Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An un-forgotten classic: the nitro-Mannich reaction between nitrones and silyl nitronates catalysed by B(C6F5)3†
Michael G.
Guerzoni
 a,
Yara
van Ingen
a,
Rasool
Babaahmadi
a,
Yara
van Ingen
a,
Rasool
Babaahmadi
 a,
Thomas
Wirth
a,
Thomas
Wirth
 b,
Emma
Richards
b,
Emma
Richards
 *ab and
Rebecca L.
Melen
*ab and
Rebecca L.
Melen
 *a
*a
aCardiff Catalysis Institute, School of Chemistry, Cardiff University, Translational Research Hub, Maindy Road, Cathays, Cardiff, CF24 4HQ, Cymru/Wales, UK. E-mail: RichardsE10@cardiff.ac.uk; MelenR@cardiff.ac.uk
bSchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, Cymru/Wales, UK
First published on 12th January 2024
Abstract
Herein we report the B(C6F5)3-catalysed nitro-Mannich reaction between nitrones and silyl nitronates, affording silyl-protected α-nitro hydroxylamines with yields up to 99% and diastereoselectivities up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Crucially, the obtained products can be converted into 1,2-diamines under simple reductive conditions. This work provides a new orthogonal method to the existing routes for the instalment of a nitro moiety under Lewis acid catalysed conditions, and expands the state-of-the-art substrate scope with respect to the silyl nitronates.
1. Crucially, the obtained products can be converted into 1,2-diamines under simple reductive conditions. This work provides a new orthogonal method to the existing routes for the instalment of a nitro moiety under Lewis acid catalysed conditions, and expands the state-of-the-art substrate scope with respect to the silyl nitronates.
Introduction
Amongst all small molecule drug candidates approved from the US FDA until 2014, 84% of them possessed at least one nitrogen atom.1 The frequent occurrence of nitrogen-containing moieties in drug candidates necessitates continuous development of new methods to install them.2,3 Nitrogen in drug scaffolds can take on several different oxidation states (e.g. as an amine or as a nitro group), and may be present in an open chain or embedded in a heterocycle. Skeletal editing as an approach for the synthesis of nitrogen-containing heterocycles has recently gained traction,4–6 however, the classical nucleophilic addition to activated electrophiles remains the most viable and exploited method for aliphatic substrates. The Mannich reaction is a well-established method to synthesise α-functionalised amines starting from an imine (or iminium ion) and a suitable nucleophile, generally an enolate.7,8 A direct evolution from the Mannich reaction is the nitro-Mannich reaction (also known as Aza-Henry reaction), where the nucleophile is a nitronate, the “enol” form of a nitro group.9–11 The importance of the nitro-Mannich reaction lies in its utility to synthesise 1,2-nitroamine products, where two nitrogen atoms in different oxidation states are in close proximity (Scheme 1a, see Venetoclax). This motif can be leveraged for the selective manipulation of one of the two nitrogen centers,12–14 and provides an accessible platform to the 1,2-diamine functionality (Scheme 1a).9,15–19 Furthermore, despite the genotoxicity associated with the nitro group,20 the possibility to introduce it as a masked carbonyl (cf. Nef reaction21) makes it a valuable handle for the synthesis of drug candidate libraries.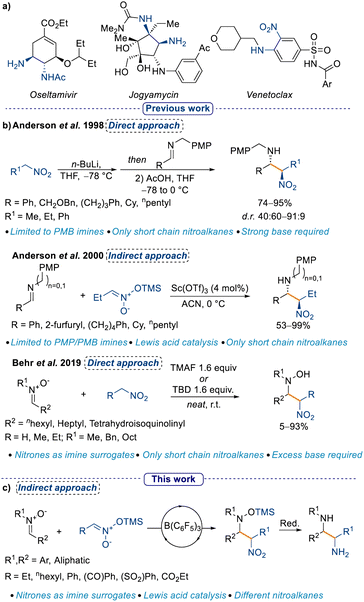 | ||
| Scheme 1 (a) 1,2-Diamine and 1,2-nitroamine containing drugs and natural products, (b) previous examples of the nitro-Mannich reaction, and (c) this work. | ||
However, the nitro-Mannich reaction possesses some intrinsic limitations such as the instability of some of the nitroamine products and the unfavorable thermodynamics associated with the addition of a nitronate anion to an imine without an acidic catalyst,10 and it has thus been defined as a “forgotten classic” of chemistry.9 In one of the earliest reports of the nitro-Mannich reaction in 1998 Anderson et al. described the use of stoichiometric amounts of n-BuLi in a direct approach to the synthesis of the 1,2-nitroamine moiety which, upon reduction with SmI2 affords the 1,2-diamine derivative in good yields and diastereoselectivities.10 Later, the same group developed the first Lewis acid catalysed indirect nitro-Mannich reaction with pre-formed silyl nitronates and PMB/PMP-protected imines (PMB = p-methoxybenzyl; PMP = p-methoxyphenyl; Scheme 1b).22 The authors noticed that the PMP protection was crucial for obtaining good diastereoselectivities, hence the reaction was limited to only a few imines. Moreover, the scope with respect to different silyl nitronates had not been explored. This protocol was further improved in 2005 using a Cu catalyst and a chiral ligand, which allowed the formation of nitroamines in yields up to 91% with almost full enantiocontrol (up to 94% ee).23 However, the scope was again limited to the simple 1-nitropropane. A direct approach for the nitro-Mannich reaction, that is, making the nitronate in situ, would be more appealing, yet the only reports of this used an excess of nitro alkane and it was restricted to specifically designed imines.24–28 A turning point occurred in 2019, when Behr et al. showed the possibility to use nitrones as imine surrogates, however, the substrate scope was limited to short chain nitro alkanes and required excess base, which in turn limited the functional group tolerability (Scheme 1b).29 In addition, a large excess of the nitro nucleophile was required, as the authorsobserved that the process was reversible and following formation of the first nitro-Mannich product, a retro-nitro-Mannich addition occured, redelivering the starting material. The use of nitrones as imine surrogates is a longstanding approach in a variety of transformations in organic chemistry.30 Moreover, nucleophilic addition to nitrones affords hydroxylamine products, which have recently been used as nitrogen radical precursors, which can increase the synthetic utility of the products.31 Additionally, the use of catalytic amounts of a Lewis acid instead of stoichiometric amounts of base is desirable in many regards, ranging from a higher functional group tolerance to less downstream waste. Finally, the possibility to use chiral Lewis acids can open up an avenue to establish an enantioselective synthesis of the 1,2-nitroamine products.32–35
Tris(pentafluorophenyl)borane [B(C6F5)3] is a well-established Lewis acid that has previously been shown to activate a wide range of substrates. Recently, we have shown that B(C6F5)3 can catalyse the Mukaiyama-Mannich addition of nitrones with silylenol diazo esters,36 and we envisioned that we could similarly employ it to catalyse the nitro-Mannich reaction, providing an orthogonal method to existing procedures. Based on this premise, we decided to expand the applicability of the nitro-Mannich reaction by using nitrones as imine surrogates, and by investigating different silyl nitronates under Lewis acid catalysis conditions. Crucially, our work would deliver silyl protected hydroxylamines, which in turn would be more stable to oxidation in comparison to the hydroxylamine products described in Behr's work. To the best of our knowledge, this is the first example of a Lewis acid catalysed indirect nitro-Mannich reaction using nitrones as imine surrogates.
Results and discussion
We began our investigation by reacting nitrone 1aa and silyl nitronate 2a in toluene at room temperature for 6 hours using 20 mol% of B(C6F5)3 (Table S1, see ESI† for full optimisation table). NMR analysis of the crude reaction mixture using 1 equivalent of 1,3,5-trimethoxybenzene as an internal standard showed clean formation of compound (±)-3 as mixture of diastereoisomers ((±)-3a and (±)-3a′) in 80% NMR spectroscopic yield and with a d.r. of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
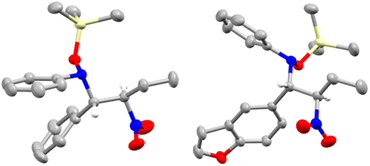
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. The diastereoisomers were separated, allowing the growth of crystals of the minor diastereoisomer suitable for single crystal X-ray diffraction, which revealed an (±)-(R,S) configuration of the two chiral centers (Fig. 1, left). This led us to assign by extension the absolute configuration of the major diastereoisomer as (±)-(R,R).
15. The diastereoisomers were separated, allowing the growth of crystals of the minor diastereoisomer suitable for single crystal X-ray diffraction, which revealed an (±)-(R,S) configuration of the two chiral centers (Fig. 1, left). This led us to assign by extension the absolute configuration of the major diastereoisomer as (±)-(R,R).
We then set out to optimise the conditions for the nitro-Mannich reaction giving the best conditions as: 1 equivalent of nitrone (1), 2 equivalents of silyl nitronate (2), 10 mol% of B(C6F5)3, dichloromethane as the solvent (0.1 M), at room temperature for 3 hours (Table 1).
| Entry | Variation from standard conditions | NMR yielda (%) | d.r.b (±)-(R,R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R,S) R,S) |
|---|---|---|---|
| a NMR yield calculated from the crude reaction mixture using 0.1 mmol of 1,3,5-trimethoxybenzene as an internal standard. b Diastereomeric ratio calculated from the 1H NMR of the crude reaction mixture. | |||
| 1 | None | 90 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 2 | BPh3 instead of B(C6F5)3 | n.r. | n.d. |
| 3 | BF3·Et2O instead of B(C6F5)3 | n.r. | n.d. |
| 4 | 1 equiv. of TFA | Decomp. | n.d. |
| 5 | B(C6F5)3 5 mol% | 71 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 6 | B(C6F5)3 20 mol%, 1.5 equiv. of 2a | 77 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 7 | B(C6F5)3 20 mol% at 0 °C | 79 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 8 | B(C6F5)3 20 mol% at –41 °C | 54 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 9 | B(C6F5)3 20 mol% at –78 °C | 44 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
Weaker Lewis acids such as BPh3 did not promote the reaction (Table 1, entry 2) presumably due to the inability to increase the electrophilicity of the carbon of the nitrone upon coordination with the Lewis basic oxygen atom. Surprisingly, Lewis acids of similar strength37,38 to B(C6F5)3 such as BF3·Et2O did not promote the reaction either (Table 1, entry 3), giving only unreacted starting material. Using Brønsted acids such as TFA (trifluoroacetic acid) instead afforded only decomposition products (Table 1, entry 4). Lowering the catalyst loading had a detrimental effect on the yield (Table 1, entry 5), similarly to when the equivalents of the silyl nitronate were decreased (Table 1, entry 6). We also attempted to improve the overall diastereoselectivity of the process by lowering the temperature, but unfortunately such improvement did not occur (Table 1, entries 7–9) (see the ESI† for the full optimisation table).
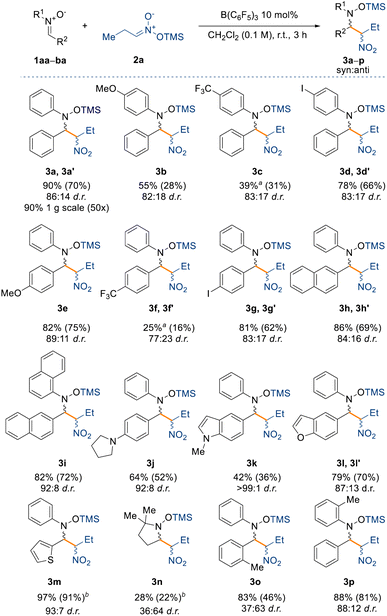
With the optimised reaction conditions in hand, we set out to explore the substrate scope for the reaction (Scheme 2). First, we assessed the effect of electron donating and withdrawing groups on the nitrone (1aa–ba, see ESI†) on the yield and diastereoselectivity of the reaction, whilst keeping the silyl nitronate as 2a. Electron donating groups (e.g. –OMe, –NR2) were well tolerated affording products 3b, 3e, and 3j in NMR spectroscopic yields between 55% and 82% and up to 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 d.r. For the methoxy-substituted product 3b the isolated yield was low (28%) merely due to the instability of the product, which readily decomposed.
11 d.r. For the methoxy-substituted product 3b the isolated yield was low (28%) merely due to the instability of the product, which readily decomposed.
On the other hand, electron withdrawing groups such as CF3 slowed the reaction down requiring longer reaction times (24 h) to obtain an appreciable amount of product 3c and 3f (39% and 25% NMR spectroscopic yields, respectively) albeit not changing the d.r. significantly (83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and 77
17 and 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23, respectively). Indeed, strongly deactivated nitrones 1ar (R1 = Ph; R2 = C6F5), 1aw (R1 = Ph; R2 = Py), or 1ay (R1 = tBu; R2 = pyridine 1-oxide) did not react at all under the optimised reaction conditions (see unsuccessful product list in the ESI†). Halogens in the para position, such as iodine, were well tolerated giving 3d (78% yield) and 3g (81% yield), making these compounds useful for possible further manipulation under cross-coupling conditions.39 Larger aromatic systems such as naphthyl did not have a negative impact on the yield of the products (3h and 3i), however, we observed an improved diastereoselectivity with compound 3i (92
23, respectively). Indeed, strongly deactivated nitrones 1ar (R1 = Ph; R2 = C6F5), 1aw (R1 = Ph; R2 = Py), or 1ay (R1 = tBu; R2 = pyridine 1-oxide) did not react at all under the optimised reaction conditions (see unsuccessful product list in the ESI†). Halogens in the para position, such as iodine, were well tolerated giving 3d (78% yield) and 3g (81% yield), making these compounds useful for possible further manipulation under cross-coupling conditions.39 Larger aromatic systems such as naphthyl did not have a negative impact on the yield of the products (3h and 3i), however, we observed an improved diastereoselectivity with compound 3i (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 d.r.), perhaps hinting at a π⋯π interaction between the catalyst and the nitrone being responsible for the diastereocontrol of the reaction. The reaction was also tolerant to heterocycles generating 3j–3m, which all gave improved diastereoselectivities relative to compound 3a. Indeed, for compound 3k the process reached almost full diastereocontrol (>99
8 d.r.), perhaps hinting at a π⋯π interaction between the catalyst and the nitrone being responsible for the diastereocontrol of the reaction. The reaction was also tolerant to heterocycles generating 3j–3m, which all gave improved diastereoselectivities relative to compound 3a. Indeed, for compound 3k the process reached almost full diastereocontrol (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.).
1 d.r.).
Next, we were interested in understanding the effect of aliphatic moieties on the nitrone. Nitrones bearing an aliphatic group on the β-position are usually unstable and hence are synthesised in situ and used immediately,40 however this produces a stoichiometric amount of water which could in turn poison our catalyst. For this reason, we synthesised nitrone 1au (R1 = Ph; R2 = Cy), which can be isolated but unfortunately the reaction did not occur, even under longer reaction times and higher temperatures (up to 40 °C). The same result was observed when we used nitrones bearing an aliphatic moiety on the α-position as in the case of 1as (R1 = Me; R2 = Ph) and 1at (R1 = tBu; R2 = Ph). Surprisingly, with benzyl protected nitrone 1av (R1 = Bn; R2 = Ph) the reaction did not occur either.
However, in the case of compound 3n, derived from the commercially available nitrone DMPO (5,5-dimethyl-1-pyrroline N-oxide), the reaction did occur, albeit in lower yield (28%) and with the opposite diastereoselectivity (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 d.r.). This result prompted us to further investigate the effect on the diastereoselectivity with sterically demanding groups on the nitrone. To this end, increasing the steric bulk around the electrophilic carbon instead shuts down the reactivity completely, as observed for penta-methyl substituted 1aq (R1 = Ph; R2 = C6Me5) and 1ba (R1 = Ph; R2 = 2-BrC6H4). Interestingly, we observed that an o-tolyl group at R2 of the nitrone also inverted the diastereoselectivity in the product (3o) (vide infra) and drastically decreased it (36
64 d.r.). This result prompted us to further investigate the effect on the diastereoselectivity with sterically demanding groups on the nitrone. To this end, increasing the steric bulk around the electrophilic carbon instead shuts down the reactivity completely, as observed for penta-methyl substituted 1aq (R1 = Ph; R2 = C6Me5) and 1ba (R1 = Ph; R2 = 2-BrC6H4). Interestingly, we observed that an o-tolyl group at R2 of the nitrone also inverted the diastereoselectivity in the product (3o) (vide infra) and drastically decreased it (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63), but this was not the case in the formation of product 3p where the o-tolyl group was at R1 position of the nitrone. We propose that the reversal of diastereoselectivity observed for compound 3o might be controlled by the large steric demand of the borane catalyst.
63), but this was not the case in the formation of product 3p where the o-tolyl group was at R1 position of the nitrone. We propose that the reversal of diastereoselectivity observed for compound 3o might be controlled by the large steric demand of the borane catalyst.
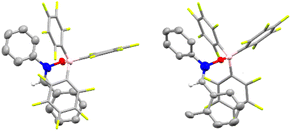
To gain further insight into this process, we undertook DFT calculations to explore the transition state structures. As expected, the reactions between 1aa (R1 = R2 = Ph) and 1ao (R1 = Ph, R2 = o-tol) with B(C6F5)3 lead to very stable adducts, in which the Lewis acid catalyst is bound to the Lewis basic oxygen of the nitrone, thus increasing the electrophilicity of the carbon and thereby facilitating the subsequent nucleophilic addition. Indeed, the reaction of equimolar amounts of B(C6F5)3 and nitrone 1aa or 1ao in dichloromethane led to the formation of 1aa·B(C6F5)3 and 1ao·B(C6F5)3. Slow evaporation of the reaction mixture led to the formation of crystals suitable for single crystal X-ray diffraction analysis (Fig. 2). In the subsequent transition state detailing the reaction between the adduct and the nitronate, two possible conformations arise which would subsequently lead to the observed syn (or (±)-(R,R)) and anti (or (±)-(R,S)) products (Scheme 3). Consistent with the experimental results, the transition state TS-3a is 5.3 kcal mol−1 lower in energy than TS-3a′, accounting for the preferential formation of the syn product over the anti with 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 d.r. The corresponding calculations for 3o still indicated a slight preference for the syn isomer over the anti, albeit with a much smaller energy gap between TS-3o and TS-3o′ (1.2 kcal mol−1).
14 d.r. The corresponding calculations for 3o still indicated a slight preference for the syn isomer over the anti, albeit with a much smaller energy gap between TS-3o and TS-3o′ (1.2 kcal mol−1).
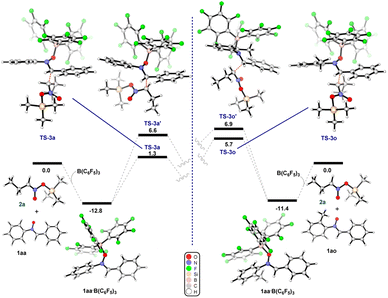 | ||
| Scheme 3 (Left) Energy diagram for the formation of product 3a. (Right) Energy diagram for the formation of product 3o. The relative free energies obtained from SMD/BP86-D3/def2-TZVP//BP86/6-31G* in dichloromethane (see ESI† for full details). Energies are given in kcal mol−1. | ||
Hence, whilst this computational result does not fully account for the opposite diastereoselectivity, within computational error it clearly indicates that the presence of an o-CH3 group on the nitrone greatly affects the stability of the conformers in the TS, and this agrees with the reduced diastereoselectivity for compound 3o (37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 d.r.). Further studies into the effects governing the diastereoselectivity of reactions catalysed by sterically encumbered Lewis acids are still ongoing in our laboratory.
63 d.r.). Further studies into the effects governing the diastereoselectivity of reactions catalysed by sterically encumbered Lewis acids are still ongoing in our laboratory.
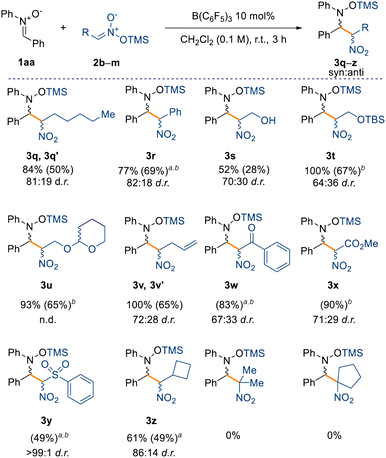
Finally, we have also observed that the reaction is scalable, as evidenced from the synthesis of compound 3a, obtained in 90% isolated yield on a gram scale. Having assessed a variety of nitrones for the nitro-Mannich reaction, we then explored the substrate scope with respect to different silyl nitronates (2b–m) with nitrone 1aa (Scheme 4). Increasing the chain length of the silyl nitronate did not alter the reactivity nor the diastereoselectivity as 3q was obtained in 84% NMR yield and in 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 d.r. Nitromethyl aromatics are also amenable to the B(C6F5)3-catalysed nitro-Mannich reaction, as observed for compound 3r (77% yield, 82
19 d.r. Nitromethyl aromatics are also amenable to the B(C6F5)3-catalysed nitro-Mannich reaction, as observed for compound 3r (77% yield, 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 d.r.). Starting from the TMS (TMS = trimethylsilyl) protected commercially available 2-nitroethanol (2d), we successfully synthesised the alcohol 3s in 52% NMR spectroscopic yield. In this case, partial cleavage of the TMS group occurred during purification on silica, and only the major isomer of 3s was obtained in an unsatisfactory 28% isolated yield. Consequently, we repeated the reaction with a more acid-stable protecting group to better validate the protocol. Pleasingly, the reaction proceeded smoothly affording the –OTBS (TBS = tert-butyldimethylsilyl) and –OTHP (THP = 2-tetrahydropyranyl) protected nitro groups, 3t and 3u respectively, in 67% and 65% isolated yield.
18 d.r.). Starting from the TMS (TMS = trimethylsilyl) protected commercially available 2-nitroethanol (2d), we successfully synthesised the alcohol 3s in 52% NMR spectroscopic yield. In this case, partial cleavage of the TMS group occurred during purification on silica, and only the major isomer of 3s was obtained in an unsatisfactory 28% isolated yield. Consequently, we repeated the reaction with a more acid-stable protecting group to better validate the protocol. Pleasingly, the reaction proceeded smoothly affording the –OTBS (TBS = tert-butyldimethylsilyl) and –OTHP (THP = 2-tetrahydropyranyl) protected nitro groups, 3t and 3u respectively, in 67% and 65% isolated yield.
Moreover, the reaction tolerates unsaturated moieties at peripheral positions of the silyl nitronate, as evidenced by compound 3v obtained in quantitative NMR spectroscopic yield. This substrate could potentially engage in a Heck-type coupling in a downstream process. Crucially, we also obtained the α-nitro ketone, ester, and sulfone derivatives (3w–3y) in very good yields (up to 90% isolated yield) and moderate diastereoselectivities (up to 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29). Interestingly, sulfone 3y was obtained without any detectable formation of the minor diastereoisomer. The synthesis of cyclobutyl compound 3z in moderate NMR spectroscopic yields and good diastereoselectivity (61% yield and 86
29). Interestingly, sulfone 3y was obtained without any detectable formation of the minor diastereoisomer. The synthesis of cyclobutyl compound 3z in moderate NMR spectroscopic yields and good diastereoselectivity (61% yield and 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
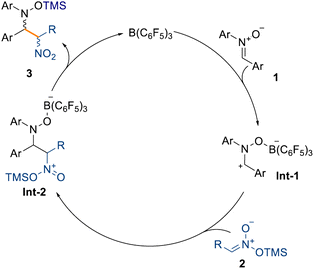
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 d.r.) showed that sp3-rich fragments can also be incorporated. The formation of a quaternary carbon center, starting from silyl nitronates 2l and 2m (see ESI†), prevents any reactivity and is the main limitation in this scope. Based on these results and the computational details highlighted in Scheme 3, we propose that the mechanism takes place through initial coordination between the catalyst B(C6F5)3 and the prochiral nitrone 1, affording the zwitterionic intermediate Int-1 (Scheme 5). This possesses enhanced electrophilicity at the α-carbon and can then undergo nucleophilic addition with the silyl nitronate 2, affording Int-2. Lastly, intramolecular silyl migration occurs affording product 3 and reinstating the active catalyst.
14 d.r.) showed that sp3-rich fragments can also be incorporated. The formation of a quaternary carbon center, starting from silyl nitronates 2l and 2m (see ESI†), prevents any reactivity and is the main limitation in this scope. Based on these results and the computational details highlighted in Scheme 3, we propose that the mechanism takes place through initial coordination between the catalyst B(C6F5)3 and the prochiral nitrone 1, affording the zwitterionic intermediate Int-1 (Scheme 5). This possesses enhanced electrophilicity at the α-carbon and can then undergo nucleophilic addition with the silyl nitronate 2, affording Int-2. Lastly, intramolecular silyl migration occurs affording product 3 and reinstating the active catalyst.
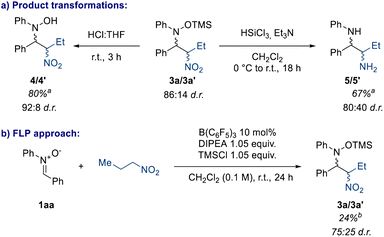
The nitro group possesses an ambiguous position in medicinal chemistry: although it has been proven to have increased genotoxicity and mutagenicity properties,20 its synthetic utility is undeniable. The most well-known reactions of the nitro group are the Nef reaction which converts the NO2 group to a carbonyl functionality, and NO2 reduction to the corresponding amine under several conditions, such as the Béchamp reaction.12,13,41,42 Therefore, we screened the product 3a towards different conditions to transform the NO2 group to other useful functional groups (Scheme 6a). Firstly, we observed that under very mild conditions the TMS group can be cleaved affording the corresponding nitro hydroxylamine (4/4′) in quantitative yield (80% isolated yield) (Scheme 6a).433a can also be reduced to the corresponding 1,2-diamine (5/5′) and the product can be obtained by a simple aqueous work-up, showing that this methodology could be a useful way to make unsymmetrical 1,2-diamines.
In this way we show that the nitrones employed in this work are readily accessible imine surrogates, which provides an alternative method to the classic nitro-Mannich reaction. Unfortunately, attempts at the Nef reaction under different conditions, such as acidic, reducing, and oxidative were unsuccessful.44 We propose that the presence of a nucleophilic group in close proximity to the nitronate intermediate interrupts the Nef reaction affording several side products.45
Finally, we were interested in a direct nitro-Mannich reaction, where the silyl nitronate starting material 2 is synthesised in situ, as the instability of these compounds pose a limitation to the work. With this in mind, we sought to apply the concept of cooperative catalysis derived from Frustrated Lewis Pair (FLP) chemistry.46,47 By mixing nitrone 1aa, 1-nitropropane, B(C6F5)3 and a suitable base we could in principle activate the nitrone with the Lewis acid, and form the nitronate with the base, whilst preventing acid–base inhibition due to the sterics of the catalysts (cf. FLP). In this case the FLP system chosen was B(C6F5)3/DIPEA (DIPEA = N,N-diisopropylethylamine). Pleasingly, preliminary results show that this protocol is possible, generating 3a in 24% yield, and this is currently under exploration in our lab (Scheme 4b).
Conclusions
In conclusion, this work highlights the ability of B(C6F5)3 to catalyse the nitro-Mannich reaction between nitrones and silyl nitronates in good to high yields (up to 91%) and with good control over the diastereoselectivity (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 10 mol% catalyst loading and under very mild conditions. The reaction is also scalable up to 50 times without any loss in reactivity or diastereoselectivity. The substrate scope (26 examples) shows a high degree of variability of both the nitrone and the silyl nitronate, rendering this protocol amenable to the synthesis of a wide range of products. Crucially, this methodology allows the formation of silyl-protected α-nitro hydroxylamines, which can be easily converted into the corresponding 1,2-diamine or hydroxylamine in high yields. This approach also overcomes previous limitations with the use of imines for the classic nitro-Mannich reaction by replacing them with nitrones. Moreover, it significantly expands the scope of silyl nitronates, which has never been applied to this transformation before. Finally, we have shown that by leveraging the concept of FLPs in cooperative catalysis, we can carry out the direct nitro-Mannich of which we will describe our future endeavours in due course.
1) with 10 mol% catalyst loading and under very mild conditions. The reaction is also scalable up to 50 times without any loss in reactivity or diastereoselectivity. The substrate scope (26 examples) shows a high degree of variability of both the nitrone and the silyl nitronate, rendering this protocol amenable to the synthesis of a wide range of products. Crucially, this methodology allows the formation of silyl-protected α-nitro hydroxylamines, which can be easily converted into the corresponding 1,2-diamine or hydroxylamine in high yields. This approach also overcomes previous limitations with the use of imines for the classic nitro-Mannich reaction by replacing them with nitrones. Moreover, it significantly expands the scope of silyl nitronates, which has never been applied to this transformation before. Finally, we have shown that by leveraging the concept of FLPs in cooperative catalysis, we can carry out the direct nitro-Mannich of which we will describe our future endeavours in due course.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
Conceptualisation: M. G. G.; data curation: M. G. G.; formal analysis: M. G. G.; funding acquisition: T. W., E. R., R. L. M.; investigation: M. G. G.; methodology: M. G. G.; crystal structure analysis: Y. v. I.; DFT calculations: R. B.; writing – original draft: M. G. G.; writing – review and editing: all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. G. G., E. R., and R. L. M. would like to thank the Leverhulme Trust (grant number: RPG-2020-016) for funding. R. B, T. W. and R. L. M. would like to thank the Royal Society for an International Newton Fellowship (NIF/R1/21130) and the support of the Supercomputing Wales project, which is part-funded by the European Regional Development Fund (ERDF).Notes and references
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS.
- L. D. Pennington, P. N. Collier and E. Comer, Med. Chem. Res., 2023, 32, 1278–1293 CrossRef CAS.
- L. D. Pennington and D. T. Moustakas, J. Med. Chem., 2017, 60, 3552–3579 CrossRef CAS.
- B. W. Joynson and L. T. Ball, Helv. Chim. Acta, 2023, 106, e202200182 CrossRef CAS.
- J. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein and B. Morandi, Science, 2022, 377, 1104–1109 CrossRef CAS PubMed.
- J. Wang, H. Lu, Y. He, C. Jing and H. Wei, J. Am. Chem. Soc., 2022, 144, 22433–22439 CrossRef CAS PubMed.
- F. F. Blicke, Org. React., 1991, 10, 303–341 Search PubMed.
- M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044–1070 CrossRef.
- A. Noble and J. C. Anderson, Chem. Rev., 2013, 113, 2887–2939 CrossRef CAS PubMed.
- H. Adams, J. C. Anderson, S. Peace and A. M. K. Pennell, J. Org. Chem., 1998, 63, 9932–9934 CrossRef CAS.
- A. M. Faisca Phillips, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Front. Chem., 2020, 8, 1–27 CrossRef.
- L. Zhao, C. Hu, X. Cong, G. Deng, L. L. Liu, M. Luo and X. Zeng, J. Am. Chem. Soc., 2021, 143, 1618–1629 CrossRef CAS PubMed.
- M. Orlandi, F. Tosi, M. Bonsignore and M. Benaglia, Org. Lett., 2015, 17, 3941–3943 CrossRef CAS.
- M. Orlandi, M. Benaglia, F. Tosi, R. Annunziata and F. Cozzi, J. Org. Chem., 2016, 81, 3037–3041 CrossRef CAS PubMed.
- Z. Lu, J. Wang, B. Han, S. Li, Y. Zhou and B. Fan, Adv. Synth. Catal., 2015, 357, 3121–3125 CrossRef CAS.
- E. Bogatcheva, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, F. Barbosa, L. Einck, C. A. Nacy and M. Protopopova, J. Med. Chem., 2006, 49, 3045–3048 CrossRef CAS PubMed.
- C. Y. Cai, X. M. Shu and H. C. Xu, Nat. Commun., 2019, 10, 1–7 CrossRef.
- D. Lucet, T. Le Gall and C. Mioskowski, Angew. Chem., Int. Ed., 1998, 37, 2580–2627 CrossRef CAS.
- G. Tan, M. Das, R. Kleinmans, F. Katzenburg, C. Daniliuc and F. Glorius, Nat. Catal., 2022, 5, 1120–1130 CrossRef CAS.
- K. Nepali, H. Y. Lee and J. P. Liou, J. Med. Chem., 2019, 62, 2851–2893 CrossRef CAS PubMed.
- W. E. Noland, Chem. Rev., 1955, 55, 137–155 CrossRef CAS.
- J. C. Anderson, S. Peace and S. Pih, Synlett, 2000, 850–852 CAS.
- J. C. Anderson, G. P. Howell, R. M. Lawrence and C. S. Wilson, J. Org. Chem., 2005, 70, 5665–5670 CrossRef CAS PubMed.
- G. Blay, A. Escamilla, V. Hernandez-Olmos, J. R. Pedro and A. Sanz-Marco, Chirality, 2012, 24, 441–450 CrossRef CAS.
- C. Palomo, M. Oiarbide, R. Halder, A. Laso and R. López, Angew. Chem., Int. Ed., 2005, 45, 117–120 CrossRef PubMed.
- B. M. Trost and D. W. Lupton, Org. Lett., 2007, 9, 2023–2026 CrossRef CAS PubMed.
- C. J. Wang, X. Q. Dong, Z. H. Zhang, Z. Y. Xue and H. L. Teng, J. Am. Chem. Soc., 2008, 130, 8606–8607 CrossRef CAS PubMed.
- L. Zhang, H. Wu, S. Su and S. Wang, Chin. J. Chem., 2009, 27, 2061–2065 CrossRef CAS.
- G. Messire, F. Massicot, A. Vallée, J. L. Vasse and J. B. Behr, Eur. J. Org Chem., 2019, 1659–1668 CrossRef CAS.
- M. Lombardo and C. Trombini, Synthesis, 2000, 759–774 CrossRef CAS.
- J. Davies, S. P. Morcillo, J. J. Douglas and D. Leonori, Chem.–Eur. J., 2018, 24, 12154–12163 CrossRef CAS.
- W. Meng, X. Feng and H. Du, Chin. J. Chem., 2020, 38, 625–634 CrossRef CAS.
- X. Li, J. J. Tian, N. Liu, X. S. Tu, N. N. Zeng and X. C. Wang, Angew. Chem., Int. Ed., 2019, 58, 4664–4668 CrossRef CAS PubMed.
- Y. Liu and H. Du, J. Am. Chem. Soc., 2013, 135, 6810–6813 CrossRef CAS PubMed.
- X. S. Tu, N. N. Zeng, R. Y. Li, Y. Q. Zhao, D. Z. Xie, Q. Peng and X. C. Wang, Angew. Chem., Int. Ed., 2018, 57, 15096–15100 CrossRef CAS PubMed.
- K. Stefkova, M. G. Guerzoni, Y. Van Ingen, E. Richards and R. L. Melen, Org. Lett., 2023, 25, 500–505 CrossRef CAS PubMed.
- P. Erdmann, J. Leitner, J. Schwarz and L. Greb, ChemPhysChem, 2020, 21, 987–994 CrossRef CAS.
- J. R. Gaffen, J. N. Bentley, L. C. Torres, C. Chu, T. Baumgartner and C. B. Caputo, Chem, 2019, 5, 1567–1583 CAS.
- B. P. Fors, N. R. Davis and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 5766–5768 CrossRef CAS PubMed.
- S. R. Sandler and W. Karo, in Organic Functional Group Preparations, ed. S. R. Sandler and W. Karo, Academic Press, 2nd edn, 1989, pp. 351–376 Search PubMed.
- H. Lu, Z. Geng, J. Li, D. Zou, Y. Wu and Y. Wu, Org. Lett., 2016, 18, 2774–2776 CrossRef CAS.
- X. Li, R. R. Thakore, B. S. Takale, F. Gallou and B. H. Lipshutz, Org. Lett., 2021, 23, 8114–8118 CrossRef CAS PubMed.
- D. W. Nelson, J. Owens and D. Hiraldo, J. Org. Chem., 2001, 66, 2572–2582 CrossRef CAS PubMed.
- S. Umemiya, K. Nishino, I. Sato and Y. Hayashi, Chem.–Eur. J., 2014, 20, 15753–15759 CrossRef CAS PubMed.
- A. Y. Sukhorukov, Molecules, 2023, 28, 686 CrossRef CAS PubMed.
- M. G. Guerzoni, A. Dasgupta, E. Richards and R. L. Melen, Chem Catal., 2022, 2, 2865–2875 CrossRef CAS.
- G. C. Welch, R. R. San Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124–1126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, crystallographic data, DFT data. CCDC 2301585–2301589. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc05672d |
| This journal is © The Royal Society of Chemistry 2024 |