Catalytic Mannich reaction of acrylic acid polymers and their application in leather retanning
Jianzhong
Ma
 *ab,
Jiamin
Zhao
a,
Hui
Zhang
a,
Zhenhua
Tian
*a,
Qiwu
Liu
a,
Na
Yang
a and
Wenbo
Zhang
bc
*ab,
Jiamin
Zhao
a,
Hui
Zhang
a,
Zhenhua
Tian
*a,
Qiwu
Liu
a,
Na
Yang
a and
Wenbo
Zhang
bc
aCollege of Bioresources Chemical and Materials Engineering, Shaanxi University of Science & Technology, Xi'an 710021, P.R. China. E-mail: majz@sust.edu.cn; Tel: +86 029 8613 2559
bCollege of Chemistry and Chemical Engineering, Shaanxi University of Science & Technology, Xi'an 710021, P.R. China
cShaanxi Collaborative Innovation Center of Industrial Auxiliary Chemistry and Technology, Shaanxi University of Science & Technology, Xi'an 710021, P.R. China
First published on 16th October 2023
Abstract
Acrylic polymers are widely used in pharmaceutical, leather, textile and other industries, and are prepared via the polyreaction of acrylic monomers, such as acrylic acid, acrylamide and acrylic esters. Acrylic acid and acrylamide have been proven to participate in the Mannich reaction to achieve cationization; however, the Mannich reaction of methyl acrylate has not been reported. At present, the main role of catalysts in the Mannich reaction is to provide products of specific spatial configuration, and there are few studies on improving the degree of cationic modification. The participation of acrylic acid, acrylamide and methyl acrylate in the Mannich reaction by means of the catalyst NaH was studied in this work. The optimal reaction conditions for the homopolymers were obtained via orthogonal experiments. At room temperature, the optimal reaction conditions for polyacrylic acid were as follows: the molar ratio of polyacrylic acid, glutaraldehyde and diethanolamine was 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, and the amount of catalyst was 7%. The optimal reaction conditions for polyacrylamide were as follows: the molar ratio of polyacrylamide, glutaraldehyde and diethanolamine was 1.1
1.0, and the amount of catalyst was 7%. The optimal reaction conditions for polyacrylamide were as follows: the molar ratio of polyacrylamide, glutaraldehyde and diethanolamine was 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, and the amount of catalyst was 9%. The optimum reaction conditions for methyl polyacrylate were as follows: the molar ratio of methyl polyacrylate, glutaraldehyde and diethanolamine was 1.1
1.2, and the amount of catalyst was 9%. The optimum reaction conditions for methyl polyacrylate were as follows: the molar ratio of methyl polyacrylate, glutaraldehyde and diethanolamine was 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, and the amount of catalyst was 9%. As a result, the maximum degrees of modification of polyacrylic acid, polyacrylamide and methyl polyacrylate were 63.0%, 33.5% and 39.0%, respectively. The range analysis of the orthogonal experiments showed that the effects on the modification degree of vinyl homopolymers, in sequence from strong to weak, were as follows: amount of catalyst > amount of amine > amount of aldehyde. Subsequently, polyacrylate–acrylamide–methyl acrylate (P(AA–AM–MA)) was synthesized using acrylic acid, acrylamide and methyl acrylate as monomers, and then modified via the catalyzed Mannich reaction. A novel amphoteric acrylic acid copolymer retanning agent (HCP(AA–AM–MA)) with a 50.2% degree of modification was obtained, and its properties as a retanning agent in leather-making were investigated. The dye-uptake and K/S value of the dyed leather retanned with HCP(AA–AM–MA) were 91.5% and 18.5, respectively, an increase of 18.4% and 3.4 in comparison with those of dyed leather retanned with P(AA–AM–MA). The results indicated that the dyeing-assistant performance of HCP(AA–AM–MA) was improved. Moreover, the elongation at break and tensile strength of the retanned leather were 82.0% and 31.9 MPa, respectively, which were higher than those of P(AA–AM–MA)-retanned leather (78.9% and 27.4 MPa).
1.0, and the amount of catalyst was 9%. As a result, the maximum degrees of modification of polyacrylic acid, polyacrylamide and methyl polyacrylate were 63.0%, 33.5% and 39.0%, respectively. The range analysis of the orthogonal experiments showed that the effects on the modification degree of vinyl homopolymers, in sequence from strong to weak, were as follows: amount of catalyst > amount of amine > amount of aldehyde. Subsequently, polyacrylate–acrylamide–methyl acrylate (P(AA–AM–MA)) was synthesized using acrylic acid, acrylamide and methyl acrylate as monomers, and then modified via the catalyzed Mannich reaction. A novel amphoteric acrylic acid copolymer retanning agent (HCP(AA–AM–MA)) with a 50.2% degree of modification was obtained, and its properties as a retanning agent in leather-making were investigated. The dye-uptake and K/S value of the dyed leather retanned with HCP(AA–AM–MA) were 91.5% and 18.5, respectively, an increase of 18.4% and 3.4 in comparison with those of dyed leather retanned with P(AA–AM–MA). The results indicated that the dyeing-assistant performance of HCP(AA–AM–MA) was improved. Moreover, the elongation at break and tensile strength of the retanned leather were 82.0% and 31.9 MPa, respectively, which were higher than those of P(AA–AM–MA)-retanned leather (78.9% and 27.4 MPa).
1 Introduction
Acrylic acid and its derivatives (e.g., acrylamide and acrylic ester), containing vinyl groups (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), are necessary industrial raw materials. Acrylic acid and acrylic esters can be homopolymerized and copolymerized and can also be copolymerized with styrene, butadiene, vinyl chloride and maleic anhydride monomers.1–3 Such polymers are used in synthetic rubber, synthetic fibers, super-absorbent resins, pharmaceuticals, leather, textiles, building materials, water treatment, oil exploitation, coatings, and other industries.4–10
CH2), are necessary industrial raw materials. Acrylic acid and acrylic esters can be homopolymerized and copolymerized and can also be copolymerized with styrene, butadiene, vinyl chloride and maleic anhydride monomers.1–3 Such polymers are used in synthetic rubber, synthetic fibers, super-absorbent resins, pharmaceuticals, leather, textiles, building materials, water treatment, oil exploitation, coatings, and other industries.4–10
However, acrylic polymers are a type of anionic polymer, which has restricted their application range. Cationic modification can give them unusual properties. For example, acrylamide polymer flocculants for wastewater treatment have better absorbability after cationization than the products without cationization; acrylate coatings after cationization have excellent antibacterial activity.11,12 Cationic acrylic polymer tanning agents have good retanning and auxiliary dyeing properties.13–15 Therefore, acrylic-polymer cationization is necessary. One of the cationic modification methods is the Mannich reaction, a three-component (acid component, aldehyde component, and amine component) reaction.16 The method is operationally simple, the raw materials are cheap and the reaction is stable.
The main acrylic monomers used in industries are acrylic acid, acrylamide, acrylonitrile and acrylate monomers.17,18 Acrylate monomers are common raw materials due to their advantages of low cost, easy preparation and easy function adjustment. They play a pivotal role in the processing and production of industrial chemicals.19,20 Acrylic acid and acrylamide have been proven to participate in the Mannich reaction to achieve cationization in previous studies, but the same for methyl acrylate has not been reported.21 The difficulty here is that the α-H of methyl acrylate is too inert to participate in the reaction. At present, the main role of catalysts in the Mannich reaction is to provide products of specific spatial configuration, and there are few studies on improving the degree of cationic modification.22,23
Can the degree of cationic modification of acrylic polymers in the Mannich reaction be improved by a catalyst? Wang et al. utilized toluene sulfonic acid as an acid catalyst for the organic chemical reactions in the synthesis of α-substituted N-amino aryl acetals. The experimental results showed that after the addition of catalyst Sc(OTf)3, glyoxal dimethyl acetal, aryl amines and ketones were successfully reacted with 1,3-dicarbonyl compounds in a Mannich reaction, which allowed the synthesis of a number of heterocyclic compounds such as indoles.24 Li et al. carried out the Mannich reaction in anhydrous ethanol using acetophenone, diphenylamine and benzaldehyde as the reaction raw materials and tin tetrachloride (SnCl4) as the reaction catalyst, and the experimental results showed that catalyzing the Mannich reaction by using SnC14 could effectively reduce the reaction time, increase the reaction yield, and enhance the reaction efficiency.25 Yamashita et al. used potassium bis(methylsilyl)diamine (KHMDS) as a base catalyst for the Mannich reaction using N-o-methoxyphenylbenzenecarboximide and tert-butyl isobutyrate as the raw materials. When KHMDS was used as a catalyst, the tert-butyl isobutyrate monomer was deprotonated by KHMDS to produce a hydrogen molecule and the corresponding potassium enol compound. The reaction continued with N-o-methoxyphenylbenzaldehyde imine to produce a Mannich-base intermediate with a potassium ion, and then the intermediate was protonated by reaction with the tert-butyl isobutyrate monomer to get the desired products, the Mannich base and a potassium enol compound. The catalytic cycle was completed by the reaction of this enol with the tert-butyl isobutyrate monomer to remove the proton and obtain the Mannich base, which then further gives the desired product as well as the potassium enol compound. The results show that these catalysts can successfully catalyze the Mannich reaction to obtain the desired products, extending the scope of application of the Mannich reaction.26 In this work, acrylic acid, acrylamide and methyl acrylate were used as acid components, glutaraldehyde and diethanolamine were used as the aldehyde component and the amine component, and NaH was used as a catalyst to participate in the Mannich reaction. An amphoteric acrylic acid retanning agent was prepared and used in leather-making, where the retanning and dyeing-assistant properties were investigated.
2 Experimental
2.1 Materials
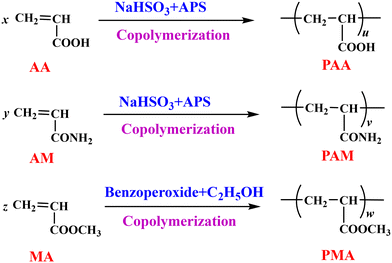
The wet blue leather was purchased from Liquan Shunji Leather Industry Co. Ltd. Acrylamide (AM), methyl acrylate (MA), acrylic acid (AA), ammonium persulfate (APS), sodium hydrogen sulfite, diethanolamine (DEA), glutaraldehyde (GA, 50 wt%), benzoperoxide, and tetrahydrofuran (THF) were purchased from Tianjin Kermel Chemical Reagent Co., Ltd. NaH was purchased from Aladdin Chemical Co., Ltd. Commercial acrylic retanning agent (MTA) was purchased from Zhejiang Sanmen Zhongxin Industrial Co., Ltd.2.2 Synthesis of acrylic acid homopolymers
MA and benzoperoxide were added to a 250 mL flask equipped with a nitrogen gas system and stirred at 80 °C. A certain amount of ethanol was added dropwise into the flask over the course of 1 h and the mixture was stirred for 4 h. PMA was prepared. The reactions are summarised in Fig. 1.
The optimized conditions in terms of GA, DEA and NaH were obtained by orthogonal experiments. The specific parameters are shown in Table 1. Fixing the number of moles of acid components for the Mannich reaction as 1, the molar ratio of the amount of glutaraldehyde, the molar ratio of the amount of diethanolamine, and the percentage of catalyst relative to the overall solids content were chosen as the key factors for optimization.
| Level of factor |
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PAA) n(PAA) |
n(DEA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PAA) n(PAA) |
Amount of catalyst |
|---|---|---|---|
| 1 | 0.8 | 0.8 | 3% |
| 2 | 1.0 | 1.0 | 5% |
| 3 | 1.2 | 1.2 | 7% |
| 4 | 1.4 | 1.4 | 9% |
An orthogonal table, L16 (43), with 3 factors and 4 levels, was selected; the specific experimental parameters are shown in Table 2.
| Entry | A | B | C |
|---|---|---|---|
| 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 |
| 3 | 1 | 3 | 3 |
| 4 | 1 | 4 | 4 |
| 5 | 2 | 1 | 2 |
| 6 | 2 | 2 | 1 |
| 7 | 2 | 3 | 4 |
| 8 | 2 | 4 | 3 |
| 9 | 3 | 1 | 3 |
| 10 | 3 | 2 | 4 |
| 11 | 3 | 3 | 1 |
| 12 | 3 | 4 | 2 |
| 13 | 4 | 1 | 4 |
| 14 | 4 | 2 | 3 |
| 15 | 4 | 3 | 2 |
| 16 | 4 | 4 | 1 |
The range size of the orthogonal test data is usually analyzed, including the total response value Ki, the average response value ki and the range value R. Ki means that when the row value in any column of the orthogonal table is i, the sum of the corresponding test results. ki is equal to Ki/s, where s is the number of occurrences at each level in a given column. R is equal to the maximum value of ki minus the minimum value of ki, indicating the degree of influence of each factor on the experimental results.
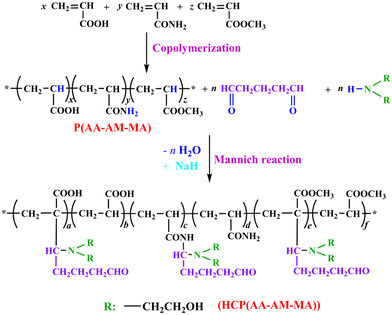
2.3 Preparation of acrylic acid copolymer (HCP(AA–AM–MA))
AA, AM and MA with a weight ratio of 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.54
0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
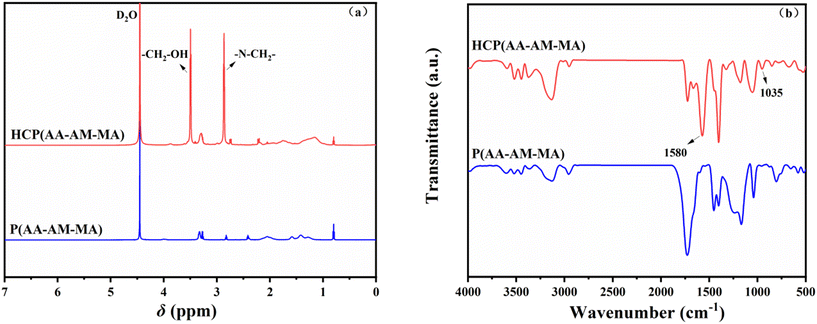
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.14 were added into a flask. Then, a certain amount of NaHSO3 and APS were added dropwise to the flask and the mixture was stirred for 4 h at 45 °C to obtain a prepolymer (P(AA–AM–MA)). The prepolymer was spray-dried at 160 °C. Subsequently, the prepolymer, GA and DEA were added to a flask with THF, and reacted with NaH as the catalyst for 18 h. Finally, HCP(AA–AM–MA) was obtained by vacuum extraction for 30 min. The reaction is summarized in Fig. 2.
0.14 were added into a flask. Then, a certain amount of NaHSO3 and APS were added dropwise to the flask and the mixture was stirred for 4 h at 45 °C to obtain a prepolymer (P(AA–AM–MA)). The prepolymer was spray-dried at 160 °C. Subsequently, the prepolymer, GA and DEA were added to a flask with THF, and reacted with NaH as the catalyst for 18 h. Finally, HCP(AA–AM–MA) was obtained by vacuum extraction for 30 min. The reaction is summarized in Fig. 2.
2.4 Detection and characterization of retanning agents
2.5 Preparation and characterization of retanned leather
| Process | Chemicals | Dosaged (%) | Temperature (°C) | Time (min) | Remarks |
|---|---|---|---|---|---|
| a Sodium bicarbonate was diluted with 10 times water (relative to the quality of sodium bicarbonate). b Retanning agent and formic acid were respectively dissolved in 20 times water (relative to the quality of Retanning agent and formic acid). c Synthetic fatliquor was emulsified with hot water. d Based on tare weight of wet-blue. | |||||
| Pretreatment | Water | 200 | 45 | 120 | |
| Degreasing agent | 0.2 | ||||
| Wetting agent | 0.5 | ||||
| Wash, drain | |||||
| Neutralization | Water | 150 | 30 | 30 | |
| Sodium formate | 1.0 | ||||
| Neutralizing syntan | 2.0 | ||||
| Sodium bicarbonatea | 1.5 | 30 × 2 | pH 5.5+; drain | ||
| Wash, drain | |||||
| Retanning | Water | 100 | 30 | 15 × 4 + 60 | |
| Retanning agentsb | 10 | ||||
| Formic acidb | 1.0 | 20 × 3 + 30 | pH ∼ 3.8 | ||
| Stop operation for 12 h | |||||
| Dyeing and fatliquoring | Water | 150 | 50 | ||
| Sodium bicarbonatea | pH ∼ 5.0 | ||||
| Black dye | 3 | 60 | |||
| Synthetic fatliquorc | 12 | 60 | |||
| Fixing | Formic acidb | 2.0 | 20 × 3 | pH ∼ 3.5; drain | |
a Leather surface color depth (K/S). The dyed leather samples were treated in constant-temperature and -humidity equipment for 48 h, and then the K/S value of the leather was determined using a colorimeter (i7800, X-Rite, USA).
b Dye uptake. The dye solution was analysed using an ultraviolet-visible spectrophotometer (Cary 5000, Agilent, Malaysia), and the absorption wavelength of dye appeared at 503 nm. Then the absorbances of the original dye solution (A) and the dyeing effluent (A0) were determined and dye uptake was calculated using the formula shown in eqn (1).
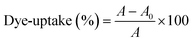 | (1) |
c Washing fastness. The leather samples were washed several times with water. Then, the blackness of the leather before and after washing was determined using a colorimeter (i7800, X-Rite, America) to evaluate the color fastness of the leather to washing.
d Rubbing fastness. The leather was rubbed with white non-woven fabrics under a certain pressure and the color was transferred from the leather surface to the fabrics. The whiteness of the non-woven fabrics was tested using a colorimeter (i7800, X-Rite, America) to evaluate the rubbing color fastness of the leather.
e Thickening rate. The thickness of retanned leather samples was measured using a leather thickness gauge (MH-YDI, Shaanxi University of Science and Technology, China). The thickening rate was calculated using the following equation:
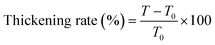 | (2) |
f Softness. The softness of the leather samples was examined using a softness tester (GT303, Gotech Testing Machines Limited, China).
g Mechanical properties. The elongation at break and tensile strength of the leather samples were measured using a universal material testing machine (AI-7000-NGD, Goodtechwill, China).
3 Results and discussion
3.1 Research on Mannich-modified polyacrylic acid
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(GA)
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(DEA) and the amount of catalyst were 1.0
n(DEA) and the amount of catalyst were 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 and 7%, respectively.
1.0 and 7%, respectively.
| Entry |
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PAA) n(PAA) |
n(DEA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PAA) n(PAA) |
Amount of catalyst | Degree of modification |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 50.50% |
| 2 | 1 | 2 | 2 | 52.00% |
| 3 | 1 | 3 | 3 | 58.00% |
| 4 | 1 | 4 | 4 | 55.00% |
| 5 | 2 | 1 | 2 | 54.00% |
| 6 | 2 | 2 | 1 | 52.00% |
| 7 | 2 | 3 | 4 | 61.00% |
| 8 | 2 | 4 | 3 | 62.50% |
| 9 | 3 | 1 | 3 | 54.50% |
| 10 | 3 | 2 | 4 | 62.00% |
| 11 | 3 | 3 | 1 | 50.50% |
| 12 | 3 | 4 | 2 | 61.50% |
| 13 | 4 | 1 | 4 | 55.50% |
| 14 | 4 | 2 | 3 | 63.00% |
| 15 | 4 | 3 | 2 | 60.00% |
| 16 | 4 | 4 | 1 | 51.50% |
| k 1 | 53.88% | 53.63% | 51.13% | |
| k 2 | 54.88% | 57.25% | 56.88% | |
| k 3 | 57.13% | 57.38% | 59.50% | |
| k 4 | 57.50% | 57.63% | 58.38% | |
| R | 3.62% | 4.00% | 8.37% |
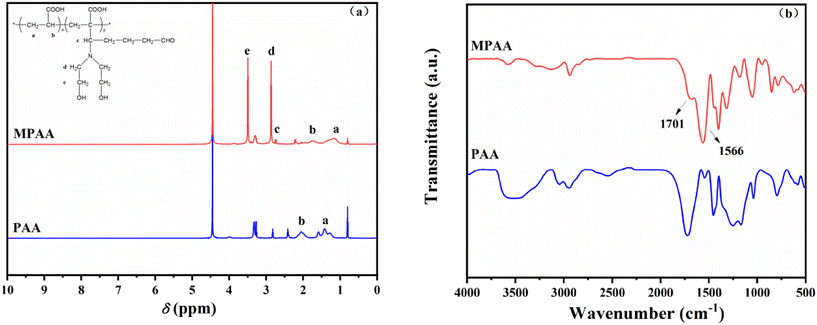
As shown in Fig. 3b, the C–H stretching vibration peak of –CH2– and –CH3– appears at 2851 cm−1, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak appears at 1701 cm−1. There is a strong absorption peak at 1566 cm−1 in the FT-IR spectrum of MPAA, which was attributed to the stretching vibration of C–N. The C–N structures that appear in the FT-IR spectrum indicated that PAA was modified successfully by the Mannich reaction.
O stretching vibration peak appears at 1701 cm−1. There is a strong absorption peak at 1566 cm−1 in the FT-IR spectrum of MPAA, which was attributed to the stretching vibration of C–N. The C–N structures that appear in the FT-IR spectrum indicated that PAA was modified successfully by the Mannich reaction.
3.2 Research on Mannich-modified polyacrylamide
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(GA)
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(DEA) and the amount of catalyst were 1.0
n(DEA) and the amount of catalyst were 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 and 9%, respectively.
1.2 and 9%, respectively.
| Entry |
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PAM) n(PAM) |
n(DEA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PAM) n(PAM) |
Amount of catalyst | Degree of modification |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 22.50% |
| 2 | 1 | 2 | 2 | 26.00% |
| 3 | 1 | 3 | 3 | 29.50% |
| 4 | 1 | 4 | 4 | 29.00% |
| 5 | 2 | 1 | 2 | 27.00% |
| 6 | 2 | 2 | 1 | 25.00% |
| 7 | 2 | 3 | 4 | 33.50% |
| 8 | 2 | 4 | 3 | 33.00% |
| 9 | 3 | 1 | 3 | 28.50% |
| 10 | 3 | 2 | 4 | 32.50% |
| 11 | 3 | 3 | 1 | 27.00% |
| 12 | 3 | 4 | 2 | 32.50% |
| 13 | 4 | 1 | 4 | 29.00% |
| 14 | 4 | 2 | 3 | 33.00% |
| 15 | 4 | 3 | 2 | 32.00% |
| 16 | 4 | 4 | 1 | 28.50% |
| k 1 | 26.75% | 26.75% | 25.75% | |
| k 2 | 29.63% | 29.13% | 29.38% | |
| k 3 | 30.13% | 30.50% | 31.00% | |
| k 4 | 30.63% | 30.75% | 31.00% | |
| R | 3.88% | 4.00% | 4.25% |
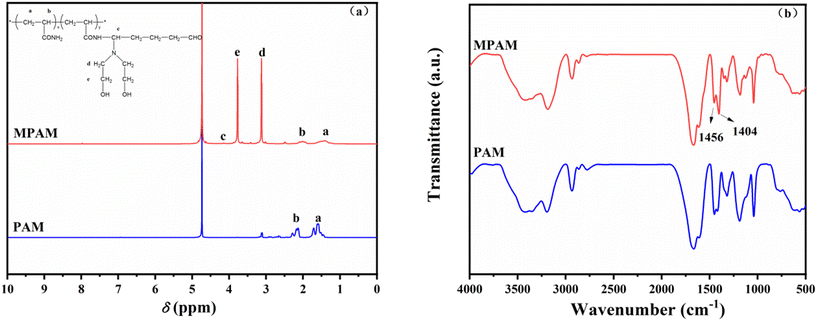
As shown in Fig. 4b, the O–H stretching vibration peak appears at 3250–3400 cm−1, the N–H stretching vibration peak appears at 3186 cm−1. The peaks at 1456 cm−1 and 1404 cm−1 in the FT-IR spectrum of MPAM were attributed to stretching vibration peaks of –CH– and –CH2– connected to N. The C–N structures appearing in the FT-IR spectrum indicated that PAM was modified successfully by the Mannich reaction.
3.3 Research on Mannich-modified polymethyl acrylate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(GA)
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(DEA) and the amount of catalyst were 1.0
n(DEA) and the amount of catalyst were 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 and 9%, respectively.
1.0 and 9%, respectively.
| Entry |
n(GA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PMA) n(PMA) |
n(DEA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(PMA) n(PMA) |
Amount of catalyst | Degree of modification |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 31.50% |
| 2 | 1 | 2 | 2 | 35.00% |
| 3 | 1 | 3 | 3 | 37.50% |
| 4 | 1 | 4 | 4 | 38.00% |
| 5 | 2 | 1 | 2 | 35.00% |
| 6 | 2 | 2 | 1 | 33.50% |
| 7 | 2 | 3 | 4 | 38.00% |
| 8 | 2 | 4 | 3 | 38.50% |
| 9 | 3 | 1 | 3 | 37.50% |
| 10 | 3 | 2 | 4 | 39.00% |
| 11 | 3 | 3 | 1 | 32.50% |
| 12 | 3 | 4 | 2 | 38.50% |
| 13 | 4 | 1 | 4 | 37.00% |
| 14 | 4 | 2 | 3 | 37.50% |
| 15 | 4 | 3 | 2 | 36.00% |
| 16 | 4 | 4 | 1 | 33.00% |
| k 1 | 35.50% | 35.25% | 32.63% | |
| k 2 | 36.25% | 36.25% | 36.13% | |
| k 3 | 36.88% | 36.00% | 37.75% | |
| k 4 | 35.88% | 37.00% | 37.00% | |
| R | 1.38% | 1.75% | 5.12% |
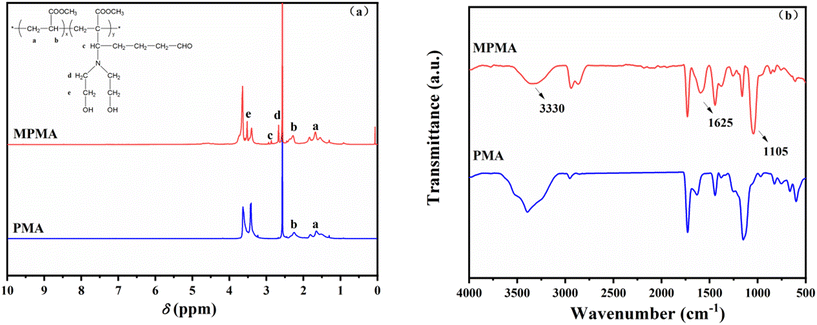
As shown in Fig. 5b, the asymmetrical stretching vibration peak of –CH2– appears at 2969 cm−1, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak appears at 1739 cm−1. There is a stretching vibration peak of N–H at 3330 cm−1 in the FT-IR spectrum of MPMA, and a tensile vibration peak of C–N appears at 1105 cm−1, which indicated that PMA was modified successfully by the Mannich reaction.
O stretching vibration peak appears at 1739 cm−1. There is a stretching vibration peak of N–H at 3330 cm−1 in the FT-IR spectrum of MPMA, and a tensile vibration peak of C–N appears at 1105 cm−1, which indicated that PMA was modified successfully by the Mannich reaction.
The α-H of poly(methyl acrylate) was abstracted by NaH, which was used as the catalyst, to form H2 and the corresponding sodium enolate. Then, sodium enolate reacted with the imine, which was formed by diethanolamine reacting with glutaraldehyde, to form the required Mannich base.
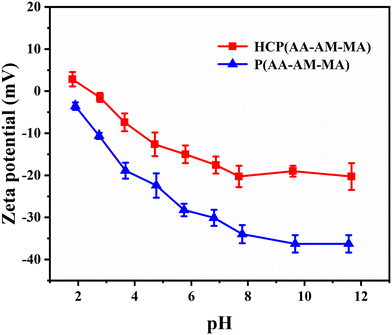
3.4 Amphoteric acrylic acid polymer retanning agents
| Sample | Mn | Mw | Mp | Mz | Mz + 1 | PDI |
|---|---|---|---|---|---|---|
| P(AA–AM–MA) | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 701 701 |
173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 718 |
218![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831 831 |
249![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 045 |
1.56 |
| HCP(AA–AM–MA) | 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912 912 |
221![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377 377 |
265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 762 |
276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 532 |
314![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019 019 |
1.57 |
3.5 Retanned leather
| P(AA–AM–MA) | HCP(AA–AM–MA) | MTA | |
|---|---|---|---|
| Elongation at break (%) | 78.9 ± 4.3 | 82.0 ± 3.7 | 73.6 ± 6.1 |
| Tensile strength (MPa) | 27.4 ± 1.6 | 31.9 ± 2.1 | 22.8 ± 4.3 |
| Softness (mm) | 8.1 ± 0.1 | 8.0 ± 0.1 | 21.4 ± 0.6 |
| Thickening rate (%) | 19.7 ± 0.3 | 21.4 ± 0.6 | 22.1 ± 0.8 |
| Dye uptake (%) | 77.3 ± 0.4 | 91.5 ± 0.2 | 85.5 ± 0.7 |
| K/S value | 15.1 ± 0.5 | 18.5 ± 0.15 | 16.5 ± 0.2 |
The elongation at break and tensile strength of HCP(AA–AM–MA)-retanned leather were 82% and 31.9 MPa, respectively, which are higher than those of P(AA–AM–MA)-retanned leather (78.9% and 27.4 MPa). The elongation at break and tensile strength of MTA-retanned leather (73.6% and 22.8 MPa) were poor. The molecular weight of HCP (AA–AM–MA) is higher than that of P (AA–AM–MA), eliminating site differences in leather. Therefore, the physical and mechanical properties of HCP(AA–AM–MA)-retanned leather were better than those of P(AA–AM–MA)-retanned leather.
There was no obvious change in the softness of the leather after retanning with the three retanning agents. As for the thickening rate of crust leather, a higher molecular weight of the retanning agent leads to a higher thickening rate. HCP(AA–AM–MA) has a higher molecular weight, so its thickening rate for leather was higher than that of P(AA–AM–MA).
3.6 Cost accounting of HCP
Table 10 shows the preparation costs of HCP(AA–AM–MA) (1 kg) and a conventional Mannich-reaction-modified acrylic retanning agent (1 kg). It was found that the difference in preparation costs was not significant, but the retanning effect of the HCP(AA–AM–MA) retanning agent was better than that of the conventional Mannich-reaction-modified acrylic retanning agent.| HCP(AA–AM–MA) (1 kg) | Traditional Mannich reaction modified acrylic retanning agent (1 kg) | |||||
|---|---|---|---|---|---|---|
| Chemicals | Input (g) | Cost (US $) | Chemicals | Input (g) | Cost (US $) | |
| AA | 229.25 | 2.83 | AA | 229.25 | 2.83 | |
| AM | 27.25 | 0.40 | AM | 27.25 | 0.40 | |
| MA | 7.06 | 0.08 | AN | 55.00 | 4.08 | |
| APS | 0.50 | 0.01 | APS | 0.50 | 0.01 | |
| NaHSO3 | 36.00 | 0.48 | NaHSO3 | 36.00 | 0.48 | |
| NaH | 23.72 | 1.04 | GA | 71.47 | 1.39 | |
| THF | 151.60 | 2.98 | Diethanolamine | 93.82 | 0.80 | |
| GA | 71.47 | 1.39 | NaOH | 58.00 | 0.51 | |
| Diethanolamine | 93.82 | 0.80 | ||||
| Total | 10.01 | 10.50 | ||||
4 Conclusions
The Mannich reaction of methyl acrylate successfully occurred by virtue of catalyst NaH; meanwhile, the modification degree of acrylic acid and acrylamide was obviously enhanced. The optimal conditions for the vinyl homopolymers were obtained through optimization analysis via orthogonal experiments. Additionally, the range analysis of the orthogonal experiments showed that the effects on the modification degree of vinyl homopolymers, in sequence from strong to weak, were as follows: amount of catalyst > amount of amine > amount of aldehyde. On the basis of the above work, a new amphoteric acrylic acid copolymer, HCP(AA–AM–MA), was prepared and used as a retanning agent in leather making, and its application properties were investigated. Application evaluation demonstrated that HCP(AA–AM–MA) had favorable tanning effects and could endow crust leather with satisfactory physical and organoleptic properties. At the same time, acrylic polymers modified via the catalytic Mannich reaction could be used as a drug carrier in the field of medicine, in biomedical engineering for the preparation of biocompatible and biodegradable materials, and also for the preparation of conductive polymer materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Key Program of the National Natural Science Foundation of China (No. 21838007), the Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 21JK0549) and the Innovation Capability Support Program of Shaanxi (No. 2021TD-16).References
- E. V. Chernikova, S. D. Zaitsev, A. V. Plutalova, K. O. Mineeva, O. S. Zotova and D. V. Vishnevetsky, Control over the relative reactivities of monomers in RAFT copolymerization of styrene and acrylic acid, RSC Adv., 2018, 8(26), 14300–14310 RSC.
- M. J. Cho and S. Y. Park, Preparation of poly (styrene)-b-poly (acrylic acid)-coupled carbon sots and their applications, ACS Appl. Mater. Interfaces, 2017, 9(28), 24169–24178 CrossRef CAS.
- M. C. Carter, L. Chen, R. S. Moglia, P. Luo, T. S. Ratani, M. Janco, J. Gu, J. N. Ngunjiri, W. Gao, C. L. Jackson and R. C. Even, Design and fabrication of polyolefin-acrylic hybrid latex particles, ACS Appl. Polym. Mater., 2019, 1(11), 3185–3195 CrossRef CAS.
- X. H. Liu, W. N. Wang, X. C. Wang, S. W. Sun and C. Wei, A “Taiji-Bagua” inspired multi-functional amphoteric polymer for ecological chromium-free organic tanned leather production: Integration of retanning and fatliquoring, J. Cleaner Prod., 2021, 319, 128658 CrossRef CAS.
- A. Koltsakidou, Z. Terzopoulou, E. V. Liakos, E. Evgenidou, D. A. Lambropoulou, D. N. Bikiaris and G. Z. Kyzas, Acrylic acid copolymers as adsorbent materials for the removal of anti-inflammatory pharmaceuticals from synthetic biomedical wastewaters, Colloids Surf., A, 2021, 629, 127382 CrossRef CAS.
- W. Xu, X. Y. Chai, G. H. Zhao, J. Li and X. C. Wang, Preparation of acrylic silicon dioxide nanoparticles as a binder for leather finishing, J. Appl. Polym. Sci., 2019, 37(8), 3276–3286 Search PubMed.
- S. Fang, G. Wang, P. Li, R. Xing, S. Liu, Y. Qin and K. Li, Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent, Int. J. Biol. Macromol., 2018, 115, 754–761 CrossRef CAS PubMed.
- I. A. Duceac, L. Verestiuc, C. D. Dimitriu, V. Maier and S. Coseri, Design and preparation of new multifunctional hydrogels based on chitosan/acrylic polymers for drug delivery and wound dressing applications, Polymers, 2020, 12(7), 1473 CrossRef CAS PubMed.
- E. Udabe, A. Somers, M. Forsyth and D. Mecerreyes, Design of polymeric corrosion inhibitors based on ionic coumarate groups, ACS Appl. Polym. Mater., 2021, 3(4), 1739–1746 CrossRef CAS PubMed.
- L. Q. Jin, W. B. Xu, H. M. Wen, Y. L. Wang and F. F. Zhang, Imparting waterproofing properties to leather by polymer nanoemulsion based on long-chain alkyl acrylate, Materials, 2023, 16(4), 1464 CrossRef CAS PubMed.
- J. C. Duan, Q. Lu, R. W. Chen, Y. Q. Duan, L. F. Wang, L. Gao and S. Y. Pan, Synthesis of a novel flocculant on the basis of crosslinked konjac glucomannan-graft-polyacrylamide-co-sodium xanthate and its application in removal of Cu2+ ion, Carbohydr. Polym., 2010, 80(2), 436–441 CrossRef CAS.
- B. Wang, F. W. Wang, Y. D. Kong, Z. M. Wu, R. M. Wang, P. F. Song and Y. F. He, Polyurea-crosslinked cationic acrylate copolymer for antibacterial coating, Colloids Surf., A, 2018, 549, 122–129 CrossRef CAS.
- Z. H. Tian, J. Z. Ma, Q. W. Liu and H. Zhang, Preparation and application of novel amphoteric acrylic retanning agents to improve dye absorption, React. Chem. Eng., 2023, 8(3), 645–655 RSC.
- Q. J. Li, Y. N. Wang, B. Shi, Y. D. Yi and J. Li, Effect of cationic monomer structure on the aggregation behavior of amphoteric acrylic polymer around isoelectric point, J. Leather Sci. Eng., 2022, 4, 4 CrossRef CAS.
- W. Ebabu, M. I. Hossain, M. E. El-Naggar, A. Kechi, S. S. Hailemariam and F. E. Ahmed, Exploration of functional polymers for cleaner leather industry, J. Inorg. Organomet. Polym. Mater., 2022, 32, 1–14 CrossRef CAS.
- S. Shi, W. T. Qiu, P. N. Miao, R. N. Li, X. F. Lin and Z. K. Sun, Three-component radical homo Mannich reaction, Nat. Commun., 2021, 12(1), 1006 CrossRef CAS PubMed.
- K. M. Meek, T. R. Eaton, N. A. Rorrer, D. G. Brandner, L. P. Manker, E. M. Karp, M. J. Biddy, A. D. Bratis, G. T. Beckham and A. K. Naskar, Emulsion polymerization of acrylonitrile in aqueous methanol, Green Chem., 2018, 20(23), 5299–5310 RSC.
- D. S. Constantino, R. P. Faria, A. M. Ribeiro and A. E. Rodrigues, Butyl acrylate production: A review on process intensification strategies, Chem. Eng. Process., 2019, 142, 107563 CrossRef CAS.
- I. Daiki, O. Yusuke, S. Mitsuo and T. Takaya, Acrylate-selective transesterification of methacrylate/acrylate copolymers: postfunctionalization with common acrylates and alcohols, ACS Macro Lett., 2018, 7(8), 997–1002 CrossRef.
- X. L. Sun, Z. Y. Qin, L. Q. Luo, Q. P. Yuan, X. M. Guo, L. Tao, Y. Wei and X. Wang, Antibacterial adhesion of poly(methyl methacrylate) modified by borneol acrylate, ACS Appl. Mater. Interfaces, 2016, 8(42), 28522–28528 CrossRef CAS PubMed.
- C. J. McDonald and R. H. Beaver, The Mannich reaction of poly(acrylamide), Macromolecules, 1979, 12(2), 203–208 CrossRef CAS.
- H. Y. Bae, M. J. Kim, J. H. Sim and C. E. Song, Direct catalytic asymmetric Mannich reaction with dithiomalonates as excellent Mannich donors: organocatalytic synthesis of (R)-sitagliptin, Angew. Chem., Int. Ed., 2016, 55(36), 10825–10829 CrossRef CAS.
- J. Iwanejko, E. Wojaczyńska and T. K. Olszewski, Green chemistry and catalysis in Mannich reaction, Curr. Opin. Green Sustainable Chem., 2018, 10, 27–34 CrossRef.
- X. Wang and Y. Gu Bronsted acid-catalyzed synthesis of α-substituted N-aminoaryl acetals and pyrroles[C]. Chinese Chemical Society, Proceedings of the 16th National Conference on Homogeneous Catalysis, Dalian.
- X. Li, L. Shi and Y. Hua, Catalytic one-pot Mannich reaction of ketones, amines and aldehydes by tin tetrachloride, Journal of Taiyuan College, 2018, 36(02), 68–71 Search PubMed.
- Y. Yamashita, H. Suzuki and S. Kobayashi, Development of strong Brønsted base catalysis: catalytic direct-type Mannich reactions of non-activated esters via a product-base mechanism, Org. Biomol. Chem., 2012, 10(30), 5750–5752 RSC.
- J. Z. Ma, Q. Liu, M. X. Wu and Z. H. Tian, Preparation and assistant-dyeing of formaldehyde-free amphoteric acrylic retanning agent, J. Leather Sci. Eng., 2021, 3, 26 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |