Open Access Article
Open Access ArticleRealizing highly efficient deep-blue organic light-emitting diodes towards Rec.2020 chromaticity by restricting the vibration of the molecular framework†
Chuan
Li
a,
Kai
Zhang
b,
Yanju
Luo
c,
Yang
Yang
a,
Yong
Huang
a,
Mengjiao
Jia
a,
Yuling
He
a,
Yue
Lei
a,
Jian-Xin
Tang
*b,
Yan
Huang
 a and
Zhiyun
Lu
a and
Zhiyun
Lu
 *a
*a
aKey Laboratory of Green Chemistry and Technology (Ministry of Education), College of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: luzhiyun@scu.edu.cn
bMacau Institute of Materials Science and Engineering (MIMSE), Faculty of Innovation Engineering, Macau University of Science and Technology, Taipa, Macau SAR 999078, P. R. China. E-mail: jxtang@must.edu.mo
cAnalytical & Testing Center, Sichuan University, Chengdu 610064, P. R. China
First published on 22nd February 2024
Abstract
Deep-blue organic light-emitting diodes (OLEDs) with narrow emission spectra and high efficiency, meeting the Rec.2020 standard, hold significant promise in the realm of 4K/8K ultrahigh-definition displays. However, the development of light-emitting materials exhibiting both narrowband emission and high efficiency, particularly in the realm of deep-blue thermally activated delayed fluorescence (TADF), confronts substantial challenges. Herein, a novel deep-blue TADF emitter, named BOC-PSi, was designed by integrating a rigid B-heterotriangulene acceptor (A) with a rigid phenazasiline donor (D). The replacement of a sp3 carbon atom with a sp3 silicon atom in the D moiety helps to restrict the low-frequency bending vibration throughout the entire D–A molecular backbone, while concurrently accelerating the multi-channel reverse intersystem crossing (RISC) processes. Notably, OLEDs using the BOC-PSi emitter exhibit exceptional performance, with a high maximum external quantum efficiency (EQEmax) approaching 20%, and a superior color purity closely aligning with the Rec.2020 blue standard.
Introduction
Narrow-emission and highly efficient blue thermally activated delayed fluorescence (TADF) materials approaching the Rec.2020 standard for color gamut (CIE coordinates: (0.131, 0.046), as recommended by the International Telecommunication Union) have attracted significant research attention due to their immense potential in the field of 4K/8K ultrahigh-definition displays.1,2 However, the development of such materials has encountered considerable challenges, as it requires concurrent optimization of the high photoluminescence (PL) efficiency (ΦPL), narrow full width at half maximum (FWHM), fast reverse intersystem crossing (RISC) process, and wide bandgap. Therefore, only a handful of reports exist on blue TADF organic light-emitting diodes (OLEDs) with a CIEy value close to 0.046 and a maximum external quantum efficiency (EQEmax) surpassing 15%.3–7To achieve TADF materials with a narrow emission bandwidth, it is essential to impart a rigid framework to the fluorophore. A notable example of this concept is multiple resonance (MR) B,N-heteroarenes, known for exhibiting narrow FWHM and high ΦPL due to their highly rigid molecular scaffolds.8–10 Nevertheless, their robust planar molecular backbones significantly hinder the realization of strong spin-orbit coupling (SOC).11 As a result, most MR-TADF dyes experience relatively slow RISC processes, posing challenges for their implementation in conventional TADF-OLEDs without the need supplementary TADF sensitizers.
On the other hand, due to the compensating effect between their variations in orbital angular momentum and spin angular momentum,12,13 TADF molecules with highly distorted donor–acceptor (D–A) structures are more likely to exhibit a relatively fast RISC process. However, as the D and A moieties are chemically linked only through a fragile single bond, these D–A dyads generally suffer from poor framework rigidity, leading to a relatively wide FWHM and an accelerated non-radiative process. Moreover, to minimize the singlet–triplet energy gap (ΔEST), these D–A TADF compounds often possess a nearly orthogonal orientation between their D and A units, resulting in a reduced radiative transition rate. Consequently, the accelerated non-radiative process poses a significant obstacle to achieving a high ΦPL.
In addition, to obtain TADF D–A dyads with deep-blue emission performance approaching the Rec.2020 standard, it is necessary to carefully select D and A moieties not only having a deep highest occupied molecular orbital (HOMO) and a shallow lowest unoccupied molecular orbital (LUMO), respectively, but also possessing a local triplet excited state (3LE) energy level higher than 3.0 eV. However, such structural units are scarce, which further adds to the challenge of constructing TADF emitters with a wide bandgap.14
Herein, we report a high-performance deep-blue TADF D–A dyad, namely BOC-PSi, which utilizes a rigid B-heterotriangulene derivative (BOC) as the A moiety, and 10,10-diphenyl-5,10-dihydrodibenzo[b,e][1,4]azasiline (PSi), the sila-product of 9,9-diphenyl-9,10-dihydroacridine (PC), as the D moiety (Fig. 1a). BOC-PSi-based OLEDs demonstrated an EQEmax of 19.6% under a CIEy value of 0.049, establishing BOC-PSi as an advanced Rec.2020 blue OLED emitter (Table S3†). Comparative studies between BOC-PSi and BOC-PC (a reference compound using PC as the D unit) revealed that the better electroluminescence (EL) performance of BOC-PSi can be mainly attributed to its narrower FWHM and higher ΦPL, which arise from the enhanced rigidity of PSi compared to PC. These findings demonstrated that D–A TADF dyads with exceptional blue color gamut can be acquired by employing rigid D and A subunits.
Molecular design rationale & characterization
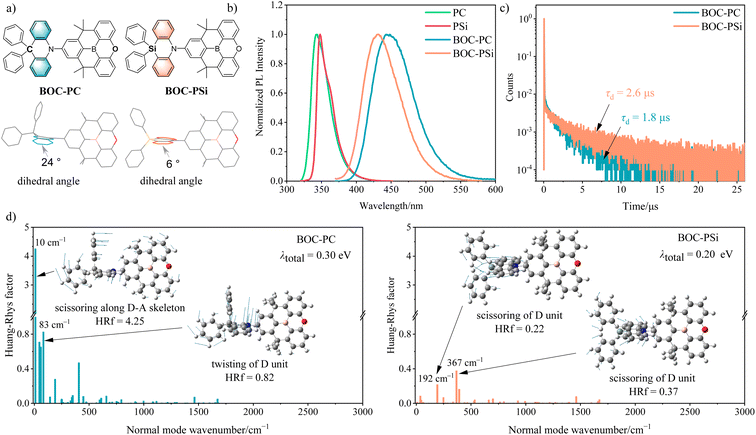
The design rationale for BOC-PSi is as follows. (1) To endow the dyad with a highly twisted geometry, PC composed of merely six-membered polycyclic ring systems was chosen as the parent D scaffold.15 (2) A sila-modification was performed on PC, because the resulting PSi shows better framework rigidity compared to PC, as evidenced by its narrower and more structured fluorescence and phosphorescence spectra (Fig. 1b and S8†). (3) The 3LE energy level of PSi is as high as 3.10 eV, and its calculated HOMO (−5.60 eV) is deeper than that of PC, which may be attributed to the weaker hyperconjugation effects in PSi (Fig. S11†).16,17 These conditions facilitate the acquisition of a TADF D–A dyad with a wide bandgap. (4) Based on the HOMO energy level data of PSi, BOC was screened out as the A moiety due to its integrated rigid molecular skeleton, high 3LE energy level of 3.17 eV, and shallow LUMO level of −2.36 eV.18The synthetic routes of BOC-PSi and BOC-PC are illustrated in the ESI (Scheme S1).† The molecular structure of BOC-PSi and BOC-PC was confirmed by nuclear magnetic resonance spectroscopy (NMR), high-resolution mass spectroscopy (MS), and single crystal X-ray diffraction (XRD).
The single crystal structures of BOC-PSi and BOC-PC are depicted in Fig. S10,† and the corresponding crystal parameters are listed in Table S1.† As expected, BOC-PSi and BOC-PC both show a nearly perpendicular orientation between their D and A segments, with torsion angles measuring 86.4° and 84.0°, respectively. This highly twisted geometry offers advantages in promoting efficient RISC by facilitating enhanced vibronic coupling between the charge-transfer triplet excited state (3CT) and 3LE state. However, the two phenyl substituents of D units in the two compounds have quite different relative positions. As shown in Fig. 1a, the two phenyls of BOC-PSi are nearly symmetrically distributed above and below the azasiline plane, whereas those of BOC-PC exhibit distinct conformations, with one quasi-axial and the other quasi-equatorial. The disparity in the relative positions of the phenyls of BOC-PSi and BOC-PC can be attributed to the more planar configuration of the azasiline ring compared to the acridine ring (dihedral angle: ∼6° vs. ∼24°, Fig. 1a and S10†), which is due to the significantly longer Csp2–Sisp3 bonds than the corresponding Csp2–Csp3 bonds (∼1.8 vs. ∼1.5 Å, Table S1†), resulting from the larger atomic radius of silicon than carbon.
Electrochemical & thermal stability properties
Through cyclic voltammetry (CV) measurements (Fig. S5†), the HOMO energy level values of BOC-PC and BOC-PSi were determined to be −5.41 eV and −5.53 eV, respectively. The deeper HOMO level of BOC-PSi than BOC-PC indicates the weaker electron-donating ability of PSi compared to PC, which ultimately benefits the realization of wider bandgap emission. Subsequently, the LUMO energy level values were calculated from the corresponding optical bandgap and HOMO energy level data, and were determined to be −2.32 eV and −2.37 eV for BOC-PC and BOC-PSi, respectively. The slight disparity in the LUMO energy level values between the two compounds could potentially stem from the variation in the D–A dihedral angles in their optimized S0 geometrical structures (Fig. S12†).Based on the results of thermal gravimetry analysis (TGA) and differential scanning calorimetry (DSC) characterizations (Fig. S6†), both compounds demonstrate excellent thermal stability, as evidenced by their high decomposition temperatures (Td) exceeding 340 °C at 5% initial weight loss. In addition, only BOC-PC exhibits a notably high melt temperature (Tm) of 327 °C, while no significant endothermic signal ascribed to glass transition could be observed in both emitters.
Photoluminescence properties
The photophysical properties of these two emitters were investigated in dilute solution (10−5 M) and doped film states (15 wt% in bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO)). As illustrated in Fig. 1b and Table 1, BOC-PSi and BOC-PC both emit deep-blue PL in toluene, but BOC-PSi exhibits a superior blue color gamut to BOC-PC due to its >10 nm blue-shifted PL emission band (λPL: 432 vs. 445 nm) and much narrowed FWHM (61 vs. 70 nm). These findings provide clear evidence for the effectiveness of this strategy in the rational design of deep-blue emitters. Besides, with increasing solvent polarity from hexane to acetonitrile, both BOC-PSi and BOC-PC display red-shifted and broadened PL spectra, manifesting the CT character of their S1 states (Fig. S7†). Notably, BOC-PSi consistently shows a narrower PL spectrum compared to BOC-PC in every solvent, suggesting that a more rigid D subunit can indeed induce a narrower FWHM of the corresponding 1CT-featured emission in a D–A dyad.| Compound | λ PL [nm] | Φ PL | Φ PF | Φ DF | Φ ISC | τ PF [ns] | τ DF [μs] | k F [s−1] | k ISC [s−1] | k RISC [s−1] | k NR S [s−1] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BOC-PC | 454 | 80% | 49% | 31% | 39% | 14.2 | 1.8 | 3.45 × 107 | 2.73 × 107 | 9.07 × 105 | 8.63 × 106 |
| BOC-PSi | 439 | 92% | 26% | 66% | 72% | 9.4 | 2.6 | 2.77 × 107 | 7.63 × 107 | 1.36 × 106 | 2.41 × 106 |
In terms of the two film samples, they both exhibit slightly narrowed PL spectra compared to their corresponding toluene solutions (FWHM: 55 vs. 61 nm for BOC-PSi; 66 vs. 70 nm for BOC-PC), which can be attributed to the restricted rotation of the C–N single bond within a more rigid matrix. In comparison to BOC-PC, BOC-PSi also shows a blue-shifted (λPL: 439 vs. 454 nm) PL spectrum with a narrowed FWHM (55 vs. 66 nm). Additionally, BOC-PSi shows a higher ΦPL compared to BOC-PC (92% vs. 80%), indicative of the existence of additional exciton loss pathways in BOC-PC, potentially arising from the vibration relaxation of the fluorophore.
Further transient PL measurements revealed the presence of delayed fluorescence (DF) behavior in both film samples. Notably, the DF lifetime (τDF) is as short as 2.6 μs for BOC-PSi and 1.8 μs for BOC-PC (Fig. 1c), indicative of the occurrence of fast RISC processes in both BOC-PSi and BOC-PC. With respect to the prompt fluorescence (PF), the average lifetime (τPF) is determined to be 9.4 ns for BOC-PSi and 14.2 ns for BOC-PC (Fig. S9†). Based on the τDF, τPF, ΦPF and ΦDF data (Table 1), the rate constants for key photophysical processes, including fluorescence decay (kF), intersystem crossing (kISC), RISC (kRISC), and non-radiative decay of the S1 state (kNRS), were calculated for the two compounds.19,20
Exciton dynamics process
As shown in Table 1, BOC-PSi has a significantly smaller kNRS than BOC-PC (2.41 × 106vs. 8.63 × 106 s−1). To elucidate the underlying cause of the suppressed non-radiative process resulting from the sila-modification, theoretical calculations were conducted to determine the total reorganization energy (λtotal) between the S1 and S0 states for both compounds as well as the A moiety of BOC. The results indicated that the λtotal of BOC-PSi is slightly smaller than that of BOC-PC (0.20 vs. 0.30 eV, vide Fig. 1d), implying that BOC-PSi possesses superior molecular skeleton rigidity to BOC-PC,21,22 while the λtotal of BOC is only 0.17 eV, indicative of its excellent rigidity (Fig. S14†). In line with this deduction, the root mean square deviation (RMSD) value of the superposition of the optimized S0 and S1 geometries of BOC-PSi is much smaller than that of BOC-PC (0.123 vs. 0.316 Å, Fig. S15†).24 These findings suggest a stronger suppression of non-radiative decay for the S1 state of BOC-PSi than BOC-PC.To elucidate the reason for the disparity in FWHM between the two emitters, the Huang–Rhys factors (HRf) at various vibration modes were calculated for BOC-PSi, BOC-PC and BOC. For BOC-PC, a low-frequency scissoring swing of the entire molecular framework was observed at a normal mode wavenumber of 10 cm−1 (Fig. 1d and S13†), accompanied by a large HRf of 4.25.23 Detailed vibration mode analysis revealed that the scissoring motion along the D–A skeleton in BOC-PC can be ascribed to the top-heavy nature of its PC moiety during the bending vibration of the C–N bond. Additionally, the vibration mode in BOC-PC that exhibits the second-largest HRf (0.82) also arises from the twisting of the PC moiety. In contrast, due to the well-balanced character of its PSi moiety, all HRfs calculated at the low-frequency region below 200 cm−1 are significantly smaller than those of BOC-PC, and no obvious vibrational motions throughout the whole D–A scaffold of BOC-PSi were observed. Therefore, the BOC-PSi exhibits a smaller overall HRf compared to BOC-PC, manifesting a significantly suppressed structural relaxation thus narrowing the FWHM.24 In the case of BOC, no detectable vibrations were found contributing to its HRf in the low-frequency range below 200 cm−1, indicative of its excellent skeletal rigidity. Therefore, it can be inferred that the severe non-radiative process and larger FWHM in BOC-PC should be mainly ascribed to its PC subunit.
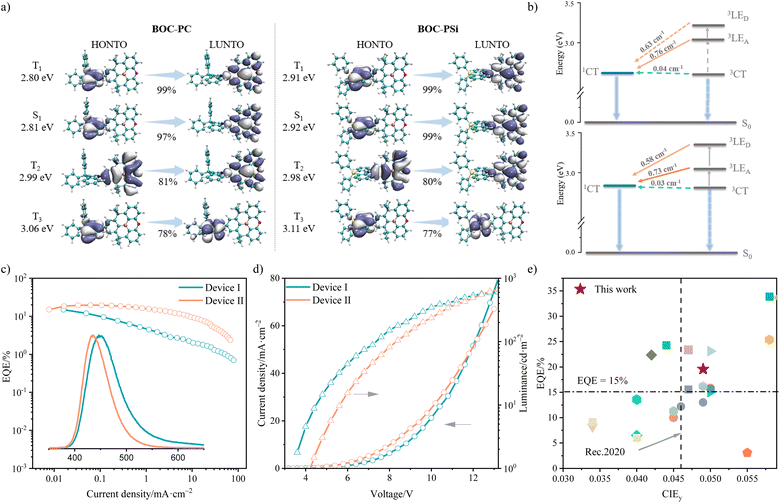
Excitingly, the kISC and kRISC values of BOC-PSi are also both larger than those of BOC-PC (kISC: 7.63 × 107vs. 2.73 × 107 s−1; kRISC: 1.36 × 106vs. 9.07 × 105 s−1), indicating a stronger SOC effect and/or a smaller energy difference between the S1 and T1/Tn states in BOC-PSi. To understand the reason behind the larger kRISC value of BOC-PSi than BOC-PC, the PL and phosphorescence (Phos) spectra of both compounds were recorded at 77 K. The structureless and red-shifted PL and Phos spectra of the two compounds relative to their corresponding D/A fragments manifest the 1CT and 3CT features of their S1 and T1 states, respectively (Fig. S8†). The 1CT/3CT energy levels, according to the onset of the PL and Phos spectra, were estimated to be 3.06/2.99 eV for BOC-PC and 3.11/3.05 eV for BOC-PSi. Although the singlet-triplet splitting of the CT excited states in BOC-PC and BOC-PSi is quite similar (0.07 vs. 0.06 eV), there is an evident difference in the energy splitting between their 1CT and 3LED/3LEA states. As depicted in Fig. S8,† the 3LE energy levels of PC and PSi were both calculated to be approximately 3.20 eV, while that of BOC was estimated to be 3.35 eV. Therefore, the absolute values of ΔEST (1CT–3LED) and ΔEST (1CT–3LEA) of BOC-PC are 0.14 eV and 0.29 eV, respectively, whereas those for BOC-PSi are 0.09 eV and 0.24 eV respectively. Considering that D–A dyads with highly twisted molecular geometries typically exhibit a much stronger SOC effect between a 3LE and a 1CT state compared to that between a 3CT and a 1CT state,13 the faster RISC process in BOC-PSi, as compared to BOC-PC, may be attributed to the smaller absolute values of ΔEST (1CT–3LE).
This deduction was supported by theoretical computations. As depicted in Fig. 2a, the S1 and T1 states of BOC-PC and BOC-PSi were both calculated to show a CT feature, and the energy splitting between the two states is 0.01 eV for both compounds. In line with our conjecture, the calculated SOC constants for the T1 → S1 process are 0.04 cm−1 for BOC-PC and 0.03 cm−1 for BOC-PSi, both are too small to trigger fast RISC processes. Nevertheless, the T2 states of BOC-PC and BOC-PSi are both dominated by the BOC unit, displaying a 3LEA character. Despite having an identical T2 energy level (∼3.0 eV), the lower S1 state of BOC-PC results in a larger absolute value of ΔEST (1CT–3LEA) compared to BOC-PSi (0.18 vs. 0.06 eV). Considering that the SOC constants between the T2 and S1 states of BOC-PC and BOC-PSi are relatively large (>0.70 cm−1), both compounds are expected to undergo a relatively fast T2 → S1 RISC process. However, BOC-PSi is likely to achieve a larger kRISC due to its much smaller ΔEST (1CT–3LEA) value compared to BOC-PC. Additionally, for BOC-PSi, its T3 state (exhibiting a 3LED character) was found to be close to its S1 state (ΔEST = 0.20 eV), and the calculated SOC constant for the T3 → S1 process was also substantial at 0.58 cm−1, implying the presence of a fast T3 → S1 RISC process in BOC-PSi. Consequently, the large kRISC of BOC-PSi is believed to stem from its effective multi-channel RISC processes.
Therefore, through the substitution of the sp3-C atom within the 9,10-diphenylacridine segment of BOC-PC with a sp3-Si, we have acquired BOC-PSi, which has a better-balanced and robust molecular framework, a slightly deepened HOMO energy level, and a maintained high 3LE energy level. As a result, in comparison to BOC-PC, BOC-PSi shows a wider emission bandgap, a narrower FWHM, a more suppressed non-radiative process and hence a higher ΦPL, as well as a faster RISC process. Consequently, it is expected that BOC-PSi will demonstrate superior EL performance to BOC-PC.
Electroluminescence performance
Subsequently, OLEDs were fabricated using BOC-PSi or BOC-PC as the doping guest (15 wt% in DPEPO): ITO/PEDOT: PSS (30 nm)/TAPC (30 nm)/TCTA (10 nm)/mCP (10 nm)/DPEPO: emitters (15 wt%, 40 nm)/DPEPO (5 nm)/TmPyPB (35 nm)/LiF (1.2 nm)/Al (120 nm). Consistent with our conjecture, the BOC-PSi-based device II shows significantly superior EL performance to the BOC-PC-based device I. As depicted in Table 2, device I displayed an inferior EL color purity than device II (CIEy: 0.085 vs. 0.049) due to the red-shifted EL band (λEL: 450 vs. 433 nm) and wide FWHM (62 vs. 52 nm). Besides, device I also exhibited a lower EQEmax (14.8% vs. 19.6%) together with more pronounced efficiency roll-off. These disadvantages can be ascribed to the narrower emission bandgap, wider FWHM, lower ΦPL and slower RISC process of BOC-PC than BOC-PSi. All these findings confirm the potential of BOC-PSi as a more promising deep-blue OLED emitter than BOC-PC. It is noteworthy that the BOC-PSi-based OLEDs stand as one of the top-performing examples among reported deep blue TADF OLEDs whose color purity approaches the Rec.2020 blue standard.| Dopant | Device | λ EL (nm) | FWHM (nm) | CEmax (cd A−1) | EQEa/EQEb (%) | Roll-offc | CIE1931 (x, y) |
|---|---|---|---|---|---|---|---|
| a Device maximum external quantum efficiency. b External quantum efficiency at 100 cd m−2. c Efficiency roll off from 1 cd m−2 to 100 cd m−2. | |||||||
| BOC-PC | I | 450 | 62 | 11.12 | 14.8/2.8 | 85% | (0.148, 0.085) |
| BOC-PSi | II | 433 | 53 | 9.10 | 19.6/14.8 | 24% | (0.154, 0.049) |
Conclusion
In conclusion, we demonstrated that by employing rigid D and A structural units, TADF D–A dyads with narrow FWHM and high ΦPL can be developed. Using BOC-PSi as an example, we proved that 10,10-diphenyl-5,10-dihydrodibenzo[b,e][1,4]azasiline (PSi) is a more promising D unit than 9,9-diphenyl-9,10-dihydroacridine (PC) when constructing deep blue TADF D–A dyads with good color gamut. The reason for this is that the replacement of a sp3-C with a sp3-Si helps to restrict the low-frequency bending vibration along the whole D–A molecular backbone, thus minimizing exciton energy loss, while concurrently accelerating the multi-channel RISC processes. OLEDs using BOC-PSi as the emitter exhibited not only an impressive EQEmax of nearly 20% and a narrow FWHM of 53 nm, but also a superior color purity approaching the Rec.2020 blue standard. This work would provide a new avenue in developing highly efficient, narrow-emission and wide-bandgap blue TADF-OLED materials.Data availability
Experimental procedures, details of the calculations, and additional data can be found in the ESI.†Author contributions
C. L., K. Z. and Y. L. contributed equally to this work. C. L. – conceptualization, synthesis, investigation, visualization, writing – original draft; K. Z. – OLED devices, investigation; Y. L. – investigation, funding acquisition, writing – review & editing; Y. Y. – investigation (photophysics); Y. H. – investigation (photophysics); M. J. – investigation (photophysics); Y. H. – investigation (photophysics); Y. L. – synthesis; J. T. – funding acquisition, OLED devices, review & editing; Y. H. – review & editing; Z. L. – funding acquisition, project administration, writing – review & editing, conceptualization, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 22105136 and 22075187), the Science and Technology Development Fund (FDCT), Macau SAR (no. 0008/2022/AMJ), the China Postdoctoral Science Foundation (no. 2021M702324) and Fundamental Research Funds for the Central Universities. The authors thank Dr Peng Wu and Dr Yang Feng of the Analytical & Testing Center and Dr Meng Yang of the College of Chemistry, Sichuan University for photophysical and molecular structure characterizations.References
- J. M. Ha, S. H. Hur, A. Pathak, J.-E. Jeong and H. Y. Woo, Recent advances in organic luminescent materials with narrowband emission, NPG Asia Mater., 2021, 13, 53 CrossRef CAS.
- R. K. Konidena and K. R. Naveen, Boron-Based Narrowband Multiresonance Delayed Fluorescent Emitters for Organic Light-Emitting Diodes, Adv. Photonics Res., 2022, 3, 2200201 CrossRef CAS.
- H. J. Kim, H. Kang, J. E. Jeong, S. H. Park, C. W. Koh, C. W. Kim, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Ultra-Deep-Blue Aggregation-Induced Delayed Fluorescence Emitters: Achieving Nearly 16% EQE in Solution-Processed Nondoped and Doped OLEDs with CIEy < 0.1, Adv. Funct. Mater., 2021, 31, 2102588 CrossRef CAS.
- V. V. Patil, H. L. Lee, I. Kim, K. H. Lee, W. J. Chung, J. Kim, S. Park, H. Choi, W. J. Son, S. O. Jeon and J. Y. Lee, Purely Spin-Vibronic Coupling Assisted Triplet to Singlet Up-Conversion for Real Deep Blue Organic Light-Emitting Diodes with Over 20% Efficiency and y Color Coordinate of 0.05, Adv. Sci., 2021, 8, 2101137 CrossRef CAS PubMed.
- H.-J. Tan, G.-X. Yang, Y.-L. Deng, C. Cao, J.-H. Tan, Z.-L. Zhu, W.-C. Chen, Y. Xiong, J. X. Jian, C.-S. Lee and Q.-X. Tong, Deep-Blue OLEDs with Rec.2020 Blue Gamut Compliance and EQE over 22% Achieved by Conformation Engineering, Adv. Mater., 2022, 34, 2200537 CrossRef CAS PubMed.
- J. Kang, S. O. Jeon, H. L. Lee, J. Lim, U. Jo and J. Y. Lee, Expanded multiple-resonance structure for highly efficient narrowband deep-blue organic light-emitting diodes, Mater. Today, 2023, 69, 88–96 CrossRef CAS.
- C. Cao, J.-H. Tan, Z.-L. Zhu, J.-D. Lin, H.-J. Tan, H. Chen, Y. Yuan, M.-K. Tse, W.-C. Chen and C.-S. Lee, Intramolecular Cyclization: A Convenient Strategy to Realize Efficient BT.2020 Blue Multi-Resonance Emitter for Organic Light-Emitting Diodes, Angew. Chem., Int. Ed., 2023, 62, e202215226 CrossRef CAS PubMed.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO-LUMO Separation by the Multiple Resonance Effect, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- D. H. Ahn, S. W. Kim, H. Lee, I. J. Ko, D. Karthik, J. Y. Lee and J. H. Kwon, Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors, Nat. Photonics, 2019, 13, 540–546 CrossRef CAS.
- H. J. Kim and T. Yasuda, Narrowband Emissive Thermally Activated Delayed Fluorescence Materials, Adv. Opt. Mater., 2022, 10, 2201714 CrossRef CAS.
- X. Wu, B.-K. Su, D.-G. Chen, D. Liu, C.-C. Wu, Z.-X. Huang, T.-C. Lin, C.-H. Wu, M. Zhu, E. Y. Li, W.-Y. Hung, W. Zhu and P.-T. Chou, The role of host–guest interactions in organic emitters employing MR-TADF, Nat. Photonics, 2021, 15, 780–786 CrossRef CAS.
- P. K. Samanta, D. Kim, V. Coropceanu and J.-L. Bredas, Up-Conversion Intersystem Crossing Rates in Organic Emitters for Thermally Activated Delayed Fluorescence: Impact of the Nature of Singlet vs Triplet Excited States, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Revealing the spin-vibronic coupling mechanism of thermally activated delayed fluorescence, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- X. Fan, X. Hao, F. Huang, J. Yu, K. Wang and X. Zhang, RGB Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes toward Realizing the BT.2020 Standard, Adv. Sci., 2023, 10, 2303504 CrossRef CAS PubMed.
- H. Lim, H. J. Cheon, S.-J. Woo, S.-K. Kwon, Y.-H. Kim and J.-J. Kim, Highly Efficient Deep-Blue OLEDs using a TADF Emitter with a Narrow Emission Spectrum and High Horizontal Emitting Dipole Ratio, Adv. Mater., 2020, 32, 2004083 CrossRef CAS PubMed.
- J. W. Sun, J. Y. Baek, K.-H. Kim, C.-K. Moon, J.-H. Lee, S.-K. Kwon, Y.-H. Kim and J.-J. Kim, Thermally Activated Delayed Fluorescence from Azasiline Based Intramolecular Charge-Transfer Emitter (DTPDDA) and a Highly Efficient Blue Light Emitting Diode, Chem. Mater., 2015, 27, 6675–6681 CrossRef CAS.
- K. T. Shigehiro Yamaguchi, Silole-containing σ- and π-conjugated compounds, J. Chem. Soc., Dalton Trans., 1998, 3693–3702 RSC.
- Y. H. Lee, W. Lee, T. Lee, D. Lee, J. Jung, S. Yoo and M. H. Lee, Blue TADF Emitters Based on B-Heterotriangulene Acceptors for Highly Efficient OLEDs with Reduced Efficiency Roll-Off, ACS Appl. Mater. Interfaces, 2021, 13, 45778–45788 CrossRef CAS PubMed.
- C. Li, Y. Luo, Y. Huang, H. Qiu and Z. Lu, A Low-Cost Test Method for Accurate Detection of Different Excited-State Species with a Lifetime Span over 5 Orders of Magnitude in One Time Window, Anal. Chem., 2023, 95, 8150–8155 CrossRef CAS PubMed.
- J. Hwang, H. Kang, J.-E. Jeong, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Donor engineered Deep-Blue emitters for tuning luminescence mechanism in Solution-Processed OLEDs, Chem. Eng. J., 2021, 416, 129185 CrossRef CAS.
- S. Jiang, Y. Yu, D. Li, Z. Chen, Y. He, M. Li, G.-X. Yang, W. Qiu, Z. Yang, Y. Gan, J. Lin, Y. Ma and S. J. Su, Sulfone-Embedded Heterocyclic Narrowband Emitters with Strengthened Molecular Rigidity and Suppressed High-Frequency Vibronic Coupling, Angew. Chem., Int. Ed., 2023, 62, e202218892 CrossRef CAS PubMed.
- J. Liu, Y. Zhu, T. Tsuboi, C. Deng, W. Lou, D. Wang, T. Liu and Q. Zhang, Toward a BT.2020 green emitter through a combined multiple resonance effect and multi-lock strategy, Nat. Commun., 2022, 13, 4876 CrossRef CAS PubMed.
- H.-T. Feng, J. Zeng, P.-A. Yin, X.-D. Wang, Q. Peng, Z. Zhao, J. W. Y. Lam and B. Z. Tang, Tuning molecular emission of organic emitters from fluorescence to phosphorescence through push-pull electronic effects, Nat. Commun., 2020, 11, 2617 CrossRef CAS PubMed.
- H. Lee, R. Braveenth, J. D. Park, C. Y. Jeon, H. S. Lee and J. H. Kwon, Manipulating Spectral Width and Emission Wavelength towards Highly Efficient Blue Asymmetric Carbazole Fused Multi-Resonance Emitters, ACS Appl. Mater. Interfaces, 2022, 14, 36927–36935 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, computational details, 1H and 13C NMR spectra, HRMS, and single-crystal XRD structures of the target compounds. CCDC 2307420–2307421. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc06763g |
| This journal is © The Royal Society of Chemistry 2024 |