Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cross-coupling of CO and an isocyanide mediated by a tetrameric magnesium hydride cluster†
Wenbang
Yang
 ,
Andrew J. P.
White
and
Mark R.
Crimmin
,
Andrew J. P.
White
and
Mark R.
Crimmin
 *
*
Department of Chemistry, Molecular Sciences Research Hub, 82 Wood Lane, Shepherds Bush, London, W12 0BZ, UK. E-mail: m.crimmin@imperial.ac.uk
First published on 24th June 2024
Abstract
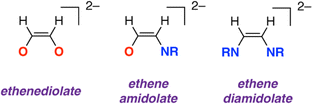
Sequential addition of CNXyl (Xyl = 2,6-dimethylphenyl) and CO to a tetrametallic magnesium hydride cluster results in stepwise reduction and cross-coupling of these substrates. Cross-coupling results in the formation of an ethene amidolate ligand [OC1(H1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2(H2)NAr]2− a previously unknown entity which contains a 1,2-difunctionalised carbon chain reminiscent of those found in aminoalcohols and amino acids. To the best of our knowledge, this is the first example of such reactivity with metal hydride precursors. DFT calculations support a mechanism that parallels that established for coupling of CO to form ethenediolate ligands, with the key carbon–carbon bond step occurring by nucleophilic attack of a putative azamethylene intermediate on CO. The cluster plays a key role in templating the synthesis, providing kinetic control over each of the steps. The ethene amidolate ligand can be transferred to other metals (Al) and semi-metals (B) through onwards metathesis reactions.
C2(H2)NAr]2− a previously unknown entity which contains a 1,2-difunctionalised carbon chain reminiscent of those found in aminoalcohols and amino acids. To the best of our knowledge, this is the first example of such reactivity with metal hydride precursors. DFT calculations support a mechanism that parallels that established for coupling of CO to form ethenediolate ligands, with the key carbon–carbon bond step occurring by nucleophilic attack of a putative azamethylene intermediate on CO. The cluster plays a key role in templating the synthesis, providing kinetic control over each of the steps. The ethene amidolate ligand can be transferred to other metals (Al) and semi-metals (B) through onwards metathesis reactions.
Introduction
Carbon monoxide (CO) is an attractive C1 building block for the construction of more complex organic molecules. In the past few years there has been increased interest in reactions that combine two (or more) molecules of CO to construct carbon chains.1–5 Controlling selectivity in these reactions is important; both the chain length and the ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O are key factors. Focusing on C2 products, it has been shown that two equivalents of CO can be combined at reducing metal centres to form ethynediolates,6–12 ethenediolates,13–19 ethanediolates,20 ketenes,21 an acetaldehyde enolate,22 and ethene.23 Isocyanides (CNR, R = alkyl, aryl) are isoelectronic with CO. Related reactions that combine two or more equivalents of isocyanide to form homocoupled products are widely reported (Fig. 1).24–38 When considering these studies, an obvious question arises as to whether it might be possible to combine CO and CNR to form cross-coupled products. For the C2 series this would potentially lead to the formation of products containing a 1,2-difunctionalised carbon chain with vicinal oxygen and nitrogen containing functional groups. Such substitution patterns are found in amino acids, β-aminoketones, and 1,2-aminoalcohols and are common across natural products, medicinally relevant compounds, and privileged ligand structures.
O are key factors. Focusing on C2 products, it has been shown that two equivalents of CO can be combined at reducing metal centres to form ethynediolates,6–12 ethenediolates,13–19 ethanediolates,20 ketenes,21 an acetaldehyde enolate,22 and ethene.23 Isocyanides (CNR, R = alkyl, aryl) are isoelectronic with CO. Related reactions that combine two or more equivalents of isocyanide to form homocoupled products are widely reported (Fig. 1).24–38 When considering these studies, an obvious question arises as to whether it might be possible to combine CO and CNR to form cross-coupled products. For the C2 series this would potentially lead to the formation of products containing a 1,2-difunctionalised carbon chain with vicinal oxygen and nitrogen containing functional groups. Such substitution patterns are found in amino acids, β-aminoketones, and 1,2-aminoalcohols and are common across natural products, medicinally relevant compounds, and privileged ligand structures.
Precedent for the cross-coupling of CO with CNR is limited to a handful of examples. Stepwise reaction of CO and CNR with a zirconium(IV) dialkyl complex to form a substituted ethene amidolate was reported as early as 1985.39 This type of reactivity has been observed at group 4 metal dialkyl complexes supported by alkoxide,40 tropocoronand,41 and N-heterocyclic carbene ligands.42 Related titanocyclobutane complexes,43 and seven coordinate niobium and tantalum complexes have also been reported to react with CO and CNR to form substituted ethene amidolates.44,45 In contrast, a thorium alkyl complex was shown to react with CO and CNR to form a ketenimine species.46,47 Binuclear silylene compounds are known to cross-couple and deoxygenated CO and CNR to form functionalised ketenimine products.48 To the best of our knowledge, despite nearly 40 years of interest in these types of reactions, cross-coupling reactions of CO and CNR at main group metals are unknown. All examples of cross-coupling reported to date employ low-oxidation state compounds or metal alkyl complexes. There are no examples of reactions that employ metal hydrides, meaning the parent ethene amidolate ligand [OCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNR]2− (R = alkyl, aryl) is an unknown chemical entity.
CHNR]2− (R = alkyl, aryl) is an unknown chemical entity.
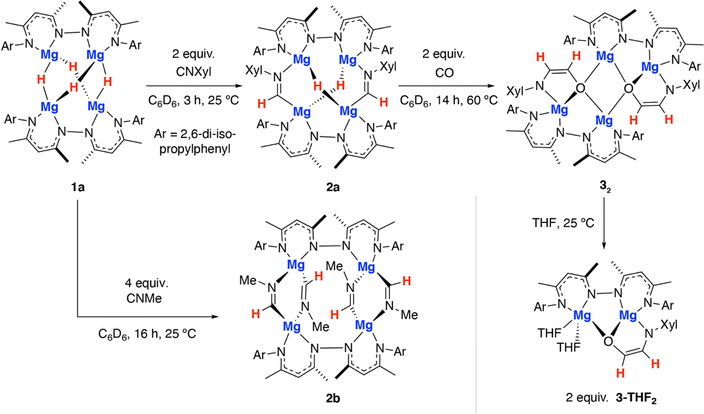
In this paper, we report the stepwise cross-coupling of CO and CNXyl (Xyl = 2,6-dimethylphenyl) using a tetrametallic magnesium hydride complex. This reaction results in the generation of the simplest known ethene amidolate ligand. A dimeric magnesium hydride complex was also investigated in CO and CNXyl cross-coupling, but did not lead to the isolation of ethene amidolate product, suggesting that the tetrameric metal cluster plays an important role in templating reactivity. DFT calculations are used to probe the electronic structure of the coordinated ethene amidolate fragment and rationalise the most likely mechanism of its formation. Finally, we show that the ethene amidolate ligand is labile and can be transferred to alternative metals and semi-metals (e.g. Al, B) providing access to a unique 1,2-difunctionalised C2 ligand derived from CO.
Results and discussion
Cross-coupling of CO and CNXyl
The tetrametallic magnesium hydride cluster 1a was originally reported by Harder and co-workers.49 Related species have recently been documented from Kretschmer's group.50 Previously we showed that 1a reacts with two equiv. CO by a deoxygenative pathway leading to the formation of a coordinated acetaldehyde enolate.22Reaction of 1a with 2-6-dimethylphenylisocyanide for 3 h at 25 °C in benzene led to selective formation of the bis(imine) complex 2a derived from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction stoichiometry (Scheme 1). Insertion of the isocyanide occurs exclusively at only one of the two possible types of hydride sites in 1a. 2a was characterised by diagnostic resonances in the 1H NMR spectrum (C6D6) at δ = 8.83 (s) and 3.38 (s) ppm each integrating to 2H and assigned to the CH
1 reaction stoichiometry (Scheme 1). Insertion of the isocyanide occurs exclusively at only one of the two possible types of hydride sites in 1a. 2a was characterised by diagnostic resonances in the 1H NMR spectrum (C6D6) at δ = 8.83 (s) and 3.38 (s) ppm each integrating to 2H and assigned to the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr imine proton and unreacted hydride ligands respectively. The former resonance shows a cross-peak in the HSQC data to a 13C resonance at δ = 157.7 ppm assigned to the CH
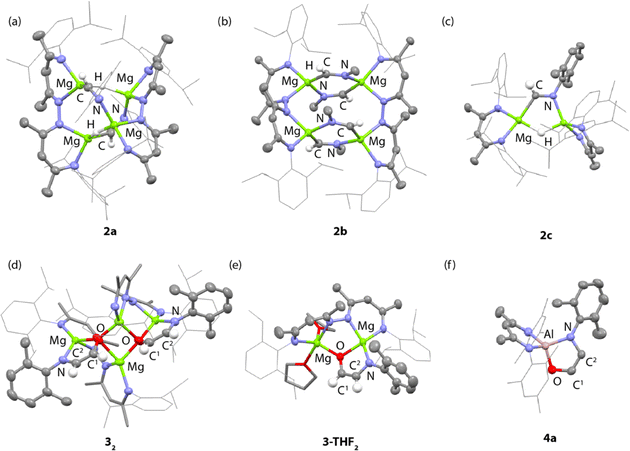
NAr imine proton and unreacted hydride ligands respectively. The former resonance shows a cross-peak in the HSQC data to a 13C resonance at δ = 157.7 ppm assigned to the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr imine carbon atom. In the solid-state, 2a retains a tetrametallic structure with the dinucleating ligands templating a close arrangement of four magnesium atom in an approximate tetrahedral arrangement (Fig. 2a). The newly formed imine ligands adopt a κ2-C, N coordination mode, bridging two of the magnesium sites of the tetrahedra, the remaining two metals are bridged by the unreacted hydrides. The Mg–C bond lengths of the metalated imine are 2.1778(17) and 2.1830(17) Å, while the Mg–N bond lengths are 2.1185(15) and 2.1428(14) Å.
NAr imine carbon atom. In the solid-state, 2a retains a tetrametallic structure with the dinucleating ligands templating a close arrangement of four magnesium atom in an approximate tetrahedral arrangement (Fig. 2a). The newly formed imine ligands adopt a κ2-C, N coordination mode, bridging two of the magnesium sites of the tetrahedra, the remaining two metals are bridged by the unreacted hydrides. The Mg–C bond lengths of the metalated imine are 2.1778(17) and 2.1830(17) Å, while the Mg–N bond lengths are 2.1185(15) and 2.1428(14) Å.
In contrast, addition of 4 equiv. of methylisocyanide to 1 led to the formation of 2b, derived from exhaustive insertion of the unsaturated substrate into all four hydride sites (Scheme 1, Fig. 2b). It remains likely that variation of the sterics of the substituent of the isocyanide directly influences the outcome.
Onwards reaction of 2a with CO for 14 h at 60 °C in benzene led to 32 (Scheme 1, Fig. 2d). 32 could also be prepared in a single-pot reaction from 1a by stepwise addition of CNXyl then CO. 32 contains two ethene amidolate ligands derived from cross-coupling of an isocyanide and CO. Each new C2 fragment in 32 originates from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of hydride
1 reaction of hydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO
CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNXyl. This is the first example of this reactivity at a metal hydride complex and as such the first time a simple unsubstituted ethene amidolate ligand, [OC1(H1)
CNXyl. This is the first example of this reactivity at a metal hydride complex and as such the first time a simple unsubstituted ethene amidolate ligand, [OC1(H1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2(H2)NAr]2−, has been observed to form from CO. In C6D6 solution, 32 was characterised by diagnostic, non-equivalent, protons of the ethene amidolate moiety H1 and H2 which resonate at δ = 5.03 (d, 3JH–H = 2.2 Hz) and 6.29 (d, 3JH–H = 2.2 Hz) ppm. These correlate with 13C environments C1 and C2 at δ = 102.9 and 152.2 ppm respectively. Reaction of 2a with 13CO allowed synthesis of 13C-32. 13C-32 is monotopically labelled at the C1 position and there is no evidence for inclusion of the isotope at the C2 position of the ligand or elsewhere. The 1JC–H and 2JC–H coupling constants take values of 139.2 and 10.8 Hz respectively. In the solid-state, 32 again retains a tetrametallic structure albeit with loss of the tetrahedral arrangement of the magnesium sites. The structure of 32 is perhaps best conceptualised as a dimer of two dimagnesium fragments related through an inversion symmetry operation. Each dimagnesium fragment is coordinated by the dinucleating bis(β-diketiminate) ligand and the ethene amidolate which coordinates one magnesium atom κ2-O,N and the other in a κ1-O fashion. The two dimagnesium fragments dimerise to form an open tetrametallic cluster with the four magnesium sites orientated in a butterfly-like configuration. Dimerisation occurs via bridging oxygen atoms of the ethene amidolate, such that these end up adopting a μ3-bridging mode. Consistent with the small coupling constant between the H1 and H2 positions, the ethene amidolate adopts a cis-configuration. The ethene amidolate is formally a dianionic ligand, bond lengths to Mg are unremarkable. The C1–O, C2–N, and C1
C2(H2)NAr]2−, has been observed to form from CO. In C6D6 solution, 32 was characterised by diagnostic, non-equivalent, protons of the ethene amidolate moiety H1 and H2 which resonate at δ = 5.03 (d, 3JH–H = 2.2 Hz) and 6.29 (d, 3JH–H = 2.2 Hz) ppm. These correlate with 13C environments C1 and C2 at δ = 102.9 and 152.2 ppm respectively. Reaction of 2a with 13CO allowed synthesis of 13C-32. 13C-32 is monotopically labelled at the C1 position and there is no evidence for inclusion of the isotope at the C2 position of the ligand or elsewhere. The 1JC–H and 2JC–H coupling constants take values of 139.2 and 10.8 Hz respectively. In the solid-state, 32 again retains a tetrametallic structure albeit with loss of the tetrahedral arrangement of the magnesium sites. The structure of 32 is perhaps best conceptualised as a dimer of two dimagnesium fragments related through an inversion symmetry operation. Each dimagnesium fragment is coordinated by the dinucleating bis(β-diketiminate) ligand and the ethene amidolate which coordinates one magnesium atom κ2-O,N and the other in a κ1-O fashion. The two dimagnesium fragments dimerise to form an open tetrametallic cluster with the four magnesium sites orientated in a butterfly-like configuration. Dimerisation occurs via bridging oxygen atoms of the ethene amidolate, such that these end up adopting a μ3-bridging mode. Consistent with the small coupling constant between the H1 and H2 positions, the ethene amidolate adopts a cis-configuration. The ethene amidolate is formally a dianionic ligand, bond lengths to Mg are unremarkable. The C1–O, C2–N, and C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2 bond lengths take values of 1.421(3), 1.357(3) and 1.375(3) Å respectively.
C2 bond lengths take values of 1.421(3), 1.357(3) and 1.375(3) Å respectively.
Dissolving crystalline samples of 32 in THF followed by analysis of the resultant sample by 1H NMR spectroscopy, either in C6D6 or THF, led to significant changes of the resonances assigned to the ethene amidolate ligand, with H1 and H2 now resonating at δ = 5.42 (d, 3JH–H = 3.4 Hz) and 5.32 (d, 3JH–H = 3.4 Hz) ppm respectively. Recrystallisation revealed the formation of 3-THF2, a product of fragmentation of the tetrametallic structure into a single dimagnesium motif (Scheme 1, Fig. 2e). In the solid state, THF preferentially solvates only one of the magnesium centres, leading to an asymmetric structure containing both 4- and 5-coordinate metals. The C1–O, C2–N, and C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2 bond lengths of the ethene amidolate are near identical to those observed in 32. The reaction is non-reversible and attempts to remove THF from 3-THF2 under high vacuum (1 × 10−4 mbar) did not lead to reformation of 32.
C2 bond lengths of the ethene amidolate are near identical to those observed in 32. The reaction is non-reversible and attempts to remove THF from 3-THF2 under high vacuum (1 × 10−4 mbar) did not lead to reformation of 32.
DFT calculations were conducted. Geometries were optimised with the B3PW91-D3 functional using a hybrid basis-set comprised of 6-31G** (C,H,N,O) and SDDAll (Mg). Solvation was taken into account in the optimisation using the polarizable continuum model (benzene). Single point corrections to energies were conducted with triple-zeta basis set 6-311+G** applied to all energies. These calculations suggest that the fragmentation of 32 to 2 equiv. 3 is disfavoured in the absence of a coordinating solvent (ΔG298 K° = +46.2 kcal mol−1; ΔH° = +66.0 kcal mol−1). Deaggregation becomes more favourable in the presence of THF with conversion of 32 + 4 THF → 2 3-THF2 being only modestly endergonic (ΔG298 K° = +11.5 kcal mol−1; ΔH° = −19.2 kcal mol−1). In the presence of an excess THF (solvent) the equilibrium is likely displaced to the products, allowing the isolation of 3-THF2 (see ESI, Fig. S19†). DOSY NMR studies suggest that the nuclearity of 32 and 3-THF2 observed in the solid-state is potentially retained in solution. In C6D6 at 298 K the diffusion coefficient of 32 was measured as 7.8 × 10−10 m2 s−1 while 3-THF2 in THF-d8 solution diffused at a substantial faster rate of 1.1 × 10−9 m2 s−1 (see ESI, Fig. S9 and S10†). The data correspond to estimated hydrodynamic radii of 9.3 Å for 32 and 7.2 Å for 3-THF2 consistent with 32 retaining its tetrametallic structure in solution.
Electronic structure of the ethene amidolate ligand
The new ethene amidolate motif [OC1(H1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2(H2)NAr]2− fills a gap between ethenediolate [OC(H)
C2(H2)NAr]2− fills a gap between ethenediolate [OC(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)O]2− and ethenediamidolate [ArNC(H)
C(H)O]2− and ethenediamidolate [ArNC(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)NAr]2− ligands (Fig. 1). NBO calculations (see ESI† Section 4.3) were undertaken on 32 and 3-THF2 to probe the electronic structure of this species and nature of its binding to magnesium (Fig. 3). For comparison, the parent [OC1(H1)
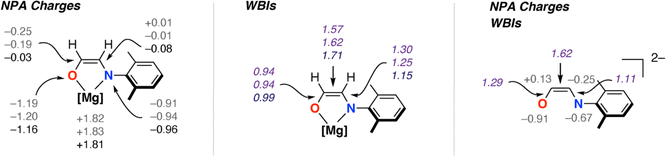
C(H)NAr]2− ligands (Fig. 1). NBO calculations (see ESI† Section 4.3) were undertaken on 32 and 3-THF2 to probe the electronic structure of this species and nature of its binding to magnesium (Fig. 3). For comparison, the parent [OC1(H1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2(H2)NAr]2− was calculated as a non-coordinated dianion. The ethene amidolate adopts a conjugated structure. For 32 the Wiberg Bond Indices (WBIs) take values of C1
C2(H2)NAr]2− was calculated as a non-coordinated dianion. The ethene amidolate adopts a conjugated structure. For 32 the Wiberg Bond Indices (WBIs) take values of C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2 (1.57, 1.62), C1–O (0.94, 0.94) and C2–N (1.25, 1.30). While in the broadest sense, these data can be interpreted in terms of an alternating array of single and double bonds for the ethene amidolate structure, the C–N WBIs are slightly greater than expected for a single bond suggesting a degree of delocalisation of the N lone-pair into the conjugated system. Binding to magnesium is primarily ionic with NPA charges on Mg (+1.82, +1.83) being highly positive and those on O (−1.19, −1.20) and N (−0.91, −0.94) uniformly negative. There also appears to be some differences in the degree of this delocalisation across the different amidoenolates in the calculated structures 32 and 3-THF2 suggesting the ligand can respond and augment its polarisation dependent on the local environment. The molecular orbitals of [OC1(H1)
C2 (1.57, 1.62), C1–O (0.94, 0.94) and C2–N (1.25, 1.30). While in the broadest sense, these data can be interpreted in terms of an alternating array of single and double bonds for the ethene amidolate structure, the C–N WBIs are slightly greater than expected for a single bond suggesting a degree of delocalisation of the N lone-pair into the conjugated system. Binding to magnesium is primarily ionic with NPA charges on Mg (+1.82, +1.83) being highly positive and those on O (−1.19, −1.20) and N (−0.91, −0.94) uniformly negative. There also appears to be some differences in the degree of this delocalisation across the different amidoenolates in the calculated structures 32 and 3-THF2 suggesting the ligand can respond and augment its polarisation dependent on the local environment. The molecular orbitals of [OC1(H1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2(H2)NAr]2− are consistent with its potential to act as both a σ- and π-donor ligand, with the HOMO, HOMO-1 and HOMO-2 showing character of both oxygen and nitrogen-based lone-pairs in the plane and orthogonal to the site of metal coordination (see ESI, Fig. S20–S22†).
C2(H2)NAr]2− are consistent with its potential to act as both a σ- and π-donor ligand, with the HOMO, HOMO-1 and HOMO-2 showing character of both oxygen and nitrogen-based lone-pairs in the plane and orthogonal to the site of metal coordination (see ESI, Fig. S20–S22†).
Mechanism
Further calculations were undertaken to shed light on the mechanism that leads to the formation of 32. For simplicity, these calculations were initiated from 2a as the reactant. We have previously calculated insertion of CO into two of the Mg–H bonds of 1a and proposed structures similar to 2a in the homologation and deoxygenation of CO using this tetrametallic cluster.22 A plausible pathway for ethene amidolate formation was found (Fig. 4). This pathway parallels the most common pathway described for ethenediolate formation in reactions of metal hydrides with CO.51,52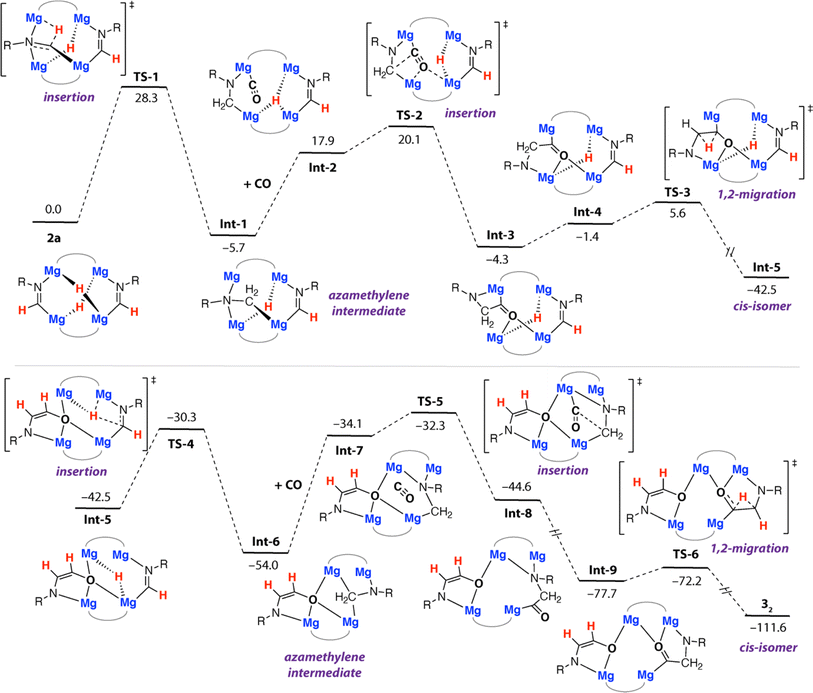 | ||
| Fig. 4 Calculated pathway for the formation of 32 from 2a. R = Xyl. B3PW91-D3/6-311+G**/PCM (benzene)//B3PW91-D3/6-31G** (C,H,N,O)/SDDAll (Mg)/PCM (benzene). Gibbs energies, values in kcal mol−1. | ||
The pathway is initiated by an intramolecular hydromagnesiation (or hydride transfer) to one of the metallated imine ligands of 2a. This step occurs by TS-1 (ΔG‡298 K = 28.3 kcal mol−1) and leads directly to an azamethylene intermediate Int-1. The structure of Int-1 parallels established oxymethylene intermediates in CO homologation. CO coordination to Int-1 forms Int-2, with subsequent insertion into a Mg–C bond of the azamethylene motif occurring viaTS-2 (ΔG‡298 K = 25.8 kcal mol−1). TS-2 leads directly to Int-3 and is the key carbon–carbon bond forming step that creates the 1,2-difunctionalised carbon chain. TS-2 appears late on the potential energy surface, C1⋯C2 bond forming is advanced and occurs with nucleophilic attack of the azamethylene carbon C2 on C1 of the coordinated CO ligand. 1,2-Hydride migration can then occur from Int-3. Int-3 can isomerise to Int-4 through rotation about the C1–C2 bond, this step necessarily occurs with decoordination of the nitrogen atom from one Mg site and recoordination to another. Despite our best efforts we were unable to identify a transition state for this process, based on scans of the potential energy surface we suggest the rearrangement is extremely facile. 1,2-Hydride migration from Int-4 can occur by the low energy TS-3 and leads directly to the cis-isomer of the ethene amidolate ligand, Int-5. The sequence of hydromagnesiation, CO insertion, and 1,2-hydride migration then repeats ultimately leading to the experimentally determined product 32. Analysis of the complete pathway for formation of 32 reveals that the formation of the 1st and 2nd ethene amidolate ligands occurs with similar barriers, consistent with the lack of observable intermediates in the conversion of 2a to 32. The global barrier is associated with the 1st hydride transfer step from 2a and is ΔG‡298 K = 28.3 kcal mol−1 which is in reasonable agreement with the experimental conditions for the reaction (14 h, 60 °C).
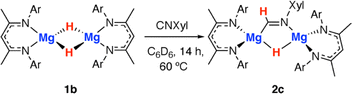
Throughout the calculated pathway the tetrametallic cluster templates bond making and breaking events and adapts its geometry to ultimately accommodating the ligands in the most thermodynamic favourable binding sites. Curious as to whether the tetrametallic structure was essential for the observed reactivity, we revisited the reactions of dimeric magnesium hydrides with isocyanides. Previously it has been shown that 1b reacts with cyclohexyl isocyanide and tert-butyl isocyanide to form double insertion products.13,53 In our hands, reaction of 1b with 2-6-dimethylphenylisocyanide in C6D6 for 14 h at 60 °C allowed the isolation of the mono-insertion product 2c (Scheme 2, Fig. 2c). Like the tetrametallic analogue 2a, 2c still contains a single unreacted hydride site and hence based on our postulated mechanism, could potentially react with CO to generate an ethene amidolate ligand. Addition of CO to 2c and monitoring the reaction for 14 h at 25 °C provided no evidence for carbon–carbon bond formation. Over this time, 2c was fully consumed and a complex mixture was formed, suggesting that if an ethene amidolate ligand is generated it is only transient and reacts further under the conditions of the experiment.
Transfer of the ethene amidolate ligand
The amidoenolate ligand of 32 could be transferred to alternative metals and semi-metal through metathesis reactions (Scheme 3, Fig. 2f). These reactions generated products in which the amidoenolate is coordinated to aluminium (4a) and boron (5). Transfer of the amidoenolate is driven by the highly ionic interaction with magnesium and thermodynamically favourable formation of the corresponding magnesium chloride byproduct, which can be separated by fractional crystallisation. NMR spectroscopic data along with single crystal X-ray diffraction studies suggest that the amidoenolate remains bound as a κ2-O,N ligand in both 4a and 5.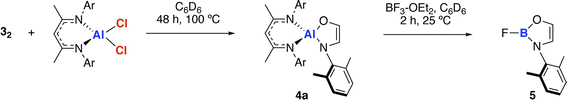 | ||
| Scheme 3 Transfer of the ethene amidolate ligand from 32 to aluminium forming 4a and boron forming 5. | ||
Conclusions
In summary, stepwise reaction of CNXyl and CO with a tetrametallic magnesium hydride cluster results in reduction and cross coupling of the substrates to form a unique ethene amidolate ligand. This new C2 fragment contains a 1,2-difunctionalised motif. Prior work has established that this type of reactivity is possible with early transition metal alkyl complexes or low-valent silicon complexes, but to the best of our knowledge this is the first example involving a metal hydride precursor. A metalated imine complex (2a) was identified as an intermediate in cross-coupling. Data suggest the formation of this species at the magnesium cluster is under strict kinetic control as variation of either the isocyanide or magnesium complex leads to alternative products that are non-productive in cross-coupling. DFT calculations support a mechanism that evolves from the metalated imine to an azamethylene intermediate by a second hydride transfer. Carbon–carbon bond formation can occur by nucleophilic attack of the azamethylene on CO, with subsequent 1,2-hydrogen migration establishing the ethene amidolate structure. This pathway closely parallels that established for ethenediolate formation from CO and metal hydride complexes. Onwards reaction of the cross-coupled product (32) has established that the ethene amidolate ligands can be transferred to alternative fragments by metathesis reactions. The discovery that metal hydride complexes are competent in cross-coupling of isocyanides and CO could inspire new approaches toward the synthesis of amino alcohols and amino acids from inexpensive and readily available C1 building block.Data availability
Synthetic procedures, kinetic experiments, NMR spectra of all compounds, crystal structures of 2a, 2b, 2c, 32, 3-THF2, and 4a, crystallographic data, and computational methods (PDF). Cartesian coordinates of the DFT-optimized structures (XYZ) are available as part of the ESI. CCDC 2289252, 2308757–2308759, 2313439 and 2328581 contain the supplementary crystallographic data for this paper.†Author contributions
WY conducted all experimental and computational work. AJPW collected and refined single crystal data. All authors were involved in writing the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The EPSRC is thanked for funding (EP/S036628/1).References
- N. M. West, A. J. M. Miller, J. A. Labinger and J. E. Bercaw, Coord. Chem. Rev., 2011, 255, 881–898 CrossRef CAS.
- J. A. Labinger, J. Organomet. Chem., 2017, 847, 4–12 CrossRef CAS.
- R. Y. Kong and M. R. Crimmin, Dalton Trans., 2020, 49, 16587–16597 RSC.
- S. Fujimori and S. Inoue, J. Am. Chem. Soc., 2022, 144, 2034–2050 CrossRef CAS PubMed.
- J. M. Parr and M. R. Crimmin, Angew. Chem., Int. Ed., 2023, 62, e202219203 CrossRef CAS PubMed.
- R. J. Ward, I. del Rosal, S. P. Kelley, L. Maron and J. R. Walensky, Chem. Sci., 2023, 14, 2024–2032 RSC.
- T. Simler, K. N. McCabe, L. Maron and G. Nocton, Chem. Sci., 2022, 13, 7449–7461 RSC.
- B. M. Gardner, J. C. Stewart, A. L. Davis, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9265–9270 CrossRef CAS PubMed.
- S. M. Mansell, N. Kaltsoyannis and P. L. Arnold, J. Am. Chem. Soc., 2011, 133, 9036–9051 CrossRef CAS PubMed.
- P. L. Arnold, Z. R. Turner, R. M. Bellabarba and R. P. Tooze, Chem. Sci., 2011, 2, 77–79 RSC.
- N. Tsoureas, O. T. Summerscales, F. G. N. Cloke and S. M. Roe, Organometallics, 2013, 32, 1353–1362 CrossRef CAS.
- A. S. Frey, F. G. N. Cloke, P. B. Hitchcock, I. J. Day, J. C. Green and G. Aitken, J. Am. Chem. Soc., 2008, 130, 13816–13817 CrossRef CAS PubMed.
- R. Lalrempuia, C. E. Kefalidis, S. J. Bonyhady, B. Schwarze, L. Maron, A. Stasch and C. Jones, J. Am. Chem. Soc., 2015, 137, 8944–8947 CrossRef CAS PubMed.
- M. D. Anker, M. S. Hill, J. P. Lowe and M. F. Mahon, Angew. Chem., Int. Ed., 2015, 54, 10009–10011 CrossRef CAS PubMed.
- E. L. Werkema, L. Maron, O. Eisenstein and R. A. Andersen, J. Am. Chem. Soc., 2007, 129, 2529–2541 CrossRef CAS PubMed.
- W. J. Evans, J. W. Grate and R. J. Doedens, J. Am. Chem. Soc., 1985, 107, 1671–1679 CrossRef CAS.
- D. A. Katahira, K. G. Moloy and T. J. Marks, Organometallics, 1982, 1, 1723–1726 CrossRef CAS.
- J. M. Manriquez, D. R. McAlister, R. D. Sanner and J. E. Bercaw, J. Am. Chem. Soc., 1978, 100, 2716–2724 CrossRef CAS.
- M. D. Ankar, C. E. Kefalidis, Y. Yang, J. Fang, M. S. Hill, M. F. Mahon and L. Maron, J. Am. Chem. Soc., 2017, 139, 10036–10054 CrossRef PubMed.
- D. M. Roddick, M. D. Fryzuk, P. F. Seidler, G. L. Hillhouse and J. E. Bercaw, Organometallics, 1985, 4, 97–104 CrossRef CAS.
- N. S. Radu, M. P. Engeler, C. P. Gerlach, T. D. Tilley and A. L. Rheingold, J. Am. Chem. Soc., 1995, 117, 3621–3622 CrossRef CAS.
- W. Yang, A. J. P. White and M. R. Crimmin, Angew. Chem., Int. Ed., 2024, 63, e202319626 CrossRef CAS PubMed.
- T. Shima and Z. Hou, J. Am. Chem. Soc., 2006, 128, 8124–8125 CrossRef CAS PubMed.
- M. J. Evans, M. D. Anker, C. L. McMullin and M. P. Coles, Chem. Sci., 2023, 14, 6278–6288 RSC.
- C. Zhang, F. Dankert, Z. Jiang, B. Wang, D. Munz and J. Chu, Angew. Chem., Int. Ed., 2023, 62, e202307352 CrossRef CAS PubMed.
- S. L. Staun, G. T. Kent, A. Gomez-Torres, G. Wu, S. Fortier and T. W. Hayton, Organometallics, 2021, 40, 2934–2938 CrossRef CAS.
- S. Hasegawa, Y. Ishida and H. Kawaguchi, Chem. Commun., 2021, 57, 8296–8299 RSC.
- W. Chen, Y. Zhao, W. Xu, J.-H. Su, L. Shen, L. Liu, B. Wu and X.-J. Yang, Chem. Commun., 2019, 55, 9452–9455 RSC.
- W. Lu, H. Hu, Y. Li, R. Ganguly and R. Kinjo, J. Am. Chem. Soc., 2016, 138, 6650–6661 CrossRef CAS PubMed.
- K. Nagata, T. Agou, T. Sasamori and N. Tokitoh, Chem. Lett., 2015, 44, 1610–1612 CrossRef CAS.
- J. Shen, G. P. A. Yap and K. H. Theopold, J. Am. Chem. Soc., 2014, 136, 3382–3384 CrossRef CAS PubMed.
- M. Ma, A. Stasch and C. Jones, Chem.–Eur. J., 2012, 18, 10669–10676 CrossRef CAS PubMed.
- X. Li, X. Cheng, H. Song and C. Cui, Organometallics, 2007, 26, 1039–1043 CrossRef CAS.
- D. Rehder, C. Böttcher, C. Collazo, R. Hedelt and H. Schmidt, J. Organomet. Chem., 1999, 585, 294–307 CrossRef CAS.
- P. J. Shapiro, A. Vij, G. P. A. Yap and A. L. Rheingold, Polyhedron, 1995, 14, 203–209 CrossRef CAS.
- F. A. Cotton, S. A. Duraj and W. J. Roth, J. Am. Chem. Soc., 1984, 106, 6987–6993 CrossRef CAS.
- J. C. Dewan, C. M. Giandomenico and S. J. Lippard, Inorg. Chem., 1981, 20, 4069–4074 CrossRef CAS.
- C. T. Lam, P. W. R. Corfield and S. J. Lippard, J. Am. Chem. Soc., 1977, 99, 617–618 CrossRef CAS.
- A. K. McMullen, I. P. Rothwell and J. C. Huffman, J. Am. Chem. Soc., 1985, 107, 1072–1073 CrossRef CAS.
- L. R. Chamberlain, L. D. Durfee, P. E. Fanwick, L. M. Kobriger, S. L. Latesky, A. K. McMullen, B. D. Steffey, I. P. Rothwell, K. Foltin and J. C. Huffman, J. Am. Chem. Soc., 1987, 109, 6068–6076 CrossRef CAS.
- M. J. Scott and S. J. Lippard, J. Am. Chem. Soc., 1997, 119, 3411–3412 CrossRef CAS.
- L. P. Spencer and M. D. Fryzuk, J. Organomet. Chem., 2005, 690, 5788–5803 CrossRef CAS.
- G. Greidanus-Strom, C. A. G. Carter and J. M. Stryker, Organometallics, 2002, 21, 1011–1013 CrossRef CAS.
- E. M. Carnahan and S. J. Lippard, J. Am. Chem. Soc., 1990, 112, 3230–3231 CrossRef CAS.
- E. M. Carnahan and S. J. Lippard, J. Am. Chem. Soc., 1992, 114, 4166–4174 CrossRef CAS.
- K. G. Moloy, T. J. Marks and V. W. Day, J. Am. Chem. Soc., 1983, 105, 5696–5698 CrossRef CAS.
- K. G. Moloy, P. J. Fagan, J. M. Manriquez and T. J. A. Marks, J. Am. Chem. Soc., 1986, 108, 56–67 CrossRef CAS.
- Y. Wang, A. Kostenko, T. J. Hadlington, M. -P Luecke, S. Yao and M. Driess, J. Am. Chem. Soc., 2019, 141, 626–634 CrossRef CAS PubMed.
- J. Intemann, J. Spielmann, P. Sirsch and S. Harder, Chem.–Eur. J., 2013, 19, 8478–8489 CrossRef CAS PubMed.
- P. Rinke, H. Görls, P. Liebing and R. Kretschmer, Organometallics, 2023, 42, 2287–2292 CrossRef CAS.
- J. M. Manriquez, D. R. McAlister, R. D. Sanner and J. E. Bercaw, J. Am. Chem. Soc., 1976, 98, 6733–6735 CrossRef CAS.
- C. P. Casey and S. M. Neumann, J. Am. Chem. Soc., 1976, 98, 5395–5396 CrossRef CAS.
- C. Weetman, M. S. Hill and M. F. Mahon, Chem. Commun., 2015, 51, 14477–14480 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2289252, 2308757–2308759, 2313439 and 2328581. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02638a |
| This journal is © The Royal Society of Chemistry 2024 |