Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Covalent bidentate ligand-enabled regioselective Wacker-type oxidation of olefins†
Liping Chenb,
Shuai Zhanga,
Yuchen Yanga,
Xue Wanga,
Wenjie Lana,
Zhijie Chena,
Wang Gonga,
Qingqing Niea,
Wenqiang Caoa and
Ziyan Meng *a
*a
aGanzhou Polytechnic College, Ganzhou, 341000, Jiangxi province, China. E-mail: mengzy0724@163.com
bGanzhou People's Hospital, Ganzhou, 341000, Jiangxi province, China
First published on 28th November 2024
Abstract
The utilization of Pd(II)-catalyzed oxidation for the transformation of terminal olefins into methyl ketones has emerged as a particularly intriguing and versatile strategy in organic synthesis. Herein we report a novel Pd(II)-catalyzed Wacker-type oxidation with covalent bidentate ligands. The ligand, 1-(pyridin-2-yl)-1,2-dihydro-3H-indazol-3-one, exhibits excellent performance in converting olefins to ketones. The optimized reaction conditions include the use of TBHP as oxidant, EtOH or MeCN as solvent and short reaction time. The substrate scope includes various substituted olefins, which undergo the desired oxidation reaction with high efficiency.
Introduction
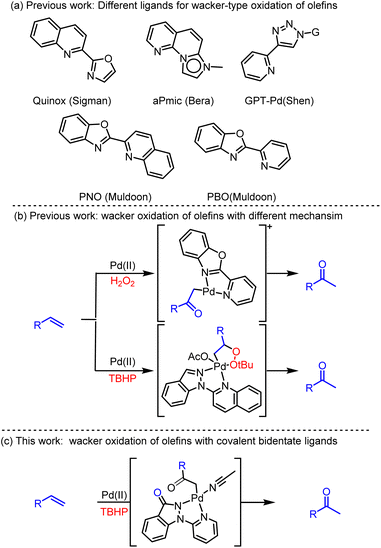
The oxidation of terminal olefins catalyzed by palladium(II) to methyl ketones is a highly valuable chemical process, widely utilized in the synthesis of natural products and fine chemicals.1 Among these, the Wacker-type oxidation, typically facilitated by PdCl2 and CuCl2 under aerobic conditions, has garnered significant attention.2 However, the application of Wacker-type oxidation is hindered by limitations in the rate and selectivity of ketone and aldehyde product formation, particularly with olefins containing proximal heteroatoms.3In 2009, Sigman's team innovatively introduced the tertiary butyl hydroperoxide (TBHP)-mediated Wacker-type oxidation method, in which they successfully demonstrated the high selectivity towards ketone products by utilizing Pd(II) complexes containing the bidentate ligand quinoxaline-2-oxazoline (Quinox) (Scheme 1a).4 Building upon this foundation, they further reported the effective oxidation of various challenging substrates, such as protected allyl alcohols, allylamines, homoallyl alcohols, and internal olefins, into methyl ketone products.5 Subsequently, a novel TBHP-mediated Wacker-type oxidation method was also developed by Bera and colleagues, ingeniously utilizing fused pyridinyl-mesoionic carbenes (aPmic) as ligands (Scheme 1a).6 More recently, the glycosylpyridyltriazole palladium (GPT Pd) complex had emerged as an environmentally friendly catalyst for selective conversions in water.7
Notably, Muldoon's group pioneered the 2-(pyridin-2-yl)naphtho[1,2-d]oxazole (PNO) as the coupling ligand to achieve a Pd(II)-catalyzed oxidation methodology, using O2 as the sole oxidant.8 Their subsequent studies devised innovative hydrogen peroxide (H2O2)-mediated Wacker-type oxidation utilizing a cationic palladium(II) complex, [(PBO)Pd(NCMe)2][OTf]2, where PBO represents 2-(pyridin-2-yl)benzo[d]oxazole (Scheme 1a).9 To unravel the intricate catalytic oxidation mechanism, in situ high-resolution mass spectrometry analyses and exhaustive isotopic labeling experiments were undertaken (Scheme 1b).10,11 Their investigations revealed that he synthesis of methyl ketones was achieved via the formation of a Pd(II)-enolate intermediate, followed by its subsequent protonolysis. Recently, Zou's team has reported an innovative TBHP-mediated, Pd(II)-catalyzed, Wacker-type oxidation that utilizes 2-(1H-indazol-1-yl)quinolinone as ligand. This advanced methodology involves the acquisition of methyl ketones through a 1,2-hydride shift mechanism of the Pd(II)-alkylperoxide complex.11
Herein we reported a TBHP-mediated Pd(II)-catalyzed Wacker-type oxidation with covalent bidentate ligands (Scheme 1c). Although numerous related ligands have been reported and the catalytic efficiency is relatively high, the potential side reactions and the presence of impurities during the reaction process continue to pose significant challenges. The ligand, 1-(pyridin-2-yl)-1,2-dihydro-3H-indazol-3-one, features an electron-deficient covalent indazol-3-one moiety and electron-rich pyridin rings, enhancing the resilience of the catalytic system to harsh oxidative conditions. This Pd(II) catalytic system exhibits exceptional performance in converting olefins to ketones, demonstrating remarkable versatility and stability even in the presence of interfering molecules such as Ac2O, MeOH, EtOH, NaOAc, and NaBr. To gain deeper insights into the intricate selective reaction mechanism, experimental studies were conducted, revealing that the dissociation of Pd(II) intermediates and the subsequent regeneration of Pd(OAc)2 are primary contributors to impurity formation.
Results and discussion
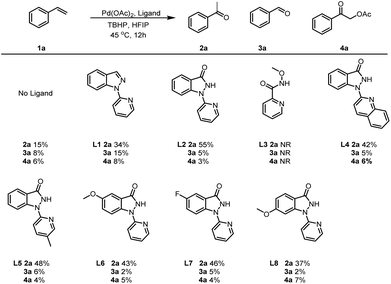
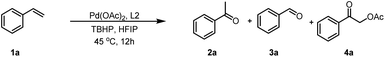
We began our studies by examining the oxidative progress of styrene (1a) in the presence of various of ligands (Table 1). A simple mixture of 1a with 5 mol% of Pd(OAc)2, and 3.0 equivalents of TBHP(decan) in hexafluoroisopropanol (HFIP) at 45 °C for 12 h obtained acetophenone (2a) and benzaldehyde (3a), with an yield of 15% and 8%, respectively(Table 1). Moreover, 2-oxo-2-phenylethyl acetate (4a) could also be detected with a 6% yield in the presence of interfering ions AcO− ions.. Interestingly, the assistance of 5 mol% of 1-(pyridin-2-yl)-1H-indazole (L1) resulted in the production of 2a, 3a and 4a with yields of 34%, 15% and 8%, respectively. Furthermore, covalent bidentate ligands (L2) could also enhance this transformation to 2a at higher rates, while simultaneously decreasing the rates towards 3a and 4a. Other covalent bidentate ligands, such as N-methoxypicolinamide (L3), failed to initiate this transformation, highlighting the importance of Pyridin-indazol-3-one in Pd-catalyzed Wacker-type oxidations. Notably, the two coordinating modules of this ligand exhibit distinct relative capabilities, with the indazol-3-one moiety forming a robust covalent bond, while the pyridyl unit functions as a σ-donor. Additionally, when quinolone rings (L4) or pyridine rings bearing different substituents (L5) were employed, the yields of 2a were inferior to those achieved with L2. Further ligands screening revealed that the presence of electron-withdrawing and electron-donating group on the benzene ring of indazol-3-one (L6-L8) could facilitating this reaction, albeit at lower rates. Consequently, 1-(pyridin-2-yl)-1,2-dihydro-3H-indazol-3-one (L2) was identified to be the optimal ligands for the Wacker-type oxidation of 1a.With the optimal ligands in hand, we continued to meticulously screen the reaction conditions by testing a diverse array of reaction parameters, including oxidants, solvents, temperatures and reaction time(Table 2). Alternative peroxide oxidants, such as TBHP (water), H2O2, m-CPBA, PhI(OAc)2 and K2S2O8 also facilitated the reaction, with reduced yields of 2a (entries 2–6). Further solvent screening revealed that both EtOH and MeCN could also promote the reaction, with diminished transformation rates of 2a (entries 7–8). Notably, elevating the reaction temperature to 60 °C significantly enhanced the yield of 2a to 86% (entry 9). However, a subsequent increase in temperature to 75 °C led to a slight decrease in yield (entry 10), underscoring the crucial role of temperature optimization in achieving successful transformation. Meanwhile, increasing the quantity of L2 and reducing the reaction duration have negligible influence on the conversion efficiency to 2a, yet they contribute to diminishing the formation of impurities (entry 11). Further solvent screening showed that a mixed solvent of HFIP and MeCN was capable of increasing the yield of 2a and reducing the formation of impurities (entry 12). Furthermore, the incorporation of additional MeCN enables the similar yield of 2a with a reduced quantity of Pd(OAc)2 and L2 (entries 13–14). Oxidant equivalents were also investigated, with reduced yields of 2a using 1 eq. or 2eq. of oxidant (entries 15–16) and approximate yield use 5eq. of oxidant.
| Entry | Oxidant | Solvent | Temp (°C) | Time (h) | Yieldc (%) |
|---|---|---|---|---|---|
| a For entries 1–14: reaction was conducted with 1a (1.0 mmol), Pd(OAc)2 (0.10 mmol), L2 (0.10 mmol), oxidant (3.0 mmol), and solvent (6 mL).b The yields of 2a, 3a and 4a were determined by HPLC.c For entry 2: TBHP(70% in water) (3.0 mmol).d For entry 8: L2 (0.15 mmol).e For entry 9: HFIP (5.5 mL) and MeCN (0.5 mL).f For entries 13–14: reaction was conducted with 1a (1.0 mmol), Pd(OAc)2 (0.02 mmol), L2 (0.025 mmol), oxidant (3.0 mmol), HFIP (5.5 mL) and MeCN (0.5 mL).g xidant (1.0 mmol).h xidant (2.0 mmol).i xidant (5.0 mmol). | |||||
| 1 | TBHP | HFIP | 45 | 12 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 2c | TBHP | HFIP | 45 | 12 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 3 | H2O2 | HFIP | 45 | 12 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | m-CPBA | HFIP | 45 | 12 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 5 | PhI(OAc)2 | HFIP | 45 | 12 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6 | K2S2O8 | HFIP | 45 | 12 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 7 | TBHP | EtOH | 45 | 12 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 8 | TBHP | MeCN | 45 | 12 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 9 | TBHP | HFIP | 60 | 12 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 10 | TBHP | HFIP | 75 | 12 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 11d | TBHP | HFIP | 60 | 6 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 12e | TBHP | HFIP + MeCN | 60 | 6 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 13f | TBHP | HFIP + MeCN | 60 | 6 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0 0 |
| 14f | TBHP | HFIP + MeCN | 60 | 4 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 15g | TBHP | HFIP + MeCN | 60 | 6 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 16h | TBHP | HFIP + MeCN | 60 | 6 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 17i | TBHP | HFIP + MeCN | 60 | 6 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
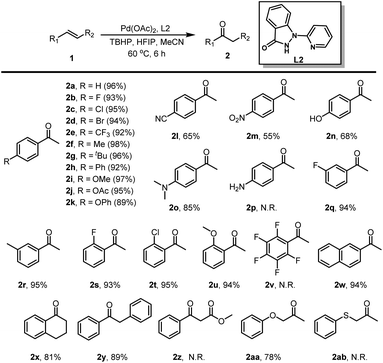
With the optimized reaction conditions established, we embarked on investigating the olefin scope of the Wacker-type oxidation reaction (Table 3). Styrenes with diverse mono-substituents, such as fluorine, chlorine, bromine, trifluoromethyl, methyl, tertiary butyl, phenyl, methoxy, acetoxy, and phenoloxy groups, underwent the desired oxidation reaction with remarkable efficiency, yielding the products in excellent isolated yields ranging from 89% to 98% (2a–2k). Notably, styrenes featuring strong electron-withdrawing group or harboring reactive phenolichydroxyl and dimethylamino groups were compatible with the reaction conditions, smoothly affording the target products in moderate to good isolated yields (2l–2o). Furthermore, the presence of electron-withdrawing or electron-donating groups at the meta or ortho positions of styrenes still permitted the formation of the corresponding methyl ketones in good yields (2q–2u). 1,2,3,4,5-Pentafluoro-6-vinylbenzene remained inert under the reaction conditions, likely hindered by its highly electron-deficient benzene ring, resulting in no observable product formation (2v). Additionally, more sterically hindered naphthyl-substituted olefins furnished 1-(naphthalen-2-yl)ethan-1-one in a yield of 94% (2w). Remarkably, the oxidation of internal alkenes, such as 1,2-dihydronaphthalene and (E)-1,2-diphenylethene, yielded their respective ketones in moderate yields (2x, 2y). Moderate yields of the corresponding product 1-phenoxypropan-2-one was achieved (2aa). Intriguingly, 4-vinylaniline, methyl cinnamate and allyl(phenyl)sulfane failed to yield any product, presumably due to their heightened susceptibility to oxidation (2p, 2z, 2ab).
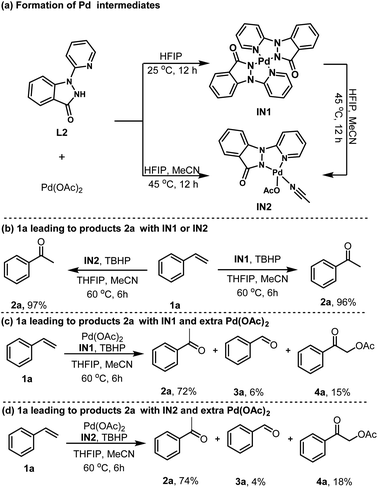
To gain insights into the reaction mechanism, we conducted a comprehensive mechanistic study. Initially, exposing L2 to Pd(OAc)2 in HFIP at ambient temperature yielded the corresponding dimeric Pd(II) intermediate IN1 (Scheme 2a). Interestingly, substitution HFIP with MeCN/HFIP (8.3%, w/w) at 45 °C exclusively produced another monomer palladacycle IN2 under otherwise similar reaction conditions (Scheme 2a). Furthermore, IN1 could be easily converted into IN2 with the assistance of MeCN in HFIP. Additional experiments were conducted to substantiate the significance of these isolated intermediates. As anticipated, using a catalytic amount of IN1 or IN2 as a surrogate for Pd(OAc)2 successfully yielded 2a with yields of 97% and 96%, respectively (Scheme 2b). These observations highlight the role of IN1 and IN2 as activating intermediate in the synthesis of the targeted ketone products. To further elucidate the reaction mechanism, a series of studies were carried out by adding extra Pd(OAc)2. Specifically, the addition of extra Pd(OAc)2 beyond the amount required for IN1 or IN2 explicitly led to the formation of impurities 3a and 4a, suggesting that the presence of unbound Pd(OAc)2 in the system is the predominant factor contributing to the generation of these impurities (Scheme 2c and d).
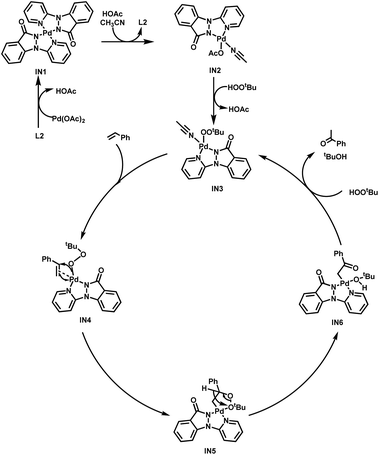
Based on the experimental results and previous research reports,11 a plausible mechanism is outlined here, utilizing 1a as an illustrative example (Scheme 3). The initial step involves the coordination of L2 with Pd(OAc)2, resulting in formation of the dimeric Pd(II) intermediate IN1. Subsequently, the transition to the MeCN-coordinated monomer Pd(II) intermediate IN2 is facilitated by the dissociation of the Pd–N (L2) interaction and the concurrent establishment of Pd–N (MeCN) and Pd–O (AcO−) bonds. Following this, a reaction with TBHP occurs, leading to the release of MeCN and the generation of the Pd–OOtBu intermediate IN3. It is postulated that the subsequent interaction of IN3 with styrene (1a) generates the alkylperoxide intermediate IN4, which undergoes an oxygen insertion at the Markovnikov position, yielding IN5. IN5 then undergoes hydrogen-atom abstraction of the α-H and homolysis of O–O bond, culminating in the production of intermediate IN6. Ultimately, the protonolysis of the novel Pd-enolate intermediate IN6 gives rise to acetophenone (2a) and the activating intermediate IN3, thereby completing the catalytic cycle.
Conclusions
In conclusion, this study presents a novel and efficient method for the Wacker-type oxidation of olefins to methyl ketones using covalent bidentate ligands. The method exhibits remarkable versatility and stability even in the presence of interfering molecules such as Ac2O, MeOH, EtOH, NaOAc, and NaBr. The ligand identified in this study plays a crucial role in enhancing the resilience of the catalytic system to harsh oxidative conditions and promotes the conversion of olefins to ketones through a series of key intermediates. The resulting diverse ketones from the olefin demonstrate this protocol's potential for further structural manipulation of functional molecules.Data availability
The data supporting this article has been uploaded as part of the ESI.†Author contributions
L. Chen, S. Zhang, Y. Yang and X. Wang performed reaction experiments and syntheses of substrates, W. Lan, Z. Chen, W. Gong, revised the manuscript. Q. Nie and W. Cao helped to prepare the manuscript. Z. Meng supervised the project and prepared the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This study was supported by grants from the Natural Science Foundation of Jiangxi Provincine – Youth Fund Project – Direct support(20242BAB21036).Notes and references
- (a) R. Jira, Acetaldehyde from ethylene—A retrospective on the discovery of the Wacker process, Angew. Chem., Int. Ed., 2009, 48, 9034–9037 CrossRef CAS PubMed; (b) Z. K. Wickens, K. Skakuj, B. Morandi and R. H. Grubbs, Catalyst-Controlled Wacker-Type Oxidation: Facile Access to Functionalized Aldehydes, J. Am. Chem. Soc., 2014, 136, 890–893 CrossRef CAS PubMed; (c) M. S. Sigman and E. W. Werner, Imparting catalyst control upon classical palladium-catalyzed alkenyl C–H bond functionalization reactions, Accounts Chem. Res., 2012, 45, 874–884 CrossRef CAS PubMed; (d) V. B. Corless, A. Holownia, H. Foy, R. Mendoza-Sanchez, S. Adachi, T. Dudding and A. K. Yudin, Synthesis of α-borylated ketones by regioselective Wacker oxidation of alkenylboronates, Org. Lett., 2018, 20, 5300–5303 CrossRef CAS PubMed; (e) R. A. Fernandes, A. K. Jha and P. Kumar, Recent advances in Wacker oxidation: from conventional to modern variants and applications, Catal. Sci. Technol., 2020, 10, 7448–7470 RSC; (f) P. Rajeshwaran, J. Trouvé, K. Youssef and R. Gramage Doria, Sustainable Wacker-Type Oxidations, Angew. Chem., Int. Ed., 2022, 61, e202211016 CrossRef CAS PubMed; (g) J. Sietmann, M. Tenberge and J. M. Wahl, Wacker Oxidation of Methylenecyclobutanes: Scope and Selectivity in an Unusual Setting, Angew. Chem., Int. Ed., 2023, 62, e202215381 CrossRef CAS PubMed.
- (a) J. Lai and M. A. Pericàs, Manganese/copper co-catalyzed electrochemical Wacker–Tsuji-type oxidation of aryl-substituted alkenes, Org. Lett., 2020, 22, 7338–7342 CrossRef CAS PubMed; (b) J. Imbao, J. A. van Bokhoven and M. Nachtegaal, Optimization of a heterogeneous Pd–Cu/zeolite Y Wacker catalyst for ethylene oxidation, Chem. Commun., 2020, 56, 1377–1380 RSC; (c) M. Miyazaki and Y. Ura, Palladium/Iron-Catalyzed Wacker-Type Oxidation of Aliphatic Terminal and Internal Alkenes Using O2, ACS Omega, 2023, 8, 41983–41990 CrossRef CAS PubMed.
- Q. Huang, Y. Li, X. Ning, G. Jiang, X. Zhang, J. Qu and Y. Kang, Regioselective Wacker-type oxidation of internal olefins in t BuOH using oxygen as the sole oxidant and t BuONO as the organic redox cocatalyst, Org. Lett., 2020, 22, 965–969 CrossRef CAS PubMed.
- B. W. Michel, A. M. Camelio, C. N. Cornell and M. S. Sigman, A general and efficient catalyst system for a Wacker-Type oxidation using TBHP as the terminal oxidant: application to classically challenging substrates, J. Am. Chem. Soc., 2009, 131, 6076–6077 CrossRef CAS PubMed.
- (a) B. W. Michel, J. R. Mccombs, A. Winkler and M. S. Sigman, Catalyst-controlled Wacker-type oxidation of protected allylic amines, Angew. Chem., Int. Ed., 2010, 49, 7312 CrossRef CAS PubMed; (b) J. R. Mccombs, B. W. Michel and M. S. Sigman, Catalyst-controlled Wacker-type oxidation of homoallylic alcohols in the absence of protecting groups, J. Org. Chem., 2011, 76, 3609–3613 CrossRef CAS PubMed.
- S. Saha, S. Yadav, N. U. D. Reshi, I. Dutta, S. Kunnikuruvan and J. K. Bera, Electronic asymmetry of an annelated pyridyl–mesoionic carbene scaffold: application in Pd (II)-Catalyzed wacker-type oxidation of olefins, ACS Catal., 2020, 10, 11385–11393 CrossRef CAS.
- K. Zheng, H. Wu, H. Xu, W. Yu, N. Sun and C. Shen, Catalyst-Controlled Selectivity in Oxidation of Olefins: Highly Facile Success to Functionalized Aldehydes and Ketones, Catal. Lett., 2022, 152, 3332–3337 CrossRef CAS.
- H. Chai, Q. Cao, L. M. Dornan, N. L. Hughes, C. L. Brown, P. Nockemann, J. Li and M. J. Muldoon, Cationic palladium (II) complexes for catalytic Wacker-type oxidation of styrenes to ketones using O2 as the sole oxidant, Eur. J. Inorg. Chem., 2017, 2017, 5604–5608 CrossRef CAS.
- Q. Cao, D. S. Bailie, R. Fu and M. J. Muldoon, Cationic palladium (II) complexes as catalysts for the oxidation of terminal olefins to methyl ketones using hydrogen peroxide, Green Chem., 2015, 17, 2750–2757 RSC.
- K. L. Walker, L. M. Dornan, R. N. Zare, R. M. Waymouth and M. J. Muldoon, Mechanism of catalytic oxidation of styrenes with hydrogen peroxide in the presence of cationic palladium (II) complexes, J. Am. Chem. Soc., 2017, 139, 12495–12503 CrossRef CAS PubMed.
- S. Zhang, J. Zhang and H. Zou, Pd-Catalyzed TBHP-Mediated Selective Wacker-Type Oxidation and Oxo-acyloxylation of Olefins Using a 2-(1 H-Indazol-1-yl) quinoline Ligand, Org. Lett., 2023, 25, 1850–1855 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07296k |
| This journal is © The Royal Society of Chemistry 2024 |