Open Access Article
Open Access ArticleModulation of the binding sites for an adaptable DNA interactive probe: efficient chromo-fluorogenic recognition of Al3+ and live cell bioimaging†
Atanu
Maji
,
Debarpan
Mitra
,
Amitav
Biswas
,
Moumita
Ghosh
,
Rahul
Naskar
,
Saswati
Gharami Nabendu Murmu
 and
Tapan K.
Mondal
and
Tapan K.
Mondal
 *
*
Department of Chemistry, Jadavpur University, Kolkata-700032, India. E-mail: tapank.mondal@jadavpuruniversity.in
First published on 18th September 2024
Abstract
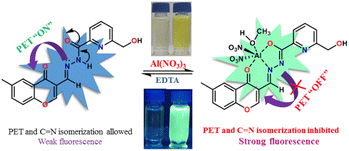
Herein, a chromone-based simple reversible fluorescent “turn-on” probe, HMCP [6-(hydroxymethyl)-N′-((6-methyl-4-oxo-4H-chromen-3-yl)methylene)picolinohydrazide], was successfully utilized to detect Al3+ over a group of other coexisting metal cations in MeOH/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (HEPES buffer, pH = 7.2). The “turn on” emission response along with the effective enhancement of the fluorescence intensity upon addition of Al3+ can be attributed to the inhibition of photo-induced electron transfer (PET) and C
1, v/v) (HEPES buffer, pH = 7.2). The “turn on” emission response along with the effective enhancement of the fluorescence intensity upon addition of Al3+ can be attributed to the inhibition of photo-induced electron transfer (PET) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, as well as the initiation of chelation-enhanced-fluorescence (CHEF). The HMCP sensor binds Al3+ in a 1
N isomerization, as well as the initiation of chelation-enhanced-fluorescence (CHEF). The HMCP sensor binds Al3+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with an excellent binding constant and good detection limit on the orders of 103 M−1 and 10−7 M, respectively. The mode of binding interaction between HMCP with Al3+ was evidenced by 1H NMR titration, HRMS, and Job's plot analyses. Theoretical calculations and molecular logic gate applications were also used to demonstrate the binding mode. A DNA binding study was also executed to elucidate the possible bioactivity of the probe and found that HMCP interacts with DNA more effectively than the other analogues studied. Furthermore, the applicability of the probe in a live cell imaging study indicated that HMCP is highly efficient for the detection of exogenous Al3+ in living cells. In addition, real water sample analysis and a dip-stick experiment demonstrate that the probe can be used in a wide range of practical and convenient applications.
1 stoichiometry with an excellent binding constant and good detection limit on the orders of 103 M−1 and 10−7 M, respectively. The mode of binding interaction between HMCP with Al3+ was evidenced by 1H NMR titration, HRMS, and Job's plot analyses. Theoretical calculations and molecular logic gate applications were also used to demonstrate the binding mode. A DNA binding study was also executed to elucidate the possible bioactivity of the probe and found that HMCP interacts with DNA more effectively than the other analogues studied. Furthermore, the applicability of the probe in a live cell imaging study indicated that HMCP is highly efficient for the detection of exogenous Al3+ in living cells. In addition, real water sample analysis and a dip-stick experiment demonstrate that the probe can be used in a wide range of practical and convenient applications.
Introduction
Materials comprising of multiple applications are important and an emerging field in modern science. A Schiff base is one such kind of material. The low cost, user-friendliness and speed of use of Schiff bases has led to attempts to use them for sustainable development in pharmacy, medicine, molecular memory storage, chemical synthesis and analysis, photochromic materials and in colorimetric and fluorometric chemosensors.1–6 Several analytical scientific techniques have been developed for the recognition of metal ions including spectrometry, chromatography, spectrophotometry, titrimetry and electrochemical strategies.7,8 The above mentioned methods are complicated, lengthy and costly. Of the various techniques utilized for metal ion recognition, fluorescence signaling is one of the first preferences as it is reliable, simple, rapid as well as profoundly sensitive for the recognition of environmentally and biologically important metal ions.9,10Aluminum is the third most abundant metal of all elements (after oxygen and silicon) and the most plentiful (8.3% by weight) metallic element in the Earth's crust. The extensive use of aluminum in our daily life appears in diverse areas, for example, in cosmetics, food packaging, medicine, water purification systems, food additives and electronic devices. Thus, the probability of human exposure to aluminum has increased, leading to a serious concern in recent years.11 In addition, the concentration of Al3+ in soil and water resources has dramatically increased due to acid rain and thus inhibits the growth of plant and aquatic ecosystems.12–20 As directed by the WHO (World Health Organization), the weekly permissible intake limit of Al3+ by human beings is around 7 mg kg−1 of body weight.21 On the other hand, long-term intake of excess Al3+ could cause major diseases, for example, Alzheimer's, Parkinson's, kidney stones, osteoporosis, cardiac arrest, anemia, headaches, rickets, dementia and many more.22–27 Doctors refers to Al3+ as the “silent killer” in human body because of possible connection to the brain.28 Therefore, because of the negative impact of the excessive presence of Al3+ in the human body and environment, it is of great importance to develop some noteworthy tools with excellent properties in order to specifically recognise Al3+, and to therefore regulate its environmental effects. DNA is one of the most important biomacromolecules in living beings that records hereditary traits. It controls the biosynthesis of various enzymes and proteins via replication and transcription. Therefore, DNA is the prime intracellular target of most hazardous substances and drugs. In general, duplex DNA binds with small molecules through various noncovalent interactions.29 Due to the non-fluorescent nature of DNA, the main physiochemical experiments to investigate the interaction of small molecules with DNA are absorption and emission spectroscopy. A better understanding of the interactions of small molecules with DNA are in great demand in recent times and further encourage the pursuit of applications such as DNA molecular probes as well as chemotherapeutic reagents.30–32
The construction of semiconductors based on molecular logic gate functions is of specific interest, and they are extensively used in modern computing, in which the relationship between the input and output might be demonstrated by a truth table, where “1” denotes an active input/output and “0” as inactive one. Since, de Silva et al. developed the molecular AND logic circuit for the first time,33 many research groups have utilized molecular logic gates in different applications.34,35 For applications in data storage, many molecular logic gates have been constructed based on the specific recognition and detection of cations and anion.36
To date, many research groups have reported fluorescent sensors based on different fluorophore units for the specific recognition of Al3+. For example, S. L. Hu et al. developed a simple pyrazoline-based fluorescent probe for Al3+ in aqueous solution that exhibited fluorescence quenching.37 Zhang et al. developed a highly sensitive naphthalimide-based sensor (NPP) which detects Al3+ with a significant enhancement in fluorescence intensity and a detection limit that is determined to be 39 nM. Meanwhile, Wang et al. fabricated a tetrastyrene based fluorescence probe (L) showing a low detection limit of 1.587 × 10−7 M for Al3+ in a methanol–water (9/1, v/v) mixed solvent. Both probes show strong fluorescence emission in living cells.38,39 Recently, Hwang et al. reported a simple Schiff base probe for live cell imaging studies which selectively binds with aluminum with a very low detection limit of 290 nM.40 In 2022, Das et al. reported a chromone-based fluorescent switch for multi-analyte detection which displayed a prominent “turn-on” fluorescence response for Al3+ (LOD = 6.9 μM) in an aqueous medium.41 Liu et al. synthesized a chromone derivative for the sole detection of Al3+ in EtOH with a detection limit of 0.214 μM.42
Considering the HSAB (hard and soft acids and bases) principle where the hard acid Al3+ always prefer to bind with the hard coordination sphere of donor atoms like “O” or “N” at suitable position to form stable chelate complex, we intend to introduce a very simple and low cost novel chromone-based probe, HMCP. The probe HMCP shows higher sensitivity over its analogues towards Al3+ in MeOH/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (HEPES buffer, pH = 7.2) based on PET and C
1, v/v) (HEPES buffer, pH = 7.2) based on PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization as well as the CHEF mechanism. Upon titration with EDTA, the probe, HMCP, shows a reversible nature due to the dissociation of the HMCP-Al3+ complex followed by formation of a stable complex Na[Al(EDTA)]. Experimental outcomes are well verified using theoretical calculations. The possible bioactivity of HMCP was also examined through DNA binding studies. Cell imaging using breast cancer cells as well as real sample analysis have also been carried out to display some extensive potential practical utilization of the probe (HMCP).
N isomerization as well as the CHEF mechanism. Upon titration with EDTA, the probe, HMCP, shows a reversible nature due to the dissociation of the HMCP-Al3+ complex followed by formation of a stable complex Na[Al(EDTA)]. Experimental outcomes are well verified using theoretical calculations. The possible bioactivity of HMCP was also examined through DNA binding studies. Cell imaging using breast cancer cells as well as real sample analysis have also been carried out to display some extensive potential practical utilization of the probe (HMCP).
Results and discussion
Synthesis of HMCP and its analogues
The synthetic path towards HMCP and its analogues (MCMP and MCMB) are depicted in Scheme 1. 6-(Hydroxymethyl)picolinohydrazide, picolinohydrazide and benzohydrazide were synthesized using reported literature procedures.43–46 Reflux condensation of 6-methyl-4-oxo-4H-chromene-3-carbaldehyde and 6-(hydroxymethyl)picolinohydrazide in ethanol for 4 h affords the desired compound HMCP with a high yield (86%). In the same way, the analogues MCMP and MCMB were fabricated through the reflux condensation of 6-methyl-4-oxo-4H-chromene-3-carbaldehyde with picolinohydrazide and 6-methyl-4-oxo-4H-chromene-3- carbaldehyde with benzohydrazide in ethanol for 3 h with yields of 83% and 85%, respectively. The chemical structures of HMCP, MCMP and MCMB were confirmed using 1H NMR, 13C NMR and ESI mass spectrometry (Fig. S1–S9†).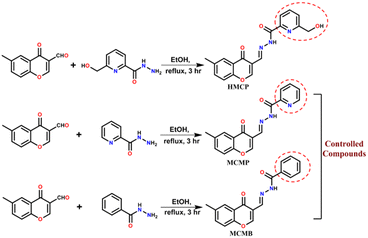 | ||
| Scheme 1 Synthetic scheme of the synthesis of the investigated probe, HMCP, and its analogues (MCMP & MCMB). | ||
Sensing studies of HMCP and its analogues using UV-vis spectroscopy
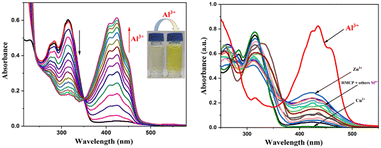
In order to investigate the interaction of HMCP with Al3+ in depth, UV-visible and emission studies were carried out in MeOH/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at 25 °C at physiological pH (10 mM HEPES buffer, pH = 7.2). The sensor HMCP displays a moderate absorbance band at 315 nm along with a shoulder peak at 283 nm. Upon incremental addition of Al3+ into the solution of HMCP, the intensity of the former absorbance peaks at 315 nm and 283 nm were gradually decreased and the simultaneous emergence of a new absorption band at 424 nm along with two closely situated bands at 408 nm and 448 nm were noticed with the formation of a distinct isosbestic point at 351 nm (Fig. 1). The appearance of new absorption bands at 424 nm along with two small shoulders at 408 nm and 448 nm and the distinct color change is supporting evidence of the complexation of Al3+ to HMCP. To establish the selectivity of the probe (HMCP), the UV-visible spectra of HMCP were recorded in presence of other metal cations such as Na+, Mg2+, Mn2+, Ba2+, Bi3+, La3+, Ce3+, Ga3+, In3+, Fe3+, Cr3+, Co2+, Ni2+, Hg2+, Zn2+, Cu2+, Pb2+ and Cd2+. No significant absorbance change was observed in the presence of any of the other metal cations, except only for Zn2+ and Cu2+ (Fig. 1). The absorption spectra of MCMP and MCMB were also studied. The probe MCMP itself shows a strong absorption band at 313 nm along with a small shoulder at 281 nm. In presence of Al3+, the absorption intensity at 313 nm and 281 nm gradually decreases followed by the appearance of a new absorption band at 422 nm with two closely situated small humps (Fig. S10†). Similarly, upon addition of Al3+ into the probe solution of MCMB, the absorption band at 310 nm for the free probe MCMB gradually decreases along with the appearance of a new band at 412 nm with a small hump at 398 nm. These outputs confirm the coordination of Al3+ to the analogues (MCMP and MCMB). Two distinct isosbestic points at 347 nm and 354 nm were also noticed for MCMP and MCMB, respectively. In the presence of other metal ions, the absorption spectra of both the analogues were recorded and it was noted that none of the abovementioned metal ions caused any noteworthy changes in the emission profile of the analogues, except Zn2+ and Cu2+. Therefore, HMCP and its analogues (MCMP and MCMB) are highly selective towards Al3+.
1, v/v) at 25 °C at physiological pH (10 mM HEPES buffer, pH = 7.2). The sensor HMCP displays a moderate absorbance band at 315 nm along with a shoulder peak at 283 nm. Upon incremental addition of Al3+ into the solution of HMCP, the intensity of the former absorbance peaks at 315 nm and 283 nm were gradually decreased and the simultaneous emergence of a new absorption band at 424 nm along with two closely situated bands at 408 nm and 448 nm were noticed with the formation of a distinct isosbestic point at 351 nm (Fig. 1). The appearance of new absorption bands at 424 nm along with two small shoulders at 408 nm and 448 nm and the distinct color change is supporting evidence of the complexation of Al3+ to HMCP. To establish the selectivity of the probe (HMCP), the UV-visible spectra of HMCP were recorded in presence of other metal cations such as Na+, Mg2+, Mn2+, Ba2+, Bi3+, La3+, Ce3+, Ga3+, In3+, Fe3+, Cr3+, Co2+, Ni2+, Hg2+, Zn2+, Cu2+, Pb2+ and Cd2+. No significant absorbance change was observed in the presence of any of the other metal cations, except only for Zn2+ and Cu2+ (Fig. 1). The absorption spectra of MCMP and MCMB were also studied. The probe MCMP itself shows a strong absorption band at 313 nm along with a small shoulder at 281 nm. In presence of Al3+, the absorption intensity at 313 nm and 281 nm gradually decreases followed by the appearance of a new absorption band at 422 nm with two closely situated small humps (Fig. S10†). Similarly, upon addition of Al3+ into the probe solution of MCMB, the absorption band at 310 nm for the free probe MCMB gradually decreases along with the appearance of a new band at 412 nm with a small hump at 398 nm. These outputs confirm the coordination of Al3+ to the analogues (MCMP and MCMB). Two distinct isosbestic points at 347 nm and 354 nm were also noticed for MCMP and MCMB, respectively. In the presence of other metal ions, the absorption spectra of both the analogues were recorded and it was noted that none of the abovementioned metal ions caused any noteworthy changes in the emission profile of the analogues, except Zn2+ and Cu2+. Therefore, HMCP and its analogues (MCMP and MCMB) are highly selective towards Al3+.
Sensing studies of HMCP and its analogues using emission spectroscopy
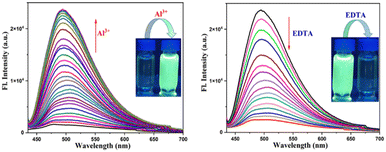
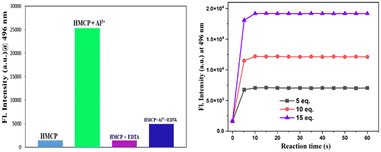
The fluorescence spectral pattern revealed that upon excitation at 351 nm, the HMCP probe displays a broad centered emission band at 471 nm with very low intensity. This very low fluorescence intensity can be ascribed to the combined effects of PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization via non-radiative decay processes from the excited state. The sequential addition of Al3+ into the probe solution resulted in a significant enhancement in the fluorescence intensity (∼14 fold) at around 496 nm with an increase in the quantum yield (φ) from 0.03 to 0.21 (Fig. 2). The “turn-on” emission response of HMCP towards Al3+ could be explained on the basis of the strong binding affinity of Al3+ with HMCP, which prohibited both the C
N isomerization via non-radiative decay processes from the excited state. The sequential addition of Al3+ into the probe solution resulted in a significant enhancement in the fluorescence intensity (∼14 fold) at around 496 nm with an increase in the quantum yield (φ) from 0.03 to 0.21 (Fig. 2). The “turn-on” emission response of HMCP towards Al3+ could be explained on the basis of the strong binding affinity of Al3+ with HMCP, which prohibited both the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and PET processes in the receptor, thereby initiating increasing rigidity in the complex resulting in chelation enhanced fluorescence (CHEF). The change in the fluorescence properties of HMCP was examined in the presence of other coexisting metal ions, but there is hardly any change in the fluorescence intensity of HMCP (Fig. 2). Therefore, the receptor, HMCP is highly efficient at detecting Al3+ fluorimetrically with a significant emission enhancement followed by a prominent fluorescence color change from colorless to intense cyan. This fluorescence enhancement falls under the category of strong “OFF–ON” fluorescence properties. Further, on sequential addition of EDTA to the solution of HMCP-Al3+, the fluorescence intensities at 484 nm as well as at 466 nm reverted back to the original emission intensity of the probe (HMCP) itself which clearly establishes the fact that HMCP has returned to its free state (Fig. 3) thereby making the sensor a reversible fluorescent switch. Hence, HMCP can serve as an “OFF–ON–OFF” fluorescence signaling sensor.
N isomerization and PET processes in the receptor, thereby initiating increasing rigidity in the complex resulting in chelation enhanced fluorescence (CHEF). The change in the fluorescence properties of HMCP was examined in the presence of other coexisting metal ions, but there is hardly any change in the fluorescence intensity of HMCP (Fig. 2). Therefore, the receptor, HMCP is highly efficient at detecting Al3+ fluorimetrically with a significant emission enhancement followed by a prominent fluorescence color change from colorless to intense cyan. This fluorescence enhancement falls under the category of strong “OFF–ON” fluorescence properties. Further, on sequential addition of EDTA to the solution of HMCP-Al3+, the fluorescence intensities at 484 nm as well as at 466 nm reverted back to the original emission intensity of the probe (HMCP) itself which clearly establishes the fact that HMCP has returned to its free state (Fig. 3) thereby making the sensor a reversible fluorescent switch. Hence, HMCP can serve as an “OFF–ON–OFF” fluorescence signaling sensor.
From the fluorescence spectral changes, the detection limit of HMCP for Al3+ was determined using the equation, LOD = K × SD/S, where “SD” is the standard deviation of the blank solution of the probe and “S” in the slope of the curve. Thus, the LOD was found to be (3.12 ± 0.12) × 10−7 (M) (Fig. S13†). This low detection limit evidently demonstrates the fact that HMCP is highly efficient in detecting Al3+ at very minute concentrations. The association constant (Ka) of HMCP for Al3+ was also calculated using the Benesi–Hildebrand equation and found to be (9.34 ± 0.45) × 103 M−1 (Fig. S14†), which signifies that the HMCP-Al3+ complex is notably stable.
To better comprehend the excited state stability of this fluorescent switch, the lifetimes of HMCP and HMCP-Al3+ were also measured. The fluorescence lifetime of the free probe (HMCP) shows a mono-exponential decay pattern and was found to be 1.12 ns while the HMCP-Al3+ complex exhibits a bi-exponential decay (measured at λem = 351 nm) with a lifetime value of 4.24 ns. The increase in lifetime is attributed to the decrease in non-radiative decay due to the structural rigidity of the complex relative to the comparatively more flexible free probe. On the other hand, after the addition of EDTA, the lifetime of HMCP-Al3+ again decreases and was found to be 1.53 ns, suggesting that the dissociation of the complex to the free HMCP had occurred (Fig. S15 and Table S4†).
In order to investigate the specificity of the probe towards Al3+, the fluorescence intensity of the probe solution containing other metal cations was recorded separately before and after the addition of the Al3+ ions. It is observed from the competitive experiment that the fluorescence enhancement of HMCP upon addition of Al3+ is not significantly affected by the presence of other metal cations (Fig. S16†). Therefore, HMCP is highly specific towards Al3+.
pH study
pH titration experiments were performed on HMCP using a fluorescence method and the titration indicated the fact that the newly developed fluorescent probe HMCP does not experience any major change in its fluorescence profile at 496 nm. This finding suggests that the sensor, HMCP, operates independently of the pH. Now, with the presence of Al3+ in the HMCP solution, the fluorescence intensity increased gradually with an increase in the pH value, achieving maximum intensity at pH 7.2. This reveals that the probe is compatible for sensing Al3+ at neutral pH (Fig. S20†). After pH 7.2 was reached, the emission intensity gradually decreased due to the deprotonation of the –OH group, causing the dissociation of the complex, which eventually results in rendering HMCP unable to sense Al3+. Hence, the newly designed probe HMCP is highly capable of detecting Al3+ in the neutral pH range with an excellent efficacy.Recyclability and reusability
An ideal chemosensor must have two essential properties, recyclability and reusability. To examine the reversibility, a solution of HMCP was prepared in MeOH/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (HEPES buffer, pH = 7.2). Fig. S14† reveals that upon the addition of Al3+ to the HMCP solution, a “turn on” emission was observed. Subsequently, the addition of EDTA solution into the HMCP-Al3+ complex solution gives a “turn off” emission as the complex is dissociated due to the formation of a stable Na[Al(EDTA)] complex, releasing a free receptor. This observation signifies that the sensing capability of HMCP is reversible. To further confirm the reversible nature, the experiment was performed using a MeOH solution of HMCP for a second time. Upon further addition of Al3+ ions, the solution again gives a “turn on” emission and the sequential addition of EDTA shows a “turn off” emission response. Thus, an increase in the emission intensity of the HMCP solution upon addition of Al3+ and a quenching in the fluorescence intensity of the HMCP-Al3+ complex in the presence of EDTA was observed over four cycles, with little loss in sensing efficacy (Fig. S17†). Such reversibility and regeneration are important for the design of devices to recognize Al3+ ions.
1, v/v) (HEPES buffer, pH = 7.2). Fig. S14† reveals that upon the addition of Al3+ to the HMCP solution, a “turn on” emission was observed. Subsequently, the addition of EDTA solution into the HMCP-Al3+ complex solution gives a “turn off” emission as the complex is dissociated due to the formation of a stable Na[Al(EDTA)] complex, releasing a free receptor. This observation signifies that the sensing capability of HMCP is reversible. To further confirm the reversible nature, the experiment was performed using a MeOH solution of HMCP for a second time. Upon further addition of Al3+ ions, the solution again gives a “turn on” emission and the sequential addition of EDTA shows a “turn off” emission response. Thus, an increase in the emission intensity of the HMCP solution upon addition of Al3+ and a quenching in the fluorescence intensity of the HMCP-Al3+ complex in the presence of EDTA was observed over four cycles, with little loss in sensing efficacy (Fig. S17†). Such reversibility and regeneration are important for the design of devices to recognize Al3+ ions.
A chemosensor with a rapid fluorescence response is always highly preferable for practical purposes. Time-dependent experiments were executed in order to interpret the instant emission response of the HMCP sensor. At different time intervals, the emission intensity was monitored in the presence of different concentrations of Al3+ (5 μM, 10 μM, and 15 μM). Fig. 3 shows that our investigated probe HMCP is highly efficient at detecting Al3+ within a very short time span (0–10 seconds) after the addition of the Al3+ ions. 10 seconds after Al3+ addition, the emission intensity at 496 nm almost reached its saturation level, which signifies completion of the reaction. Hence HMCP can serve as a powerful and reliable sensor for the recognition of aluminum ions within a short time period (0–10 s).
Photosensitivity study
For fluorescence based sensing applications, a probe that has high photo-stability whilst exhibiting steady analytical signals is highly preferable. Therefore, in order to evaluate the photosensitivity of the newly designed sensor, HMCP and the HMCP-Al3+ complex were irradiated over 2 min in day light under optimal conditions according to the previously reported literature.47,48 Fig. S22† indicates that the sensor HMCP and complex HMCP-Al3+ had high photostability throughout the experiment (2 min) with negligible changes in emission intensity. Therefore, these experimental results suggest that HMCP has a highly photostable “turn-on” fluorescence signal response in the presence of Al3+, making it highly competent for the spectrofluorimetric analysis of real samples.Probable sensing mechanism of HMCP
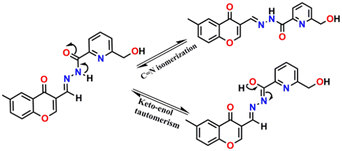
The enhancement in the emission intensity of HMCP and its analogues in the presence of Al3+ is possibly accredited to the combined effect of two important mechanisms, PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 5). These mechanisms are on in the free probes, but after binding with Al3+, these two mechanisms were inhibited and rigidity in the complex is induced, thus accounting for the enhancement in fluorescence intensity. In addition, due to the presence of several oxygen and nitrogen donor centres, it was anticipated that the probe and its analogues have strong affinity for chelation with the hard acid Al3+, according to the HSAB principle.
N isomerization (Fig. 5). These mechanisms are on in the free probes, but after binding with Al3+, these two mechanisms were inhibited and rigidity in the complex is induced, thus accounting for the enhancement in fluorescence intensity. In addition, due to the presence of several oxygen and nitrogen donor centres, it was anticipated that the probe and its analogues have strong affinity for chelation with the hard acid Al3+, according to the HSAB principle.
The nature of the binding mode of the chemosensor HMCP towards Al3+ was investigated via a 1H NMR titration study. The signal at δ = 12.98 ppm can be assigned to the proton (Hb) of the –NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit (Fig. 4). Upon interaction with the Al3+ ions, the Hb proton of the amide unit gradually shortens and meanwhile a new peak is noticed at 9.23 ppm due to the conversion of the amide into an imidic acid tautomer (–(N
O unit (Fig. 4). Upon interaction with the Al3+ ions, the Hb proton of the amide unit gradually shortens and meanwhile a new peak is noticed at 9.23 ppm due to the conversion of the amide into an imidic acid tautomer (–(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OH))–).45 Furthermore, the imine proton (Ha) peak at 6.78 ppm underwent a slight down-field shift, thereby confirming the participation of the imine nitrogen atom in complex formation. The HRMS spectrum of HMCP exhibits a peak at m/z 360.0491, possibly due to the [HMCP + Na]+ ion, whereas the HMCP-Al3+ complex exhibits a peak at m/z 542.0648 indicating the formation of a mononuclear complex between HMCP and Al3+ (Fig. S21†). The binding stoichiometry of HMCP to Al3+ (1
C(OH))–).45 Furthermore, the imine proton (Ha) peak at 6.78 ppm underwent a slight down-field shift, thereby confirming the participation of the imine nitrogen atom in complex formation. The HRMS spectrum of HMCP exhibits a peak at m/z 360.0491, possibly due to the [HMCP + Na]+ ion, whereas the HMCP-Al3+ complex exhibits a peak at m/z 542.0648 indicating the formation of a mononuclear complex between HMCP and Al3+ (Fig. S21†). The binding stoichiometry of HMCP to Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was further supported by a Job's plot analysis (Fig. S18†). Therefore, the 1H NMR, HRMS, and Job's plot analyses gave strong evidence for the proposed binding mode depicted in Fig. 6.
1) was further supported by a Job's plot analysis (Fig. S18†). Therefore, the 1H NMR, HRMS, and Job's plot analyses gave strong evidence for the proposed binding mode depicted in Fig. 6.
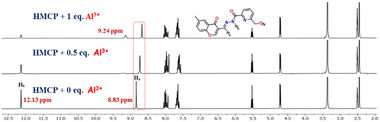 | ||
| Fig. 4 1H NMR titration experiments to elucidate the sensing mechanism of HMCP upon addition of different concentrations of Al3+. | ||
The cyan fluorescence response of MCMB in the presence of Al3+ established the fact that the pyridinium nitrogen atoms of HMCP and MCMB do not participate in the complex formation and further supported the binding mechanism depicted in Fig. 6. The greater emission intensity enhancement observed for HMCP and MCMP compared to MCMB upon addition of Al3+ can be attributed to the –I effect of the pyridine moiety which favours the formation of the enol form of both HMCP and MCMP (Table S1†). Again, the large emission intensity enhancement of HMCP compared to MCMP in presence of Al3+ is due to the presence of the additional aliphatic hydroxyl group (–CH2OH) which increases the solubility of HMCP through H-bonding, thereby displaying a higher emission intensity.
DNA binding study
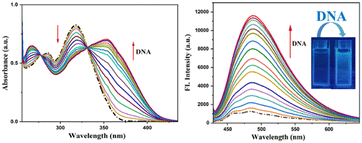
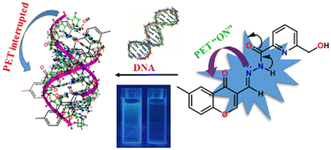
The binding of DNA with sensors is in high demand for the design and development of new targeted drugs. Ligands can bind with DNA through various non-covalent interactions. The affinity of HMCP and its analogues toward ct-DNA were examined through the changes in absorption and emission spectra (Fig. 7). Generally, a change in the absorption spectra, whereby a new peak appears at a new location, is observed when a complex is formed by the interaction of a small molecule/ligand with ct-DNA. The absorption spectra of HMCP was recorded upon addition of ct-DNA. Fig. 7 reveals the fact that upon incremental addition of ct-DNA into the HMCP probe solution, the inherent absorption band of HMCP at 315 nm gradually diminishes along with the appearance of two new absorption bands at 356 nm and 266 nm. Here, ct-DNA absorption is insignificant, suggesting the existence of an effective interaction between the π electron cloud of the interfacing sensor HMCP and the base pairs of DNA along with H-bonding.49Upon excitation at 331 nm, the HMCP sensor displays an emission band at 471 nm. After successive addition of ct-DNA into the HMCP solution, about an 8-fold enhancement in the fluorescence intensity was detected at 476 nm (Fig. 7). This significant rise in fluorescence intensity with a distinct fluorescence color change upon association with ct-DNA implies that an effective non-covalent interaction exists between the HMCP sensor and the DNA. The magnitude of the apparent equilibrium constant KBH between HMCP and ct-DNA was assessed spectrofluorimetrically (Benesi–Hildebrand plot) and was found to be 2.66 × 104 M−1 (Fig. S23†). The turn on fluorescence response can be attributed to the PET process, which is interrupted upon binding with DNA and may be due to the interaction of the lone pair of the HMCP imine nitrogen with the π-electron clouds of the DNA (Fig. 8). The “turn-on” emission enhancement of MCMP and MCMB upon addition of ct-DNA was also recorded and the outcomes are summarized in Table S2.† The higher enhancement in the fluorescence intensity for HMCP compared to MCMP may be due to the presence of an aliphatic –OH group in HMCP which initiates additional H-bonding interactions followed by stronger binding with DNA compared to that of MCMP. Again, the higher emission intensity of MCMP compared to MCMB may be because the presence of the additional lone pair of electrons on the pyridinium nitrogen atom allows for a more extensive interaction with the DNA base pairs as compared to MCMB.
Molecular logic gate
The emission properties of the HMCP sensor compelled us to investigate its application in numerous logic gates by successive addition of inputs, like metal cations such as Al3+ and anionic species like EDTA, whilst recording its fluorescence at 496 nm as the output. Inputs are denoted as “1” for their presence and their absence are assumed to be “0”. Outputs are denoted as “1” when the fluorescence intensity is above a certain threshold value (25% of the maximum). When no input is present, no characteristic fluorescence is noted and is considered to be “0” (off state). From the truth table, it is observed that HMCP shows an emission intensity output signal with Al3+ (input A) in such a manner that it seems to realise the requirements of an “AND” operation. In the presence of EDTA (input B), HMCP realizes a “NOT” operation (Fig. 9). Furthermore, when both inputs (Al3+ and EDTA) are present, the output emission intensity decreases, indicating the off state according to the truth table. These data are related to an “INHIBIT” logic gate via a particular arrangement of the logical functions AND and NOT.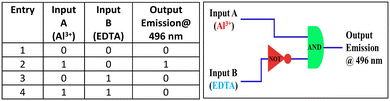 | ||
| Fig. 9 Truth table for advance-level molecular logic gate (left side) and corresponding circuit diagram (right side). | ||
Molecular memory device
The techniques for data storing are well known and can be combined by successive logic circuits. The combination of these circuits creates the following response loop where one of the output signals is assumed to be the input of the memory device and is considered as the “memory element”. The function of the memory device is built on a binary logic; either 1 or 0 alternates the two crisp states. In our device, for establishing a suitable mimic of the memory component, we consider Al3+ and EDTA as the set (S) and reset (R) inputs respectively and the fluorescence intensity at 496 nm as the output signal. In the memory device function, input A (Al3+) is considered as the set (S) and binary state 1 appears. However, the written data is erased under the reset input (EDTA) and binary state “0” is recorded. So, by applying a binary logic function, we have successfully established a consecutive logic circuit displaying a “write–read–erase–read” feature (Fig. 10). It is worth mentioning that the write–erase cycles could be reversed repeatedly several times with the same complex solution without a significant decrease in the fluorescence intensity. Thus, the circuits developed in this manner function comparably to logic memory devices of conventional semiconductors and can be considered as a better technique that can be possibly explored in the near future for the setup of molecular microprocessors of integrated circuits.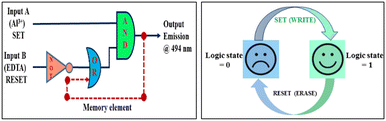 | ||
| Fig. 10 Consecutive logic gate of a memory unit (left side) and schematic representation of the reversible logic operation of the memory element with a write–read–erase–read function (right side). | ||
Practical application as a dip-stick: detection of Al3+ using a TLC plate
For the qualitative identification of Al3+, we have carried out an experiment known as a dip-stick experiment where the sensor can act as a portable fluorescence kit, also exhibiting its sensing capability towards the specific metal ion in the solid state without the aid of any sophisticated instruments. In order to execute this experiment, a few thin-layer chromatography (TLC) plates were prepared and they were immersed into a solution of HMCP (2 × 10−4 M) in MeOH and then dried by evaporating the solvent. Next, the dried probe-coated TLC plates were immersed into an Al3+ solution (2 × 10−3 M) and then the solvent was evaporated again in order to dry the plates. The visual colour change of the probe-loaded TLC plates in the absence and presence of Al3+ was prominent and distinguishable in day light as well as under UV light (Fig. 11). This method helps one to thoroughly and easily identify the presence of Al3+ using the naked eye.Real sample analysis
In order to validate the practical utility of our approach, the feasibility of the probe was investigated for the detection of aluminium ions in different water samples (tap and lake water) via fluorescence method according to previously reported methods.50,51 The samples were first filtered and then used for further experiment. These natural water samples were not subjected to the detection method. Now various concentrations of Al3+ ion were mixed into these natural water samples and the HMCP probe was successfully employed for the determination of aluminium content in these real water samples. From the experimental outcomes summarized in Table S3,† it is concluded that the HMCP sensor is highly proficient at detecting the concentration of Al3+ in contaminated natural water samples with excellent recovery rates (96.5–100.2%).Application in the biological field of live cell imaging in human breast cancer cells
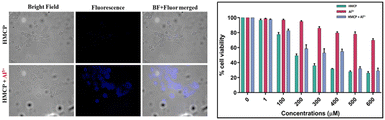
From the MTT experiments, it can be concluded that the receptor, HMCP, has negligible toxicity towards human breast cancer cells (MDA-MB-231) at lower concentrations (Fig. 12). The IC50 value of HMCP was determined and found to be 189.6 μM and therefore we have preferred the dose, when conducting this experiment, to be 15 μM as the chosen dose should be less than the IC50 value (Fig. S19†). Now, the fluorescence imaging experiment under the microscope revealed that the treatment of the breast cancer cells with HMCP itself exhibits no fluorescence, but following incubation of the MDA-MB-231 cells with 15 μM of aluminum and HMCP, a bright blue emission in the intracellular region was noticed (Fig. 12). This experiment established the fact that the HMCP sensor can easily penetrate the cell membrane in order to bind with the intracellular aluminum ion. Further, there is no physical change observed for the studied cells in bright field images after incubation with Al3+, implying that the MDA-MB-231 cells are viable and HMCP is not toxic at that specific concentration (Fig. 12). Therefore, our present probe has a chance to serve as a cell membrane-penetrable fluorescence kit that has excellent biological applications in living cells for analyzing exogenous Al3+ ion content.Theoretical calculations
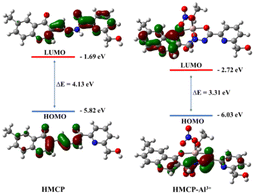
In order to understand the structural changes of HMCP upon binding with Al3+, DFT calculations were executed using the B3LYP/6-31+G(d) method of the Gaussian 09 program. Fig. S24 and S25† represent the optimized structures of HMCP and its complex. Fig. S26 and S27† display the contour plots of some selected molecular orbitals (HOMOs) of HMCP and the HMCP-Al3+ complex, respectively. The HOMO–LUMO energy gap of HMCP is 4.13 eV, while for the HMCP-Al3+ complex, the HOMO–LUMO energy gap is decreased to 3.31 eV (Fig. 13). This effective change in the HOMO–LUMO energy gap is well supported by the bathochromic shift observed in the absorption spectrum upon addition of Al3+ into the probe solution. To interpret the electronic spectra, vertical electronic transitions were determined using time-dependent density-functional theory (TDDFT) calculations (solvent correction was incorporated by the CPCM model and MeOH was chosen as the solvent) and are summarized in Table S6.† The calculated vertical excitations of HMCP show strong transitions at 335 nm (f = 0.7610) due to the HOMO → LUMO transition. However for HMCP-Al3+, the HOMO → LUMO transition shifted to 380 nm (f = 0.0868) (Table S6†).Conclusion
In conclusion, we have designed and successfully fabricated a chromone based OFF–ON–OFF reversible chemosensor HMCP, which is more efficient for detecting Al3+ than its analogues. The affinity of HMCP towards Al3+ rather than a group of other coexisting metal ions was accredited to the HSAB principle, where the hard centred N and O atoms in the probe prefer to bind with the hard acid Al3+ ions. The detection limit was determined based on fluorescence changes and found to be 3.12 ± 0.12 × 10−7 M. The “turn-on” chromo-fluorogenic response after binding with Al3+ was due to the restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N rotation and the inhibition of a PET process along with the initiation of chelation-enhanced-fluorescence (CHEF). The proposed binding mode of HMCP to Al3+ was supported by 1H NMR titration, HRMS, and Job's plot analyses as well as DFT calculations. DNA binding studies were also performed to demonstrate the higher bioactivity of HMCP compared to its analogues. In addition, the synthesized HMCP probe was utilised in the selective detection of Al3+ in real water samples. Moreover, cellular imaging studies were also conducted to establish the Al3+ bio-sensing ability of HMCP in living cells (MDA-MB-231).
N rotation and the inhibition of a PET process along with the initiation of chelation-enhanced-fluorescence (CHEF). The proposed binding mode of HMCP to Al3+ was supported by 1H NMR titration, HRMS, and Job's plot analyses as well as DFT calculations. DNA binding studies were also performed to demonstrate the higher bioactivity of HMCP compared to its analogues. In addition, the synthesized HMCP probe was utilised in the selective detection of Al3+ in real water samples. Moreover, cellular imaging studies were also conducted to establish the Al3+ bio-sensing ability of HMCP in living cells (MDA-MB-231).
Experimental section
Materials and instrumentation
All the reagents and organic compounds used in this synthesis were purchased from Sigma-Aldrich. All the other solvents and inorganic salts used in the synthetic procedures were purchased from available commercial sources. DMSO-d6 and CDCl3 were used as the solvents for the NMR experiments without further distillation. Thin layer chromatography (TLC) was carried out using Merck 60 F254 plates with a thickness of 0.25 mm. A PerkinElmer Lambda 750 spectrophotometer was used to obtain the absorbance spectra and the emission properties were measured using a Shimadzu RF-6000 fluorescence spectrophotometer at room temperature (298 K). Lifetimes were measured using a time-resolved spectrofluorometer from IBH, UK.Synthesis of 6-(hydroxymethyl)-N′-((6-methyl-4-oxo-4H-chromen-3-yl)methylene)picolinohydrazide (HMCP)
6-(Hydroxymethyl)picolinohydrazide (0.167 g, 1 mmol) was added to an ethanolic solution of 6-methyl-4-oxo-4H-chromene-3-carbaldehyde (0.19 g, 1 mmol) and the mixture was then refluxed for about 7 hours. After completion of the reaction, a white precipitate was obtained which was purified via column chromatography to yield the desired product (HMCP). The yield was 0.29 g, 86%.Synthesis of (E)-N′-((6-methyl-4-oxo-4H-chromen-3-yl)methylene)picolinohydrazide (MCMP)
Picolinohydrazide (0.14 g, 1 mmol) was added to an ethanolic solution of 6-methyl-4-oxo-4H-chromene-3-carbaldehyde (0.19 g, 1 mmol) and the reaction mixture was refluxed for about 8 hours. After completion of the reaction, a white product appeared which was then subjected to column chromatography to get a pure white solid. The yield was 0.25 g, 83%.Synthesis of (E)-N′-((6-methyl-4-oxo-4H-chromen-3-yl)methylene) benzohydrazide (MCMB)
An ethanolic mixture of benzohydrazide (0.14 g, 1 mmol) and 6-methyl-4-oxo-4H-chromene-3-carbaldehyde (0.19 g, 1 mmol) was refluxed carefully for about 7 hours. After completion of the reaction, a white precipitate was obtained which was subjected to column chromatography to yield the pure final product. The yield was 0.26 g, 85%.General method for UV-vis and fluorescence titration
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (HEPES buffer, pH = 7.2) (at 25 °C). The solutions of all the competitive cations were prepared at concentration on the order of 1 × 10−5 M, in deionized water. Solutions of different concentrations of HMCP and all the metal cations were prepared separately. The UV-vis spectra of these sample solutions were monitored.
1, v/v) (HEPES buffer, pH = 7.2) (at 25 °C). The solutions of all the competitive cations were prepared at concentration on the order of 1 × 10−5 M, in deionized water. Solutions of different concentrations of HMCP and all the metal cations were prepared separately. The UV-vis spectra of these sample solutions were monitored.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (HEPES buffer, pH = 7.2). Job's plots were then plotted as a function of the emission intensity change at 496 nm versus the mole fraction of Al3+.
1, v/v) (HEPES buffer, pH = 7.2). Job's plots were then plotted as a function of the emission intensity change at 496 nm versus the mole fraction of Al3+.
Live cell imaging studies
DNA-binding experiments
Data availability
The data that support the findings of this study are available on request from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank CSIR (No. 01(2992)/19/EMR-II) and SERB (No. EEQ/2018/000266), New Delhi, India for financial support.Notes and references
- R. Emas, The Concept of Sustainable Development: Definition and Defining Principles, in Brief for GSDR, Florida International University, 2015, pp. 1–3 Search PubMed.
- A. Frio, R. Janeiro, S. M. Viana, C. S. Valladares and B. P. Duarte, Rev. Bras. Geocienc., 2001, 6 Search PubMed.
- K. Brodowska and E. Łodyga-Chruscińska, Chemik, 2014, 68, 129–134 CAS.
- S. Kumar, D. N. Dhar and P. N. Saxena, J. Sci. Ind. Res., 2009, 68, 181–187 CAS.
- A. M. Abu-Dief and I. M. A. Mohamed, J. Basic Appl. Sci., 2015, 4, 119–133 Search PubMed.
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410–421 RSC.
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS.
- F. J. Hayes, H. B. Halsall and W. R. Heineman, Anal. Chem., 1994, 66, 1860–1865 CrossRef CAS.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS.
- L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Chem. Rev., 2000, 205, 59–83 CAS.
- G. C. Woodson, Bone, 1998, 22, 695–698 CrossRef CAS.
- C. S. Cronan, W. J. Walker and P. R. Bloom, Nature, 1986, 324, 140–143 CrossRef CAS.
- T. P. Flaten, Brain Res. Bull., 2001, 55, 187–196 CrossRef CAS PubMed.
- G. Berthon, Coord. Chem. Rev., 1996, 149, 241–280 CrossRef CAS.
- M. G. Soni, S. M. White, W. G. Flamm and G. A. Burdock, Regul. Toxicol. Pharmacol., 2001, 33, 66–79 CrossRef CAS PubMed.
- E. Gauthier, I. Fortier, F. Courchesne, P. Pepin, J. Mortimer and D. Gauvreau, Environ. Res., 2000, 84, 234–246 CrossRef CAS PubMed.
- G. D. Fasman, Coord. Chem. Rev., 1996, 149, 125–165 CrossRef CAS.
- D. Krewski, R. A. Yokel, E. Nieboer, D. Borchelt, J. Cohen, S. Kacew, J. Lindsay, A. M. Mahfouz and V. Rondeau, J. Toxicol. Environ. Health, Part B, 2009, 10, 1–269 Search PubMed.
- G. C. Woodson, Bone, 1998, 22, 695–698 CrossRef CAS PubMed.
- J. Barcelo and C. Poschenrieder, Environ. Exp. Bot., 2002, 48, 75–92 CrossRef CAS.
- WHO, Guidelines for Drinking-Water Quality, World Health Organization, 4th edn, 2017 Search PubMed.
- T. P. Flaten, Brain Res. Bull., 2001, 55, 187–196 CrossRef CAS.
- G. Berthon, Coord. Chem. Rev., 1996, 149, 241–280 CrossRef CAS.
- M. G. Soni, S. M. White, W. G. Flamm and G. A. Burdock, Regul. Toxicol. Pharmacol., 2001, 33, 66–79 CrossRef CAS PubMed.
- E. Gauthier, I. Fortier, F. Courchesne, P. Pepin, J. Mortimer and D. Gauvreau, Environ. Res., 2000, 84, 234–246 CrossRef CAS.
- G. D. Fasman, Coord. Chem. Rev., 1996, 149, 125–165 CrossRef CAS.
- D. Krewski, R. A. Yokel, E. Nieboer, D. Borchelt, J. Cohen, S. Kacew, J. Lindsay, A. M. Mahfouz and V. Rondeau, J. Toxicol. Environ. Health, Part B, 2009, 10, 1–269 Search PubMed.
- B. Das, M. Dolai, A. Ghosh, A. Dhara, A. Das Mahapatra, D. Chattopadhyay, S. Mabhai, A. Jana, S. Dey and A. Misra, Anal. Methods, 2021, 13, 4266 RSC.
- C. V. Kumar and E. H. Asuncion, J. Am. Chem. Soc., 1993, 115, 8547–8553 CrossRef CAS.
- X. Chen, L. Zhang, K. Zhou, E. Davies, K. Sugden, I. Bennion, M. Hughes and A. Hine, Opt. Lett., 2007, 32, 2541–2543 CrossRef CAS PubMed.
- W. A. Durai and A. Ramu, ChemistrySelect, 2020, 5, 4778–4785 CrossRef.
- S. K. Sheeta, B. Sena, R. Thounaojamb, K. Aguanb and S. Khatua, J. Photochem. Photobiol., A, 2017, 332, 101–111 CrossRef.
- A. P. de Silva, Molecular Logic-Based Computation, Royal Society of Chemistry, Cambridge, UK, 2012 Search PubMed.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- J. Andreasson and U. Pischel, Chem. Soc. Rev., 2018, 47, 2266–2279 RSC.
- B. Das, S. Dey, G. P. Maiti, A. Bhattacharjee, A. Dhara and A. Jana, New J. Chem., 2018, 42, 9424–9435 RSC.
- S. L. Hu, J. J. Song, G. Y. Wu, C. X. Cheng and Q. Gao, Spectrochim. Acta, Part A, 2015, 136, 1188–1194 CrossRef CAS PubMed.
- S. Zhang, Y. Wang and H. Xu, Spectrochim. Acta, Part A, 2022, 275, 121193 CrossRef CAS.
- J. Wang, L. Feng, J. Chao, Y. Wang and S. Shuang, Anal. Methods, 2019, 11, 5598 RSC.
- I. H. Hwang, Y. W. Choi, K. B. Kim, G. J. Park, J. J. Lee, L. Nguyen, I. Noh and C. Kim, New J. Chem., 2016, 40, 171–178 RSC.
- A. Das and G. Das, New J. Chem., 2022, 46, 19002–19008 RSC.
- C. Liu, L. Liu, T. Li, K. Liu and Z. Yang, Inorg. Chim. Acta, 2020, 502, 119327 CrossRef CAS.
- N. Behera and V. Manivannan, J. Photochem. Photobiol., A, 2018, 353, 77–85 CrossRef CAS.
- A. Maji, R. Naskar, D. Mitra, S. Gharami, N. Murmu and T. K. Mondal, J. Fluoresc., 2023, 33, 2403–2414 CrossRef CAS PubMed.
- J. Wang, L. Feng, J. Chao, Y. Wang and S. Shuang, Anal. Methods, 2019, 11, 5598 RSC.
- S. Gharami, K. Aich, D. Sarkar, P. Ghosh, N. Murmu and T. K. Mondal, New J. Chem., 2019, 43, 1857–1863 RSC.
- S. OğuzTümay, A. Şenocak and A. Mermer, New J. Chem., 2021, 45, 18400–18411 RSC.
- A. Maji, A. Biswas, B. Bera and T. K. Mondal, Anal. Methods, 2023, 15, 6417–6424 RSC.
- U. Saha, B. Das, M. Dolai, J. Butcher and G. Suresh Kumar, ACS Omega, 2020, 5(29), 18411–18423 CrossRef CAS.
- S. O. Tümay, A. Şenocak and A. Mermer, New J. Chem., 2021, 45, 18400–18411 RSC.
- A. Saravanan, S. Shyamsivappan, N. K. Kalagatur, T. Suresh, N. Maroli, N. Bhuvanesh, P. Kolandaivel and P. S. Mohan, Spectrochim. Acta, Part A, 2020, 241, 118684 CrossRef CAS PubMed.
- S. Gharami, K. Aich, D. Sarkar, P. Ghosh, N. Murmu and T. K. Mondal, New J. Chem., 2019, 43, 1857–1863 RSC.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- F. Furche and R. Ahlrichs, J. Chem. Phys., 2002, 117, 7433 CrossRef CAS.
- R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett., 1996, 256, 454–464 CrossRef CAS.
- R. E. Stratmann, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 1998, 109, 8218–8224 CrossRef CAS.
- M. E. Casida, C. Jamorski, K. C. Casida and D. R. Salahub, J. Chem. Phys., 1998, 108, 4439–4449 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and MS spectra of all new compounds, limit of detection determination, quantum yield calculations, CCDC no. 1535847 & 1535849etc. See DOI: https://doi.org/10.1039/d4sd00242c |
| This journal is © The Royal Society of Chemistry 2024 |