Open Access Article
Open Access ArticleFusion of two homoleptic truncated tetrahedra into a heteroleptic truncated octahedron†
Haifei
Liu‡
a,
Chenxing
Guo‡
 b,
Yujuan
Huang‡
a,
Zilin
Zhou
a,
Shijin
Jian
a,
Zeyuan
Zhang
b,
Yujuan
Huang‡
a,
Zilin
Zhou
a,
Shijin
Jian
a,
Zeyuan
Zhang
 a,
Yali
Hou
a,
Chaoqun
Mu
*c and
Mingming
Zhang
a,
Yali
Hou
a,
Chaoqun
Mu
*c and
Mingming
Zhang
 *a
*a
aState Key Laboratory for Mechanical Behavior of Materials, Shaanxi International Research Center for Soft Matter, School of Materials Science and Engineering, Xi'an Jiaotong University, Xi'an 710049, P. R. China. E-mail: mingming.zhang@xjtu.edu.cn
bCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518055, P. R. China
cSchool of Chemistry and Chemical Engineering, Xi'an University of Architecture and Technology, Xi'an 710055, Shaanxi, P. R. China. E-mail: chaoqunmu@xauat.edu.cn
First published on 9th August 2024
Abstract
The exploration of novel structures and structural transformation of supramolecular assemblies is of vital importance for their functions and applications. Herein, based on coordination-driven self-assembly, we prepare a neutral truncated tetrahedron and a heteroleptic truncated octahedron, whose structures are unambiguously confirmed by X-ray diffraction analysis. More importantly, the truncated tetrahedron is quantitatively transformed into the truncated octahedron through its fusion with another cationic truncated tetrahedron, as evidenced by fluorescence, mass and NMR spectroscopy. This study not only deepens our understanding of the process of supramolecular fusion but also opens up possibilities for the subsequent preparation of advanced supramolecular assemblies with complex structures and integrated functions.
Introduction
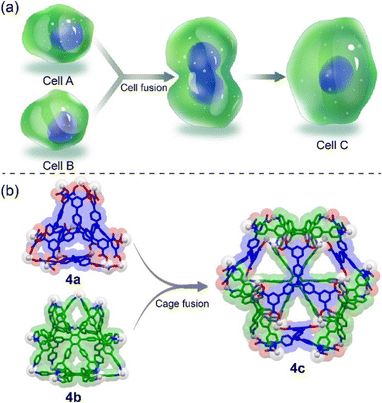
In natural systems, the structural transformation of biomolecules plays a crucial role in biological processes like enzymatic catalysis, signal transduction, allosteric regulation, etc.1–3 Inspired by this, chemists have constructed a series of artificial systems to mimic biological transformations based on supramolecular self-assembly,4–8 expecting to prepare advanced materials with sophisticated functions comparable to biomolecules. In this regard, fusion-based structural transformation has received much attention because it can integrate all the building blocks of precursors into the final constructs, forming assemblies with improved structural and functional complexity (Fig. 1).9,10 However, such a process is highly challenging because elegant ligand design and tedious synthesis are generally required to prevent the formation of thermodynamic mixtures and meet the requirements of quantitative supramolecular transformation.Owing to their moderate bond strength and fixed directionality, metal-coordination bonds have been widely used for the construction of two-dimensional metallacycles and three-dimensional metallacages,11–23 which are further employed in molecular recognition, stabilization of active substances, catalysis, etc.24–29 Moreover, these metal–organic assemblies are promising candidates for the construction of transformable supramolecular systems based on their dynamic and moderate stability. Although some progress has been made in this field,9 fusion-based structural transformation, especially the fusion of two geometrically similar metallacages into a new structure, has been rarely reported, which is probably because it is difficult to design and prepare two complementary three-dimensional coordination assemblies for quantitative fusion. Such a study will not only deepen our understanding of the mechanism of biological and abiological fusion, but also offer new opportunities for the direct functional integration of different supramolecular assemblies. Therefore, the development of fusion-based supramolecular systems from three-dimensional self-assembled structures is highly demanding, yet challenging.
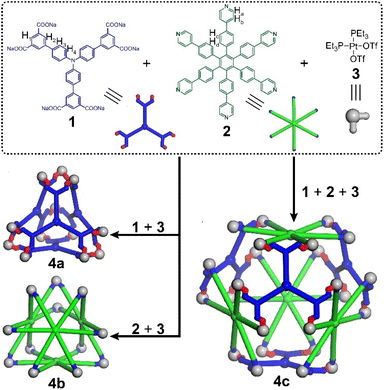
The Stang and Mukherjee groups pioneered the integrative self-assembly of pyridyl and carboxylic donors with metal acceptors via charge separation,30–32 demonstrating its efficiency in preparing multicomponent metallacages. The heteroleptic self-assembly of platinum nodes with both multiple pyridyl and carboxylic ligands has also proved to be an efficient strategy for the construction of multicomponent metallacages in our group.33–44 Moreover, the homoleptic self-assembly of platinum nodes with multiple pyridyl ligands also provides a series of two-component metallacages.45,46 However, the preparation of metallacages via the self-assembly of platinum nodes with multiple carboxylic ligands has never been reported, although some metallacycles have been reported by this strategy,30 which is due to the decreased solubility and difficult structural characterization of such neutral, three-dimensional supramolecular structures. Herein, based on metal-coordination-driven self-assembly, we prepare a neutral, truncated tetrahedral metallacage (4a) and a heteroleptic truncated octahedral metallacage (4c) (Fig. 2), whose structures are unambiguously confirmed by X-ray diffraction analysis. Interestingly, further assembly of 4a with a previously reported cationic, truncated tetrahedron (4b)45 leads to the quantitative formation of 4c, due to the energy of the heteroleptic metallacage significantly being lower than that with two homoleptic metallacages,30 representing a fusion-based supramolecular transformation from geometrically the same truncated tetrahedron-shaped metallacages. This study not only contributes to the construction of novel coordination structures but also explores their fusion-based structural transformation, which will promote the development of supramolecular assemblies with tunable structures and integrated functions.
Results and discussion
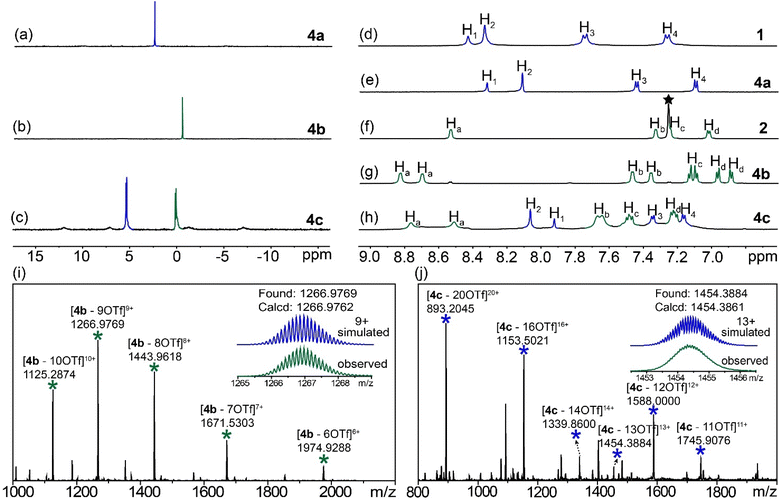
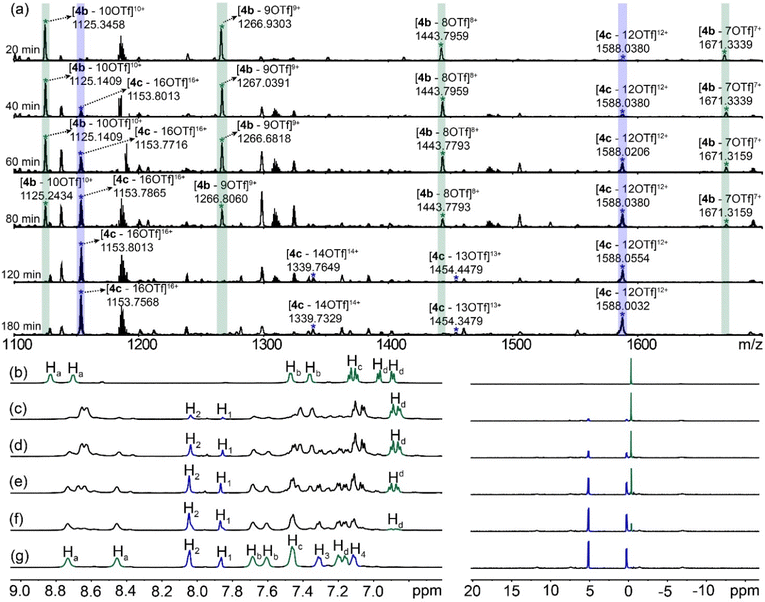
Metallacage 4a or 4b was prepared by the self-assembly of hexatopic carboxylic ligand 1 (L1) or pyridyl ligand 2 (L2) with cis-Pt(PEt3)2(OTf)23 (M), while metallacage 4c was synthesized by the heteroleptic self-assembly of 1, 2 and 3 (Fig. 2, see the ESI† for synthetic details). All the metallacages were characterized by 1D multinuclear (31P{1H} and 1H) NMR, 2D diffusion-ordered NMR spectroscopy (DOSY) and electrospray ionization time-of-flight mass spectroscopy (ESI-TOF-MS), as well as X-ray diffraction analysis. In the 31P{1H} NMR spectra (Fig. 3a and b), only one single peak was observed for metallacages 4a (M12(L1)4) at 2.52 ppm and 4b (M12(L2)4) at 0.47 ppm, agreeing well with the single phosphorus environment in their symmetric structures. For metallacage 4c (M24(L1)4(L2)4), two doublet peaks with equal intensities were found at 5.35 and 0.10 ppm (Fig. 3c), which is consistent with the different phosphorus environments after the coordination. In the 1H NMR spectra, upfield chemical shifts were observed for protons H1, H2, H3 and H4 of 4a compared with its carboxylic precursor 1 (Fig. 3d and e). For metallacages 4b and 4c, downfield chemical shifts were observed for α-pyridyl protons Ha and β-pyridyl protons Hb compared with ligand 2 (Fig. 3f–h). Both Ha and Hb split into two sets of signals, which correspond to the protons inside and outside of the metallacages. In the 2D DOSY spectra, all the protons of metallacages 4b and 4c displayed a single diffusion coefficient (Fig. S13 and S17†), respectively, suggesting the formation of single discrete supramolecular structures. ESI-TOF-MS was carried out to afford the coordination stoichiometries of metallacages 4a–4c. For metallacage 4a (Fig. S7†), peaks at m/z = 1035.5970, 1180.2861, 1373.1576 and 1643.2198 were observed, corresponding to [4a + 8Na]8+, [4a + 7Na]7+, [4a + 6Na]6+ and [4a + 5Na]5+, respectively. For metallacages 4b and 4c, multiple prominent peaks of [M–x(OTf)]x+ (x = 6–20) were observed, owing to the successive loss of counterions (OTf−) (Fig. 3i and j). For instance, peaks at m/z = 1266.9769 and 1454.3884 were found, corresponding to [4b–9OTf]9+ and [4c–13OTf]13+, respectively. All the peaks were isotopically resolved and matched well with their theoretical distributions. These results are consistent with previous reports,33–44 suggesting the successful formation of metallacages 4a–4c.Single crystals of metallacages 4a and 4c were obtained through the slow diffusion of dioxane into a DMF (for 4a) or ethyl acetate into a DMSO (for 4c) solution of the samples for more than two months. In the crystal structure of 4a, four hexatopic carboxylic ligands were connected by 12 platinum(II) nodes, forming a truncated tetrahedron (Fig. 4a). The diameter of 4a was 3.4 nm and the shortest Pt⋯Pt distance was 0.82 nm. The angle of O–Pt–O was ca. 84.1°, while the angle of P–Pt–P was ca. 97.8°. The size of 4a is close to that of 4b (Fig. 4b), whose diameter reached 3.6 nm,45 which would benefit their supramolecular fusion. For metallacage 4c, four hexacarboxylic ligands (1) and four hexapyridyl units (2) were connected by 24 Pt nodes, forming a truncated octahedron with a diameter of 4.4 nm (Fig. 4c). Each carboxylic ligand was connected with its neighboring pyridyl unit through two O–Pt–N coordination bonds. The shortest Pt⋯Pt distance was 1.09 nm. The angle of O–Pt–N was ca. 84.4°, while the angle of P–Pt–P was ca. 96.0°. In the crystal packing of 4c (Fig. S21†), regular packing was facilitated by non-covalent interactions between neighboring molecules, such as van der Waals force between the peripheral PEt3 units and C–H⋯O hydrogen bonds. Due to the propeller-shaped structure of hexaphenylbenzene (HPB), the metallacages constructed with HPB as the main building block exhibit weak host–guest interactions with the common polycyclic aromatic hydrocarbon guest molecule (Fig. S30 and S31†). To the best of our knowledge, metallacage 4a represents the first neutral metallacage self-assembled from Pt nodes and multiple carboxylic ligands, while metallacage 4c is the largest metallacage formed by the heteroleptic assembly of Pt nodes with both pyridyl and carboxylic ligands, which will promote the design and preparation of metallacages with advanced geometric structures.
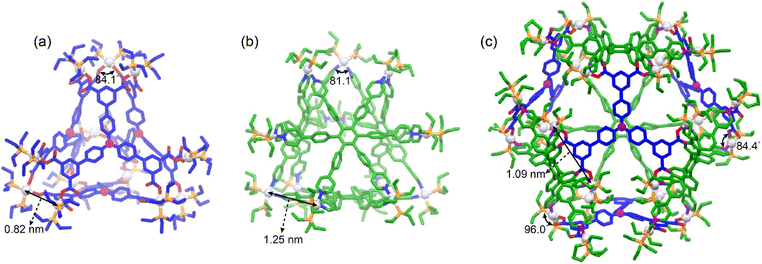 | ||
| Fig. 4 (a)–(c) Crystal structures of metallacages 4a, 4b (ref. 45) and 4c. Hydrogen atoms, counterions, and solvent molecules are omitted for clarity. | ||
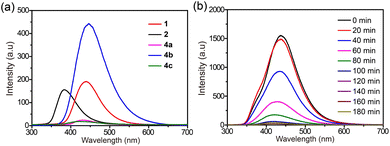
UV/Vis absorption spectroscopy and fluorescence spectroscopy were conducted to investigate the photophysical properties of these ligands and metallacages. Ligands 1 and 2 displayed weak absorption bands centered at ca. 247 nm and 256 nm, with molar absorption coefficients (ε) of 3.67 × 104 and 1.50 × 104 M−1 cm−1, respectively. Metallacages 4a and 4b exhibited dense absorption bands centered at ca. 354 nm and 276 nm, with ε of 9.27 × 104 and 1.83 × 105 M−1 cm−1, respectively (Fig. S22†). Metallacage 4c showed two absorption bands centered at ca. 297 and 342 nm (Fig. S22†), with ε of 3.07 × 105 and 1.09 × 105 M−1 cm−1, respectively, which were derived from the absorption of ligands 1 and 2. Metallacages 4a and 4c showed weak emission centered at ca. 433 and 420 nm, respectively, while bright emission centered at ca. 450 nm was observed for metallacage 4b (Fig. 5a). The weak emission of 4a and 4c was due to the photoinduced electron transfer,47 while the bright emission of 4b was because of the restriction of molecular motions by metal-coordination bonds,48,49 which has also been observed in other supramolecular systems.50,51
The distinct fluorescence characteristics of these metallacages provide a visual method to monitor their assembly and fusion processes by using the changes in fluorescence as output signals. Mixing 4a and 4b in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio resulted in a gradually decreased emission over time, accompanied by a blueshift of the maximum emission (Fig. 5b). After 120 min, negligible changes were observed from the fluorescence spectra (Fig. S23†), suggesting that the reaction reached an equilibrium state and that the supramolecular fusion of geometrically similar 4a and 4b into 4c was complete in 120 min.
1 molar ratio resulted in a gradually decreased emission over time, accompanied by a blueshift of the maximum emission (Fig. 5b). After 120 min, negligible changes were observed from the fluorescence spectra (Fig. S23†), suggesting that the reaction reached an equilibrium state and that the supramolecular fusion of geometrically similar 4a and 4b into 4c was complete in 120 min.
ESI-TOF-MS was also carried out to study the kinetics of the fusion (Fig. 6a). Acetonitrile solutions containing the same amount of 4a and 4b were stirred at 60 °C and the ESI-TOF-MS spectra were collected at different time points. After 20 min, the peaks corresponding to 4c started to appear, suggesting that the fusion process began to take place at this time point. As time progressed, the signals of 4b gradually decreased, while those of 4c increased. After 120 min, only the signals of 4c were observed from the spectra, which agreed well with the fluorescence data, indicating that the fusion of 4a and 4b was complete.
The concentration-dependent fusion between 4a and 4b was investigated by NMR spectroscopy. The gradual addition of 4a into the acetonitrile solution of 4b resulted in significant changes in the NMR spectra (Fig. 6b–g). The peaks of protons Ha shifted upfield. while downfield chemical shifts were found for protons Hb, Hc and Hd. Two new peaks, derived from protons H1 and H2 of 4a, gradually emerged. After the addition of 1.0 equivalent of 4a into 4b, the 1H NMR spectrum matched well with that of 4c, suggesting a quantitative transformation from the mixture of 4a and 4b to 4c. In the 31P{1H} NMR spectra, the single peak of 4b slowly disappeared, while two doublet peaks corresponding to 4c were observed over time. Moreover, the variations in solvent and counterions showed little influence on the formation of 4c (Fig. S26–29†). Therefore, the fusion process of these assemblies was fully tracked and supported by the combination of fluorescence, mass and NMR spectroscopy, which provides a good example for the construction of supramolecular assemblies with improved structural complexity via supramolecular fusion. To understand the mechanism of the fusion from two truncated tetrahedron-shaped metallacages, we compared the energies of the sum of the two homoleptic M12L4 metallacages with that of 4c using the semiempirical GFN1-xTB method. According to the comparison between the energy values of metallacages, the energy of 4c is significantly lower than the sum of the energies of the homoleptic metallacages (Fig. S32†), suggesting that the assembly of 4c is enthalpically more favorable.
Conclusions
In summary, a neutral truncated tetrahedron and a giant truncated octahedron were successfully prepared by metal-coordination-driven self-assembly. Interestingly, the neutral truncated tetrahedron could be quantitatively transformed into the truncated octahedron via its further assembly with another cationic truncated tetrahedron, representing a supramolecular fusion at the three-dimensional level from geometrically the same truncated tetrahedron-shaped metallacages. The fundamental knowledge obtained from the current research not only shows the process of supramolecular fusion, but also provides a simple and efficient strategy for preparing supramolecular assemblies with enhanced structural complexity and integrated functions.Data availability
The data supporting this article have been included in the ESI.† Crystallographic data for 4a (2314346) and 4c (2314347) have been deposited at the Cambridge Crystallographic Data Centre.Author contributions
M. Zhang and H. Liu conceived the project. H. Liu and Y. Huang carried out the synthesis and some characterization experiments of the compounds. C. Guo carried out the ESI-TOF-MS tests and analyzed the data. Z. Zhou, S. Jian, Z. Zhang, and Y. Hou assisted in the synthesis and characterization of the compounds. The manuscript was written by H. Liu, C. Mu, and M. Zhang and all authors contributed to the final draft of the paper.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171219 and 22222112), the Innovation Talent Promotion Plan of Shaanxi Province for Science and Technology Innovation Team (2023-CX-TD-51), and the Fundamental Research Funds for the Central Universities. We thank Dr Gang Chang and Dan He at the Instrument Analysis Center of Xi'an Jiaotong University for the NMR and fluorescence measurements. We also thank Dr Zhenyi Zhang from Bruker (Beijing) Scientific Technology in Shanghai for the X-ray crystal structure analysis.References
- Y. Yuan, M. F. Tam, V. Simplaceanu and C. Ho, Chem. Rev., 2015, 115, 1702–1724 CrossRef CAS PubMed.
- G. P. Lisi and J. P. Loria, Chem. Rev., 2016, 116, 6323–6636 CrossRef CAS PubMed.
- K. W. Chen, T. Y. Sun and Y. D. Wu, Chem. Rev., 2023, 123, 9940–9981 CrossRef CAS PubMed.
- J. J. Vittal, Coord. Chem. Rev., 2007, 251, 1781–1795 CrossRef CAS.
- W. Wang, Y. X. Wang and H. B. Yang, Chem. Soc. Rev., 2016, 45, 2656–2693 RSC.
- Y. Gong, Y. Zhang, C. Qin, C. Sun, X. Wang and Z. Su, Angew. Chem., Int. Ed., 2019, 58, 780–784 CrossRef CAS PubMed.
- D. Zhang, Q. Gan, A. J. Plajer, R. Lavendomme, T. K. Ronson, Z. Lu, J. D. Jensen, B. W. Laursen and J. R. Nitschke, J. Am. Chem. Soc., 2022, 144, 1106–1112 CrossRef CAS PubMed.
- A. Walther, I. Regeni, J. J. Holstein and G. H. Clever, J. Am. Chem. Soc., 2023, 145, 25365–25371 CrossRef CAS PubMed.
- G. Li, Z. Zhou, C. Yuan, Z. Guo, Y. Liu, D. Zhao, K. Liu, J. Zhao, H. Tan and X. Yan, Angew. Chem., Int. Ed., 2020, 59, 10013–10017 CrossRef CAS PubMed.
- C. F. Espinosa, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2023, 145, 9965–9969 CrossRef CAS PubMed.
- E. Zangrando, M. Casanova and E. Alessio, Chem. Rev., 2008, 108, 4979–5013 CrossRef CAS PubMed.
- T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed.
- T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang and K. W. Chi, Acc. Chem. Res., 2019, 46, 2464–2474 CrossRef PubMed.
- M. Yoshizawa, M. Tamura and M. Fujita, Science, 2006, 312, 251–254 CrossRef CAS PubMed.
- M. D. Wise, J. J. Holstein, P. Pattison, C. Besnard, E. Solari, R. Scopelliti, G. Bricogne and K. Severin, Chem. Sci., 2015, 6, 1004–1010 RSC.
- T. Tsutsui, L. Catti, K. Yoza and M. Yoshizawa, Chem. Sci., 2020, 11, 8145–8150 RSC.
- T. Z. Xie, K. J. Endres, Z. Guo, J. M. Ludlow III, C. N. Moorefield, M. J. Saunders, C. Wesdemiotis and G. R. Newkome, J. Am. Chem. Soc., 2016, 138, 12344–12347 CrossRef CAS PubMed.
- J. E. M. Lewis, E. L. Gavey, S. A. Cameron and J. D. Crowley, Chem. Sci., 2012, 3, 778–784 RSC.
- R. L. Spicer, A. D. Stergiou, T. A. Young, F. Duarte, M. D. Symes and P. J. Lusby, J. Am. Chem. Soc., 2020, 142, 2134–2139 CrossRef CAS PubMed.
- P. Howlader, S. Mondal, S. Ahmed and P. S. Mukherjee, J. Am. Chem. Soc., 2020, 142, 20968–20972 CrossRef CAS PubMed.
- D. N. Yan, L. X. Cai, P. M. Cheng, S. J. Hu, L. P. Zhou and Q. F. Sun, J. Am. Chem. Soc., 2021, 143, 16087–16094 CrossRef CAS PubMed.
- R.-J. Li, A. Marcus, F. Fadaei-Tirani and K. Severin, Chem. Commun., 2021, 57, 10023–10026 RSC.
- Y. Li, J. Dong, W. Gong, X. Tang, Y. Liu, Y. Cui and Y. Liu, J. Am. Chem. Soc., 2021, 143, 20939–20951 CrossRef CAS PubMed.
- A. Kumar, S. S. Sun and A. J. Lees, Coord. Chem. Rev., 2008, 252, 922–939 CrossRef CAS.
- M. D. Ward, C. A. Hunter and N. H. Williams, Acc. Chem. Res., 2018, 51, 2073–2082 CrossRef CAS PubMed.
- K. Niki, T. Tsutsui, M. Yamashina, M. Akita and M. Yoshizawa, Angew. Chem., Int. Ed., 2020, 59, 10489–10492 CrossRef CAS PubMed.
- Y. Tamura, H. Takezawa and M. Fujita, J. Am. Chem. Soc., 2020, 142, 5504–5508 CrossRef CAS PubMed.
- P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed.
- G. R. Genov, H. Takezawa, H. Hayakawa and M. Fujita, J. Am. Chem. Soc., 2023, 145, 17013–17017 CrossRef CAS PubMed.
- Y. R. Zheng, Z. Zhao, M. Wang, K. Ghosh, J. B. Pollock, T. R. Cook and P. J. Stang, J. Am. Chem. Soc., 2010, 132, 16873–16882 CrossRef CAS PubMed.
- A. K. Bar, G. Mostafa and P. S. Mukherjee, Inorg. Chem., 2010, 49, 7647–7649 CrossRef CAS PubMed.
- A. K. Bar, S. Raghothama, D. Moon and P. S. Mukherjee, Chem.–Eur. J., 2012, 18, 3199–3209 CrossRef CAS PubMed.
- Y. Hou, Z. Zhang, S. Lu, J. Yuan, Q. Zhu, W. P. Chen, S. Ling, X. Li, Y. Z. Zheng, K. Zhu and M. Zhang, J. Am. Chem. Soc., 2020, 142, 18763–18768 CrossRef CAS PubMed.
- C. Mu, Z. Zhang, Y. Hou, H. Liu, L. Ma, X. Li, S. Ling, G. He and M. Zhang, Angew. Chem., Int. Ed., 2021, 60, 12293–12297 CrossRef CAS PubMed.
- Y. Hou, R. Shi, H. Yuan and M. Zhang, Chin. Chem. Lett., 2023, 34, 107688 CrossRef CAS.
- Z. Zhang, L. Ma, F. Fang, Y. Hou, C. Lu, C. Mu, Y. Zhang, H. Liu, K. Gao, M. Wang, Z. Zhang, X. Li and M. Zhang, JACS Au, 2022, 2, 1479–1487 CrossRef CAS PubMed.
- Q. Feng, T. Yang, L. Ma, X. Li, H. Yuan, M. Zhang, Y. Zhang and L. Fan, ACS Appl. Mater. Interfaces, 2022, 14, 38594–38603 CrossRef CAS PubMed.
- Y. Hou, Z. Zhang, L. Ma, R. Shi, S. Ling, X. Li and M. Zhang, CCS Chem., 2022, 4, 2604–2611 CrossRef CAS.
- H. Liu, Z. Zhang, C. Mu, L. Ma, H. Yuan, S. Ling, H. Wang, X. Li and M. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202207289 CrossRef CAS PubMed.
- Q. Feng, N. Li, Z. Zhang, K. Gao, K. Wang, S. Ling, H. Yuan, Y. Zhang and M. Zhang, Chin. Chem. Lett., 2023, 34, 108439 CrossRef CAS.
- H. Liu, C. Guo, Z. Zhang, C. Mu, Q. Feng and M. Zhang, Chem.–Eur. J., 2023, 29, e202203926 CrossRef CAS PubMed.
- H. Duan, T. Yang, Q. Li, F. Cao, P. Wang and L. Cao, Chin. Chem. Lett., 2024, 3, 108878 CrossRef.
- R. Zhang, D. Hu, Y. Fu, Q. Feng, C. Mu, K. Gao, H. Ma, M. Liu and M. Zhang, Aggregate, 2024, 5, e408 CrossRef CAS.
- Y. Hou, C. Mu, Y. Shi, Z. Zhang, H. Liu, Z. Zhou, S. Ling, B. Shi, X. Duan, C. Yang and M. Zhang, Aggregate, 2024, e628 CrossRef.
- Y. R. Zheng, Z. Zhao, H. Kim, M. Wang, K. Ghosh, J. B. Pollock, K. W. Chi and P. J. Stang, Inorg. Chem., 2010, 49, 10238–10240 CrossRef CAS PubMed.
- Z. Guo, G. Li, H. Wang, J. Zhao, Y. Liu, H. Tan, X. Li, P. J. Stang and X. Yan, J. Am. Chem. Soc., 2021, 143, 9215–9221 CrossRef CAS PubMed.
- J. L. Zhu, L. Xu, Y. Y. Ren, Y. Zhang, X. Liu, G. Q. Yin, B. Sun, X. Cao, Z. Chen, X. L. Zhao, H. Tan, J. Chen, X. Li and H. B. Yang, Nat. Commun., 2019, 10, 4285 CrossRef PubMed.
- J. Mei, N. L. Leung, R. T. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS PubMed.
- L. J. Chen, Y. Y. Ren, N. W. Wu, B. Sun, J. Q. Ma, L. Zhang, H. Tan, M. Liu, X. Li and H. B. Yang, J. Am. Chem. Soc., 2015, 137, 11725–11735 CrossRef CAS PubMed.
- P. Das, A. Kumar, P. Howlader and P. S. Mukherjee, Chem.–Eur. J., 2017, 23, 12565–12574 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2314346 (for 4a) and 2314347 (for 4c). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02736a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |