and the obtained data are to be published in the near future. In short, ALMP-4-Ag displayed the highest antibacterial activity against both Gram-positive bacteria and Gram-negative bacteria, with lower MICs compared to previous reports.10,13
3.4. Antibacterial mechanism of the AgNPs
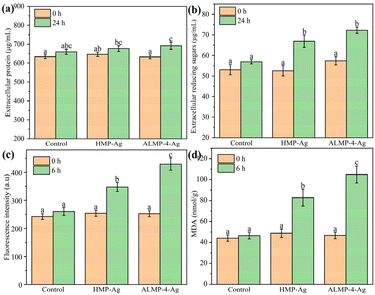
The disruption of bacterial cell membranes and the subsequent leakage of intracellular components, such as proteins and sugars, is a well-documented antibacterial mechanism of AgNPs.35 We determined the leakages of intracellular protein and reducing sugar by treating E. coli cells with AgNPs at their MIC value of 533 μg mL−1. As shown in Fig. 6a, only a small amount of protein leaked incrementally from the E. coli after treatment with HMP-Ag and ALMP-4-Ag for 24 h. Nevertheless, the leakage of reducing sugar reached 66.9 and 72.3 μg mL−1 after treatment with HMP-Ag and ALMP-4-Ag for 24 h, respectively, which were much higher than the control sample (Fig. 6b). All of these results are consistent with previous studies.35,36Generally, ROS are regarded as a collective marker of the superoxide anion, hydroxyl radical, and hydrogen peroxide, which acts as an indicator of oxidative stress.37 Oxidative stress plays an important role in mediating toxicity in bacterial cells, which can damage bacterial DNA, the cell membrane, and cellular proteins, leading to cell death.4 Fig. 6c shows that the fluorescence intensity of ROS in each group was basically the same at 0 h, but ROS increased significantly after 6 h of treatment by AgNPs. It is worth noting that the HMP-Ag- and ALMP-4-Ag-treated cells produced increases of ROS of up to 36.9% and 70.0%, respectively, but the untreated treated cells increased by only 7.5%.
Furthermore, oxidative stress can lead to the peroxidation of polyunsaturated lipids, resulting in the production of peroxy radicals and single oxygen.35,38 MDA is a well-known product of lipid peroxidation and is often used as an important biomarker to assess the extent of oxidative damage to lipids within cells. As shown in Fig. 6d, the levels of MDA in AgNPs-treated cells were significantly higher compared to the untreated cells. The MDA levels were 2.3- and 1.7-folds higher in HMP-Ag- and ALMP-4-Ag-treated E. coli, respectively. These findings highlight the antibacterial potential of AgNPs, especially ALMP-4-Ag, as effective antibacterial agents that disrupt bacterial cells through oxidative stress and lipid peroxidation mechanisms.
4. Conclusions
Pectin-based AgNPs were synthesized using HMP and ALMP as both reducers and stabilizers. Compared to HMP, the ALMPs exhibited distinctive features, including higher GA content, lower DE values, higher DA, and lower Mw. Furthermore, ALMP-Ag demonstrated several advantages over HMP-Ag, such as having less negative surface charges, smaller particle size, higher silver content, and lower ratio of Ag0 to Ag+ on the nanoparticle surface. Importantly, the antibacterial activity of ALMP-Ag exhibited a positive correlation with the DA of pectin and was inversely correlated with the Mw of pectin. Among all the tested AgNPs, ALMP-4-Ag had the strongest antibacterial effect against both E. coli and S. aureus (MIC 33 μg mL−1), which were much lower than HMP-Ag (MIC 533 μg mL−1). In brief, the experimental data provided valuable insights into the enhanced antibacterial efficiency of modified pectin-based AgNPs. These findings have significant implications for potential applications in the food and medical industries, where the demand for effective and safe antibacterial agents is high.Data availability
Data will be made available on request.Author contributions
P. J. L. and R. S. X. conceived the concept and experiments. R. S. X. and J. J. P. carried out the materials synthesis, characterizations, data curation, writing-original draft, writing-review & editing. P. J. L. carried out the writing-review & editing, supervision, project administration and funding acquisition. Y. Q. J. and X. L. co-wrote the paper and revised the paper. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the Guangxi Natural Science Foundation (2020GXNSFAA297198), the Shaoguan Government-supported Project for Scientific Researchers in 2023 (230324098034896), the Talent Introduction (Training) Project of Shaoguan University (142-9900064701), and the Open Fund of the Guangdong Provincial Key Laboratory of Utilization and Conservation of Food and Medicinal Resources in Northern Region (FMR2022004M).References
- Y. Wang, Y. Yang, Y. Shi, H. Song and C. Yu, Adv. Mater., 2020, 32, 1904106 CrossRef CAS.
- J. M. V. Makabenta, A. Nabawy, C.-H. Li, S. Schmidt-Malan, R. Patel and V. M. Rotello, Nat. Rev. Microbiol., 2021, 19, 23–36 CrossRef CAS PubMed.
- A. Roy, O. Bulut, S. Some, A. K. Mandal and M. D. Yilmaz, RSC Adv., 2019, 9, 2673–2702 RSC.
- Z.-Y. Zhao, P.-J. Li, R.-S. Xie, X.-Y. Cao, D.-L. Su and Y. Shan, Int. J. Biol. Macromol., 2022, 214, 220–229 CrossRef CAS PubMed.
- L. Xu, Y.-Y. Wang, J. Huang, C.-Y. Chen, Z.-X. Wang and H. Xie, Theranostics, 2020, 10, 8996–9031 CrossRef CAS.
- J. R. Nakkala, R. Mata and S. R. Sadras, J. Colloid Interface Sci., 2017, 499, 33–45 CrossRef CAS PubMed.
- H. E. Emam and H. B. Ahmed, Carbohydr. Polym., 2016, 135, 300–307 CrossRef CAS PubMed.
- P. Pallavicini, C. R. Arciola, F. Bertoglio, S. Curtosi, G. Dacarro, A. D'Agostino, F. Ferrari, D. Merli, C. Milanese, S. Rossi, A. Taglietti, M. Tenci and L. Visai, J. Colloid Interface Sci., 2017, 498, 271–281 CrossRef CAS.
- K. Devasvaran and V. Lim, Pharm. Biol., 2021, 59, 494–503 CrossRef CAS PubMed.
- K. Hileuskaya, A. Ladutska, V. Kulikouskaya, A. Kraskouski, G. Novik, I. Kozerozhets, A. Kozlovskiy and V. Agabekov, Colloids Surf., A, 2020, 585, 124141 CrossRef CAS.
- S. Shankar, N. Tanomrod, S. Rawdkuen and J.-W. Rhim, Int. J. Biol. Macromol., 2016, 92, 842–849 CrossRef CAS PubMed.
- N. Ardjoum, S. Shankar, N. Chibani, S. Salmieri and M. Lacroix, Food Hydrocolloids, 2021, 121, 107000 CrossRef CAS.
- D.-L. Su, P.-J. Li, M. Ning, G.-Y. Li and Y. Shan, Mater. Lett., 2019, 244, 35–38 CrossRef CAS.
- Z.-Y. Zhao, P.-J. Li, X.-Y. Cao, R.-S. Xie and H.-Y. Li, Cellulose, 2023, 30, 9425–9437 CrossRef CAS.
- J. Chen, W. Liu, C. M. Liu, T. Li, R. H. Liang and S. J. Luo, Crit. Rev. Food Sci. Nutr., 2015, 55, 1684–1698 CrossRef CAS PubMed.
- S. A. Elnawawi and Y. A. Heikal, Carbohydr. Polym., 1995, 27, 191–195 CrossRef CAS.
- D.-L. Su, P.-J. Li, S. Y. Quek, Z.-Q. Huang, Y.-J. Yuan, G.-Y. Li and Y. Shan, Food Chem., 2019, 286, 1–7 CrossRef CAS PubMed.
- R. Xu and W.-J. Wu, Food Res. Dev., 2012, 33, 23–26 CAS.
- P.-J. Li, J.-L. Xia, Z.-Y. Nie and Y. Shan, Food Sci. Technol., 2016, 69, 203–210 CAS.
- P.-J. Li, J.-Y. Liang, D.-L. Su, Y. Huang, J.-J. Pan, M.-F. Peng and Y. Shan, Bioprocess Biosyst. Eng., 2020, 43, 2017–2026 CrossRef CAS PubMed.
- G. Wang, W. Jin, A. M. Qasim, A. Gao, X. Peng, W. Li, H. Feng and P. K. Chu, Biomaterials, 2017, 124, 25–34 CrossRef CAS PubMed.
- P.-J. Li, J.-L. Xia, Z.-Y. Nie and Y. Shan, Bioprocess Biosyst. Eng., 2016, 39, 485–492 CrossRef CAS.
- H. Shi, Experimental Guidance on Plant Stress Physiology, Science Press, 2016 Search PubMed.
- S. A. El-Nawawi and Y. A. Heikal, Carbohydr. Polym., 1995, 27, 191–195 CrossRef CAS.
- P.-J. Li, J.-J. Pan, L.-J. Tao, X. Li, D.-L. Su, Y. Shan and H.-Y. Li, Molecules, 2021, 26, 4479 CrossRef CAS.
- J. Yang, Y. Chen, L. Zhao, Z. Feng, K. Peng, A. Wei, Y. Wang, Z. Tong and B. Cheng, Composites, Part B, 2020, 197, 108139 CrossRef CAS.
- K. Zheng, M. I. Setyawati, D. T. Leong and J. Xie, Coord. Chem. Rev., 2018, 357, 1–17 CrossRef CAS.
- N. K. Sharma, J. Vishwakarma, S. Rai, T. S. Alomar, N. AlMasoud and A. Bhattarai, ACS Omega, 2022, 7, 27004–27020 CrossRef CAS PubMed.
- S. Vijayaram, H. Razafindralambo, Z.-y. Sun, S. Vasantharaj, H. Ghafarifarsani, S. H. Hoseinifar and M. Raeeszadeh, Biol. Trace Elem. Res., 2024, 202, 360–386 CrossRef CAS.
- D. Wang, W. Geng, Q. Li, G. Li, D. Zhang and H. Zhang, Arabian J. Chem., 2022, 15, 103500 CrossRef CAS.
- M. K. A. Al-Muhanna, K. S. Hileuskaya, V. I. Kulikouskaya, A. N. Kraskouski and V. E. Agabekov, Colloid J., 2015, 77, 677–684 CrossRef CAS.
- N. Yu, X. Wang, F. Ning, C. Jiang, Y. Li, H. Peng and H. Xiong, Carbohydr. Polym., 2019, 217, 58–68 CrossRef CAS PubMed.
- S. A. Ibraheem, E. A. Audu, A. J. Atabat, B. F. Tanimu, J. Y. Yahaya and J. T. Barminas, Inorg. Chem. Commun., 2023, 158, 111500 CrossRef CAS.
- G. Wang, X. Yang, X. Chen, J. Huang, R. He, R. Zhang and Y. Zhang, Int. J. Biol. Macromol., 2024, 276, 133798 CrossRef CAS.
- E. Z. Gomaa, J. Gen. Appl. Microbiol., 2017, 63, 36–43 CrossRef CAS.
- Y. G. Yuan, Q. L. Peng and S. Gurunathan, Int. J. Mol. Sci., 2017, 18, 569 CrossRef PubMed.
- A. J. Kora and R. B. Sashidhar, Arabian J. Chem., 2018, 11, 313–323 CrossRef CAS.
- A. Rahal, A. Kumar, V. Singh, B. Yadav, R. Tiwari, S. Chakraborty and K. Dhama, BioMed Res. Int., 2014, 2014, 761264 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07060g |
| This journal is © The Royal Society of Chemistry 2024 |