Syngas conversion to biofuels and biochemicals: a review of process engineering and mechanisms
Habiba
Khalid
 ab,
Farrukh Raza
Amin
abc,
Lian
Gao
ab,
Limei
Chen
ab,
Wuxi
Chen
ab,
Sundus
Javed
d and
Demao
Li
*ab
ab,
Farrukh Raza
Amin
abc,
Lian
Gao
ab,
Limei
Chen
ab,
Wuxi
Chen
ab,
Sundus
Javed
d and
Demao
Li
*ab
aTianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin 300308, China. E-mail: li_dm@tib.cas.cn; Tel: +86-22-84861932
bNational Center of Technology Innovation of Synthetic Biology, Tianjin 300308, China
cDepartment of Chemistry, COMSATS University Islamabad, Park Road, Tarlai Kalan, 45550, Islamabad, Pakistan
dDepartment of Biosciences, COMSATS University Islamabad, Park Road, Tarlai Kalan, 45550, Islamabad, Pakistan
First published on 27th November 2023
Abstract
Syngas is generated by thermochemical conversion of a wide range of organic wastes, or it is directly produced as an industry off-gas. The syngas components are converted into alcohols and other high-value-added bioproducts by acetogenic bacteria primarily via the Wood–Ljungdahl pathway. The feasibility of the syngas fermentation process to produce ethanol and other biochemicals is affected by many factors, such as microorganisms, fermentation strategies, gas–liquid mass transfer, and reactor types and design. This study offers new perspectives on the fermentation of syngas by focusing on all these factors to achieve commercialization of these value-added products. Moreover, it includes concepts regarding industrial applications by focusing on metabolic engineering and life cycle assessment for evaluating alternative sustainability dimensions and optimizing the production of ethanol and other biochemicals. This review paper lays a foundation for comparative studies that can be carried out to improve the technological, environmental, and socioeconomic aspects of bioethanol production.
1. Introduction
Syngas is composed of different gases, such as carbon monoxide (CO), carbon dioxide (CO2) and hydrogen (H2) and is produced by thermal gasification of a wide range of organic wastes. The gasification process of these various types of feedstocks is executed in a gasifier at high pressure and temperature in the presence of gasifying agents, including oxygen or steam.1 According to one study, the estimated production of syngas is approximately 598 million tons per year.2 Apart from the thermal gasification process, syngas is generated as a waste gas from the chemical and steel industry, and its composition is influenced by a number of parameters, such as the type of gasifier and operational conditions.3 Syngas fermentation is meaningful for carbon emission reduction as well biofuel production.4Since the demand for energy worldwide is predicted to increase by 47% over the next 30 years and the consumption of liquid fuel is predicted to climb by 64% relative to 2020, hence, in order to reduce the demand for carbon-based fuels, increasing the production of biofuels might be a more sustainable approach.5,6
The market for biofuels will reach USD 201.21 billion by 2030, expanding at a compound annual growth rate (CAGR) of 8.3% between 2021 and 2030. Utilizing cutting-edge techniques to achieve better titers, rates, and yields is essential for the environmentally responsible, cost-effective, and sustainable production of biofuels.7,8 To efficiently ferment syngas, various studies have reported useful acetogens that have been isolated and are capable of utilizing syngas as the sole carbon source for their growth.
Acetogens are a phylogenetically and physiologically varied group of anaerobic bacteria, with over 100 species dispersed over 23 bacterial genera. The common characteristic of all acetogens is that they utilize the Wood–Ljungdahl pathway (WLP) to fix C1 gases. During gas fermentation, CO or CO2 is employed as a carbon source, while CO or H2 offers reducing equivalents. The condensation of the methyl group, a carbonyl group by CO or CO2, and coenzyme A at the expense of one adenosine triphosphate (ATP) molecule occurs in the WLP over the course of many steps to produce one molecule of acetyl-CoA, which eventually leads to the generation of biomass, and other metabolites are produced from the generated acetyl-CoA.9
Among these, several species of Clostridium have been widely reported for syngas fermentation, such as Clostridium carboxidivorans, which is capable of producing hexanol, butanol, and ethanol from syngas using the WLP. Moreover, Clostridium species have carbon monoxide dehydrogenase (CODH), which participates in catabolic CO oxidation. Since acetogens utilize H2/CO2 as the main substrates for growth, therefore, CO toxicity remains a major issue.10 Carbon monoxide binds metal clusters in enzymes and prevents the binding of the enzymes' natural substrates. Therefore, to avoid fully depleting the ferredoxin pool or inhibiting important enzymes, carboxydotrophic organisms should effectively limit the toxicity of carbon monoxide. CODH catalyzes the oxidation of CO to CO2 upon reduction of ferredoxin at a high specific activity and detoxifies carbon monoxide. This is why most Clostridium species are used as biocatalysts for syngas fermentation; nonetheless, some studies have also identified other species that can potentially utilize C1 gases and convert them directly into acetate and butyrate. The most common products of syngas fermentation are ethanol, acetate, n-butanol, 2,3-butanediol and hexanol, which have vast industrial applications, including their usage as solvents and fuels. They are also used in the textile industry for dyeing, rubber production and polyurethane production; the detailed applications of the major syngas fermentation products are presented in Table 1.11
| Name | Applications | Ref. |
|---|---|---|
| Acetate (C2H4O2) | Textile industry (dyeing), rubber production, concrete sealant, food, and flavoring | 12 |
| Ethanol (C2H6O) | Precursor of acetic acids, diethyl ether, and ethyl halides | 13 |
| n-Butanol (C4H10O) | Precursor of butyl esters, acetates, pharmaceuticals, polymers, plastics, and herbicides | 14 and 15 |
| 2,3-Butanediol (C4H10O) | Polyurethane production, solvent, glycerin substitute, and sanitary products | 16 |
| Hexanol (C6H14O) | Solvent, shellac, resins, and hormones | 17 |
| Single-cell protein | Used as animal feed | LanzaTech Inc., (http://www.lanzatech.com) |
The production of biofuels and biochemicals from syngas has gained increasing attention over the last few years, but understanding and efficient execution of the process is still far from being achieved. The latest reviews on biofuel production studied syngas fermentation; however, process engineering has seldom been reported. Moreover, the feasibility of the syngas fermentation process to produce ethanol is influenced by many factors, including microorganisms, fermentation strategies, gas–liquid mass transfer, and reactor types and design. These limitations are an impediment to syngas fermentation technology commercialization. This review offers new perspectives on the fermentation of syngas by focusing on all these factors to achieve commercialization of ethanol and other biochemicals. This paper sheds light on metabolic engineering, which can be applied to improve process engineering and eventually lead to improvements in reaction conditions, thus providing smart product recovery strategies. In addition, this paper also discusses scale-up strategies, commercialization, and future research directions while providing a technological road map.
2. Microorganisms and metabolic pathways
Biofuel and biochemical production by syngas fermentation has several benefits compared to metal catalytic conversion, for instance, higher biocatalyst specificity, lower cost of energy, independence of a fixed H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio, and greater resistance to catalyst poisoning.18 Research over the past several years has reported anaerobes that utilize CO and H2 for acetate, butyrate, butanol, ethanol, and formate production, as shown in Table 2. Most syngas-fermenting microorganisms develop autotrophically by utilizing the components of syngas as the sole source of carbon and energy. This involves the reductive acetyl-CoA pathway, in which carbon from CO and CO2 is assimilated into biomass and CO and H2 are utilized as energy sources.3,19 This pathway is adopted by several microbes including Moorella thermoacetica that utilizes CO2.20 Several other anaerobic microbes that adopt the Wood–Ljungdahl pathway (Fig. 1) to produce acetic acid as a major product have been displayed in Table 2.
CO ratio, and greater resistance to catalyst poisoning.18 Research over the past several years has reported anaerobes that utilize CO and H2 for acetate, butyrate, butanol, ethanol, and formate production, as shown in Table 2. Most syngas-fermenting microorganisms develop autotrophically by utilizing the components of syngas as the sole source of carbon and energy. This involves the reductive acetyl-CoA pathway, in which carbon from CO and CO2 is assimilated into biomass and CO and H2 are utilized as energy sources.3,19 This pathway is adopted by several microbes including Moorella thermoacetica that utilizes CO2.20 Several other anaerobic microbes that adopt the Wood–Ljungdahl pathway (Fig. 1) to produce acetic acid as a major product have been displayed in Table 2.
| Species | Temp. (°C) | pH | Doubling time (h) | Substrates | Products | Ref. | |
|---|---|---|---|---|---|---|---|
| Pure cultures | Acetobacterium woodii | 30 | 6.8 | 13 | CO/H2/CO2 | Acetate | 27 |
| Butyribacterium methylotrophicum | 37 | 5.5–6.0 | 12–20 | CO | Acetate, butyrate, butanol & ethanol | 28 | |
| C. autoethanogenum | 20–44 | 4.5–6.5 | NA | CO/H2/CO2 | Acetate, ethanol, and 2,3-butanediol | 16 | |
| C. carboxidivorans | 24–42 | 4.4–7.6 | 6.25 | CO/CO2/H2 | Acetate, ethanol, butyrate, butanol, caproate, and hexanol | 29 | |
| C. drakei | 18–42 | 4.6–7.8 | 8.3 | CO/CO2/H2 | Acetate, ethanol, butyrate, and butanol | 30 | |
| C. ljungdahli | 30–40 | 4.0–6.0 | 3.8 | CO/H2/CO2/N2 | Acetate, ethanol, 2,3-butanediol, and formic acid | 31 | |
| C. ragsdalei | 25–40 | 5.0–7.5 | 5.7 | CO/H2/CO2 | Acetate, ethanol, and 2,3-butanediol | 17 | |
| C. aceticum | 30–37 | 7.7 | NA | CO/CO2 | Acetate | 32 | |
| Eubacterium limosum | 38–39 | 7.0–7.2 | 7.0–18.2 | CO2/H2 | Acetate and butyrate | 27 | |
| M. thermoacetica | 55 | 7 | 18 | CO | Acetate | 33 | |
| Sporomusa ovata | 15–45 | 5.0–8.1 | 13 | CO2/H2 | Acetate and ethanol | 34 | |
| Co-cultures | Alkalibaculum bacchi and C. propionicum | 37 | 6.0–8.0 | NA | Syngas | Ethanol, n-propanol, and n-butanol | 14 |
| C. ljungdahlii and C. kluyveri | 35 | 5.7–6.4 | NA | Syngas | Acetate, butyrate, caproate, ethanol, butanol, hexanol, 2,3-butanediol, and octanol | 35 | |
| C. kluyveri and C. autoethanogenum | 37 | 5.5–6.5 | NA | CO/CO2/H2 | Acetate, butyrate, caproate, ethanol, butanol, and hexanol | 36 | |
| Multi-stage culture | C. ljungdahlii (reactor 1) | 37 | 5.9 | NA | CO/H2/CO2/N2 | Ethanol and acetate | 37 |
| Aspergillus oryzae (reactor 2) | 35 | 6.5 | NA | Fermentation effluent | Malic acid | 7 | |
| C. autoethanogenum (stage 1) | 30 | 5.75 | NA | CO/H2/CO2/N2 | Acetate, ethanol, and 2,3-butanediol | ||
| Mixed microbial consortia (MMC) (stage 2) | 30 | NA | NA | Fermentation effluent | PHA | ||
| Genetically engineered | C. coskatii [p83_tcb] | 37 | NA | 3-Hydroxybutyrate (3-HB) | 38 | ||
| C. autoethanogenum PHB | 37 | 5 | NA | Poly-3-hydroxybutyrate (PHB) | 39 |
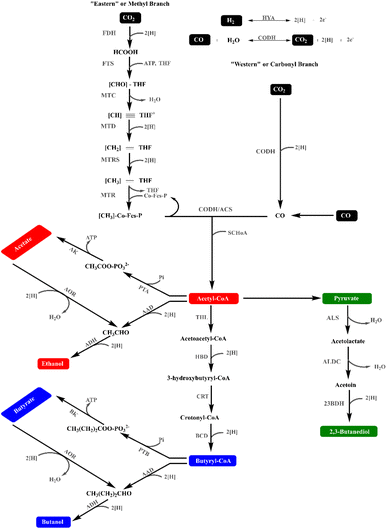 | ||
| Fig. 1 Wood–Ljungdahl pathway, “Adapted from ref. 21”. | ||
The initial discovery of the metabolic acetogenesis process, or the reduction of two molecules of CO2 with hydrogen into one molecule of acetate, in a microbial community from sewage was made in 1932.22 In 1936, Clostridium aceticum was successfully isolated from soil for the first time. The following stoichiometry was used to determine how much acetate would be produced from CO2 and H2.23
| 2CO2 + 4H2 → CH3COOH + 2H2O |
Later, Moorella thermoacetica was isolated which converted one molecule of glucose to nearly three molecules of acetate.24
| C6H12O6 → 3CH3COOH |
In the 1980s, further research was carried out to determine how CO2 turned into acetate. Thus, the WLP gave insights into the metabolism of acetic acid production by Moorella thermoacetica while utilizing CO2.20,25 The ideal temperature for growth is between 55 and 60 °C. It can grow on a variety of other substrates, including methanol and ethanol. Sugars and methoxylated aromatic compounds are other substrates employed in addition to CO, CO2, and hydrogen. M. thermoacetica is not dependent on the Na+ ion.26 CO dehydrogenase/acetyl-CoA synthase (CODH/ACS), which forms a C–C bond between CO and a methyl group, is the key enzyme in this route. The active site of this core enzyme contains cobalt and nickel. Acetogenic bacteria, which can convert two moles of CO2 with four moles of hydrogen into acetate, were the primary organisms used to study WLPs. With −94 kJ mole−1 acetate, this reaction is exergonic.
One of the most thoroughly researched acetogens in recent years is Acetobacterium woodii. It is a Gram-positive, mesophilic (30 °C), quinone- and cytochrome-deficient bacterium. The presence of Na+ ions is necessary for A. woodii development.
The Gram-positive, chemolithotrophic, motile anaerobe C. autoethanogenum is regarded as another model acetogen with established industrial applications. Its main natural products when grown autotrophically on syngas are acetate, ethanol, 2,3-butandiol, and lactate. The WLP, which dates back thousands of years, is thought to be the main mechanism that promotes autotrophic production of fermentative products and biomass.26
Microorganisms show a variety of metabolic pathways that can be engineered to overcome the obstacles in the biofuel production. These pathways have revolutionized the norms for producing both conventional and advanced biofuels (bioethanol, biobutanol, etc.). drop-in fuel, biohydrogen, biodiesel, etc. By changing the molecular mechanisms connected to the metabolic pathways that produce fuel, this method tries to improve the metabolic performance of microorganisms. The availability of cutting-edge research tools such as CRISPR/Cas9, MAGE, and other ‘omics’ platforms and databases, such as genomics, transcriptomics, proteomics, and metabolomics, has accelerated metabolic engineering-based approaches and made biofuel production efficient and labor intensive.40 Several metabolic engineering techniques have been used to modify metabolic pathways for better biofuel generation, including improvement of carbon fluxes toward the target pathway, incorporation of heterologous pathways, introduction of consolidated pathways, engineering redox balance for adequate supply of NADH/NAD(P)H (cofactor engineering), and directed enzyme evolution.41–45
3. Gas–liquid mass transfer mechanism
The gas–liquid mass transfer of CO and H2 in the aqueous phase is one of the main bottlenecks of syngas fermentation.46,47 Various approaches, including increased pressure, different reactor configurations, high flow rates, large specific gas–liquid interfacial areas, modified fluid flow patterns, innovative impeller designs, use of microbubble dispersers and varying mixing times and speeds, have been investigated for enhancing the solubility of gas in the aqueous phase of the fermentation medium.48–51 Most of these methods enhance the power-to-volume ratio that increases the breakup of the bubbles, thereby enhancing the surface area for mass transfer.52Generally, mass transfer takes place either via diffusion or convection. The diffusion of mass occurs at a different concentration gradient of a component that moves through a mixture from a high concentration gradient to a low concentration gradient. On the other hand, convection is mass transfer due to the bulk motion of a fluid, where the component movement requires mixing. There are three phases of mass transfer: gas segregation within the bulk phase, gas diffusion from the gaseous to the aqueous phase, and gas diffusion into the cells of microbes. The bulk segregation is instantaneous, and the microbial cell specific surface area causes the liquid film resistance to be negligible. Therefore, gas diffusion into the aqueous medium results in limited mass transfer.53,54 Diffusion is described in two ways. The first is called Fick's law, which helps study diffusion with respect to position.
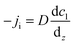 | (1) |
The second mathematical representation comprises a mass transfer coefficient.
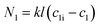 | (2) |
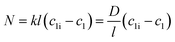 | (3) |
Here, N denotes the mass flux/unit area, kl is the coefficient of mass transfer, c1i is the interface concentration and c1 is the bulk concentration. This is a steady-state model; however, to evaluate diffusion with time, a time-dependent model would be more appropriate. One such example is Fick's second law:
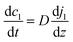 | (4) |
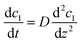 | (5) |
The concentration is dependent on time and the flux is dependent on the distance of diffusion. Nevertheless, the limitations of the film model are that it overlooks undefined film turbulence. Another model that describes mass transfer is the penetration model, where the mass transfer is related to the contact time.
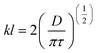 | (6) |
This model is also called “Higbie's model”, which assumes that each liquid element at the gas–liquid interface is exposed to the gas for a short time. The three main assumptions of this model are (1) mass transfer from the gas into a liquid element occurs under unsteady-state conditions, (2) each liquid element stays in contact with the gas for the same duration of time, and (3) equilibrium occurs at the gas–liquid interface. This model is an improvement on the previously mentioned film model, as mass transfer takes place under unsteady-state conditions in different processes at the industrial scale. Various models have been reported in the published works, however, the selection of the most suitable model depends on different factors including bioreactor design and fermentation medium.
Alternative bioreactor configurations have also been employed to achieve efficient mass transfer.48,52 The most consistent factor to investigate the efficient mass transfer rate is the gas–liquid volumetric mass transfer coefficient, which indicates the hydrodynamic reactor conditions. According to a study, a maximum kl of 190 and 75 L h−1 was achieved for H2 gas in a STR at 300 rpm using a mixed culture of sulfate-reducing bacteria as a biocatalyst.55 Another study stated kl values of 0.4 to 91 L h−1 for CO for seven types of bioreactors.48 It has been reported that multilayered composite hollow fiber, nonporous, ultrathin membranes made from polyethylene potentially increase the gas–liquid mass transfer in the liquid phase by preventing permeation of liquid and only allowing gases to pass through. If the inlet gas pressure is increased, higher levels of gas saturation might be achieved.56 Many studies have focused on using composite hollow fiber (CHF) membranes to enhance CO mass transfer by investigating the impacts of recirculation flow rates and inlet gas pressure on the solubility of gas.48,57 These studies indicated that employing CHF membranes is advantageous and increases CO transfer into the liquid phase. Another way to solve this problem is to employ a multipronged strategy for an efficient syngas fermentation process that relies on three basic approaches: pathway and strain optimization and development of the process. Nonetheless, efficient strain development has been negatively impacted by the lack of high-throughput strain engineering workflows, and there is not a well-defined pathway for industrial scale-up. Therefore, more work can be done to integrate high-throughput strain engineering workflows, omics, cell-free systems, kinetic modeling, fermentation scale-up and life-cycle assessments. Different heterologous pathway enzymes can be optimized to achieve the desired molecular transformations; later the strains can be optimized to increase the production of the targeted products.58
The above-mentioned strategies may lead to enhancement in the gas–liquid mass transfer rates of poorly soluble gases into the liquid phase. Thus, it is clear that further advancements will be made in the near future.
4. Factors affecting syngas fermentation and optimization
4.1. Medium composition
A syngas fermentation medium must contain essential nutrients for supporting the metabolic growth of syngas-fermenting acetogens, which include carbon, organic and inorganic nitrogen sources, amino acids, and trace metals. The most essential medium component is carbon, which plays a key role in the growth and production of primary and secondary metabolites. Carbon sources are metabolized at different rates, which also affects biomass formation. According to a previous study, it was reported that the gradual assimilation of carbon sources usually enhances secondary metabolite production.59,60 Different carbon sources along with their roles are summarized in Table 3. Notably, in fermentation processes where medium components contribute significantly to the product cost, the choice of carbon sources in the medium is very important. Apart from carbon source assimilation, the nature of the carbon source also influences the amount and type of the product. Therefore, it is important to study carbon source dynamics to determine their role as substrates in the process of fermentation. Inorganic nitrogen sources such as ammonium ions (NH4+) have been reported to significantly influence cell mass and ethanol production by C. ragsdalei.61 Organic nitrogen sources such as tryptone and peptone help in the synthesis of core proteins during metabolite production and enhancement of carbon uptake.62 Amino acids are building blocks of different metalloenzymes and proteins in the Wood–Ljungdahl pathway. According to research that studied the effects of trace elements on syngas fermentation, it was reported that if nickel (Ni+2) was removed from the C. ragsdalei medium, no cell growth or acetic acid and ethanol production was observed.63 Other trace elements, such as WO4−2, Fe+2, Co+2, and Mo+6, were also removed from the medium to study the effects on ethanol formation, and it was seen that the ethanol concentration was reduced by 97%, 82%, 24% and 38%, respectively. In the same research study, the effect of adding Ni+2, Zn+2, and SeO4− was also observed. Ethanol production increased with the addition of these trace elements along with an increase in FDH activity. The activity of CODH and hydrogenase was also enhanced by the addition of Ni; nonetheless, Fe removal inhibited these enzymes. Another study explored the impacts of W on S. ovata grown with a mixture of CO2 and H2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 by volume). It was observed that there was a 19% enhancement in cell growth, whereas the conversion of propionate and butyrate increased by 60% and butyrate by 32% along with the production of alcohols.64 The effect of Zn concentration (7–280 μM) on the growth of C. carbooxidivorans was also studied, and it was observed that Zn concentration not only doubled cell growth but also increased ethanol, butanol, and hexanol production.65 In conclusion, an optimized medium that has a balanced concentration of nutrients is very important to support the growth of microbes and the production of high value-added bioproducts.
20 by volume). It was observed that there was a 19% enhancement in cell growth, whereas the conversion of propionate and butyrate increased by 60% and butyrate by 32% along with the production of alcohols.64 The effect of Zn concentration (7–280 μM) on the growth of C. carbooxidivorans was also studied, and it was observed that Zn concentration not only doubled cell growth but also increased ethanol, butanol, and hexanol production.65 In conclusion, an optimized medium that has a balanced concentration of nutrients is very important to support the growth of microbes and the production of high value-added bioproducts.
| Composition/source | Biological catalyst | Effects on the production | Ref. | |
|---|---|---|---|---|
Syngas (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4) CH4) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
C. carboxidivorans | 1.9 g L−1 butanol; 2.7 g L−1 ethanol; 0.85 g L−1 hexanol | 66 and 67 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
C. carboxidivorans | 1 g L−1 butanol; 2 g L−1 ethanol; 0.5 g L−1 hexanol | 66 and 67 | |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
C. carboxidivorans | 1.8 g L−1 ethanol, 0.66 g L−1 butanol, 3.35 g L−1 acetic acid, and 0.38 g L−1 hexanol | 67 and 68 | |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
C. carboxidivorans | 1.76 g L−1 ethanol; 1.32 g L−1 acetic acid; 0.43 g L−1 butanol | 67 and 69 | |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
C. ragsdalei | Significant reduction in hydrogenase activity with NH3 in the syngas | 67 and 70 | |
32.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.5 32.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (without impurities) 0 (without impurities) |
C. ljungdahlii | 2.47 g L−1 ethanol; 16.75 g L−1 acetic acid | 67 and 71 | |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0:20 0:20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (without impurities) 0 (without impurities) |
C. carboxidivorans | 1.17 g L−1 ethanol; 0.96 g L−1 acetic acid; 0.56 g L−1 butanol | 67 and 68 | |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0:20 0:20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (0.1 g L−1 H2S) 0 (0.1 g L−1 H2S) |
C. carboxidivorans | 3.2 g L−1 ethanol, 0.8 g L−1 acetic acid, and 0.38 g L−1 hexanoic acid | 67 and 68 | |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (0.1 g L−1 NaNO3) 0 (0.1 g L−1 NaNO3) |
C. carboxidivorans | 1.1 g L−1 ethanol, 0.38 g L−1 acetic acid, and 2.04 g L−1 butyric acid | 67 and 68 | |
| Inorganic nitrogen | NH4+ | C. ragsdalei | Cell mass and ethanol production reduced by 33% and 41%, respectively, if removed | 61 |
| Organic nitrogen | Tryptone | C. autoethanogenum | 45.9% enhancement in ethanol production with 0.5 g L−1 tryptone | 62 |
| Peptone | C. autoethanogenum | 44.3% enhancement in ethanol with 0.5 g L−1 peptone | 62 | |
| Amino acids | L-Cysteine | C. autoethanogenum | Cysteine addition did not impact ethanol production. Conc. higher than 0.5 g L−1 negatively impacts the biomass | 72 |
| Trace elements | Fe | C. ragsdalei | Ethanol reduced by 82% on eliminating Fe | 63 and 73 |
| Co | C. ragsdalei | Ethanol reduced by 24% on eliminating Co | 63 and 73 | |
| Mn | C. ragsdalei | No effect on cell growth, ethanol, and acetate production | 63 and 73 | |
| Ni | C. ragsdalei | No effect on cell growth, ethanol, and acetate production | 63 and 73 | |
| Mo | C. ragsdalei | Ethanol decreased by 51% with 20% less Mo | 17, 63 and 73 | |
| Se | C. ragsdalei | Ethanol production increased by 52% on adding 10.6 μM Se | 63 and 73 | |
| W | C. ragsdalei | Ethanol increased by 102% on adding 6.8 μM W | 63, 64 and 73 | |
| S. ovata | Ethanol increased by 206% on adding 0.1 μM W | 63 and 73 | ||
| Zn | C. ragsdalei | Ethanol increased 4.2-fold with 66.9 μM Zn | 63 and 73 | |
| C. carboxidivorans | 3.0-Fold, 7.6-fold, and 44-fold increase in ethanol, butanol and hexanol on adding 280 μM Zn | 73 and 74 |
Different medium optimization techniques can be employed to enhance the productivity of ethanol and other value-added bioproducts. These techniques include single factor studies, artificial neural networks (ANNs), Plackett–Burman design, response surface methodology and advanced synthetic biology techniques. Employing them either as a single technique or in combination may give desirable results.
4.2. Fermentation conditions
Syngas fermentation is greatly influenced by the process operating conditions, including pH, temperature, and pressure. The pH of the medium leads to a shift between the acetogenesis and solventogenesis phases of fermentation, as volatile fatty acid (VFA) accumulation during the former causes a reduction in pH, which is then followed by the latter, resulting in the conversion of acid to alcohols.35 Many studies have reported this phenomenon while studying the impacts of pH on the syngas fermentation process using different biocatalysts, such as C. carboxidivorans, anaerobic mixed cultures, C. ljungdahlii and C. ragsdalei.75–79 These studies have indicated that a pH drop ranging between 4.5 and 5.0 leads to solventogenesis, whereas operating at a relatively higher pH (5.0–6.0) favors acetogenesis, which is related to cell growth and VFA production. A study was carried out using mixed cultures of C. ljungdahlii and C. kluyveri, and a narrow pH range was reported for the production of elongated chain alcohols.80 A study developed a novel method to control syngas supply based on pH that shows the activity of the cell. This method has resulted in the stability of the process and enhanced ethanol production during the continuous fermentation of syngas.79 Temperature is another important factor for the growth and metabolism of different acetogens, as shown in Table 2. However, it should be noted that the optimal temperature needed for growth and VFA accumulation might not be favorable for the production of alcohol. For instance, a study reported that C. carboxidivorans exhibits enhanced production of alcohol and chain elongation at 25 °C.81 Another study reported higher production of acetate, butanol, butyrate, ethanol and hexanol with C. carboxidivorans when the temperature was decreased from 37 °C to 25 °C.82 The pressure of the system is another important parameter that affects the fermentation process. The main obstacle of syngas fermentation is the rate of mass transfer, which can be overcome by increasing the pressure. A study specifically focused on studying the impacts of moderately elevated pressures (5–10 bar) on the process.83 Previous researchers have laid a strong foundation for syngas fermentations carried out at higher pressures to increase mass transfer rates; however, moderate elevation of pressures using substrate gases, including CO2, CO, and H2, has not been fully explored. Although the growth rate of acetogens and formation of the product are improved at higher pressures, the titers must be increased to facilitate industrialization in the future. Therefore, further investigations into the optimization of operating parameters are needed. A summary of the operating conditions is given in Table 4.| Substrate gas | Biocatalyst | Operating conditions | Conversion efficiency of gas | Impact on product formation | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| pH | Temp. °C | Pressure | Reactor type | |||||
Syngas CO/CO2/N2(25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
C. carboxidivorans | 5.2 | 37 | NR | BCR | CO: 60% | 0.33 mol ethanol/mol CO; 0.04 mol acetate/mol CO | 75 |
| CO | C. carboxidivorans | 5.75(*) | 33 | NR | Continuous gas-fed | CO: 50% | 1.04 g L−1 acetic acid; ethanol: 7.52 g L−1 | 76 |
| CO | C. carboxidivorans | 4.75(**) | 33 | NR | Continuous gas-fed | CO: 50% | 0.06 g L−1 acetic acid; ethanol: 4.21 g L−1 | 76 |
CO/H2/CO2 (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
Anaerobic mixed cultures | 6.9 | 37 | 1.0 atm | This experiment was carried out in vials | NR | 0.92 mol VFA/mol syngas | 77 |
| CO and H2 | C. ljungdahlii | 5.9 | 37 | 1,4, and 7 bar | STR (batch) | NR | At 7 bar 4 g L−1 of VFAs produced comprising 15.6% acetic acid, 1.7% ethanol, and 82.7% formic acid | 78 |
4.3. Reactors
One of the most crucial factors that should be considered for the optimal growth of microorganisms during syngas fermentation is the bioreactor configuration that will significantly influence the entire process, whether it is batch, continuous, semicontinuous, or a combination of processes. Therefore, at an industrial scale, different factors influence the final choice of the bioreactor and the operation mode. Different types of reactors, including stirred vessels, bubble columns, trickle bed or membrane reactors, and gas lift and loop reactors, have been studied and compared by scientists for syngas fermentation.54,84 The most suitable reactors for syngas fermentation are those that achieve high cell concentrations and mass transfer in an energy saving manner.54,55 The reactor types presented in Fig. 2 have been widely studied in different laboratory settings.4,28,85,86 A schematic diagram of different types of reactors is illustrated in Fig. 2.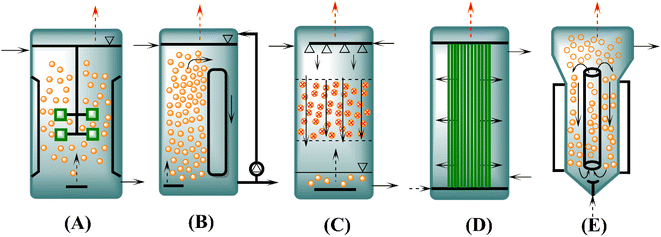 | ||
| Fig. 2 Main types of syngas fermentation reactors. (A) continuous stirred tank reactor (CSTR), (B) bubble column reactor (BCR), (C) trickle bed reactor (TBR), (D) hollow fiber membrane bioreactor (HFMBR), and (E) gas lift bioreactor (GLR); “adapted from ref. 4”. | ||
A CSTR is shown in Fig. 2A, where mechanical agitation is performed for mixing and the desired reaction conditions are adjusted easily. However, in high volume (>500 m3) fermenters, an increased energy input is needed for sufficient mixing. Many reaction-engineering studies have been reported on CSTRs, both in continuous and batch modes. Nonetheless, most of the published research work is basic and only investigated different parameters affecting the syngas fermentation process, but the development of an optimized reactor has not been reported. Although the CSTR is commonly used for syngas fermentation, it is not preferable for industrial-scale usage because it is energy intensive.48,55,87,88 As a high energy input is an impediment to economic scale-up for obtaining value-added products, the simplest and most attractive reactors with regards to industrial application are bubble columns (Fig. 2B) due to their low cost and energy requirements.89 There is a possibility of large volumes in bubble columns, but liquid phase mixing is limited. Bubble column reactors (BCRs) have complex flow systems and axial gradients in gas pressure, biomass concentrations, pH of the fermentation medium and height of the reactor. Hence, to enhance efficiency, BCRs have been modified as gas lift reactors (GLRs), in which the gas enters via the lower end, thus causing upward flow of the gas bubbles and liquid phase.90–92 One of the most common problems with reactors is the availability of biocatalysts. To ensure a higher cell concentration, biofilm reactors, such as trickle bed reactors (TBRs) (Fig. 2C), have been developed to prevent a loss of cells while achieving high gas–liquid transport rates. However, the stability of the biofilm is a gray area, and very little research has been performed on it. Therefore, biofilm reactors with immobilized cells are of interest to scientists in further research.93 Another type of reactor is the HFMBR (Fig. 2D), which is a unique reactor that comprises microporous hollow fiber membranes as gas distributors. The diffusion of gas molecules to the liquid takes place via the membrane, where the biocatalyst sticks to the surface as a biofilm; therefore, the membrane works as a gas input as well as a carrier for useful microorganisms.88,94 HFMBRs are gaining increasing attention because of their low energy input requirements and high mass transfer rates.88 Nonetheless, the selection of a suitable membrane material is an important factor because the membrane surface strongly impacts mass transfer. Furthermore, special HFMBRs for gas fermentation are not easily available in the market. Moreover, membrane stability under real fermentation conditions is also a matter of concern.88,95 Although the membrane module offers a great advantage with regard to mass transfer, this configuration is relatively intricate.88,96 As shown in Fig. 2E, GLRs result in improved mixing and a more defined flow profile.90 Nonetheless, in GLRs, there is a problem of substrate limitation due to gas voids in the downcomer area. To solve these bottlenecks in conventional BCRs and GLRs, an external pump is attached to the BCR, which improves liquid phase mixing and achieves higher kLa values.91 In the literature, this reactor configuration is known as a forced circulation loop reactor.92
Data on syngas fermentation carried out at the pilot scale using different types of reactors have rarely been reported. However, the information extracted from published patents and articles indicates that the most famous companies, including BRI/Ineos Bio and LanzaTech, use the CSTR and loop reactor, respectively, for fermentation. Coskata has published a report stating the use of an HFM module reactor, but no information on reactors with a volume >10 L has been published.97 Additionally, Ineos Bio and Coskata went out of business, and LanzaTech is solely operating at a larger scale.89
To overcome the challenges of using different reactor types at the industrial scale, a bubble column with liquid circulation is expected to be a sound method for the successful commercialization of syngas fermentation. A standard column with circulation is not capital intensive, and the forced circulation facilitates sufficient mixing of the aqueous phase and gas–liquid mass transfer (GLMT) rates. Two main factors that determine the power input are the pump flow rate and the volume of substrate gas. For energy efficiency, GLMT can be optimized by installing internal packings, improving gas delivery systems, or using other supporting technologies.46,98 Additionally, moderate pressurization is advisable to enhance mass transfer, as high solubility at higher pressure makes the substrate gas available for the biocatalyst.46,89 Generally, simple design of the reactor and continuous fermentation have been proven to be better strategies to achieve higher kl values.31,55,84,89,99 A thorough comparison of different reactor types with regard to their performance and scale-up cost is given in Table 5. Furthermore, a two- or multistage system is preferable because it has improved process control: if acidogenesis and solventogenesis phases are separated, then process parameters can be optimized to enhance reactor productivity.80,84,100 A summary of different types of reactors, such as a CSTR (160 L), TBR (144 L), HFMBR (10 L), HFMBR (7.5 L), loop reactor (71 L), GLR (50 L), forced-circulation loop reactor with a secondary loop (390 L and 9800 L), MBBR (36 m3) with biocatalysts such as Clostridium ljungdahlii ERI2 ATCC 55380, Clostridium ljungdahlii ERI2 ATCC 55380, Clostridium ragsdalei ATCC BAA-622, Clostridium ragsdalei ATCC BAA-622, Clostridium autoethanogenum DSMZ 19630, and Clostridium autoethanogenum DSMZ 10061, with different specifications for each reactor is given in a previously published work.4 However, the most important parameters to choose a suitable reactor include achievable gas–liquid mass transfer rates, investment, operational costs, application perspective, scale-up and commercialization.
4.4. Culture strategies
Microbes interact with one another and have complementary roles in ecological networks where they coexist.101 Two-phase systems and cocultures have gained increasing attention for use in syngas fermentation, as they resemble the microflora of a real fermenter, which can promote the accumulation of value-added bioproducts. Cross-feeding processes create this synergy; for instance, the metabolic products formed by one or many organisms serve as substrates for the others.102 Mixed culture fermentation between Alkalibaculum bacchi and C. propionicum has been reported. The use of Alkalibaculum bacchi in a monoculture produced only ethanol from syngas, whereas the mixed culture produced additional bioproducts, including n-propanol and n-butanol, in addition to ethanol.14 According to another study, C. ljungdahlii and C. kluyveri were cross-fed to yield butanol and hexanol.35 Cocultures of C. kluyveri and C. autoethanogenum were employed in the chain elongation of organic acids and alcohols.103 For C. ljungdahlii alone, CO was lethal, but when employed in a coculture with C. autoethanogenum, the two microbes exhibited sound growth and produced butanol, butyric acid, hexanol and hexanoic acid.36 Syngas was used as the primary substrate to develop a two-stage fermentation system to produce malic acid.37 In the first reactor, syngas was converted into acetic acid using C. ljungdahlii as a biocatalyst that was later used as a carbon source for the growth of Aspergillus oryzae in the second reactor. This implies that syngas can be converted into a variety of value-added bioproducts and can be used as an alternative substrate to produce malic acid, which generally grows on sugars and glycerol. Another study proposed a two-stage fermentation in which syngas (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2
CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2
H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2, 30
N2, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) fixation occurred in the first stage by utilizing C. autoethanogenum under anaerobic conditions to obtain 2.66 g L−1 acetic acid, ethanol and 2,3 butanediol.7 In the second stage, mixed microbial consortia (MMCs) were employed to produce PHA in a fed-batch culture utilizing first-stage products as substrates. Recently, the genetic editing tool CRISPR/Cas9 has been employed for deleting the target genes in C. coskatii, C. ljungdahlii and C. autoethanogenum to modify the genome of these acetogens to make them more efficient in increasing the production of desired products.38,39,104 Among all strategies mentioned above, cocultures of microorganisms are a promising approach to expand syngas utilization to obtain a broad spectrum of high value-added bioproducts. This is because they are potentially more resilient than monoculture approaches. Furthermore, apart from in vivo studies, many researchers are making efforts to model synthetic cocultures in silico on the basis of kinetic or genomic data.105 Microbial coculture can be combined with experimental work and in silico simulations to improve and accelerate the ecology of consortia, leading to advanced microbial cultivation. While cross-feeding seems to be a promising approach for designing synthetic communities, microbial interactions, including metabolite secretion and competition, can most likely lead to lower efficiency of the coculture. Sometimes ‘unwanted interactions’ can be helpful in creating ‘artificial’ interdependencies to make the cultures more robust. For instance, some strains that generate toxins to kill an undesired contaminant or have a metabolic activity that triggers a specific metabolism can be exploited. However, microbial interactions and the effects of prolonged coevolution during this process still need to be explored. These topics require further research to gain deep insights into the applicability of the most suitable culture strategies in the biotechnology industry.
40) fixation occurred in the first stage by utilizing C. autoethanogenum under anaerobic conditions to obtain 2.66 g L−1 acetic acid, ethanol and 2,3 butanediol.7 In the second stage, mixed microbial consortia (MMCs) were employed to produce PHA in a fed-batch culture utilizing first-stage products as substrates. Recently, the genetic editing tool CRISPR/Cas9 has been employed for deleting the target genes in C. coskatii, C. ljungdahlii and C. autoethanogenum to modify the genome of these acetogens to make them more efficient in increasing the production of desired products.38,39,104 Among all strategies mentioned above, cocultures of microorganisms are a promising approach to expand syngas utilization to obtain a broad spectrum of high value-added bioproducts. This is because they are potentially more resilient than monoculture approaches. Furthermore, apart from in vivo studies, many researchers are making efforts to model synthetic cocultures in silico on the basis of kinetic or genomic data.105 Microbial coculture can be combined with experimental work and in silico simulations to improve and accelerate the ecology of consortia, leading to advanced microbial cultivation. While cross-feeding seems to be a promising approach for designing synthetic communities, microbial interactions, including metabolite secretion and competition, can most likely lead to lower efficiency of the coculture. Sometimes ‘unwanted interactions’ can be helpful in creating ‘artificial’ interdependencies to make the cultures more robust. For instance, some strains that generate toxins to kill an undesired contaminant or have a metabolic activity that triggers a specific metabolism can be exploited. However, microbial interactions and the effects of prolonged coevolution during this process still need to be explored. These topics require further research to gain deep insights into the applicability of the most suitable culture strategies in the biotechnology industry.
5. Fermentation system modeling
5.1. Kinetic modeling
Thermodynamics govern the course and potential size of the events that take place along the production route, whereas kinetics characterizes the speed of those reactions as well as the overall rates of CO and H2 consumption, acetic acid and ethanol build-up, and cell growth. Overall rates are anticipated to be proportional to the number of cells in the fermenter (XVL), with the specific growth rate (μ) for growth and the specific absorption rate (qCO for CO and qH2 for H2) serving as the coefficients of proportionality. A kinetic model, such as Michaelis–Menten for enzyme-mediated processes, is used to connect individual reaction rates to the concentrations of the reactants and products. In a kinetic model such as the Monod equation, the specific growth and uptake rates are also associated with the concentration of substrates, such as CO and H2, inside the cell.101Basically, kinetic models aim to describe certain features of cellular function or production. Biotechnology is interested in any procedure that could affect how well a cell factory functions. The scope and concentration of potentially pertinent kinetic models are broad due to the variety of cell factories and the abundance of interesting products that can be created by them. Depending on the issue, they may focus on internal or environmental factors such as temperature, pH, osmolality, product and byproduct toxicity, and the type and mode of the fermenter. These include cellular processes such as metabolism, protein maturation and secretion, signaling, gene regulation, stress responses, cell cycle progression, and apoptosis.106
Various kinetic models, such as spatiotemporal metabolic models, have also been employed to solve the bottlenecks of syngas fermentation. A study developed a model for a BCR using C. ljungdahlii as a model organism for syngas fermentation. This modeling approach comprised combining a genome-scale reconstruction of C. ljungdahlii metabolism with multiphase transport equations that consider both the process of convection and dispersion occurring in the column. The reactor model equations and programs were solved using the MATLAB-based code DFBA lab. Simulations were carried out to analyze the significant parameters, including the ethanol-to-acetate ratio, ethanol titer, and conversion of CO and H2. The model predictions obtained from this study might be helpful in cellular and process engineering to overcome the main bottlenecks for ethanol production in a BCR.107 Another study developed a model for predicting the performance of a syngas-fermenting BCR for the recovery of anhydrous ethanol. An optimization framework was developed that employed artificial neural networks and genetic algorithms to enhance various process conditions and design variables, including the MESP, cost, energy efficiency and reactor productivity.108 Another study employed a spatiotemporal metabolic model by using CO fermentation data assembled from a laboratory-scale BCR using MATLAB. This modeling approach comprised combining a genome-scale reconstruction of C. autoethanogenum metabolism with multiphase transport equations that consider both the convective and dispersive processes that impact CO transport, biomass, and secreted byproducts. The simulated values were in conformity with the measured values for acetate, ethanol, and biomass concentrations at a single gas flow rate. Later, the predictions were carried out at three different gas flow rates. Spatiotemporal modeling has the potential to assist in the commercial-scale design of processes involved in syngas fermentation to produce high value-added bioproducts.109 Other kinetic models have been developed to study ethanol and acetate production from syngas fermentation. For example, a study employed kinetic modeling to investigate the parameters affecting syngas fermentation in different pressurized batch bioreactors, such as substrate uptake, intrinsic growth, and product recovery, for anaerobic bacteria using C. ljungdahlii as the model organism. A dual substrate such as CO and H2 growth kinetic model using Luong and Monod was developed for describing the growth of C. ljingdahlii. A maximum specific growth rate of 0.195 h−1 and Monod constants of 0.855 atm and 0.412 atm for CO and H2 were achieved. Furthermore, the impacts of CO on the growth of the cell at high pressures were studied, and it was found that if the CO pressure exceeded 0.743 atm, no significant growth was observed. Other kinetic models that were employed to study the above-mentioned parameters include the Volterra model, Andrews model, and modified Gompertz model.110
Conclusively, a model that describes the aforementioned characteristics of cell factories may be created specifically for a given application, such as a particular pathway for the manufacture of a particular metabolite, or it may describe more generic characteristics. The vast range of timeframes of often modeled processes reflects the diversity of kinetic model objectives and domains in biotechnology.
5.2. Reactor modeling
Computational fluid dynamics (CFD) is a computer-assisted technique that is generally employed to simulate complex fluid flow using numerical approaches to predict fluid mechanics and heat transfer. Its calculation is based on model division of a real piece in various cells, which is followed by applying differential equations to each individual cell to improve the flow models by obtaining the evolution of physical properties and flow variables, including temperature, pressure, velocity, and turbulence.111 A recent study employed the CFD technique to investigate syngas fermentation in a 125 m3 BCR using C. ljungdahlii DSM 13528 as a model organism. The factors that were investigated included short- and long-term responses, circulation time and bacterial motion. A pseudostationary gas gradient was conducted with CFD in a Euler–Euler approach, whereas the microorganism movement was simulated as Lagrangian massless particles. Lagrangian trajectory analysis of the gas gradients of a BCR is helpful in predicting performance and therefore minimizing the risk in scale-up. Although CFD has been employed in different studies for reactor modeling, there are some disadvantages to CFD simulations, including accurate modeling of the composition. Therefore, scientists have developed alternative models to study the desired thermodynamic properties in the virtual setting and to better address the complexity of the process.1125.3. Process modeling
One of the most popular chemical engineering tools is the commercially available software called Aspen Plus®, designed by AspenTech®. It is commonly used for simulating real processes by investigating any compound and mixture under various operational conditions. Many studies have employed Aspen modeling to optimize reactor performance. A study developed a simulation model with ASPEN Plus for the gasification of lignocellulosic biomass combined with syngas fermentation to produce ethanol.113 The simulation was carried out in three modules: biomass gasification, syngas fermentation, and ethanol recovery. The model predicted that a gasification temperature of 700–1000 °C and an equivalence ratio from 0.2–0.4 were optimal for high ethanol production.113 Another study also proposed a novel simulation model using Aspen Plus® based on the biorefinery concept for the conversion of 1200 tons per day of switchgrass to anhydrous ethanol.114 The simulation model in this study was based on the same three modules as mentioned above and predicted an annual production of approximately 36.5 million gallons of anhydrous ethanol. Another study modeled an HFMBR to solve the problem of gas–liquid mass transfer during syngas fermentation by optimizing three operational factors: membrane surface area per working volume (A/v), water velocity (VL), and specific gas flow rate (Vg). The maximum kl of 385.0 h−1 in water was observed for the mass transfer of CO at an A/v of 0.56 cm−1, a VL of 2.20 cm s−1, and a Vg of 1.02 min−1, which is relatively higher than the kl values given by other studies using different models. A three-factor quadratic model and a dimensionless model were developed from experimental data with high correlation coefficients to facilitate the scale-up process. The most significant factor with regard to kl is the membrane surface area, which positively impacts the process.94 The membrane surface area is an important parameter considered in process modeling because CO and H2 are less soluble in water than O2 (104 g g−1) at 37 °C, and therefore, gas–liquid mass transfer is difficult. To improve gas–liquid mass transfer, numerous studies have examined various reactor designs and configurations including hollow fiber membrane reactors (HFMRs), bubble column reactors, trickling bed reactors, CSTRs, and gas lift reactors.According to Yasin et al., the HFMR volumetric mass transfer coefficient kLa increases as the membrane surface area per unit working volume and pressure increases. In microbial syngas systems, researchers have suggested utilizing a submerged HFMR, which results in high CO mass transfer rates. As the fluctuations in the pressure through the hollow fiber lumen and the membrane's surface area per unit working volume of the liquid influence the gas–liquid kLa,115 a study has reported that with a lower pressure of 37.23 kPa and a larger membrane surface area per unit working volume AS/VL of 62.5 m−1, a higher volumetric mass transfer coefficient kLa of 155.16 h−1 can be achieved.116 In other types of reactors, such as BCRs, the most important parameters that are considered include gas and liquid phase concentrations, whereas in CSTRs, the specific gas uptake, cell retention time, ethanol and acetic acid concentration, cell concentration, CO2 conversion efficiency, and H2 conversion efficiency are important parameters.
Different studies have been carried out to use different modeling approaches to study these parameters to solve the main bottlenecks of syngas fermentation. A summary of these is given in Table 6.
| Reactor type | Parameters and modeling approaches | Main findings | Microbial catalyst | Ref. |
|---|---|---|---|---|
| Bubble column reactors | Gas and liquid phase concentrations | Mass transfer coefficient of 500 h−1 for CO; predicted ethanol (130 g L−1, predicted conversion of H2 and CO of 89% and 34%) | C. ljungdahlii | 107 |
| Modeling approach: MATLAB based code DFBA lab | ||||
| Down-draft reactors | Low heating value (LHV), temperature, equivalence ratio (ER), cold gas efficiency (CGE), and quantity of bioethanol | Optimal gasification temperature (700–1000 °C). Ethanol 0.114 kg h−1 per 1 kg h−1 of garden waste | C. ljungdahlii | 113 |
| Modeling approach: ASPEN plus V.10; the non-random-two-liquid (NRTL) model; HCOALGEN and DCOALIGT models; MCINCPSD stream, three sub streams of the MIXED, CIPSD, and NCPSD classes | ||||
| CSTR | Specific gas uptake, cell retention time, ethanol & acetic acid concentration, cell concentration, CO2 conversion efficiency, H2 conversion efficiency | Predicted ethanol production of 36.5 million gallons per year | C. ragsdalei | 114 |
| Modeling approach: Aspen Plus V. 8.2; NRTL model | ||||
| External microporous hollow fiber membrane diffuser | CO transfer, membrane surface area per working volume, water velocity, and specific gas flow rate | Higher kl of CO using an HFM diffuser, the membrane area positively impacts CO kl | NR | 94 |
| Modeling approach: empirical (quadratic and dimensionless) regressions | ||||
| Pressurized batch bioreactors | Growth kinetics of bacteria with CO and H2 at high partial pressures, substrate uptake rate & product formation | Maximum CO uptake rate is 34.364 mmol g−1 cell h−1, maximum production rate of ethanol and acetate is 0.172 and 0.096 mmol L−1 h−1, respectively, at a P(CO) of 0.598 atm and 0.539 atm, respectively. For H2 the maximum growth rate was achieved at 0.412 atm | C. ljungdahlii | 110 |
| Modeling approach: luong + monod; double-experimental + monod; luong + tessier; double-experimental + moser; luong + moser; double-experimental + tessier; andrew + tessier; edwards + monod; edwards + tessier; andrew + monod; haldane + moser; haldane + monod | ||||
| CSTR | Gas composition, dilution rate, gas flow rates, and cell recycling | The predicted ethanol production was 2 g L−1 h−1 with a substrate gas composition of 54% CO and 46% H2 and a dilution rate of 0.06 h−1 | C. ljungdahlii | 108 |
| Modeling approach: MATLAB | ||||
| Bench-scale bubble column reactor | CO and CO2 gas–liquid mass transfer, cellular consumption of CO and CO2, and production of ethanol, acetate, 2,3-butanediol, lactate and CO2 | Ethanol production of 18.7 g L−1 d−1 and acetate production of 10.2 g L−1 d−1 at a gas flow rate of 700 ml min−1 at 53.5% conversion of inlet CO | C. autoethanogenum | 109 |
| Modeling approach: MATLAB code DFBA lab | ||||
| Bubble column reactor | Bacterial motion patterns, circulation time, qnd short- and long-term responses | Substrate limitations (97% of all cells) while 84% react to CO limitations when exposed to the stress zone for more than 70 s | C. ljungdahlii | 112 |
| Modeling approach: computational fluid dynamics |
5.4. Life cycle assessment
The environmental performance of biofuels requires evaluation that helps in technology and policy development for their sustainable production.117 Recently, the assessment of environmental performance has been carried out through life cycle assessment (LCA), which is a standard tool given by the International Organization for Standardization (ISO) for quantifying the environmental impacts of natural resource utilization and emissions.118 This is done by collecting relevant information about the material and energy requirements and outputs of each step of the product life cycle, as shown in Fig. 3.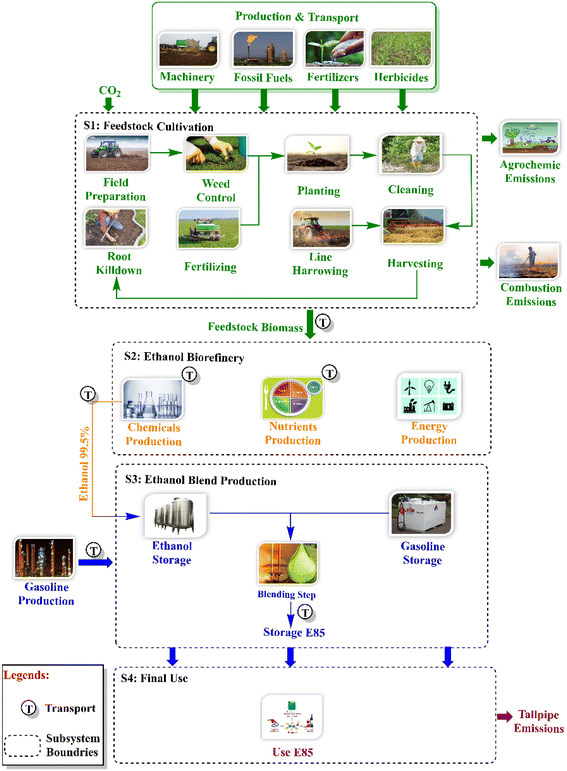 | ||
| Fig. 3 Life cycle assessment; reproduced from119, with permission from Elsevier, copyright 2023. | ||
According to preliminary life-cycle assessment (LCA) research on the environmental effects of China's steel mill waste petrol conversion to ethanol, there is a possibility for a low-emission fuel product. This article provides preliminary LCA results for ethanol made from biogenic (corn stover, forestry residues, and switch grass) feedstock and updates data for syngas conversion to ethanol based on design enhancements from LanzaTech's scale-up work and application of the technology to a domestic United States market. GHG emissions are calculated for each phase of the ethanol life cycle and contrasted with those of other ethanol-producing methods and conventional transportation fuels.120
Various studies have carried out life cycle assessments of bioethanol and improvements have been observed in environmental performance with regard to greenhouse gas (GHG) emissions and consumption of energy compared to gasoline, which are summarized in Table 7. Considering industries, a study has used the LCA method to quantify the global warming potential of different scenarios for bioethanol with the LanzaTech process. Different scenarios were considered in which ethanol was produced from industry off gases as well as biomass such as corn stover, forest residue, or switchgrass, extracting input data from published reports, databases, and estimates of LanzaTech engineering processes. According to the standard LCA method, ethanol production via LanzaTech fermentation has reported a 60% reduction in GHG emissions compared with that of conventional gasoline, and biomass-based ethanol achieves approximately 90% reductions in emissions. All the input parameters have been reported in the published work.120 It was reported that the fermentation technology reported by LanzaTech is a viable option to produce next-generation biofuels that meet the standard policy requirements of United States Renewable Fuels regarding the fuels with the lowest footprint. Conclusively, LCA proposes a unique biorefinery design that is energy efficient and mitigates the environmental effects of biomass-derived ethanol production.
| Object of the assessment | Experimental inputs | Output products | System boundaries | Ref. | |
|---|---|---|---|---|---|
| Biomasses | Processing technologies | ||||
| Switchgrass | Biorefinery | Pretreatment, enzymatic hydrolysis, fermentation, recovery, and purification | Ethanol, electricity, furfural, acetic, and formic acid | Switchgrass cultivation, switchgrass transportation to the production facility, ethanol and co-product production, and ethanol distribution | 118 |
| Rice straw | Ethanol plant | Extraction process including water and varying alkali concentrations, dilute acid pretreatment, enzymatic hydrolysis, and ethanol production | Ethanol | Feedstock acquisition and ethanol production and transport | 121 |
| Wheat straw | Biochemical conversion | An advanced second-generation technology | Ethanol | WS cultivation, transportation, ethanol production, ethanol blending and distribution, and use in E10 and E85 passenger cars | 122 |
| Miscanthus | Biorefinery | Pretreatment, enzymatic hydrolysis, fermentation, and distillation/separation | Ethanol | Miscanthus cultivation, production, and transport of needed inputs such as fertilizers, pesticides, propagation material, management and harvesting operations | 123 |
| Cassava straw | Biorefinery | Improved hydrothermal pretreatment, enzymatic hydrolysis, fermentation, distillation, and adsorption dehydration | Bioethanol | Cultivation, transportation, preparation, and bioethanol conversion | 124 |
| Biomasses of first- and second-generation type | Biochemical conversion | Pretreatment, fermentation and ethanol recovery, wastewater treatment plant (WWTP), anaerobic digestion (AD) and combined heat and power (CHP) system | Ethanol; electricity surplus | (Well-to-wheel) bioethanol production from biomass at the plant gate to the ethanol end use | 125 |
| Corn | Biorefinery | Cleaning/milling, liquefaction/fiber separation, fermentation, and distillation | Ethanol | (Cradle-to-gate) analysis including infrastructure and fertilizer, pesticide and diesel use and the production of anhydrous ethanol at the plant | 126 |
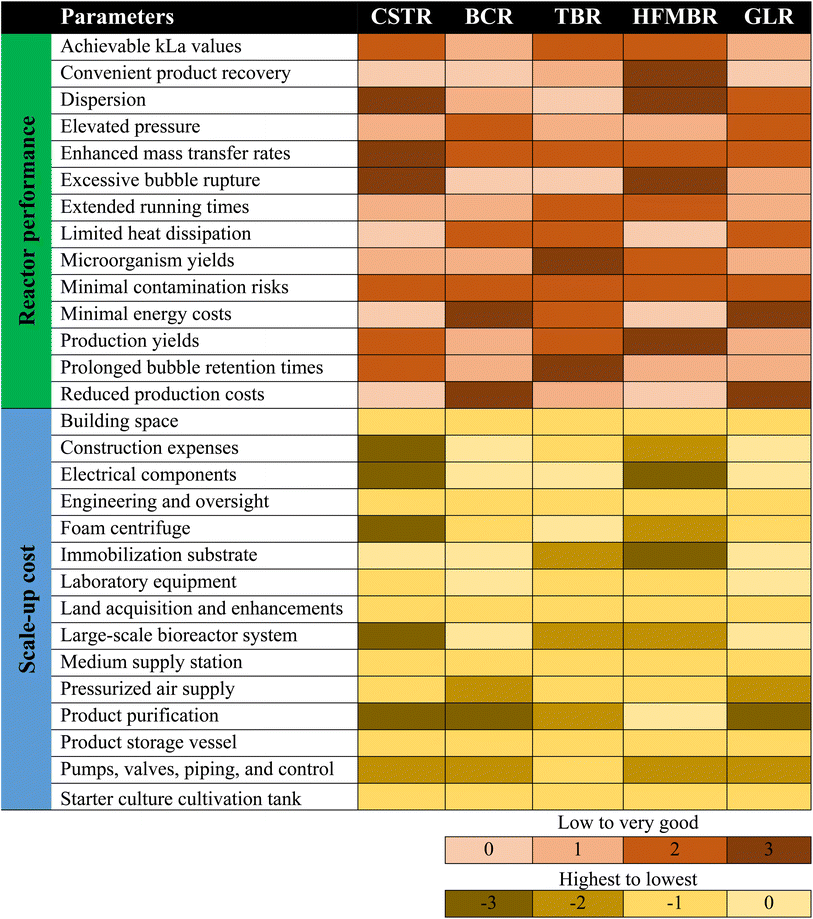
6. Scale up and commodification: a global perspective
Generally, the most efficient syngas bioreactor achieves enhanced mass transfer rates and high cell concentrations and is economical at the same time.54 For the evaluation of syngas reactors, a ranking system was proposed in previously published studies, which mainly focused on reactor performance and scale-up costs.112 The main parameters for scale-up include distribution, bubble breakage, bubble retention times, mass transfer rates, low production costs, low energy costs, high pressure, low contamination risks, low heat dissipation, microorganism yields, easy product recovery, and kl values. Due to the evaluation system, it was established by a previous study that the best performing reactors for gas fermentation include the microbubble STR combination and MBBR. The scale-up approximation factors include land acquisition and improvements, building area, laboratory instruments, large-scale bioreactor system, medium supply station, starter culture cultivation tank, immobilization material, product purification, product storage tank, centrifuge (foam) pumps, valves, piping and control, electrical engineering and supervision and construction expenses. It was reported that the most cost-efficient reactors in scale-up are the BCR, ALR and TBR. Considering the design and scale-up parameters, the most appropriate reactor configuration for syngas fermentation includes the BCR, ALR, MBR and TBR.Until 2012, only three companies named INEOS Bio, Coskata and LanzaTech were dealing with the commercialization of syngas fermentation.21 However, the expansion of this industry has taken place over the last decade, and this section introduces all current industrial initiatives along with the substrates and products summarized in Table 8. INEOS Bio (2013) is a subsidiary of INEOS that utilizes propriety isolates of C. ljungdahlii. Their pilot-scale demonstration reported a production of 380 L ethanol per ton of dry feedstock. First, the gasification of the feedstock is carried out, and later, it is fermented to bioethanol and other high value-added bioproducts. Another company is Coskata, which uses C. ragsdalei and C. carboxidivorans as licensed biocatalysts from Oklahoma. Other strains, such as C. coskatii, have also been reported to be used for ethanol production. Coskata has reportedly produced 380 L of ethanol per ton of dry softwood in Madison and Pennsylvania. In 2015, Coskata ran out of business, and Synata Bio acquired the technology in 2016. LanzaTech (2005) has a diverse product range primarily focusing on ethanol and 2,3-butanediol production. Apart from syngas, LanzaTech also utilizes CO-rich industrial off-gases for producing chemicals, including 2,3-butanediol, butanol and ethanol. A demonstration plant has been set up in Auckland, New Zealand, that generates 380 L ethanol per year utilizing off-gases from steel mills. Industrial scale facilities have been established in China since 2017 with an annual production of 16 M gallons and in Gent (Belgium) with ArcelorMittal since 2018 with an annual production of 21 M gallons (LanzaTech, 2018). More commercial scale projects have been established in South Africa (Swayana), India (Indian Oil) and California (Aemetis) utilizing ferroalloy off-gases, refinery off-gases and gasified orchard biomass, respectively.127
| Country | Company | Feedstock for syngas production | Products |
|---|---|---|---|
| USA | Coskata Inc., (http://www.coskata.com) | Non-renewable sources (natural gas, and coal), other gases include industrial gases; renewable sources such as lignocellulosic biomass | Butanol, butanediol, ethanol, propanol, hexanol, and fatty acids |
| OPX Biotechnologies Inc., (http://www.opxbio.com) | CO2 and H2 | Fatty acids | |
| LanzaTech Inc., (http://www.lanzatech.com) | Steel mill off gas and coal producers; lignocellulosic biomass such as wood residues | Ethanol and 2,3-butanediol | |
| Syngas Biofuels Energy Inc., (http://www.syngasbiofuelsenergy.com) | CO2 and H2 | Fuel n-butanol | |
| BRI Energy Inc., USA (http://www.brienergy.com) | Organic waste such as municipal solid waste (MSW), agricultural residues and coal | Ethanol and electrical energy | |
| Aemetis Inc (https://www.aemetis.com/) | Orchard wood and nutshells | Ethanol | |
| Switzerland | INEOS Bio, (http://www.ineos.com/businesses/ineos-bio/company) | Organic waste | Ethanol |
| Netherland | BioMethanol Chemie Nederland B.V., (http://www.biomcn.eu/) | Bio-feedstock like biogas & feedstock such as glycerine | Methanol |
| EU | SYNPOL project platform, EU (http://www.synpol.org) | MSW, sewage sludge and agricultural residues | Polyhydroxyalkanoates, hydroxybutyrate, butanediol, and succinate |
| China | Beijing Shougang Co., Ltd. (http://www.shougang.com.cn/) | Syngas produced as steel mill-off gas | Ethanol |
| Jupeng Biotech Ltd. China (http://www.jupengbio.com/) | Organic waste | Ethanol | |
| Luxembourg | ArcelorMittal (http://corporate.arcelormittal.com) | Steel mill-off gas (syngas) | Ethanol |
| South Africa | Swayana http://www.swayana.co.za | Ferroalloy off-gases | Ethanol |
| India | IndianOil https://iocl.com | Refinery off-gases | Ethanol |
The White Dog Lab was established to work on mixotrophic fermentation by utilizing sugars and syngas to produce acetone and isopropanol, which was also exhibited by LanzaTech (2017). Although many companies that ferment syngas are currently operating, their efficiency and profitability are not clear, and more research is required to study the commercialization aspect. To make the business more profitable, new fermentation products can be introduced with higher market prices while proposing cost-effective scale-up technologies. Conclusively, syngas fermentation has high potential with a steadily growing market.
7. Innovative strategies to optimize the syngas fermentation process
With the above-mentioned detailed review of the recent advancements in syngas fermentation technologies for biofuel and biochemical production, it is important to indicate that with the main bottlenecks of syngas fermentation, such as mass transfer and cell factories, multiple advanced synthetic biology techniques can be employed, rather than individual applications, to determine the solutions to the current problems for the production of biofuels and biochemicals, as shown in Fig. 4. The behavior of the cell in the reactor flow field can be analyzed by employing CFD modeling. Multiomics analysis can be used to enhance the mechanism of substrate utilization, product metabolism and metabolic migration in the gas fermentation process. It is expected that if genomic, metagenomic, and metabolic information is widely available, it can allow the development of precise computational algorithms that are useful in predicting the most efficient biosynthetic pathways. Moreover, optimized pathways when combined with the latest experimental tools reduce efforts and facilitate the construction and optimization of metabolic pathways. Reactor optimization should be based on the combination of metabolism and reactor simulation. It is not a traditional optimization method based on trial and error. Hence, reactor optimization, CFD modeling, and other related technologies can be integrated to establish efficient metabolic flow control methods for efficient conversion to ethanol. As these technologies advance, it is expected that the goal of enhancing syngas fermentation will be achieved soon while overcoming the shortcomings of the process.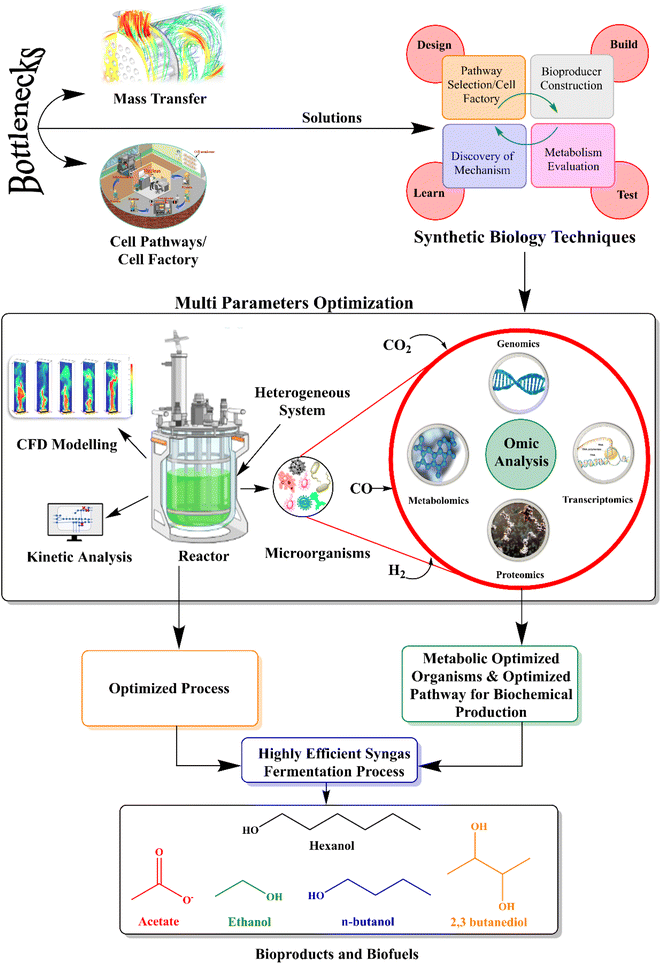 | ||
| Fig. 4 Innovative strategies to enhance biofuel and biochemical production from syngas fermentation. | ||
8. Conclusions
Syngas fermentation is a convenient method to convert CO2, H2 and CO gaseous streams into fuels and chemicals. However, the main factors that contribute to enhance the fermentation process are the modifications of the bioreactor design for increasing mass transfer rates, enhancing the concentration of microbial cells in the bioreactor, optimization of economical media, genetic engineering of syngas-fermenting microbes with relatively higher rates of gas uptake and product yield, and process control and modeling. Although significant advancements in syngas fermentation have been reported for making it a commercially feasible technology, more research is needed for the advanced development of syngas fermentation technology to improve the productivity of high value-added bioproducts at low costs. If genetically modified microorganisms are developed by employing advanced synthetic biology techniques, they will lead to enhancements in process efficiency and control. Conclusively, syngas fermentation technology has the potential for utilizing abundantly available organic waste to produce biofuels and other value-added chemicals with no adverse environmental impacts; therefore, the development of this technology can significantly contribute to sustainable development.Abbreviations
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| H2 | Hydrogen |
| CAGR | Compound annual growth rate |
| WLP | Wood–Ljungdahl pathway |
| ATP | Adenosine triphosphate |
| CODH | Carbon monoxide dehydrogenase |
| ACS | Acetyl-CoA synthase |
| CSTR | Continuous stirred-tank reactor |
| LCA | Life cycle assessment |
| CHF | Composite hollow fiber |
| ANN | Artificial neural network |
| VFA | Volatile fatty acid |
| BCR | Bubble column reactor |
| TBR | Trickle bed reactor |
| HFMBR | Hollow fiber membrane bioreactor |
| GLR | Gas lift bioreactor |
| FBR | Fiber bioreactor |
| GLMT | Gas–liquid mass transfer |
| MMC | Mixed microbial consortia |
| ISO | International Organization for Standardization |
| CFD | Computational fluid dynamics |
Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This project was financially supported by the National Key Research and Development Program of China (2018YFA0901500), Science and Technology Partnership Program, Ministry of Science and Technology of China (KY202001017), and Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-IJCP-001-04 and TSBICIP-BRFI-002).References
- N. L. Slatter, B. Vichanpol, J. Natakaranakul, K. Wattanavichien, P. Suchamalawong, K. Hashimoto, N. Tsubaki, T. Vitidsant and W. Charusiri, ACS Omega, 2022, 7, 44951–44961 CrossRef CAS PubMed.
- A. Schreiber, A. Peschel, B. Hentschel and P. Zapp, Front. Energy Res., 2020, 8, 1–17 CrossRef.
- Y. He, C. Kennes and P. N. L. Lens, Chemosphere, 2022, 299, 134425 CrossRef CAS.
- I. K. Stoll, N. Boukis and J. Sauer, Chem.-Ing.-Tech., 2020, 92, 125–136 CrossRef CAS.
- F. Regis, A. H. A. Monteverde and D. Fino, Energy, 2023, 274, 127318 CrossRef CAS.
- V. Ahuja, A. K. Bhatt, B. Ravindran, Y. H. Yang and S. K. Bhatia, Sustain, 2023, 15, 1–21 Search PubMed.
- B. Lagoa-Costa, H. N. Abubackar, M. Fernández-Romasanta, C. Kennes and M. C. Veiga, Bioresour. Technol., 2017, 239, 244–249 CrossRef CAS PubMed.
- T. Janković, A. J. J. Straathof and A. A. Kiss, Sep. Purif. Technol., 2023, 322, 124320 CrossRef.
- J. Bae, Y. Song, H. Lee, J. Shin, S. Jin, S. Kang and B. K. Cho, Chem. Eng. J., 2022, 428, 131325 CrossRef CAS.
- S. Shen, G. Wang, M. Zhang, Y. Tang, Y. Gu, W. Jiang, Y. Wang and Y. Zhuang, Bioresour. Bioprocess., 2020, 7, 1–13 CrossRef.
- M. T. Allaart, M. Diender, D. Z. Sousa and R. Kleerebezem, Microb. Biotechnol., 2023, 16, 697–705 CrossRef CAS.
- P. Hu, S. Chakraborty, A. Kumar, B. Woolston, H. Liu, D. Emerson and G. Stephanopoulos, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 3773–3778 CrossRef CAS PubMed.
- K. Liu, H. K. Atiyeh, B. S. Stevenson, R. S. Tanner, M. R. Wilkins and R. L. Huhnke, Bioresour. Technol., 2014, 151, 69–77 CrossRef CAS.
- K. Liu, H. K. Atiyeh, B. S. Stevenson, R. S. Tanner, M. R. Wilkins and R. L. Huhnke, Bioresour. Technol., 2014, 152, 337–346 CrossRef CAS.
- P. Dürre, FEMS Microbiol. Lett., 2016, 363, 1–7 CrossRef PubMed.
- M. Köpke, C. Mihalcea, F. M. Liew, J. H. Tizard, M. S. Ali, J. J. Conolly, B. Al-Sinawi and S. D. Simpson, Appl. Environ. Microbiol., 2011, 77, 5467–5475 CrossRef.
- J. R. Phillips, H. K. Atiyeh, R. S. Tanner, J. R. Torres, J. Saxena, M. R. Wilkins and R. L. Huhnke, Bioresour. Technol., 2015, 190, 114–121 CrossRef CAS.
- A. M. Henstra, J. Sipma, A. Rinzema and A. J. Stams, Curr. Opin. Biotechnol., 2007, 18, 200–206 CrossRef CAS PubMed.
- A. Katsyv and V. Müller, Front. Bioeng. Biotechnol., 2020, 8, 1–23 Search PubMed.
- S. W. Ragsdale and E. Pierce, Biochim. Biophys. Acta - Proteins Proteom., 2008, 1784, 1873–1898 CrossRef CAS.
- J. Daniell, M. Köpke and S. D. Simpson, Energies, 2012, 5, 5372–5417 CrossRef CAS.
- F. Fischer, R. Lieske and K. Winzer, Biochem. Z., 1932, 245, 1–12 CAS.
- K. T. Wieringa, Antonie Van Leeuwenhoek, 1940, 6, 251–262 CrossRef.
- F. E. Fontaine, W. H. Peterson, E. McCOY, M. J. Johnson and G. J. Ritter, J. Bacteriol., 1941, 43, 701–715 CrossRef.
- G. Pregnon, Characterization of the metabolism of Eubacterium limosum by a quantitative systems biology approach, Université de Toulouse, 2023 Search PubMed.
- A. Mickan, Central dissimilatory pathways of the thermophilic acetogen Thermacetogenium phaeum, Universität Konstanz, 2019 Search PubMed.
- B. R. S. Genthner and M. P. Bryant, Appl. Environ. Microbiol., 1987, 53, 471–476 CrossRef PubMed.
- A. J. Grethlein, R. M. Worden, M. K. Jain and R. Datta, Appl. Biochem. Biotechnol., 1990, 24–25, 875–884 CrossRef CAS.
- J. S. C. Liou, D. L. Balkwill, G. R. Drake and R. S. Tanner, Int. J. Syst. Evol. Microbiol., 2005, 55, 2085–2091 CrossRef CAS.
- A. S. Gößner, F. Picardal, R. S. Tanner and H. L. Drake, FEMS Microbiol. Lett., 2008, 287, 236–242 CrossRef PubMed.
- J. L. Gaddy, US Pat., US006136577A, 1996, vol. 136, pp. 423–464 Search PubMed.
- K. Arslan, B. Bayar, H. Nalakath Abubackar, M. C. Veiga and C. Kennes, Bioresour. Technol., 2019, 292, 121941 CrossRef CAS PubMed.
- R. Kerby and J. G. Zeikus, Curr. Microbiol., 1983, 8, 27–30 CrossRef CAS.
- P. L. Tremblay, D. Höglund, A. Koza, I. Bonde and T. Zhang, Sci. Rep., 2015, 5, 1–11 Search PubMed.
- H. Richter, B. Molitor, M. Diender, D. Z. Sousa and L. T. Angenent, Front. Microbiol., 2016, 7, 1–13 Search PubMed.
- S. Benito-Vaquerizo, M. Diender, I. Parera Olm, V. A. P. Martins dos Santos, P. J. Schaap, D. Z. Sousa and M. Suarez-Diez, Comput. Struct. Biotechnol. J., 2020, 18, 3255–3266 CrossRef CAS PubMed.
- F. Oswald, S. Dörsam, N. Veith, M. Zwick, A. Neumann, K. Ochsenreither and C. Syldatk, Front. Microbiol., 2016, 7, 1–12 Search PubMed.
- S. Flüchter, S. Follonier, B. Schiel-Bengelsdorf, F. R. Bengelsdorf, M. Zinn and P. Dürre, Biomacromolecules, 2019, 20, 3271–3282 CrossRef PubMed.
- R. S. P. de Lemgruber, K. Valgepea, R. Tappel, J. B. Behrendorff, R. W. Palfreyman, M. Plan, M. P. Hodson, S. D. Simpson, L. K. Nielsen, M. Köpke and E. Marcellin, Metab. Eng., 2019, 14–23 CrossRef PubMed.
- S. Joshi and S. D. Mishra, Bioresour. Technol., 2022, 352, 127037 CrossRef CAS.
- F. Liew, A. M. Henstra, M. Kӧpke, K. Winzer, S. D. Simpson and N. P. Minton, Metab. Eng., 2017, 40, 104–114 CrossRef CAS PubMed.
- S. Dusséaux, W. T. Wajn, Y. Liu, C. Ignea and S. C. Kampranis, Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 31789–31799 CrossRef PubMed.
- G. Bokinsky, P. P. Peralta-Yahya, A. George, B. M. Holmes, E. J. Steen, J. Dietrich, T. S. Lee, D. Tullman-Ercek, C. A. Voigt, B. A. Simmons and J. D. Keasling, Proc. Natl. Acad. Sci. U.S.A., 2011, 108, 19949–19954 CrossRef CAS PubMed.
- J. Zhou, C. Wang, L. Yang, E. S. Choi and S. W. Kim, Enzyme Microb. Technol., 2015, 68, 50–55 CrossRef CAS PubMed.
- J. Abatemarco, A. Hill and H. S. Alper, Biotechnol. J., 2013, 8, 1397–1410 CrossRef CAS PubMed.
- M. Yasin, N. Jang, M. Lee, H. Kang, M. Aslam, A. A. Bazmi and I. S. Chang, Bioresour. Technol. Rep., 2019, 7, 100207 CrossRef.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- P. C. Munasinghe and S. K. Khanal, Bioresour. Technol., 2010, 101, 5013–5022 CrossRef CAS.
- A. J. Ungerman and T. J. Heindel, Biotechnol. Prog., 2007, 23, 613–620 CrossRef CAS PubMed.
- D. Birch and N. Ahmed, Powder Technol., 1996, 88, 33–38 CrossRef CAS.
- J. A. Kaster, D. L. Michelsen and W. H. Velander, Appl. Biochem. Biotechnol., 1990, 24–25, 469–484 CrossRef CAS.
- M. D. Bredwell and R. M. Worden, Biotechnol. Prog., 1998, 14, 31–38 CrossRef CAS.
- S. Achinas, J. Mulder and G. J. W. Euverink, in Handbook of Biofuels, INC, 2022, pp. 511–527 Search PubMed.
- K. T. Klasson, M. D. Ackerson, E. C. Clausen and J. L. Gaddy, Resour., Conserv. Recycl., 1991, 5, 145–165 CrossRef.
- M. D. Bredwell, P. Srivastava and R. M. Worden, Biotechnol. Prog., 1999, 15, 834–844 CrossRef CAS PubMed.
- K. C. Lee and B. E. Rittmann, Water Res., 2002, 36, 2040–2052 CrossRef CAS.
- P. C. Munasinghe and S. K. Khanal, Bioresour. Technol., 2012, 122, 130–136 CrossRef CAS.
- F. E. Liew, R. Nogle, T. Abdalla, B. J. Rasor, C. Canter, R. O. Jensen, L. Wang, J. Strutz, P. Chirania, S. De Tissera, A. P. Mueller, Z. Ruan, A. Gao, L. Tran, N. L. Engle, J. C. Bromley, J. Daniell, R. Conrado, T. J. Tschaplinski, R. J. Giannone, R. L. Hettich, A. S. Karim, S. D. Simpson, S. D. Brown, C. Leang, M. C. Jewett and M. Köpke, Nat. Biotechnol., 2022, 40, 335–344 CrossRef CAS PubMed.
- V. Singh, S. Haque, R. Niwas, A. Srivastava, M. Pasupuleti and C. K. M. Tripathi, Front. Microbiol., 2017, 7, 1–16 CAS.
- C. F.-B. R. Robles-, M. C. Veiga, C. Kennes and C. Naveira-, Microb. Biotechnol., 2023, 1, 1–16 Search PubMed.
- J. Saxena and R. S. Tanner, World J. Microbiol. Biotechnol., 2012, 28, 1553–1561 CrossRef CAS PubMed.
- H. Im, T. An, R. Kwon, S. Park and Y. K. Kim, Biotechnol. Bioprocess Eng., 2021, 26, 476–482 CrossRef CAS.
- J. Saxena and R. S. Tanner, J. Ind. Microbiol. Biotechnol., 2011, 38, 513–521 CrossRef CAS PubMed.
- F. Ammam, P. L. Tremblay, D. M. Lizak and T. Zhang, Biotechnol. Biofuels, 2016, 9, 1–10 CrossRef PubMed.
- D. Li, C. Meng, G. Wu, B. Xie, Y. Han, Y. Guo, C. Song, Z. Gao and Z. Huang, J. Ind. Microbiol. Biotechnol., 2018, 45, 61–69 CrossRef CAS PubMed.
- Á. Fernández-Naveira, M. C. Veiga and C. Kennes, J. Chem. Technol. Biotechnol., 2017, 92, 1178–1185 CrossRef.
- C. Benevenuti, P. Amaral, T. Ferreira and P. Seidl, Reactions, 2021, 2, 391–407 CrossRef.
- K. Doll, A. Rückel, P. Kämpf, M. Wende and D. Weuster-Botz, Bioprocess Biosyst. Eng., 2018, 41, 1403–1416 CrossRef CAS PubMed.
- C. Benevenuti, M. Branco, M. Do Nascimento-Correa, A. Botelho, T. Ferreira and P. Amaral, Fermentation, 2021, 7, 1–13 CrossRef.
- D. Xu and R. S. Lewis, Biomass Bioenergy, 2012, 45, 303–310 CrossRef CAS.
- A. Infantes, M. Kugel, K. Raffelt and A. Neumann, Fermentation, 2020, 6, 1–26 Search PubMed.
- H. N. Abubackar, M. C. Veiga and C. Kennes, Bioresour. Technol., 2012, 114, 518–522 CrossRef CAS PubMed.
- X. Sun, H. K. Atiyeh, R. L. Huhnke and R. S. Tanner, Bioresour. Technol. Rep., 2019, 7, 100279 CrossRef.
- D. Li, C. Meng, G. Wu, B. Xie, Y. Han, Y. Guo, C. Song, Z. Gao and Z. Huang, J. Ind. Microbiol. Biotechnol., 2018, 45, 61–69 CrossRef CAS PubMed.
- US Pat., US 200702275447A1, 2007, pp. 1–8 Search PubMed.
- Á. Fernández-Naveira, H. N. Abubackar, M. C. Veiga and C. Kennes, Appl. Microbiol. Biotechnol., 2016, 100, 3361–3370 CrossRef PubMed.
- F. M. Pereira, M. M. Alves and D. Z. Sousa, in 13th World Congress on Anaerobic Digestion, 2013, vol. 36, pp. 1–4 Search PubMed.
- F. Oswald, I. K. Stoll, M. Zwick, S. Herbig, J. Sauer, N. Boukis and A. Neumann, Front. Bioeng. Biotechnol., 2018, 6, 1–9 Search PubMed.
- US Pat., US 10,017,789 B2, 2018 Search PubMed.
- H. Richter, B. Molitor, H. Wei, W. Chen, L. Aristilde and L. T. Angenent, Energy Environ. Sci., 2016, 9, 2392–2399 RSC.
- S. Ramió-Pujol, R. Ganigué, L. Bañeras and J. Colprim, Bioresour. Technol., 2015, 192, 296–303 CrossRef PubMed.
- S. Shen, Y. Gu, C. Chai, W. Jiang, Y. Zhuang and Y. Wang, Bioresour. Technol., 2017, 239, 236–243 CrossRef CAS PubMed.
- W. Van Hecke, R. Bockrath and H. De Wever, Bioresour. Technol., 2019, 293, 1–10 CrossRef PubMed.
- US Pat., US009834792B2, 2017, pp. 1–13 Search PubMed.
- M. Farcasiu, M. K. Jain, R. M. Worden and H. E. Grethlein, Bioconversion of Coal-Derived Synthesis Gas to Liquid Fuels, USA, 1995 Search PubMed.
- Y. Shen, R. C. Brown and Z. Wen, Appl. Energy, 2017, 187, 585–594 CrossRef CAS.
- B. Acharya, P. Roy and A. Dutta, Biofuels, 2014, 5, 551–564 CrossRef CAS.
- M. Yasin, Y. Jeong, S. Park, J. Jeong, E. Y. Lee, R. W. Lovitt, B. H. Kim, J. Lee and I. S. Chang, Bioresour. Technol., 2015, 177, 361–374 CrossRef CAS PubMed.
- R. Takors, M. Kopf, J. Mampel, W. Bluemke, B. Blombach, B. Eikmanns, F. R. Bengelsdorf, D. Weuster-Botz and P. Dürre, Microb. Biotechnol., 2018, 11, 606–625 CrossRef CAS PubMed.
- V. M. Y. Chisti, Chem.-Ing.-Tech., 1989, 61, 931–932 Search PubMed.
- K. Schügerl, Chem. Ing. Tech., 1980, 52, 951–965 CrossRef.
- A. Fadavi and Y. Chisti, Chem. Eng. J., 2005, 112, 73–80 CrossRef CAS.
- K. Asimakopoulos, H. N. Gavala and I. V. Skiadas, Chem. Eng. J., 2018, 348, 732–744 CrossRef CAS.
- P. H. Lee, S. Q. Ni, S. Y. Chang, S. Sung and S. H. Kim, Chem. Eng. J., 2012, 184, 268–277 CrossRef CAS.
- US Pat., US008198055B2, 2012, vol. 2, pp. 1–10 Search PubMed.
- Y. Shen, R. Brown and Z. Wen, Biochem. Eng. J., 2014, 85, 21–29 CrossRef CAS.
- K. Bullis, How Coskata Makes Biofuels A versatile new process for making biofuels could slash their cost, https://www.technologyreview.com/2008/02/19/221678/how-coskata-makes-biofuels/ Search PubMed.
- US Pat., US 8,178,330 B2, 2012, vol. 2, pp. 1–5 Search PubMed.
- A. J. Grethlein, R. M. Worden, M. K. Jain and R. Datta, J. Ferment. Bioeng., 1991, 72, 58–60 CrossRef CAS.
- H. Richter, M. E. Martin and L. T. Angenent, Energies, 2013, 6, 3987–4000 CrossRef CAS.
- Y. Cui, K. L. Yang and K. Zhou, Trends Biotechnol., 2021, 39, 914–926 CrossRef CAS PubMed.
- L. Goers, P. Freemont and K. M. Polizzi, J. R. Soc., Interface, 2014, 11, 1–13 CrossRef PubMed.
- M. Diender, A. J. M. Stams and D. Z. Sousa, Biotechnol. Biofuels, 2016, 9, 1–11 CrossRef PubMed.
- H. Huang, C. Chai, N. Li, P. Rowe, N. P. Minton, S. Yang, W. Jiang and Y. Gu, ACS Synth. Biol., 2016, 5, 1355–1361 CrossRef CAS PubMed.
- M. Diender, I. Parera Olm and D. Z. Sousa, Curr. Opin. Biotechnol., 2021, 67, 72–79 CrossRef CAS PubMed.
- J. Almquist, M. Cvijovic, V. Hatzimanikatis, J. Nielsen and M. Jirstrand, Metab. Eng., 2014, 24, 38–60 CrossRef CAS PubMed.
- J. Chen, J. A. Gomez, K. Höffner, P. I. Barton and M. A. Henson, Biotechnol. Biofuels, 2015, 8, 1–12 CrossRef PubMed.
- E. M. De Medeiros, J. A. Posada, H. Noorman and R. M. Filho, Biotechnol. Bioeng., 2019, 116, 2473–2487 CrossRef CAS PubMed.
- J. Chen, J. Daniell, D. Griffin, X. Li and M. A. Henson, Biochem. Eng. J., 2018, 129, 64–73 CrossRef CAS.
- M. Mohammadi, A. R. Mohamed, G. D. Najafpour, H. Younesi and M. H. Uzir, Sci. World J., 2014, 2014, 1–8 Search PubMed.
- L. Vaquerizo and M. J. Cocero, Comput. Chem. Eng., 2018, 113, 152–161 CrossRef CAS.
- F. Siebler, Scale-up of gas fermentations - Modelling tools for risk minimisation, Doctor of Engineering thesis, University of Stuttgart, 2020.
- S. Safarian, R. Unnthorsson and C. Richter, Fermentation, 2020, 6(3), 68 CrossRef CAS.
- O. Pardo-planas, H. K. Atiyeh, J. R. Phillips, C. P. Aichele and S. Fermentation, Bioresour. Technol., 2017, 245, 925–932 CrossRef CAS PubMed.
- M. Yasin, M. Cha, I. S. Chang, H. K. Atiyeh, P. Munasinghe and S. K. Khanal, in Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, ed. A. Pandey, C. Larroche and S. Ricke, Elsevier, 2nd edn, 2019, pp. 301–327 Search PubMed.
- A. Owoade, A. S. Alshami, D. Levin, S. Onaizi and Z. O. Malaibari, Biofuels, Bioprod. Biorefin., 2023, 10, 25–32 Search PubMed.
- H. L. MacLean and S. Spatari, Environ. Res. Lett., 2009, 4, 1–10 Search PubMed.
- V. Larnaudie, M. D. Ferrari and C. Lareo, Renewable Energy, 2021, 176, 606–616 CrossRef CAS.
- S. González-García, M. T. Moreira, G. Feijoo and R. J. Murphy, Biomass Bioenergy, 2012, 39, 378–388 CrossRef.
- R. M. Handler, D. R. Shonnard, E. M. Griffing, A. Lai and I. Palou-Rivera, Ind. Eng. Chem. Res., 2016, 55, 3253–3261 CrossRef CAS.
- S. Soam, M. Kapoor, R. Kumar, R. P. Gupta, S. K. Puri and S. S. V. Ramakumar, J. Cleaner Prod., 2018, 197, 732–741 CrossRef CAS.
- A. Zucaro, A. Forte and A. Fierro, J. Cleaner Prod., 2018, 194, 138–149 CrossRef CAS.
- J. Lask, M. Wagner, L. M. Trindade and I. Lewandowski, GCB Bioenergy, 2019, 11, 269–288 CrossRef CAS.
- H. Lyu, S. Yang, J. Zhang, Y. Feng and Z. Geng, Bioresour. Technol., 2021, 323, 124586 CrossRef CAS PubMed.
- J. Sadhukhan, E. Martinez-Hernandez, M. A. Amezcua-Allieri, J. Aburto and J. A. Honorato S, Bioresour. Technol. Rep., 2019, 7, 100230 CrossRef.
- D. Gerrior, K. Delsoz Bahri, A. Kermanshahi-pour, M. J. Eckelman and S. K. Brar, Sustain. Prod. Consum., 2022, 30, 359–376 CrossRef.
- L. Axelsson, M. Franzén, M. Ostwald, G. Berndes, L. Gopakumar and N. H. Ravindranath, Biofuels, Bioprod. Biorefin., 2012, 6, 246–256 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |