Open Access Article
Open Access ArticleComparative study of the antiproliferative activity of heterometallic carbene gold(I)–platinum(II) and gold(I)–palladium(II) complexes in cancer cell lines†
Martin C.
Dietl
 a,
Melina
Maag
a,
Melina
Maag
 a,
Sophia
Ber
a,
Frank
Rominger
a,
Matthias
Rudolph
a,
Isabella
Caligiuri
b,
Pacome K.
Andele
a,
Sophia
Ber
a,
Frank
Rominger
a,
Matthias
Rudolph
a,
Isabella
Caligiuri
b,
Pacome K.
Andele
 bc,
Ibraheem A. I.
Mkhalid
d,
Flavio
Rizzolio
bc,
Ibraheem A. I.
Mkhalid
d,
Flavio
Rizzolio
 be,
Pablo A.
Nogara
be,
Pablo A.
Nogara
 f,
Laura
Orian
f,
Laura
Orian
 g,
Thomas
Scattolin
g,
Thomas
Scattolin
 *g and
A. Stephen K.
Hashmi
*g and
A. Stephen K.
Hashmi
 *ad
*ad
aOrganisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: hashmi@hashmi.de
bPathology Unit, Centro di Riferimento Oncologico di Aviano (C.R.O.) IRCCS via Franco Gallini 2, 33081, Aviano, Italy
cDipartimento di Scienze Molecolari e Nanosistemi, Università Ca’ Foscari, Campus Scientifico Via Torino 155, 30174 Venezia-Mestre, Italy
dChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
eDipartimento di Scienze Molecolari e Nanosistemi, Università Ca’ Foscari, Campus Scientifico Via Torino 155, 30174 Venezia-Mestre, Italy
fInstituto Federal de Educação, Ciência e Tecnologia Sul-rio-grandense (IFSul), Av. Leonel de Moura Brizola, 2501, 96418-400, Bagé, RS, Brazil
gDipartimento di Scienze Chimiche, Università degli Studi di Padova, via Marzolo 1, 35131 Padova, Italy. E-mail: thomas.scattolin@unipd.it
First published on 29th August 2024
Abstract
The stepwise, one-pot synthesis of heterobimetallic carbene gold(I) platinum(II) complexes from readily available starting materials is presented. The protecting group free methodology is based on the graduated nucleophilicities of aliphatic and aromatic amines as linkers between both metal centers. This enables the selective, sequential installation of the metal fragments. In addition, the obtained complexes were tested as potential anticancer agents and directly compared to their gold(I) palladium(II) counterparts.
Introduction
Encompassing both early, as well as late transition metals,1,2 heterobimetallic complexes have emerged as an intriguing subclass of coordination, as well as organometallic compounds. In these structures, the metal centers can be connected either by a direct metal–metal bond3–6 or via bridging ligands.7–9 Depending on the ligands and the coordination sphere of the metals used, supramolecular,10,11 oligo-12 and polymeric13,14 or monomolecular15–17 structures are attainable, that find application in catalysis,18,19 in materials chemistry,20,21 as well as in medicinal chemistry.22–26 Regarding acyclic diaminocarbenes however, only a limited number of studies about the synthesis and application of homo-27 as well as heterometallic complexes28 are found.In our pursuit to synthesize metal carbene complexes,29–36 inspired by the pioneering works of Chugaev37 and Shaw,38 Bonati and Minghetti,39,40 we have recently succeeded in obtaining heterobimetallic complexes,28 containing the pharmacologically relevant metal centers gold(I)41 and palladium(II).42 In this study, we extended our synthetic methodology to introduce platinum(II) as another medicinally important metal center42,43 into the heterobimetallic complex scaffold. Given that there are only a few comparative studies between palladium and platinum complexes bearing the same organometallic fragments,44,45 we regard this concept as an ideal tool for a direct comparison of bimetallic systems by changing only one parameter, namely the central metal atom attached to the aryl amine subunit.
It should be stressed that the main advantage of heterobimetallic compounds in medicinal chemistry is their ability to harness the complementary properties of diverse metal centers. For instance, combining transition metal centers with redox-active properties alongside inert or biocompatible metal centers can introduce redox-switchable behaviour to the compounds.46–61 This attribute proves valuable in crafting agents for targeted drug delivery, enabling controlled release of therapeutic payloads triggered by external stimuli like alterations in pH or the application of electromagnetic fields.62,63 Moreover, the incorporation of different metal centers in heterobimetallic compounds with multifunctionality, allows the concurrent modulation of multiple biological pathways associated with a specific disease.46–55 By leveraging the distinct reactivities of different metal centers, researchers can engineer compounds capable of exerting synergistic effects on intricate biological systems, thus enhancing therapeutic outcomes.46–61
Additionally, in the realm of drug resistance, heterobimetallic compounds present a promising approach to overcoming challenges associated with multidrug-resistant pathogens or cancer cells.46–65 By capitalizing on the unique mechanisms of action facilitated by the synergistic interactions between different metal ions, these compounds can evade resistance mechanisms that render conventional monometallic drugs ineffective.
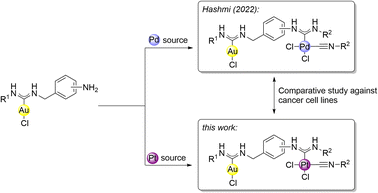
Herein we were able to test and compare both series of heterobimetallic carbene gold(I) palladium(II) and gold(I) platinum(II) complexes against various cancer cell lines, as well as their cytotoxicity against non-cancerous cells (Scheme 1).
 | ||
| Scheme 1 Expansion of the synthetic protocol of heterobimetallic gold(I) palladium(II) complexes28 towards heterobimetallic gold(I) platinum(II) complexes and testing their in vitro anticancer activity in a comparative study. | ||
Results and discussion
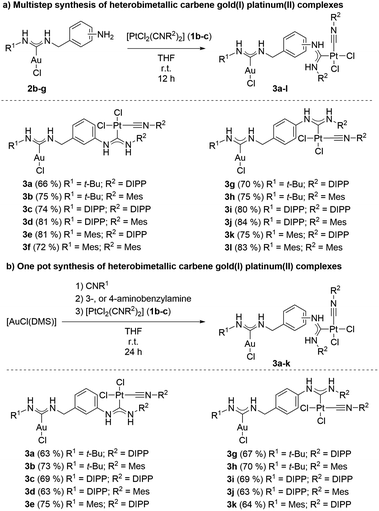
Our research group has previously demonstrated the synthesis of heterobimetallic gold(I) palladium(II) complexes through the nucleophilic attack of aminobenzylamines on isonitrile metal complexes.28 This methodology relies on the addition of primary or secondary amines to isonitrile metal complexes. Depending on the nature of the amine, this template-controlled reaction yields either cyclic or acyclic carbene metal complexes.29–36Aminobenzylamines possess both aliphatic and aromatic amine functionalities. Due to the higher nucleophilicity of aliphatic amines compared to aromatic amines, the reaction between aminobenzylamine and isonitrile gold(I) complexes selectively yields amino functionalized carbene gold(I) complexes. The yet unreacted free amino group originating from the aromatic core of the aminobenzylamine can then serve as a nucleophile for a subsequent addition to another isonitrile metal complex, resulting in the formation of heterobimetallic carbene complexes. Consequently, we synthesized a series of isonitrile platinum(II) complexes from the precursor complex [PtCl2(COD)], which is readily available from the commercially available K2PtCl4 and cyclooctadiene.66 [PtCl2(COD)] was reacted with 2.20 equiv. of isonitrile in chloroform at reflux to furnish the corresponding isonitrile platinum(II) complexes 1a–c in excellent yields (Scheme 2a). Purification was simply achieved by crystallization of the desired products by addition of n-pentane to the reaction mixture. The amino functionalized carbene gold(I) complexes 2a–g, encompassing aliphatic and aromatic substituents, as well as the free amino group at different positions on the phenyl ring were synthesized according to our previous report (Scheme 2b).28
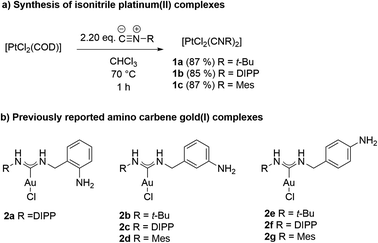 | ||
| Scheme 2 (a) Synthesis of isonitrile platinum(II) complexes from [PtCl2(COD)]. Conditions: 2 (668 μmol, 1.00 eq.), isonitrile (1.47 mmol, 2.20 eq.), CHCl3, 70 °C, 1 h; (b) previously reported amino carbene gold(I) complexes.28 | ||
These complexes were subsequently used as nucleophiles for the generation of heterobimetallic carbene gold(I) platinum(II) complexes.
Hence, one equivalent of an amino carbene gold(I) complex 2a–g was brought to reaction with one equivalent of an isonitrile platinum(II) complex 1a–c. Firstly, the solvent of choice was dichloromethane, since in our previous report the reaction between amino carbene gold(I) complexes and isonitrile palladium(II) complexes performed well.28 Notably, in the case of platinum(II) isonitriles, performing the reaction under these conditions did not lead to the formation of any product and both starting materials were fully recovered. In another report from our group, we discovered that the addition of aromatic amines to isonitrile metal complexes proceeded faster, if tetrahydrofuran was used as solvent.35 In line with these findings, the reaction between amino functionalized carbene gold(I) complexes 2b–g and the isonitrile platinum(II) complexes 1b–c in tetrahydrofuran at room temperature showed full conversion of the starting material after a reaction time of 24 hours. Purification of the heterobimetallic carbene gold(I) platinum(II) complexes 3a–l was simply achieved by addition of n-pentane to the reaction mixture, which led to the precipitation of the desired product. The reaction protocol generally worked for most combinations of amino functionalized carbene gold(I) complexes and isonitrile platinum(II) complexes in moderate to good yields (Scheme 3a). In general, there was no great influence of the substitution pattern at the amino functionalized carbene gold(I) complexes on the yield of the reaction. Also, no great differences in yield were observed if the free amino group was found at the 3- or 4-position of the aromatic ring of the amino functionalized carbene gold(I) complex. However, if complex 2a was used, in which the free amino group was located at the 2-position, no reaction was observed. This is most likely attributed to the 2-position of the free amino group, furnishing a sterically too demanding reaction site. Furthermore, no reaction occurred if the aliphatic isonitrile platinum(II) complex 1a was used. These unsuccessful reactions indicate a general limitation of the reaction to aromatic isonitrile platinum(II) complexes and sterically less demanding amino carbene gold(I) complexes. Increasing the reaction temperature overall led to the decomposition of the amino functionalized carbene gold(I) complex. It was therefore not possible to run the reaction at elevated temperatures to form any product. All reaction products were characterized spectroscopically to verify the formation of the desired heterobimetallic carbene gold(I) platinum(II) complexes. In the IR spectra of the isonitrile platinum(II) complexes 1b–c, the isonitrile stretching mode at ṽ = 2195 to 2193 cm−1 vanished, while a new isonitrile stretching mode in the complexes 3a–l at a slightly lower wavenumber at ṽ = 2185 to 2192 cm−1 appeared. This indicates, that one of the isonitrile ligands was converted to a carbene ligand, while the other coordinated isonitrile ligand remained intact. Furthermore, in the 13C NMR spectrum the formation of the new carbene unit coordinated to the platinum(II) metal center was observed as a resonance in the 13C NMR spectrum at δ = 170 to 159 ppm. By 195Pt NMR spectroscopy the change of the chemical surroundings of the platinum(II) center could be studied. While the isonitrile platinum(II) complexes showed a resonance of the 195Pt nucleus at δ = −3754 to −3750 ppm, after the reaction it shifted to δ = −3496 to −3551 ppm. Additionally, the experimental data from high-resolution mass spectra of the [M–Cl] fragments and their isotopic patterns match their calculated values. Furthermore, the bulk purity was confirmed by elemental analysis.
The rotation of substituents around the N–CCarbene bond of the acyclic carbene units leads to four different possible conformers per metal center, the so called rotamers. Since there are two metals centers present in the complexes 3a–l, theoretically 16 different rotamers are possible. However, not all 16 possible rotamers were observed. For all complexes with 4-aminobenzylamine as the linker between both metal centers 3g–l, no rotational isomerism was observed at all, since in the 1H, 13C, as well as 195Pt NMR spectra only one species was present. If, on the other hand, 3-aminobenzylamine was employed as the linker between the gold(I) and platinum(II) metal centers, in some cases two rotamers were observed. This is especially evident in the 195Pt NMR spectra, because for the complexes 3b–f, two distinct resonances in the range of δ = −3488 to −3501 ppm and δ = −3523 to −3530 ppm appear, indicating the presence of two species. After expanding the multistep synthesis of heterobimetallic complexes to gold(I) and platinum(II) as metal centers, we were curious, if also a one-pot protocol was applicable. The direct access to heterobimetallic complexes would not only save time but would also abbreviate the multiple purification steps, leading to a more efficient and environmentally friendly protocol. Hence, the reactions were carried out with the commercially available [AuCl(DMS)] which was firstly reacted in tetrahydrofuran at room temperature with one equivalent of isonitrile and one equivalent of either 3-, or 4-aminobenzylamine to form the corresponding amino functionalized carbene gold(I) complex in situ. Finally the addition of one equivalent of a isonitrile platinum(II) complex 1b–c led to the formation of the desired heterobimetallic carbene gold(I) palladium(II) complexes 3a–k in moderate to good yields (Scheme 3b). Overall, the yields stemming from the one-pot protocol did not deviate significantly from the yields from the multistep approach. Therefore, a one-pot protocol offers an elegant and direct path to heterobimetallic carbene gold(I) platinum(II) complexes.
Slow diffusion of n-pentane in saturated solutions of 3c in 1,2-dichloroethane furnished single crystals suitable for single crystal X-ray diffraction. The obtained molecular structure in the solid state reveals the proper connectivity of all atoms (Fig. 1). The gold(I) center shows the typical linear coordination sphere, while the platinum(II) metal center exhibits a square planar molecular geometry. Furthermore, no metallophilic interactions were observed.
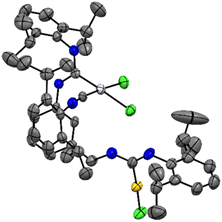 | ||
| Fig. 1 Molecular structure of 3c (CCDC 2339445) in the solid state thermal ellipsoids are shown at a 50% probability. The carbon atoms are shown in grey, nitrogen atoms in blue, the chlorine atoms in green, platinum in silver and gold in yellow. Selected bond distances (Å) and angles (°): d(CCarbene–Au) = 1.964(10), d(Au–Cl) = 2.281(3), ∠(N, CCarbene, N) = 116.3(9), ∠(CCarbene, Au, Cl) = 176.3(3), d(CCarbene–Pd) = 1.973(9), ∠(N, CCarbene, N) = 114.8(8), d(CIsonitrile–Pd) = 1.855(9). | ||
Despite the plethora of platinum(II)– and palladium(II) based metal drugs against cancer, there is still a scarcity of comparative studies, in which the metal complexes only differ in the metal center while the organometallic fragment remains the same. Our established reaction protocol allows the introduction of platinum(II), as well as palladium(II) into the gold(I) containing heterobimetallic scaffold. This offers the possibility for the synthesis of similar complexes, only differing in one metal center. For that, we aimed to compare the activity of the synthesized heterobimetallic carbene gold(I) platinum(II) complexes 3a–k with their gold(I) and palladium(II) containing counterparts 5a–j, that were synthesized according to our previous work from amino functionalized carbene gold(I) complexes 2b–g and the corresponding palladium isonitriles 4a–b (Scheme 4).28
With the aim of exploring the potential anticancer effects of the heterobimetallic carbene gold(I) platinum(II) complexes object of this work, as well as the previously reported gold(I) palladium(II) congeners, we subjected a selection of human tumor cell lines (ovarian cancer A2780, high-grade serous ovarian cancer OVCAR-3, colon cancer HCT116, lung cancer A549 and triple-negative breast cancer MDA-MB231) to a 96 h treatment with both the synthesized compounds and cisplatin (utilized as a positive control). For the sake of completeness, we also tested some isonitrile platinum(II)– and palladium(II)– and amino functionalized carbene gold(I) complexes (1b–c, 4a–b and 2b–g, respectively). The stability of the newly synthesized heterobimetallic gold platinum complexes in the cell media was tested exemplarily with complex 3hvia1H NMR spectroscopy (see ESI†). For this sake, the exemplary complex 3h was dissolved in a mixture of a 100 mM saline solution in D2O and DMSO-d6. After 48 h at room temperature, 3h did not depict any decomposition.
The results dealing with the antiproliferative activity of the tested compounds are reported in Table 1, presented as half inhibitory concentrations (IC50) values.
| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| A2780 | OVCAR-3 | HCT116 | A549 | MDA-MB-231 | MRC-5 | |
| a Data after 96 h of incubation. Stock solutions in DMSO for all complexes; stock solutions in H2O for cisplatin. A2780 (cisplatin-sensitive ovarian cancer cells), OVCAR-3 (high-grade serous ovarian cancer cells), HCT116 (colon cancer cells), MDA-MB-231 (triple-negative breast cancer cells), A549 (lung cancer cells), MRC-5 (non-cancerous lung fibroblasts). | ||||||
| Cisplatin | 0.6 ± 0.2 | 3.1 ± 0.2 | 7.9 ± 0.8 | 11 ± 1 | 15 ± 1 | 2 ± 1 |
| 1b | >100 | >100 | 0.6 ± 0.5 | >100 | 0.7 ± 0.5 | >100 |
| 1c | >100 | 60 ± 30 | 1.0 ± 0.7 | >100 | 0.24 ± 0.02 | >100 |
| 2b | >100 | >100 | 4.4 ± 0.2 | >100 | >100 | >100 |
| 2c | 2.8 ± 0.2 | 5 ± 1 | 4.4 ± 0.3 | >100 | 3.9 ± 0.5 | >100 |
| 2d | >100 | >100 | >100 | >100 | >100 | >100 |
| 2e | >100 | >100 | >100 | >100 | >100 | >100 |
| 2f | 3.6 ± 0.2 | >100 | >100 | >100 | >100 | >100 |
| 2g | 1.9 ± 0.2 | >100 | >100 | >100 | >100 | >100 |
| 3a | >100 | >100 | >100 | >100 | >100 | >100 |
| 3b | >100 | >100 | >100 | >100 | >100 | >100 |
| 3c | >100 | >100 | >100 | >100 | >100 | >100 |
| 3d | >100 | >100 | >100 | >100 | >100 | >100 |
| 3e | >100 | >100 | >100 | >100 | 0.05 ± 0.04 | >100 |
| 3f | >100 | >100 | >100 | >100 | >100 | >100 |
| 3g | >100 | >100 | 12 ± 7 | >100 | >100 | >100 |
| 3h | >100 | >100 | 0.5 ± 0.4 | 0.36 ± 0.01 | >100 | >100 |
| 3i | 13.4 ± 0.2 | >100 | >100 | >100 | >100 | >100 |
| 3j | >100 | >100 | >100 | >100 | 0.2 ± 0.1 | >100 |
| 3k | >100 | >100 | >100 | >100 | >100 | >100 |
| 3l | >100 | >100 | 14 ± 5 | >100 | >100 | >100 |
| 4a | >100 | >100 | >100 | >100 | >100 | >100 |
| 4b | >100 | >100 | >100 | >100 | 24 ± 4 | >100 |
| 5a | >100 | 40 ± 4 | >100 | >100 | >100 | >100 |
| 5b | >100 | >100 | 5.2 ± 0.2 | >100 | >100 | >100 |
| 5c | >100 | 20 ± 3 | 40 ± 20 | 12 ± 1 | 6 ± 1 | >100 |
| 5d | 12 ± 2 | 26 ± 2 | 7.2 ± 0.8 | 30 ± 4 | 6 ± 1 | >100 |
| 5e | >100 | 80 ± 10 | >100 | >100 | >100 | >100 |
| 5f | >100 | >100 | 33 ± 4 | >100 | >100 | >100 |
| 5g | 5 ± 1 | >100 | >100 | >100 | >100 | >100 |
| 5h | >100 | >100 | 0.3 ± 0.2 | >100 | >100 | >100 |
| 5i | 30 ± 10 | >100 | >100 | >100 | 0.25 ± 0.02 | 6 ± 1 |
| 5j | >100 | 40 ± 10 | >100 | 23 ± 4 | 17 ± 1 | >100 |
Although many of the tested compounds did not exhibit cytotoxicity towards most of the examined tumor cell lines, it is nonetheless possible to make interesting observations regarding the active compounds.
In the A2780 and OVCAR-3 ovarian cancer cell lines, among the tested amino functionalized carbene gold(I) complexes, 2c emerged as the most active, displaying IC50 values comparable to cisplatin in both cell lines. Conversely, complexes 2f and 2g were active only in the A2780 line. Among the heterobimetallic complexes, the majority of active compounds, albeit exhibiting moderate cytotoxicity, feature gold(I) and palladium(II) metal centers (5d, 5g, and 5i). Their IC50 values generally ranged between 5 and 40 μM. Of particular interest is compound 5d, which showed activity in both cell lines, albeit with lower cytotoxicity than cisplatin.
The promising antiproliferative activity of organopalladium complexes towards ovarian cancer cells is a recent and interesting topic in medicinal chemistry, since some derivatives reported in the literature have shown mechanisms of action markedly different from that of classical platinum-based anticancer agents.67,68 Many derivatives have indeed demonstrated excellent activity against cisplatin-resistant ovarian cancer cell lines, as well as promising data in ex vivo (patient-derived organoids) and in vivo models.69–74
In the HCT116 line (colon cancer), a significant number (11) of compounds were active. The most active derivatives exhibited IC50 values comparable to or even an order of magnitude lower than that obtained with cisplatin. Among these, noteworthy are the amino functionalized carbene gold(I) complexes 2b–c, the isonitrile platinum(II) complexes 1b–c, the heterobimetallic carbene gold(I) platinum(II) complex 3h, and the heterobimetallic carbene gold(I) palladium(II) complexes 5b, 5d, and 5h. Interestingly, the heterobimetallic carbene gold(I) platinum(II) complex 3h and the heterobimetallic carbene gold(I) palladium(II) complex 5h, both sharing a para substituted arene linker, exhibited remarkable cytotoxicity in the sub-micromolar range.
In the case of the MDA-MB-231 cells (triple-negative breast cancer), a substantial number of compounds were active against this aggressive subtype of breast cancer. All active compounds exhibited significantly higher cytotoxicity, up to two orders of magnitude, compared to cisplatin. Specifically, unlike the cell lines previously discussed, the most active compounds (IC50 < 1 μM) contained at least one platinum(II) center (1b, 1c, 3e, and 3j), with the exception of the heterobimetallic carbene gold(I) palladium(II) complex 5i. Remarkably, the heterobimetallic complexes 3e, 3j and 5i, sharing DIPP and Mes residues while differing in meta- and para-substituted arene linkers, showed low nanomolar IC50 values.
In the A549 cell line (lung cancer), only four compounds among those tested were active (3h, 5c, 5d, and 5j). Remarkably, the heterobimetallic carbene gold(I) platinum(II) compound 3h showed an IC50 value one order of magnitude lower than that of cisplatin. All the other three complexes have gold(I) and palladium(II) as central atoms, with IC50 values comparable to that of cisplatin.
Overall, although compounds 5c and 5d are interesting as they are moderately active in at least four out of the five tested cell lines, we strongly believe that the most promising candidate warranting further investigation in future studies is the heterobimetallic carbene gold(I) platinum(II) complex 3h. This compound exhibits a specific and potent antiproliferative activity against colon and lung cancer cells, with IC50 values markedly lower than those obtained with cisplatin.
Notably, complex 3h, as well as most of the tested compounds, was inactive towards non-cancerous MRC-5 lung fibroblasts, thus suggesting an interesting in vitro selectivity towards cancer cells. In contrast, cisplatin did not exhibit in vitro selectivity, as the IC50 value determined on MRC-5 normal cells (2 ± 1 μM) is comparable to the IC50 values obtained in the five tumor lines.
To the best of our knowledge, this is one of the few contributions systematically comparing the activity of platinum and palladium complexes bearing the same organometallic fragments.75,76
This comparison is often difficult to make as it is not always obvious to synthesize organopalladium complexes and their organoplatinum congeners while maintaining the same chemical environment.
More specifically, the synthetic protocol developed by our group allowed us to evaluate this effect by incorporating a carbene gold(I) moiety into the structure of the complexes under examination. Given the significant importance of gold in medicinal chemistry,77–79 especially in cancer therapy, we firmly believe that the structures and data proposed in this work represent an added value in this fruitful research area.
Moreover, intrigued by the promising antiproliferative activity of the heterobimetallic carbene gold(I) platinum(II) complex 3h towards colon and lung cancer cells, we investigated both its potential interaction with DNA, which is typical of platinum-based anticancer agents, as well as the cellular uptake.
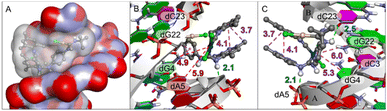
In particular, the structure of complex 3h was fully optimized at ZORA-BLYP-D3(BJ)/TZ2P level of theory (see Computational details)80–83 to generate a good structure as well as reliable partial atomic charges for the docking simulations. The binding poses and interactions between 3h and DNA were studied by molecular docking simulations. Complex 3h binds in the major groove region of the DNA (sequence d(CGCGAATTCGCG)2), where H-bonds and hydrophobic interactions with nucleotide residues (Fig. 2) are established, in a similar way as previously reported for Au and Pt complexes.84 The binding of complex 3h is thermodynamically favourable (ΔG = −7.4 kcal mol−1) indicating that the ligand–receptor complex is stable. The dA5 and dC23 residues are involved in H-bonds with complex 3h, while the dG4 and dG22 interact by hydrophobic (π–π stacking) interactions. The simulations also indicate that only the Pt atom of the complex interacts with the nucleotide bases (Pt⋯N interactions with dG4 and dA5), suggesting a possible covalent bond formation, in a similar way to cisplatin (Fig. S49†). Although tempting, it is not possible to compare directly the binding energies of 3h and cisplatin (docking simulation is shown in the ESI†) because for small compounds the energy predictions is strongly influenced by their size.85
Finally, with the aim of explaining the higher cytotoxicity of complex 3h compared to other heterobimetallic carbene gold(I) platinum(II) complexes, we opted to study the cellular uptake of 3h and 3e (chosen as model compound) in A549 cancer cells. Specifically, we treated A549 cancer cells with a concentration of 1 μM of each complex and monitored the cellular uptake of gold at three time points (3, 6, and 24 hours) using ICP-MS. The results are summarized in Table 2.
| Au (ng/2 × 106 cells) | ||
|---|---|---|
| Time (h) | 3h | 3e |
| 3 | 54.3 ± 0.8 | 5.9 ± 0.1 |
| 6 | 74.0 ± 0.4 | 10.44 ± 0.05 |
| 24 | 81 ± 1 | 78 ± 1 |
The cellular uptake data suggest that the superior cytotoxicity of complex 3h may be linked, among several factors, to a faster uptake (ca. one order of magnitude difference after 3 hours). However, since the uptake of the two compounds is very similar after 24 hours, we believe that the distinct structural characteristics of the two complexes also contribute to this marked difference in antiproliferative activity.
Conclusions
Based on the isonitrile route to carbene complexes, we were able to synthesize a range of heterobimetallic carbene gold(I) platinum(II) complexes in a straightforward and protecting group free approach. Also, a one pot protocol was employed, furnishing the desired complexes equally efficient compared to the corresponding multistep procedure. All complexes were characterized spectroscopically and were tested as potential anticancer agents against different cell lines and compared to their gold(I) palladium(II) counterparts.In particular, the most promising complex is the heterobimetallic carbene gold(I) platinum(II) complex 3h, which has demonstrated potent antiproliferative activity towards colon and lung cancer cells. Notably, this compound, as well as most of the active complexes, exhibited an interesting in vitro selectivity towards cancer cells over normal ones.
Moreover, the interaction between complex 3h and DNA was studied in silico, demonstrating a thermodynamically favourable binding. Specifically, the interaction involves only the Pt atom of the complex with the nucleotide bases (dG4 and dA5), thus suggesting a possible covalent bond formation.
Further investigations concerning the synthesis of heterobimetallic complexes with other metal centers are ongoing in our laboratories.
Data availability
The authors declare that all the data used for this manuscript can be found in its ESI.† The single crystal structure used in this manuscript has been assigned the CCDC number 2339445.Author contributions
Martin C. Dietl: conceptualization, investigation, methodology formal analysis and validation of analytical data, visualisation, writing-original draft; Melina Maag: investigation; Sophia Ber: investigation; Frank Rominger: data curation, formal analysis, validation and visualisation of X-ray Single Crystal Data; Matthias Rudolph: project administration, writing-review and editing; Isabella Caligiuri: investigation; Pacome Kossivi Andele: investigation; Ibraheem A. I. Mkhalid: conceptualization; Flavio Rizzolio: investigation, methodology formal analysis and validation of analytical data; Pablo A. Nogara: data curation, formal analysis, validation and visualisation of molecular docking data, writing-review and editing; Laura Orian: data curation, formal analysis, validation and visualisation of DFT data, writing-review and editing; Thomas Scattolin: supervision, writing-original draft, data curation; A. Stephen K. Hashmi: supervision, project administration, resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University under grant (14-130-35-HiCi). The authors therefore, acknowledge technical and financial support of KAU.References
- B. G. Cooper, J. W. Napoline and C. M. Thomas, Catal. Rev.: Sci. Eng., 2012, 54, 1–40 CrossRef CAS.
- N. Wheatley and P. Kalck, Chem. Rev., 1999, 99, 3379–3420 CrossRef CAS PubMed.
- A. M. Chapman, S. R. Flynn and D. F. Wass, Inorg. Chem., 2016, 55, 1017–1021 CrossRef CAS PubMed.
- S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741–2760 RSC.
- S. Deolka, O. Rivada-Wheelaghan, S. L. Aristizábal, R. R. Fayzullin, S. Pal, K. Nozaki, E. Khaskin and J. R. Khusnutdinova, Chem. Sci., 2020, 11, 5494–5502 RSC.
- G. Dübek, F. Hanusch, D. Munz and S. Inoue, Angew. Chem., Int. Ed., 2020, 132, 5872–5878 CrossRef.
- H. Molaee, S. M. Nabavizadeh, M. Jamshidi, M. Vilsmeier, A. Pfitzner and M. Samandar Sangari, Dalton Trans., 2017, 46, 16077–16088 RSC.
- K. J. Cluff, N. Bhuvanesh and J. Blümel, Chem.–Eur. J., 2015, 21, 10138–10148 CrossRef CAS PubMed.
- H. E. Wagner, S. Hohnstein, M. G. Schußmann, L. A. Steppe and F. Breher, Dalton Trans., 2019, 48, 15397–15407 RSC.
- W.-X. Gao, H.-N. Zhang and G.-X. Jin, Coord. Chem. Rev., 2019, 386, 69–84 CrossRef CAS.
- M. L. Saha, X. Yan and P. J. Stang, Acc. Chem. Res., 2016, 49, 2527–2539 CrossRef CAS PubMed.
- R. Clérac, H. Miyasaka, M. Yamashita and C. Coulon, J. Am. Chem. Soc., 2002, 124, 12837–12844 CrossRef PubMed.
- C. L. Cahill, D. T. de Lill and M. Frisch, CrystEngComm, 2007, 9, 15–26 RSC.
- X. Feng, Y.-Q. Feng, L. Liu, L.-Y. Wang, H.-L. Song and S.-W. Ng, Dalton Trans., 2013, 42, 7741–7754 RSC.
- K. Kawakita, Y. Kakiuchi, E. P. Beaumier, I. A. Tonks, H. Tsurugi and K. Mashima, Inorg. Chem., 2019, 58, 15155–15165 CrossRef CAS PubMed.
- S. Termühlen, L. F. B. Wilm, P. D. Dutschke, A. Hepp and F. E. Hahn, Organometallics, 2021, 40, 1565–1570 CrossRef.
- P. D. Dutschke, S. Bente, C. G. Daniliuc, J. Kinas, A. Hepp and F. E. Hahn, Dalton Trans., 2020, 49, 14388–14392 RSC.
- J. A. Mata, F. E. Hahn and E. Peris, Chem. Sci., 2014, 5, 1723–1732 RSC.
- P. Buchwalter, J. Rosé and P. Braunstein, Chem. Rev., 2015, 115, 28–126 CrossRef CAS PubMed.
- P. Shukla, S. Das, P. Bag and A. Dey, Inorg. Chem. Front., 2023, 10, 4322–4357 RSC.
- J.-L. Liu, J.-Y. Wu, Y.-C. Chen, V. Mereacre, A. K. Powell, L. Ungur, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, Angew. Chem., Int. Ed., 2014, 53, 12966–12970 CrossRef CAS PubMed.
- L. Ma, L. Li and G. Zhu, Inorg. Chem. Front., 2022, 9, 2424–2453 RSC.
- A. van Niekerk, P. Chellan and S. F. Mapolie, Eur. J. Inorg. Chem., 2019, 2019, 3432–3455 CrossRef CAS.
- A. Jain, Coord. Chem. Rev., 2019, 401, 213067 CrossRef CAS.
- M. Redrado, V. Fernández-Moreira and M. C. Gimeno, ChemMedChem, 2021, 16, 932–941 CrossRef CAS PubMed.
- Y.-A. Deng, S.-J. Tang, M.-F. Wang, X. Ren, X.-L. Li, L.-Z. Zeng, D.-N. Ren, M.-R. Wang, W.-L. Xiao, Z.-Y. Cai, D. Zhang, H. Zhang and F. Gao, Inorg. Chem. Front., 2023, 10, 4552–4561 RSC.
- G. M. D. M. Rúbio, T. T. Y. Tan, A. Prado-Roller, J. M. Chin and M. R. Reithofer, Inorg. Chem., 2022, 61, 7448–7458 CrossRef PubMed.
- M. C. Dietl, V. Vethacke, A. Keshavarzi, F. F. Mulks, F. Rominger, M. Rudolph, I. A. I. Mkhalid and A. S. K. Hashmi, Organometallics, 2022, 41, 802–810 CrossRef CAS.
- C. Hubbert, M. C. Dietl, D. Zahner, F. Rominger, M. Rudolph and A. S. K. Hashmi, Organometallics, 2023, 42, 2762–2770 CrossRef CAS.
- D. Riedel, T. Wurm, K. Graf, M. Rudolph, F. Rominger and A. S. K. Hashmi, Adv. Synth. Catal., 2015, 357, 1515–1523 CrossRef CAS.
- A. S. K. Hashmi, T. Hengst, C. Lothschütz and F. Rominger, Adv. Synth. Catal., 2010, 352, 1315–1337 CrossRef CAS.
- T. Wurm, F. Mulks, C. R. N. Böhling, D. Riedel, P. Zargaran, M. Rudolph, F. Rominger and A. S. K. Hashmi, Organometallics, 2016, 35, 1070–1078 CrossRef CAS.
- P. Zargaran, T. Wurm, D. Zahner, J. Schiessl, M. Rudolph, F. Rominger and A. S. K. Hashmi, Adv. Synth. Catal., 2018, 360, 106–111 CrossRef CAS.
- C. Lothschütz, T. Wurm, A. Zeiler, A. F. v. Falkenhausen, M. Rudolph, F. Rominger and A. S. K. Hashmi, Chem.–Asian J., 2016, 11, 342–346 CrossRef PubMed.
- V. Vethacke, V. Claus, M. C. Dietl, D. Ehjeij, A. Meister, J. F. Huber, L. K. Paschai Darian, M. Rudolph, F. Rominger and A. S. K. Hashmi, Adv. Synth. Catal., 2022, 364, 536–554 CrossRef CAS.
- A. S. K. Hashmi, C. Lothschütz, C. Böhling, T. Hengst, C. Hubbert and F. Rominger, Adv. Synth. Catal., 2010, 352, 3001–3012 CrossRef CAS.
- L. Tschugajeff, M. Skanawy-Grigorjewa and A. Posnjak, Z. Anorg. Allg. Chem., 1925, 148, 37–42 CrossRef.
- G. Rouschias and B. L. Shaw, J. Chem. Soc. D, 1970, 183 RSC.
- F. Bonati and G. Minghetti, Synth. Inorg. Met.-Org. Chem., 1971, 1, 299–302 CrossRef CAS.
- F. Bonati and G. Minghetti, J. Organomet. Chem., 1973, 59, 403–410 CrossRef CAS.
- I. Ott, Coord. Chem. Rev., 2009, 253, 1670–1681 CrossRef CAS.
- M. Fanelli, M. Formica, V. Fusi, L. Giorgi, M. Micheloni and P. Paoli, Coord. Chem. Rev., 2016, 310, 41–79 CrossRef CAS.
- B. W. Harper, A. M. Krause-Heuer, M. P. Grant, M. Manohar, K. B. Garbutcheon-Singh and J. R. Aldrich-Wright, Chem.–Eur. J., 2010, 16, 7064–7077 CrossRef CAS PubMed.
- C. Cullinane, G. B. Deacon, P. R. Drago, A. P. Erven, P. C. Junk, J. Luu, G. Meyer, S. Schmitz, I. Ott and J. Schur, Dalton Trans., 2018, 47, 1918–1932 RSC.
- M. A. Bernd, E. B. Bauer, J. Oberkofler, A. Bauer, R. M. Reich and F. E. Kühn, Dalton Trans., 2020, 49, 14106–14114 RSC.
- E. J. Anthony, E. M. Bolitho, H. E. Bridgewater, O. W. L. Carter, J. M. Donnelly, C. Imberti, E. C. Lant, F. Lermyte, R. J. Needham, M. Palau, P. J. Sadler, H. Shi, F.-X. Wang, W. Zhang and Z. Zhang, Chem. Sci., 2020, 11, 12888–12917 RSC.
- G. Gasser, I. Ott and N. Metzler-Nolte, J. Med. Chem., 2011, 54, 3–25 CrossRef CAS PubMed.
- P. Zhang and P. J. Sadler, J. Organomet. Chem., 2017, 839, 5–14 CrossRef CAS.
- E. Bortolamiol, F. Visentin and T. Scattolin, Appl. Sci., 2023, 13, 5561 CrossRef CAS.
- J. E. López-Hernández, N. Nayeem, J. P. Cerón-Carrasco, A. Lahad, A. Hafeed, I. E. León and M. Contel, Chem.–Eur. J., 2023, 29, e202302045 CrossRef PubMed.
- N. Curado, N. Giménez, K. Miachin, M. Aliaga-Lavrijsen, M. Cornejo, A. A. Jarzęcki and M. Contel, ChemMedChem, 2019, 14, 1086–1095 CrossRef CAS PubMed.
- R. A. Khan, A. Asim, R. Kakkar, D. Gupta, V. Bagchi, F. Arjmand and S. Tabassum, Organometallics, 2013, 32, 2546–2551 CrossRef CAS.
- A. Herman, J. M. Tanski, M. F. Tibbetts and C. M. Anderson, Inorg. Chem., 2007, 47, 274–280 CrossRef PubMed.
- J. E. López-Hernández and M. Contel, Curr. Opin. Chem. Biol., 2023, 72, 102250 CrossRef PubMed.
- B. Bertrand, A. Citta, I. L. Franken, M. Picquet, A. Folda, V. Scalcon, M. P. Rigobello, P. L. Gendre, A. Casini and E. Bodio, JBIC, J. Biol. Inorg. Chem., 2015, 20, 1005–1020 CrossRef CAS PubMed.
- J. Banfić, A. A. Legin, M. A. Jakupec, M. Galanski and B. K. Keppler, Eur. J. Inorg. Chem., 2014, 484–492 CrossRef.
- K. Mitra, U. Basu, I. Khan, B. Maity, P. Kondaiah and A. R. Chakravarty, Dalton Trans., 2014, 43, 751–763 RSC.
- C. M. Anderson, S. S Jain, L. Silber, K. Chen, S. Guha, W. Zhang, E. C. McLaughlin, Y. Hu and J. M. Tanski, J. Inorg. Biochem., 2015, 145, 41–50 CrossRef CAS PubMed.
- C. Mu, S. W. Chang, K. E. Prosser, A. W. Y. Leung, S. Santacruz, T. Jang, J. R. Thompson, D. T. T. Yapp, J. J. Warren, M. B. Bally, T. V. Beischlag and C. J. Walsby, Inorg. Chem., 2016, 55, 177–190 CrossRef CAS PubMed.
- D. Aucamp, S. V. Kumar, D. C. Liles, M. A. Fernandes, L. Harmse and D. I. Bezuidenhout, Dalton Trans., 2018, 47, 16072–16081 RSC.
- T. A. C. A. Bayrakdar, T. Scattolin, X. Ma and S. P. Nolan, Chem. Soc. Rev., 2020, 49, 7044–7100 RSC.
- W. D. J. Tremlett, T. Söhnel, J. D. Crowley, L. J. Wright and C. G. Hartinger, Inorg. Chem., 2023, 62, 3616–3628 CrossRef CAS PubMed.
- A. S. Braegelman and M. J. Webber, Theranostics, 2019, 9, 3017–3040 CrossRef CAS PubMed.
- A. Van Niekerk, P. Chellan and S. F. Mapolie, Eur. J. Inorg. Chem., 2019, 2019, 3432–3455 CrossRef CAS.
- A. P. M. Guedes, F. Mello-Andrade, W. C. Pires, M. A. M. De Sousa, P. F. F. Da Silva, M. Camargo, H. Gemeiner, M. A. Amauri, C. G. Cardoso, P. R. De Melo Reis, E. De Paula Silveira-Lacerda and A. A. Batista, Metallomics, 2020, 12, 547–561 CrossRef CAS PubMed.
- T. B. Peters, J. C. Bohling, A. M. Arif and J. A. Gladysz, Organometallics, 1999, 18, 3261–3263 CrossRef CAS.
- T. Scattolin, V. A. Voloshkin, F. Visentin and S. P. Nolan, Cell Rep. Phys. Sci., 2021, 2, 100446 CrossRef CAS.
- A. R. Kapdi and I. J. S. Fairlamb, Chem. Soc. Rev., 2014, 43, 4751–4777 RSC.
- T. Scattolin, I. Pessotto, E. Cavarzerani, V. Canzonieri, L. Orian, N. Demitri, C. Schmidt, A. Casini, E. Bortolamiol, F. Visentin, F. Rizzolio and S. P. Nolan, Eur. J. Inorg. Chem., 2022, e202200103 CrossRef CAS.
- T. Scattolin, E. Bortolamiol, F. Visentin, S. Palazzolo, I. Caligiuri, T. Perin, V. Canzonieri, N. Demitri, F. Rizzolio and A. Togni, Chem.–Eur. J., 2020, 26, 11868–11876 CrossRef CAS PubMed.
- T. Scattolin, E. Bortolamiol, S. Palazzolo, I. Caligiuri, T. Perin, V. Canzonieri, N. Demitri, F. Rizzolio, L. Cavallo, B. Dereli, M. V. Mane, S. P. Nolan and F. Visentin, Chem. Commun., 2020, 56, 12238–12241 RSC.
- T. T.-H. Fong, C.-N. Lok, C. Y.-S. Chung, Y.-M. E. Fung, P.-K. Chow, P.-K. Wan and C.-M. Che, Angew. Chem., Int. Ed., 2016, 55, 11935–11939 CrossRef CAS PubMed.
- T. Scattolin, G. Tonon, E. Botter, S. G. Guillet, N. V. Tzouras and S. P. Nolan, Chem.–Eur. J., 2023, 29, e202301961 CrossRef CAS PubMed.
- T. Scattolin, A. A. Logvinov, N. V. Tzouras, C. S. J. Cazin and S. P. Nolan, Organometallics, 2023, 42, 2692–2730 CrossRef CAS.
- T. Scattolin, G. Valente, L. Luzietti, M. Piva, N. Demitri, I. Lampronti, R. Gambari and F. Visentin, Appl. Organomet. Chem., 2021, 35, e6438 CrossRef CAS.
- J. E. López-Hernández and M. Contel, Curr. Opin. Chem. Biol., 2023, 72, 102250 CrossRef PubMed.
- G. Moreno-Alcántar, P. Picchetti and A. Casini, Angew. Chem., Int. Ed., 2023, 62, e202218000 CrossRef PubMed.
- P. J. Sadler and R. van Eldik, Advances in Inorganic Chemistry: Medicinal Chemistry, Academic Press, 2020 Search PubMed.
- R. P. Herrera and M. C. Gimeno, Chem. Rev., 2021, 121, 8311–8363 CrossRef CAS PubMed.
- A. Madabeni, P. A. Nogara, M. Bortoli, J. B. T. Rocha and L. Orian, Inorg. Chem., 2021, 60, 4646–4656 CrossRef CAS PubMed.
- M. Monticelli, M. Baron, C. Tubaro, S. Bellemin-Laponnaz, C. Graiff, G. Bottaro, L. Armelao and L. Orian, ACS Omega, 2019, 4, 4192–4205 CrossRef CAS PubMed.
- P. A. Nogara, A. Madabeni, M. Bortoli, J. B. T. Rocha and L. Orian, Chem. Res. Toxicol., 2021, 34, 1655–1663 Search PubMed.
- A. Madabeni, T. Scattolin, E. Bortolamiol, F. Visentin and L. Orian, Organometallics, 2024, 43, 954–962 CrossRef CAS.
- M. Sankarganesh, J. Dhaveethu Raja, K. Sakthikumar, R. V. Solomon, J. Rajesh, S. Athimoolam and V. Vijayakumar, Bioorg. Chem., 2018, 81, 144–156 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data and spectra. CCDC 2339445. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04585h |
| This journal is © The Royal Society of Chemistry 2024 |