Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spectroscopic and photochemical evaluation of stereochemically biased 3′-substituted spiropyran photoswitches†
Vojtěch Boháčeka,
Tereza Erbenováa,
Jakub Dávid Malinaa,
Marie Kloubcováa,
Michal Šmahela,
Václav Eigner b and
Jiří Tůma
b and
Jiří Tůma *a
*a
aDepartment of Organic Chemistry, University of Chemistry and Technology Prague, Technická 5, Prague 6, Prague, 166 28, Czech Republic. E-mail: tumaa@vscht.cz
bDepartment of Solid State Chemistry, University of Chemistry and Technology Prague, Technická 5, Prague 6, Prague, 166 28, Czech Republic
First published on 21st November 2024
Abstract
Three series of spiropyran photoswitches with an auxiliary chiral centre at position 3′ of the indoline unit were synthesized. Using one example, a novel methodology for synthesis of an optically active spiropyran photoswitch with a defined chirality at position 3′ is demonstrated. Furthermore, a new acid-mediated strategy for spiropyran purification affording moderate to excellent yields (up to 96%) is reported herein. Relative diastereomeric ratios of the prepared spiropyrans were evaluated using NMR spectroscopy in five different solvents (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti up to 21
anti up to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79) and their photoswitching properties determined by UV-vis spectroscopy. It was found that substitution at position 8 of the chromene subunit notably accelerates the photoswitching process.
79) and their photoswitching properties determined by UV-vis spectroscopy. It was found that substitution at position 8 of the chromene subunit notably accelerates the photoswitching process.
Introduction
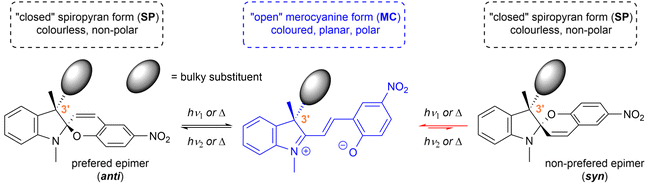
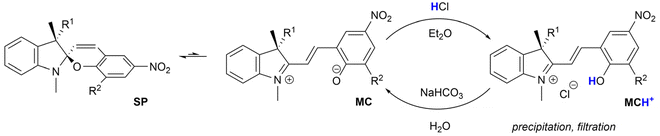
Spiropyrans are well-known photoswitchable compounds with unique properties. The key characteristic of spiropyrans is the very nature of their photoswitching transformation. In contrast to common photoswitches (e.g., stilbenes and azobenzenes), upon irradiation, they not only change their geometry, but also undergo a dramatic transformation in their polarity. The non-polar, colourless spiropyran (SP) arrangement with two perpendicular subunits (indoline and chromene part) can be easily turned into a planar, conjugated, zwitterionic merocyanine form (MC), which is deeply coloured (Scheme 1).1,2 Alongside photoinduced activation, spiropyran switching can also be triggered by other stimuli, such as thermochromic,3 acidochromic,4 electrochromic,5 or mechanochromic6 impulse. Thanks to this fast and wide tunability of their molecular scaffold, spiropyrans have found numerous applications in materials science7 including, but not limited to, modern drug delivery systems,8 materials facilitating molecular motion,9 photochromic fluorescent probes,10 chemosensors of metal ions,11 photochromic liquid crystals,12 and modification of solid surfaces.13The very nature of the currently emerging applications of spiropyrans calls for optically pure compounds that would govern the next evolutionary step of spiropyran-based materials. An optically pure spiropyran represents a cornerstone of a new dimension of spiropyran research. Materials based on such photoswitch could facilitate numerous new applications, such as tunable stereoselective sensing, formation of switchable oriented chiral domains in bulk materials, optically active modifiable surfaces, light-driven selective recognition of chiral molecules, and likely many others.7
Generally, the scaffold of the spiropyran photoswitch is chiral due to the presence of the spiro-carbon. It is, therefore, theoretically feasible to isolate their respective enantiomers, e.g., by dynamic enantioselective crystallization,14 or via HPLC with a chiral stationary phase.15,16 In solution, however, spiropyrans undergo a dynamic equilibrium of the spiropyran and merocyanine form, which leads to undesired spontaneous racemization16,17 leaving these compounds unreliable for the outlined applications requiring optically pure species.
In order to stabilize the optical purity of the spiropyran backbone, it is possible to incorporate a defined centre of chirality in the position 3′ of the indoline subunit, i.e., using two different substituents in this position (e.g., methyl and bulky alkyl or aryl groups). This way, the overall structure, due to the presence of two chiral centres, shifts from a racemic mixture to diastereomeric equilibrium undergoing between two epimers (Scheme 1). Such stereoisomers differ in their free energy and, therefore, their relative ratio. For example, if the energy difference between the two epimers is 12 kJ mol−1, their ratio is equal to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 25 °C. This concept was originally investigated by Gruda et al. in 1978 (ref. 18) and later by Eggers et al. in 1997.19,20 However, only seven compounds in total, exhibiting rather modest diasteromeric excess (syn
1 at 25 °C. This concept was originally investigated by Gruda et al. in 1978 (ref. 18) and later by Eggers et al. in 1997.19,20 However, only seven compounds in total, exhibiting rather modest diasteromeric excess (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = up to 29
anti = up to 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71), were reported. To the best of our knowledge, this approach to optically biased spiropyrans remained unexplored any further until 2018, when Perry et al. published their work on microwave-promoted synthesis of spiropyrans with varying substitution in the position 3′.21 They reported a library of 15 compounds bearing several alkyl and arylmethyl groups mostly with respect to their steric hindrance on the individual spiropyran epimers. The epimeric ratio was evaluated using 1H NMR in CDCl3 and the stereochemical preference (syn
71), were reported. To the best of our knowledge, this approach to optically biased spiropyrans remained unexplored any further until 2018, when Perry et al. published their work on microwave-promoted synthesis of spiropyrans with varying substitution in the position 3′.21 They reported a library of 15 compounds bearing several alkyl and arylmethyl groups mostly with respect to their steric hindrance on the individual spiropyran epimers. The epimeric ratio was evaluated using 1H NMR in CDCl3 and the stereochemical preference (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti) was determined by NMR NOESY experiments that (in most cases) showed clear prevalence of the anti-configuration over syn-. The best epimeric ratio at ambient temperature was found for a combination of methyl and 2-bromophenylmethyl moieties in the position 3′ (syn
anti) was determined by NMR NOESY experiments that (in most cases) showed clear prevalence of the anti-configuration over syn-. The best epimeric ratio at ambient temperature was found for a combination of methyl and 2-bromophenylmethyl moieties in the position 3′ (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = 14
anti = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86). In their follow-up work in 2020 (ref. 22) and 2023,23 respectively, Perry et al. reported further progress on the synthesis of non-symmetrically substituted spiropyrans but put very little emphasis on the stereochemical aspect of the additional chiral centre in the position 3′.
86). In their follow-up work in 2020 (ref. 22) and 2023,23 respectively, Perry et al. reported further progress on the synthesis of non-symmetrically substituted spiropyrans but put very little emphasis on the stereochemical aspect of the additional chiral centre in the position 3′.
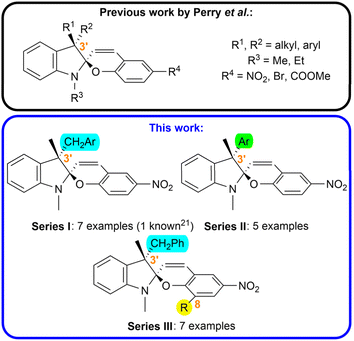
In this work, we focused on the synthesis of a library of spiropyran compounds with varying substitution in the position 3′. We prepared three series of photoswitches (Fig. 1): (i) we broadened the scope of arylmethyl-substituted compounds investigated by Perry et al.21 studying the effect of electron donating and electron withdrawing groups (CH3, CH3O, CF3) in the position 3 and 4 of the aromatic moiety (Series I); (ii) we synthesized a series possessing an aryl unit in the position 3′ instead of more flexible arylmethyl functionality (Series II); (iii) we introduced a new structural pattern by incorporation of an auxiliary bulky group (tBu, Ar) in the position 8 of the chromene subunit alongside a benzylic moiety in the position 3′ to increase the steric hindrance between these two groups in the syn-epimer (Series III). To the best of our knowledge, all the prepared compounds except for Ia have not been reported up to date.
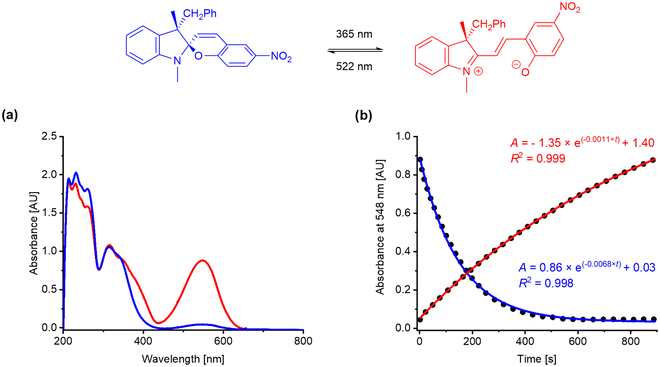
The kinetic rates of photochemical switching of all the prepared spiropyrans were evaluated using UV-vis spectroscopy. The molecular isomerization from SP to MC form was initiated by UV light (λ = 365 nm) while the backwards process was triggered by green light (λ = 522 nm). The syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio of the prepared spiropyrans was determined by NMR spectroscopy at 25 °C in various solvents.
anti ratio of the prepared spiropyrans was determined by NMR spectroscopy at 25 °C in various solvents.
As the syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio reflects only relative stereochemical arrangement, all the target compounds have been synthesized in a non-stereoselective manner, i.e., without specification of absolute configuration in the position 3′. Thus, every compound prepared holds a total sum of four diastereomers. However, we also report one compound where we successfully applied our own developed method of stereoselective separation using co-crystallization of a corresponding 3H-indole precursor with (–)-camphor-10-sulfonic acid. This way, pure (R)-enantiomer of the 3H-indole was yielded and subsequently transformed into the target spiropyran. The absolute configuration of the compound was proven by X-ray crystallography. We also developed and successfully applied a new purification method for isolation of spiropyrans using their acidochromic properties. We believe that both of our separation/isolation methods represent a strong standpoint that can serve the spiropyran research community going forward.
anti ratio reflects only relative stereochemical arrangement, all the target compounds have been synthesized in a non-stereoselective manner, i.e., without specification of absolute configuration in the position 3′. Thus, every compound prepared holds a total sum of four diastereomers. However, we also report one compound where we successfully applied our own developed method of stereoselective separation using co-crystallization of a corresponding 3H-indole precursor with (–)-camphor-10-sulfonic acid. This way, pure (R)-enantiomer of the 3H-indole was yielded and subsequently transformed into the target spiropyran. The absolute configuration of the compound was proven by X-ray crystallography. We also developed and successfully applied a new purification method for isolation of spiropyrans using their acidochromic properties. We believe that both of our separation/isolation methods represent a strong standpoint that can serve the spiropyran research community going forward.
Results and discussion
Synthesis
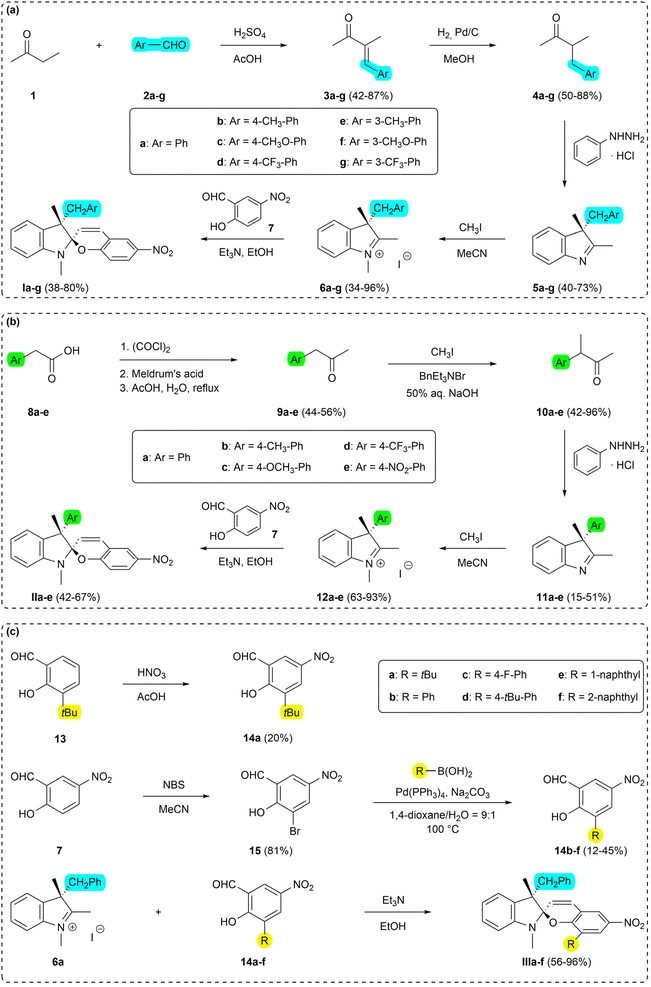
This chapter summarizes the applied synthetic pathways towards the target spiropyrans of the Series I–III, and the description of the outlined isolation/purification methods. Detailed experimental protocols and spectroscopic data are available in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with (–)-camphor-10-sulfonic acid (1.16 g; 5.0 mmol) in EtOH. Upon evaporation, crystalline compound was obtained, which was subsequently recrystallized from EtOAc/MeCN mixture. The progress of the recrystallization was followed by 1H NMR. Two isolated peaks for the two diastereomers were found for the methyl group in the position 2 of the 3H-indole (singlet at δ = 2.84 and 2.85 ppm, respectively) in CD3CN. Other solvents tested (CDCl3, DMSO-d6, acetone-d6, CD3OD) showed no baseline differentiation of signals at 400 MHz. After the first recrystallization, 702 mg of 74
1 ratio with (–)-camphor-10-sulfonic acid (1.16 g; 5.0 mmol) in EtOH. Upon evaporation, crystalline compound was obtained, which was subsequently recrystallized from EtOAc/MeCN mixture. The progress of the recrystallization was followed by 1H NMR. Two isolated peaks for the two diastereomers were found for the methyl group in the position 2 of the 3H-indole (singlet at δ = 2.84 and 2.85 ppm, respectively) in CD3CN. Other solvents tested (CDCl3, DMSO-d6, acetone-d6, CD3OD) showed no baseline differentiation of signals at 400 MHz. After the first recrystallization, 702 mg of 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 diastereomeric mixture was found. Second recrystallization using the same solvent mixture yielded 225 mg (19%) of optically pure salt (Fig. 2). The free base of 3H-indole 5a was subsequently released by treatment with aq. NaHCO3 followed by extraction into dichloromethane. 101 mg (90%) of optically pure 3H-indole 5a was obtained this way. Experimental details are available in the ESI.†
26 diastereomeric mixture was found. Second recrystallization using the same solvent mixture yielded 225 mg (19%) of optically pure salt (Fig. 2). The free base of 3H-indole 5a was subsequently released by treatment with aq. NaHCO3 followed by extraction into dichloromethane. 101 mg (90%) of optically pure 3H-indole 5a was obtained this way. Experimental details are available in the ESI.†
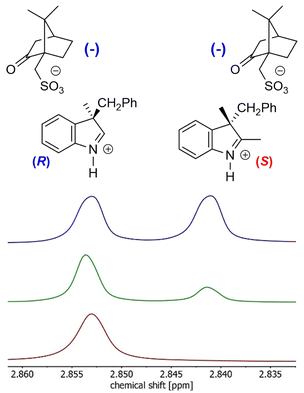 | ||
Fig. 2 NMR analysis of diastereomeric co-crystallization: (i) initial 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture (blue); (ii) 74 50 mixture (blue); (ii) 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 mixture after the 1st recrystallization (green); (iii) optically pure mixture after the 2nd recrystallization (maroon). Full NMR spectra are shown in the ESI.† 26 mixture after the 1st recrystallization (green); (iii) optically pure mixture after the 2nd recrystallization (maroon). Full NMR spectra are shown in the ESI.† | ||
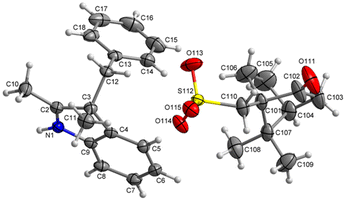
The recrystallized optically pure salt of 3H-indole 5a and (–)-camphor-10-sulfonic acid was studied using X-ray crystallography and the absolute configuration of the isolated 3H-indole was assigned as (3-R) (Fig. 3). Details of the X-ray crystallography measurements are enclosed in the ESI.† The optically pure 3H-indole 5a was subsequently transformed into the corresponding spiropyran Ia using the synthetic protocol for the Series I. The reaction yields and spectroscopic data are in agreement with those of the spiropyran Ia using racemic 3H-indole 5a. To the best of our knowledge, we are the first to report a working protocol for synthesis and characterization of a spiropyran photoswitch with a defined stereochemistry in the position 3′ using purely chemical methods. Despite the modest yield, we believe the process can serve as a basis for further progress in search for optically pure spiropyrans.
This method of purification was successfully applied to the synthesis of the Series III and provided moderate to excellent yields (56–96%; for details see ESI†). The undisputable advantage of this process is low solvent consumption as well as overall cost as opposed to chromatographic methods, thus being potentially attractive for large-scale synthesis. This protocol serves as a convenient and practical methodology for purification of spiropyrans and can be widely used alongside traditionally utilized procedures.
Diastereomeric preference of the target spiropyrans
The diastereomeric preference of the target spiropyrans was determined by 1H NMR. The NMR samples were not subjected to photoisomerization prior to or during the measurement. The influence of five different solvents per each compound was studied using moderately polar chloroform as a benchmark solvent while two polar (acetone, DMSO) and two non-polar (toluene, benzene) solvents were tested as well. Each spectrum is a superposition of both relative stereoisomers (syn- and anti-) and, in some cases, also the open merocyanine form. The syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio was estimated based on the integral values corresponding to the H-3 proton in the spectrum for both stereoisomers. For compounds IIIb–d, doublet signals H-5 and H-7 were utilized instead as there was an overlap of the H-3 signals with other proton multiplets. The syn
anti ratio was estimated based on the integral values corresponding to the H-3 proton in the spectrum for both stereoisomers. For compounds IIIb–d, doublet signals H-5 and H-7 were utilized instead as there was an overlap of the H-3 signals with other proton multiplets. The syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
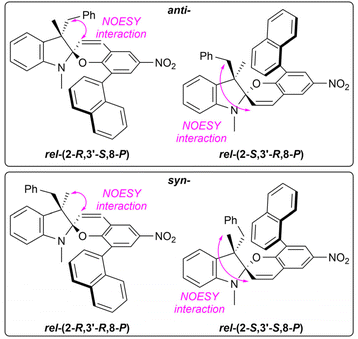
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti assignment of signals was performed using NMR NOESY experiments as demonstrated by Perry et al. (Fig. 4).21 The obtained results are summarized in Table 1.
anti assignment of signals was performed using NMR NOESY experiments as demonstrated by Perry et al. (Fig. 4).21 The obtained results are summarized in Table 1.
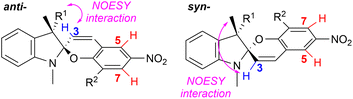 | ||
Fig. 4 Signal assignment of the syn- and anti-epimers based on NMR NOESY experiments; the syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratios were calculated using signals of the H-3 protons or H-5/H-7 protons from 1H NMR spectra. anti ratios were calculated using signals of the H-3 protons or H-5/H-7 protons from 1H NMR spectra. | ||
| Entry | Compound | 3′-Substitution | 8-Substitution | CDCl3 | Acetone-d6 | DMSO-d6 | Toluene-d8 | Benzene-d6 |
|---|---|---|---|---|---|---|---|---|
a Percentage of merocyanine with respect to the content of the whole sample (spiropyran + merocyanine form) is shown in the brackets.b Syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratios quantified using H-5 and H-7 proton signals.c Total four relative stereoisomers found due to axial chirality of the 1,1′-binaphthalene-like structure (see Fig. 5).d Syn anti ratios quantified using H-5 and H-7 proton signals.c Total four relative stereoisomers found due to axial chirality of the 1,1′-binaphthalene-like structure (see Fig. 5).d Syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio not determined due to a signal overlap. anti ratio not determined due to a signal overlap. |
||||||||
| 1 | Ia | PhCH2 | H | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 (5)a 74 (5)a |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 77 |
| 2 | Ib | 4-CH3-PhCH2 | H | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 (8)a 72 (8)a |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
| 3 | Ic | 4-OCH3-PhCH2 | H | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 76 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 (4)a 72 (4)a |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79 79 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
| 4 | Id | 4-CF3-PhCH2 | H | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 (4)a 71 (4)a |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79 79 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 77 |
| 5 | Ie | 3-CH3-PhCH2 | H | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 (5)a 65 (5)a |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
| 6 | If | 3-OCH3-PhCH2 | H | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (4)a 70 (4)a |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
| 7 | Ig | 3-CF3-PhCH2 | H | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 (4)a 69 (4)a |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
| 8 | IIa | Ph | H | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 9 | IIb | 4-CH3-Ph | H | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
| 10 | IIc | 4-OCH3-Ph | H | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
| 11 | IId | 4-CF3-Ph | H | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
| 12 | IIe | 4-NO2-Ph | H | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 13 | IIIa | PhCH2 | tBu | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 (6)a 69 (6)a |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
| 14 | IIIb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
PhCH2 | Ph | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (21)a 70 (21)a |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
| 15 | IIIc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
PhCH2 | 4-F-Ph | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 (31)a 71 (31)a |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
| 16 | IIId![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
PhCH2 | 4-tBu-Ph | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 (23)a 72 (23)a |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
| 17 | IIIe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
PhCH2 | 1-Naphthyl | n.d.d | n.dd | n.d.d | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
| 18 | IIIf | PhCH2 | 2-Naphthyl | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 (33)a 72 (33)a |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
The compounds of the Series I and III exhibited varying concentration of merocyanine in DMSO (4–33%). All other combinations of compounds and solvents showed either no merocyanine peaks at all or only small hints of these signals that could not be reliably quantified. Therefore, we estimate that the concentration of merocyanine in these samples was 0–2%. This observation is in agreement with the expected trend that highly polar solvents promote the formation of merocyanine. Surprisingly, no compound of the Series II exhibited notable amount of merocyanine regardless the solvent. This indicates that the materials IIa–e are not prone to the spiropyran opening process. This supposition is further supported by the obtained photokinetic data (see below). In the case of the Series III, notable increase of the merocyanine form was observed with respect to the analogous compound Ia with no substitution in the position 8. While the tert-butyl-substituted compound IIIa yielded comparable amount of merocyanine to Ia, the aryl-substituted spiropyrans IIIb–f reached up to 33% of merocyanine in DMSO. The amount of merocyanine for IIIe was not determined due to signal overlap. However, the spectrum clearly indicated substantial concentration of the merocyanine form. These results indicate that the presence of an aryl moiety in the position 8 supports the formation of merocyanine in strongly polar media, such as DMSO. This effect is likely not a result of steric hindrance as no such increase in the merocyanine concentration was found for IIIa.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio and solvent polarity. Chloroform solutions exhibit syn
anti ratio and solvent polarity. Chloroform solutions exhibit syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = 25
anti = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 on average for all the compounds Ia–g. More polar solvents (acetone, DMSO) decrease the anti-epimer prevalence up to 35
75 on average for all the compounds Ia–g. More polar solvents (acetone, DMSO) decrease the anti-epimer prevalence up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 (Ie; Table 1, entry 5). DMSO (the most polar solvent used) provides the lowest syn
65 (Ie; Table 1, entry 5). DMSO (the most polar solvent used) provides the lowest syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio except for Ia (Table 1, entry 1) where the lowest anti-abundance was found in acetone. On the other hand, low-polar solvents (benzene, toluene) yield the highest anti-preference (syn
anti ratio except for Ia (Table 1, entry 1) where the lowest anti-abundance was found in acetone. On the other hand, low-polar solvents (benzene, toluene) yield the highest anti-preference (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti up to 21
anti up to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79). We also tested cyclohexane-d12 as a non-polar and non-polarizable solvent. However, the prepared spiropyrans were insoluble thereof. Despite low syn
79). We also tested cyclohexane-d12 as a non-polar and non-polarizable solvent. However, the prepared spiropyrans were insoluble thereof. Despite low syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti differences across the whole Series I, the observed trend shows steady increase of the diastereomeric ratio with decreasing solvent polarity.
anti differences across the whole Series I, the observed trend shows steady increase of the diastereomeric ratio with decreasing solvent polarity.Within the Series I, two structural patterns were studied: 4-substituted, and 3-substituted benzylic units in the position 3′ of the spiropyran scaffold (for examples of 2-substituted analogues see ref. 21). In polar environment (DMSO, acetone), slight preference of the anti-epimer was observed for the 4-substituted phenyl derivatives (Ib–d; Table 1, entries 2–4) over the 3-substituted materials (Ie–g; Table 1, entries 5–7). The same trend is prevalent in toluene as well. In chloroform and benzene, only minor differences were found. Overall, substitution in the position 4 of the benzylic arm yields comparable or slightly higher anti-preference than the non-substituted compound Ia. On the other hand, substitution in the position 3 generally delivers similar or slightly lower anti-abundance than Ia.
The electronic effects of the substituents used (CH3, OCH3, CF3) appear to have very limited influence on the stereochemical preference. The strongly electron withdrawing CF3 group and electron donating OCH3 group have a slightly positive effect on the syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio with respect to the CH3 substituted compounds (Ib, Ie). This effect is mostly prevalent in benzene and, for the 3-substituted compounds Ie–g also in chloroform, acetone, and DMSO. For the remaining combinations of compounds and solvents, the electronic effect is mostly negligible.
anti ratio with respect to the CH3 substituted compounds (Ib, Ie). This effect is mostly prevalent in benzene and, for the 3-substituted compounds Ie–g also in chloroform, acetone, and DMSO. For the remaining combinations of compounds and solvents, the electronic effect is mostly negligible.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti preference regardless the type of solvent (Table 1, entry 8). Other compounds (IIb–e), that hold a substituent in the position 4 of the aromatic side arm, exhibit minor bias towards the anti-epimer in polar solvents (acetone, DMSO). In chloroform, benzene, and toluene, only subtle differences from equal syn
anti preference regardless the type of solvent (Table 1, entry 8). Other compounds (IIb–e), that hold a substituent in the position 4 of the aromatic side arm, exhibit minor bias towards the anti-epimer in polar solvents (acetone, DMSO). In chloroform, benzene, and toluene, only subtle differences from equal syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratios were found. Analogously to the Series I, strong electron withdrawing and, to minor extent, electron donating effects (R = CF3, NO2, OCH3) provide higher diastereomeric preference over weaker or no electronic effects (R = H, CH3). This factor implies a broader trend that substituents exerting strong polarization have partial positive influence on the energy differentiation of the spiropyran epimers thus increasing their diastereomeric ratio.
anti ratios were found. Analogously to the Series I, strong electron withdrawing and, to minor extent, electron donating effects (R = CF3, NO2, OCH3) provide higher diastereomeric preference over weaker or no electronic effects (R = H, CH3). This factor implies a broader trend that substituents exerting strong polarization have partial positive influence on the energy differentiation of the spiropyran epimers thus increasing their diastereomeric ratio.Despite low effect of less polar solvents (chloroform, toluene, benzene), the NO2-substituted compound IIe yields minor syn-preference in toluene and benzene (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = 55
anti = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45). Lesser, but still notable, syn-bias was found for the methyl-substituted compound IIb. Despite the low diastereomeric excesses found for the Series II, it is worth noting that a change of solvent can lead to opposite epimeric preference. This highlights the significance of solvent selection while contemplating potential applications.
45). Lesser, but still notable, syn-bias was found for the methyl-substituted compound IIb. Despite the low diastereomeric excesses found for the Series II, it is worth noting that a change of solvent can lead to opposite epimeric preference. This highlights the significance of solvent selection while contemplating potential applications.
Compound IIIa exhibits by far the largest span of syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti values throughout the series ranging from 31
anti values throughout the series ranging from 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 in DMSO to 22
69 in DMSO to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 in toluene (Table 1, entry 13). This finding further supports the trend found in the Series I, reflecting the influence of the solvent polarity on the stereochemical preference. The sole effect of the tert-butyl group in the position 8, however, brings no conclusive improvement over the unsubstituted compound Ia. In acetone and toluene, IIIa yields higher anti-preference than Ia, whereas in chloroform, DMSO, and benzene, the opposite is true. Compounds IIIb–f each provide almost identical diastereomeric preference (syn
78 in toluene (Table 1, entry 13). This finding further supports the trend found in the Series I, reflecting the influence of the solvent polarity on the stereochemical preference. The sole effect of the tert-butyl group in the position 8, however, brings no conclusive improvement over the unsubstituted compound Ia. In acetone and toluene, IIIa yields higher anti-preference than Ia, whereas in chloroform, DMSO, and benzene, the opposite is true. Compounds IIIb–f each provide almost identical diastereomeric preference (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = approx. 30
anti = approx. 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) no matter the solvent used (Table 1, entries 14–18). The only exception from this trend is the 4-fluorophenyl-substituted compound IIIc in benzene where syn
70) no matter the solvent used (Table 1, entries 14–18). The only exception from this trend is the 4-fluorophenyl-substituted compound IIIc in benzene where syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = 35
anti = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 ratio was found. The unravelled trend suggests that the auxiliary aromatic ring in the position 8 stabilizes the syn
65 ratio was found. The unravelled trend suggests that the auxiliary aromatic ring in the position 8 stabilizes the syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio regardless the solvent used. The effect of the substitution, however, does not improve the results found for the compound Ia and is, in fact, slightly detrimental (syn
anti ratio regardless the solvent used. The effect of the substitution, however, does not improve the results found for the compound Ia and is, in fact, slightly detrimental (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti max. up to 27
anti max. up to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73). The two functionalities in the position 3′ and 8, respectively, are likely too far away from each other to cause a notable steric hindrance in the syn-isomer. Thus, no major improvement over the compound Ia was found.
73). The two functionalities in the position 3′ and 8, respectively, are likely too far away from each other to cause a notable steric hindrance in the syn-isomer. Thus, no major improvement over the compound Ia was found.
The compound IIIe bears a 1-naphthyl group in the position 8 of the chromene unit. Therefore, it acts as a 1,1′-binaphthalene system that appears in two rotameric forms. The spiropyran IIIe thus holds three elements of chirality instead of two as the other compounds. As a result, eight diastereomers, i.e., four relative stereoisomers, are formed. The relative ratio of these stereoisomers could not be elucidated using 400 MHz NMR in chloroform, acetone, and DMSO due to overlapping signals. Thus, only data for toluene and benzene are shown. If the effect of axial chirality is neglected, the syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio equals to 30
anti ratio equals to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 for toluene, and 31
70 for toluene, and 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 for benzene, respectively (Table 1, entry 17). The actual ratio of the four relative stereoisomers is, however, 13
69 for benzene, respectively (Table 1, entry 17). The actual ratio of the four relative stereoisomers is, however, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 for toluene, and 12
27 for toluene, and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for benzene, respectively. The signal assignment for the syn- and anti-stereoisomers was carried out using NMR NOESY (Fig. 5).
30 for benzene, respectively. The signal assignment for the syn- and anti-stereoisomers was carried out using NMR NOESY (Fig. 5).
Photokinetic studies
The photokinetic properties of the target spiropyrans were studied using UV-vis spectroscopy of methanol solutions at concentration 10−4 mol L−1 in quartz glass fluorescent cuvettes (path length 1 cm, λ = 200–800 nm). The photoswitching processes were triggered by a UV diode (λ = 365 nm, 175 mW) for spiropyran opening and a green diode (λ = 522 nm, 60 mW) for spiropyran closing, respectively. The progress of both the opening (kSP-MC) and closing (kMC-SP) transition was monitored at wavelength corresponding to the absorption maximum of the merocyanine band (λmax ca. 540–580 nm). The rate constants kSP-MC and kMC-SP were calculated based on the assumption of first order kinetics. The results are summarized in Table 2. The values Amax correspond to the maximal absorbance found at λmax during the photoswitching measurements, i.e., in the photostationary state upon UV light irradiation (at maximal merocyanine concentration). UV-vis spectra and kinetic plots for Ia are shown in Fig. 6. Experimental details, UV-vis spectra and kinetic plots for the remaining spiropyrans are available in the ESI.† To the best of our knowledge, we are the first to report any data on photoswitching of optically biased spiropyrans with an auxiliary chiral centre in the position 3′.| Entry | Compound | 3′-Substitution | 8-Substitution | λmax (nm) | Amax (AU) | kSP-MC (10−3 s−1) | kMC-SP (10−3 s−1) |
|---|---|---|---|---|---|---|---|
| 1 | Ia | PhCH2 | H | 548 | 0.88 | 1.1 | 6.8 |
| 2 | Ib | 4-CH3-PhCH2 | H | 548 | 0.56 | 2.5 | 3.2 |
| 3 | Ic | 4-OCH3-PhCH2 | H | 548 | 0.77 | 2.2 | 3.0 |
| 4 | Id | 4-CF3-PhCH2 | H | 548 | 0.79 | 3.0 | 3.0 |
| 5 | Ie | 3-CH3-PhCH2 | H | 547 | 0.62 | 2.5 | 3.2 |
| 6 | If | 3-OCH3-PhCH2 | H | 548 | 0.78 | 2.6 | 2.7 |
| 7 | Ig | 3-CF3-PhCH2 | H | 558 | 0.44 | 5.6 | 5.7 |
| 8 | IIa | Ph | H | 549 | 0.36 | 8.1 | 5.6 |
| 9 | IIb | 4-CH3-Ph | H | 544 | 0.17 | 7.6 | 8.8 |
| 10 | IIc | 4-OCH3-Ph | H | 550 | 0.06 | 9.3 | 10.4 |
| 11 | IId | 4-CF3-Ph | H | 552 | 0.10 | 30.8 | 27.1 |
| 12 | IIe | 4-NO2-Ph | H | 559 | 0.05 | 15.4 | 13.6 |
| 13 | IIIa | PhCH2 | tBu | 578 | 2.76 | 146.2 | 22.1 |
| 14 | IIIb | PhCH2 | Ph | 577 | 2.20 | 56.1 | 11.4 |
| 15 | IIIc | PhCH2 | 4-F-Ph | 577 | 2.33 | 37.9 | 11.9 |
| 16 | IIId | PhCH2 | 4-tBu-Ph | 570 | 2.95 | 64.1 | 4.9 |
| 17 | IIIe | PhCH2 | 1-Naphthyl | 573 | 1.00 | 63.4 | 60.9 |
| 18 | IIIf | PhCH2 | 2-Naphthyl | 573 | 1.70 | 35.3 | 8.0 |
The compounds of the Series I exhibit the slowest photoswitching overall, not exceeding 10−3 s−1 rate. The non-substituted material Ia has a notable discrepancy between the kinetic rate of spiropyran opening and closing (Table 2, entry 1). This suggests that Ia prefers to adopt its closed spiropyran form even in polar solvents, such as methanol, where the merocyanine form is more stabilized in contrast to less polar media. In comparison to Ia, the compounds Ib–g exhibit faster spiropyran opening and slower closing. Similar kinetic rates for both opening and closing processes were found with the ring closing being only slightly faster (Table 2, entries 2–7). The compound Ig with the electron withdrawing CF3 group in the position 3 of the benzylic side arm provides double kinetic rates for both processes over the other substituted compounds from the Series I (Ib–f). The analogous compound Id with a CF3 group in the position 4 of the benzyl moiety exhibits minor increase in spiropyran opening rate over other compounds bearing electron donating moieties (CH3, OCH3). This indicates that a presence of an electron withdrawing group generally supports the photoswitching rate.
The compounds of the Series I provide similar values of Amax. The highest Amax = 0.88 was found for Ia, while the lowest Amax = 0.44 for Ig. Assuming the molar extinction coefficients of the observed absorption bands of Ia–g are comparable, the Amax values indicate that any substitution on the benzylic side arm in the position 3′ partly shifts the SP-MC photostationary state equilibrium towards the spiropyran form.
The Series II, bearing aromatic moieties in the position 3′ instead of benzylic groups, exhibits substantially faster photoswitching than the Series I. The kinetic rates of both processes (opening, closing) are similar for each compound with the rate of opening being slightly prevalent for IIa, IId, and IIe. For IIb and IIc, the opposite is true. While compounds IIa–c either non-substituted or substituted with an electron donating group show comparable kinetic rates (ca. 5–10 × 10−3 s−1), the CF3-substituted material IId exhibits tripled values for both opening and closing. This finding supports the trend of the influence of electron withdrawing moieties on the kinetic rates outlined for the Series I.
The Amax values of the compounds of the Series II are considerably lower than for the other series. This indicates that the spiropyrans IIa–e exhibit very little change upon external light stimulus. Therefore, direct aryl-substitution in the position 3′ stabilizes the spiropyran form to the extent that, at given conditions, it acts as a poor photoswitch.
All the compounds of the Series III show highly accelerated spiropyran opening kinetics in comparison to the unsubstituted compound Ia. The highest photoswitching rates of opening were found for IIIa with a tert-butyl group (Table 2, entry 13). The fastest switching for closing was found for the 1-naphthyl-substituted material IIIe (Table 2, entry 17). In the case of other materials (IIIb–d, IIIf), dramatic difference between the opening and closing rates were observed similarly to IIIa. While the rates of closing are comparable to the non-substituted material Ia, the rates of opening are increased by an order of magnitude. In general, based on the variability of the substituents in the Series III, we assume that any bulky group in the position 8 of the chromene unit promotes spiropyran opening.
All the compounds from the Series III show higher λmax than those from the two other series including the tert-butyl-substituted material IIIa. Therefore, the observed band shift is not a result of the aromatic character of the substituents in the position 8 (IIIb–f), but rather a general auxochromic effect of these functionalities. Throughout the whole series, notably high values of Amax were found in contrast to the other series. Therefore, it is safe to assume that the compounds of this series provide the highest merocyanine concentrations upon irradiation. This is especially true for the compounds IIIa–d, where Amax ≥ 2.2 AU. In the case of the naphthyl-substituted compounds IIIe–f, the Amax is partially decreased, however, still surpasses the values for the compounds of the Series I and II. Overall, the collected data for the Series III shows that the substitution in the position 8 of the spiropyran scaffold supports both fast photoswitching and high concentration changes of the spiropyran and merocyanine form. Such feature is a necessary precondition for potential applications, e.g., in materials chemistry of low-molecular sensors and light-tunable liquid crystals.12
Conclusions
Three series of spiropyran photoswitches (18 compounds) with an additional element of chirality in the position 3′ of the indoline subunit were synthesized. The target materials were yielded in racemic form with no stereocontrol over the chiral centre in the position 3′. One compound (Ia), however, was also prepared with a defined chirality in this position using co-crystallization of its 3H-indole precursor with optically pure (–)-camphor-10-sulfonic acid. The absolute configuration of this intermediate was determined using X-ray crystallography. To the best of our knowledge, this is an unprecedented method for synthesis of optically active spiropyrans thus far. Moreover, a novel approach to spiropyran purification using its acid–base equilibrium was developed. This method represents a complementary protocol to conventional techniques (e.g., column chromatography) for purification of such compounds and can serve as a cheap alternative for scale-up synthesis.The stereochemical bias of the prepared compounds in various solvents (chloroform, acetone, DMSO, toluene, benzene) was evaluated using 1H NMR spectroscopy at 25 °C. The assignment of the NMR signals to the corresponding relative stereoisomers (syn-, anti-) was performed using NOESY. Series I exhibited syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratios up to 21
anti ratios up to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79 with notable influence of the solvent used. In general, non-polar solvents were found to yield the highest stereochemical preference. The compounds of the Series II showed little to no stereochemical bias. In the case of the compound IIe, rather uncommon (minor) syn-preference was observed. All photoswitches throughout the Series III (excluding IIIa) afforded comparable syn
79 with notable influence of the solvent used. In general, non-polar solvents were found to yield the highest stereochemical preference. The compounds of the Series II showed little to no stereochemical bias. In the case of the compound IIe, rather uncommon (minor) syn-preference was observed. All photoswitches throughout the Series III (excluding IIIa) afforded comparable syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti ratio (ca. 30
anti ratio (ca. 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) regardless of the substitution pattern or the solvent applied. The compounds of the series I and III also exhibited varying amounts of the merocyanine form in DMSO, which were notably increased for IIIb–f. It was thereby postulated that a presence of an aromatic unit in the position 8 promotes the spiropyran opening in polar solvents.
70) regardless of the substitution pattern or the solvent applied. The compounds of the series I and III also exhibited varying amounts of the merocyanine form in DMSO, which were notably increased for IIIb–f. It was thereby postulated that a presence of an aromatic unit in the position 8 promotes the spiropyran opening in polar solvents.
The photochemical switching of the target compounds was monitored by UV-vis spectroscopy. Throughout Series I and II, a presence of an electron withdrawing group in the aromatic side arm in the position 3′ accelerated the photoswitching kinetics by a factor of up to 5 with respect to the non-substituted compounds Ia and IIa, respectively. The main difference between the photokinetics of the Series I and II is very low maximal abundance of the merocyanine form for IIa–e. The substitution pattern of the Series II (direct aryl substitution in the position 3′) thus appears to stabilize the spiropyran form considerably. Compounds of the Series III exhibited one to two orders of magnitude higher kinetic rates of spiropyran opening than the unsubstituted benchmark compound Ia. For IIIa and IIIe, the same was true for the kinetics of spiropyran closing, whereas IIIb–d and IIIf showed similar closing rate constants as Ia. The Series III also provided the highest concentration of merocyanine upon irradiation with UV light, especially in the case of the compounds IIIa–d. This indicates that substitution of the spiropyran scaffold in the position 8 has a dramatic effect on both the kinetics and thermodynamics of the photoswitching process.
Data availability
Detailed synthesis and characterization of the prepared compounds, X-ray crystallographic data, NMR spectra, and photokinetic plots are included in ESI.† The X-ray structure was deposited into the Cambridge Structural Database under number CCDC 2393565.†Author contributions
Vojtěch Boháček, Tereza Erbenová, Jakub Dávid Malina and Marie Kloubcová – performed the experiments, characterized the compounds and interpreted the data. Václav Eigner – performed the X-ray crystallography experiment and interpreted the data. Michal Šmahel – supervised the project, interpreted the data and edited the manuscript. Jiří Tůma – designed and supervised the project, wrote the manuscript and provided funding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Dagmar Procházková Fund (Project SALSA, 2023–2024).Notes and references
- R. Klajn, Chem. Soc. Rev., 2014, 73, 148 RSC.
- L. Kortekaas and W. R. Browne, Chem. Soc. Rev., 2019, 48, 3406 RSC.
- L. Hu, Z. Wang, Y. Yang, Y. Gao, Y. Wei, J. Zhu and W. Wu, Surf. Interfaces, 2024, 46, 104116 CrossRef CAS.
- L. Wimberger, S. K. K. Prasad, M. D. Peeks, J. Andréasson, T. W. Schmidt and J. E. Beves, J. Am. Chem. Soc., 2021, 143, 20758 CrossRef CAS PubMed.
- J. D. Steen, D. R. Duijnstee, A. S. Sardjan, J. Martinelli, L. Kortekaas, D. Jacquemin and W. R. Browne, J. Phys. Chem. A, 2021, 125, 3355 CrossRef CAS PubMed.
- Y. Lin, T. B. Kouznetsova, A. G. Foret and S. L. Craig, J. Am. Chem. Soc., 2024, 146, 3920 CrossRef CAS PubMed.
- A. S. Kozlenko, I. V. Ozhogin, A. D. Pugachev, M. B. Lukyanova, I. M. El-Sewify and B. S. Lukyanov, Top. Curr. Chem., 2023, 381, 8 CrossRef CAS PubMed.
- A. Fagan, M. Bartkowski and S. Giordani, Front. Chem., 2021, 9, 720087 CrossRef CAS PubMed.
- A. S. Kozlenko, A. D. Pugachev, I. V. Ozhogin, I. M. El-Sewify and B. S. Lukyanov, Chem. Heterocycl. Compd., 2021, 57, 984 CrossRef CAS.
- M. Mandal, D. Banik, A. Karak, S. K. Manna and A. K. Mahapatra, ACS Omega, 2022, 7, 36988 CrossRef CAS PubMed.
- S. Chatterjee, B. Liu and H.-S. Peng, Coord. Chem. Rev., 2024, 508, 215779 CrossRef CAS.
- J. Malinčík, M. Kohout, J. Svoboda, S. Stulov, D. Pociecha, Z. Bohmova and V. Novotná, J. Mol. Liq., 2022, 346, 117842 CrossRef.
- B. Zhou, A. Govyadinov, P. Kornilovitch and V. T. Remcho, ACS Omega, 2024, 9, 29401 CrossRef CAS PubMed.
- H. Ishikawa, N. Uemura, R. Saito, Y. Yoshida, T. Mino, Y. Kasashima and M. Sakamoto, Chem.–Eur. J., 2019, 25, 9758 CrossRef CAS PubMed.
- S.-R. Keum, M.-J. Lee, S. Swansburg, E. Buncel and R. P. Lemieux, Dyes Pigm., 1998, 39, 383 CrossRef CAS.
- S. Swansburg, E. Buncel and R. P. Lemieux, J. Am. Chem. Soc., 2000, 122, 6594 CrossRef CAS.
- Y. Sheng and J. Leszczynski, Struct. Chem., 2014, 25, 667 CrossRef CAS.
- I. Gruda, R. M. Leblanc and J. Sochanski, Can. J. Chem., 1978, 56, 1296 CrossRef CAS.
- L. Eggers and V. Buss, Angew. Chem., Int. Ed., 1997, 36, 881 CrossRef CAS.
- L. Eggers and V. Buss, Tetrahedron: Asymmetry, 1999, 10, 4485 CrossRef CAS.
- A. Perry, K. Davis and L. West, Org. Biomol. Chem., 2018, 16, 7245 RSC.
- H. Swinson and A. Perry, Tetrahedron, 2020, 76, 131219 CrossRef CAS.
- C. A. Hughes-Whiffing and A. Perry, Eur. J. Org Chem., 2023, 26, e202201245 CrossRef CAS.
- P. C. Too, T. Noji, Y. J. Lim, X. Li and S. Chiba, Synlett, 2011, 19, 2789 Search PubMed.
- X. Li, L. Li, Y. Tang, L. Zhong, L. Cun, J. Zhu, J. Liao and J. Deng, J. Org. Chem., 2010, 75, 2981 CrossRef CAS PubMed.
- D. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. Van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martínez, S. R. White, J. S. Moore and N. R. Sottos, Nature, 2009, 459, 68 CrossRef CAS PubMed.
- X. Ji, C. Zhou, K. Ji, R. E. Aghoghovbia, Z. Pan, V. Chittavong, B. Ke and B. Wang, Angew. Chem., Int. Ed., 2016, 55, 15846 CrossRef CAS PubMed.
- G. Capozzi, S. Roelens and S. Talami, J. Org. Chem., 1993, 58, 7932 CrossRef CAS.
- S. Hamilakis and A. Tsolomitis, Heterocycl. Commun., 2005, 11, 149 CAS.
- C. Kong, N. Jana, C. Jones and T. G. Driver, J. Am. Chem. Soc., 2016, 138, 13271 CrossRef CAS PubMed.
- B. Manfred, R. Fleischer, B. Mai, M.-A. Schneider and S. Lachenicht, Adv. Synth. Catal., 2004, 346, 474 CrossRef.
- Z. Zhiming, G. Daxin, C. Shoujun, W. Zhiheng, CN108623615A, 2018.
- C.-T. Chen, J.-Q. Kao, S. B. Salunke and Y.-H. Lin, Org. Lett., 2011, 13, 26 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2393565. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra07750d |
| This journal is © The Royal Society of Chemistry 2024 |