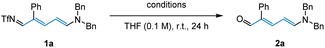Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitrogen-to-functionalized carbon atom transmutation of pyridine†
Fu-Peng
Wu
a,
Madina
Lenz
a,
Adhya
Suresh‡
b,
Achyut R.
Gogoi‡
b,
Jasper L.
Tyler
 a,
Constantin G.
Daniliuc
a,
Constantin G.
Daniliuc
 a,
Osvaldo
Gutierrez
a,
Osvaldo
Gutierrez
 *b and
Frank
Glorius
*b and
Frank
Glorius
 *a
*a
aOrganisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149, Münster, Germany. E-mail: glorius@uni-muenster.de
bDepartment of Chemistry, Texas A&M University, 3255 TAMU, 580 Ross St, 77843 College Station, TX, USA. E-mail: og.labs@tamu.edu
First published on 26th August 2024
Abstract
The targeted and selective replacement of a single atom in an aromatic system represents a powerful strategy for the rapid interconversion of molecular scaffolds. Herein, we report a pyridine-to-benzene transformation via nitrogen-to-carbon skeletal editing. This approach proceeds via a sequence of pyridine ring-opening, imine hydrolysis, olefination, electrocyclization, and aromatization to achieve the desired transmutation. The most notable features of this transformation are the ability to directly install a wide variety of versatile functional groups in the benzene scaffolding, including ester, ketone, amide, nitrile, and phosphate ester fragments, as well as the inclusion of meta-substituted pyridines which have thus far been elusive for related strategies.
Introduction
Single-atom skeletal editing has recently gained unprecedented amounts of attention as a unique strategy for drug design and development.1 This is most prominently demonstrated in structure–activity relationship (SAR) studies where it can be observed that replacing a single atom in the core scaffold of a compound can have an enormous effect on molecular properties.2 For example, the replacement of the nitrogen atom in pyridine for a carbon atom has been shown in certain systems to increase metabolic stability and bioavailability, reducing the risk of adverse side-reactions (Fig. 1A).3 Additionally, nitrogen-containing heterocycles, such as pyridine, represent some of the most common scaffolds in pharmaceuticals4 and, as a consequence, related analogues of these structures are constantly sought-after. Although the concept of atom transmutation is simple, the practical barriers to achieving this transformation are considerable, principally due to the inherent difficulty associated with arene dearomatization and cleaving strong σ-bonds. Nevertheless, single-atom skeletal editing reactions have been reported with increasing frequency over recent years.5 For example, transformations such as oxygen-to-nitrogen swapping,6 nitrogen-atom deletion7 and single carbon atom insertion8 have all been demonstrated across a variety of systems. Furthermore, the conversion of functionalized benzene rings to pyridine, via atom transmutation, has become established as a result of pioneering work, most notably by the groups of Sundberg,9 Burns,10 and Levin.11 However, our attention was drawn to the reverse of this transformation, as the extra available bond associated with the replacement of a nitrogen atom for carbon provides the potential to install additional functionality at this position (Fig. 1B). This fundamental transformation has been previously achieved via intramolecular rearrangements12 and multi-atom replacement reactions,13 however, single-atom transmutations14 are noticeably rarer.In 1977, Hamada and Takeuchi15 discovered that benzo[h]quinoline N-oxide could be converted to anthracene using DMSO as the source of carbon atoms, but this strategy was found to be unsuitable for other substrates (Fig. 1C). Recently, Sorensen and coworkers improved this method by employing n-butyllithium, effectively enhancing conversion efficiency and expanding the substrate scope.16a Similarly, Kano and Morofuji17 reported the synthesis of 4-aryl substituted benzene compounds by reacting Zincke-imines, obtained via pyridine ring-opening, with methyl ketone derived enolates. Although impressive, this two-step approach requires the isolation of the intermediate Zincke-imine in order to facilitate the desired electrocyclization process. In addition, the functionality installed was inherently restricted to ketones and only 4-substituted benzene products could be generated. A further recent report from Greaney and coworkers disclosed a pyridine-to-benzene transformation using malonate nucleophilic addition, although this was limited to para-substituted pyridines.16b Finally, this strategy of ring-opening and ring-closing has been applied to the isotopic labelling of pyridine nitrogen atoms.18
Encouraged by the potential of this emerging field, we set out to identify novel ways to achieve a pyridine-to-benzene transformation via N-to-C atom swapping. Inspired by the work of McNally and coworkers on pyridine halogenation19 and the studies of Juttz and Wagner on electrocyclization,20 we speculated that Tf2O promoted pyridine ring-opening could be followed by the selective olefination of the resulting NTf-imine to access Zincke alkenes (Fig. 1D). These intermediates would be predisposed towards 6π-electrocyclization21 to access a single benzene isomer. In this article, we focus on the reaction pathways of meta-substituted electrocyclizations and the insertion of a new functional group on the newly installed carbon atom (–CFG). Thus, we report the successful development of a new nitrogen-to-functionalized carbon (N–CFG) atom reaction to convert pyridine rings to benzene analogues. Depending on the substituents appended to the olefination reagent, various functionalized benzenes can be effectively accessed using this protocol.
Results and discussion
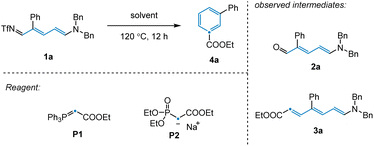
To validate our reaction design, 3-phenyl-pyridine was used as a model substrate, since meta-substituted pyridines have thus far been elusive for related synthetic strategies. We employed Tf2O, amine, and KOtBu to achieve ring-opening of 3-phenyl-pyridine to afford 3-phenyl-NTf-Zincke imine intermediate 1a (Table 1).18 We initially observed that olefination of 1a with phosphaneylidene P1 as the carbon source provided direct access to the desired benzene product 4a in 14% yield (Table 1, entries 1–2). Unfortunately, an extensive screen of reaction solvents was unable to improve the reaction outcome (Table 1, entry 3). However, when olefination reagent P2 was employed in combination with THF, the product yield was improved to 22% (Table 1, entry 4). As well as 4a, Zincke aldehyde 2a and Zincke alkene 3a were also observed under these conditions, suggesting that the reaction mechanism likely involves imine hydrolysis followed by olefination and electrocyclization. With this in mind, we attempted to probe these individual steps in isolation in order to gain a better understanding of the transformation.| Entry | Solvent | Reagent | 4a, yield (%) |
|---|---|---|---|
| a Reaction conditions: Zincke imine (0.05 mmol), P1 (1.5 eq.) or P2 (1.5 eq., prepared from ethyl 2-(diethoxyphosphoryl)acetate and NaH), stirred at 120 °C for 12 h. Yields were determined by 1H NMR using CH2Br2 as an internal standard. | |||
| 1 | Toluene (r.t.) | P1 | Trace |
| 2 | Toluene | P1 | 14 |
| 3 | EtOAc, CH2Cl2, THF, DMF, MeCN, or EtOH | P1 | <10 |
| 4 | THF | P2 | 22 |
Despite being reported as an effective method for imine hydrolysis in previous transformations,22 silica gel was found to be entirely unsuitable in this case (Table 2, entry 1). Additionally, employing HCl and NaOH respectively, failed to access the desired aldehyde 2a (Table 2, entries 2–3). Conversely, when imine 1a was subjected to NaOtBu and H2O, we observed full conversion and obtained 99% of the Zincke aldehyde (Table 2, entry 4). Subsequent control experiment showed that imine quickly decomposes in the absence of H2O (Table 2, entry 5). It was later discovered that hydrolysis could also be efficiently achieved after just 15 minutes upon heating the imine under basic conditions (Table 2, entry 6).
| Entry | Conditions | RSM (%) | 4a, yield (%) |
|---|---|---|---|
| a Reaction conditions: Zincke imine (0.05 mmol), base or acid, and THF (0.1 M) stirred at room temperature for 24 h. b 120 °C for 15 min. | |||
| 1 | Silica gel (50 mg mL−1) | 100 | 0 |
| 2 | HCl (1 M) | 100 | 0 |
| 3 | NaOH (1 M) | 100 | 0 |
| 4 | NaOtBu, H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.1 eq.) 1, 1.1 eq.) |
0 | 99 |
| 5 | NaOtBu (1.1 eq.) | 0 | 0 |
| 6b | NaOtBu, H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.1 eq.) 1, 1.1 eq.) |
0 | 99 |
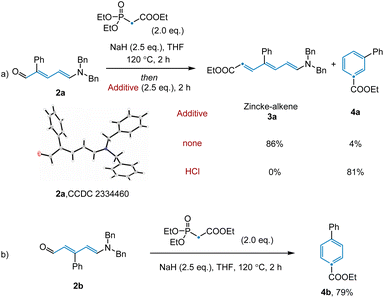
Having achieved this next step of the transformation, we turned our attention to the olefination and subsequent ring closure of Zincke aldehyde 2a (Scheme 1a). Although the thermal Horner–Wadsworth–Emmons reaction (HWE) was expected to promote both of these steps, only trace desired product 4a was observed under standard olefination conditions. Upon scanning additives, we found that basic conditions inhibited electrocyclization, while acidic conditions promoted reactivity for this substrate (see ESI†). Notably, in the synthesis of para-substituted pyridine 4b, no additive was required to access the desired product (Scheme 1b, see below for discussion). Given that the conditions employed for the individual transformations in this sequence were relatively simple, we hypothesized that all steps, including Tf2O promoted pyridine ring-opening, could be combined into a convenient protocol. Indeed, this was found to be the case, with desired benzene product 4a being prepared in 70% yield from the corresponding substituted pyridine.
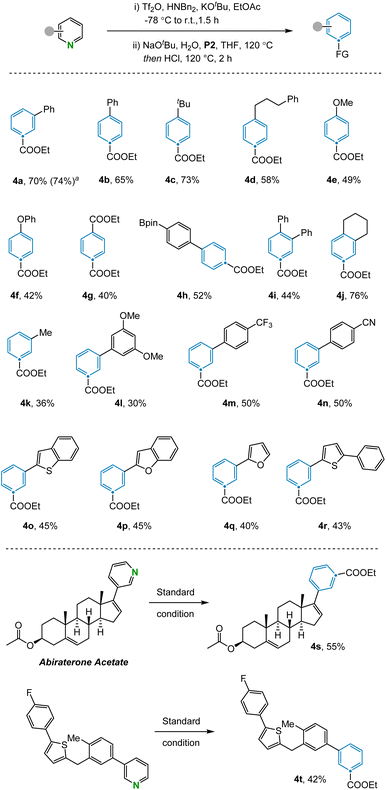
With the optimized reaction conditions in hand, the scope of the N-to-C(CO2Et) atom transmutation of pyridine was explored (Fig. 2). A range of para-substituted pyridines bearing various substituents including aryl (4b) and alkyl groups (4c, 4d), ethers (4e, 4f), esters (4g), and boronic esters (4h), performed well in the reaction. The method could also be used to access trisubstituted benzene compounds (4i, 4j) from disubstituted pyridines in moderate to good yields. Various aromatic systems containing meta-substituents such as trifluoromethyl (4m) and cyano (4n) groups were compatible. Furthermore, pyridines containing heterocyclic rings like benzothiophene (4o), benzofuran (4p), furan (4q) and thiophene (4r) were efficiently converted to the corresponding benzene products. Despite the success of utilizing meta- and para-substituted pyridines, subjecting ortho-substituted pyridines failed to afford the desired benzene products due to the inability of the corresponding Zincke ketones to engage in the olefination reaction. Pyrimidines are not compatible with this reaction as the corresponding aza-Zincke imine intermediates undergo preferential hydrolysis of the non-terminal imine under basic conditions.23 In addition, some sensitive functional groups such as phenols, anilines and halogens were not tolerated under the reaction conditions (see ESI Section 4†). To illustrate the potential of the transformation in late-stage modification, we applied it to complex compounds. Abiraterone acetate and bioactive fragment molecule are readily converted to the corresponding arenes (4s, 4t) in moderate yields.
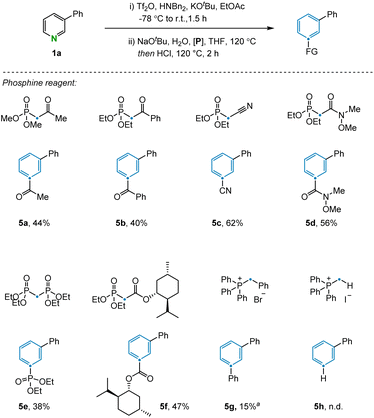
The transformation was further examined by exploring alternative olefination partners that could be used to install diverse functionality on the benzene products (Fig. 3). A range of synthetically useful functionalized carbon atoms could undergo transmutation with the pyridine nitrogen to deliver decorated benzene compounds that contain both aliphatic (5a) and aryl (5b) ketones as well as nitrile (5c), amide (5d), phosphate ester (5e), and ester (5f) functional groups. However, when the reagent benzyltriphenylphosphonium was examined under the reaction condition, only 15% yield was obtained due to incomplete conversion in the electrocyclization step (5g). The same issue was also observed during attempts to install the corresponding C–H bond in the product, with no indication of benzene formation in this case. However, transmutation of N-to-CH can still be conceptually be achieved via a sequence of ester hydrolysis and subsequent decarboxylation.24 Alternative phosphonium reagents bearing electron-donating groups were evaluated but failed to produce the desired products, presumably because electron-rich 6π-systems are not conducive to cyclization (see ESI Section 4.2†).
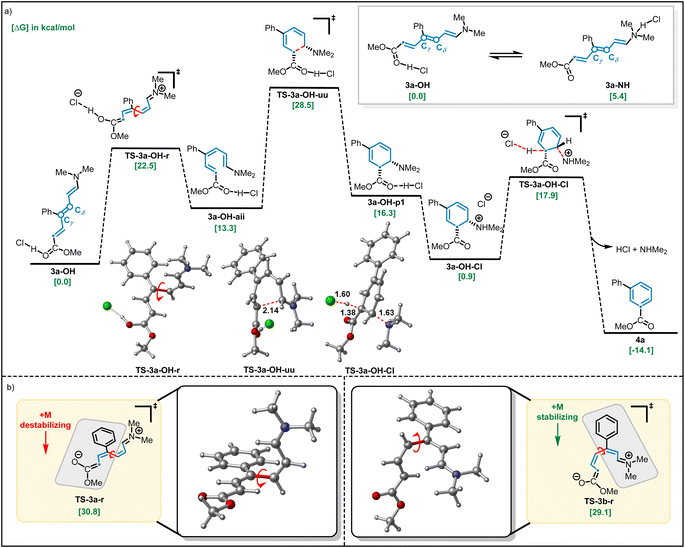
As discussed above (Scheme 1), we observed that the use of basic or acidic conditions had a profound effect on the reactivity of meta-aryl substituted pyridines while the corresponding para-substituted pyridine system was considerably less sensitive. To shed light on the mechanism, and particularly the effect of the substitution pattern (meta vs. para) and additives on reactivity, we employed dispersion-corrected density functional theory calculations (see ESI†). We initiated our studies by exploring the propensity of Zincke intermediates to undergo electrocyclization using 3a as model system. As shown in Fig. 4, our calculations suggest that under acidic conditions, carbonyl protonation (3a-OH) is significantly more energetically favored (5.4 kcal mol−1) over enamine-protonation (3a-NH), presumably due to the disruption of charge delocalization in 3a-NH upon protonation (see S8 and S9† for more details). Notably, upon protonation under acidic conditions, 3a-OH could rapidly undergo a Cγ–Cδ bond rotation viaTS-3a-OH-r (22.5 kcal mol−1) to get to the productive (3a-OH-aii) isomer required for subsequent electrocyclization (28.5 kcal mol−1viaTS-3a-OH-uu). Finally, a proton transfer leads to the thermodynamically favorable 3a-OH-Cl intermediate which, after deprotonation, restores aromaticity and leads to the final product 4a.
Consistent with the lower reactivity observed for the ring-closing step in the absence of HCl (4% vs. 81% yield; Scheme 1), calculations show that when model Zincke intermediate 3a is not protonated, a much higher (30.8 kcal mol−1) barrier for the rate-limiting Cγ–Cδ rotation (barrier for electrocyclization is lower, 28.6 kcal mol−1, than bond rotation, see Fig. S14†) is observed. These results suggest that the role of strong acid is to catalyze the Cγ–Cδ isomerization and, in turn, allow for the formation of the productive isomer to undergo electrocyclization. Furthermore, under basic conditions, meta-substituted phenyl pyridine is observed to give a much lower yield when compared to the analogous para-substituted pyridine (4% vs. 79% respectively, see Scheme 1). Consistent with the experiments, dispersion-corrected DFT calculations predict that under basic conditions, the rate for Cγ–Cδ rotation (see Fig. S14 and S15† for the full profile) is ∼10× faster (i.e., 1.7 kcal mol−1 lower in energy) for para-substituted pyridine (3b) as compared to meta-substituted pyridine (3a) (see Fig. 4b). This lower barrier is attributed to the positive mesomeric effect (+M effect) of the phenyl ring which destabilizes the Cγ–Cδ rotation transition state of 3a because the phenyl ring is coplanar with the ester group predominantly containing negative charge (TS-3a-r; Fig. 4b). In contrast, the positive mesomeric effect (+M effect) of the phenyl ring stabilizes the Cγ–Cδ rotation transition state of 3b (TS-3b-r) as the phenyl ring is coplanar with the amine moiety predominantly containing positive charge. We sought to validate this hypothesis by theoretically introducing electron-donating as well as electron-withdrawing groups at the para-position of the phenyl ring that would create a strong +M or −M effect. The predicted barriers for the para-substituted substrates support our hypothesis about the crucial role played by the +M effect of the phenyl ring (see Fig. S16† for a detailed analysis).
Conclusions
In summary, we have developed a convenient N-to-C single-atom transmutation reaction to convert pyridines to benzenes. This method exhibits remarkable functional group tolerance and could effectively convert both para- and meta-substituted pyridines. This strategy also allows a variety of functionalized carbon atoms to be installed in place of nitrogen to directly edit the molecular scaffold of these heterocycles. The DFT results reveal the effect of acidic or basic conditions on the electrocyclization of para- and meta-substituents. We anticipate that this strategy will provide new avenues for single-atom transmutation as a strategy to directly manipulate the core of heteroaromatic scaffolds.Data availability
General information, detailed experimental procedures, characterization data for compounds, and NMR, HPLC spectra are available in the ESI.†Author contributions
F. G. and F.-P. W. conceived the project. F.-P. W. performed the initial screening experiments. F.-P. W. and M. L. performed synthetic experiments. A. S. and A. R. G. conducted computations. C. G. D. analysed X-ray structures. F.-P. W. and F. G. supervised research. F.-P. W., J. L. T., O. G. and F. G. wrote the manuscript with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Generous financial support was provided by the Deutsche Forschungsgemeinschaft (Leibniz Award) and the Alexander von Humboldt Foundation (J. L. T.). O. G. acknowledges support from the National Institutes of Health (R35GM137797) for funding and Texas A&M University HPRC resources (https://hprc.tamu.edu) for computational resources. We thank the analytical departments of the Institute of Organic Chemistry (University of Münster) for their assistance.Notes and references
- (a) J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong and M. D. Levin, Nat. Synth., 2022, 1, 352–364 CrossRef PubMed; (b) C. Hui, Z. Wang, S. Wang and C. Xu, Org. Chem. Front., 2022, 9, 1451–1457 RSC; (c) B. W. Joynson and L. T. Ball, Helv. Chim. Acta, 2023, 106, e202200182 CrossRef CAS; (d) Z. Liu, P. Sivaguru, Y. Ning, Y. Wu and X. Bi, Chem.–Eur. J., 2023, 29, e202301227 CrossRef CAS.
- (a) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529–2591 CrossRef CAS PubMed; (b) D. J. St. Jean Jr and C. Fotsch, J. Med. Chem., 2012, 55, 6002–6020 CrossRef PubMed; (c) Y. Hu, D. Stumpfe and J. Bajorath, J. Med. Chem., 2017, 60, 1238–1246 CrossRef CAS PubMed.
- Y. Yin, K. Zheng, N. Eid, S. Howard, J. H. Jeong, F. Yi, J. Guo, C. M. Park, M. Bibian, W. Wu, P. Hernandez, H. Park, Y. Wu, J. L. Luo, P. V. LoGrasso and Y. Feng, J. Med. Chem., 2015, 58, 1846–1861 CrossRef CAS PubMed.
- A. Mermer, T. Keles and Y. Sirin, Bioorg. Chem., 2021, 114, 105076 CrossRef CAS PubMed.
- (a) X. Qiu, Y. Sang, H. Wu, X. S. Xue, Z. Yan, Y. Wang, Z. Cheng, X. Wang, H. Tan, S. Song, G. Zhang, X. Zhang, K. N. Houk and N. Jiao, Nature, 2021, 597, 64–69 CrossRef CAS; (b) J. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein and B. Morandi, Science, 2022, 377, 1104–1109 CrossRef CAS PubMed; (c) J. Wang, H. Lu, Y. He, C. Jing and H. Wei, J. Am. Chem. Soc., 2022, 144, 22433–22439 CrossRef CAS PubMed; (d) G. L. Bartholomew, F. Carpaneto and R. Sarpong, J. Am. Chem. Soc., 2022, 144, 22309–22315 CrossRef CAS PubMed; (e) H. Wang, H. Shao, A. Das, S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houk and F. Glorius, Science, 2023, 381, 75–81 CrossRef CAS; (f) B. A. Wright, A. Matviitsuk, M. J. Black, P. Garcia-Reynaga, L. E. Hanna, A. T. Herrmann, M. K. Ameriks, R. Sarpong and T. P. Lebold, J. Am. Chem. Soc., 2023, 145, 10960–10966 CrossRef CAS PubMed; (g) B. W. Joynson, G. R. Cumming and L. T. Ball, Angew. Chem., Int. Ed., 2023, 62, e202305081 CrossRef PubMed; (h) J. Woo, C. Stein, A. H. Christian and M. D. Levin, Nature, 2023, 623, 77–82 CrossRef CAS PubMed; (i) P. Finkelstein, J. C. Reisenbauer, B. B. Botlik, O. Green, A. Florin and B. Morandi, Chem. Sci., 2023, 14, 2954–2959 RSC; (j) H. Li, N. Li, J. Wu, T. Yu, R. Zhang, L. P. Xu and H. Wei, J. Am. Chem. Soc., 2023, 144, 22433–22439 Search PubMed; (k) E. Boudry, F. Bourdreux, J. Marrot, X. Moreau and C. Ghiazza, J. Am. Chem. Soc., 2024, 146, 2845–2854 CrossRef CAS PubMed; (l) H. M. H. Nguyen, D. C. Thomas, M. A. Hart, K. R. Steenback, J. N. Levy and A. McNally, J. Am. Chem. Soc., 2024, 146, 2944–2949 CrossRef CAS PubMed; (m) G. L. Bartholomew, S. L. Kraus, L. J. Karas, F. Carpaneto, R. Bennett, M. S. Sigman, C. S. Yeung and R. Sarpong, J. Am. Chem. Soc., 2024, 146, 2950–2958 CrossRef CAS; (n) Z. A. Tolchin and J. M. Smith, J. Am. Chem. Soc., 2024, 146, 2939–2943 CrossRef CAS PubMed.
- H. Lyu, I. Kevlishvili, X. Yu, P. Liu and G. Dong, Science, 2021, 372, 175–182 CrossRef CAS PubMed.
- (a) S. H. Kennedy, B. D. Dherange, K. J. Berger and M. D. Levin, Nature, 2021, 593, 223–227 CrossRef CAS PubMed; (b) H. Qin, W. Cai, S. Wang, T. Guo, G. Li and H. Lu, Angew. Chem., Int. Ed., 2021, 60, 20678–20683 CrossRef CAS PubMed.
- (a) D. Ma, B. S. Martin, K. S. Gallagher, T. Saito and M. Dai, J. Am. Chem. Soc., 2021, 143, 16383–16387 CrossRef CAS PubMed; (b) B. D. Dherange, P. Q. Kelly, J. P. Liles, M. S. Sigman and M. D. Levin, J. Am. Chem. Soc., 2021, 143, 11337–11344 CrossRef CAS; (c) E. E. Hyland, P. Q. Kelly, A. M. McKillop, B. D. Dherange and M. D. Levin, J. Am. Chem. Soc., 2022, 144, 19258–19264 CrossRef CAS; (d) F.-P. Wu, C. C. Chintawar, R. Lalisse, P. Mukherjee, S. Dutta, J. Tyler, C. G. Daniliuc, O. Gutierrez and F. Glorius, Nat. Catal., 2024, 7, 242–251 CrossRef CAS.
- R. J. Sundberg, S. R. Suter and M. Brenner, J. Am. Chem. Soc., 1972, 94, 513–520 CrossRef CAS.
- S. C. Patel and N. Z. Burns, J. Am. Chem. Soc., 2022, 144, 17797–17802 CrossRef CAS PubMed.
- T. J. Pearson, R. Shimazumi, J. L. Driscoll, B. D. Dherange, D.-I. Park and M. D. Levin, Science, 2023, 381, 1474–1479 CrossRef CAS PubMed.
- (a) R. S. Sagitullin, S. P. Gromov and A. N. Kost, Tetrahedron, 1978, 34, 2213–2216 CrossRef CAS; (b) T. Morofuji, K. Inagawa and N. Kano, Org. Lett., 2021, 23, 6126–6130 CrossRef CAS PubMed; (c) T. Morofuji, S. Nagai, A. Watanabe, K. Inagawa and N. Kano, Chem. Sci., 2023, 14, 485–490 RSC.
- (a) Q. Cheng, D. Bhattacharya, M. Haring, H. Cao, C. Muck-Lichtenfeld and A. Studer, Nat. Chem., 2024, 16, 741–748 CrossRef CAS PubMed; (b) B. R. Boswell, Z. Zhao, R. L. Gonciarz and K. M. Pandya, J. Am. Chem. Soc., 2024, 146, 19660–19666 CrossRef CAS PubMed.
- A. R. Fout, B. C. Bailey, J. Tomaszewski and D. J. Mindiola, J. Am. Chem. Soc., 2007, 129, 12640–12641 CrossRef CAS PubMed.
- Y. Hamada and I. Takeuchi, J. Org. Chem., 1977, 42, 4209–4213 CrossRef CAS.
- When we were preparing the manuscript, Sorensen and Greaney reported pyridine-to-benzene reactions. (a) N. A. Falcone, S. He, J. F. Hoskin, S. Mangat and E. J. Sorensen, Org. Lett., 2024, 26, 4280–4285 CrossRef CAS PubMed; (b) A. Conboy and M. F. Greaney, Chem, 2024, 10, 1940–1949 CrossRef CAS.
- T. Morofuji, H. Kinoshita and N. Kano, Chem. Commun., 2019, 55, 8575–8578 RSC.
- (a) M. Feng, M. Norlöff, B. Guichard, S. Kealey, T. D'Anfray, P. Thuéry, F. Taran, A. Gee, S. Feuillastre and D. Audisio, Nat. Commun., 2024, 15, 6063 CrossRef CAS PubMed; (b) H. M. H. Nguyen, D. C. Thomas, M. A. Hart, K. R. Steenback, J. N. Levy and A. McNally, J. Am. Chem. Soc., 2024, 146, 2944–2949 CrossRef CAS PubMed; (c) G. L. Bartholomew, S. L. Kraus, L. J. Karas, F. Carpaneto, R. Bennett, M. S. Sigman, C. S. Yeung and R. Sarpong, J. Am. Chem. Soc., 2024, 146, 2950–2958 CrossRef CAS.
- B. T. Boyle, J. N. Levy, L. d. Lescure, R. S. Paton and A. McNally, Science, 2022, 378, 773–779 CrossRef CAS PubMed.
- (a) C. Jutz and R. M. Wagner, Angew. Chem., Int. Ed., 1972, 11, 315–318 CrossRef CAS; (b) C. Jutz, R.-M. Wagner, A. Krautz and H.-G. Löbering, Ann. Chem., 1975, 5, 874–900 CrossRef; (c) P. Näding and J. Mäding, J. Labelled Compd. Radiopharm., 1998, 647–656 Search PubMed.
- (a) T.-Q. Yu, Y. Fu, L. Liu and Q.-X. Guo, J. Org. Chem., 2006, 71, 6157–6164 CrossRef CAS PubMed; (b) L. M. Bishop, J. E. Barbarow, R. G. Bergman and D. Trauner, Angew. Chem., Int. Ed., 2008, 47, 8100–8103 CrossRef CAS PubMed; (c) H. Kinoshita, T. Tohjima and K. Miura, Org. Lett., 2014, 16, 4762–4765 CrossRef CAS PubMed; (d) J. M. Percy, H. Emerson, J. W. Fyfe, A. R. Kennedy, S. Maciuk, D. Orr, L. Rathouska, J. M. Redmond and P. G. Wilson, Chem.–Eur. J., 2016, 22, 12166–12175 CrossRef CAS PubMed; (e) T. Itoh, T. Abe, T. Choshi, T. Nishiyama, R. Yanada and M. Ishikura, Eur. J. Org. Chem., 2016, 2016, 2290–2299 CrossRef CAS; (f) X. Li, H. Yu and Y. Huang, Adv. Synth. Catal., 2017, 359, 1379–1387 CrossRef CAS; (g) P. Qin, L. A. Wang, J. M. O'Connor, K. K. Baldridge, Y. Li, B. Tufekci, J. Chen and A. L. Rheingold, Angew. Chem., Int. Ed., 2020, 59, 17958–17965 CrossRef CAS PubMed; (h) G. A. Barcan, A. Patel, K. N. Houk and O. Kwon, Org. Lett., 2012, 14, 5388–5391 CrossRef CAS PubMed.
- S. Ito, R. Yokoyama, T. Okujima, T. Terazono, T. Kubo, A. Tajiri, M. Watanabe and N. Morita, Org. Biomol. Chem., 2003, 1, 1947–1952 RSC.
- B. J. H. Uhlenbruck, C. M. Josephitis, L. d. Lescure, R. S. Paton and A. McNally, Nature, 2024, 631, 87–93 CrossRef CAS PubMed.
- (a) L. J. Gooßen, C. Linder, N. Rodríguez, P. P. Lange and A. Fromm, Chem. Commun., 2009, 7173–7175 RSC; (b) T. Patra, S. Mukherjee, J. Ma, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 10514–10520 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2334460. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04413d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |