Open Access Article
Open Access ArticleTrityl isocyanide as a general reagent for visible light mediated photoredox-catalyzed cyanations†
Irene
Quirós
a,
María
Martín
 a,
Carla
Pérez-Sánchez
a,
Thomas
Rigotti
a,
Carla
Pérez-Sánchez
a,
Thomas
Rigotti
 *a and
Mariola
Tortosa
*a and
Mariola
Tortosa
 *abc
*abc
aOrganic Chemistry Department, Faculty of Science, Autonomous University of Madrid (UAM), Madrid, 28049, Spain. E-mail: mariola.tortosa@uam.es
bCenter for Innovation in Advanced Chemistry (ORFEO-CINQA), Autonomous University of Madrid (UAM), Madrid, 28049, Spain
cInstitute for Advanced Research in Chemical Sciences (IAdChem), Autonomous University of Madrid (UAM), Madrid, 28049, Spain
First published on 6th August 2024
Abstract
A photoredox catalytic strategy has been developed to enable the functionalization of a variety of commercially available, structurally different radical precursors by the use of a bench-stable isonitrile as an efficient cyanating reagent. Specifically, a radical-based reaction has provided a mild and convenient procedure for the cyanation of primary, secondary and tertiary radicals derived from widely accessible sp3-hybridized carboxylic acids, alcohols and halides under visible light irradiation. The reaction tolerates a variety of functional groups and it represents a complementary method for the cyanation of structurally different scaffolds that show diverse native functionalities, expanding the scope of previously reported methodologies.
Introduction
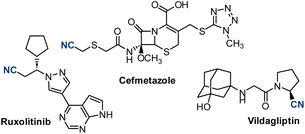
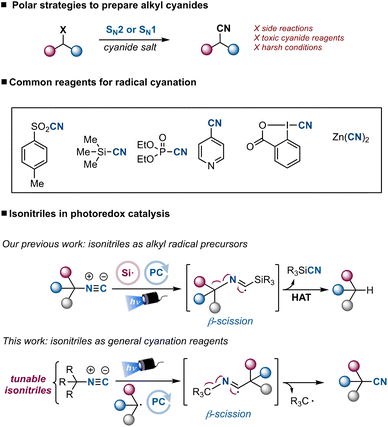
Aliphatic nitriles are ranked among the most common functional groups in bioactive molecules.1 The anticancer agent ruxolitinib, the antihyperglycemic vildagliptin and the antibacterial cefmetazole are examples of commercialized drugs containing alkyl cyanides. Additionally, nitriles are versatile synthetic handles used to introduce a broad variety of functional groups in organic molecules, and prevalent intermediates in the synthesis of heterocycles (Fig. 1).2Traditionally, alkyl halides have been used to prepare aliphatic nitriles through a SN2 or SN1 reaction. Some drawbacks of this approach are the need to prepare the alkyl halides, the use of toxic cyanide salts, high temperatures and competing elimination reactions.3 To complement this polar strategy different radical approaches have been developed, based on the generation of a nucleophilic radical from a suitable precursor followed by reaction with a cyanating reagent. With the expansion of photoredox catalysis4 and electrochemistry,5 radical approaches have become attractive ways to prepare alkyl nitriles from native and abundant functional groups under extremely mild conditions,6 complementing the use of transition metals and avoiding high temperatures.7 Carboxylic acids,8–10 redox-active esters,11 alkyl halides,12,13 trifluoroborate salts,14 and specific C–H bonds10,15 have been used as carbon-centered alkyl radical precursors in photoredox-catalyzed and electrochemical cyanation reactions. The cyanating reagents used in these transformations include tosyl and trimethylsilyl cyanide, cyanobenziodoxolone, 4-cyano pyridine and inorganic salts such as sodium and potassium cyanides (Scheme 1). All of them are cyanide-containing reagents often used in superstochiometric amounts. The introduction of cyanide-free reagents that could promote general photoredox catalyzed cyanations would be a convenient addition to the toolbox that chemists have at disposal to prepare aliphatic nitriles.
 | ||
| Scheme 1 Common reagents used for radical cyanation of aliphatic precursors. Dual use of isonitriles in photoredox catalysis: alkyl radical precursors and cyanation reagents. | ||
Our group recently demonstrated that isonitriles can unlock hydro- and deuterodeamination reactions under extremely mild conditions.16 Indeed, isonitriles can intercept visible light-generated silyl radicals to give an imidoyl radical intermediate that enables a β-scission, provoking the C–N bond fragmentation. Based on these results, we reasoned that a tunable isonitrile could intercept carbon-centered radicals generated under photoredox catalysis to provide a unified strategy for the cyanation of common functional groups.
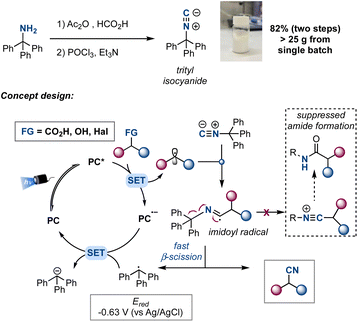
Although isonitriles have been used as efficient radical traps17 and suitable reagents in radical cyanations,18 the nitrile product usually competes with the formation of an amide, through the in situ generation of a nitrilium ion or a ketenimine.19 We envisioned that trityl isocyanide, a bench stable solid easily prepared from trityl amine,20 could be used as a selective cyanating reagent for precursors capable to provide alkyl radicals through a photoredox reductive quenching cycle (Scheme 2). Among the class of compounds that could generate alkyl radicals through a reductive quenching cycle, carboxylic acids, alcohols and alkyl halides (via an alpha-amino radical-mediated halogen atom transfer) attracted our interest as they are commercially available abundant building blocks.21 Once generated, the alkyl radical would add to trityl isocyanide to form an imidoyl radical.22 Subsequent β-fragmentation would afford the nitrile product and a trityl radical that could be easily reduced to the stabilized trityl anion through a single-electron reduction [Ered = −0.63 V vs. Ag/AgCl],23 regenerating the photocatalyst. Key in our design is the fact that the stability of the trityl radical would favour a fast β-scission, therefore avoiding the nitrilium ion formation through single-electron oxidation,24 and shifting the selectivity towards the nitrile formation. During the preparation of this manuscript Procter reported the synthesis of aliphatic nitriles from alkyl iodides using a sulfonium salt as a halogen atom transfer reagent precursor and an α-amide isocyanide as cyanating reagent.25 Herein, we present the use of trityl isocyanide as a general cyanating reagent for alkyl carboxylic acids, alcohols and halides. This reagent allows the use of widely accessible sp3-hybridized building blocks, providing a straightforward access to structurally different nitriles under mild conditions (Scheme 2).
Results and discussion
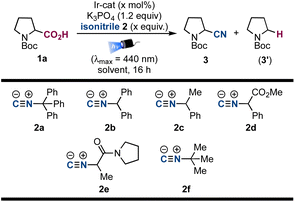
We started our investigations choosing carboxylic acids 1 as building blocks that could generate carbon-centered radicals by established photoredox catalysis via the corresponding carboxylates [Eox ≈ +1.2 V vs. SCE] upon deprotonation and subsequent decarboxylation.26,27As preliminary conditions, we employed [Ir(dF(CF3)ppy)2(dtbbpy)PF6] (Ir-cat) as the photoredox catalyst 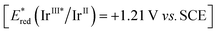 ,28 K3PO4 as base, acetonitrile as solvent and we irradiated the reaction with a blue 440 nm Kessil PR160L LED lamp. On the other hand, isonitrile 2a (3.0 equiv.) was chosen since we envisioned that the corresponding trityl radical that would be obtained after β-scission would be easily reduced to regenerate the ground state photocatalyst. Indeed, we were delighted to observe an efficient decarboxylation of carboxylic acid 1a and the concomitant formation of product 3 in 68% yield (Table 1, entry 1). Next, we evaluated different isonitriles (2b–f) as alternative cyanating reagents (entries 2–6), observing a diminished reactivity which indicated that the stability and redox potential of the intermediate isocyanide-derived radical play a crucial role. Moreover, different amounts of decarboxylative product 3′ were obtained, observing a lower ratio with isonitrile 2a, presumably due to the easy β-scission that leads to the highly stabilized trityl radical. Switching the solvent to DMSO led to an improvement of the yield (entry 7) and after some further optimization (see ESI† for additional details) the desired product could be obtained in 82% yield, employing 1.0 mol% of catalyst loading, 2.5 equiv. of isonitrile and performing the reaction on a 0.20 mmol scale (entry 11).
,28 K3PO4 as base, acetonitrile as solvent and we irradiated the reaction with a blue 440 nm Kessil PR160L LED lamp. On the other hand, isonitrile 2a (3.0 equiv.) was chosen since we envisioned that the corresponding trityl radical that would be obtained after β-scission would be easily reduced to regenerate the ground state photocatalyst. Indeed, we were delighted to observe an efficient decarboxylation of carboxylic acid 1a and the concomitant formation of product 3 in 68% yield (Table 1, entry 1). Next, we evaluated different isonitriles (2b–f) as alternative cyanating reagents (entries 2–6), observing a diminished reactivity which indicated that the stability and redox potential of the intermediate isocyanide-derived radical play a crucial role. Moreover, different amounts of decarboxylative product 3′ were obtained, observing a lower ratio with isonitrile 2a, presumably due to the easy β-scission that leads to the highly stabilized trityl radical. Switching the solvent to DMSO led to an improvement of the yield (entry 7) and after some further optimization (see ESI† for additional details) the desired product could be obtained in 82% yield, employing 1.0 mol% of catalyst loading, 2.5 equiv. of isonitrile and performing the reaction on a 0.20 mmol scale (entry 11).
| Entry | Solvent | 2 (equiv.) | Ir-cat (mol%) | Yield 3 (3′) % |
|---|---|---|---|---|
| a The reactions were performed on a 0.10 mmol scale and the yields were determined by 1H NMR with CH2Br2 as an internal standard. b The reaction was performed on a 0.20 mmol scale. c Yield determined after isolation by column chromatography purification. d The reaction was performed in the absence of light. e The reaction was performed in the absence of photocatalyst. See ESI for further details. | ||||
| 1 | MeCN | 2a (3.0) | 2 | 68 (14) |
| 2 | MeCN | 2b (3.0) | 2 | 55 (35) |
| 3 | MeCN | 2c (3.0) | 2 | 30 (5) |
| 4 | MeCN | 2d (3.0) | 2 | 48 (52) |
| 5 | MeCN | 2e (3.0) | 2 | 24 (0) |
| 6 | MeCN | 2f (3.0) | 2 | 4 (12) |
| 7 | DMSO | 2a (3.0) | 2 | 74 (12) |
| 8 | DMF | 2a (3.0) | 2 | 60 (14) |
| 9 | DCM | 2a (3.0) | 2 | 0 (5) |
| 10 | PhCH3 | 2a (3.0) | 2 | 36 (30) |
| 11b,c | DMSO | 2a (2.5) | 1 | 82 (8) |
| 12d | DMSO | 2a (2.5) | 1 | 0 |
| 13e | DMSO | 2a (2.5) | 1 | 0 |
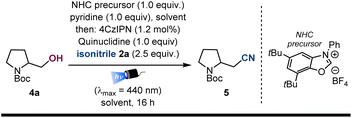
To enhance the synthetic utility of the isonitrile-enabled photocatalytic cyanation, we decided to expand the scope of the reaction by tackling a similar transformation starting from widely accessible aliphatic alcohols 4 as radical precursors. To achieve the required radical deoxygenation, we relied on a recently developed strategy that, upon oxidation of an alcohol-NHC (N-heterocyclic carbene) adduct [Eox ≈ +0.6 V vs. SCE], allows an efficient C–O bond homolysis.29 Slightly adapting this methodology to our envisioned cyanation strategy, we obtained a promising 30% yield (Table 2, entry 1) when 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) was used as the photoredox catalyst 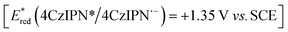 .30 The use of MTBE or 1,4-dioxane as solvents improved the yields (entries 2 and 3), obtaining the product in 75% yield when a 1
.30 The use of MTBE or 1,4-dioxane as solvents improved the yields (entries 2 and 3), obtaining the product in 75% yield when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dioxane and DMSO was employed (entry 4). After some additional optimization (see ESI† for further details) the reaction could be scaled up to 0.2 mmol with a 2.0 mol% of photocatalyst, delivering the desired nitrile 5 in a slightly diminished yield (60%, entry 5).
1 mixture of dioxane and DMSO was employed (entry 4). After some additional optimization (see ESI† for further details) the reaction could be scaled up to 0.2 mmol with a 2.0 mol% of photocatalyst, delivering the desired nitrile 5 in a slightly diminished yield (60%, entry 5).
| Entry | Solvent | 4CzIPN (mol%) | Yield % |
|---|---|---|---|
| a The reactions were performed on a 0.10 mmol scale and the yields were determined after isolation by column chromatography purification. b The reaction was performed on a 0.20 mmol scale. c The reaction was performed in the absence of light. d The reaction was performed in the absence of photocatalyst. See ESI for further details. | |||
| 1 | MTBE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMA (1 DMA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.2 | 30 |
| 2 | MTBE | 1.2 | 46 |
| 3 | Dioxane | 1.2 | 52 |
| 4 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.2 | 75 |
| 5b | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.2 | 60 |
| 6c | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.2 | 0 |
| 7d | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 0 |
Next, with the aim of developing a complementary method that could provide an alternative to nucleophilic substitution reactions, we evaluate the possibility to exploit a halogen atom transfer (XAT) photocatalytic strategy to generate a carbon-centered radical from alkyl halides 6 (Table 3).31 Specifically, the photoredox catalyst engages in a SET with triethylamine [Eox = +0.77 V vs. SCE] by a reductive quenching cycle to deliver an α-amino alkyl radical as a powerful XAT reagent that abstract the iodine atom to deliver a new carbon-centered radical.
| Entry | Solvent | 4CzIPN (mol%) | Yield % |
|---|---|---|---|
| a The reactions were performed on a 0.20 mmol scale and the yields were determined after isolation by column chromatography purification. b The reaction was performed in the absence of triethylamine. c The reaction was performed in the absence of light. d The reaction was performed in the absence of photocatalyst. See ESI for further details. | |||
| 1 | MeCN | 5.0 | 88 |
| 2 | MeCN | 2.0 | 91 |
| 3 | DMSO | 2.0 | 50 |
| 4 | Acetone | 2.0 | 69 |
| 5b | MeCN | 2.0 | 0 |
| 6c | MeCN | 2.0 | 0 |
| 7d | MeCN | — | 0 |
Gratifyingly, adding isonitrile 2a to the reaction mixture, the transiently generated radical could be efficiently trapped to furnish the corresponding isonitrile 7 in 88% yield (Table 3, entry 1). Decreasing the photocatalyst loading to 2.0 mol% afforded the product in 91% yield (entry 2), whereas different solvents than acetonitrile led to diminished yields (entries 3 and 4). Even in this case, the presence of all the reaction components was necessary for a successful reaction since it did not proceed in the absence of XAT reagent, light or photocatalyst.
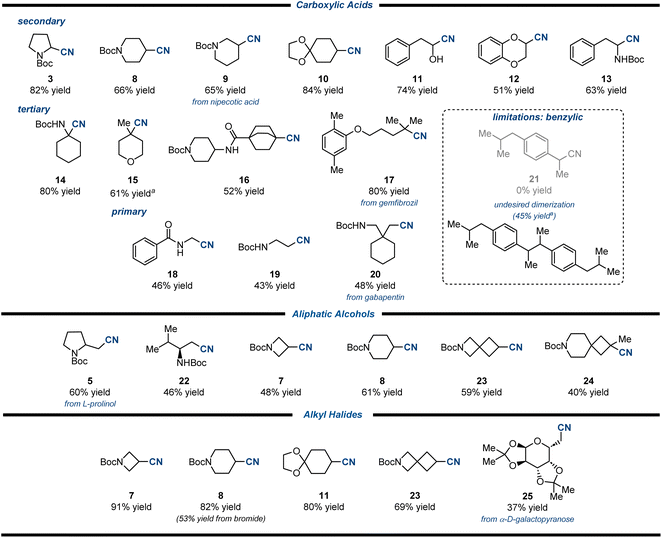
With the optimized conditions in hand for the three different radical precursors, we evaluated the scope of the photocatalytic cyanation reaction, employing a variety of carboxylic acids 1,8–10 aliphatic alcohols 4, and alkyl halides 6 (Table 4).12,13,25N-Boc-piperidine-containing secondary carboxylic acids delivered the corresponding nitriles 8 and 9 in 66% and 65% yield. Acetal-, hydroxy- and catechol-containing products (10–12) were obtained in moderate to high yields, highlighting the functional group compatibility of the method. On the other hand, N-boc phenylalanine derived nitrile 13 was isolated in 63% yield, showcasing that N-protected α-amino acids are suitable substrates. Next, we employed tertiary carboxylic acids to study the influence of the radical stability and of its steric hindrance on the outcome of the reaction. We were pleased to observe high reactivity in all the cases, indicating an efficient β-scission of the imidoyl radical upon radical addition on isonitrile 2a. Indeed, products 14–17 were obtained, allowing the introduction of a nitrile functionality at the bridgehead position of a bicyclo[2.2.2]octane and the modification of the lipid-regulating agent gemfibrozil. Stabilized and not stabilized primary radicals could be employed, resulting in the formation of nitriles 18–20, albeit with slightly lower yields. The general reactivity observed with primary, secondary and tertiary aliphatic carboxylic acids is striking as most previous examples are limited to carboxylic acids carrying an α-heteroatom.8–10 On the other hand, benzylic carboxylic acids were not suitable for this reaction. Indeed, although efficiently decarboxylated, the corresponding nitrile 21 was not observed and only a dimeric compound was detected (45% NMR yield of a 50% maximum theoretical yield), presumably due to a reluctance of the (more stable) benzyl radical to undergo radical addition to the isonitrile or to a more favourable and undesired α-scission of the transiently formed imidoyl radical.
Subsequently, we evaluated the scope of the reaction employing aliphatic alcohols as radical precursors. Different β-amino alcohols, which are derived from ubiquitous α-amino acids and that present a primary alcohol as functional group, could be employed, delivering products 5 and 22 in 60% and 46% yield, respectively. Secondary aliphatic alcohols furnished a variety of cyclic nitriles (7, 8, and 23) with different ring size or a spirocyclic scaffold. Moreover, a tertiary alcohol was amenable to this transformation, delivering the spirocyclic tertiary nitrile 24 in 40% yield. To the best of our knowledge our protocol represents the first example of photoredox-catalyzed direct cyanation of aliphatic alcohols.
Next, we explored the possibility to carry out a photocatalytic cyanation starting from alkyl halides 6.12,13,25 A variety of structurally different secondary iodides could be employed, obtaining nitriles 7, 8, 11, and 23 in excellent yields. In addition, the same reaction could be performed over secondary bromides, despite a diminished yield in comparison with the parent iodide (53% vs. 82% yield). Primary halides were also suitable for this transformation, as exemplified by the α-D-galactopyranose-containing product 25, which demonstrated the feasibility to employ more complex scaffolds and natural product cores. These results are comparable to those obtained using TMS-CN as cyanating reagent.12
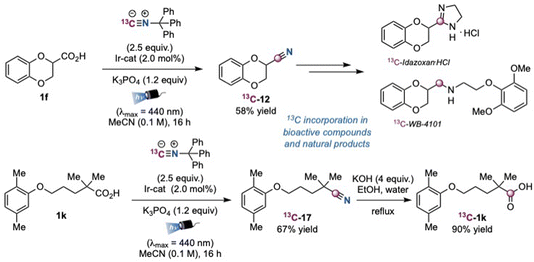
Moreover, we decided to prepare the 13C-labelled analogous of trityl isocyanide 2a, employing 13C-formic acid for its synthesis. Indeed, the use of this 13C-labelled cyanating reagent would enable access to the corresponding isotopic nitrile analogues through functionalization of widely available carboxylic acids, aliphatic alcohols and alkyl halides, allowing the efficient incorporation of a carbon isotope starting from diversified starting materials. As representative examples, we chose to prepare the isotopic analogues of products 12 and 17. When the photocatalytic reaction was performed in the presence of isonitrile 13C-2a as a trapping agent, we smoothly observed the formation of compounds 13C-12 and 13C-17 in 58% and 70% yield (Scheme 3). Therefore, this labelling strategy could enable the synthesis of 13C-labelled analogous of bioactive compounds such as idazoxan hydrochloride and WB-4101.32 Moreover, the potential of this methodology was showcased by the facile hydrolysis of 13C-17, which allowed to access a 13C-labelled gemfibrozil analogue 13C-1k in 90% yield.
Conclusion
In summary, we have developed a general photocatalytic cyanation reaction employing trityl isocyanide as selective cyanating reagent. The use of a photoredox reductive quenching strategy, along with a judicious choice of the more appropriate isonitrile, avoids the formation of undesired nitrilium ions, shifting the transformation towards the nitrile product. The suitability of carboxylic acids, aliphatic alcohols and alkyl halides as radical precursors enables a straightforward transformation from widely accessible building blocks. Besides enabling access to alkyl nitriles from common and diversified precursors, this methodology represents a valuable alternative to polar cyanation strategies that allows the easy preparation of isotopic analogues, avoiding the use of cyanide-containing reagents.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
M. T. conceived and designed the project. I. Q., M. M., C. P.-S., and T. R. performed all optimization studies and photocatalytic reactions. M. T. and T. R. wrote the manuscript with the contribution of all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the European Research Council (ERC Consolidator Grant – 101002715 – SCAN) and the Spanish Ministry of Science and Innovation (MICINN) [PID2022-142594NB-I00] for financial support. M. M. and C. P.-S. acknowledge Ministerio de Universidades and MICINN for FPU (FPU20/06320) and for FPI (PREP2022-000243) fellowships, respectively.Notes and references
- (a) F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, Nitrile-containing pharmaceuticals: Efficacious roles of the nitrile pharmacophore, J. Med. Chem., 2010, 53, 7902 CrossRef CAS PubMed; (b) J. Wang and H. Liu, Application of nitrile in drug design, Chin. J. Org. Chem., 2012, 32, 1643 CrossRef CAS.
- (a) The Chemistry of the Cyano Group, ed. Z. Rappoport and S. Patai, John Wiley & Sons, 1970 Search PubMed; (b) Science of Synthesis, ed. S.-I. Murahashi, Georg Thieme Verlag, Stuttgart, Germany, 2004, p. 19 Search PubMed; (c) R. C. Larock, Comprenhensive Organic Transformations: A Guide to Functional Group Preparations, VCH, New York, 2nd edn, 1999 Search PubMed.
- (a) M. T. Reetz, I. Chatziiosifidis, H. Künzer and H. Müller-Starke, Tetrahedron, 1983, 39, 961–965 CrossRef CAS; (b) M. T. Reetz and I. Chatziiosifidis, Angew. Chem., Int. Ed., 1981, 20, 1017–1018 CrossRef.
- (a) M. D. Kärkäs, J. A. Porco Jr and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef PubMed; (b) Visible Light Photocatalysis in Organic Chemistry, ed. C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, Wiley-VCH, 2018 Search PubMed; (c) T. Rigotti and J. Alemán, Chem. Commun., 2020, 56, 11169–11190 RSC.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed.
- R. I. Patel, S. Sharma and A. Sharma, Org. Chem. Front., 2021, 8, 3166–3200 RSC.
- (a) F. Yan, J.-F. Bai, Y. Dong, S. Liu, C. Li, C.-X. Du and Y. Li, JACS Au, 2022, 2, 2522–2528 CrossRef CAS PubMed; (b) J. Xu, J. C. Twitty and M. P. Watson, Org. Lett., 2021, 23, 6242–6245 CrossRef CAS PubMed; (c) A. Xia, X. Xie, H. Chen, J. Zhao, C. Zhang and Y. Liu, Org. Lett., 2018, 20, 7735–7739 CrossRef CAS PubMed.
- (a) F. Le Vaillant, M. D. Wodrich and J. Waser, Chem. Sci., 2017, 8, 1790–1800 RSC; (b) N. P. Ramirez, B. König and J. C. Gonzalez-Gomez, Org. Lett., 2019, 21, 1368–1373 CrossRef CAS PubMed.
- For a photoelectrochemical asymmetric cyanation, see: X.-L. Lai, M. Chen, Y. Wang, J. Song and H.-C. Xu, J. Am. Chem. Soc., 2022, 144, 20201–20206 CrossRef CAS PubMed.
- For an electrochemical cyanation of carboxylic acids and activated C–H bonds, see: G. S. Kumar, P. S. Shinde, H. Chen, K. Muralirajan, R. Kancherla and M. Rueping, Org. Lett., 2022, 24, 6357–6363 CrossRef CAS PubMed.
- (a) D. Wang, N. Zhu, P. Chen, Z. Lin and G. Liu, J. Am. Chem. Soc., 2017, 139, 15632–15635 CrossRef CAS PubMed; (b) H.-W. Chen, F.-D. Lu, Y. Cheng, Y. Jia, L.-Q. Lu and W.-J. Xiao, Chin. J. Chem., 2020, 38, 1671–1675 CrossRef CAS; (c) For a pioneering photochemical reaction employing Barton esters, see: D. H. R. Barton, J. C. Jaszberenyi and E. A. Theodorakis, Tetrahedron, 1992, 48, 2613–2626 CrossRef CAS.
- For a halogen atom transfer-enabled strategy via metallaphotoredox, see: L. Caiger, H. Zhao, T. Constantin, J. J. Douglas and D. Leonori, ACS Catal., 2023, 13, 4985–4991 CrossRef CAS.
- For a photochemical cyanation of unactivated alkyl chlorides that proceed through the use of UV light, see: T. S. Ratani, S. Bachman, G. C. Fu and J. C. Peters, J. Am. Chem. Soc., 2015, 137, 13902–13907 CrossRef CAS PubMed.
- J.-J. Dai, W.-M. Zhang, Y.-J. Shu, Y.-Y. Sun, J. Xu, Y.-S. Feng and H.-J. Xu, Chem. Commun., 2016, 52, 6793–6796 RSC.
- For examples of photocatalytic hydrogen atom transfer-enabled C–H cyanations, see: (a) S. Kamijo, T. Hoshikawa and M. Inoue, Org. Lett., 2011, 13, 5928–5931 CrossRef CAS PubMed; (b) K. Kim, S. Lee and S. H. Hong, Org. Lett., 2021, 23, 5501–5505 CrossRef CAS PubMed; (c) For a photoredox-catalyzed C–H cyanation enabled by deprotonation of a radical cation, see: I. Robb and J. A. Murphy, Org. Lett., 2024, 26, 2218–2222 CrossRef CAS PubMed; (d) For an electrochemical cyanation of an activated C–H bond, see: G. S. Kumar, P. S. Shinde, H. Chen, K. Muralirajan, R. Kancherla and M. Rueping, Org. Lett., 2022, 24, 6357–6363 CrossRef CAS PubMed.
- I. Quirós, M. Martín, M. Gomez-Mendoza, M. J. Cabrera-Afonso, M. Liras, I. Fernández, L. Nóvoa and M. Tortosa, Angew. Chem., Int. Ed., 2024, 63, e202317683 CrossRef PubMed.
- (a) D. Nanni, Isonitriles: a Useful Trap in Radical Chemistry, in Radicals in Organic Synthesis, ed. P. Renaud and M. P. Sibi, Wiley-VCH Verlag GmbH, 2001 Search PubMed; (b) R. Leardini, D. Nanni and G. Zanardi, J. Org. Chem., 2000, 65, 2763–2772 CrossRef CAS PubMed; (c) J. Lei, D. Li and Q. Zhu, Synthesis of Nitrogen-Containing Heterocycles via Imidoyl or Iminyl Radical Intermediates, In Free-Radical Synthesis and Functionalization of Heterocycles, Topics in Heterocyclic Chemistry 54, ed. Y. Landais, Springer, Cham, Switzerland, 2018 Search PubMed; (d) B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505–3521 RSC.
- (a) G. Stork and P. M. Sher, J. Am. Chem. Soc., 1983, 105, 6765–6766 CrossRef CAS; (b) Y. Shan, X. Zhang, G. Liu, J. Li, Y. Liu, J. Wang and D. Chen, Chem. Commun., 2024, 60, 1546–1562 RSC.
- (a) S. Tang, R. Guillot, L. Grimaud, M. R. Vitale and G. Vincent, Org. Lett., 2022, 24, 2125–2130 CrossRef CAS PubMed; (b) W. Huang, Y. Wang, Y. Weng, M. Shrestha, J. Qu and Y. Chen, Org. Lett., 2020, 22, 3245–3250 CrossRef CAS PubMed; (c) For a chemoselective one-pot sequence towards benzylic nitriles using BF3OEt2 to promote dehydration: X. Jia, Z. Zhang and V. Gevorgyan, ACS Catal., 2021, 11, 13217–13222 CrossRef CAS PubMed.
- (a) R. C. Cioc, P. Schuckman, H. D. Preschel, T. Vlaar, E. Ruijter and R. V. A. Orru, Org. Lett., 2016, 18, 3562–3565 CrossRef CAS PubMed; (b) R. C. Cioc, H. D. Preschel, G. Heijden, E. Ruijter and R. V. A. Orru, Chem.–Eur. J., 2016, 22, 7837–7842 CrossRef CAS PubMed; (c) For a Mn(III) mediated oxidative radical cyanation of arylboronic acids with trityl isocyanide, see: Z. Xu, X. Liang and H. Li, Org. Lett., 2022, 24, 9403–9407 CrossRef CAS PubMed.
- Y. Zabolotna, D. M. Volochnyuk, S. V. Ryabukhin, D. Horvath, K. S. Gavrilenko, G. Marcou, Y. S. Moroz, O. Oksiuta and A. Varnek, J. Chem. Inf. Model., 2022, 62, 2171–2185 CrossRef CAS PubMed.
- (a) M. Minozzi, D. Nanni and P. Spagnolo, Curr. Org. Chem., 2007, 11, 1366–1384 CrossRef CAS; (b) J. Lei, J. Huang and Q. Zhu, Org. Biomol. Chem., 2016, 14, 2593–2602 RSC; (c) S. Sharma, A. P. Pandey and A. Sharma, Adv. Synth. Catal., 2020, 362, 5196–5218 CrossRef CAS.
- M. Poncelet, B. Driesschaert, A. A. Bobko and V. V. Khramtsov, Free Radical Res., 2017, 52, 373–379 CrossRef PubMed.
- For selected examples of nitrilium ion formation through oxidation of an imidoyl radical, see: (a) C. Russo, F. Brunelli, G. C. Tron and M. Giustiniano, Eur. J. Org Chem., 2023, 26, e202300743 CrossRef CAS; (b) S. Pelliccia, A. I. Alfano, P. Luciano, E. Novellino, A. Massarotti, G. C. Tron, D. Ravelli and M. Giustiniano, J. Org. Chem., 2020, 85, 1981–1990 CrossRef CAS PubMed; (c) R. Cannalire, J. Amato, V. Summa, E. Novellino, G. C. Tron and M. Giustiniano, J. Org. Chem., 2020, 85, 14077–14086 CrossRef CAS PubMed; (d) Y. Lv, P. Bao, H. Yue, J.-S. Li and W. Wei, Green Chem., 2019, 21, 6051–6055 RSC; (e) W. Wei, P. Bao, H. Yue, S. Liu, L. Wang, Y. Li and D. Yang, Org. Lett., 2018, 20, 5291–5295 CrossRef CAS PubMed; (f) M. Chen, Y. Li, H. Tang, H. Ding, K. Wang, L. Yang, C. Li, M. Gao and A. Lei, Org. Lett., 2017, 19, 3147–3150 CrossRef CAS PubMed; (g) Z. Guan, Y. Peng, D. Yang, S. Zhu, H. Zhang and A. Lei, Green Chem., 2022, 24, 3964–3968 RSC.
- H. Zhao, V. D. Cuomo, J. A. Rossi-Ashton and D. J. Procter, Chem, 2024, 10, 1–12 Search PubMed.
- (a) L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886–10889 CrossRef CAS PubMed; (b) S. B. Beil, T. Q. Chen, N. E. Intermaggio and D. W. C. MacMillan, Acc. Chem. Res., 2022, 55, 3481–3494 CrossRef CAS PubMed.
- (a) M. Galicia and F. J. Gonzalez, J. Electrochem. Soc., 2002, 149, D46 CrossRef CAS; (b) H. G. Roth, N. A. Romero and D. A. Nicewicz, Synlett, 2016, 27, 714–723 CAS.
- M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal, G. G. Malliaras and S. Bernhard, Chem. Mater., 2005, 17, 5712–5719 CrossRef CAS.
- Z. Dong and D. W. C. MacMillan, Nature, 2021, 598, 451–456 CrossRef CAS PubMed.
- J. Luo and J. Zhang, ACS Catal., 2016, 6, 873–877 CrossRef CAS.
- T. Constantin, M. Zanini, A. Regni, N. S. Sheikh, F. Juliá and D. Leonori, Science, 2020, 367, 1021–1026 CrossRef CAS PubMed.
- (a) C. B. Chapleo, P. L. Myers, R. C. M. Butler, J. C. Doxey, A. G. Roach and C. F. C. Smith, J. Med. Chem., 1983, 26, 823–831 CrossRef CAS PubMed; (b) D. Giardina, P. Angeli, L. Brasili, U. Gulini, C. Melchiorre and G. Strappaghetti, Eur. J. Med. Chem., 1984, 19, 411–414 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization data for all starting materials and products. See DOI: https://doi.org/10.1039/d4sc04199b |
| This journal is © The Royal Society of Chemistry 2024 |