Open Access Article
Open Access ArticleFrom π-conjugated macrocycles to heterocycloarenes based on benzo[2,1-b:3,4-b′]dithiophene (BDTh): size- and geometry-dependent host–guest properties†
Dongyue
An
,
Rong
Zhang
,
Jiangyu
Zhu
,
Teng
Wang
,
Yan
Zhao
,
Xuefeng
Lu
 * and
Yunqi
Liu
* and
Yunqi
Liu

Department of Materials Science, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China. E-mail: luxf@fudan.edu.cn
First published on 20th February 2024
Abstract
π-Conjugated macrocycles have been highly attractive due to their challenging synthesis, fascinating aesthetic structure and unique physical and chemical properties. Although some progress has been made in synthesis, the study of π-macrocycles with different structural characteristics and supramolecular interactions still faces major challenges. In this paper, two new single-bond linked macrocycles (MS-4T/MS-6T) were reported, and the corresponding vinyl-bridged heterocycloarenes (MF-4T/MF-6T) were synthesized by the periphery fusion strategy. Further studies have indicated that the structure of these four macrocycles is determined by both size and curvature, showing unique variations from nearly planar to bowl and then to saddle. Interestingly, the nearly planar MS-4T with a small size and the rigid saddle-shaped MF-6T show no obvious response to fullerenes C60 or C70, while the bowl-shaped MS-6T and MF-4T demonstrate a strong binding affinity towards fullerenes C60 and C70. What's more, two kinds of co-crystals with capsule-like configurations, MS-6T@C60 and MS-6T@C70, have been successfully obtained, among which the former shows a loose columnar arrangement while the latter displays a unique three-dimensional honeycomb arrangement that is extremely rare in supramolecular complexes. This work systematically studies the π-conjugated macrocycles and provides a new idea for the development of novel host–guest systems and further multifunctional applications.
Introduction
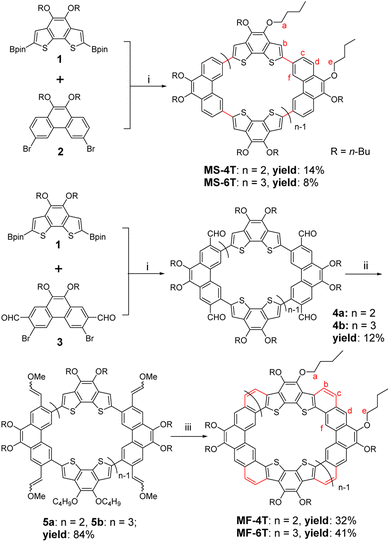
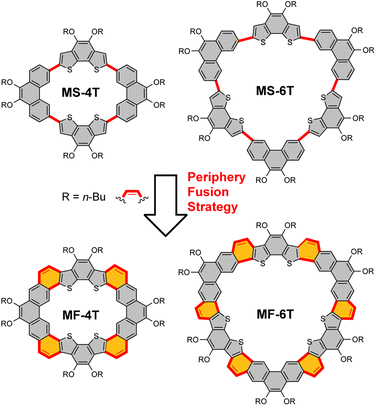
π-Conjugated macrocycles1 are an important kind of organic conjugated materials with special geometric structures, physicochemical properties and good electron transport characteristics, demonstrating broad application prospects in organic electronic devices,2 supramolecular assembly3 and chemical sensors.4 They can be roughly divided into single bond linked macrocycles and fully fused cycloarenes according to different structural characteristics. Among them, the single bond linked macrocycles have an inner cavity formed by single bonds and aromatic rings5 or double6/triple7 bonds, while fully fused cycloarenes8 have clear internal and external rings constructed by aromatic rings with common edges. Their geometric configurations, optoelectronic properties as well as supramolecular assembly behaviours can be controlled by adjusting the building blocks and conjugation length of π-conjugated systems. [n]Cyclo-meta-phenylene9 (CMP) is a kind of typical single bond linked macrocycle whose structure and optoelectronic properties are closely related to the number of phenylene blocks. In fact, for [6]CMP, if we assume that it can be fully periphery-fused, the basic cycloarene kekulene10 can be obtained, which is composed of 12 benzenoid rings prepared by Stab and Diederich in 1978. And more than 30 years later, septulene11 with 14 benzenoid rings and octulene12 with 16 benzenoid rings were successfully synthesized. The conjugated extension significantly transforms these cycloarenes from a planar structure (kekulene) into a twisted saddle structure (octulene), and their supramolecular assembly properties are quite different.13 In addition to conjugate extension, the introduction of heteroatoms can also greatly change the various properties of macrocycles, as it is conducive to enhancing intramolecular charge transfer, which has a great influence on photophysical and electrochemical properties, as well as device performance. For single bond linked macrocycles, aromatic heterocycles14 can be used as building blocks, such as thiophene,15 pyridine,16 pyrazine,17etc. Heteroatoms containing lone electron pairs can also be used directly as bridging units to realize the heteroatomization of macrocycles and achieve the purpose of regulating the performance of the final π-conjugated macrocycles.18 For fully fused cycloarenes, heteroatoms are generally introduced through aromatic heterocycles.19 At present, some heterocycloarenes that fuse thiophene,2e carbazole20 and other aromatic heterocycles21 have been successfully synthesized and show great application prospects in organic optoelectronic devices and supramolecular chemistry.However, due to the internal strain caused by special topological structures, the accurate construction of π-conjugated macrocycles is not an easy task. What's more, we have found that periphery fusion to build a vinyl bridge for macrocycles and the relationship between single bond linked macrocycles and fully fused cycloarenes have been less investigated. The effect of periphery fusion on the geometrical structure, physicochemical properties and supramolecular assembly behaviours of macrocycles is still an open question. Herein, we report a nearly planar single bond linked π-conjugated macrocycle MS-4T and a bowl-shaped macrocycle MS-6T, both of which are composed of benzo[2,1-b:3,4-b′]dithiophene (BDTh) and phenanthrene (Phen) units. In order to further study the effect of periphery fusion on macrocycles, the bowl-shaped heterocycloarene MF-4T and saddle-shaped heterocycloarene MF-6T on the basis of a single bond linked macrocycle are subsequently synthesized. Thus, the electronic structure, aromaticity, as well as optoelectronic properties of both the single bond linked π-conjugated macrocycles MS-4T/MS-6T and heterocycloarenes MF-4T/MF-6T are of great interest. In addition, such types of π-conjugated macrocycles may show selective host–guest interactions with fullerenes. In this article, we will report the synthesis, geometric configurations, optoelectronic properties and selective supramolecular interactions with fullerenes of these single bond linked π-conjugated macrocycles and fully fused heterocycloarenes (Fig. 1).
 | ||
| Fig. 1 The design of single bond linked macrocycles and corresponding vinyl-bridged heterocycloarenes. | ||
Results and discussion
Synthetic procedures
The nearly planar single bond linked π-conjugated macrocycle MS-4T, bowl-shaped macrocycle MS-6T, bowl-shaped heterocycloarene MF-4T and saddle-shaped hetero-cycloarene MF-6T were synthesized according to Scheme 1. The Suzuki coupling reaction between equimolar amounts of 1 (see synthesis in the ESI†) and 2 (ref. 22) under optimal conditions gave a mixture of MS-4T and MS-6T, which were further purified by recycling preparative gel permeation chromatography (GPC) with yields of 14% and 8%, respectively.In order to achieve the fused cyclization of single bond linked π-conjugated macrocycles, compounds 1 and 323 were used for the Suzuki coupling reaction, and the macrocyclic intermediates 4a and 4b with four and six aldehyde groups were synthesized. It should be noted that although GPC did not show two distinct product peaks, the single peak was maldistributed (Fig. S7†), indicating that the product was actually a mixture, which was very hard to further separate completely. In addition, two peaks of different molecular weight appeared in the MALDI-TOF mass spectrum (Fig. S51 and 52†), corresponding to 4a and 4b respectively. The yield of the mixture of 4a and 4b was about 18%. Then, the aldehyde groups in the intermediates 4a and 4b were converted to methoxyethenyl groups by a Wittig reaction to obtain the corresponding macrocyclic precursors 5a and 5b with four and six vinyl ether groups. Similar to the macrocyclic intermediates 4a and 4b, 5a and 5b were also hard to isolate, and the yield of the mixture of 5a and 5b was about 84%. Finally, the periphery fusion of macrocyclic precursors 5a and 5b was realized by the Friedel–Crafts reaction catalyzed by Bi(OTf)3 in 1,2-dichloroethane at room temperature, which afforded the expected fully fused heterocycloarenes MF-4T and MF-6T. The large size difference between the two molecules allowed them to be easily separated by recycling preparative gel permeation chromatography (GPC) with yields of 32% and 37% respectively. All new products were confirmed using 1H/13C NMR and mass spectra (see the ESI†).
Ground-state geometry
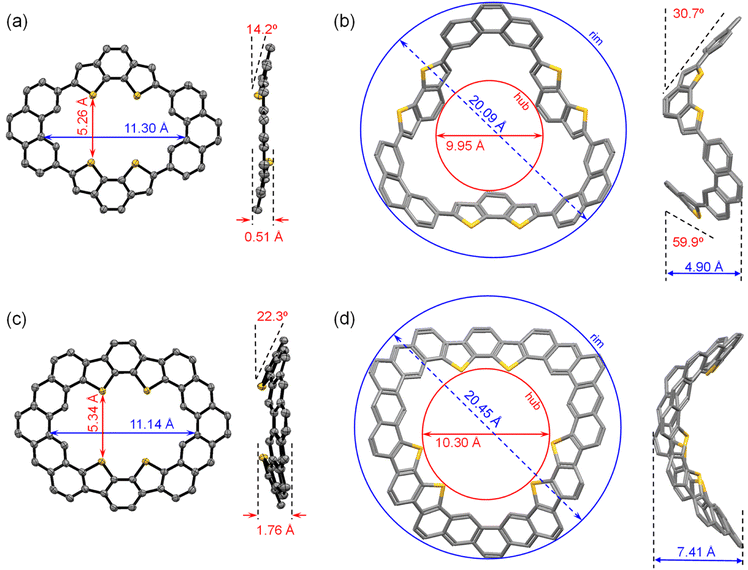
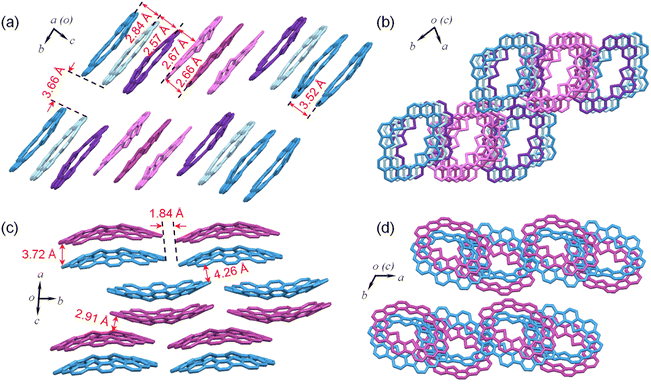
Single crystals of MS-4T were obtained as yellow needle-shaped crystals by slow diffusion of methanol into the chloroform solution under ambient conditions. X-ray crystallography reveals that MS-4T adopts a nearly planar structure, in which the two benzo[2,1-b:3,4-b′]dithiophene (BDTh) building blocks twist slightly out of the plane of phenanthrene (Phen) blocks in opposite directions with a dihedral angle of 14.2°. The long axial length of the inner cavity of MS-4T is 11.30 Å and the short axial length is 5.26 Å (Fig. 2a). It is worth noting that MS-4T exhibits polymorphism and the configuration of the conjugated skeleton is varied in the same unit cell. As is shown in Fig. 4a, there are nine macrocycles distributed in parallel along the body diagonal of the unit cell, which are centrosymmetric with respect to the central molecule. A detailed crystallographic analysis reveals that the central molecule has a standard symmetrical structure (Fig. 2a), while the other eight molecules exhibit four kinds of conformations (Fig. 3) with different degrees of distortion, in which the dihedral angle between phenanthrene (Phen) blocks varies from 2.2° to 10.1° and the dihedral angle between benzo[2,1-b:3,4-b′]dithiophene (BDTh) blocks varies from 3.0° to 21.6°.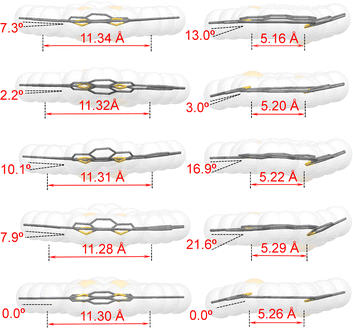 | ||
| Fig. 3 Five kinds of different crystal structures of polymorphs of MS-4T, with the main view on the left and the corresponding side view on the right. All alkyl chains have been omitted. | ||
In addition, the size of the inner cavity of MS-4T also changes slightly. The long axial length increases from 11.28 Å to 11.34 Å, while the short axial length decreases from 5.29 Å to 5.16 Å. The π–π distance between two adjacent molecules is 2.84/2.57/2.67/2.66 Å (Fig. 4a) and MS-4T molecules pack along the body diagonal of the cell to form columnar stacking with the spacing of layers and columns being 3.52 A and 3.66 A, respectively (Fig. 4a and b). This rare nonamer structure may be related to the tight arrangement of MS-4T in the solid phase due to the existence of flexible single bonds, promoting multiple configuration changes to achieve the densest stacking in long-range space. To further study the aromaticity, harmonic oscillator model of aromaticity (HOMA)38 value calculation was performed on MS-4T, which was a method to measure aromaticity based on the geometric structure. The closer HOMA is to 1, the stronger the aromaticity is. If HOMA approaches 0, it is non-aromatic. If it is negative, the bond length is extremely unbalanced, reflecting the anti-aromatic characteristics. According to the results of HOMA value calculations on individual rings, the benzenes on the side of phenanthrene blocks have a larger HOMA value (0.86) than the middle benzenes (0.47). And for the benzo[2,1-b:3,4-b′]dithiophene (BDTh) blocks, the HOMA value of benzenes (0.85) is larger than that of thiophene (0.57). All the calculation results of macrocycle MS-4T are consistent with those of monomers, implying that the formation of the single bond linked π-conjugated macrocycle does not change the aromaticity of each construction unit.
 | ||
| Fig. 4 3D packing structures for MS-4T: (a) side view, (b) top view and MF-4T: (c) side view, (d) top view. | ||
The single crystals of MS-6T were also obtained and tested by SC-XRD. However, due to the weak diffraction and complex stacking of the crystal, the structure analysis could not be carried out accurately. Alternatively, the structure of MS-6T was optimized by DFT at the B3LYP/6-31G(d,p) level of theory (Fig. 2b). With the increase of building blocks, the geometrical configurations of macrocycles change significantly. According to DFT calculations, MS-6T adopts a bowl-shaped structure, in which the diameter of the inner hub and the outer rim is about 9.95 Å and 20.09 Å, respectively. The depth of the bowl can also be obtained by measuring the distance between the mean planes of the inner hub and the outer rim, which is accurate to 4.90 Å. In addition, the sidewall composed of Phen blocks and the opposite sidewall composed of BDTh blocks are tilted about 30.7° and 59.9° relative to the mean plane of the inner rim, respectively. Noticeably, in contrast to MS-4T, the BDTh units in MS-6T are reversed, with the sulfur atoms oriented toward the outer rim, which may be due to large strain caused by the special bowl-shaped structure. To further verify this change, MS-4T and MS-6T were tested using 2D NOESY NMR spectra which can reveal the spatial proximity relationship between all protons and protons within the molecule and all aromatic protons have been assigned well. As is shown in Fig. S9,† for MS-4T, there is no obvious correlation between the inner-hub proton f and the outer-rim proton b, indicating that the two are far apart in space. But in MS-6T, there is a significant correlation between the proton f and b (Fig. S10†), so it can be inferred that BDTh blocks of MS-6T are actually flipped relative to the structural formula shown in Scheme 1, which is also consistent with the results of our DFT calculations. In addition, bond length analysis and HOMA value calculations of MS-6T are similar to those of MS-4T.
To study the effect of periphery fusion on the macrocyclic molecular structure, single crystals of MF-4T were obtained as yellow rhombic crystals by slow diffusion of methanol into the toluene solution. As is shown in Fig. 2c, MF-4T adopts a bowl-shaped structure with an approximately rectangular inner cavity, in which the long axial length and the short axial length of the inner cavity are about 11.14 Å and 5.34 Å. The bowl depth of MF-4T is 1.76 Å, and the side-wall plane is tilted about 22.3° relative to the mean plane containing four sulfur atoms. Interestingly, MF-4T exists in the form of a bilayer dimer with the π–π stacking distance of 3.72 Å. The molecules are packed into a unique bilayer-wavy layer stacking due to the bowl-shaped conformation and the distance of two adjacent “waves” formed by dimers is 1.84 Å. The distance of close π–π contacts between the slipped layer molecules is measured to be 2.91 and 4.26 Å respectively (Fig. 4c and d). To further study the new six-membered rings constructed by periphery fusion, bond length analysis and harmonic oscillator model of aromaticity (HOMA) value calculations on individual rings were performed (Fig. S8†). In the hexagons fused from vinyl ethers, the bonds linking the BDTh units and the bonds linking the Phen units (1.43 Å (5)) are close to the typical C(sp2)–C(sp2) single bond (1.45 Å), while the bond b (1.37 Å (5)) along the outer periphery is slightly shorter than that of a typical C(sp2)–C(sp2) double bond in benzene (1.39 Å). The HOMA value calculations suggest that the newly constructed six-membered rings of MF-4T exhibit strong aromatic character (large HOMA values), while the HOMA values of the middle benzene in Phen units decrease significantly compared with MS-4T, indicating that the periphery fusion has significantly changed the aromatic properties of the macrocycles. We also tried many methods to grow the single crystals of heterocycloarene MF-6T but did not succeed, and the structure of MF-6T was optimized by DFT calculations at the B3LYP/6-31G(d,p) level of theory (Fig. 2d). According to DFT calculations, MF-6T adopts the shape of a very deep saddle with a maximum bending depth of 7.41 Å, and the inner hub and the outer rim diameters of 10.30 Å and 20.45 Å respectively. In contrast to the sulfur atoms in MS-6T, which are oriented toward the outer edge of the bowl-shaped molecule, the sulfur atoms in MF-6T are oriented toward the inner edge of the heterocycloarene to realize the periphery fusion of the macrocycle, resulting in its distorted saddle-shaped structure. It was also verified using 2D NOESY NMR spectra (Fig. S14†). Bond length analysis and HOMA value calculations imply that MF-6T has a local aromatic character, which is similar to that of MF-4T.
Optical and electrochemical properties
Both MS-4T and MS-6T are yellowish in the solid state, while MF-4T and MF-6T are deep yellow to yellowish-brown. These four compounds all have good solubility and can be dissolved in common organic solvents, such as toluene, tetrahydrofuran and chloroform, with the solubility varying between 50 and 70 mg ml−1. The absorption spectra and normalized fluorescence emission spectra of MS-4T, MS-6T, MF-4T and MF-6T in toluene (ca. 10−5 M) are shown in Fig. 5. Compounds MS-4T and MS-6T display intense absorption bands with peaks at 356 and 358 nm respectively, which can be mainly attributed to a combination of HOMO−m → LUMO and HOMO → LUMO+n electronic transitions.24MF-4T shows a major absorption with maximum wavelength (λmax) at 338 nm and several vibrational peaks in the 350−450 nm region, while MF-6T displays a maximum absorption peak at 339 nm with two vibronic shoulders at 324 and 353 nm and several weaker peaks in the 370−450 nm region. The strong absorption in the high energy region can be attributed to a combination of HOMO-m → LUMO and HOMO → LUMO+n electronic transitions, while the lowest absorption band can be attributed to HOMO → LUMO transitions, which is remarkably similar to that observed in cycloarenes25 and heterocycloarenes.3c The absorption onsets (λonset) of the four compounds have also been estimated to be 450 nm for MS-4T, 463 nm for MS-6T, 452 nm for MF-4T and 466 nm for MF-6T. This slight difference may be the result of a combination of the extension of the π-conjugated system and the twisted geometry of the molecule.3b,e According to the lowest-energy absorption onset, the optical energy gap (Eoptg) was estimated to be 2.73 eV, 2.68 eV, 2.74 eV, and 2.66 eV for MS-4T, MS-6T, MF-4T and MF-6T respectively.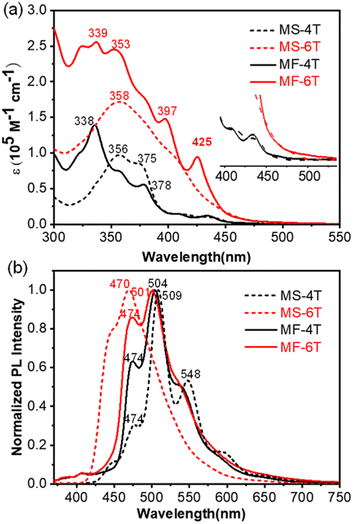 | ||
| Fig. 5 (a) UV-vis spectra and (b) normalized fluorescence emission spectra of MS-4T, MS-6T, MF-4T and MF-6T in toluene solution; insets show the magnified onset absorption bands. | ||
The normalized fluorescence emission spectra show that the maximum emission wavelength (λem) of MS-4T, MF-4T and MF-6T is 509 nm, 504 nm, and 501 nm respectively. These similar peaks can be explained by the fact that the redshift caused by extended π-conjugation is offset to some extent by the negative effect of the distorted geometry. However, the fluorescence emission spectra show a significantly blue-shifted maximum emission wavelength (λem = 470 nm) for MS-6T compared with MS-4T (λem = 509 nm), indicating that the Stokes shift of MS-6T and the energy loss from the excited state back to the ground state are small. In addition, cyclic voltammetry measurements of MS-4T, MS-6T, MF-4T and MF-6T in dry DCM (Fig. S13†) generally revealed multiple irreversible redox waves, which can also be observed in many expanded kekulenes and polycyclic aromatic hydrocarbons containing thiophenes.26MS-4T, MS-6T, MF-4T and MF-6T exhibit several similar oxidation peaks with initial oxidation potentials at 0.94 V, 0.62 V, 0.73 V and 0.69 V (vs. Fc+/Fc), respectively.
Theoretical calculations
In order to further evaluate the electronic properties of single bond linked π-conjugated macrocycles and fully fused heterocycloarenes, density functional theory (DFT) calculations were conducted by the B3LYP method and 6-31G(d,p) basis set. The calculations were minimized by substituting methyl groups for alkyl chains. As shown in Fig. 6, the frontier HOMO and LUMO coefficients of the nearly planar MS-4T and bowl-shaped MF-4T are well delocalized along the whole conjugated backbone, which is conducive to intramolecular charge transfer. However, the bowl-shaped MS-6T shows two nearly degenerated HOMO and LUMO, with coefficients distributed in half of the molecule. And the saddle-shaped MF-6T also shows similar electron cloud distribution in which the HOMO and LUMO are mainly distributed at half of the molecule. The special distributions in MS-6T and MF-6T can be attributed to their twisted geometric configuration. In addition, the distribution of HOMOs is obvious on the inner and outer edges of the middle benzene rings of Phens in the single bond linked π-conjugated macrocycles MS-4T/MS-6T. In contrast, the distribution of HOMOs is more obvious on the outer edges but negligible on the inner edges of the middle benzene rings of Phens in the heterocycloarene MF-4T/MF-6T, suggesting that the periphery fusion may affect the charge distribution on the π-conjugated backbones. What's more, the HOMO and LUMO energy levels of these four macrocycles can be obtained by DFT calculations, from which the energy gaps can also be calculated (Fig. 6). In general, the energy gaps of MS-4T, MF-4T and MF-6T gradually decrease with the increase of size and conjugation degree, which is consistent with the trend of optical band gaps measured from the UV-vis absorption spectrum (Fig. 5a). However, the energy gap of MS-6T calculated by DFT theory is quite different from the optical band gap measured by UV-vis absorption spectroscopy, which may be due to the distorted geometry constructed through flexible single bonds.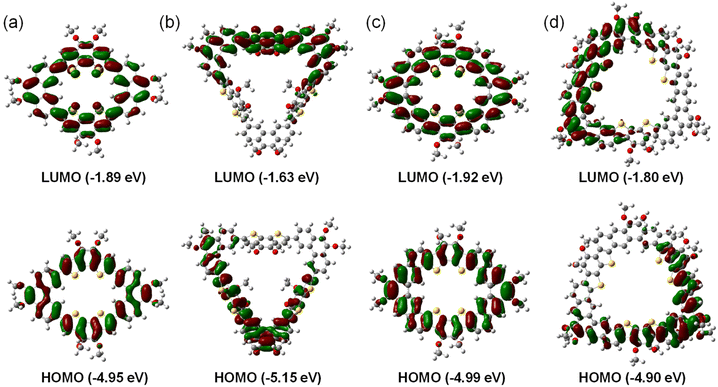 | ||
| Fig. 6 Calculated frontier molecular orbital profiles (isovalue = 0.02) of the macrocycles: (a) MS-4T, (b) MS-6T, (c) MF-4T, and (d) MF-6T. | ||
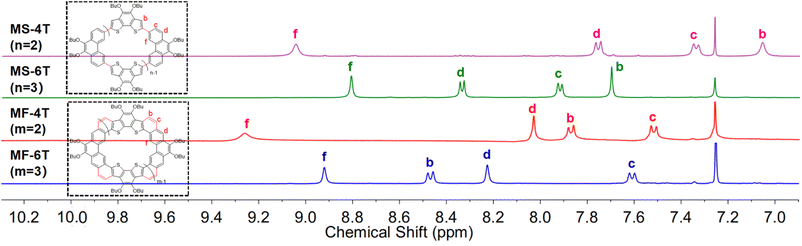
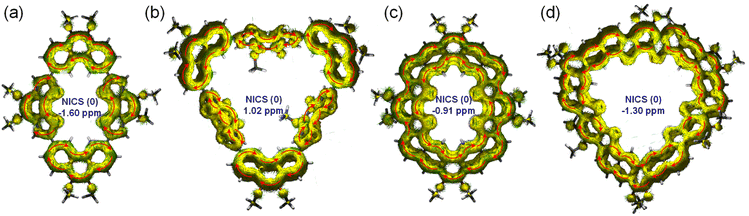
The 1H NMR spectra (Fig. 7) depict the chemical shifts of the four macrocycles, while calculations were conducted at B3LYP/6-31G(d,p) levels to determine the anisotropy of the induced current density (ACID)27 and nucleus independent chemical shift (NICS).28 As shown in Fig. 8, MS-4T and MS-6T exhibit four and six local clockwise ring current flows respectively, while the C(sp2)–C(sp2) bonds formed by the Suzuki reaction show no ring current flows, which are consistent with the results of bond length analysis, indicating that the single bond linked π-conjugated macrocycles MS-4T and MS-6T have local aromatic characteristics. The NICS (0) values for MS-4T and MS-6T are −1.60 and 1.02 ppm respectively. What's more, ACID plots reveal that MF-4T and MF-6T show local aromatic characteristics and the NICS (0) values are −0.91 and −1.30 ppm respectively, which are similar to the single bond linked π-conjugated macrocycles. However, the distributions of ring current flows in fully fused heterocycloarenes have changed significantly. As shown in Fig. 8, both the C(sp2)–C(sp2) bonds formed by the Suzuki reaction and the hexagons fused from vinyl ethers all show heavy ring current, implying obvious aromatic characteristics, which are also reflected in bond length analysis and HOMA value calculations. In addition, compared with typical cycloarenes of the carbon conjugated framework,25 ring current flows over the C(sp2)–C(sp2) double bonds at each corner of MF-4T/MF-6T can be observed in all cases (seen in Fig. S18−S21 in the ESI†), indicating that the introduction of sulfur atoms significantly changes the distribution of ring current and the aromatization of local rings in heterocycloarenes.
Binding behaviour with fullerenes
Considering that both the single bond linked π-conjugated macrocycles MS-4T/MS-6T and fully fused heterocycloarenes MF-4T/MF-6T have well-defined cavities, they may behave as hosts for some typical guests such as fullerenes.3a,e Therefore, C60 was dropped into the solution of MS-4T, MS-6T, MF-4T and MF-6T respectively and then tested using the 1H NMR spectrum at 298 K, in which the feasibility of macrocycles as host molecules was judged by the movement of characteristic peaks.29 The specific procedure of 1H NMR titration can be seen in the ESI.† For the single bond-linked conjugated macrocycles, no appreciable change was observed when MS-4T was mixed with C60, indicating that there was no obvious supramolecular binding30 (Figure S26a†). However, the mixture of MS-6T and C60 displayed unusual changes. As shown in Fig. 9a, upon addition of 0.3 equivalent C60 to MS-6T in CD2Cl2, two sets of resonances for the protons of macrocycles appeared in the 1H NMR spectrum, implying a slow-exchange complexation21,31 between the host MS-6T and the guest C60. Affected by the ring currents of aromatic surfaces of spherical C60, the inner hub protons (b and f) and the outer rim protons (c) all were shifted to high field, while the outer-most rim protons (d) were shifted to low field, which had been confirmed by 2D NOESY NMR spectroscopy (Fig. S11†). After the addition of C60 to half an equivalent of MS-6T, the proton signals of free MS-6T disappeared while the four new groups of proton signals remained and the integration ratio of the four characteristic peaks was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then no further spectral changes were observed even with increased amounts of C60, indicating that the binding stoichiometry between MS-6T and C60 tended to be 2
1. Then no further spectral changes were observed even with increased amounts of C60, indicating that the binding stoichiometry between MS-6T and C60 tended to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was further confirmed by subsequent fluorescence titration tests (Fig. S28 and 29†).
1, which was further confirmed by subsequent fluorescence titration tests (Fig. S28 and 29†).
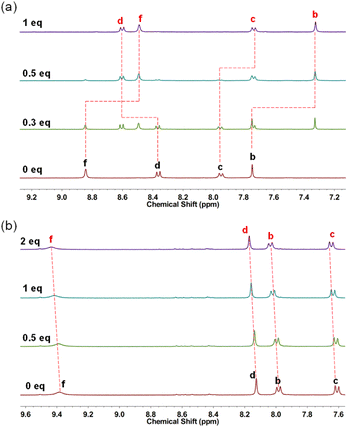 | ||
| Fig. 9 1H NMR spectral change of (a) MS-6T (in CD2Cl2, 1.0 mM) and (b) MF-4T (in CDCl3, 1.0 mM) with the addition of C60 (in the same solvent with host, 8.0 mM) at 298 K. | ||
On the other hand, a completely different phenomenon occurred when C60 was dropped into the solution of the fully fused heterocycloarenes MF-4T and MF-6T. With the increase of C60, the aromatic proton signals of MF-4T shifted to the downfield and the signal peak of the inner hub proton f widened significantly (Fig. 9b), indicating that there is an obvious supramolecular interaction between the host MF-4T and guest C60. In contrast, the trimer heterocycloarene MF-6T exhibited no pronounced chemical shift changes, implying that MF-6T didn't bind with C60 effectively (Fig. S26b†). These entirely different supramolecular binding behaviours can be attributed to the different sizes and geometry of the four macrocycles. For the single bond-linked conjugated macrocycles, the rigid and nearly planar configuration prevents MS-4T from effectively encapsulating spherical C60 molecules, while MS-6T which has a flexible bowl-shaped structure and a large internal cavity can bind with C60 strongly. However, after the periphery fusion, it is found that the dimer heterocycloarene MF-4T has become a bowl-shaped configuration which is conducive to its binding with C60, while the trimer heterocycloarene MF-6T adopts a rigid and highly twisted saddle-shaped structure that no longer has the ability to encapsulate C60. In addition, the ellipsoidal C70 was also added to the solution of the four macrocycles as guest molecules, and their supramolecular binding behaviors were similar to those of C60 (Fig. S27†).
In addition to the 1H NMR titration spectra, fluorescence titration spectra have also been obtained and the supramolecular assembly properties of hosts (MS-6T and MF-4T) and guests (C60 and C70) in toluene were quantitatively analyzed by the degree of fluorescence quenching. As shown in Fig. S28,† the fluorescence emission intensity of MS-6T at 469 nm decreased rapidly with the increase of fullerenes C60 and C70, implying the formation of supramolecular complexes. Similarly, the fluorescence emission intensity of MF-4T at 504 nm was also quenched obviously when C60 and C70 were added to the solution. By fitting the data of multiple fluorescence titrations into different binding models (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and analyzing the residual plots,32 we found that for MS-6T, 2
1) and analyzing the residual plots,32 we found that for MS-6T, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is a more ideal stoichiometry (Fig. S28 and 29†), while for MF-4T, only the 1
1 is a more ideal stoichiometry (Fig. S28 and 29†), while for MF-4T, only the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model can obtain an effective binding constant, which proves that 1
1 model can obtain an effective binding constant, which proves that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 may be a more appropriate stoichiometry. And the binding constants Ka and associated data of four macrocycles and fullerenes C60/C70 were obtained by nonlinear fitting of fluorescence titration data, which have been detailed in Tables 1 and S6.†
1 may be a more appropriate stoichiometry. And the binding constants Ka and associated data of four macrocycles and fullerenes C60/C70 were obtained by nonlinear fitting of fluorescence titration data, which have been detailed in Tables 1 and S6.†
| K 11 [M−1] | K 21 [M−1] | |
|---|---|---|
| MS-6T@C60 | (8.71 ± 0.54) × 105 | (1.04 ± 0.04) ×105 |
| MS-6T@C70 | (5.10 ± 0.14) × 105 | (2.44 ± 0.42) ×105 |
| MF-4T@C60 | (2.09 ± 0.18) × 104 | |
| MF-4T@C70 | (1.62 ± 0.25) × 105 |
To study the supramolecular assembly behaviors of macrocycles and fullerenes more directly, co-crystals of MS-6T@C60 and MS-6T@C70 were obtained by slow diffusion of methanol into a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
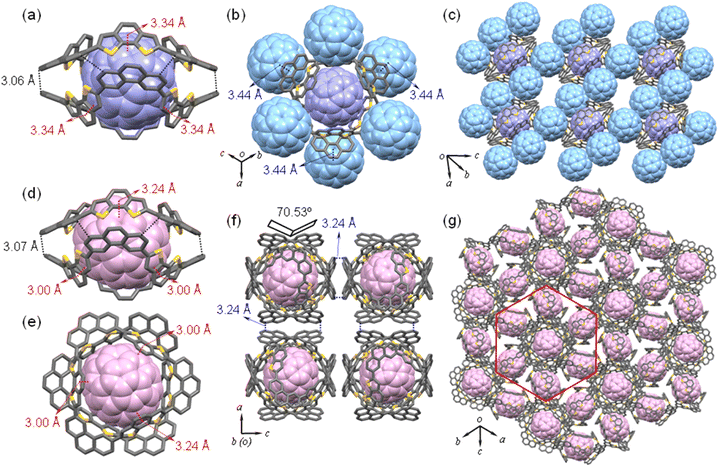
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of MS-6T and C60 or C70 in toluene. The structures of these complexes were analyzed by single crystal XRD. As shown in Fig. 10, both C60 and C70 can bind well to the bowl-shaped macrocycle MS-6T. For co-crystals MS-6T@C60, there is a molecule of C60 (purple) fully encapsulated in the large cavity of a capsule, which is composed of two MS-6Ts through twelve C–H⋯π interactions (shown in Fig. 10a as black dashed lines) between the phenanthrene (Phen) and the conjugated framework of the outer rim of the other macrocycle MS-6T (dC–H⋯π = 3.06 Å). And the supramolecular assembly is achieved by π–π interactions (shown in Fig. 10a as red dashed lines) between the spherical C60 and benzodithiophene (BDTh) units33 at the inner edge of the bowl-shaped molecule MS-6T at a distance of 3.34 Å. Interestingly, in addition to the C60 located in the inner cavity of the molecular capsule, six other C60 molecules fill the outer part of the capsule through π–π interactions (shown in Fig. 10b as blue dashed lines) between the outer rim of MS-6T and spherical fullerenes with a distance of 3.44 Å, forming a symmetrical hexagonal arrangement. Analysis of the stacking pattern of MS-6T@C60 shows that there are multiple π–π interactions (Fig. 10c) between the six C60 molecules on the outside of one dimer capsule and neighboring capsules with a distance of 3.44 Å, which are conducive to the regular arrangement of co-crystal molecules in long-range space.
1 mixture of MS-6T and C60 or C70 in toluene. The structures of these complexes were analyzed by single crystal XRD. As shown in Fig. 10, both C60 and C70 can bind well to the bowl-shaped macrocycle MS-6T. For co-crystals MS-6T@C60, there is a molecule of C60 (purple) fully encapsulated in the large cavity of a capsule, which is composed of two MS-6Ts through twelve C–H⋯π interactions (shown in Fig. 10a as black dashed lines) between the phenanthrene (Phen) and the conjugated framework of the outer rim of the other macrocycle MS-6T (dC–H⋯π = 3.06 Å). And the supramolecular assembly is achieved by π–π interactions (shown in Fig. 10a as red dashed lines) between the spherical C60 and benzodithiophene (BDTh) units33 at the inner edge of the bowl-shaped molecule MS-6T at a distance of 3.34 Å. Interestingly, in addition to the C60 located in the inner cavity of the molecular capsule, six other C60 molecules fill the outer part of the capsule through π–π interactions (shown in Fig. 10b as blue dashed lines) between the outer rim of MS-6T and spherical fullerenes with a distance of 3.44 Å, forming a symmetrical hexagonal arrangement. Analysis of the stacking pattern of MS-6T@C60 shows that there are multiple π–π interactions (Fig. 10c) between the six C60 molecules on the outside of one dimer capsule and neighboring capsules with a distance of 3.44 Å, which are conducive to the regular arrangement of co-crystal molecules in long-range space.
On the other hand, the crystal structure of MS-6T@C70 has also been thoroughly studied. Similar to MS-6T@C60, the macrocycles in MS-6T@C70 also appear as capsules created by the dimerization of two MS-6Ts through C–H⋯π interaction (shown in Fig. 10d as black dashed lines) between the phenanthrene (Phen) and the other macrocycle MF-6T (dC–H⋯π = 3.07 Å), whose dimensions are identical to those of capsules in MS-6T@C60. One ellipsoidal C70 resides in the center of the capsule by π–π interactions (shown in Fig. 10d and e as red dashed lines) between the C70 and benzodithiophene (BDTh) units of the bowl-shaped molecule MS-6T. Due to the larger ellipsoidal structure of C70, the maximum distance of π–π interactions is 3.24 Å and the minimum is 3.00 Å, indicating a stronger supramolecular interaction between C70 and MS-6T than that between C60 and MS-6T. However, unlike MS-6T@C60, fullerene C70 was not found outside the capsules. Further crystallographic analysis showed that there are eight co-crystal molecules in the cube cell (Fig. 10f), which are distributed on eight vertices of the cube, and the angle between the two adjacent molecules is 70.53°. In addition, the C–H⋯π interactions (dC–H⋯π = 3.24 Å, shown in Fig. 10f as blue dashed lines) are formed between two adjacent molecular capsules through the phenanthrene (Phen) and the conjugated framework. And due to these molecular capsules rotating at different angles and balanced C–H⋯π interactions, MS-6T@C70 exhibits a particularly complex hexagonal windmill-shaped packing,34 which can also be called a honeycomb35 arrangement. In fact, although some honeycomb structures have been reported in polymers36 or non-planar graphene,37 self-assembled honeycomb lattices of supramolecular complexes with special molecular capsules structures are rare and we may be the first to report supramolecular honeycomb structures supported by explicit crystallographic data. As shown in Fig. 10g, six molecular capsules perpendicular to the central capsule are distributed around the central molecular capsule and rotate at the same angle, thus forming a set of hexagonal phases, which can be considered as the smallest unit to construct a honeycomb lattice. It is also worth noting that due to the inherent three-dimensional structure of the molecular capsules, MS-6T@C70 molecules can realize honeycomb stacking in other directions to form a complex three-dimensional honeycomb lattice.
Conclusions
In summary, single bond linked π-conjugated macrocycles with different sizes (MS-4T and MS-6T) and corresponding fully fused heterocycloarenes (MF-4T and MF-6T) were successfully synthesized by effective macrocycle formation strategy and the periphery fusion strategy. X-ray crystallographic analysis and DFT calculations reveal that the geometric configurations of macrocycles have been greatly changed by the periphery fusion, in which the single bond linked π-conjugated macrocycle MS-4T presents a rigid and nearly planar geometry while the heterocycloarene MF-4T adopts a bowl-shaped structure and the larger size macrocycle MS-6T presents a bowl-shaped structure while the heterocycloarene MF-6T shows a saddle-shaped geometric configuration. In addition, all four macrocycles exhibited local aromatic properties. The conjugation length and geometric configuration of these macrocycles greatly affect their optical and electrochemical properties. Moreover, MS-6T and MF-4T exhibit special supramolecular assembly behaviours with spherical C60 and ellipsoidal C70, which can be attributed to their suitable size and unique bowl configuration. Among them, MS-6T and fullerenes can form unique molecular capsules and different spatial arrangements. In particular, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex molecular capsule MS-6T@C70 shows a very special honeycomb packing in long-range space, which may be the first three-dimensional honeycomb lattice found in a supramolecular complex, giving insights into the development of novel host–guest systems based on bowl-shaped macrocycles.
1 complex molecular capsule MS-6T@C70 shows a very special honeycomb packing in long-range space, which may be the first three-dimensional honeycomb lattice found in a supramolecular complex, giving insights into the development of novel host–guest systems based on bowl-shaped macrocycles.
Data availability
Synthetic procedures and characterization data of new compounds, X-ray crystallographic data, details of all characterization studies and theoretical calculations, and additional spectra have been provided in the ESI File.† CCDC 2272202, 2272207, 2327360 and 2272225 contain the supplementary crystallographic data for this paper.Author contributions
X. L. conceived and supervised the project. D. A. contributed to all experiments, data analysis, and manuscript writing. R. Z. and J. Z. participated in the experimental operations and data analysis. T. W. contributed to manuscript revision. Y. Z., Y. L. and X. L. were responsible for funding acquisition and manuscript revision. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22322502, 52073063 and 61890940), the National Key R&D Program of China (2023YFB3609000), the Natural Science Foundation of Shanghai (22ZR1405800 and 23ZR1405100), and the Program for Professor of Special Appointment (Eastern Scholar) at the Shanghai Institutions of Higher Learning. Thanks to crystallographer Pengfei Zhou from Beijing Zhongkebaice Technology Service Co. for his help in the analysis of the single crystal structure.Notes and references
- (a) S. Eisler, R. McDonald, G. R. Loppnow and R. R. Tykwinski, Structural, Vibrational, and Electronic Characteristics of Enyne Macrocycles as a Function of Ring Strain, J. Am. Chem. Soc., 2000, 122, 6917–6928 CrossRef CAS; (b) J. Kromer, I. Rios-Carreras, G. Fuhrmann, C. Musch, M. Wunderlin, T. Debaerdemaeker, E. Mena-Osteritz and P. Bauerle, Synthesis of the first fully alpha-conjugated macrocyclic oligothiophenes: Cyclo[n]thiophenes with tunable cavities in the nanometer regime, Angew. Chem., Int. Ed., 2000, 39, 3481–3486 CrossRef CAS; (c) V. Martí-Centelles, M. D. Pandey, M. I. Burguete and S. V. Luis, Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization, Chem. Rev., 2015, 115, 8736–8834 CrossRef PubMed; (d) B. Szyszko, M. J. Białek, E. Pacholska-Dudziak and L. Latos-Grażyński, Flexible Porphyrinoids, Chem. Rev., 2017, 117, 2839–2909 CrossRef CAS PubMed.
- (a) Q. Chen, M. T. Trinh, D. W. Paley, M. B. Preefer, H. Zhu, B. S. Fowler, X. Y. Zhu, M. L. Steigerwald and C. Nuckolls, Strain-Induced Stereoselective Formation of Blue-Emitting Cyclostilbenes, J. Am. Chem. Soc., 2015, 137, 12282–12288 CrossRef CAS PubMed; (b) B. Zhang, M. T. Trinh, B. Fowler, M. Ball, Q. Xu, F. Ng, M. L. Steigerwald, X. Y. Zhu, C. Nuckolls and Y. Zhong, Rigid, Conjugated Macrocycles for High Performance Organic Photodetectors, J. Am. Chem. Soc., 2016, 138, 16426–16431 CrossRef CAS PubMed; (c) Y. Yang, M. Chu and Q. Miao, From Phenanthrylene Butadiynylene Macrocycles to S-Heterocycloarenes, Org. Lett., 2018, 20, 4259–4262 CrossRef CAS PubMed; (d) M. Ball, B. Zhang, Y. Zhong, B. Fowler, S. Xiao, F. Ng, M. Steigerwald and C. Nuckolls, Conjugated Macrocycles in Organic Electronics, Acc. Chem. Res., 2019, 52, 1068–1078 CrossRef CAS PubMed; (e) N. Zhang, L. Yang, W. Li, J. Zhu, K. Chi, D. Chang, Y. Qiao, T. Wang, Y. Zhao, X. Lu and Y. Liu, Alkyl-Substituted N,S-Embedded Heterocycloarenes with a Planar Aromatic Configuration for Hosting Fullerenes and Organic Field-Effect Transistors, J. Am. Chem. Soc., 2022, 144, 21521–21529 CrossRef CAS PubMed; (f) J. Liu, W. Hu and L. Jiang, Monolayer molecular crystals and devices, Sci. Bull., 2023, 68, 1474–1477 CrossRef CAS PubMed.
- (a) T. Kawase and H. Kurata, Ball-, Bowl-, and Belt-Shaped Conjugated Systems and Their Complexing Abilities: Exploration of the Concave−Convex π−π Interaction, Chem. Rev., 2006, 106, 5250–5273 CrossRef CAS PubMed; (b) X. Lu, T. Y. Gopalakrishna, Y. Han, Y. Ni, Y. Zou and J. Wu, Bowl-Shaped Carbon Nanobelts Showing Size-Dependent Properties and Selective Encapsulation of C70, J. Am. Chem. Soc., 2019, 141, 5934–5941 CrossRef CAS PubMed; (c) Y. Ni, F. Gordillo-Gámez, M. Peña Alvarez, Z. Nan, Z. Li, S. Wu, Y. Han, J. Casado and J. Wu, A Chichibabin's Hydrocarbon-Based Molecular Cage: The Impact of Structural Rigidity on Dynamics, Stability, and Electronic Properties, J. Am. Chem. Soc., 2020, 142, 12730–12742 CrossRef CAS PubMed; (d) J. Wang, Y.-Y. Ju, K.-H. Low, Y.-Z. Tan and J. Liu, A Molecular Transformer: A π-Conjugated Macrocycle as an Adaptable Host, Angew. Chem., Int. Ed., 2021, 60, 11814–11818 CrossRef CAS PubMed; (e) J. Zhu, W. Li, N. Zhang, D. An, Y. Zhao, X. Lu and Y. Liu, Size-dependent properties and unusual reactivity of novel nonplanar heterocycloarenes, Chem. Sci., 2022, 13, 11174–11182 RSC.
- (a) V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Carbon Nanotube Chemical Sensors, Chem. Rev., 2019, 119, 599–663 CrossRef CAS PubMed; (b) D. T. McQuade, A. E. Pullen and T. M. Swager, Conjugated Polymer-Based Chemical Sensors, Chem. Rev., 2000, 100, 2537–2574 CrossRef CAS PubMed; (c) X. Dong, X. Dai, G. Li, Y.-M. Zhang, X. Xu and Y. Liu, Conformationally Confined Emissive Cationic Macrocycle with Photocontrolled Organelle-Specific Translocation, Adv. Sci., 2022, 9, 2201962 CrossRef CAS PubMed.
- (a) H. Thakellapalli, S. Li, B. Farajidizaji, N. N. Baughman, N. G. Akhmedov, B. V. Popp and K. K. Wang, Synthesis and Properties of Conjugated Macrocycles Containing 2,7-Bis(2-thienyl)-9H-fluoren-9-one Units, Org. Lett., 2017, 19, 2674–2677 CrossRef CAS PubMed; (b) K. Kato, Y. Kurakake, S. Ohtani, S. Fa, M. Gon, K. Tanaka and T. Ogoshi, Discrete Macrocycles with Fixed Chirality and Two Distinct Sides: Dipole-Dependent Chiroptical Response, Angew. Chem., Int. Ed., 2022, 61, e202209222 CrossRef CAS PubMed; (c) W. Xu, Y. Nagata and N. Kumagai, TEtraQuinolines: A Missing Link in the Family of Porphyrinoid Macrocycles, J. Am. Chem. Soc., 2023, 145, 2609–2618 CrossRef CAS PubMed.
- (a) J. S. Reddy and V. G. Anand, Aromatic Expanded Isophlorins: Stable 30π Annulene Analogues with Diverse Structural Features, J. Am. Chem. Soc., 2009, 131, 15433–15439 CrossRef CAS PubMed; (b) Y. Pareek, M. Ravikanth and T. K. Chandrashekar, Smaragdyrins: Emeralds of Expanded Porphyrin Family, Acc. Chem. Res., 2012, 45, 1801–1816 CrossRef CAS PubMed; (c) L. Andreo, G. Volpi, F. Rossi, P. Benzi and E. Diana, Two-step Synthesis of a New Twenty-Membered Macrocycle: Spectroscopic Characterization and Theoretical Calculations, ChemistrySelect, 2022, 7, e202202564 CrossRef CAS.
- (a) J. M. W. Chan, J. R. Tischler, S. E. Kooi, V. Bulović and T. M. Swager, Synthesis of J-Aggregating Dibenz[a,j]anthracene-Based Macrocycles, J. Am. Chem. Soc., 2009, 131, 5659–5666 CrossRef CAS PubMed; (b) K. Miki, M. Fujita, Y. Inoue, Y. Senda, T. Kowada and K. Ohe, Synthesis of Strained Pyridine-Containing Cyclyne via Reductive Aromatization, J. Org. Chem., 2010, 75, 3537–3540 CrossRef CAS PubMed.
- (a) U. Beser, M. Kastler, A. Maghsoumi, M. Wagner, C. Castiglioni, M. Tommasini, A. Narita, X. Feng and K. Müllen, A C216-Nanographene Molecule with Defined Cavity as Extended Coronoid, J. Am. Chem. Soc., 2016, 138, 4322–4325 CrossRef CAS PubMed; (b) G.-F. Huo, T. M. Fukunaga, X. Hou, Y. Han, W. Fan, S. Wu, H. Isobe and J. Wu, Facile Synthesis and Chiral Resolution of Expanded Helicenes with up to 35 cata-Fused Benzene Rings, Angew. Chem., Int. Ed., 2023, 62, e202218090 CrossRef CAS PubMed.
- (a) K. Ikemoto, A. Yoshii, T. Izumi, H. Taka, H. Kita, J. Y. Xue, R. Kobayashi, S. Sato and H. Isobe, Modular Synthesis of Aromatic Hydrocarbon Macrocycles for Simplified, Single-Layer Organic Light-Emitting Devices, J. Org. Chem., 2016, 81, 662–666 CrossRef CAS PubMed; (b) K. Ikemoto, T. Tokuhira, A. Uetani, Y. Harabuchi, S. Sato, S. Maeda and H. Isobe, Fluorescence Enhancement of Aromatic Macrocycles by Lowering Excited Singlet State Energies, J. Org. Chem., 2020, 85, 150–157 CrossRef CAS PubMed.
- (a) F. Diederich and H. A. Staab, Benzenoid versus Annulenoid Aromaticity: Synthesis and Properties of Kekulene, Angew. Chem., Int. Ed., 1978, 17, 372–374 CrossRef; (b) C. Krieger, F. Diederich, D. Schweitzer and H. A. Staab, Molecular Structure and Spectroscopic Properties of Kekulene, Angew. Chem., Int. Ed., 1979, 18, 699–701 CrossRef; (c) H. A. Staab and F. Diederich, Cycloarenes, a New Class of Aromatic Compounds, I. Synthesis of Kekulene, Chem. Ber., 1983, 116, 3487–3503 CrossRef CAS.
- B. Kumar, R. L. Viboh, M. C. Bonifacio, W. B. Thompson, J. C. Buttrick, B. C. Westlake, M.-S. Kim, R. W. Zoellner, S. A. Varganov, P. Mörschel, J. Teteruk, M. U. Schmidt and B. T. King, Septulene: The Heptagonal Homologue of Kekulene, Angew. Chem., Int. Ed., 2012, 51, 12795–12800 CrossRef CAS PubMed.
- M. A. Majewski, Y. Hong, T. Lis, J. Gregoliński, P. J. Chmielewski, J. Cybińska, D. Kim and M. Stępień, Octulene: A Hyperbolic Molecular Belt that Binds Chloride Anions, Angew. Chem., Int. Ed., 2016, 55, 14072–14076 CrossRef CAS PubMed.
- H. Miyoshi, S. Nobusue, A. Shimizu and Y. Tobe, Non-alternant non-benzenoid kekulenes: the birth of a new kekulene family, Chem. Soc. Rev., 2015, 44, 6560–6577 RSC.
- G. R. Newkome, J. D. Sauer, J. M. Roper and D. C. Hager, Construction of synthetic macrocyclic compounds possessing subheterocyclic rings, specifically pyridine, furan, and thiophene, Chem. Rev., 1977, 77, 513–597 CrossRef CAS.
- B. V. Phulwale, S. K. Mishra, M. Nečas and C. Mazal, Phenanthrylene-butadiynylene and Phenanthrylene-thienylene Macrocycles: Synthesis, Structure, and Properties, J. Org. Chem., 2016, 81, 6244–6252 CrossRef CAS PubMed.
- M. Kuritani, S. Tashiro and M. Shionoya, Heterodinuclear Metal Arrangement in a Flat Macrocycle with Two Chemically- Equivalent Metal Chelating Sites, Inorg. Chem., 2012, 51, 1508–1515 CrossRef CAS PubMed.
- M. Taniguchi and J. S. Lindsey, Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins, Chem. Rev., 2017, 117, 344–535 CrossRef CAS PubMed.
- (a) L. Zhang, J. Mu, Z. Jiang, H. Zhang and X. Yue, Fully aromatic macrocycle-terminated polyimide: synthesis and cross-linking, Angew. Chem., Int. Ed., 2013, 24, 415–420 CAS; (b) R. Inoue, M. Hasegawa, T. Nishinaga, K. Yoza and Y. Mazaki, Efficient Synthesis, Structure, and Complexation Studies of Electron-Donating Thiacalix[n]dithienothiophene, Angew. Chem., Int. Ed., 2015, 54, 2734–2738 CrossRef CAS PubMed; (c) R. Kurosaki, H. Hayashi, M. Suzuki, J. Jiang, M. Hatanaka, N. Aratani and H. Yamada, A remarkably strained cyclopyrenylene trimer that undergoes metal-free direct oxygen insertion into the biaryl C–C σ-bond, Chem. Sci., 2019, 10, 6785–6790 RSC.
- (a) A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed; (b) T. Luo, Y. Wang, J. Hao, P.-A. Chen, Y. Hu, B. Chen, J. Zhang, K. Yang and Z. Zeng, Furan-Extended Helical Rylenes with Fjord Edge Topology and Tunable Optoelectronic Properties, Angew. Chem., Int. Ed., 2023, 62, e202214653 CrossRef CAS PubMed.
- D. Myśliwiec and M. Stępień, The Fold-In Approach to Bowl-Shaped Aromatic Compounds: Synthesis of Chrysaoroles, Angew. Chem., Int. Ed., 2013, 52, 1713–1717 CrossRef PubMed.
- J. Xie, X. Li, S. Wang, A. Li, L. Jiang and K. Zhu, Heteroatom-bridged molecular belts as containers, Nat. Commun., 2020, 11, 3348 CrossRef CAS PubMed.
- (a) H. Gregolińska, M. Majewski, P. J. Chmielewski, J. Gregoliński, A. Chien, J. Zhou, Y.-L. Wu, Y. J. Bae, M. R. Wasielewski, P. M. Zimmerman and M. Stępień, Fully Conjugated [4]Chrysaorene. Redox-Coupled Anion Binding in a Tetraradicaloid Macrocycle, J. Am. Chem. Soc., 2018, 140, 14474–14480 CrossRef PubMed; (b) Z. Luo, X. Yang, K. Cai, X. Fu, D. Zhang, Y. Ma and D. Zhao, Toward Möbius and Tubular Cyclopolyarene Nanorings via Arylbutadiyne Macrocycles, Angew. Chem., Int. Ed., 2020, 59, 14854–14860 CrossRef CAS PubMed.
- (a) X. Lu, T. Y. Gopalakrishna, H. Phan, T. S. Herng, Q. Jiang, C. Liu, G. Li, J. Ding and J. Wu, Global Aromaticity in Macrocyclic Cyclopenta-Fused Tetraphenanthrenylene Tetraradicaloid and Its Charged Species, Angew. Chem., Int. Ed., 2018, 57, 13052–13056 CrossRef CAS PubMed; (b) B. Prajapati, D.-K. Dang, P. J. Chmielewski, M. A. Majewski, T. Lis, C. J. Gómez-García, P. M. Zimmerman and M. Stępień, An Open-Shell Coronoid with Hybrid Chichibabin–Schlenk Conjugation, Angew. Chem., Int. Ed., 2021, 60, 22496–22504 CrossRef CAS PubMed.
- N. M. O'boyle, A. L. Tenderholt and K. M. Langner, cclib: A library for package-independent computational chemistry algorithms, J. Comput. Chem., 2008, 29, 839–845 CrossRef PubMed.
- W. Fan, Y. Han, X. Wang, X. Hou and J. Wu, Expanded Kekulenes, J. Am. Chem. Soc., 2021, 143, 13908–13916 CrossRef CAS PubMed.
- R. Dong, M. Pfeffermann, D. Skidin, F. Wang, Y. Fu, A. Narita, M. Tommasini, F. Moresco, G. Cuniberti, R. Berger, K. Müllen and X. Feng, Persulfurated Coronene: A New Generation of “Sulflower”, J. Am. Chem. Soc., 2017, 139, 2168–2171 CrossRef CAS PubMed.
- (a) D. Geuenich, K. Hess, F. Köhler and R. Herges, Anisotropy of the Induced Current Density (ACID), a General Method To Quantify and Visualize Electronic Delocalization, Chem. Rev., 2005, 105, 3758–3772 CrossRef CAS PubMed; (b) B. Li, C. Yang, X. Wang, G. Li, W. Peng, H. Xiao, S. Luo, S. Xie, J. Wu and Z. Zeng, Synthesis and Structural Elucidation of Bisdibenzocorannulene in Multiple Redox States, Angew. Chem., Int. Ed., 2021, 60, 19790–19796 CrossRef CAS PubMed.
- (a) Y. Ruiz-Morales, The Agreement between Clar Structures and Nucleus-Independent Chemical Shift Values in Pericondensed Benzenoid Polycyclic Aromatic Hydrocarbons: An Application of the Y-Rule, J. Phys. Chem. A, 2004, 108, 10873–10896 CrossRef CAS; (b) Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- P. Thordarson, Determining association constants from titration experiments in supramolecular chemistry, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- Y. Tian, Y. Guo, X. Dong, X. Wan, K.-H. Cheng, R. Chang, S. Li, X. Cao, Y.-T. Chan and A. C. H. Sue, Synthesis of covalent organic pillars as molecular nanotubes with precise length, diameter and chirality, Nat. Synth., 2023, 2, 395–402 CrossRef.
- Y. Shi, K. Cai, H. Xiao, Z. Liu, J. Zhou, D. Shen, Y. Qiu, Q.-H. Guo, C. Stern, M. R. Wasielewski, F. Diederich, W. A. Goddard III and J. F. Stoddart, Selective Extraction of C70 by a Tetragonal Prismatic Porphyrin Cage, J. Am. Chem. Soc., 2018, 140, 13835–13842 CrossRef CAS PubMed.
- (a) D. Brynn Hibbert and P. Thordarson, The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis, Chem. Commun., 2016, 52, 12792–12805 RSC; (b) N. Grabicki, S. Fisher and O. Dumele, A Fourfold Gold(I)−Aryl Macrocycle with Hyperbolic Geometry and its Reductive Elimination to a Carbon Nanoring Host, Angew. Chem., Int. Ed., 2023, 62, e202217917 CrossRef CAS PubMed; (c) K. Li, Z. Xu, H. Deng, Z. Zhou, Y. Dang and Z. Sun, Dimeric Cycloparaphenylenes with a Rigid Aromatic Linker, Angew. Chem., Int. Ed., 2021, 60, 7649–7653 CrossRef CAS PubMed; (d) Non-linear least-squares curve fitting were carried out with the online software Bindfit. http://supramolecular.org Search PubMed.
- H. Shimizu, J. D. Cojal González, M. Hasegawa, T. Nishinaga, T. Haque, M. Takase, H. Otani, J. P. Rabe and M. Iyoda, Synthesis, Structures, and Photophysical Properties of π-Expanded Oligothiophene 8-mers and Their Saturn-Like C60 Complexes, J. Am. Chem. Soc., 2015, 137, 3877–3885 CrossRef CAS PubMed.
- T. Nishikawa, H. Narita, S. Ogi, Y. Sato and S. Yamaguchi, Hydrophobicity and CH/π-interaction-driven self-assembly of amphiphilic aromatic hydrocarbons into nanosheets, Chem. Commun., 2019, 55, 14950–14953 RSC.
- Z. Zhang, A. Kutana, Y. Yang, N. V. Krainyukova, E. S. Penev and B. I. Yakobson, Nanomechanics of carbon honeycomb cellular structures, Carbon, 2017, 113, 26–32 CrossRef CAS.
- Q. Zhang, X. Yang, P. Li, G. Huang, S. Feng, C. Shen, B. Han, X. Zhang, F. Jin, F. Xu and T. J. Lu, Bioinspired engineering of honeycomb structure – Using nature to inspire human innovation, Prog. Mater. Sci., 2015, 74, 332–400 CrossRef.
- D. Meng, G. Liu, C. Xiao, Y. Shi, L. Zhang, L. Jiang, K. K. Baldridge, Y. Li, J. S. Siegel and Z. Wang, Corannurylene Pentapetalae, J. Am. Chem. Soc., 2019, 141, 5402–5408 CrossRef CAS PubMed.
- (a) G. Portella, J. Poater and M. Solà, Assessment of Clar's aromatic π-sextet rule by means of PDI, NICS and HOMA indicators of local aromaticity, J. Phys. Org. Chem., 2005, 18, 785–791 CrossRef CAS; (b) D. Jan Cz, Three Queries about the HOMA Index, ACS Omega, 2019, 4, 18699–18710 CrossRef PubMed; (c) D. Jan Cz and O. Sławomir, HOMA Index Establishes Similarity to a Reference Molecule, J. Chem. Inf. Model., 2023, 63, 7744–7754 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2272202, 2272207, 2327360 and 2272225. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc05074b |
| This journal is © The Royal Society of Chemistry 2024 |