Open Access Article
Open Access ArticleSmart membranes for separation and sensing
Xin
Liu
 a,
Gengwu
Zhang
a,
Khozama Bader
Al Mohawes
ab and
Niveen M.
Khashab
a,
Gengwu
Zhang
a,
Khozama Bader
Al Mohawes
ab and
Niveen M.
Khashab
 *a
*a
aSmart Hybrid Materials Laboratory (SHMs), Department of Chemistry, Division of Physical Science and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
bDepartment of Chemistry, College of Science, Princess Nourah bint Abdulrahman University (PNU), Riyadh 11671, Kingdom of Saudi Arabia
First published on 17th October 2024
Abstract
Self-assembled membranes are extensively applied across various fields due to their non-thermal and low-carbon footprint characteristics. Recently, smart membranes with stimuli responsiveness have garnered significant attention for their ability to alter physical and chemical properties in response to different stimuli, leading to enhanced performance and a wider range of applications compared to traditional membranes. This review highlights the recent advancements in self-assembled smart membranes, beginning with widely used membrane preparation strategies such as interfacial polymerization and blending. Then it delves into the primary types of stimuli-responses, including light, pH, and temperature, illustrated in detail with relevant examples. Additionally, the review explores the latest progress in the use of smart membranes for separation and sensing, addressing the challenges and opportunities in both fields. This review offers new insights into the design of novel smart membrane platforms for sustainable development and provides a broader perspective on their commercial potential.
1. Introduction
As the most sophisticated system, biological membranes have demonstrated their capabilities as smart membranes for transporting water, ions, and proteins with open and close switching behavior in response to diverse environmental stimuli in their surroundings.1–3 Inspired by natural systems, the development of artificial smart membranes with similar properties has attracted increasing attention in the fields of separation and sensing.4–8 In recent decades, a wide variety of materials have been utilized as building blocks for preparing smart membranes, including polymers,9 graphene oxide (GO),10 metal–organic frameworks (MOFs),11 covalent organic frameworks (COFs),8,12 macrocycles,13–15 and porous organic cages (POCs).16,17 Moreover, extensive efforts have been devoted to designing smart membranes that are capable of altering their physical or chemical properties in response to various external stimuli such as light, pH, temperature, humidity, mechanical force, electrical fields, and magnetic fields.18–20 Recent progress in smart membrane technology has been remarkable, leading to significant impacts on many aspects of human life. For instance, a thermochromic and photochromic smart film serves as a suitable substrate for smart windows with dual functionality,21 and a pH-responsive smart membrane enables efficient recovery of certain ions from brine.22 Advanced smart membranes are now designed and intensively applied in areas such as nanofiltration, molecular sieving, ion separation, actuators, self-healing, biosensors, shape-memory, and electro-devices.23–27Membrane-based separation technologies are increasingly crucial in various industrial applications, such as water treatment and valuable resource recovery.28,29 However, due to the fixed structural properties, the application of traditional membranes for separation in complex and dynamic environments faces significant limitations.30 For instance, solute blockage within membrane pores and contamination during the separation process can lead to a decline in membrane performance.31 Despite recent efforts to improve traditional membrane performance, there is an urgent need for the development of smart membranes with adjustable functions to better address these challenges. Smart membranes with unique physiochemical properties hold the potential to significantly enhance membrane performance by offering adjustable separation selectivity and permeability.32 Precise control over permeance and selectivity are key factors in fabricating high-performance membranes. These advanced membranes are functionalized with various stimuli-responsive motifs, enabling them to selectively and effectively alter their pore structure and surface properties in response to specific environmental stimuli. This dynamic adaptability allows smart membranes to flexibly regulate permeability and separation performance, achieving results unattainable by traditional membranes. Moreover, smart membranes maintain their mechanical strength and stability, and in many cases, they even enhance the separation process, thus expanding their application prospects in complex operating conditions.33,34
Biotechnology is another essential area where smart membranes are in great demand for human health, defense, and security. Traditional smart materials usually respond to external stimuli with structural deformation or color change. However, they mostly exist as powders, which is greatly limited for practical applications.35,36 In contrast, smart membranes with high mechanical properties and good processability offer substantial advantages when used for sensing and actuators.37 For example, a photoresponsive and vapor-responsive smart membrane can function like artificial muscles, enabling complex motions.38 As a new generation of stimuli-responsive smart materials, smart membranes with abundant responsive sites ensure faster and more effective sensing to meet diverse application demands compared to traditional powder materials.17,39 Moreover, the processability of smart membranes allows for the construction of practical stimuli-responsive systems, such as sensor devices and smart actuators. Thus, there is an urgent need to develop new strategies to fabricate multifunctional smart membranes for practical sensing applications. These advancements will not only enhance the performance of sensing and actuation systems but also broaden their scope of application in various biotechnological fields.
This review aims to provide an overview of the recent progress in smart membrane technology (Fig. 1). We start by summarizing some widely used membrane preparation methods, discussing mechanisms of responsiveness, and the relationship and difference among them. Then we present state-of-the-art examples of smart membranes in advanced separation and sensing applications. Finally, by exploring these current achievements of smart membranes, we highlight the role of smart membranes in addressing critical global challenges and advancing technical innovation.
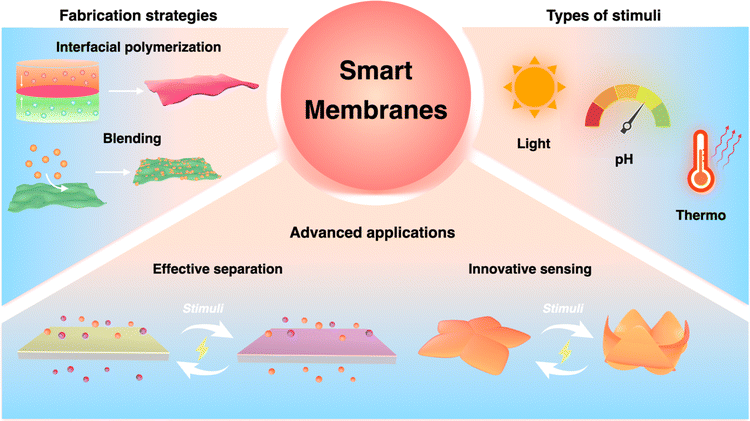 | ||
| Fig. 1 Schematic illustration of the smart membrane fabrication strategies, types of stimuli, and advanced applications in separations and sensing. | ||
2. Preparation of smart membranes
The well-established membrane fabrication strategies are essential for producing membranes with stable mechanical strength and excellent performance. The preparation methods of smart membranes share certain similarities to traditional membranes. However, the difference is the addition of functional motifs that can respond to environmental stimuli. By incorporating these stimuli-responsive motifs, smart membranes are expected to combine the advantages of both smart materials and conventional membranes for enhanced separation and sensing applications. The preparation methods for smart membranes are well-known as one-step and two-step methods, which involve incorporating the stimuli-responsive components either during the membrane preparation or after the membrane formation. This section mainly introduces and discusses two important and widely applied strategies for preparing smart membranes, including interfacial polymerization (IP) and blending methods. Then we will briefly discuss some other methods in smart membrane fabrication.2.1 Interfacial polymerization
IP is an ingenious technique for the preparation of ultrathin film at the interface between two immiscible phases.40 In general, the IP process is a polycondensation reaction between two highly reactive monomers, in which two active monomers (or monomers and catalyst) are distributed in an aqueous phase and an organic phase, respectively. Then two monomers come into contact and are covalently bonded at the interface.41 The resulting film or membrane prepared by the IP method is inherently defect-free, thin, and uniform, which greatly promotes the membrane performance in the application of various separations.42 Moreover, the pore size, thickness, and surface properties of the membrane can be easily manipulated by adjusting the reaction parameters, such as monomer concentration, reactivity, solvent, temperature, and reaction time.43Recently, the IP method has emerged as a powerful and practical fabrication technique to produce smart membranes with unique structures and properties.44,45 In designing smart membranes, functional units are usually introduced into the active layer via the IP method to enhance membrane performance. For instance, Bruggen and co-workers prepared a high-performance flexible aliphatic-aromatic polyamide thin-film composite (TFC) membrane through the IP method.46 The flexible aliphatic chains in the polyamide network provide the selective layer with an adjustable free volume via solvent activation (Fig. 2a). This designed membrane exhibited high stability, permeability, and precise selectivity for organic solvent nanofiltration (OSN) applications. However, membrane fouling remains a significant obstacle to practical separation. To address this issue, Bruggen's group developed another TFC membrane with thermoresponsive properties for nanofiltration.47 This smart membrane was fabricated by grafting poly(N-isopropylacrylamide) (PNIPAM) chains onto a bromine-containing polyamide (Br-PA) layer (Fig. 2b). PNIPAM chain is a typical smart material with a hydrophilic coil structure that shrinks when the water temperature exceeds the lower critical solution temperature (LCST). When the temperature rose above the LCST, the PNIPAM-PA membrane exhibited improving fouling resistance and cleaning efficiency during the filtration and cleaning stages. Meanwhile, a buffer layer formed, eliminating the interaction energy between the membrane surface and pollutants, further enhancing the recovery of flux. This finding demonstrates that incorporating functional units or stimuli-responsive motifs into membranes via the IP method can significantly boost their performance in nanofiltration.
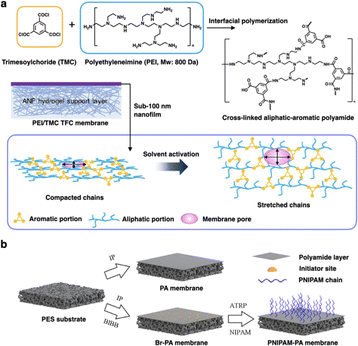 | ||
| Fig. 2 (a) Schematic illustration of the fabrication of PEI/TMC TFC membrane fabrication via interfacial polymerization and the solvent activation process. Reproduced with permission from ref. 46. Copyright 2020, American Chemical Society. (b) Schematic illustration of the preparation of PA, Br-PA, and PNIPAM-PA membrane. BIBB and ATRP are α-bromoisobutyryl bromide and atom-transfer radical polymerization. Reproduced with permission from ref. 47. Copyright 2022, American Chemical Society. | ||
The IP method can also be utilized to prepare smart membranes based on macrocycles and porous organic cages,48–52 offering a novel approach in the field of nanofiltration and separation. Peinemann et al. fabricated ultrathin layered macrocycle membranes for molecular separation using cyclodextrin and its derivatives as monomers via the IP method.53,54 The cyclodextrin or its derivative molecules were crosslinked to form a layered structure. The channel-like cavities of cyclodextrins generated numerous defined pores in the separation layer, demonstrating outstanding performance in effectively discriminating molecules of different shapes. Recently, Livingston and co-workers synthesized selectively functionalized macrocycles with differentiated reactivities that preferentially aligned to create well-defined pores across an ultrathin nanofilm.55 By tailoring the size to angstrom precision through varying the macrocycle identity, these aligned macrocycle membranes exhibited twice the methanol permeance and higher selectivity compared to their disordered counterparts. This approach offers a feasible strategy for creating smart membranes with subnanometer channels. Sun and co-workers utilized a cucurbit[7]uril (CB[7])-viologen complex as the monomer to prepare a voltage-gated nanofiltration membrane via the IP method (Fig. 3a).56 The voltage-gated smart membranes exhibited enhanced water permeability and superior molecular separation efficiency by controlling the applied voltage. When the membrane worked as the cathode with the voltage increased from 0–20 V, the rejection of methyl green increased from 20% to nearly 100% (Fig. 3b).
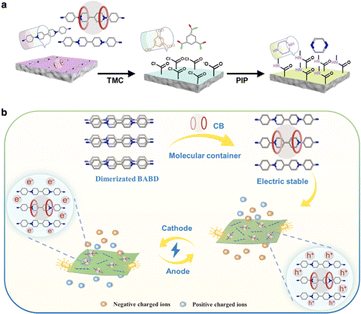 | ||
| Fig. 3 (a) Schematic preparation of a (CB[7])-viologen membrane via interfacial polymerization. (b) Schematic illustration of smart separation achieved by the voltage-gated membrane in the electric field. Reproduced with permission from ref. 56. Copyright 2022, American Chemical Society. | ||
2.2 Blending
Mixed-matrix membranes (MMMs) technique has been widely used to prepare membranes because of its simple process, easy operation, and controllable membrane properties.57 In this method, various fillers, such as nanoparticles, zeolites, MOFs, and POCs, are dispersed within a continuous organic polymer matrix.58–62 The process of preparing smart MMMs involves blending stimuli-responsive materials, used as fillers, with an organic polymer matrix to form a homogenous solution. This mixture is then transformed into a composite membrane by controlled solvent evaporation or phase inversion.63 Such methods ensure that the fillers are evenly distributed throughout the polymer matrix, leading to a membrane that can respond to external stimuli, making them highly versatile for applications in separation and sensing.Shen and co-workers developed a composite membrane by incorporating a poly(N-isopropylacrylamide-methacrylic acid) (PNIPAM-MAA) hydrogel into the GO layer, resulting in thermo- and pH-responsive water channels (Fig. 4a).64 The responsive properties of this membrane stem from the regulated nanochannels and nanopores of GO membranes, achieved by tuning the shape features of the hydrogel. This smart gating membrane with tunable channels exhibits superior molecular sieving performance compared to traditional nanofiltration membranes. MOFs have also emerged as promising candidates for preparing MMMs. Recently, Jin and Liu reported a solid-solvent processing strategy to prepare an ultrathin MMM with MOF filler loading up to 80 volume %.65 In contrast to the current method, the polymer matrix acts as a solid solvent to dissolve and immobilize metal salts after evaporation of the aqueous solution. After a ligand vapor treatment, the metal salts in the precursor layer undergo in situ conversion to nanoporous MOF crystals, resulting in an ultrathin and highly loaded MOF@polymer MMM (Fig. 4b). A polyMOF-based MMMs were developed by using intrinsically microporous ligand coordinated with metals as fillers. These polyMOFs have better dispersion in polymer matrix when casting into membranes.66 Despite many MOF-based MMMs being fabricated, the scale-up and widespread of MOF-based membranes are still challenging. Elimelech and An reported a nanoreactor-confined crystallization strategy that enables rapid and roll-to-roll fabrication of high-performance ultra-thin (∼25 nm) MOF hybrid membranes with scalable size (0.33 m × 35 m).67
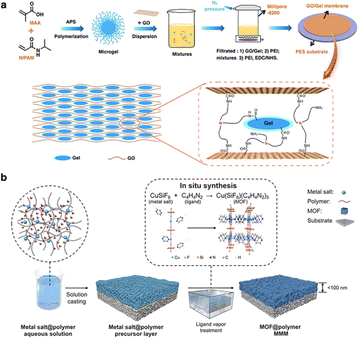 | ||
| Fig. 4 (a) Schematic illustration of the fabrication process of GO/hydrogel composite membranes. Reproduced with permission from ref. 64. Copyright 2019, Elsevier. (b) Schematic illustration of the mixed-matrix membrane fabricated by a solid-solvent processing strategy. Reproduced with permission from ref. 65. Copyright 2023, American Association for the Advancement of Science. | ||
POCs are also a suitable selection of porous filler that can be easily incorporated into the polymer matrix to produce MMMs.68 These intriguing properties of POCs greatly enhance the membrane performance. Jansen and co-workers reported a novel method to prepare MMMs through the in situ crystallization of POC molecules from an intrinsic porous polymer (PIM-1) solution.69 The incorporation of porous organic cages into polymer significantly increases the gas permeation and provides better resistance to physical aging compared to pure polymer membrane. Moreover, Lively's group developed molecularly mixed composite membranes by integrating amorphous scrambled porous organic cages into Matrimid, which can be used in a wide range of separation applications, such as gas separation and nanofiltration.70
2.3 Other methods
In addition to the IP and blending methods, various other approaches are also employed to prepare smart membranes. Such as interpenetrating polymer networks (IPNs), layer-by-layer (LBL) self-assembly, and chemical vapor deposition (CVD).IPNs are composed of two or more polymers through covalent cross-linking to form a penetrating polymer network. With this unique mixing method and network structure, stimuli-responsive monomers are easily blended into the membrane.71,72 Moreover, covalent cross-linking is used to increase the stability of membranes under demanding environments. Thus, highly cross-linked IPNs exhibit good mechanical strength in membrane formation.73
The LBL self-assembly is commonly used in smart nanofiltration membrane fabrication.74,75 It is a method of assembling molecularly thin layers composed of different molecules into ordered structures through various intermolecular interactions, which can form ultrathin membranes on the surface of the porous support. Therefore, the LBL method could precisely control the membrane thickness and surface properties.76 Thus, one or more stimuli-responsive layers can be assembled into nanofiltration membranes, achieving improved separation performance.77 However, due to the weak interaction between layers, membranes formed by the LBL method have poor stability in harsh conditions.
The CVD method is used to prepare polymer composite films by directly transporting gas monomers on the substrate surface with functional sites.78,79 The functional sites on polymer layers provide an opportunity to finely control the surface properties of composite membranes. Moreover, this method provides an alternative for polymer membranes, especially for those insoluble and infusible films, which can be formed using this simple step.80,81
Given the complexity of smart membrane structures, a single preparation method may not be sufficient to meet the demands. Thus, optimizing existing methods or combining multiple techniques is crucial to achieve membranes with superior performance and stimuli-responsive properties.
3. Stimuli-responsive behaviors in smart membranes
With the increasing demand for advanced membranes across different fields, various stimuli-responsive membranes have been developed. These smart membranes are engineered to respond to specific external stimuli, allowing them to adapt their properties and functionalities dynamically. In this section, we will discuss recent advancements in smart membranes and their response to different external stimuli such as light, pH, and temperature.3.1 Light-responsive membranes
Light is a highly controllable stimulus that can be precisely modulated, making light-responsive membranes a promising candidate for various applications such as sensing, actuating, drug delivery, and smart separation systems.82–84 Light-responsive membranes generally have rapid and specific responses by altering molecular conformation and morphology, such as isomerization, energy excitation, and bond dissociation, under illumination with different wavelengths. The ability to fine-tune light wavelength and exposure duration provides a high degree of control over these membranes' functional response. In general, photochromic units can be integrated into membranes through physical blending, chemical bonding within the membrane matrix, or immobilization on the membrane surface.85 The choice of integration method depends on the desired application and the specific properties required from the membrane. Reversible physical and chemical changes in light-responsive membranes are primarily attributed to the isomerization of photochromic units, which lead to significant changes in membrane properties, including pore size and surface characteristics.86Light-responsive chemical moieties, such as azo-benzene, spiropyran, diarylethene, peptides, and stilbene, have been extensively utilized in the development of light-responsive membranes. Among them, azobenzene and spiropyran are mainly studied in light-responsive membrane design due to their well-characterized photoisomerization properties.
Azobenzene is known for its reversible trans–cis photoisomerization process, which can directly convert light energy into mechanical motion. This process results in a size change of the azobenzene molecule from 0.9 nm in the trans form to 0.6 nm in the cis form. By incorporating the azobenzene moiety into the conjugated microporous polymer (CMP) as a monomer, Lai and co-workers have fabricated the smart membrane (azo-CMP@200-50c) with artificial light-gated ion channels.87 Using electrochemical methods, the nanoscale thickness of these membranes can be precisely tailored. This bottom-up strategy allows each building block of the membrane channels to function as a photo switch with an “on–off–on” photoisomerization response (Fig. 5a). The well-defined conjugated three-dimensional (3D) network with uniform micropores serves as smart ion channels, where geometrical changes induced by light can remotely and dynamically regulate the ion transport (Fig. 5b). Moreover, azobenzene can form a host–guest complex with cyclodextrin, which can be attached to the membrane surface.88 Bonding cyclodextrin to the membrane surface provides linking sites for azobenzene polymers and allows for the regulation of surface properties and the pore structure. The successful assembly of light-responsive functional polymer on the surface enables the membrane to enhance water permeability and excellent fouling resistance properties (Fig. 5c).
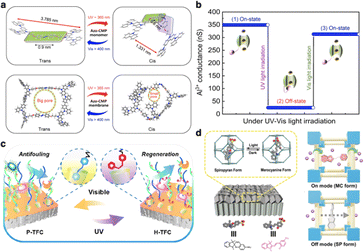 | ||
| Fig. 5 (a) Schematic representation of trans–cis–trans reversible isomerization of the azo-CMP monomer and the corresponding pore structure of the azo-CMP membrane under light irradiation. (b) The controllable Al3+ ion transport within the light-gated membrane azo-CMP@200-50c. Reproduced with permission from ref. 87. Copyright 2022, American Association for the Advancement of Science. (c) Schematic illustration of light-responsive TFC membrane with antifouling and regenerable properties. Reproduced with permission from ref. 88. Copyright 2020, American Chemical Society. (d) Illustrations of the possible proton transport mechanism through the SSP@ZIF-8-10% membrane in the dark (on mode) and under visible light (off mode). Reproduced with permission from ref. 89. Copyright 2020, Wiley-VCH. | ||
Spiropyran,90 another widely studied light-responsive moiety, undergoes reversible configuration change from ring-closed form to ring-opened form under light exposure. This transformation is accompanied by significant changes in its chemical and physical properties. Incorporating sulfonated spiropyran (SSP) into the cavity of ZIF-8, a hybrid smart membrane SSP@ZIF-8 can be developed.89 The resulting membrane exhibits high proton conductivity, outstanding switching properties, and fast response time upon visible light irradiation (Fig. 5d).
Light-responsive MOF and COF-based membranes are also investigated recently. Sun and coworkers report a photo-responsive ionic dye-sensitized COF membrane. This membrane can adeptly convert light into electrical signals through photoexcitation-triggered ion movement within the membrane channels, which outperformed the current market standard by approximately 26-fold.91 A similar concept was also utilized in the light-responsive MOF-based membrane, which also performed a high power density by light-controlled ion active transport with the leverage of photo-thermal property of MOF structure.92
Although many light-responsive membranes have been investigated for many years, the functionalization strategy and response speed of the membrane still greatly limit the performance and applications of light-responsive membranes.93 It is important to combine advanced material science and precise engineering techniques for the development of novel light-responsive membrane materials with fast response and enhanced efficiency, suitable for a wide range of applications in biology and industry.
3.2 pH-responsive membranes
pH-responsive membranes, which can dynamically modulate their permeability and selective permeation characteristics in response to variations in environmental pH, represent a prominent category of smart membranes. These advanced materials are typically engineered by integrating pH-responsive components into the membrane matrix.94–96 The acid–base moieties embedded within the membrane undergo reversible protonation and deprotonation under specific pH conditions, which often leads to conformation change, further causing changes in the surface properties or channel sizes of membranes. The incorporation of such components allows smart membranes to exhibit significant changes in their structural and functional properties under different pH conditions. For example, it has been found that block polymers can be used to prepare thin films with narrow pore size distribution for separation.97,98 The pore size can be adjusted by swelling or shrinking the pH-responsive polymer chain. Such properties of polymer membranes are highly desirable for size-based separation.Chu's group has designed a novel dual thermo- and pH-responsive composite membrane by blending a pH-responsive block copolymers poly(styrene)-block-poly(4-vinylpyridine) (PS-b-P4VP) with the thermos-responsive PNIPAM nanogels as the top and bottom layer in a simple twice-casting method, respectively.99 The pKa of P4VP is about 3.5–4.5 and the volume phase transition temperature (VPTT) of PNIPAM nanogels is about 33 °C. At pH 2.5/20 °C, the water flux reached a small value of 1.13 kg m−2 h−1 bar−1 due to the pore closure resulting from the protonation of pyridine groups in the P4VP. In contrast, at pH 6.8/45 °C, the water flux increased dramatically to 778.03 kg m−2 h−1 bar−1. The substantial variation in permeability is a direct consequence of the deprotonation of P4VP and the phase transition of PNIPAM nanogels, which caused the pores to open (Fig. 6a).
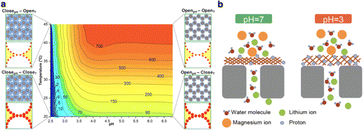 | ||
| Fig. 6 (a) The water flux contour map of the composite membrane, and four schemes near the four vertices of the contour map correspond to four switching states of the composite membrane at different pH values and temperatures. Reproduced with permission from ref. 99. Copyright 2017, American Chemical Society. (b) Schematic illustration of ion transport mechanism for pH-responsive membrane. Reproduced with permission from ref. 100. Copyright 2021, American Chemical Society. | ||
More recently, Tang's group developed a novel nonpolyamide nanofiltration membrane based on a metal-coordinated structure by fabricating a Cu-1,3-phenylenediamine (Cu-MPD) self-polymerized membrane.100 The incorporation of copper into the membrane matrix significantly enhanced both water permeability and ion selectivity, effectively overcoming the conventional MPD membranes. The low pH environment positively affected the water permeability due to the protonation of amino groups within the membrane structure, which reduced the electrostatic repulsions and loosened the membrane pores, facilitating high water flux. Conversely, increasing the pH led to the deprotonation of the amino groups, which increased electrostatic repulsions and tightened the pore structure, resulting in a lower water permeability (Fig. 6b).
pH-responsive smart membranes have been developed using various methods and exhibit promising potential in a range of applications, including separation, sewage treatment, sensors, and protein fractionation.101–104 These advanced membranes capitalize on the ability to dynamically alter their physical or chemical properties in response to changes in environmental pH, making them highly versatile for diverse industrial and environmental applications. However, there remains significant potential for improvement in the design and synthesis of pH-responsive smart membranes. Future studies should focus on enhancing the responsiveness, durability, and scalability of these membranes to transfer them to real-world applications.
3.3 Thermo-responsive membranes
In thermo-responsive membranes, temperature serves as an external trigger that induces reversible changes of microscopic or macroscopic features to achieve “on and off” behaviors, which opens a variety of application possibilities. Temperature-responsive smart composite membranes are modified by decorating them with thermochromic units that respond to temperature variations. For example, the thermal-responsive polymer membrane generally has an LCST in aqueous solution. When the temperature is higher or lower, the polymer chain will shrink or swell, causing the actuator behavior of the membrane. Some membranes exhibit an optical response when subjected to temperature fluctuations, making them highly valuable for applications requiring temperature-sensitive visual indicators, including smart filtration systems and biomedical devices. This section will discuss the latest developments in temperature-responsive smart membranes incorporating thermochromic segments.Jeon's group manufactured mechano- and thermochromism membranes (PDMS/Tdye_Al2O3 HNTs) with an ultra-large-scale size of over 300 cm2. These membranes consist of ultrathin (∼60 nm) Al2O3 hollow nanotubes (HNTs) and polydimethylsiloxane (PDMS) nanofibers (NFs), with polyoxymethylene melamine serving as the thermochromic dye. The thermochromic dye changed its color from powder pink to deep pink in response to environmental temperature increases within the ranges of 30 °C to 50 °C and 50 °C to 70 °C.105 A dual scattering effect was obtained by increasing the temperature to 70 °C and a mechanical (stretching) force was applied. This effect enhanced the visibility and accuracy of the temperature-induced color change. Furthermore, the red response in the RGB color model has been evaluated for the composite to obtain a more precise color analysis. The red response progressively increased from 1.02 to 1.13 as the temperature rose, and this change was observed before and after applying tensile stress (Fig. 7a).
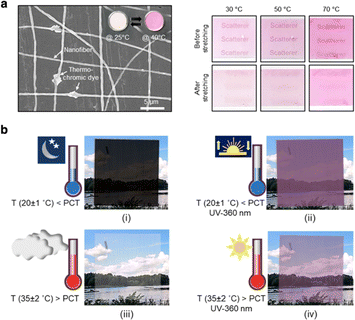 | ||
| Fig. 7 (a) SEM image of PDMS/Tdye_Al2O3 HNTs and a thermo-dependent color change of the membrane before and after stretching. Reproduced with permission from ref. 105. Copyright 2020, American Chemical Society. (b) Photographic images demonstrate a visual change in transmission and color on exposure to various conditions of temperature and/or UV light. Reproduced with permission from ref. 106. Copyright 2021, American Chemical Society. | ||
Recently, Berglund's group utilized a mixture of thermo- and photochromic components as pigments in their transparent wood (TW)-based composite, creating innovative smart windows.106 These smart windows integrated dual-stimuli-responsive chromic materials, enabling them to react to both temperature and UV light. In their study, the transmission properties of a representative sample, T50P501 (containing equal mass ratios of thermochromic and photochromic), were compared with a thiol–ene monomer sample devoid of any chromic components. The transmission data at 550 nm for the T50P501 sample showed a 41% reduction below the phase change temperature (PCT is 31 °C) and under UV exposure, while above the PCT, the transmission increased to 48%. These results indicated that T50P501 exhibited noticeable color changes in response to varying temperature and UV conditions. Cycle tests demonstrate the durability of the reversible optical properties where the chromic constituents were restricted inside the lumen of the wood of the composites (Fig. 7b).
These developments highlight the potential of temperature-responsive membranes for various applications, including temperature sensors, environmental monitoring systems, and smart separations.107 By exploring advanced fabrication techniques, novel thermo-responsive smart membranes can also be designed for energy-efficient and environmentally sustainable filtration systems.108–111
Given the current research progress, each type of smart membrane has a specific application range. Thermo-responsive membranes and light-responsive membranes are typical physical-stimuli-responsive membranes. Thermo-responsive membranes have a much higher degree of intelligence, which can respond rapidly to temperature changes, so as to achieve the purpose of real-time monitoring in nanofiltration. However, most of the thermos-responsive membranes used in the experiment are relatively monolithic. Light-responsive membranes have become a hot topic in recent years because such membranes are widely used in both separation and sensing fields. This kind of membrane can store large-capacity information with a low transmission loss, which makes it possible to apply for optical information storage and optical smart switches. Moreover, due to the realization of large-scale manufacturing of some light-responsive membranes, such membranes have been applied to self-cleaning, anti-counterfeiting, and sensors, which have broad market prospects. pH-responsive membranes, as a typical chemical-stimuli-responsive membrane, have unique advantages in fine control of membrane surface properties and pore size. In addition, pH-responsive membranes can be easily combined with other factors, like temperature and voltage, which extends its applications in complicated separation under harsh conditions.112
Although various stimuli-responsive membranes have been developed and applied in a wide application range, the currently used smart membranes are relatively single, multi-responsive membranes that are more promising when operated in a complicated environment, particularly in industrial separations. Multi-responsive membranes can alter their pore size, surface charge, or hydrophilicity in response to several stimuli, allowing for dynamic and adjustable separation performance. For instance, a membrane that responds to the changes in both pH and temperature can fine-tune its filtration properties depending on environmental conditions, offering higher selectivity and adaptability than single-responsive membranes. In the field of sensing, multi-responsive membranes can detect changes in multiple parameters, offering more detailed and comprehensive sensing capabilities. For example, a light and pH dual-responsive membrane could serve as a dual-mode sensor, improving accuracy and sensitivity in detecting analytes in biochemical or environmental monitoring applications. Above all, multiple stimuli-responsive membranes enable broader application possibilities, which is essential for cutting-edge technologies in environmental, biomedical, and industrial fields.
4. Advanced applications of smart membranes
With well-designed molecular structures, smart membranes exhibit various responsive properties that enable their efficient and flexible use in practical applications. Engineering chemical or physical changes in smart membranes is crucial for unlocking their advanced functionalities. In this section, we introduce two key applications of smart membranes: separation and sensing.4.1 Separation
With the stimuli-responsive control of pore size and surface property, smart membranes have the potential for enhanced performance in various separation processes compared to traditional membranes. Nunes and co-workers designed a smart covalent organic network (CON) membrane featuring “on–off–on” light-switchable pores for molecular separation. In this design, azobenzene moieties acted as light switches, bridging flexible cyclen building blocks.113 A chair-to-boat stereoisomerism change of cyclen rings occurred due to the isomerization of azobenzene under UV/vis light, which led to geometrical changes in the membrane pores. Initially, the original trans-form film had an open pore aperture of about 10.6 Å. Upon UV irradiation, the trans-to-cis isomerization process reduced the pore size to 7.5 Å (Fig. 8a). This resulted in a membrane with on–off–on light-switchable pores, enabling precise control of molecular sieving and high solvent permeability (Fig. 8b and c). By leveraging these dynamic and tunable properties, smart membranes can achieve precise and efficient separations, surpassing the capabilities of traditional static membranes.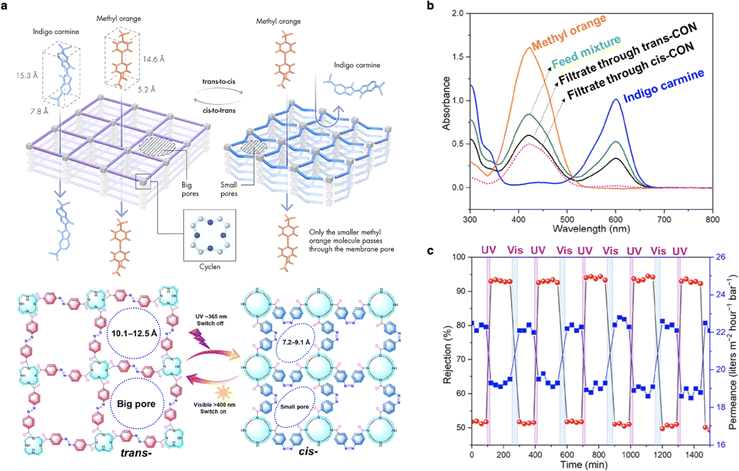 | ||
| Fig. 8 (a) Schematic illustration of the trans–cis photoisomerization and chemical structures of the light-responsive membranes. (b) Separation performance of mixed dyes through trans- and cis-CON membranes. (c) Indigo carmine separation performance in consecutive cycles under UV/vis light. Reproduced with permission from ref. 113. Copyright 2022, American Association for the Advancement of Science. | ||
The development of smart membranes with more tunable pore structures is highly demanded to perform exclusively as size-based molecular sieves rather than selective absorbents.114 Cooper and Livingston designed a smart, responsive composite membrane comprising crystalline porous organic films prepared by interfacial synthesis on polyacrylonitrile (PAN) support.115 Crystals of the organic cage (CC3) grew at the water/dichloromethane interface, resulting in the most thermodynamically stable polymorph, CC3α, which was transferred to PAN to produce a continuous CC3α-PAN membrane. When exposed to methanol, a rapid phase transition from CC3α to CC3γ′ occurred, resulting in a CC3γ′-PAN membrane with an increased effective pore size (Fig. 9a).
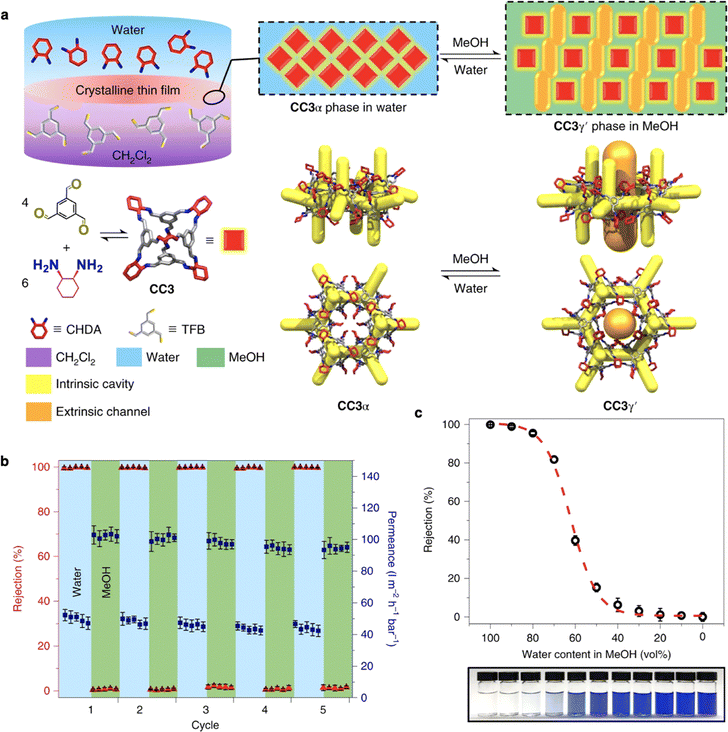 | ||
| Fig. 9 (a) Scheme shows the synthesis of a crystalline CC3 film and its two different forms by cycling the solvent between water and MeOH. (b) Reversible dye rejection of brilliant blue and solvent permeance of the CC3-PAN membrane was observed upon switching the feedstock solvent between water and MeOH. (c) Brilliant blue rejection in mixtures of water and MeOH (v/v) for CC3-PAN membrane. Reproduced with permission from ref. 115. Copyright 2022, Springer Nature. | ||
This switchable porosity is reversible, enabling the separation of three organic dyes with different sizes via graded sieving using a single membrane (Fig. 9b and c).
Very recently, Sun and co-workers fabricated a networked cage (iac-cage) nanofilm, less than 8 nm thick, comprising tunable, light-responsive organic cage-based water channels via oil/water interface crosslinking.116 The networked cage nanofilm, attached to the porous support, exhibited ultrahigh water permeance and excellent molecular sieving performance, surpassing the capabilities of most recent membranes (Fig. 10a). Notably, the introduction of the light-responsive motif “azo” allowed the membrane to display switchable water permeance and graded molecular sieving (Fig. 10b). This smart membrane was employed to separate a mixture of three organic dyes (4-nitrophenol, methyl orange, and Congo red) with gradient dimensions. Upon UV irradiation, only 4-nitrophenol was detected in the permeate, while the other two dyes were almost completely rejected. After vis irradiation, methyl orange became permeable, while the Congo red was still rejected. By alternating UV and vis irradiation, the smart membrane achieved the separation of a ternary mixture (Fig. 10c and d). The versatility of porous organic cages presents great potential for developing next-generation molecular sieve membranes with smart responsive properties for industrial separation.
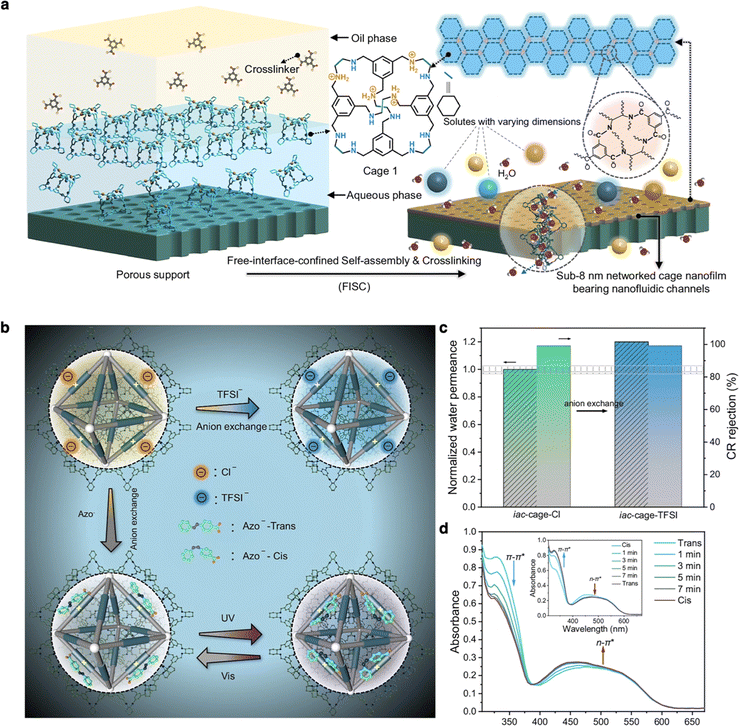 | ||
| Fig. 10 (a) Crosslinking POCs at a free interface between water and the organic phase by trimesoyl chloride and the schematic diagram of networked cage nanofilm with water channel for rapid molecular separation. (b) Schematic illustration showing the microenvironment modulation of the pore windows via anion exchange. (c) Separation performance difference of iac-cage membrane with Cl− anion and TFSI− anion. (d) Time-dependent UV/vis spectra of the iac-cage-azo membrane at 298 K, using UV and vis (inset) irradiation. Reproduced with permission from ref. 116. Copyright 2024, Springer Nature. | ||
MOF-based MMMs have also been designed as smart membranes for gas separation.117 One of the most widely studied photo-responsive membranes in MOF is azobenzene. For example, a photo-responsive MOF membrane was designed by using azobenzene and bis(4-pyridyl)ethylene. The membrane is in situ irradiated with UV and vis light, and the separation factor of an H2/CO2 mixture can be switched reversibly between 21.3 and 43.7.118 Another photoswitchable MOF membrane (SURMOF) was fabricated by incorporating azobenzene moieties as side groups into the framework. By controlling the isomerization state of the azobenzene groups by light, the flux and separation factor of H2 and CO2 can be precisely adjusted.119
Moreover, to meet the sustainable separation requirements, a smart hybrid membrane with self-healing properties was designed by incorporating hyper-cross-linked metal–organic polyhedra (HCMOPs) as nodes in hyper-cross-linked polymer networks.120 The resulting membrane exhibited enhanced mechanical properties, self-healing ability, antibacterial activity, and outstanding separation performance compared to traditional MOP-based mixed-matrix membranes.
In addition to self-healing capabilities, smart membranes with self-cleaning properties were also fabricated. Antifouling is crucial in industrial separation, especially in water treatment, pharmaceutical production, and food processing. Membranes are continuously exposed to complex mixtures containing particles, proteins, and oils, which can adhere to the membrane surface, causing decreased performance. Smart membranes could address this issue by utilizing self-cleaning mechanisms; for example, thermoresponsive membranes can undergo temperature-induced contraction or expansion, expelling contaminants from their pores, while pH-responsive membranes can alter their surface charge, repelling charged contaminants under certain pH conditions. Photoresponsive membranes can leverage light to trigger surface modifications that reduce fouling adhesion. Moreover, some smart membranes incorporate anti-fouling coatings or surface hydrophilicity adjustments, making it harder for organic and biofouling substances to adhere.
Naumov and Di Profio report a self-cleaning smart membrane (PVDF-PVA-TBB) by incorporating stimuli-responsive organic crystals onto the membrane surface (Fig. 11a).121 This design resulted in smart gating membranes that demonstrated increased water flux and enhanced fouling resistance due to the thermos-responsive properties of the incorporated crystals. Such properties enable the membrane to maintain high performance and durability under varying conditions, making it suitable for long-term applications in water treatment and purification (Fig. 11b and c). Another innovative approach involved designing a self-cleaning smart membrane (PPFM) with CO2 responsiveness for controllably separating oil/water mixtures (Fig. 11d).122 By adhering CO2-responsive copolymer to the membrane surface, a scale membrane with gas-tunable surface wettability was generated. This smart membrane can be applied to various oil/water mixtures due to its switchable transport property under alternating CO2/N2 stimulation, demonstrating high separation efficiency and adaptability to different industrial scenarios (Fig. 11e and f).
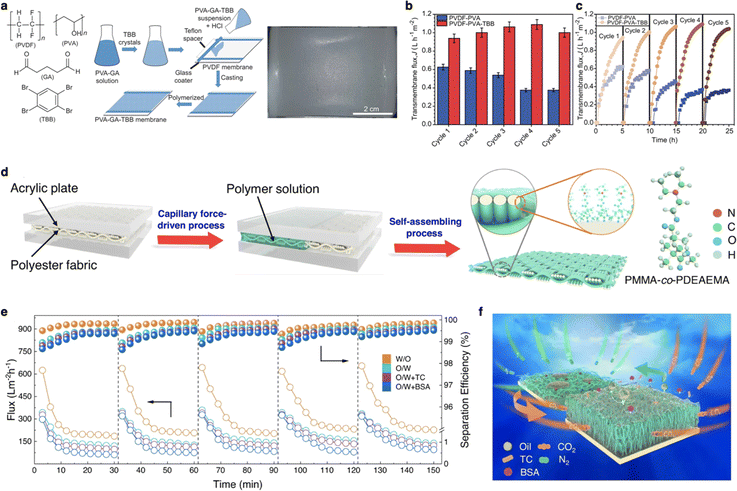 | ||
| Fig. 11 (a) Chemical structure of smart membrane component, the process of hybrid membrane preparation, and optical image of the membrane. (b) Multi-cycle osmotic distillation (OD) tests of membranes. (c) Instantaneous transmembrane flux as a function of the operating time for undoped and PVDF-PVA-TBB (doped) membranes in multiple OD cycles. Reproduced with permission from ref. 121. Copyright 2023, Springer Nature. (d) Schematic illustration of the fabrication process of PPFM. (e) Self-cleaning performance of PPFM-0.5 with a gap width of 150 μm for both water/oil and oil/water emulsion with others without organic foulants at 25 °C. (f) Schematic illustration of the gas-controlled self-cleaning mechanism. Reproduced with permission from ref. 122. Copyright 2023, Springer Nature. | ||
The development of these advanced smart membranes represents a significant leap forward in separation science and technology. The integration of dynamic and responsive elements into membrane design paves the way for next-generation materials that can meet the rigorous demands of modern industrial separation processes.
4.2 Sensing
A sensor is an integrated device that senses and transforms external stimuli into detectable signals.123,124 Sensors are widely applied in various fields, such as touch-sensitive buttons, environmental monitoring, and intelligent switches. Among smart sensing devices, humidity sensors are particularly noteworthy for their ability to detect moisture and record the variation of humidity in real time, demonstrating great potential in flexible electronic devices. Our group reported an organic caged-based humidity sensor with ultrafast response/recovery time (1 s/3 s) and remarkable stability, capable of operating for over 800 cycles.124 The humidity sensor comprises a unique organic cage film featuring carboxylic (–COOH) and protonated amine functional groups. With the incorporation of the –COOH unit, protonated cages self-assembled into hydrogen-bonding networks with highly interconnected pores, enhancing the film's efficiency for water transfer and its sensitivity to the water molecules under varying humidity conditions (Fig. 12a). Moreover, the fabricated humidity sensor was successfully applied as a touchless screen and touchless password manager, demonstrating its versatility and practical applicability in advanced electronic devices (Fig. 12b).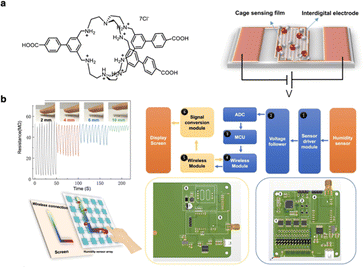 | ||
| Fig. 12 (a) Chemical structure of the cage and crystalline cage sensing film. (b) Demonstration of the smart touches HMI application based on cage humidity sensor. Reproduced with permission from ref. 124. Copyright 2024, Springer Nature. | ||
Various polymer-based smart membranes have been developed for sensor applications; and their low-cost process, high mechanical stability, and flexibility are vital for future applications in wearable/attachable devices. Zhang and co-workers reported a porous ionic membrane (PIM) with fast and reversible humidity response at room temperature, which can be used as a highly selective flexible humidity sensor. The conductivity of the PIM-based sensor changed more than 70 times as the relative humidity (RH) value increased from 10.89% to 81.75%, with rapid response (0.4 s) and recovery times (2.6 s).125,126
Smart membranes capable of dynamically sensing external stimuli have been designed to function as actuators, a key component in advanced materials science. Ma's group developed a mixed-matrix membrane that functions as artificial muscles by incorporating photomechanical molecular crystals into polymers such as polyvinylidene (PVDF).127 In this design, the photomechanical molecular crystals and polymers served as muscle fibers and connective tissues, respectively. The membrane exhibited controllable mobility, allowing it to reversibly lift/grasp objects, crawl, and swim under visible light irradiation. Similarly, Zhang's group engineered a class of rigid-flexible coupling crystalline crosslinked polymer (CCP) membranes, which exhibited a reversible and repeatable vapor-triggered actuation performance. The unique polymer structures, high vapor sorption, and anisotropic properties of these membranes induced the directional deformation, enabling precise control movement in the CCP actuators.127 Our group recently reported the first example of a mechanically responsive soft composite film based on [2 + 3] imine-based porous organic cages (Oba-cage).128,129 This actuator is vapor-responsive and possesses a shape memory, allowing it to recover its original shape after the removal of the trigger due to the reversible structural change of Oba-cage upon the vapor adsorption/desorption. The response and recovery speed of this actuator depends on the ratio of the Oba-cage used during fabrication, with the optimal performance achieved at 50 wt% (Fig. 13a). Inspired by this work, we designed another vapor-responsive composite film UC-1@PVDF that displays distinct actuation modes.130 The smart membrane was fabricated by incorporating novel urea cage molecules into a polymer matrix (Fig. 13b). The specific actuation mode shows variations in different organic vapors. In this process, host–guest interactions between urea cages and solvent molecules play a key role, as these solvent molecules can either occupy the extrinsic spaces among the urea cages or be encapsulated in the intrinsic cavities of the cages, leading to transformations between different crystalline polymorph. Thus, the urea cages can serve as smart molecular recognition units within the polymer matrix, driving the membranes to perform diverse mechanical movements in response to external stimuli (Fig. 13c).
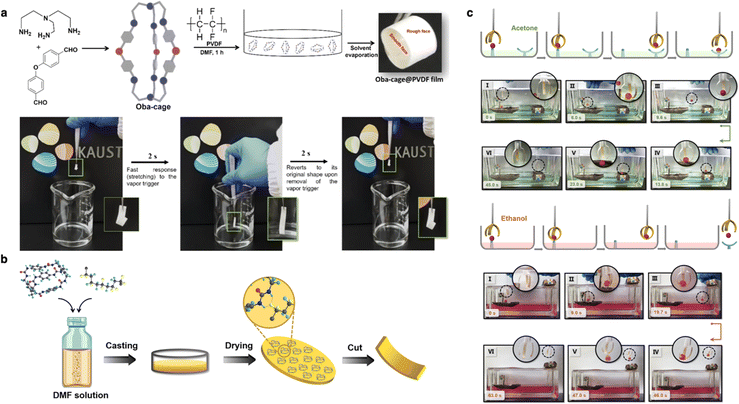 | ||
| Fig. 13 (a) Synthesis, fabrication, and operation of the Oba-cage@PVDF actuator film. Reproduced with permission from ref. 129. Copyright 2022, Wiley-VCH. (b) Schematic illustration of cage-based composite film fabrication. (c) The different actuation behavior of UC-1@PVDF (50) on exposure to acetone vapor and ethanol vapor. Reproduced with permission from ref. 130. Copyright 2024, Cell Press. | ||
Recently, COF-based composite membranes also have been designed as a new type of stimuli-responsive actuators. A smart soft polymer composite film with a 3D COF (FCOF-5) flexible building block was fabricated, which can show a reversible vapor-triggered transformation. The adsorption and desorption of tetrahydrofuran (THF) molecules within FCOF-5 can remarkably induce the expansion or contraction of the framework, indicating a breathing motion.131 Zhang and coworkers designed a humidity-responsive composite membrane by incorporating rigid hydrazone-linked COF (COF-42) with flexible polyethylene glycol (PEG) moieties. The PEG-COF-42 membrane exhibited fast water adsorption/desorption due to the asymmetric membrane structure. Due to the high sensitivity to minimal humidity fluctuations, the PEG-COF-42 membrane could achieve various actuation behaviors and continuous self-oscillation above a water surface.132
5. Conclusion and outlook
In this review, we have summarized recent advances in the field of smart membranes, covering preparation strategies, stimuli-responsive mechanisms, and their promising applications in sensing and separation. By incorporating various stimuli-responsive moieties into the structural building blocks and tuning the physical and chemical properties, diverse types of smart membranes, including polymer and molecular crystals-based composite membranes, have been developed. Therefore, smart membranes can adjust their physical or chemical properties in response to external stimuli, resulting in more efficient performance and broader application prospects, such as smart separation, advanced sensors, and soft robots. While smart membranes offer significant potential as the next-generation solutions for addressing critical global challenges and advancing technological innovation, there remains a considerable gap between fundamental research and practical industrial implementation due to numerous unresolved challenges.First, despite significant progress over the past decades, the industrial IP process for membrane fabrication has remained largely unchanged, highlighting a gap between fundamental research and real industrial applications. Enhanced fabrication methods are needed to improve membrane stability and mechanical strength. For MMMs, current porous materials are still far from being ideal fillers due to the poor affinity to the polymer matrix and long-term stability problem. Designing and synthesizing smart MMMs with good stability and excellent polymer-filler compatibility remains a significant challenge. Thus, more research is needed to explore and adapt smart membranes for advanced sensing and separation.
Looking forward, one of the future research works includes improving the scalability of smart membrane production. While there have been numerous breakthroughs in material design, the fabrication processes still lag in terms of cost-effectiveness and large-scale reproducibility. The development of high-throughput manufacturing techniques that can retain the advanced properties of smart membranes without compromising performance will be essential for their widespread adoption in the industry. Additionally, enhancing the mechanical stability and long-term durability of these membranes will be critical to ensuring their practicality for real-world applications.
Second, the practical factors of response range, speed, and reversibility in smart membranes for sensing and separation are extremely critical. Unlike traditional membranes, smart membranes can quickly respond to environmental stimuli by altering their physical or chemical properties, allowing them to adapt flexibly to complex separation environments and sensing systems. Therefore, developing novel stimuli-responsive membranes that can respond to multiple stimuli is highly demanded. Future research could focus on addressing specific problems such as improving membrane selectivity and response speed in highly sensitive applications. As industries demand increasingly sophisticated materials for applications ranging from water purification to medical diagnostics, smart membranes will need to become more versatile, efficient, and reliable. For instance, the integration of smart membranes into soft robotics or advanced medical devices could open up new pathways for innovation.
Third, it is essential to consider the practical value of smart membranes from the initial design stages. Meantime, the advantages of low production cost, low energy consumption, and wide response range should be prioritized. These undoubtedly require the intensively cooperative effort of scientists and engineers from both academia and industry. Interdisciplinary collaboration between academia and industry will be crucial in overcoming the challenges related to scaling up and adapting smart membranes for commercial applications. Partnerships that combine academic innovation with industrial expertise can help expedite the transition from bench-scale research to market-ready solutions. Collaborative efforts should also prioritize the development of smart membranes that can respond to multiple stimuli simultaneously, enabling their use in more complex and dynamic environments. By addressing the current challenges through improved fabrication methods, enhanced response characteristics, practical design considerations, and interdisciplinary collaboration, the transition from fundamental research to industrial implementation can be accelerated.
Another pressing issue is the environmental impact associated with smart membrane materials. Current production processes often rely on non-renewable resources and involve energy-intensive procedures, which may limit the sustainability of these technologies. Future research should focus on developing environmentally friendly and energy-efficient production methods. This includes exploring biodegradable or recyclable materials to reduce the ecological footprint of smart membrane technologies. Emphasizing the use of green chemistry principles in the design and fabrication of smart membranes could significantly advance their acceptance in environmentally conscious markets.
In summary, while smart membranes represent a promising solution for many contemporary challenges, there is still much work to be done to fully realize their potential. By addressing key issues such as scalability, environmental sustainability, and interdisciplinary collaboration, the next generation of smart membranes could have far-reaching impacts across multiple sectors. With continued research and innovation, these materials may soon move from the lab to large-scale industrial applications, revolutionizing fields such as environmental sensing, healthcare, and industrial separation.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Conceptualization: Xin Liu and Niveen M. Khashab; writing – original draft: Xin Liu, Gengwu Zhang, and Khomaza Bader Al Mohawes; writing – review and editing: Niveen M. Khashab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the King Abdullah University of Science and Technology (KAUST).References
- E. Gouaux and R. MacKinnon, Science, 2005, 310, 1461–1465 CrossRef PubMed.
- M. J. Thompson and J. E. Baenziger, Nat. Chem. Biol., 2020, 16, 1331–1342 CrossRef PubMed.
- F. M. Ashcroft, Nature, 2006, 440, 440–447 CrossRef PubMed.
- Q. Zhang, J. Zhang, S. Wan, W. Wang and L. Fu, Adv. Funct. Mater., 2018, 28, 1802500 CrossRef.
- G. Guan, M. Wu and M.-Y. Han, Adv. Funct. Mater., 2020, 30, 1903439 CrossRef CAS.
- M. V. González and I. Willner, Angew. Chem., Int. Ed., 2020, 59, 15342–15377 CrossRef.
- J. Zhang, B. He, Y. Hu, P. Alam, H. Zhang, J. W. Y. Lam and B. Z. Tang, Adv. Mater., 2021, 33, 2008071 CrossRef CAS.
- P. She, Y. Qin, X. Wang and Q. Zhang, Adv. Mater., 2022, 34, 2101175 CrossRef CAS.
- T. Huang, Z. Su, K. Hou, J. Zeng, H. Zhou, L. Zhang and S. P. Nunes, Chem. Soc. Rev., 2023, 52, 4173–4207 RSC.
- Z. Zhang, X. Xiao, Y. Zhou, L. Huang, Y. Wang, Q. Rong, Z. Han, H. Qu, Z. Zhu, S. Xu, J. Tang and J. Chen, ACS Nano, 2021, 15, 13178–13187 CrossRef CAS PubMed.
- Y. Cheng, S. J. Datta, S. Zhou, J. Jia, O. Shekah and M. Eddaoudi, Chem. Soc. Rev., 2022, 51, 8300–8350 RSC.
- X. Liu, J. Wang, Y. Shang, C. T. Yavuz and N. M. Khashab, J. Am. Chem. Soc., 2024, 146, 2313–2318 CrossRef.
- T. Huang, B. A. Moosa, P. Hoang, J. Liu, S. Chisca, G. Zhang, M. Alyami, N. M. Khashab and S. P. Nunes, Nat. Commun., 2020, 11, 5882 CrossRef PubMed.
- T. Huang, M. Alyami, N. M. Khashab and S. P. Nunes, J. Mater. Chem. A, 2021, 9, 18102–18128 RSC.
- G. Zhang, X. Zhu, L. O. Alimi, X. Liu, A. Chen, B. M. Moosa and N. M. Khashab, J. Mater. Chem. A, 2024, 12, 6875 RSC.
- X. Li, W. Lin, V. Sharma, R. Gorecki, M. Ghosh, B. A. Moosa, S. Aristizabal, S. Hong, N. M. Khashab and S. P. Nunes, Nat. Commun., 2023, 14, 3112 CrossRef PubMed.
- D. Yan, Z. Wang and Z. Zhang, Acc. Chem. Res., 2022, 55, 1047–1058 CrossRef PubMed.
- P. Yang, F. Zhu, Z. Zhang, Y. Cheng, Z. Wang and Y. Li, Chem. Soc. Rev., 2021, 50, 8319–8343 RSC.
- C.-Y. Shi, W.-Y. Qin and D.-H. Qu, Chem. Sci., 2024, 15, 8295–8310 RSC.
- P. Liu, F. Fang, H. Wang and N. M. Khashab, Angew. Chem., Int. Ed., 2023, 62, e202218706 CrossRef CAS.
- W. Meng, A. J. J. Kragt, X. Hu, J. S. van der Burgt, A. P. H. J. Schenning, Y. Yue, G. Zhou, L. Li, P. Wei, W. Zhao, Y. Li, J. Wang and L. Jiang, Adv. Funct. Mater., 2024, 34, 2402494 CrossRef CAS.
- L. Wang, D. Rehman, P.-F. Sun, A. Deshmukh, L. Zhang, Q. Han, Z. Yang, Z. Wang, H.-D. Park, J. H. Lienhard and C. Y. Tang, ACS Appl. Mater. Interfaces, 2021, 13, 16906–16915 CrossRef CAS.
- H. F. M. Austria, S. T. M. O. Setiawan, J. Widakdo, Y.-H. Chiao, W.-S. Hung, C.-F. Wang, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Mater. Chem. A, 2021, 9, 21510–21531 RSC.
- L. Liu, R. Yu, L. Yin, N. Zhang and G. Zhu, Chem. Sci., 2024, 15, 1924–1937 RSC.
- P. Sarkar, C. Wu, Z. Yang and C. Y. Tang, Chem. Soc. Rev., 2024, 53, 4374–4399 RSC.
- M. Sun, X. Wang, L. R. Winter, Y. Zhao, W. Ma, T. Hedtke, J.-H. Kim and M. Elimelech, ACS ES&T Eng., 2021, 1, 725–752 Search PubMed.
- S. Uredat, A. Gujare, J. Runge, D. Truzzolillo, J. Oberdisse and T. Hellweg, Phys. Chem. Chem. Phys., 2024, 26, 2732–2744 RSC.
- D. J. Miller, D. R. Dreyer, C. W. Bielawski, D. R. Paul and B. D. Freeman, Angew. Chem., Int. Ed., 2017, 56, 4662–4711 CrossRef CAS PubMed.
- H. Wang, M. Wang, X. Liang, J. Yuan, H. Yang, S. Wang, Y. Ren, H. Wu, F. Pan and Z. Jiang, Chem. Soc. Rev., 2021, 50, 5468–5516 RSC.
- D. Yu, X. Xiao, C. Shokoohi, Y. Wang, L. Sun, Z. Juan, M. J. Kipper, J. Tang, L. Huang, G. S. Han, H. S. Jung and J. Chen, Adv. Funct. Mater., 2023, 33, 2211983 CrossRef CAS.
- Z. Wang, X. Luo, Z. Song, K. Lu, S. Zhu, Y. Yang, Y. Zhang, W. Fang and J. Jin, Nat. Commun., 2022, 13, 4169 CrossRef.
- S. Kim, H. Wang and Y. M. Lee, Angew. Chem., Int. Ed., 2019, 58, 17512–17527 CrossRef PubMed.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- A. Gugliuzza, Smart Membranes and Sensors, John Wiley & Sons, 2014 Search PubMed.
- S. Qutub, I. Bhat, B. Maatouk, B. Moosa, K. Nawaz, E. D. Galicia, A. Fakim, W. Lin, R. Grünberg, S. Arold and N. M. Khashab, Angew. Chem., Int. Ed., 2024, 63, e202403647 CrossRef PubMed.
- T. D. Bennett, F.-X. Coudert, S. L. James and A. I. Cooper, Nat. Mater., 2021, 20, 1179–1187 CrossRef PubMed.
- L. Hu, Q. Zhang, X. Li and M. J. Serpe, Mater. Horiz., 2019, 6, 1774–1793 RSC.
- M. Wang, M. Wang, H.-H. Lin, M. Ballabio, H. Zhong, M. Bonn, S. Zhou, T. Heine, E. Ćanovas, R. Dong and X. Feng, J. Am. Chem. Soc., 2020, 142, 21622–21627 CrossRef PubMed.
- A. J. R. Amaral and G. Pasparakis, Polym. Chem., 2017, 8, 6464–6484 RSC.
- Y. Li, Z. Guo, S. Li and B. Van der Bruggen, Adv. Mater. Interfaces, 2021, 8, 2001671 CrossRef.
- X. Lu and M. Elimelech, Chem. Soc. Rev., 2021, 50, 6290–6307 RSC.
- M. Elimelech and W. A. Philip, Science, 2011, 333, 712–717 CrossRef PubMed.
- T. E. Culp, B. Khara, K. P. Brickey, M. Geitner, T. J. Zimudzi, J. D. Wilbur, S. D. Jons, A. Roy, M. Paul, B. Ganapathysubramanian, A. L. Zydney, M. Kumar and E. D. Gomez, Science, 2021, 371, 72–75 CrossRef PubMed.
- F. Zhang, J. Fan and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 21840–21856 CrossRef.
- M. F. J. Solomon, Q. Song, K. E. Jelfs, M. M. Ibanez and A. G. Livingston, Nat. Mater., 2016, 15, 760–767 CrossRef.
- Y. Li, J. Zhu, S. Li, Z. Guo and B. Van der Bruggen, ACS Appl. Mater. Interfaces, 2020, 12, 31962–31974 CrossRef.
- D. Xu, J. Zheng, X. Zhang, D. Lin, Q. Gao, X. Luo, X. Zhu, G. Li, H. Liang and B. Van der Bruggen, Environ. Sci. Technol., 2022, 56, 1927–1937 CrossRef.
- A. Zhao, M. Zhang, Y. Bao, L. Zhao, G. Liu, Y. Jiang, P. Zhang and X. Gao, J. Membr. Sci., 2022, 664, 121081 CrossRef CAS.
- T. Xu, B. Wu, L. Hou, Y. Zhu, F. Sheng, Z. Zhao, Y. Dong, J. Liu, B. Ye, X. Li, L. Ge, H. Wang and T. Xu, J. Am. Chem. Soc., 2022, 144, 10220–10229 CrossRef PubMed.
- L. O. Alimi, B. Moosa, W. Lin and N. M. Khashab, ACS Mater. Lett., 2024, 6, 1467–1473 CrossRef.
- B. Moosa, L. O. Alimi, W. Lin, A. Fakim, P. M. Bhatt, M. Eddaoudi and N. M. Khashab, Angew. Chem., Int. Ed., 2023, 62, e202311555 CrossRef PubMed.
- L. O. Alimi, X. Liu, G. Zhang, B. Moosa and N. M. Khashab, Chem. Commun., 2024, 60, 10768–10771 RSC.
- L. F. Villalobos, T. Huang and K.-V. Peinemann, Adv. Mater., 2017, 29, 1606641 CrossRef PubMed.
- T. Huang, T. Puspasari, S. P. Nunes and K.-V. Peinemann, Adv. Funct. Mater., 2020, 30, 1906797 CrossRef.
- Z. Jiang, R. Dong, A. M. Evans, N. Biere, M. A. Ebrahim, S. Li, D. Anselmetti, W. R. Dichtel and A. G. Livingston, Nature, 2022, 609, 58–64 CrossRef.
- Y. Wang, R.-Z. Liang, T.-Z. Jia, X.-L. Cao, Q. Wang, J.-R. Cao, S. Li, Q. Shi, L. Isaacs and S.-P. Sun, J. Am. Chem. Soc., 2022, 144, 6483–6492 CrossRef.
- Y. Cheng, Y. Ying, S. Japip, S.-D. Jiang, T.-S. Chung, S. Zhang and D. Zhao, Adv. Mater., 2018, 30, 1802401 CrossRef.
- S. Sorribas, P. Gorgojo, C. Téllez, J. Coronas and A. G. Livingston, J. Am. Chem. Soc., 2013, 135, 15201–15208 CrossRef PubMed.
- X. Tan, S. Robijns, R. Thür, Q. Ke, N. De Wittes, A. Lamaire, Y. Li, I. Aslam, D. Van Havere, T. Donckels, T. Van Assche, V. Van Speybroeck, M. Dusselier and I. Vankelecom, Science, 2022, 378, 1189–1194 CrossRef.
- L. Xiang, L. Sheng, C. Wang, L. Zhang, Y. Pan and Y. Li, Adv. Mater., 2017, 29, 1606999 CrossRef.
- C. Kong, H. Du, L. Chen and B. Chen, Energy Environ. Sci., 2017, 10, 1812–1819 RSC.
- L. Xuan, L.-J. Tian, T. Tian, X.-M. Wang, D.-H. Yang and H.-Q. Yu, Sep. Purif. Technol., 2020, 251, 117381 CrossRef.
- Y. Inoue, Y. Atsumi, A. Kawamura and T. Miyata, J. Membr. Sci., 2019, 588, 117213 CrossRef.
- H. Liu, J. Zhu, L. Hao, Y. Jiang, B. van der Bruggen, A. Sotto, C. Gao and J. Shen, J. Membr. Sci., 2019, 587, 117163 CrossRef.
- G. Chen, C. Chen, Y. Guo, Z. Chu, Y. Pan, G. Liu, G. Liu, T. Han, W. Jin and N. Xu, Science, 2023, 381, 1350–1356 CrossRef PubMed.
- T. H. Lee, B. K. Lee, S. Y. Yoo, H. Lee, W.-N. Wu, Z. P. Smith and H. B. Park, Nat. Commun., 2023, 14, 8330 CrossRef PubMed.
- Y.-L. Ji, B.-X. Gu, H.-Q. Huo, S.-J. Xie, H. Peng, W.-H. Zhang, M.-J. Yin, B. Xiong, H. Lu, L. F. Villalobos, Q. Zhao, C.-J. Gao, M. Elimelech and Q.-F. An, Nat. Water., 2024, 2, 183–192 CrossRef.
- Q. Song, S. Jiang, T. Hasell, M. Liu, S. Sun, A. K. Cheetham, E. Sivaniah and A. I. Cooper, Adv. Mater., 2016, 28, 2629–2637 CrossRef PubMed.
- A. F. Bushell, P. M. Budd, M. P. Attfield, J. T. A. Jones, T. Hasell, A. I. Cooper, P. Bernardo, F. Bazzarelli, G. Clarizia and J. C. Jansen, Angew. Chem., Int. Ed., 2013, 52, 1253–1256 CrossRef CAS.
- G. Zhu, F. Zhang, M. P. Rivera, X. Hu, G. Zhang, C. W. Jones and R. P. Lively, Angew. Chem., Int. Ed., 2019, 58, 2638–2643 CrossRef CAS PubMed.
- E. J. Gachuz, M. C. Santilán, K. J. Moreno, J. M. Cornejo, A. M. Richa, A. Andrio, V. Compañ and J. D. M. Morales, Green Chem., 2020, 22, 5785–5797 RSC.
- H. Mei, H. Liu, Q. Shang, Y. Dong, S. P. Bjergaard, C. Huang and X. Shen, Green Chem., 2021, 23, 1782–1793 RSC.
- D. Zhao, J. F. Kim, G. Ignacz, P. Pogany, Y. M. Lee and G. Szekely, ACS Nano, 2019, 13, 125–133 CrossRef CAS PubMed.
- K. Kang, K.-H. Lee, Y. Han, H. Gao, S. Xie, D. A. Muller and J. Park, Nature, 2017, 550, 229–233 CrossRef.
- G.-M. Weng, J. Li, M. Alhabeb, C. Karpovich, H. Wang, J. Lipton, K. Maleski, J. Kong, E. Shaulsky, M. Elimelech, Y. Gogotsi and A. D. taylor, Adv. Funct. Mater., 2018, 28, 1803360 CrossRef.
- F.-X. Xiao, M. Pagliaro, Y.-J. Xu and B. Liu, Chem. Soc. Rev., 2016, 45, 3088–3121 RSC.
- E. Ahn, H. Gaiji, T. Kim, M. Abderrabba, H.-W. Lee and B.-S. Kim, J. Membr. Sci., 2019, 585, 191–198 CrossRef CAS.
- P. Chaturvedi, I. V. Vlassiouk, D. A. Cullen, A. J. Rondinone, N. V. Lavrik and S. N. Smirnov, ACS Nano, 2019, 13, 12109–12119 CrossRef CAS PubMed.
- N. D. Boscher, M. Wang, A. Perrotta, K. Heinze, M. Creatore and K. K. Gleason, Adv. Mater., 2016, 28, 7479–7485 CrossRef CAS PubMed.
- B. M. E. Alf, A. Asatekin, M. C. Barr, S. H. Baxamusa, H. Chelawat, G. Ozaydin-Ince, C. D. Petruczok, R. Sreenivasan, W. E. Tenhaeff, N. J. Trujillo, S. Vaddiraju, J. Xu and K. K. Gleason, Adv. Mater., 2010, 22, 1993–2027 CrossRef PubMed.
- X. Shi, Y. Ye, H. Wang, F. Liu and Z. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 38449–38458 CrossRef CAS.
- E. Pantuso, G. De Filpo and F. P. Nicoletta, Adv. Opt. Mater., 2019, 7, 1900252 CrossRef.
- A. Díaz-Moscoso and P. Ballester, Chem. Commun., 2017, 53, 4635–4652 RSC.
- A. H. Gelebart, D. J. Mulder, G. Vantomme, A. P. H. J. Schenning and D. J. Broer, Angew. Chem., Int. Ed., 2017, 56, 13436–13439 CrossRef CAS.
- S. Hussain and X. Peng, Sep. Purif. Technol., 2022, 278, 119528 CrossRef CAS.
- M. Ikbal, R. Banerjee, S. Barman, S. Atta, D. Dhara and N. D. P. Singh, J. Mater. Chem. C, 2014, 2, 4622–4630 RSC.
- Z. Zhou, I.-C. Chen, L. M. Rehman, A. M. Aboalsaud, D. B. Shinde, L. Cao, Y. Zhang and Z. Lai, Sci. Adv., 2022, 8, eabo2929 CrossRef CAS.
- L. Ren, J. Chen, Q. Lu, J. Han and H. Wu, ACS Appl. Mater. Interfaces, 2020, 12, 52050–52058 CrossRef CAS.
- H.-Q. Liang, Y. Guo, Y. Shi, X. Peng, B. Liang and B. Chen, Angew. Chem., Int. Ed., 2020, 59, 7732–7737 CrossRef CAS.
- R. Klajn, Chem. Soc. Rev., 2014, 43, 148–184 RSC.
- Q. Guo, Z. Lai, X. Zuo, W. Xian, S. Wu, L. Zheng, Z. Dai, S. Wang and Q. Sun, Nat. Commun., 2023, 14, 6702 CrossRef CAS.
- J. Wang, Z. Song, M. He, Y. Qian, D. Wang, Z. Cui, Y. Feng, S. Li, B. Huang, X. Kong, J. Han and L. Wang, Nat. Commun., 2024, 15, 2125 CrossRef CAS PubMed.
- Y. Zhou, T. Xiong, J. Lu, P. Yu, Y. Jiang, F. Xia and L. Mao, Angew. Chem., Int. Ed., 2023, 62, e202302997 CrossRef CAS PubMed.
- F. Khamis, H. M. Hegab, F. Banat, H. A. Arafat and S. W. Hasan, Chem. Eng. J., 2023, 474, 145471 CrossRef CAS.
- A. Tufani and G. O. Ince, J. Membr. Sci., 2017, 537, 255–262 CrossRef CAS.
- P. Gao, J. Huang, E. Pakkaner, J. Wagemans, S. Eyley, W. Thielemans, R. Gijsbers, M. Smet and X. Yang, Chem. Eng. J., 2023, 476, 146700 CrossRef CAS.
- E. A. Jackson and M. A. Hillmyer, ACS Nano, 2010, 4, 3548–3553 CrossRef CAS.
- S. Rangou, K. Buhr, V. Filiz, J. I. Clodt, B. Lademann, J. Hahn, A. Jung and V. Abetz, J. Membr. Sci., 2014, 451, 266–275 CrossRef CAS.
- B. Ma, X.-J. Ju, F. Luo, Y.-Q. Liu, Y. Wang, Z. Liu, W. Wang, R. Xie and L.-Y. Chu, ACS Appl. Mater. Interfaces, 2017, 9, 14409–14421 CrossRef CAS.
- L. Wang, D. Rehman, P.-F. Sun, A. Deshmukh, L. Zhang, Q. Han, Z. Yang, Z. Wang, H.-D. Park, J. H. Lienhard and C. Y. Tang, ACS Appl. Mater. Interfaces, 2021, 13, 16906–16915 CrossRef CAS.
- X.-X. Fan, R. Xie, Q. Zhao, X.-Y. Li, X.-J. Ju, W. Wang, Z. Liu and L.-Y. Chu, J. Membr. Sci., 2018, 555, 20–29 CrossRef CAS.
- T. V. Plisko, A. V. Penkova, K. S. Burts, A. V. Bildyukevich, M. E. Dmitrenko, G. B. Melnikova, R. R. Atta, A. S. Mazur, A. A. Zolotarev and A. B. Missyul, J. Membr. Sci., 2019, 580, 336–349 CrossRef CAS.
- B. Saini, S. Khuntia and M. K. Sinha, J. Membr. Sci., 2019, 572, 184–197 CrossRef CAS.
- H. Ye, C. Gao, G. Yang, Y. Zhou, R. Jiao, Y. Zhang, L. Zhao, Q. Xin and H. Li, J. Membr. Sci., 2022, 641, 119849 CrossRef CAS.
- D. Cho, J.-S. Jang, S.-H. Nam, K. Ko, W. Hwang, J.-K. Jung, J. Lee, M. Choi, J.-W. Hong, I.-D. Kim and S. Jeon, ACS Nano, 2020, 14, 12173–12183 CrossRef CAS.
- A. Samanta, H. Chen, P. Samanta, S. Popov, I. Sychugov and L. A. Berglund, ACS Appl. Mater. Interfaces, 2021, 13, 3270–3277 CrossRef CAS.
- Z. Chen, J. Liu, Y. Chen, X. Zheng, H. Liu and H. Li, ACS Appl. Mater. Interfaces, 2021, 13, 1353–1366 CrossRef CAS PubMed.
- C. Bar, N. Cağlar, M. Uz, S. K. Mallapragada and S. A. Altinkaya, ACS Appl. Mater. Interfaces, 2019, 11, 31367–31377 CrossRef CAS PubMed.
- Z. Sun, L. Li, Q. Wu, Z. Zhang, L. Yang, G. Jiang, C. Gao and L. Xue, J. Membr. Sci., 2022, 656, 120609 CrossRef CAS.
- R. Xie, F. Luo, L. Zhang, S.-F. Guo, Z. Liu, X.-J. Ju, W. Wang and L.-Y. Chu, Small, 2018, 14, 1703650 CrossRef.
- Y. Wang, Z. Zhang, T. Li, P. Ma, H. Zhang, M. Chen, M. Du and W. Dong, ACS Appl. Mater. Interfaces, 2019, 11, 44886–44893 CrossRef CAS.
- Y. Pan, Y. Liu, S. Yang, C. Zhang and Z. Ullah, Nanotechnol. Rev., 2023, 12, 20220538 CrossRef CAS.
- J. Liu, S. Wang, T. Huang, P. Manchanda, E. Abou-Hamad and S. P. Nunes, Sci. Adv., 2020, 6, eabb3188 CrossRef CAS.
- Z. Wang, N. Sikdar, S.-Q. Wang, X. Li, M. Yu, X.-H. Bu, Z. Chang, X. Zou, Y. Chen, P. Cheng, K. Yu, M. J. Zaworotko and Z. Zhang, J. Am. Chem. Soc., 2019, 141, 9408–9414 CrossRef CAS.
- A. He, Z. Jiang, Y. Wu, H. Hussain, J. Rawle, M. E. Briggs, M. A. Little, A. G. Livingston and A. I. Cooper, Nat. Mater., 2022, 21, 463–470 CrossRef CAS.
- S.-H. Liu, J.-H. Zhou, C. Wu, P. Zhang, X. Cao and J.-K. Sun, Nat. Commun., 2024, 15, 2478 CrossRef CAS.
- J. Widakdo, H. F. M. Austria, T. M. Subrahmanya, E. Suharyadi, W.-S. Hung, C.-F. Wang, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Mater. Chem. A, 2022, 10, 16743–16760 RSC.
- C. Liu, Y. Jiang, C. Zhou, J. Caro and A. Huang, J. Mater. Chem. A, 2018, 6, 24949–24955 RSC.
- Z. Wang, A. Khebel, S. Grosjean, D. Wagner, S. Bräse, C. Wöl, J. Caro and L. Heinke, Nat. Commun., 2015, 7, 13872 CrossRef.
- J. Liu, W. Duan, J. Song, X. Guo, Z. Wang, X. Shi, J. Liang, J. Wang, P. Cheng, Y. Chen, M. J. Zaworotko and Z. Zhang, J. Am. Chem. Soc., 2019, 141, 12064–12070 CrossRef CAS.
- E. Pantuso, E. Ahmed, E. Fontananova, A. Brunetti, I. Tahir, D. Karothu, N. A. Alnji, G. Dushaq, M. Rasras, P. Naumov and G. D. Profio, Nat. Commun., 2023, 14, 5751 CrossRef CAS PubMed.
- Y. Wang, S. Yang, J. Zhang, Z. Chen, B. Zhu, J. Li, S. Liang, Y. Bai, J. Xu, D. Rao, L. Dong, C. Zhang and X. Yang, Nat. Commun., 2023, 14, 1108 CrossRef CAS.
- T. T. Tung, M. J. Nine, M. Krebsz, T. Pasinszki, C. J. Coghlan, D. N. H. Tran and D. Losic, Adv. Funct. Mater., 2017, 27, 1702891 CrossRef.
- B. L. Li, J. Wang, H. L. Zou, S. Garaj, C. T. Lim, J. Xie, N. B. Li and D. T. Leong, Adv. Funct. Mater., 2016, 26, 7034–7056 CrossRef CAS.
- J. Wang, W. Lin, Z. Chen, V. O. Nikolaeva, L. O. Alimi and N. M. Khashab, Nat. Commun., 2024, 15, 1575 CrossRef CAS.
- T. Li, L. Li, H. Sun, Y. Xu, X. Wang, H. Luo, Z. Liu and T. Zhang, Adv. Sci., 2017, 4, 1600404 CrossRef.
- Q. Yu, X. Yang, Y. Chen, K. Yu, J. Gao, Z. Liu, P. Cheng, Z. Zhang, B. Aguila and S. Ma, Angew. Chem., Int. Ed., 2018, 57, 10192–10196 CrossRef CAS PubMed.
- E. Lin, Z. Wang, X. Zhao, Z. Liu, D. Yan, F. Jin, Y. Chen, P. Cheng and Z. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202117390 CrossRef CAS.
- L. O. Alimi, F. Fang, B. Moosa, Y. Ding and N. M. Khashab, Angew. Chem., Int. Ed., 2022, 61, e202212596 CrossRef CAS.
- P. Liu, F. Fang, L. O. Alimi, B. A. Moosa, X. Zhu, X. Liu, H. Wang and N. M. Khashab, Chem, 2024, 10, 1–15 Search PubMed.
- X. Liu, J. Li, B. Gui, G. Lin, Q. Fu, S. Yin, X. Liu, J. Sun and C. Wang, J. Am. Chem. Soc., 2021, 143, 2123–2129 CrossRef CAS.
- T. Mao, Z. Liu, X. Guo, Z. Wang, J. Liu, T. Wang, S. Geng, Y. Chen, P. Cheng and Z. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202216318 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |