Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
s-Block metal complexes of superbulky (tBu3Si)2N−: a new weakly coordinating anion?†
Christian
Knüpfer
 ,
Lukas
Klerner
,
Jonathan
Mai
,
Lukas
Klerner
,
Jonathan
Mai
 ,
Jens
Langer
,
Jens
Langer
 and
Sjoerd
Harder
and
Sjoerd
Harder
 *
*
Inorganic and Organometallic Chemistry, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstraße 1, 91058, Erlangen, Germany. E-mail: sjoerd.harder@fau.de
First published on 20th February 2024
Abstract
Sterically hindered amide anions have found widespread application as deprotonation agents or as ligands to stabilize metals in unusual coordination geometries or oxidation states. The use of bulky amides has also been advantageous in catalyst design. Herein we present s-block metal chemistry with one of the bulkiest known amide ligands: (tBu3Si)2N− (abbreviated: tBuN−). The parent amine (tBuNH), introduced earlier by Wiberg, is extremely resistant to deprotonation (even with nBuLi/KOtBu superbases) but can be deprotonated slowly with a blue Cs+/e− electride formed by addition of Cs0 to THF. (tBuN)Cs crystallized as a separated ion-pair, even without cocrystallized solvent. As salt-metathesis reactions with (tBuN)Cs are sluggish and incomplete, it has only limited use as an amide transfer reagent. However, ball-milling with LiI led to quantitative formation of (tBuN)Li and CsI. Structural characterization shows that (tBuN)Li is a monomeric contact ion-pair with a relatively short N–Li bond, an unusual T-shaped coordination geometry around N and extremely short Li⋯Me anagostic interactions. Crystal structures are compared with Li and Cs complexes of less bulky amide ligands (iPr3Si)2N− (iPrN−) and (Me3Si)2N− (MeN−). DFT calculations show trends in the geometries and electron distributions of amide ligands of increasing steric bulk (MeN− < iPrN− < tBuN−) and confirm that tBuN− is a rare example of a halogen-free weakly coordinating anion.
Introduction
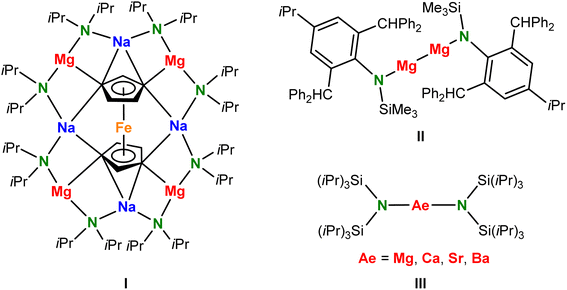
According to the formal IUPAC definition,1 metal amide complexes should not be described as organometallic compounds. However, due to their crucial role and close relationship to organometallic chemistry, they are often considered as such.2 Especially the group 1 metal amides have found widespread applications as amide ligand transfer reagents to access metal amide complexes across the periodic table. Given the considerably higher electronegativity of N compared to C, amide anions are somewhat less Brønsted basic than corresponding carbanions. Despite this fundamental difference, carefully chosen, sterically hindered amide bases like lithium diisopropylamide (LDA) or lithium 2,2,6,6-tetramethylpiperidide (LiTMP) found fame as powerful non-nucleophilic deprotonation reagents.3,4 Amide anions are also markedly different from carbanions by the presence of two lone-pairs of electrons at N, which sets them apart as great bridging ligands. Their rich coordination chemistry has led to stunning examples of their unique deprotonation power5,6 and self-assembled aggregates in which multiply deprotonated substrates are embedded in a ring of metal cations that act as an inverse crown ether7 (e.g.I in Scheme 1).Within the large range of amines, the silyl-substituted amine HN(SiMe3)2 (1,1,1,3,3,3-hexamethyldisilazane) is arguably the most common source for synthesis of metal complexes.8,9 The corresponding (Me3Si)2N− ligand, often abbreviated as HMDS or N′′, is attractive while its Me3Si-substituents offer steric protection of the metal center and stabilize the neighbouring negative charge on N by polarization and negative hyperconjugation; cf. the pKa values for HN(SiMe3)2 (25.8) and HNiPr2 (35.7).10,11 There are, however, also drawbacks of this ligand which are exemplified by N–Si bond cleavage12,13 or Me–Si deprotonation.14
In order to achieve greater stability and improve steric protection, a large range of bulkier silyl-substituted amides have been designed.15 Such bulky monodentate ligands achieved stabilization of low-oxidation-state ZnI and MgI centers (e.g. in II).16,17 They also found application in the synthesis of nearly linear lanthanide metal complexes which have been studied extensively for their magnetic properties.18 Our interest in bulky amide ligands is related to their ability to lower the aggregation state of alkaline-earth (Ae) metal complexes19,20 and reduce the nuclearity of Ae metal hydride clusters.21 For this reason, bulky Ae metal amide complexes like Ae[N(SiiPr3)2] (III) are much more reactive in hydrogenation catalysis21 than Ae[N(SiMe3)2]2 catalysts22,23 and under controlled reaction conditions even found application as catalysts for Hydrogen-Isotope-Exchange (HIE) in aromatic substrates.24 As the activities of these catalysts increase with the size of the amide ligand, we are interested in s-block metal complexes with even bulkier amide ligands. It is, however, questionable what the limitations to the bulk of the substituents are. Herein, we report on the unusual coordination chemistry of the extremely bulky (tBu3Si)2N− anion (abbreviated: tBuN−), for which there is hitherto a complete lack of knowledge, and show comparisons with the smaller (iPr3Si)2N− (iPrN−) and (Me3Si)2N− (MeN−) anions.
Results and discussion
Syntheses
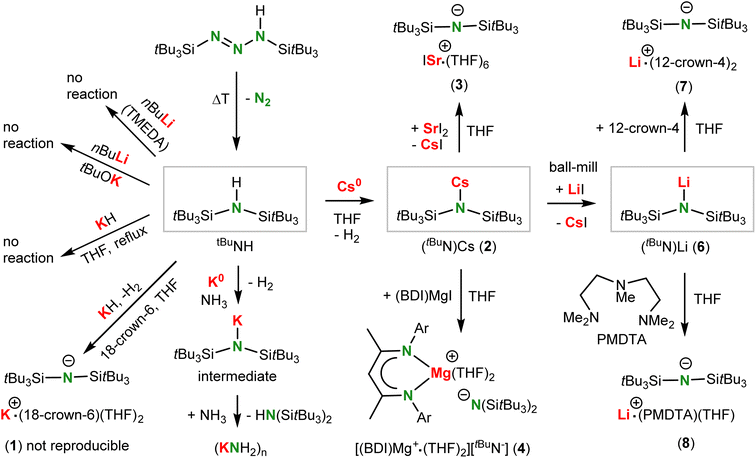
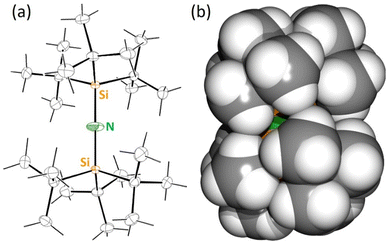
The parent amine (tBu3Si)2NH (tBuNH) was obtained by thermal decomposition of (tBu3Si)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH(SitBu3) following a method reported by Wiberg (Scheme 2).25 A slightly modified procedure gave the ligand precursor in crystalline purity (yield: 71%). Although the Si–N–Si angle in tBuNH is reported to be 167(2)°,25 a later refinement26 and our structure determination show a linear structure with N on an inversion center. There are, however, large N displacement factors in two dimensions (U1 0.072, U2 0.018, U3 0.054) which is the plane perpendicular on the Si–N–Si axis (Fig. 1a). This shows that, similar to [(Ar)5Cp]2Ae sandwich complexes,27 the molecule slightly deviates from linearity and the central N atom is disordered over a ring of positions. Due to disorder the exact location of the N–H hydrogen atom could not be determined. As the N–H functionality is fully embedded in the bulk of two very large tBu3Si-substituents (Fig. 1b), the deprotonation of tBuNH turned out to be extremely challenging.
N–NH(SitBu3) following a method reported by Wiberg (Scheme 2).25 A slightly modified procedure gave the ligand precursor in crystalline purity (yield: 71%). Although the Si–N–Si angle in tBuNH is reported to be 167(2)°,25 a later refinement26 and our structure determination show a linear structure with N on an inversion center. There are, however, large N displacement factors in two dimensions (U1 0.072, U2 0.018, U3 0.054) which is the plane perpendicular on the Si–N–Si axis (Fig. 1a). This shows that, similar to [(Ar)5Cp]2Ae sandwich complexes,27 the molecule slightly deviates from linearity and the central N atom is disordered over a ring of positions. Due to disorder the exact location of the N–H hydrogen atom could not be determined. As the N–H functionality is fully embedded in the bulk of two very large tBu3Si-substituents (Fig. 1b), the deprotonation of tBuNH turned out to be extremely challenging.
Lithiation with nBuLi in boiling hexane, or with nBuLi/TMEDA at somewhat lower temperatures to avoid TMEDA deprotonation,28 did not give any conversion. Treating tBuNH with KH in boiling THF or under microwave conditions at 180 °C did not show reaction. In contrast, the somewhat smaller amine iPrNH could be smoothly deprotonated even in toluene.18 Addition of 18-crown-6 did give deprotonation and a small batch of crystals with composition [K+·(18-crown-6)(THF)2][tBuN−] (1) was isolated in very low yields (crystal structure: Fig. S37 and S38†). Repeated attempts to improve this synthesis led to the conclusion that this procedure is irreproducible. As tBuNH did not even react with the superbase mixture nBuLi/KOtBu, this amine is particularly resistant towards deprotonation.
Analysis of the thermodynamics of the reaction by DFT calculation (B3PW91/def2tzvp including GD3BJ dispersion corrections) showed that silyl-substituents have a strong stabilizing effect on the amide anion and that deprotonation of silyl-substituted amines becomes more exergonic with increasing bulk (Scheme 3). The reluctance of tBuNH to be deprotonated is therefore exclusively due to kinetic problems related to the very poor accessibility of the N–H proton. We reasoned that application of a metal electride29 may solve this problem. Addition of tBuNH to a dark-blue solution of K0 in NH3 led to rapid decolorization, however, we were only able to isolate highly insoluble KNH2 in the form of a grey powder. The latter is likely formed by reaction of intermediate (tBuN)K with NH3 which, although contrathermodynamic (see Scheme 3), can be explained by the insolubility and precipitation of KNH2. Reaction of tBuNH with a blue K0/18-crown-6 electride solution29a led to crown ether decomposition, which is a known side-reaction for such reagents.30 However, the reaction with a blue Cs+/e− electride formed by addition of Cs0 to THF gave full conversion of the amine. The reaction is extremely slow and needs slight heating at 40 °C for four days. Under these conditions not only (tBuN)Cs (2) but also several solvent decomposition side-products are formed. As THF is prone to C–H activation in the OCH2 group and subsequent ring opening,31 we changed the solvent to the more robust tetrahydropyran (THP). Reaction of a Cs0/THP electride solution with tBuNH resulted in considerably less side-product formation and gave solvent-free (tBuN)Cs in 84% yield. Although formally a deprotonation, this procedure should be described as a redox reaction in which one equivalent of Cs0 reduces tBuNH to tBuN− and 0.5 equivalent of H2 (the latter could be detected by 1H NMR monitoring). We used excess of Cs0 to accelerate conversion and reduce the amount of side-products. This synthetic method shows that electrides, which nowadays can also be obtained simply by ball-milling,29c may have strong potential as a reagent for the formal deprotonation of challenging substrates.
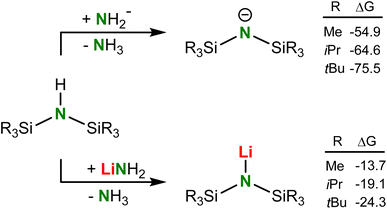 | ||
| Scheme 3 Calculated free energies (298 K, kcal mol−1) for the deprotonation of HN(SiR3)2 with NH2− or LiNH2 (B3PW91/def2tzvp including GD3BJ dispersion correction). | ||
The amide complex (tBuN)Cs (1) with a large heavy alkali metal cation could potentially be used in syntheses of Ae amide complexes by salt-metathesis. However, reactions between (tBuN)Cs and AeI2 to give (tBuN)2Ae and CsI were found to be problematic. These ligand exchange reactions in either THF or toluene are very slow, irreproducible and incomplete. This led to complex reaction mixtures from which in one case we were able to isolate some crystals of the ion-pair [ISr+·(THF)6][tBuN−] (3) for which we could determine the structure (Fig. S43 and S44†). The reaction of (tBuN)Cs with (BDI)MgI in THF was clean and reproducible and the complex [(BDI)Mg+·(THF)2][tBuN−] (4) was isolated in 55% yield (BDI = β-diketiminate ligand HC[C(Me)N-DIPP]2, DIPP = 2,6-diisopropylphenyl). Product crystallization was problematic but recrystallization from Et2O gave good quality crystals of the ion-pair [(BDI)Mg+·(THF)(Et2O)][tBuN−] (5) with Et2O/THF disorder (Fig. S45 and S46†).
Interestingly, ball-milling (tBuN)Cs and LiI and subsequent extraction with hexane cleanly led to formation of (tBuN)Li (6) which was isolated in the form of colorless crystals in 55% yield. The driving force for this exchange reaction is the formation of CsI. Note that (tBuN)Li could not be obtained directly from the amine and a Li base. Addition of either 12-crown-4 or PMDTA to a THF solution of (tBuN)Li led to crystallization of the free tBuN− anion with non-coordinating Li+·(12-crown-4)2 (7, 71% yield) or Li+·(PMDTA)(THF) (8) cations (crystal structures: Fig. S49–S52†); PMDTA = N,N′,N′,N′′,N′′-pentamethyl-diethylenetriamine.
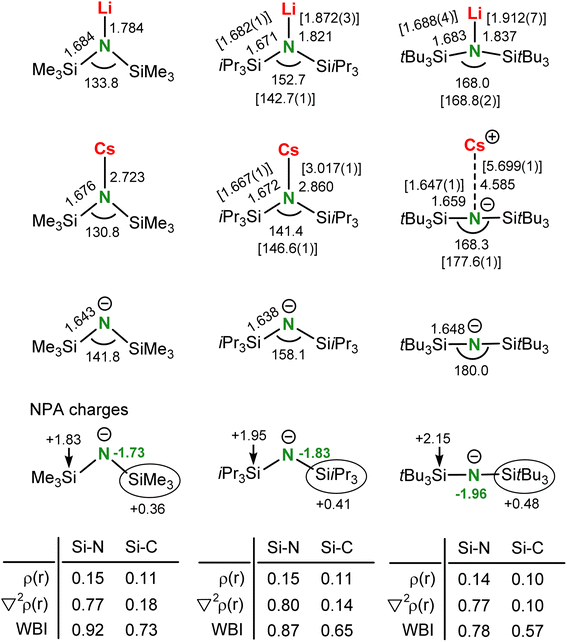
Crystal structures of Li and Cs amide complexes
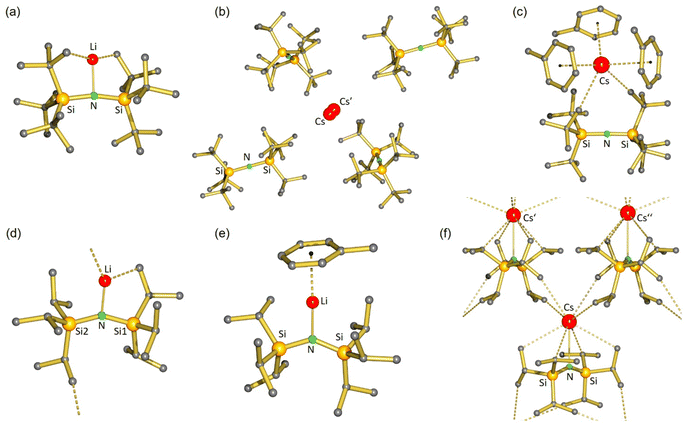
As it is questionable whether a bulky amide anion like tBuN− can coordinate at all to metal cations, we aimed to reveal the crystal structures of (tBuN)Li and (tBuN)Cs, preferably without strongly coordinating solvents. Solvent-free (tBuN)Li crystallized from hot hexane in space group P1 with four independent, partially disordered, structurally similar molecules in the unit cell (Fig. 2a). To date, it represents the only example of a monomeric LiNR2 complex without stabilizing interactions between Li+ and electron-rich ligands or substituents (e.g. O, N, P, aryl). Although the Si–N–Si units with an average angle of 167.4° (range: 165.7(2)°–168.8(2)°) are close to linear, there are distinct N–Li contacts which are surprisingly small (average: 1.913 Å, range: 1.905(7)–1.920(6) Å). For comparison, the N–Li distance of 1.965(4) Å in monomeric (MeN)Li·(12-crown-4) is considerably larger.32 The tricoordinate N atom has an unusual nearly T-shaped coordination geometry. The Li+ cation in (tBuN)Li is embedded in a surrounding of tBu groups with extremely short anagostic interactions. The shortest Li⋯CH3 distances in the non-disordered molecules range from 2.303(8) to 2.338(7) Å (average: 2.325 Å). This is considerably shorter than the shortest reported anagostic Li⋯CH3 interactions for low-coordinate Li complexes by Snaith (2.415(7)–2.823(7) Å; average: 2.607 Å) or Scherer (2.658(5) Å).33,34 The shortest Li⋯H distances in a structure determination of (tBuN)Li with freely refined H atoms vary from 1.68–2.00 Å but without neutron diffraction data, these values are not accurate.The very short Li⋯CH3 distances in (tBuN)Li indicate strong secondary bonding interactions. Since metal⋯H3C–X bonds become stronger with decreasing electronegativity of X, i.e. with increasing H3Cδ−–Xδ+ bond polarity,35 it is surprising that the Li⋯H3C–C bonds in (tBuN)Li (average: 2.325 Å) are so much shorter than the average Li⋯H3Cδ−–Siδ+ anagostic interactions in trimeric36,37 or tetrameric38 (MeN)Li of 2.888 Å and 2.955 Å, respectively. The N–Li bond in (tBuN)Li can be easily cleaved by addition of 12-crown-4 which resulted in crystallization of the ion-pair [Li+·(12-crown4)2][tBuN−] (7) (see Fig. S49 and S50†).
The amide complex with the larger Cs+ cation, (tBuN)Cs, is hardly soluble in hexane. Despite numerous attempts, it was impossible to obtain crystals from strictly nonpolar solvents. However, it does dissolve in polar but weakly coordinating chlorobenzene39 from which it crystallized solvent-free. The crystal structure (Fig. 2b) shows a nearly linear tBuN− anion (Si–N–Si 177.6(1)°) and very short Si–N bonds (1.652(2) Å). In contrast to (tBuN)Li, there is no N–metal bonding (N⋯Cs 5.520(2) Å). The Cs+ cation resides in a cavity spanned by four tBuN− anions in which there are at most weak anagostic Cs⋯CH3 interactions (3.497(3)–3.562(3) Å). The Cs+ atom is disordered over two positions separated by circa 0.45 Å. This is likely due to the extremely weak electrostatic bonding interaction between tBuN−, one of the largest amide anions, and Cs+, the largest stable metal cation.
Addition of toluene led to strong Cs+⋯toluene interactions and crystallization of monomeric (tBuN)Cs·(toluene)3 in the monoclinic space group P21/c with one molecule in the asymmetric unit (Fig. 2c). The Cs+ cation is bound to the three toluene solvent molecules in a η6-fashion with Cs-ring centroid distances ranging from 3.251 to 3.321 Å. The Cs coordination sphere is completed by two anagostic Cs⋯CH3 interactions of 3.423(2) and 3.605(2) Å. There is hardly structural information on organometallic Cs compounds. The few reported Cs⋯H3Cδ−–Siδ+ anagostic interactions in (MeN)Cs complexes, which should be stronger than Cs⋯H3C–C bonds,35 are much longer (range: 3.623(4)–3.879(5) Å).39,40 This again shows the importance for secondary bonding in metal complexes with the tBuN ligand. The Si–N–Si backbone in the anion tBuN− is close to being linear (177.6(1)°).
Although there are many structures of alkali metal complexes with bulky bis(silyl) amide ligands,42,43 a comparison is often difficult due to use of different coordinating solvents or different silyl substituents. In order to compare Li and Cs amide structures with ligands of increasing steric bulk, MeN < iPrN < tBuN, we therefore also synthesized (iPrN)Li (9, 85% yield) and (iPrN)Cs (10, 68% yield). Both could be crystallized either from apolar hexanes or slightly polar aromatic solvents like toluene.
Solvent-free (iPrN)Li (9) crystallizes monomeric with a bent Si–N–Si framework (142.73(7)°) and a very short anagostic Li⋯CH3 interaction of 2.292(3) Å to a neighbouring molecule, resulting in chain-like polymer (Fig. 2d). An intramolecular anagostic Li⋯CH3 interaction of 2.531(3) Å results in slight asymmetry (Li–N–Si1 = 104.7(1)°; Li–N–Si2 = 111.7(1)°), typically also found in complexes with the MeN-ligand.44 The Li–N bond (1.872(3) Å) is significantly shorter than in solvent-free (tBuN)Li (average: 1.913 Å). This is due to bending of the amide ligand resulting in compacter orbitals on N with more s-character. When crystallized from toluene, the complex (iPrN)Li·(toluene) (9·toluene) was isolated (Fig. 2e). The crystal structure shows capping of the Li+ cation with a η6-coordinating toluene ligand (Li-centroid: 2.391 Å) leading to disappearance of anagostic Li⋯CH3 interactions (Li⋯C > 3.0 Å) and significant elongation of the N–Li bond from 1.872(3) Å in (iPrN)Li to 1.916(3) Å in (iPrN)Li·(toluene).
Complex (iPrN)Cs (10) crystallizes from hexane or hexane/toluene mixtures as a solvent-free C2-symmetric monomer (Fig. 2f). The structure is bent (Si–N–Si 146.6(1)°) allowing for a Cs–N interaction of 3.017(2) Å which is considerably shorter than those in dimeric [(MeN)Cs]2 structures (3.016(2)–3.149(2) Å).45 Although crystallized in the presence of toluene, there is no aromatic capping ligand, resulting in a two-dimensional network of four intramolecular and four intermolecular anagostic Cs⋯C interactions in the range 3.573(2)–3.710(2) Å.
There is a clear trend in the crystal structures of solvent-free (MeN)Li, (iPrN)Li and (tBuN)Li (Scheme 4). The smallest lithium amide reagent crystallizes either as cyclic trimer or tetramer.36–38 The bulkier (iPrN)Li crystallizes as a monomer with strong anagostic interactions to neighbours resulting in a one-dimensional polymer. Addition of toluene results in formation of a solvated monomer. The bulkiest (tBuN)Li forms a discrete monomer in which Li is saturated only by intramolecular anagostic interactions. Although the N–Li bond in (tBuN)Li is easily broken by addition of 12-crown-4, crystallization of (MeN)Li with this Li+ specific crown ether gave monomeric (MeN)Li·(12-crown-4) with a short N–Li contact of 1.965(4) Å.32
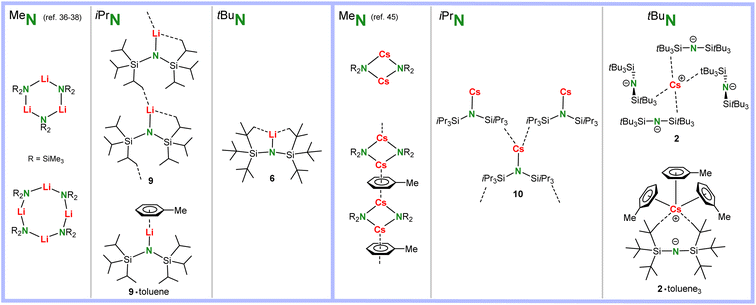 | ||
| Scheme 4 Comparison of polar solvent-free Li and Cs amide structures with ligands of increasing bulk: MeN < iPrN < tBuN. | ||
Although alkali metal complexes with large cations usually tend to form extensive coordination polymers, the structure of solvent-free (MeN)Cs is only dimeric, while addition of toluene results in a linear array of dimers bridged by Cs⋯η6-toluene interactions (Scheme 4).45 In contrast, (iPrN)Cs crystallizes from hexanes as a monomer with a short Cs–N distance and an extended network of intra- and intermolecular anagostic interactions. The complex with the bulkiest amide ligand, (tBuN)Cs, crystallized as an ion-pair in which the amide ligand functions as a weakly coordinating anion (WCA) through longer anagostic Cs⋯C interactions. From toluene the complex crystallized as (tBuN)Cs·(toluene)3 in which there is also no Cs–N contact.
The superbulky amide anion tBuN− is therefore an odd example of a halogen-free WCA. Nearly all WCAs are heavily fluorinated or halogenated46,47 and there are only few examples of halogen-free WCAs.48,49 The latter are especially desirable for their great stability as electrolytes in metal batteries.50,51
Theory
There is a strong analogy between the metal coordination of the bis(silyl) amide anion tBuN− and that of the isolobal bis(silyl) ethers R3SiOSiR3 which are notorious for their extremely poor donor ability.52–55 This is underscored by the fact that hydrogen bonds to silyl ethers are very rare.56–58 A first example of unsupported metal⋯O(SiMe3)2 bonding was only recently structurally characterized.59 It has been previously discussed that the main reason for the poor electron pair donating abilities of R3SiOSiR3 is negative hyperconjugation, i.e. delocalization of free electron pairs at the central O into the σ*(Si–R) bond, leading to wide Si–O–Si angles, short Si–O bonds and long Si–R bonds.54 However, this theory has been abandoned and its unusual geometry can also be explained with the ionic character of the Si–O bond which increases upon widening the Si–O–Si angle.52,55 Despite the strong similarities between isolobal (R3Si)2O and (R3Si)2N− species, the bonding and electronic structure of bis(silyl) amide ligands has not been described in detail. Herein we provide DFT calculations on monomeric Li and Cs complexes and free anions of increasing bulk: MeN− < iPrN− < tBuN− (Scheme 5).The structures have been optimized at the B3PW91/def2tzvp level of theory. As it was found that using Grimme's third dispersion correction with Becke–Johnson damping (GD3BJ) gave a better match with the experimental data, we only show results with dispersion correction. The coordination geometry of Li in (tBuN)Li deserves some special attention. Also in the calculated structure the Li+ cation is fully embedded in the ligand and bound by a short N–Li bond and anagostic interactions. This results in an unusually large value for the buried volume (Vbur).60 For monomeric structures the following values have been calculated: (MeN)Li 45.4%, (iPrN)Li 72.7% and (tBuN)Li 86.6% (H atoms have been included, Table S2 and Fig. S32–S34†).
Comparison of the optimized structures for (MeN)Li, (iPrN)Li and (tBuN)Li shows that the N–Li bonds slightly elongate and the Si–N–Si angles considerably widen when the ligand bulk is increased. A similar trend can be recognized for the corresponding Cs amide complexes with the difference that the Cs–N bond in (tBuN)Cs is completely cleaved, even in a calculated gas phase structure. The difference between The Si–N bond distances remain surprisingly constant when increasing the bulk of the silyl substituents.
As Li and Cs are extremes in the alkali metal series, we also calculated the structures of (tBuN)M (M = Na, K, Rb; Fig. S24†). The gradual increase in calculated N–M distances is larger than the increase in ionic radii (Table 1). The difference between these values, (N–M) – (ionic radius), steadily increases from Li to Rb and at Cs becomes extremely large. The calculated N–metal distances in (tBuN)M compare well with those in crystal structures of monomeric (MeN)M complexes in which metals have been solvated with multi-dentate ligands (Table 1). These data show that although the tBuN− anion becomes gradually less coordinating from Li+ to Rb+, it is truly weakly coordinating only for Cs+. However, it should be considered that these are gas phase calculations in which charge separation is notoriously difficult. Even weak donor ligands like aromatic solvents may induce N–M bond dissociation already for smaller metal cations.
| M | Li | Na | K | Rb | Cs |
|---|---|---|---|---|---|
| a Experimental values in monomeric (MeN)M complexes with multidentate ligands; Li: 12-crown-4, Na: (dimethoxyethane)2, K and Rb: 18-crown-6, Cs: N(CH2CH2OCH2CH2OMe)3.68 | |||||
| N–M (calcd) | 1.837 | 2.246 | 2.690 | 3.001 | 4.585 |
| Ionic radii (CN = 6) | 0.76 | 1.02 | 1.38 | 1.52 | 1.67 |
| (N–M) – (ionic radius) | 1.077 | 1.226 | 1.310 | 1.481 | 2.915 |
| N–M in (MeN)Ma | 1.965 | 2.306 | 2.760 | 3.038 | 3.086 |
Comparison of the free amide anions show a similar widening of Si–N–Si angles. Optimization of the MeN− anion without considering dispersion gave a linear minimum with a Si–N–Si angle of 179.8°. However, with correction for dispersion it optimized to a bent structure with a Si–N–Si angle of 141.8° which fits better to experimental values for free MeN− anions (128.6(1)–143.2(1)°).61–63 The value for the iPrN− anion (158.1°) also corresponds with experiment (152.8(1)°).64 The calculated Si–N–Si angle in tBuN− is truly linear (180.0) and fits the angle in the crystal structure of (tBuN)Cs (177.6(1)°) and those in structures with free amide anions [Li+·(PMDTA)(THF)][tBuN−], [Li+·(12-crown-4)2][tBuN−], [ISr+·(THF)6][tBuN−] and [(BDI)Mg+·(THF)x][tBuN−] in which the Si–N–Si angles range from 173.2(1)° to 179.3(1)°. Interestingly, also in the free anions the calculated Si–N distances hardly vary (range: 1.638–1.648 Å). This stands in stark contrast with the observed trend that the Si–O bonds in H3Si–O–SiH3 become shorter and more ionic when widening the Si–O–Si angle.52 It also contradicts with a strong decrease of Si–O bond lengths in R3Si–O–CR′3 upon becoming more linear.65 The invariance in Si–N bond lengths in MeN−, iPrN− and tBuN− is likely due to two opposing effects that counterbalance each other: (a) increasing bulk results in Si–N–Si widening and therefore Si–N bond shortening, (b) increasing bulk results in Si–N bond lengthening due to increased repulsion of the silyl substituents. In contrast to the invariance of the Si–N bond lengths, the Si–C bonds become longer with widening the Si–N–Si angle. This effect is amplified by increased repulsion of the silyl substituents. Lengthening of the Si–C bonds is in agreement with decreasing Wiberg bond indices (WBIs) (Scheme 5).
Although the Si–N bonds in the series are of similar length, the WBIs are reduced going from MeN− (0.92) to tBuN− (0.78) due to an increase of the ionic character in the Si–N bond. The increase of Si–N bond ionicity in the row MeN− < iPrN− < tBuN− is evident from the charges calculated by Natural-Population-Analysis (Scheme 5).66 Bulky substituents result in Si–N–Si widening and an increase of negative charge on N from −1.73 (MeN−) to −1.96 (tBuN−) with a concomitant increase of positive charge from +1.83 to +2.15 on Si. The ionicity of the Si–N bonds is also evident from atoms-in-molecules analysis.67 Covalent bonds typically have large electron densities ρ(r) and negative values for the Laplacian ∇2ρ(r) in the bond-critical-point (bcp). The Si–N bonds in the series have low electron densities (0.14–0.15 a.u.) and positive Laplacians (0.77–0.80 a.u.), typically observed for ionic bonding (Scheme 5).
It seems counterintuitive that the amide anion with the highest charge on N (tBuN−) shows the poorest donor ability. Although this partially may be explained by steric hindrance, poor coordination properties have also been described for linear H3Si–O–SiH3 in which sterics do not play any role.55 A simple electronic explanation can be found in differences in the spatial arrangement of electrons at the donor site. Whereas the electron density at the central O in bent ether ligands is directional and has the form of a cashew nut, the electron pairs in a linear ether are in a circular donut shape.55 Despite the high electron density at O in the latter, there is an unfavorable non-directional distribution of the charge density. Similar arguments explain the weakly coordinating behavior of the nearly linear tBuN− anion.
The weakly coordinating behavior of tBuN− is also nicely demonstrated by comparison of its electrostatic potential isosurface with those of iPrN− and MeN− (Fig. 3). The negatively charged N (in red) in tBuN− is completely buried by ligand bulk and the positively charged surface (in blue) is highly unfavorable for interactions with cations.
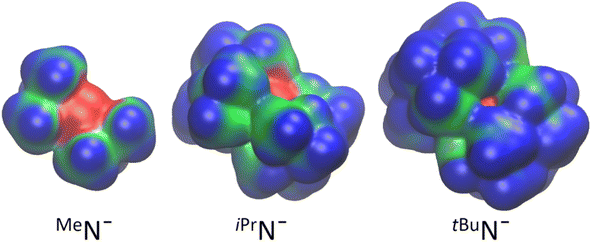 | ||
| Fig. 3 Electrostatic potential isosurfaces for bis(silyl) amide anions. Red is negatively charged, blue is positively charged. | ||
Conclusion
The superbulky amine (tBu3Si)2NH (tBuNH) can be easily obtained by a synthetic method reported by Wiberg and coworkers. However, deprotonation of the relatively acidic N–H functionality turned out to be extremely difficult and could not even be achieved with nBuLi/KOtBu superbase mixtures. This stands in complete contrast to the facile deprotonation of the somewhat smaller amine ligand (iPr3Si)2NH (iPrNH) which reacts smoothly with nBuLi or KH.18,42 The origin for its reluctance to be deprotonated lies in steric congestion and poor accessibility. However, using a blue electride solution of Cs+/e− in THF resulted in slow deprotonation and formation of (tBuN)Cs. This reagent is also the key to (tBuN)Li which could be obtained by reaction of (tBuN)Cs with LiI. However, in contrast to the facile salt metathesis reactions with (iPrN)K,20,43 the application of (tBuN)Cs in the synthesis of other metal complexes is limited.These solvent-free superbulky amide complexes could be obtained in crystalline form by recrystallization from either apolar hexanes or weakly coordinating polar solvents like chlorobenzene. Although the N atom in the anion tBuN− is completely shielded by large bulky tBu3Si-substituents, small cations like Li+ can be embedded between substituents and form relatively short N–Li bonds (1.913 Å). This only results in very slight bending of the Si–N–Si backbone (167.4°) and therefore an unusual T-shaped coordination geometry around N. Replacing Li+ for the much larger Cs+ cation led to cleavage of the metal–N bond and formation of an ion-pair, even in the absence of stabilizing solvent molecules.
Structural comparison of a range of Li and Cs amide complexes with ligands of increasing bulk (MeN < iPrN < tBuN) shows that the tBuN− anion can be considered a WCA, at least for large cations like Cs+ but not for Li+. Reduction of the ligand bulk to iPrN already results in N–Cs bonding. The presence of polar solvents like ethers or amines leads to cleavage of the tBuN-metal bond, also for metals like Li+, Mg2+ or Sr2+.
DFT analysis of a series of Li and Cs amide complexes with ligands of increasing bulk support these experimental observations: (tBuN)Li optimizes as a contact ion-pair with a short N–Li bond whereas (tBuN)Cs optimizes as a separated ion-pair with a long N⋯Cs distance. Calculations also show that increasing the Si–N–Si angle results in more ionic and shorter Si–N bonding. Although the negative charge on N is largest in the bulkiest linear amide anion, tBuN−, this is the anion showing the poorest ability to coordinate to metals. This can be explained partially by steric arguments but also finds it origin in the non-directional distribution of electron density along the N atom.
The very poor donor ability of the tBuN− anion can be exploited in the search for new non- or weakly coordinating anions. It is a rare example of a WCA that is free of halogens. We are currently investigating potential applications of tBuN− and similar bis(silyl)amide anions as WCAs.
Data availability
Crystallographic data has been deposited with the Cambridge structural database.Author contributions
C. Knüpfer: conceptualization, investigation, validation, formal analysis, writing – original draft, visualization. L. Klerner: investigation, validation, formal analysis. J. Mai: investigation, validation, formal analysis. J. Langer: formal analysis, validation. Sjoerd Harder: conceptualization, writing – original draft – review and editing, visualization, validation, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Mrs A. Roth (University of Erlangen-Nürnberg) for CHN analyses and J. Schmidt and Dr C. Färber (University of Erlangen-Nürnberg) for assistance with the NMR analyses.References
- IUPAC, Compendium of Chemical Terminology, Gold Book, ed. A. D. McNaught and A. Wilkinson, Blackwell Scientific Publications, Oxford, 2nd edn, 1997, online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8, DOI:10.1351/goldbook.
- M. Lappert, A. Protchenko, P. Power and A. Seeber, Metal Amide Chemistry, John Wiley & Sons, Ltd, Chichester, 2009 Search PubMed.
- R. E. Mulvey and S. D. Robertson, Angew. Chem., Int. Ed., 2013, 52, 11470–11487 CrossRef CAS PubMed.
- K. A. Mack and D. B. Collum, J. Am. Chem. Soc., 2018, 140, 4877–4883 CrossRef CAS PubMed.
- P. C. Andrikopoulos, D. R. Armstrong, W. Clegg, C. J. Gilfillan, E. Hevia, A. R. Kennedy, R. E. Mulvey, C. T. O'Hara, J. A. Parkinson and D. M. Tooke, J. Am. Chem. Soc., 2004, 126, 11612–11620 CrossRef CAS PubMed.
- A. J. Martínez-Martínez, A. R. Kennedy, R. E. Mulvey and C. T. O'Hara, Science, 2014, 346, 834–837 CrossRef PubMed.
- R. E. Mulvey, Organometallics, 2006, 25, 1060–1075 CrossRef CAS.
- M. P. Coles, Coord. Chem. Rev., 2015, 297–298, 2–23 CrossRef CAS.
- M. P. Coles, Coord. Chem. Rev., 2015, 297–298, 24–39 CrossRef CAS.
- R. R. Fraser and T. S. Mansour, J. Org. Chem., 1984, 49, 3442–3443 CrossRef CAS.
- R. R. Fraser, T. S. Mansour and S. Savard, J. Org. Chem., 1985, 50, 3232–3234 CrossRef CAS.
- D. R. Moore, M. Cheng, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2003, 125, 11911–11924 CrossRef CAS PubMed.
- (a) Y. Liu, L. Y. M. Eymann, E. Solari, F. Fadaei Tirani, R. Scopelliti and K. Severin, Inorg. Chem., 2018, 57, 11859–11863 CrossRef CAS PubMed; (b) M. Xu, B. Kooij, T. Wang, J. H. Lin, Z.-W. Qu, S. Grimme and D. W. Stephan, Angew. Chem., Int. Ed., 2021, 60, 16965–16969 CrossRef CAS PubMed.
- M. Wiesinger, B. Maitland, C. Färber, G. Ballmann, C. Fischer, H. Elsen and S. Harder, Angew. Chem., Int. Ed., 2017, 56, 16654–16659 CrossRef CAS PubMed.
- D. L. Kays, Chem. Soc. Rev., 2016, 45, 1004–1018 RSC.
- J. Hicks, E. J. Underhill, C. E. Kefalidis, L. Maron and C. Jones, Angew. Chem., Int. Ed., 2015, 54, 10000–10004 CrossRef CAS PubMed.
- A. J. Boutland, D. Dange, A. Stasch, L. Maron and C. Jones, Angew. Chem., Int. Ed., 2016, 55, 9239–9243 CrossRef CAS PubMed.
- N. F. Chilton, C. A. P. Goodwin, D. P. Mills and R. E. P. Winpenny, Chem. Commun., 2015, 51, 101–103 RSC.
- A. Torvisco, A. Y. O'Brien and K. Ruhlandt-Senge, Coord. Chem. Rev., 2011, 255, 1268–1292 CrossRef CAS.
- J. D. Leng, C. A. P. Goodwin, I. J. Vitorica-Yrezabal and D. P. Mills, Dalton Trans., 2018, 47, 12526–12533 RSC.
- J. Martin, C. Knüpfer, J. Eyselein, C. Färber, S. Grams, J. Langer, K. Thum, M. Wiesinger and S. Harder, Angew. Chem., Int. Ed., 2020, 59, 9102–9112 CrossRef CAS PubMed.
- H. Bauer, M. Alonso, C. Fischer, B. Rösch, H. Elsen and S. Harder, Angew. Chem., Int. Ed., 2018, 57, 15177–15182 CrossRef CAS PubMed.
- H. Bauer, K. Thum, M. Alonso, C. Fischer and S. Harder, Angew. Chem., Int. Ed., 2019, 58, 4248–4253 CrossRef CAS PubMed.
- J. Martin, J. Eyselein, S. Grams and S. Harder, ACS Catal., 2020, 10, 7792–7799 CrossRef CAS.
- N. Wiberg, E. Kühnel, K. Schurz, H. Borrmann and A. Simon, Z. Naturforsch., B: J. Chem. Sci., 1988, 43, 1075–1086 CrossRef CAS.
- H.-W. Lerner and M. Bolte, Cambridge Structural Database (private communication: CCDC 776544), 2010, reference code: SANCIT01 Search PubMed.
- L. Orzechowski, D. F.-J. Piesik, C. Ruspic and S. Harder, Dalton Trans., 2008, 37, 4742–4746 RSC.
- S. Harder and M. Lutz, Organometallics, 1994, 13, 5173–5176 CrossRef CAS.
- (a) J. L. Dye, M. G. DeBacker and V. A. Nicely, J. Am. Chem. Soc., 1970, 92, 5226–5228 CrossRef CAS; (b) J. L. Dye, Acc. Chem. Res., 2009, 42, 1564–1572 CrossRef CAS PubMed; (c) N. Davison, J. A. Quirk, F. Tuna, D. Collison, C. L. McMullin, H. Michaels, G. H. Morritt, P. G. Waddell, J. A. Gould, M. Freitag, J. A. Dawson and E. Lu, Chem, 2023, 9, 576–591 CrossRef CAS.
- Z. Jedliński, A. Stolarzewicz and Z. Grobelny, Makromol. Chem., 1986, 187, 795–799 CrossRef.
- R. E. Mulvey, V. L. Blair, W. Clegg, A. R. Kennedy, J. Klett and L. Russo, Nat. Chem., 2010, 2, 588–591 CrossRef CAS PubMed.
- P. P. Power and X. Xiaojie, Chem. Commun., 1984, 358–359 RSC.
- W. Clegg, E. Lamb, S. T. Liddle, R. Snaith and A. E. H. Wheatley, J. Organomet. Chem., 1999, 573, 305–312 CrossRef CAS.
- W. Scherer, P. Sirsch, M. Grosche, M. Spiegler, S. A. Mason and M. G. Gardiner, Chem. Commun., 2001, 2072–2073 RSC.
- O. Loveday and J. Echeverría, Nat. Commun., 2021, 12, 5030 CrossRef CAS PubMed.
- D. Mootz, A. Zinnius and B. Böttcher, Angew. Chem., 1969, 81, 398–399 CrossRef.
- J. Hämäläinen, F. Munnik, T. Hatanpää, J. Holopainen, M. Ritala and M. Leskelä, J. Vac. Sci. Technol., A, 2011, 30, 01A106 CrossRef.
- A. Koch, H. Görls, S. Krieck and M. Westerhausen, Dalton Trans., 2017, 46, 9058–9067 RSC.
- A. Friedrich, J. Pahl, J. Eyselein, J. Langer, N. van Eikema Hommes, A. Görling and S. Harder, Chem. Sci., 2021, 12, 2410–2418 RSC.
- W. Clegg, A. R. Kennedy, J. Klett, R. E. Mulvey and L. Russo, Eur. J. Inorg. Chem., 2012, 2012, 2989–2994 CrossRef CAS.
- A. I. Ojeda-Amador, A. J. Martínez-Martínez, A. R. Kennedy and C. T. O'Hara, Inorg. Chem., 2016, 55, 5719–5728 CrossRef CAS PubMed.
- H. M. Nicholas, C. A. P. Goodwin, J. G. C. Kragskow, S. J. Lockyer and D. P. Mills, Molecules, 2018, 23, 1138 CrossRef PubMed.
- C. A. P. Goodwin, K. C. Joslin, S. J. Lockyer, A. Formanuik, G. A. Morris, F. Ortu, I. J. Vitorica-Yrezabal and D. P. Mills, Organometallics, 2015, 34, 2314–2325 CrossRef CAS.
- T. X. Gentner, B. Rösch, K. Thum, J. Langer, G. Ballmann, J. Pahl, W. A. Donaubauer, F. Hampel and S. Harder, Organometallics, 2019, 38, 2485–2493 CrossRef CAS.
- S. Neander and U. Behrens, Z. Anorg. Allg. Chem., 1999, 625, 1429–1434 CrossRef CAS.
- T. A. Engesser, M. R. Lichtenthaler, M. Schleep and I. Krossing, Chem. Soc. Rev., 2016, 45, 789–799 RSC.
- I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982–14024 CrossRef CAS PubMed.
- C. A. Reed, Acc. Chem. Res., 1998, 31, 133–139 CrossRef CAS.
- C. Douvris and J. Michl, Chem. Rev., 2013, 113, PR179–PR233 CrossRef CAS PubMed.
- K. Xu, S. Zhang, T. R. Jow, W. Xu and C. A. Angell, Electrochem. Solid-State Lett., 2002, 5, A26 CrossRef CAS.
- T. Söhner, F. Braun, L. C. Over, S. Mehlhose, F. Rominger and B. F. Straub, Green Chem., 2014, 16, 4696–4707 RSC.
- R. J. Gillespie and S. A. Johnson, Inorg. Chem., 1997, 36, 3031–3039 CrossRef CAS PubMed.
- F. Weinhold and R. West, Organometallics, 2011, 30, 5815–5824 CrossRef CAS.
- F. Weinhold and R. West, J. Am. Chem. Soc., 2013, 135, 5762–5767 CrossRef CAS PubMed.
- S. Grabowsky, M. F. Hesse, C. Paulmann, P. Luger and J. Beckmann, Inorg. Chem., 2009, 48, 384–4393 CrossRef PubMed.
- C. Eaborn, P. B. Hitchcock and P. D. Lickiss, J. Organomet. Chem., 1984, 264, 119–126 CrossRef CAS.
- A. I. Gusev, M. G. Los’, Yu. M. Varezhkin, M. M. Morgunova and D. Ya. Zhinkin, J. Struct. Chem., 1976, 17, 329–331 CrossRef.
- A. Spielberger, P. Gspaltl, H. Siegl, E. Hengge and K. Gruber, J. Organomet. Chem., 1995, 499, 241–246 CrossRef CAS.
- J. Pahl, H. Elsen, A. Friedrich and S. Harder, Chem. Commun., 2018, 54, 7846–7849 RSC.
- A. C. Hillier, W. J. Sommer, B. S. Yong, J. L. Petersen, L. Cavallo and S. P. Nolan, Organometallics, 2003, 22, 4322–4326 CrossRef CAS.
- T. Kähler and F. Olbrich, CCDC, private communication, refcode: BANNAG.
- R. A. Woltornist and D. B. Collum, J. Org. Chem., 2021, 86, 2406–2422 CrossRef CAS PubMed.
- M. Xu, A. R. Jupp, Z. W. Qu and D. W. Stephan, Angew. Chem., Int. Ed., 2018, 57, 11050–11054 CrossRef CAS PubMed.
- C. A. P. Goodwin, B. L. L. Réant, G. F. Vettese, J. G. C. Kragskow, M. J. Giansiracusa, I. M. DiMucci, K. M. Lancaster, D. P. Mills and S. Sproules, Inorg. Chem., 2020, 59, 7571–7583 CrossRef CAS PubMed.
- S. Shambayati, S. L. Schreiber, J. F. Blake, S. G. Wierschke and W. L. Jorgensen, J. Am. Chem. Soc., 1990, 112, 697–703 CrossRef CAS.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
- T. A. Keith, AIMAll TK Gristmill Software, Overland Park, KS, 2017 Search PubMed.
- Li: ref 32, Na: P. Schuler, H. Görls, M. Westerhausen and S. Krieck, Dalton Trans., 2019, 48, 8966–8975 RSC . K: C. Gunnar Werncke, E. Suturina, P. C. Bunting, L. Vendier, J. R. Long, M. Atanasov, F. Neese, S. Sabo-Etienne and S. Bontemps, Chem.–Eur. J., 2016, 22, 1668–1674 CrossRef PubMed . Rb: T. Kähler and F. Olbrich, CCDC, private communication, refcode: BADQAZ, Cs: ref. 41..
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H and 13C NMR spectra, crystallographic details including ORTEP presentations, details for the DFT calculations including XYZ-files. CCDC 2321294–2321305. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc06896j |
| This journal is © The Royal Society of Chemistry 2024 |