 Open Access Article
Open Access ArticleHybrid and composite materials of organic crystals
Xuesong
Yang
a,
Marieh B.
Al-Handawi
b,
Liang
Li
bc,
Panče
Naumov
 *bdef and
Hongyu
Zhang
*bdef and
Hongyu
Zhang
 *a
*a
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: hongyuzhang@jlu.edu.cn
bSmart Materials Lab, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, UAE. E-mail: pance.naumov@nyu.edu
cDepartment of Sciences and Engineering, Sorbonne University Abu Dhabi, PO Box 38044, Abu Dhabi, UAE
dCenter for Smart Engineering Materials, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, UAE
eResearch Center for Environment and Materials, Macedonian Academy of Sciences and Arts, Bul. Krste Misirkov 2, MK-1000 Skopje, Macedonia
fMolecular Design Institute, Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003, USA
First published on 8th January 2024
Abstract
Organic molecular crystals have historically been viewed as delicate and fragile materials. However, recent studies have revealed that many organic crystals, especially those with high aspect ratios, can display significant flexibility, elasticity, and shape adaptability. The discovery of mechanical compliance in organic crystals has recently enabled their integration with responsive polymers and other components to create novel hybrid and composite materials. These hybrids exhibit unique structure–property relationships and synergistic effects that not only combine, but occasionally also enhance the advantages of the constituent crystals and polymers. Such organic crystal composites rapidly emerge as a promising new class of materials for diverse applications in optics, electronics, sensing, soft robotics, and beyond. While specific, mostly practical challenges remain regarding scalability and manufacturability, being endowed with both structurally ordered and disordered components, the crystal–polymer composite materials set a hitherto unexplored yet very promising platform for the next-generation adaptive devices. This Perspective provides an in-depth analysis of the state-of-the-art in design strategies, dynamic properties and applications of hybrid and composite materials centered on organic crystals. It addresses the current challenges and provides a future outlook on this emerging class of multifunctional, stimuli-responsive, and mechanically robust class of materials.
1. Introduction
Organic molecular crystals, composed of discrete molecules arranged in periodic, symmetrical structures, have been traditionally viewed as fragile materials that are unsuitable for applications requiring mechanical stability.1–3 However, recent studies have challenged this notion by uncovering remarkable dynamic properties of certain organic crystals, including elasticity, plasticity, stimuli-responsiveness, and even stress-induced luminescence.4–7 Unlike conventional brittle molecular solids, these dynamic and, oftentimes, mechanically compliant organic crystals can undergo reversible mechanical deformations such as bending, twisting, and coiling, oftentimes without significant loss of their crystallinity.8,9 The realization that mechanical flexibility and stimuli-induced adaptability emerge from (relatively weak) intermolecular interactions could have profound implications for their versatility and scope of applications.10,11 The ability of these dynamic organic crystals to change shape responsively makes them attractive as components in designing soft, deformable hybrid materials.12–14 When coupled with some intrinsic properties like low density, optical transparency, and diverse electronic properties, the newly uncovered mechanical compliance of organic crystals offers a tremendous yet at present, underexplored potential for next-generation smart, stimuli-responsive materials.15,16 Overcoming the challenges related to their small crystal size, slow responsiveness, and poor processability may be critical to be able to harness their potential for direct applications.17,18 In response to this challenge, researchers have initiated endeavors aimed at synthesizing hybrid materials possessing photoresponsive attributes. This involves the amalgamation of photomechanical organic nanocrystals with both polymers and inorganic substances. Optical regulators based on molecular crystals exhibit immense promise, particularly in domains like artificial musculature and the realm of soft robotics.19–24 Building upon prior research, endeavors have been pursued to broaden the array of responsive characteristics and application scopes of organic crystals. This has motivated ample efforts aimed at integrating mechanically dynamic organic crystals with polymers, metals, two-dimensional (2D) materials, and other components into multifunctional composites that have synergistically enhanced materials properties.25,26 Aimed to capitalize on the unique properties of organic crystals, while mitigating their limitations, the current efforts are geared towards exploring the structure-dynamic functions of these hybrid materials that are unavailable with the individual constituents. Based on some early successes, it is concluded that the organic crystal-based composite platform holds enormous promise as the foundational architecture for future technologies ranging from soft robotics, to sensors, to flexible electronics, and beyond.12,25,27 Realizing this vision demands a deeper understanding of the relationships between the structure and the dynamics that determines the macroscale behaviour of hybrid organic crystals. This Perspective summarizes the recent advances in the burgeoning research direction of organic crystal hybrid and composite materials. It discusses the innovative materials design strategies, their unique multifunctional properties, and the recent efforts to deploy these composites to diverse application spaces. The outlook and current challenges for further development of organic crystal hybrids are also presented. This timely overview matches the transition from fundamental discoveries of these materials to their prototypical testing in materials engineering and technological applications.2. Evolution of organic crystals: from fragility to flexibility
2.1 Fundamentals of organic molecular crystals
Organic molecular crystals comprise discrete molecules held together by non-covalent intermolecular interactions such as hydrogen bonding, π–π stacking, van der Waals interactions, and dipolar and quadrupolar forces.28,29 The crystalline lattice exhibits long-range translational and orientational order, giving rise to anisotropic physical properties.30 Small-molecule crystals represent the most widely studied category, with constituents that include aromatic hydrocarbons, heterocycles, π-conjugated architectures, and pharmaceutical compounds.31 While traditionally perceived as fragile materials, certain organic molecular crystals are now known to exhibit remarkable latent mechanical compliance and adaptability to external stimuli.32–372.2 Fragility versus latent dynamic properties
Since the earliest studies on crystalline organic compounds, organic crystals have been predominantly perceived as delicate, fragile solids.38,39 The purported mechanical fragility arises from the weak intermolecular forces, such as van der Waals, π–π and CH–π interactions, that act between the isolated molecular entities and hold together the discrete molecular components within the crystal lattice.2,28 In single crystals, these weak non-covalent interactions are oriented anisotropically in one, two, or three dimensions (1D, 2D or 3D), thereby turning certain crystallographic directions weaker compared to inorganic crystalline solids like metals.40 Consequently, organic molecular crystals were deemed mechanically rather unstable and prone to fracture when subjected to stresses.41 However, over the past decade, pioneering studies have overturned this notion for certain classes of organic crystals by uncovering their remarkable mechanical and dynamic properties, including elasticity, plasticity, stimuli-responsiveness and “mechanofluorochromic” luminescence.42–44 The perception of organic crystals as intrinsically fragile materials has been transformed drastically, and this has motived attempts to explain these exotic properties by analysis of their crystal structures (Fig. 1).11–13,45–57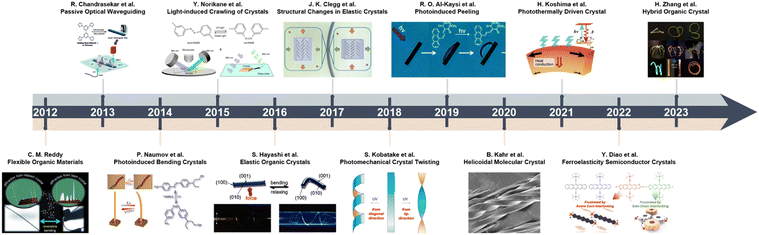 | ||
| Fig. 1 Time-line and milestones in the research on the mechanical properties and effects of molecular crystals, and evolution of the crystal adaptronics. Reproduced with permission from ref. 12, Copyright 2022 Spring Nature. Reproduced with permission from ref. 13, Copyright 2022 Wiley-VCH. Reproduced with permission from ref. 14, Copyright 2022 Wiley-VCH. Reproduced with permission from ref. 46, Copyright 2012 Wiley-VCH. Reproduced with permission from ref. 47, Copyright 2013 Royal Society of Chemistry. Reproduced with permission from ref. 48, Copyright 2014 American Chemical Society. Reproduced with permission from ref. 49, Copyright 2015 Spring Nature. Reproduced with permission from ref. 50, Copyright 2016 Wiley-VCH. Reproduced with permission from ref. 51, Copyright 2017 Spring Nature. Reproduced with permission from ref. 52, Copyright 2018 American Chemical Society. Reproduced with permission from ref. 53, Copyright 2019 Royal Society of Chemistry. Reproduced with permission from ref. 54, Copyright 2020 Wiley-VCH. Reproduced with permission from ref. 55, Copyright 2021 American Chemical Society. Reproduced with permission from ref. 56, Copyright 2023 American Chemical Society. Reproduced with permission from ref. 57, Copyright 2023 Wiley-VCH. | ||
2.3 Milestones in establishing the organic crystals' compliance
Although elastic crystals have been reported before,58–63 one of the notable reports of mechanical compliance of organic crystals was that of Reddy et al. in 2012 who showed that the molecular crystal structure of caffeine cocrystal can be elastically bent and unbent (Fig. 2a).46 Building on this finding, Hayashi et al. in 2016 demonstrated that π-conjugated organic crystals could also exhibit elastic mechanical properties, attributed to slipped π-stacking between the molecules (Fig. 2b).50 Clegg et al. in 2017 reported acicular crystals of bis(acetylacetonato)copper(II), ([Cu(acac)2]) (“acac” stands for acetylacetonate). By employing microfocus X-ray diffraction, they mapped the structural alterations that take place during the bending of the crystal, and provided direct evidence for the mechanism responsible for crystal's elasticity (Fig. 2c).51 Besides elastic bending, certain organic crystals were found to display plastic deformation, including bending, twisting, and coiling, properties that were attributed to molecular slip planes within their crystal structures.64–67 For instance, Norikane et al. in 2015 showed that irradiation with ultraviolet (UV) light could induce directional “crawling” of azobenzenes crystals on glass surfaces resulting from a solid-state photochemical reaction (Fig. 2d).47 Furthermore, the group of Naumov have extensively studied stimuli-responsive dynamic crystals that are capable of splintering, fragmentation, explosion, jumping, and bending (Fig. 2e).68,69 Recently, Chandrasekar's group noted an anomalous pseudoplastic behavior in waveguide microcrystals on silica surfaces during atomic force microscopy (AFM) manipulation. These crystals, previously classified as elastic, showcased an unexpected attribute due to the prevalence of adhesive over restorative forces upon the substrate. Leveraging this mechanical micromanipulation (termed mechanophotonics), the group proceeded to craft a range of organic crystal-based devices, such as resonators, interferometers, and others.70–77 These latter demonstrations utilized an AFM cantilever tip for mechanical micromanipulation of flexible microcrystalline waveguides (Fig. 2f). In addition, Zhang et al. pioneered the development of the flexible optical waveguide incorporating organic crystals.78 This breakthrough was further augmented by their demonstration of ultra-low temperature bending capabilities of flexible organic crystals, alongside additional properties such as their ability to act as polarization rotators (Fig. 2g–i).79,80 Simultaneously, Zhao et al. has detailed the creation of a suite of micro-optical waveguide and micro-ring resonator structures via solution-processing fabrication techniques. This mode of optical signal transmission boasts a notably low optical loss coefficient, foreseeably finding application in information recording lasers, integrated circuits, and related domains(Fig. 2j–l).81–83 The selected seminal works highlighted above have been pivotal in establishing that, contrary to traditional perceptions, organic molecular crystals can demonstrate remarkable latent dynamic properties that are unmatched by other materials.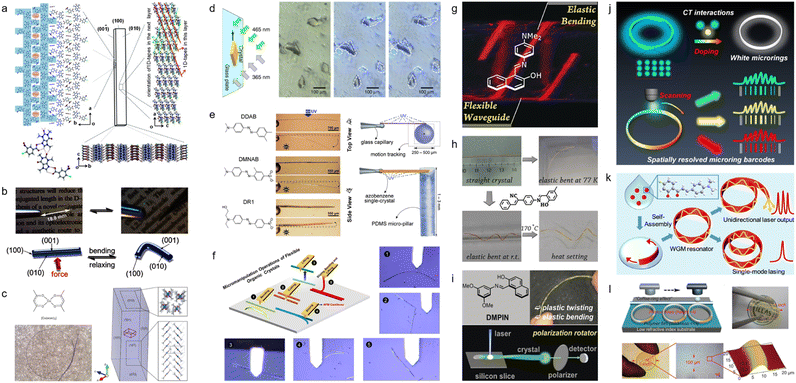 | ||
| Fig. 2 Research on flexible organic crystals. (a) Illustration of the crystal arrangement in an elastically bendable cocrystal. (a) Reproduced with permission from ref. 46, Copyright 2012 Wiley-VCH. (b) Images under both daylight and UV light, showing a single crystal of 1,4-bis[2-(4-methylthienyl)]-2,3,5,6-tetrafluorobenzene, notable for its remarkable elasticity. (b) Reproduced with permission from ref. 50, Copyright 2016 Wiley-VCH. (c) Depicts the chemical structure of [Cu(acac)2], an image of a sharply bent crystal, and its crystal structure. (c) Reproduced with permission from ref. 51, Copyright 2017 Spring Nature. (d) Diagram and optical micrographs showing 3,3′-dimethylazobenzene crystals moving vertically when exposed to ultraviolet (UV) light (365 nm) and visible light (VIS) (465 nm). Dashed lines mark the crystals' initial positions. (d) Reproduced with permission from ref. 49, Copyright 2015 Spring Nature. (e) Photoreactive behaviour of azobenzene crystals, which exert force on PDMS pillars during bending, for force measurement. (e) Reproduced with permission from ref. 68 under the terms of the CC-BY license. (f) Schematic and optical microscopic views of five micromechanical processes conducted using an atomic force microscopy (AFM) tip on a flexible crystal. (f) Reproduced with permission from ref. 72, Copyright 2020 Wiley-VCH. (g) Image of flexible optical waveguides made from organic crystals. (g) Reproduced with permission from ref. 78, Copyright 2018 Wiley-VCH. (h) Image of flexible organic crystals bent at −196 °C. (h) Reproduced with permission from ref. 79, Copyright 2020 Wiley-VCH. (i) Flexible organic single crystal having a polarization-rotation functionality. (i) Reproduced with permission from ref. 80, Copyright 2019 Wiley-VCH. (j) Doping for the creation of diverse fluorescent whispering-gallery-mode (WGM) hetero-microrings. (j) Reproduced with permission from ref. 81, Copyright 2023 Wiley-VCH. (k) WGB Lasers utilizing self-assembled organic single-crystalline microrings. (k) Reproduced with permission from ref. 82, Copyright 2019 American Chemical Society. (l) Development and production of a wafer-scale organic printed photonic chip. (l) Reproduced with permission from ref. 83 under the terms of the CC-BY license. | ||
3. Design strategies for crystal–polymer composites
Recently, there has been an effort among researchers to fabricate expansive arrays of organic micro-lasers on flexible polymer substrates. These innovations hold promise in serving as versatile mechanical sensors and exhibit substantial potential for applications in artificial skin technology.84–86 Capitalizing on the dynamic mechanical properties of responsive organic crystals, researchers have very recently proposed various design strategies to integrate these materials into multifunctional hybrids and composites. The overarching motivation is the intention to combine the desirable properties of organic crystals with those of other materials having different properties into synergistic hybrids for applications that require flexible and stimuli-responsive components. One such strategy entails integration of organic crystals with stimuli-responsive polymers that can impart motility and responsiveness.87–90 Being an intrinsically responsive macromolecules that can translate molecular-level changes in their structures into macroscopic movements, such polymers serve as ideal partners that are complementary in function to the (static) organic crystals.12,13 For instance, coating organic crystals with humidity-responsive polymers introduces capability of humidity-driven actuation, while temperature-responsive polymers impart a capability for thermally activated reshaping.27 The polymer components within these hybrids undergo reversible changes in conformation, solubility or swelling in response to stimuli, thereby transducing stresses or strains to mechanically deform physically coupled organic crystals in a dynamic and possibly reversible manner.12,13,25,26 This section details various materials integration strategies that have been devised to fabricate hybrid and composite materials of organic crystals (Fig. 3).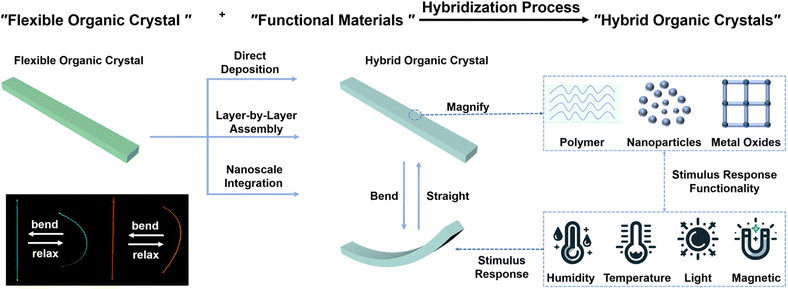 | ||
| Fig. 3 Preparation process, deposition method, classification, functionality, and other details pertinent to the hybrid organic–polymer materials. | ||
3.1 Direct deposition of stimuli-responsive polymers on crystal surfaces
A straightforward yet powerful approach for organic crystal hybridization is based on direct deposition of stimuli-responsive polymer coatings or surface layers on molecular crystals of suitable morphology. Peng et al. in 2022 exploited hydrogen bonding interactions to deposit polyvinyl alcohol (PVA) polymer coatings on 10-(trifluoromethyl)anthracene-9-carbonitrile (TFMAC) crystals and prepared robust bilayers (Fig. 4a).91 By repeated heating and cooling, the differential thermal shrinkage and expansion of the PVA layer and the crystal generates a bending moment of the resulting bilayer. Bilayer composites fabricated by this simple method are capable of thermally driven actuation at low temperature and exhibit rapid response. Such direct deposition approaches highlight the ease and versatility in the methods for integration of components with disparate functionalities into hybrid systems.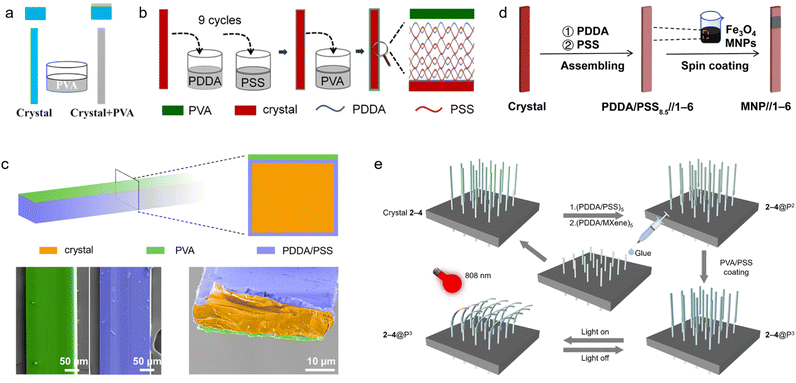 | ||
| Fig. 4 Preparation of hybrid organic crystals. (a) Diagram showing the process for creating organic/polymer hybrids that are sensitive to low temperatures. (a) Reproduced with permission from ref. 91, Copyright 2022 American Chemical Society. (b) Illustration of the construction process and structure of solvent-resistant hybrid organic crystals. (b) Reproduced with permission from ref. 92, Copyright 2021 Wiley-VCH. (c) The technique for preparation of humidity-responsive hybrid crystals, with the lower-row SEM images highlighting the crystals in orange, the polymer coating in purple, and the moisture-reactive polymer (PVA) in green. (c) Reproduced with permission from ref. 13, Copyright 2022 Wiley-VCH. (d) Procedure for fabrication of hybrid crystals that react to magnetic field. (d) Reproduced with permission from ref. 12, Copyright 2022 Spring Nature. (e) Method for developing photoresponsive arrays using hybrid organic crystals. (e) Reproduced with permission from ref. 26, Copyright 2023 Spring Nature. | ||
3.2 Layer-by-layer assembly of polymer films on crystals
While direct polymer deposition offers simplicity of preparation, the stepwise layer-by-layer assembly provides the opportunity for fine control over the multilayer structure of the organic crystal–polymer composite materials.13,14,27,92 This highly tunable and sequential surface engineering approach is based on alternating deposition of stimuli-responsive polymers and countercharged polyelectrolytes on the crystal surface. Lan, Zhang et al. in 2021 exploited electrostatic interactions to deposit poly(diallyldimethylammonium chloride) (PDDA) and poly(sodium 4-styrenesulfonate) (PSS) multilayer films, followed by deposition of a polyvinyl alcohol (PVA) layer, on aromatic hydrocarbon crystals (Fig. 4b).92 The resulting coatings, PVA-(PDDA/PSS)9, prevent the crystals from dissolution in common organic solvents like dichloromethane (DCM) and tetrahydrofuran (THF) for over 24 hours. The polymer layer imparts resistance to solvents while it maintains the optical properties of the crystal. Subsequently, the same research group used a similar approach to coat the surface of aromatic hydrocarbon crystals with multiple layers of a polyelectrolyte membrane as a binding substrate (Fig. 4c)13 followed by deposition of a stimuli-responsive PVA coatings on one side of the crystal. By alternating exposure to humidity, the PVA layer swells and exerts a bending force on the attached organic crystal. The hydrogen bonding ensures firm, reversible integration between the organic crystal and polymer phases. Bilayer composites fabricated with this approach are capable of robust humidity-driven actuations. Beyond humidity, numerous other external triggers can be introduced by selecting polymers for stepwise deposition on crystals.14,273.3 Integration of nanoscale components for response to multiple stimuli
In addition to direct polymer coatings, researchers have integrated organic crystals with nanoscale additives to construct multistimuli responsive hybrid crystals.12,25,26 Nanoparticles, metal oxides, and other inorganic materials can contribute magnetic, photothermal, and other type of responses that are otherwise absent in the nascent organic nascent organic crystal. For instance, Zhang, Naumov et al. in 2022 decorated aromatic crystal surfaces with magnetic iron oxide nanoparticles by using polyelectrolyte films as binders (Fig. 4d).12 This allowed remote manipulation of crystal morphology using external magnetic fields. In another study, the same group in 2023 coated organic crystals with titanium carbide (MXene) nanosheets capable of efficient near-infrared photothermal conversion (Fig. 4e).26 Upon light irradiation, the localized heat generated by the nanosheets induce rapid crystal bending through differential thermal expansion against the organic crystal. Such multifunctional hybrids combining organic crystals with tailored nanoscopic agents effectively expand the response capabilities and utility of organic crystals in complex environments.4. Responsive properties of hybrid organic crystals
The principal motivation behind the integration of organic crystals into multifunctional hybrid materials is to obtain rapid, reversible stimuli-responsive actuation capabilities by leveraging the dynamic properties of the partnering phases.25,27 The resultant organic crystal-based composites display unique responsive characteristics and sensitivity to diverse environmental changes. Coupling organic crystals with stimuli-responsive polymers has already provided hybrid crystals with dynamic behaviour that are responsive to temperature, humidity, light, and other external triggers.12–14,25–274.1 Activation of thermal response through polymer integration
Among various stimuli modalities, the thermal response has been widely implemented in organic crystal hybrids to enable rapid, remotely triggered actuation.14,25 In their native state, organic molecular crystals are composed of discrete molecular entities that are held together by non-covalent interactions. Combination with polymers that have defined glass transition temperatures (Tg) can result in reversible thermally activated transitions of the hybrid material. The polymer components can undergo transitions from a rigid, glassy state to a soft, rubbery state upon heating over Tg, leading to significant differential thermal strain in the polymer–crystal bilayer composites. This results in reversible bending or other mechanical deformations. Therefore, polymer integration provides a versatile route to bestow organic crystals with tuneable thermal response and rapid actuation capabilities. For example, Lan et al. in 2022 introduced thermoplastic polyurethane (TPU) layers on aromatic hydrocarbon crystals such as anthracene derivatives (Fig. 5a).14 The TPU coating provided response to low temperature; upon cooling, the TPU layer contracts, resulting in bending of the bilayer hybrid structure. By changing the temperature, rapid and reversible control over the bending was achieved. The hybrid crystals showed outstanding response from −15 to −120 °C, both in air and in vacuum.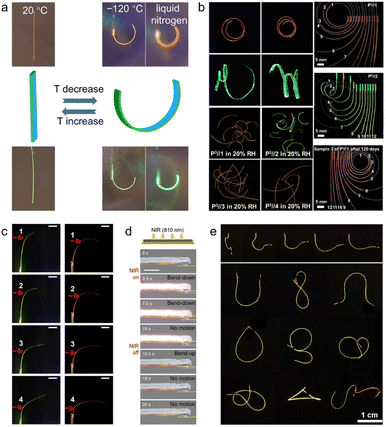 | ||
| Fig. 5 Response of hybrid organic crystals. (a) Optical images displaying the shape transformation of temperature-sensitive hybrid crystals at 20 °C, −120 °C, and when submerged in liquid nitrogen. (a) Reproduced with permission from ref. 14, Copyright 2022 Wiley-VCH. (b) Optical images of humidity-sensitive hybrid organic crystals bending in response to humidity under UV light. (b) Reproduced with permission from ref. 13, Copyright 2022 Wiley-VCH. (c) Images showing hybrid organic crystals bending when exposed to infrared light. (c) Reproduced with permission from ref. 26, Copyright 2023 Spring Nature. (d) Images of Ti3C2Tx-coated enol-1 crystal rapidly bending upon exposure to near-infrared LED light. (d) Reproduced with permission from ref. 93, Copyright 2023 Wiley-VCH. (e) Images of magnetically responsive hybrid organic crystals bending when a magnet is applied. (e) Reproduced with permission from ref. 12, Copyright 2022 Spring Nature. | ||
4.2 Hygroscopic actuation through integration of crystals and hygroresponsive polymers
In addition to thermal response, humidity-driven actuation has also been implemented in organic crystal composites by exploiting the intrinsic hygroexpansion capabilities of certain polymers.13,27 This mode of actuation driven by absorption and desorption of moisture is particularly relevant to crystal hybrids designed to operate in different humidity environments. Lan et al. in 2022 prepared humidity-responsive hybridized organic crystals by using deposition based on electrostatic interactions, and the resulting hygroresponsive hybrid material showed actuation induced by humidity (Fig. 5b).13 In response to changes in aerial humidity, the PVA layer undergoes differential swelling/contraction due to its propensity to absorb or release moisture. This generates strain mismatch at the bilayer interface, resulting in reversible macroscopic bending of the hybrid structure. The hybrids can be actuated bidirectionally between completely straight and tightly coiled shapes within minutes by using only changes in humidity.4.3 Photoactuation through integration
In addition to common stimuli like heat and moisture, light represents another widely utilized external trigger for reversible actuation of organic crystal–polymer hybrids.26 Yang et al. reported a new class of hybrid organic crystals that can be deformed by irradiation with infrared light (Fig. 5c).26 The hybrid crystals were fabricated by coating organic crystals with MXene nanosheets and polymer films using the layer-by-layer assembly approach. MXene are 2D materials that have near-infrared absorption. Upon infrared irradiation, the MXene layers converts the light to heat locally, causing thermal contraction of the polymer coating and bending of the organic crystal. By modulating the position of the infrared light, the bending point of individual hybrid crystals can be precisely controlled. This light-driven shape control was demonstrated not only with single crystals but also with 2D ordered arrays of hybrid crystals. Sitti et al. developed a novel type of hybrid crystal actuator that demonstrates rapid bending under broad-wavelength light, including UV, visible, and near-infrared (NIR) light.93 These actuators were prepared by integrating nanosheets of titanium carbide (Ti3C2Tx) with salicylideneaniline crystals. The incorporation of MXene enables rapid actuation, transforming the light energy into mechanical energy. When exposed to light, particularly in the NIR part of the spectrum, the MXene layers generate heat, causing the crystal to bend (Fig. 5d).4.4 Unconventional activation mechanisms
Beyond the common stimuli described above, researchers have engineered organic crystal hybrids responsive to magnetic fields and other unconventional stimuli by integrating suitable responsive phases through creative materials design.12 As an illustration, Zhang, Naumov et al. functionalized aromatic crystal facets with magnetic iron oxide nanoparticles using polyelectrolyte films as linkers (Fig. 5e).12 This allowed precise manipulation of crystal morphology under alternating magnetic fields using external magnets as remote controllers. Experiments showed the crystals can be repeatedly bent over 3000 cycles without compromising their integrity and with sustained precision in curvature. The actuation could be performed in both aerial environment and under water. A myriad of other unconventional actuation mechanisms can be envisioned by selecting and integrating dynamic crystal and polymer phases using the design strategies outlined previously.5. Exemplary applications of hybrid polymer–crystal materials
Leveraging their responsive properties and mechanical compliance, organic crystal-based hybrids and composites have enabled diverse applications across multiple disciplines, including soft actuators and robotics, flexible electronics, sensors, and optics.12–14,25–27,92 Their performance and scope of utility arising from the synergistic combination of desirable attributes underscore the significant technological potential of such hybrid composite materials. This section describes the key application domains that could benefit from the multifunctional capabilities of dynamic hybrid organic crystals.5.1 Soft actuators and robotics
A prominent application area of responsive organic crystal hybrids are the soft actuators and robotics, which leverage their dynamic shape-changing abilities for biomimetic motility.12,14,27 As a demonstration, Yang et al. constructed soft actuators and robots constructed from the organic polymer–crystal hybrid materials responding to humidity and temperature changes (Fig. 6a–c).27 The researchers devised an artificial “inflorescence” made of multiple hybrid crystal “petals” that open and close by alternating levels of humidity, mimicking the motion of the flowers of the rain lily plant. A hybrid crystal “tendril” was also fabricated that curls upon temperature change similar to some plant tendrils. Furthermore, a spider-like walking soft robot was developed using collective bending of multiple hybrid legs by breathing in its proximity (Fig. 6a and b). Additionally, a simple walking robot was demonstrated that directionally “walks” across surfaces through humidity/temperature-driven bending and unbending cycles (Fig. 6c). Lan et al. demonstrated a simple gripping soft robot made of these hybrid crystals (Fig. 7a and b).14 This device consists of eight similarly sized crystals which quickly clustered together to form a cage-like structure within one second as the temperature is decreased from room temperature to −110 °C. This 0.4 mg lightweight device could firmly grip a 6.9 mg object, which is 17 times heavier than the device's own weight. Even when the device was shaken, it did not release the cargo, unless the temperature was increased (Fig. 7b).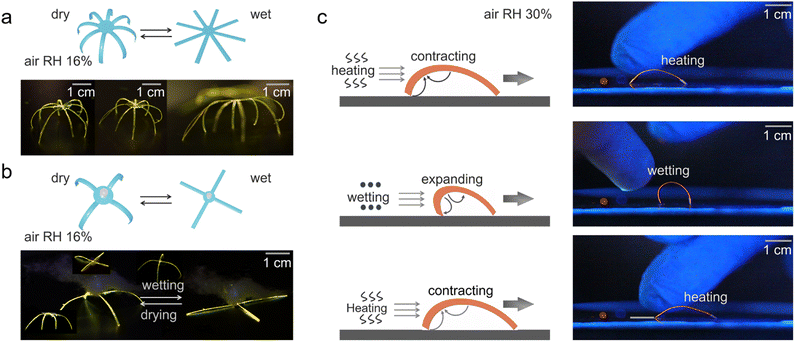 | ||
| Fig. 6 Applications of hybrid organic crystals. (a) Illustrative diagram and photographs showing a soft robot designed to mimic spider-like movements. (b) Diagram and images of a soft gripper constructed from hybrid organic crystal. (c) Schematic and photographs depicting the ‘walking’ movement of organic polymer–crystal hybrid materials on a surface. (a–c) Reproduced with permission from ref. 27, Copyright 2023 Spring Nature. | ||
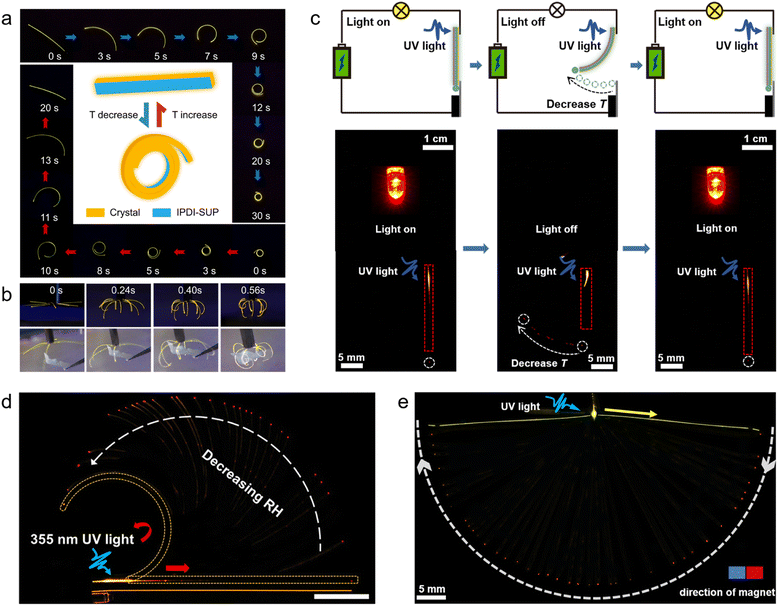 | ||
| Fig. 7 Applications of hybrid organic crystals. (a) A worm-like movement of hybrid crystal–polymer materials at high and low temperatures, with the central diagram illustrating the initial and final states of motion. (b) Shows a soft robot made from hybrid organic crystals, demonstrating its ability to grab objects at low temperatures. (a and b) Reproduced with permission from ref. 14, Copyright 2022 Wiley-VCH. (c) Illustrates a dual-function optical/electrical signal transmission circuit using a hybrid organic crystal. A bulb serves as an indicator, activated by a 355 nm laser. The waveguide output's position indicates the ambient temperature (marked with a white dashed line), with a white arrow showing output change upon cooling. The crystals are outlined with red rectangles. (c) Reproduced with permission from ref. 25, Copyright 2022 Spring Nature. (d) Depicts the spatial control of light output influenced by humidity. A crystal fixed at one end is shown bending with increased humidity (indicated by a white arrow), altering the light output's position. This bending is marked by a yellow broken line, with red arrows showing the light's path in both straight and bent states. (d) Reproduced with permission from ref. 13, Copyright 2022 Wiley-VCH. (e) A hybrid crystal functioning as a magnetically controlled optical waveguide. One end is fixed, with the other controlled by a magnet's movement. The crystal, excited by a 355 nm laser (blue arrow), directs light (yellow arrow) influenced by the magnet's movement (white arrow). The red dot marks the light output. (e) Reproduced with permission from ref. 12, Copyright 2022 Spring Nature. | ||
5.2 Flexible electronics
In addition to actuators, researchers have leveraged the advantageous electronic properties, optical transparency and mechanical compliance of organic crystals to develop flexible optoelectronic systems including transistors, photodetectors, temperature sensors and other devices.25 Zhang, Naumov et al. demonstrated flexible electronics fabricated using the electrically conductive and mechanically robust hybrid organic crystals (Fig. 7c).25 This approach overcomes the limitation with poor conductivity of organic crystals by coating the crystal surfaces with conductive metal layers and protective polymer coatings to achieve both electrical conductivity and mechanical flexibility. The authors showed that the hybrid crystals can be repeatedly bent without losing their conductivity. Such stable conductivity coupled with mechanical compliance turns them into promising components for flexible transistors, sensors, circuits, and other electronic elements or devices. The ordered crystal structures provide high charge transport characteristics, while the polymer coating contributes flexibility. This synergistic crystal–polymer combination enables emerging high-performance flexible organic electronics that combines the benefits of crystalline order and mechanical flexibility.5.3 Flexible photonics
The integration of organic crystals and polymers has enabled the emerging field of flexible photonics to harness the benefits of optical clarity, waveguiding abilities, and mechanical compliance of dynamic organic crystals.12,13 Lan et al. reported the use of humidity-responsive hybrid organic crystals as flexible optical waveguides with controllable direction of the light output by using humidity (Fig. 7d).13 The PVA-coated elastic crystals exhibited reversible bending and unbending in response to variation in humidity. This humidity-induced mechanical actuation was used to exert spatial control over the position of the light output when such hybrid crystals were used as active optical waveguides. Yang et al. demonstrated the magnetic hybrid organic crystals can be used as multidirectional, ultraprecise, and ultradurable optical waveguides that can be reconfigured in both two and three dimensions (Fig. 7e).12 The hybrid crystal was used as a waveguide whose output can be precisely controlled by using a magnet. As a proof-of-concept, the researchers have fabricated a waveguide using this material and succeeded to precisely modulate its shape with magnetic field with remote control of the direction of light output.6. Challenges and future perspectives
Despite the impressive progress made over the past few years in demonstrating organic crystal-based hybrid materials with attractive responsive properties, several challenges remain on the way to elevate this technology from a laboratory-scale research to scalable, real-world applications.6.1 Scalable and sustainable manufacturing
The first challenge lies with devising a sustainable, economical, and scalable manufacturing methods for large-scale production of organic crystal hybrids. Laboratory-scale fabrication predominately relies on multistep crystallization, surface functionalization, and manual assembly—processes that are generally difficult to directly implement in mass manufacturing. For instance, layer-by-layer polyelectrolyte deposition that is widely adopted for polymer integration provides exquisite control over composite architecture, but is time-consuming and inefficient.94–97 There is a need to develop more economical and high-throughput methods for stimuli-responsive polymer adhesion. Furthermore, upscaling the organic single-crystal growth itself requires innovative approaches, since the conventional crystallization methods are constrained by lengthy processes, small crystal sizes, and low yields that are generally commercially unfeasible.98,99 Overall, cross-disciplinary collaboration efforts between chemists, chemical engineers, and manufacturing specialists is imperative to transform the current laboratory-centric fabrication protocols into scalable, sustainable industrial manufacturing processes. Lately, Chandrasekar's group has achieved noteworthy advancements in shaping rigid crystals into diverse curved structures utilizing the “crystal photonic foundry” (a FIB-milling based) technique. This innovative approach showcases the viability of a scalable, replicable fabrication method to produce crystal-based photonic components of varying shapes for device applications, demonstrating a significant potential.100–1026.2 Property benchmarking and materials screening
The second major challenge lies in methodical quantification of the structure–property relationships guiding the selection and design of optimized material components that are selected for target applications. For instance, actuating properties such as the magnitude or range of activating stimulus, deformation curvature, and cycling lifetime vary considerably across the existing polymer–crystal composites.12–14 However, the precise effects of materials parameters (e.g. crystal structure, polymer molecular weight) on dynamic performance remain poorly understood, hindering the property improvement.25–27 Systematic benchmarking together with high-throughput screening protocols are required to populate the comprehensive property libraries and derive predictive models as guiding tools for materials selection. Property evaluation should encompass diverse modalities beyond the mechanical actuation, including electronic transport, optical response, and surface interactions. Materials screening should extend beyond polymers to encompass diverse stimuli-responsive nanoscale inclusions.103,104 Cheminformatic and machine learning methods show promise in accelerating knowledge-driven materials selection and discovery.6.3 Multi-stimuli response and spatiotemporal actuation control
A third aspect requiring enhancement lies in multi-stimuli response capabilities and spatiotemporal control over the actuation, aspects which remain largely unexplored in the existing organic crystal hybrids.13,14,25,26 Current demonstrations are primarily focused on single-stimulus response optimization and global actuation of the crystal shape.13,25 However, most of the real-world applications require response and adaptability to complex environments with spatial and temporal variations in multiple external stimuli such as chemicals, light, humidity, and temperature. This calls for expertise in dynamically coupling diverse responsive stimuli using bespoke materials combinations and interface engineering. Furthermore, actuators that can reconfigure their shapes in predetermined, spatially complex patterns in response to controlled external triggers could, in principle, expand the range of applications of the hybrid crystal–polymer materials.6.4 Device engineering and systems integration
The fourth challenge is related to device engineering efforts aimed at integration of the dynamic hybrid crystals into functional technological systems. For instance, soft actuating components demand judicious device architectures that optimally transmit or leverage the material deformations for useful functions like walking or gripping.25,27 Packaging solutions that enable integration while preserving the dynamic material properties will likely be imperative for future applications. For flexible electronic devices, bendable hybrid crystals have been incorporated into basic diode or transistor architectures as active layers, however systems-level optimization of interfaces, dielectrics and electrodes needs further development and optimization. The synergistic co-design of material, device, and system represents a collaborative endeavour necessary to transform proof-of-concept crystal hybrids into technologies that are competitive with the well-established platforms. Hybrid electronics that combine organic electronic materials with the available inorganic semiconductors also offer integration pathways to leverage the benefits of different material classes.105,1067. Conclusions
In this Perspective we provide a comprehensive analysis of the burgeoning new research field of organic crystal-based hybrid materials. We discuss the key developments in the change in the perception of organic crystals from delicate, fragile solids to elastically compliant, stimuli-responsive materials that are capable of robust reversible actuation. The emergent dynamic properties of organic crystals provide tremendous, yet unexplored opportunities for their integration as active components in multifunctional smart composites. The key materials design strategies are highlighted along with representative studies on the fabrication of soft, stimuli-responsive organic crystal–polymer hybrids that are responsive to temperature, humidity, light, and other actuation mechanisms. The unique combination of dynamic actuation and electronic, optical, and other functional attributes favors these composite materials for diverse technological applications that range from soft robotics to flexible photonics to sensing. However, translation from academic demonstrations to scalable applied systems requires addressing significant challenges in manufacturability, performance benchmarking, property optimization and collaborative convergence across materials chemistry, engineering and processing disciplines. Looking forward, this adaptable new class of stimuli-responsive crystalline hybrid materials appears to hold an enormous potential for next-generation smart technologies, given that their current limitations are overcome through interdisciplinary efforts.Author contributions
X. Yang and M. Al-Handawi performed the literature search, analyzed the published results, and wrote the manuscript. L. Li, P. Naumov, and H. Zhang provided key advice and supervised the preparation of the text.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52173164 and 52373181), the Natural Science Foundation of Jilin Province (20230101038JC), and a fund from New York University Abu Dhabi. This material is based upon works supported by Tamkeen under NYUAD RRC Grant No. CG011.Notes and references
- M. Garcia-Garibay, Angew. Chem., Int. Ed., 2007, 46, 8945–8947 CrossRef CAS PubMed
.
- P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyreva, Chem. Rev., 2015, 115, 12440–12490 CrossRef CAS PubMed
.
- C. M. Reddy, K. A. Padmanabhan and G. R. Desiraju, Cryst. Growth Des., 2006, 6, 2720–2731 CrossRef CAS
.
- N. K. Nath, M. K. Panda, S. C. Sahoo and P. Naumov, CrystEngComm, 2014, 16, 1850–1858 RSC
.
- A. Priimagi, C. J. Barrett and A. Shishido, J. Mater. Chem. C, 2014, 2, 7155–7162 RSC
.
- T. Kim, L. Zhu, R. O. Al-Kaysi and C. J. Bardeen, ChemPhysChem, 2014, 15, 400–414 CrossRef CAS PubMed
.
- M. D. Manrique-Juárez, S. Rat, L. Salmon, G. Molnár, C. M. Quintero, L. Nicu, H. J. Shepherd and A. Bousseksou, Coord. Chem. Rev., 2016, 308, 395–408 CrossRef
.
- Q. Yu, B. Aguila, J. Gao, P. Xu, Q. Chen, J. Yan, D. Xing, Y. Chen, P. Cheng, Z. Zhang and S. Ma, Chem.–Eur. J., 2019, 25, 5611–5622 CrossRef CAS PubMed
.
- S. C. Sahoo, M. K. Panda, N. K. Nath and P. Naumov, J. Am. Chem. Soc., 2013, 135, 12241–12251 CrossRef CAS PubMed
.
- P. Commins, H. Hara and P. Naumov, Angew. Chem., Int. Ed., 2016, 55, 13028–13032 CrossRef CAS PubMed
.
- Y. Chen, Z. Chang, J. Zhang and J. Gong, Angew. Chem., Int. Ed., 2021, 60, 22424–22431 CrossRef CAS PubMed
.
- X. Yang, L. Lan, L. Li, X. Liu, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 2322 CrossRef CAS PubMed
.
- L. Lan, X. Yang, B. Tang, X. Yu, X. Liu, L. Li, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200196 CrossRef CAS
.
- L. Lan, L. Li, Q. Di, X. Yang, X. Liu, P. Naumov and H. Zhang, Adv. Mater., 2022, 34, 2200471 CrossRef CAS PubMed
.
- P. Commins, A. B. Dippenaar, L. Li, H. Hara, D. A. Haynes and P. Naumov, Chem. Sci., 2021, 12, 6188–6193 RSC
.
- S. Khan, B. Dutta, S. Naaz, A. Choudhury, P. Cazade, E. Kiely, S. Guerin, R. Medishetty and M. H. Mir, Commun. Chem., 2023, 6, 150 CrossRef CAS
.
- P. Gupta, D. P. Karothu, E. Ahmed, P. Naumov and N. K. Nath, Angew. Chem., Int. Ed., 2018, 57, 8498–8502 CrossRef CAS
.
- P. Gupta, T. Panda, S. Allu, S. Borah, A. Baishya, A. Gunnam, A. Nangia, P. Naumov and N. K. Nath, Cryst. Growth Des., 2019, 19, 3039–3044 CrossRef CAS
.
- T. Lan and W. Chen, Angew. Chem., Int. Ed., 2013, 52, 6496–6500 CrossRef CAS PubMed
.
- H. Koshima, M. Matsudomi, Y. Uemura, F. Kimura and T. Kimura, Chem.
Lett., 2013, 42, 1517–1519 CrossRef CAS
.
- Q. Yu, X. Yang, Y. Chen, K. Yu, J. Gao, Z. Liu, P. Cheng, Z. Zhang, B. Aguila and S. Ma, Angew. Chem., Int. Ed., 2018, 57, 10192–10196 CrossRef CAS PubMed
.
- X. Dong, F. Tong, K. M. Hanson, R. O. Al-Kaysi, D. Kitagawa, S. Kobatake and C. J. Bardeen, Chem. Mater., 2019, 31, 1016–1022 CrossRef CAS
.
- W. Xu, D. M. Sanchez, U. Raucci, H. Zhou, X. Dong, M. Hu, C. J. Bardeen, T. J. Martinez and R. C. Hayward, Nat. Mater., 2023, 22, 1152–1159 CrossRef CAS PubMed
.
- D. Urban, N. Marcucci, C. H. Wölfle, J. Torgersen, D. R. Hjelme and E. Descrovi, Nat. Commun., 2023, 14, 6843 CrossRef CAS
.
- X. Yang, L. Lan, X. Pan, X. Liu, Y. Song, X. Yang, Q. Dong, L. Li, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 7874 CrossRef CAS PubMed
.
- X. Yang, L. Lan, L. Li, J. Yu, X. Liu, Y. Tao, Q. Yang, P. Naumov and H. Zhang, Nat. Commun., 2023, 14, 3627 CrossRef CAS PubMed
.
- X. Yang, L. Lan, X. Pan, Q. Di, X. Liu, L. Li, P. Naumov and H. Zhang, Nat. Commun., 2023, 14, 2287 CrossRef CAS PubMed
.
- G. R. Desiraju, Angew. Chem., Int. Ed., 1995, 34, 2311–2327 CrossRef CAS
.
- Y. Tsarfati, S. Rosenne, H. Weissman, L. J. W. Shimon, D. Gur, B. A. Palmer and B. Rybtchinski, ACS Cent. Sci., 2018, 4, 1031–1036 CrossRef CAS PubMed
.
- J. S. Brooks, Chem. Soc. Rev., 2010, 39, 2667–2694 CAS
.
- J. A. Newman, L. Iuzzolino, M. Tan, P. Orth, J. Bruhn and A. Y. Lee, Mol. Pharmaceutics, 2022, 19, 2133–2141 CrossRef CAS
.
- Z. Lu, Y. Zhang, H. Liu, K. Ye, W. Liu and H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 4299–4303 CrossRef CAS PubMed
.
- R. Huang, C. Wang, Y. Wang and H. Zhang, Adv. Mater., 2018, 30, 1800814 CrossRef PubMed
.
- B. Liu, Z. Lu, B. Tang, H. Liu, H. Liu, Z. Zhang, K. Ye and H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 23117–23121 CrossRef CAS PubMed
.
- E. Ahmed, D. P. Karothu and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 8837–8846 CrossRef CAS
.
- L. Catalano, D. P. Karothu, S. Schramm, E. Ahmed, R. Rezgui, T. J. Barber, A. Famulari and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 17254–17258 CrossRef CAS
.
- S. C. Sahoo, S. B. Sinha, M. S. R. N. Kiran, U. Ramamurty, A. F. Dericioglu, C. M. Reddy and P. Naumov, J. Am. Chem. Soc., 2013, 135, 13843–13850 CrossRef CAS
.
- S. H. Mir, Y. Takasaki, E. R. Engel and S. Takamizawa, Angew. Chem., Int. Ed., 2017, 56, 15882–15885 CrossRef CAS PubMed
.
- S. Takamizawa, Y. Takasaki, T. Sasaki and N. Ozaki, Nat. Commun., 2018, 9, 3984 CrossRef
.
- S. L. Price, Proc. R. Soc. A, 2018, 474, 20180351 CrossRef PubMed
.
- M. Lightowler, S. Li, X. Ou, X. Zou, M. Lu and H. Xu, Angew. Chem., Int. Ed., 2022, 61, e202114985 CrossRef CAS
.
- S. Hayashi, S. Yamamoto, D. Takeuchi, Y. Ie and K. Takagi, Angew. Chem., Int. Ed., 2018, 57, 17002–17008 CrossRef CAS PubMed
.
- Q. Di, L. Li, X. Miao, L. Lan, X. Yu, B. Liu, Y. Yi, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 5280 CrossRef CAS PubMed
.
- M. Kato, H. Ito, M. Hasegawa and K. Ishii, Chem.–Eur. J., 2019, 25, 5105–5112 CrossRef CAS PubMed
.
- D. Yan, Z. Wang and Z. Zhang, Acc. Chem. Res., 2022, 55, 1047–1058 CrossRef CAS
.
- S. Ghosh and C. M. Reddy, Angew. Chem., Int. Ed., 2012, 51, 10319–10323 CrossRef CAS PubMed
.
- S. Basak and R. Chandrasekar, J. Mater. Chem. C, 2014, 2, 1404–1408 RSC
.
- N. K. Nath, L. Pejov, S. M. Nichols, C. Hu, N. Saleh, B. Kahr and P. Naumov, J. Am. Chem. Soc., 2014, 136, 2757–2766 CrossRef CAS PubMed
.
- E. Uchida, R. Azumi and Y. Norikane, Nat. Commun., 2015, 6, 7310 CrossRef CAS PubMed
.
- S. Hayashi and T. Koizumi, Angew. Chem., Int. Ed., 2016, 55, 2701–2704 CrossRef CAS PubMed
.
- A. Worthy, A. Grosjean, M. C. Pfrunder, Y. Xu, C. Yan, G. Edwards, J. K. Clegg and J. C. McMurtrie, Nat. Chem., 2018, 10, 65–69 CrossRef CAS PubMed
.
- D. Kitagawa, H. Tsujioka, F. Tong, X. Dong, C. J. Bardeen and S. Kobatake, J. Am. Chem. Soc., 2018, 140, 4208–4212 CrossRef CAS PubMed
.
- F. Tong, M. Al-Haidar, L. Zhu, R. O. Al-Kaysi and C. J. Bardeen, Chem. Commun., 2019, 55, 3709–3712 RSC
.
- A. G. Shtukenberg, R. Drori, E. V. Sturm, N. Vidavsky, A. Haddad, J. Zheng, L. A. Estroff, H. Weissman, S. G. Wolf, E. Shimoni, C. Li, N. Fellah, E. Efrati and B. Kahr, Angew. Chem., Int. Ed., 2020, 59, 14593–14601 CrossRef CAS PubMed
.
- S. Hasebe, Y. Hagiwara, J. Komiya, M. Ryu, H. Fujisawa, J. Morikawa, T. Katayama, D. Yamanaka, A. Furube, H. Sato, T. Asahi and H. Koshima, J. Am. Chem. Soc., 2021, 143, 8866–8877 CrossRef CAS PubMed
.
- S. K. Park, H. Sun, M. Bernhardt, K. Hwang, J. E. Anthony, K. Zhao and Y. Diao, Chem. Mater., 2023, 35, 81–93 CrossRef CAS
.
- L. Lan, L. Li, X. Yang, P. Naumov and H. Zhang, Adv. Funct. Mater., 2023, 33, 2211760 CrossRef CAS
.
- M. C. Etter and A. R. Siedle, J. Am. Chem. Soc., 1983, 105, 641–643 CrossRef CAS
.
- M. Irie, T. Lifka, S. Kobatake and N. Kato, J. Am. Chem. Soc., 2000, 122, 4871–4876 CrossRef CAS
.
- T. Yamada, K. Muto, S. Kobatake and M. Irie, J. Org. Chem., 2001, 66, 6164–6168 CrossRef CAS PubMed
.
- R. O. Al-Kaysi and C. J. Bardeen, Adv. Mater., 2007, 19, 1276–1280 CrossRef CAS
.
- H. Koshima, N. Ojima and H. Uchimoto, J. Am. Chem. Soc., 2009, 131, 6890–6891 CrossRef CAS PubMed
.
- L. Zhu, A. Agarwal, J. Lai, R. O. Al-Kaysi, F. S. Tham, T. Ghaddar, L. Mueller and C. J. Bardeen, J. Mater. Chem., 2011, 21, 6258–6268 RSC
.
- Y. Yang, L. S. de Moraes, C. Ruzié, G. Schweicher, Y. H. Geerts, A. R. Kennedy, H. Zhou, S. J. Whittaker, S. S. Lee, B. Kahr and A. G. Shtukenberg, Adv. Mater., 2022, 34, 2203842 CrossRef CAS PubMed
.
- E. Ahmed, D. P. Karothu, M. Warren and P. Naumov, Nat. Commun., 2019, 10, 3723 CrossRef PubMed
.
- C. Huang, R. Huang, S. Zhang, H. Sun, H. Wang, B. Du, Y. Xiao, T. Yu and W. Huang, Research, 2021 Search PubMed.
- C. Chou, S. Nobusue, S. Saito, D. Inoue, D. Hashizume and S. Yamaguchi, Chem. Sci., 2015, 6, 2354–2359 RSC
.
- J. M. Halabi, E. Ahmed, S. Sofela and P. Naumov, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020604118 CrossRef CAS PubMed
.
- D. P. Karothu, J. Weston, I. T. Desta and P. Naumov, J. Am. Chem. Soc., 2016, 138, 13298–13306 CrossRef CAS PubMed
.
- J. Ravi, A. V. Kumar, M. Annadhasan and R. Chandrasekar, Adv. Opt. Mater., 2022, 10, 2102545 CrossRef CAS
.
- J. Ravi and R. Chandrasekar, Adv. Opt. Mater., 2021, 9, 2100550 CrossRef CAS
.
- M. Annadhasan, D. P. Karothu, R. Chinnasamy, L. Catalano, E. Ahmed, S. Ghosh, P. Naumov and R. Chandrasekar, Angew. Chem., Int. Ed., 2020, 59, 13821–13830 CrossRef CAS PubMed
.
- A. V. Kumar and R. Chandrasekar, Adv. Opt. Mater., 2023, 11, 2201009 CrossRef CAS
.
- A. V. Kumar, M. Godumala, J. Ravi and R. Chandrasekar, Adv. Opt. Mater., 2023, 2302807 CrossRef
.
- M. Annadhasan, A. Vinod Kumar, P. Giri, S. Nandy, M. K. Panda, K. V. Jovan Jose and R. Chandrasekar, Angew. Chem., Int. Ed., 2023, 62, e202302929 CrossRef CAS PubMed
.
- A. Vinod Kumar, M. Rohullah, M. Chosenyah, J. Ravi, U. Venkataramudu and R. Chandrasekar, Angew. Chem., Int. Ed., 2023, 62, e202300046 CrossRef CAS PubMed
.
- R. Chandrasekar, Chem. Commun., 2022, 58, 3415–3428 RSC
.
- H. Liu, Z. Lu, Z. Zhang, Y. Wang and H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 8448–8452 CrossRef CAS PubMed
.
- H. Liu, K. Ye, Z. Zhang and H. Zhang, Angew. Chem., Int. Ed., 2019, 58, 19081–19086 CrossRef CAS PubMed
.
- H. Liu, Z. Lu, B. Tang, C. Qu, Z. Zhang and H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 12944–12950 CrossRef CAS PubMed
.
- X. Feng, R. Lin, S. Yang, Y. Xu, T. Zhang, S. Chen, Y. Ji, Z. Wang, S. Chen, C. Zhu, Z. Gao and Y. S. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202310263 CrossRef CAS PubMed
.
- Y. Lv, X. Xiong, Y. Liu, J. Yao, Y. J. Li and Y. S. Zhao, Nano Lett., 2019, 19, 1098–1103 CrossRef CAS PubMed
.
- C. Zhang, C. Zou, Y. Zhao, C. Dong, C. Wei, H. Wang, Y. Liu, G. Guo, J. Yao and Y. S. Zhao, Sci. Adv., 2015, 1, e1500257 CrossRef PubMed
.
- H. Dong, C. Zhang, W. Zhou, J. Yao and Y. S. Zhao, Adv. Mater., 2022, 34, 2107611 CrossRef CAS PubMed
.
- C. Qiao, C. Zhang, Z. Zhou, J. Yao and Y. S. Zhao, CCS Chem., 2022, 4, 250–258 CrossRef
.
- C. Zhang, H. Dong, C. Zhang, Y. Fan, J. Yao and Y. S. Zhao, Sci. Adv., 2021, 7, eabh3530 CrossRef PubMed
.
- C. Wang, H. Dong, L. Jiang and W. Hu, Chem. Soc. Rev., 2018, 47, 422–500 RSC
.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed
.
- L. Zhai, Chem. Soc. Rev., 2013, 42, 7148–7160 RSC
.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2013, 65, 10–16 CrossRef CAS PubMed
.
- J. Peng, C. C. Han, J. K. Bai, X. M. Cao, J. Z. Wang, Y. F. Cao and J. H. Jia, Cryst. Growth Des., 2022, 22, 7187–7194 CrossRef CAS
.
- L. Lan, H. Liu, X. Yu, X. Liu and H. Zhang, Angew. Chem., Int. Ed., 2021, 60, 11283–11287 CrossRef CAS PubMed
.
- D. W. Kim, Y. Hagiwara, S. Hasebe, N. O. Dogan, M. Zhang, T. Asahi, H. Koshima and M. Sitti, Adv. Funct. Mater., 2023, 33, 2305916 CrossRef CAS
.
- E. S. Miranda, T. H. Silva, R. L. Reis and J. F. Mano, Tissue Eng., 2011, 17, 2663–2674 CrossRef CAS PubMed
.
- Y. Li, X. Wang and J. Sun, Chem. Soc. Rev., 2012, 41, 5998–6009 RSC
.
- X. Wang, Y. Wang, S. Bi, Y. Wang, X. Chen, L. Qiu and J. Sun, Adv. Funct. Mater., 2014, 24, 403–411 CrossRef CAS
.
- S. Takahashi, I. Suzuki, T. Ojima, D. Minaki and J. Anzai, Sensors, 2018, 18, 317 CrossRef PubMed
.
- Y. Li, D. Ji, J. Liu, Y. Yao, X. Fu, W. Zhu, C. Xu, H. Dong, J. Li and W. Hu, Sci. Rep., 2015, 5, 13195 CrossRef CAS PubMed
.
- S. Jiang, Y. Zeng, W. Zhou, X. Miao and Y. Yu, Sci. Rep., 2016, 6, 19313 CrossRef CAS PubMed
.
- V. V. Pradeep and R. Chandrasekar, Adv. Opt. Mater., 2022, 10, 2201150 CrossRef CAS
.
- V. V. Pradeep, M. Chosenyah, E. Mamonov and R. Chandrasekar, Nanoscale, 2023, 15, 12220–12226 RSC
.
- V. V. Pradeep, A. V. Kumar and R. Chandrasekar, Laser Photon. Rev., 2023, 17, 2300552 CrossRef CAS
.
- M. Mitsuishi, Y. Koishikawa, H. Tanaka, E. Sato, T. Mikayama, J. Matsui and T. Miyashita, Langmuir, 2007, 23, 7472–7474 CrossRef CAS PubMed
.
- S. Srivastava, M. Fukuto and O. Gang, Soft Matter, 2018, 14, 3929–3934 RSC
.
- C. Yang, Y. Sun, X. Li, C. Li, J. Tong, J. Li, P. Zhang and Y. Xia, Nanoscale Res. Lett., 2018, 13, 184 CrossRef PubMed
.
- Y. Park, I. Yun, W. G. Chung, W. Park, D. H. Lee and J. Park, Adv. Sci., 2022, 9, 2104623 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |





