Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design of nanostructured 2D (photo-)electrocatalysts for biomass valorization coupled with H2 production†
Bahareh Feizi
Mohazzab
*a,
Kiarash
Torabi
b and
Dandan
Gao
 *a
*a
aDepartment of Chemistry, Johannes Gutenberg University Mainz, Duesbergweg 10-14, 55128 Mainz, Germany. E-mail: bahareh.feizimohazzab@uni-mainz.de; dandan.gao@uni-mainz.de
bDepartment of Materials Engineering and Metallurgy, Faculty of Engineering, Arak University, Arak 3815688349, Iran
First published on 2nd October 2024
Abstract
Electrocatalytic water splitting driven by renewable energy is a promising strategy for sustainable hydrogen production. However, the slow oxygen evolution reaction (OER) kinetics severely limit the rate of the hydrogen evolution reaction (HER) and the overall energy conversion efficiency of the water electrolyzer. To overcome this challenge, hybrid water electrolysis systems have been developed which replace the sluggish OER with thermodynamically and kinetically favorable biomass (photo-)electro-oxidation. In addition, these systems allow for the simultaneous production of value-added chemical products. This review highlights the design strategies involving the host structure remodeling and structure assembly design of two-dimensional (2D) nanomaterial-based (photo-)electrocatalysts, as well as their wide application in hybrid water electrolysis. Moreover, the current challenges and emerging strategies for the development of advanced (photo-)electrocatalysts and industrial-scale systems are emphasized.
1. Introduction
Growing global energy demands and environmental crises have prompted researchers to explore cleaner and more sustainable energy technologies. In this regard, hydrogen (H2) has been considered as an attractive energy carrier with high gravimetric energy density. In particular, H2 can be produced in a carbon-neutral fashion and a sustainable energy scheme can be built to this end.1,2 For example, electrochemical (EC)3–6 or photoelectrochemical (PEC)7–9 water splitting has been widely employed for green H2 production. However, the sluggish oxygen evolution reaction (OER) with a proton-coupled four-electron transfer process is currently the main bottleneck, requiring high voltage supply (>1.8 V vs. RHE),10 suffering from instability of the applied catalyst during operation (e.g., benchmark IrO2 OER catalyst degradation under harsh alkaline or acidic conditions11,12), and thus preventing large-scale industrial deployment. In consequence, conventional water splitting for efficient H2 production with economic and ecological viability remains a challenge.13,14One approach to overcome this challenge is hybrid water electrolysis. In this context, the hydrogen evolution reaction (HER) can be alternatively coupled with industrially important (photo-)electro-oxidation reactions, which can produce high-value products while requiring lower overpotentials as compared to the OER. Over the last decade, this novel concept has thrived, and led to major breakthroughs for (photo-)electro-oxidation of organics, including alcohols,15,16 aldehydes,17,18 carboxylic acids,19,20 amines,21,22 ammonia,23,24 and hydrazine.25,26 In addition, biomass feedstock valorization is one of the most attractive (photo-)electro-oxidation reactions.27,28 For example, 5-hydroxymethyl furfural (HMF), which possesses different functional groups such as a furan ring, aldehyde group, and hydroxymethyl group, can also be valorized to chemicals and fuels with higher values (for details see the ESI, Fig. S1a†). In this regard, furandicarboxylic acid (FDCA) is one of the desired products, which is an important alternative to petroleum-derived terephthalic acid in the polymer industry.29 Moreover, glucose is considered as another ideal biomass feedstock, which is a waste product generated in the pulp and paper processing industry.30 For this reason, glucose oxidation is emerging as an industrially important valorization strategy for value-added products, such as gluconic acid (GLA), glucaric acid (GUA), formic acid (FA), tartaric acid and glycolic acid (ESI, Fig. S1b†). Another example, lignin, as one of the most abundant biomass feedstocks, can be converted into value-added products through photocatalysis, EC and PEC, under oxidative conditions.31 During the oxidation process, functional groups and chemical bonds of lignin can be oxidized through different pathways, including side-chain linkages, aromatic rings, and phenolic hydroxyl groups (ESI, Fig. S2†).32 Altogether, to give an overview of the biomass and its valorization involved in this review, a summary table is included in the ESI (Table S1).†
The establishment of sustainable systems for simultaneous H2 production and biomass valorization requires the exploration of low-cost and robust (photo-)electrocatalysts with high performance, including superior intrinsic catalytic activity, excellent selectivity and outstanding durability.33,34 In this context, two-dimensional (2D) nanomaterials have become an attractive class of (photo-)electrocatalysts which combine a variety of desirable properties including high surface area, high electrical conductivity and tunable reactivity.35 Triggered by the pioneering studies on graphene and graphene exfoliation, a multitude of 2D layered nanomaterials have been developed, including transition metal dichalcogenides (TMDs), metal oxides, metal organic frameworks (MOFs), layered double hydroxides (LDHs), transition metal carbides/nitrides (MXenes), and hexagonal boron nitride (h-BN).36,37 The emergence of 2D nanomaterials with unique electrochemical and electronic properties has sparked research interest in biomass valorization coupled with H2 production. In order to endow 2D nanomaterials with further improved catalytic performance, a wide range of design strategies have been developed including heterojunctions of two distinct materials, structure manipulation (e.g. doping and defect/vacancy engineering), and rational integration of 2D nanomaterials on three-dimensional (3D) supports.33,38
Herein, this review reports recent developments in the field of 2D nanomaterials for hybrid water electrolysis (Fig. 1). Rational 2D nanomaterial design strategies are reviewed from host structure remodeling (heteroatom doping, defect/vacancy engineering, and co-catalyst engineering) to structure assembly design (construction of heterostructures, surface engineering, and 2D nanomaterials integrated into 3D matrices). Each design strategy is followed by a review of (photo-)electro-oxidation of HMF, glucose, and lignin coupled with H2 production. Additionally, current challenges and emerging research directions have been pointed out.
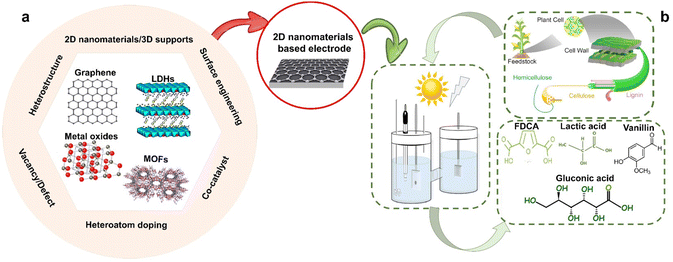 | ||
| Fig. 1 Graphical illustration of the scope of this review: (a) 2D nanomaterial categories and design strategies and (b) employment of 2D nanomaterials in hybrid water (photo-)electrolysis. The 2D nanomaterial categories and lignocellulosic biomass cell wall have been adapted from ref. 39 and 40, with permission from MDPI and Frontiers, copyright 2022, respectively. | ||
2. 2D nanomaterials: design strategies and applications in hybrid water electrolysis
2.1. Host structure remodeling
For PEC applications, in the case of semiconductors, doping is able to enhance the light absorption optical range by multiple methods such as (1) introducing mid-gap states, (2) narrowing band gaps and (3) forming impurity bands.45 Doping introduces mid-gap shallow or deep-level states which broaden the light absorption edge to longer wavelengths.46 The mid-gap deep-level states contribute to extending light absorption, and these states do not participate in visible-light photocatalytic activity and act as recombination centers mostly due to the lowest mobility of charge carriers.47 In contrast, the mid-gap shallow-level states are capable of increasing charge carrier diffusion length and mobility.48 In the case of noble metal doping, such as gold and silver, the semiconductor experiences the localized surface plasmon resonance (LSPR) effect. This LSPR effect results in enhanced light absorption in the near-infrared region which is beneficial for turning light into energy.49
For EC applications, in one instructive example, Wei et al.50 designed a Bi-doped Co3O4 nanosheet array on Ni foam (BiCoO-NA/NF) for the 5-hydroxymethyl furfural oxidation reaction (HMFOR) coupled with the HER. In the study, complete conversion of HMF to FDCA (faradaic efficiency (FE): 97.7% and yield: 362.5 μmol h−1) and promotion of H2 production (yield: 7.33 μmol h−1) can be simultaneously achieved at a low cell voltage of 1.3 V. The outstanding electrocatalytic performance is attributed to heteroatom doping which provides fast mass/electron transfer channels and thus reduces the energy consumption. In a related study, Zhang et al.51 synthesized Cu-doped Ni(OH)2 nanosheets (Ni1−xCux(OH)2), in which Cu presented an island-like elemental distribution (Fig. 2a). The optimum Cu doping led to Ni0.9Cu0.1(OH)2, deriving higher performance toward the HMFOR in comparison to the OER (Fig. 2b). Particularly, Ni0.9Cu0.1(OH)2 realized the HMFOR to FDCA with a 91.2% FE at 1.45 V vs. RHE. The high selectivity for the HMFOR is attributed to the formation of more active Ni3+ species and the coordination of unsaturated amorphous Cu species which are OER inert.
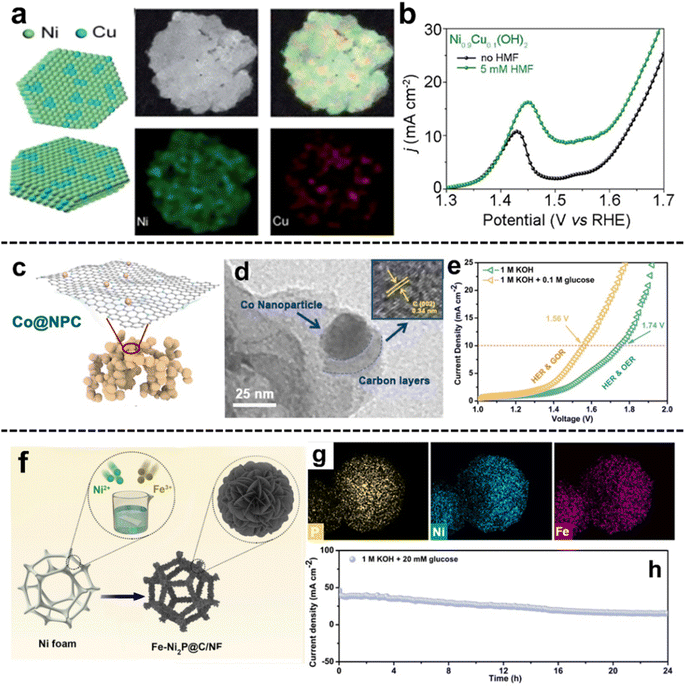 | ||
| Fig. 2 Selected examples for the Heteroatom doping subsection: (a) schematic illustration of the Ni1−xCux(OH)2 nanosheets and elemental distribution, (b) LSV curve comparison of Ni0.9Cu0.1(OH)2 with and without HMF (these figures have been reproduced from ref. 51 with permission from Royal Society of Chemistry, copyright 2021), (c) the schematic illustration and (d) TEM images of Co@NPC, (e) LSV curves of Co@NPC for the GOR and OER coupled with the HER (these figures have been reproduced from ref. 52 with permission from Elsevier, copyright 2021), (f) the schematic representation and (g) elemental distribution of the Fe–Ni2P@C/NF electrode, and (h) the chronopotentiometry curve of Fe–Ni2P@C/NF‖Fe–Ni2P@C/NF in 1 M KOH with 20 mM glucose (these figures have been reproduced from ref. 53 with permission from Elsevier, copyright 2022). | ||
In addition, Li et al.52 investigated the glucose oxidation reaction (GOR) coupled with the HER over cobalt nanoparticles supported on N-doped porous carbon (Co@NPC-T, T: calcination temperature, Fig. 2c and d). The activity of Co@NPC-800 was evaluated in a two-electrode cell, in which the presence of glucose resulted in a reduced cell voltage, for example, by 180 mV at a current density of 10 mA cm−2 (Fig. 2e). Product analysis indicated that lactic acid (LA) and FA were the primary GOR products, with smaller amounts of GLA and GUA. According to DFT calculations, N doping improved glucose adsorption and optimized H2O adsorption, thereby promoting GOR and HER performance. Furthermore, Li et al.53 synthesized bifunctional Fe-doped Ni2P nanosheets hybridized with C on Ni foam (Fe–Ni2P@C/NF) for the GOR and HER (Fig. 2f and g). Additionally, the chronoamperometry experiments conducted on the two-electrode electrolyzer with glucose addition showed that the current decreased to around half of its initial level after 24 h, indicating sustained glucose conversion during electrolysis (Fig. 2h). The electrode showed sustained stability for 24 h and achieved a high current density of 100 mA cm−2 at a cell voltage of ca. 1.55 V. The electrolysis resulted in complete glucose conversion to LA and FA with a yield of 52.1% and 35.6%, respectively. A high FE of 98.2% for the HER was observed in the hybrid water electrolysis system. DFT calculation indicated that Fe doping optimized the adsorption energy of H2O molecules and the free energy of H2 adsorption which facilitates the HER performance.
Moreover, heteroatom doping has also been widely applied for the lignin oxidation reaction (LOR). For example, Cui et al.54 reported the use of a single atom Pt catalyst anchored on an N-doped carbon nanotube (Pt1/N-CNTs) anode for selective electrocatalytic C–C bond cleavage in lignin, coupled with promoted H2 production on a glassy carbon cathode. The Pt1/N-CNTs exhibit unique electronic structures due to the increase in unsaturated coordination number, resulting in the increased active site number. Remarkably, the high dispersion degree of the Pt single atom improved atomic utilization, leading to the increased activity per unit catalyst. Altogether, the Pt1/N-CNTs demonstrate outstanding selectivity in lignin electrochemical oxidative cleavage of C–C bonds for aromatic monomer formation. In a related study, Hao et al.55 employed an electrochemical redox ion, [Fe(CN)6]3−, in a PbO2 matrix by the electro-deposition method. The [Fe(CN)6]3− doped PbO2 electrode exhibits a service lifetime of 287.25 hours, nearly triple that of standard PbO2, and a 45% higher rate of alkali lignin degradation. This is attributed to a more compact surface and larger grain size, resulting in increased average cross-sectional area of pores. These structural improvements lead to greater stability and a higher electrochemical active surface area, enabling more efficient reactions. This pioneering work opens a new avenue for next-generation 2D electrocatalyst design for robust lignin electrolysis.
In the PEC pathway, defects can be located in the bulk phase or on the surface of semiconductors, resulting in volume and planar defects, respectively. They affect the photo-electrocatalytic process in different ways (ESI, Fig. S3b†). For instance, planar defects within the photo-electrocatalyst can act as traps for holes, enhancing charge separation by preventing immediate recombination with electrons. These trapped holes can then react with electron donors on the catalyst's surface, leading to the desired chemical reactions. Conversely, volume defects may trap both electrons and holes, potentially leading to recombination, which is counterproductive for photo-electrocatalytic activity.59 Therefore, the generated charge carriers could be trapped by volume or planar defects, resulting in recombination or reaction participation, respectively.60 It should be noted that excessive vacancies can reduce reaction efficiency resulting from unfavourable charge carrier recombination. Therefore, moderate vacancies in 2D nanomaterial design are desired to rationalize visible light absorption, electron–hole pair generation, and hydrogen adsorption.61
For applications, Zhang et al.62 developed a series of ternary NiVW layered metal hydroxide electrocatalysts with abundant lattice vacancies (NiVWv-LMH) for the HMFOR (Fig. 3a). Remarkably, the NiVWv-LMH nanosheets obtained lattice-disordered phases and vacancies after 50 cyclic voltammetry (CV) cycles (Fig. 3b), and demonstrated a high current density of 193 mA cm−2 for the HMFOR to FDCA with a 100% conversion rate at 1.43 V vs. RHE (Fig. 3c). The high performance is attributed to the dissolution of V and W which changes the local coordination of Ni cations and increases the amount of unsaturated active sites. In another study, Zhu et al.63 synthesized a CuMn2O4 spinel electrocatalyst for the HMFOR using the hydrothermal deposition approach followed by ammonia etching to create oxygen vacancies. The ammonia-etched CuMn2O4 achieved a current density of 20 mA cm−2 at 1.31 V vs. RHE. A 100% conversion rate and 96% selectivity to FDCA were observed. Moreover, Tian et al.64 studied PEC hybrid water electrolysis using single-atom Pt on defective TiO2 nanorod arrays (Pt/def-TiO2 RNAs) for the GOR coupled with the HER (Fig. 3d and e). The prepared Pt/def-TiO2 RNAs reached a high photocurrent density of 1.91 mA cm−2 at 0.6 V vs. RHE (Fig. 3f). After 5.5 h of the GOR, 98.8% of glucose was converted into GLU and GLA with a yield of 84.3% and 9.2%, respectively. And a high FE of 99% was observed for the HER. The TiO2 defective structure led by oxygen vacancies promotes charge dynamics for the PEC GOR by facilitating charge movement. def-TiO2 possesses a large electron conduction band and large surface band bending which are favorable properties for charge carrier movement and extraction.
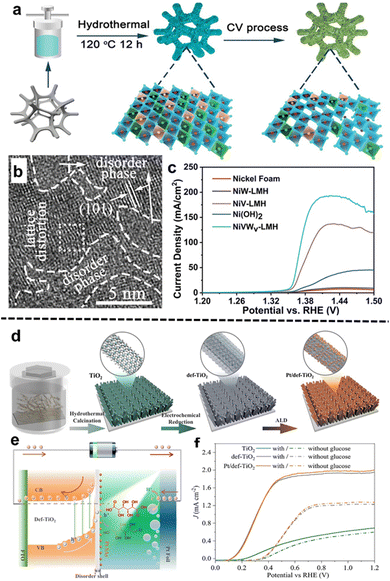 | ||
| Fig. 3 Selected examples for the Defect/vacancy engineering subsection: (a) synthetic route of NiVWv-LMH crumpled nanosheets, (b) HR-TEM image of NiVWv-LMH, and (c) LSV curves of NiVWv-LMH after the 50th CV cycle with 90% iR correction (these figures have been reproduced from ref. 62 with permission from Wiley, copyright 2023), (d) fabrication process for the TiO2, def-TiO2, and Pt/def-TiO2 NRAs, (e) energy band diagram of the Pt/def-TiO2 NRA photoanode and charge carrier movement during GOR and HER processes, and (f) LSV profiles of prepared photoanodes for the GOR under AM 1.5G, 100 mW cm−2 illumination (these figures have been reproduced from ref. 63 with permission from Nature, copyright 2023). | ||
In PEC systems, the integration of a co-catalyst is crucial for promoting charge separation and enhancing transport at the semiconductor–co-catalyst interface.66 The addition of the co-catalyst leads to the formation of a space-charge region, which in turn establishes a semiconductor/co-catalyst junction, commonly referred to as a Schottky barrier.67 At this junction, the alignment of Fermi energy levels initiates electron migration. The direction of electron movement and the resultant band bending are influenced by the relative Fermi levels of the materials in contact. Typically, for an n-type semiconductor combined with a metal co-catalyst possessing a higher work function, there is a flow of electrons from the semiconductor to the metal. This electron flow induces upward band bending, propelling electrons toward the space-charge region, while holes tend to accumulate on the photo-electrocatalyst's surface.68 The formation of the Schottky barrier is instrumental in mitigating electron–hole recombination, thereby significantly improving PEC efficiency. It is essential to recognize that the precise direction of electron flow and band bending is contingent upon the specific energy levels of the materials involved (ESI, Fig. S3c†). Despite the high activity of noble metals, earth-abundant 2D noble-metal-free co-catalysts have emerged in recent years attributed to the low cost, tunable composition, superior structural stability and tailorable reactivity.69
Liang et al.70 employed Ni3N–V2O3 as a potent co-catalyst for the HMFOR-assisted HER (Fig. 4a), which demonstrated high FDCA selectivity (98.7%) and yield (96.1%) as well as advanced HER performance (Fig. 4b and c). Co-catalyst engineering altered the electronic structure and reduced the band gap energy, leading to low cell voltage (1.40 V at 10 mA cm−2) and 100% FE of the HER in hybrid water electrolysis. For GOR application, Ru and Ir nanoclusters and their oxides have been extensively employed due to their structural and chemical stability, as well as their superior electrocatalytic activity. For example, Chen's group71 developed a novel calixarene-based {Ni18} coordination wheel entity (CIAC-123) as an efficient bifunctional electrocatalyst for the GOR and HER (Fig. 4d). Promisingly, the open-wheel structure can serve as a template for encapsulation and fabrication of tiny metal nanoclusters such as Au, Pd, Ir, Ru, Rh, Pt, and AuPd. As observed, CIAC-123 was performed as an efficient catalyst for the GOR while bimetallic AuPd@CIAC-123 exhibited significantly improved HER performance (Fig. 4e and f). Considering the pore diameter (0.9 nm) in AuPd@CIAC-123 and glucose molecule diameter (∼1 nm), it could be concluded that the presence of AuPd limits the accessibility of the inner cavity to glucose molecules, while hydrogen ions can pass through the cavity for absorbance. Therefore, based on the size of molecules, the Ni sites are responsible for the GOR while HER activity can be attributed to Pd sites with synergistic effects of Au and Ni.
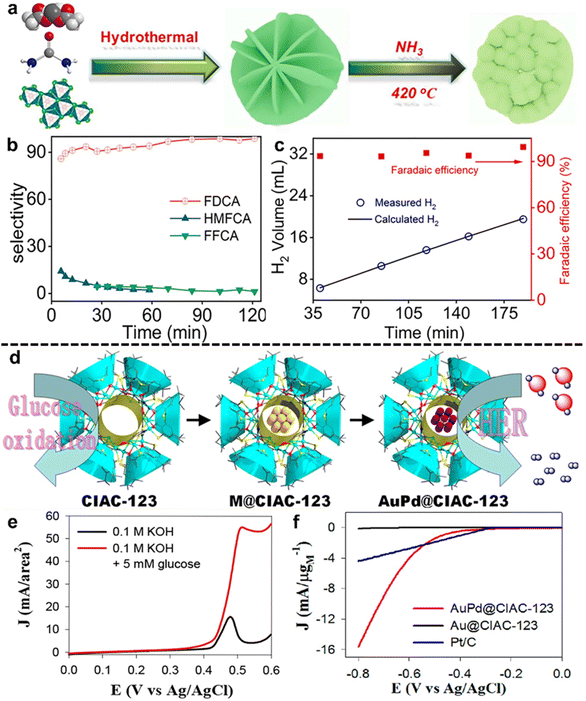 | ||
| Fig. 4 Selected examples for the Co-catalyst engineering subsection: (a) synthetic route of the Ni3N–V2O3 electrode, (b) HMFOR selectivity for FDCA, HMFCA and FFCA, (c) hydrogen production and faradaic efficiency for the HER as a function of electric charge (left axis) and electric current (right axis) (these figures have been reproduced from ref. 70 with permission from Elsevier, copyright 2021), (d) schematic illustration of the wheel structure of CIAC-123, M@CIAC-123, and AuPd@CIAC-123; M: tiny metal nanoclusters such as Au, Pd, Ir, Ru, Rh, Pt, and AuPd, (e) LSV curves of CIAC-123 in 0.1 M KOH with or without 5 mM glucose, and (f) LSV curves of AuPd@CIAC-123, Au@CIAC-123, and commercial Pt/C in 0.5 M H2SO4 for the HER (these figures have been reproduced from ref. 71 with permission from American Chemical Society, copyright 2018). | ||
Co-catalyst engineering has also been employed to explore advanced catalysts for efficient LOR. In this regard, Rauber et al.72 coated a third metal (Mn, Pd, V, or Ti) in mixed Ru–Ir oxides (Ru0.2Mn0.2Ir0.6Ox, Ru0.2Pd0.2Ir0.6Ox, Ru0.2V0.2Ir0.6Ox and Ru0.2Ti0.2Ir0.6Ox). Compared to the initial binary metal oxide (Ru0.4Ir0.6Ox), the co-existence of the third metal, particularly for Mn metal, can significantly improve the yield of monomers from the LOR. In one related investigation, the Gao group modified the NiFeO arrays fabricated on Ni foam with Ir single atoms (Ir–NiFeO@NF) for efficient 1-phenylethanol (a simple lignin model) valorization and H2 production.73 This showed that deployment of bifunctional Ir–NiFeO@NF in a two-electrode cell can reach a current density of 10 mA cm−2 at 1.33 V. Additionally, a H2 production rate of ca. 0.45 mmol h−1 cm−2 was observed with a FE close to 100% at an applied cell voltage of 1.5 V. Anodic product analysis revealed that lignin motifs were transformed into benzoic acids.
2.2. Structure assembly design
For PEC applications, primary objectives of heterostructure design are to prevent charge recombination while promoting charge movement and separating electron–hole pairs using diverse charge transfer mechanisms. The efficiency of PEC processes is enhanced through improved charge-carrier separation, enriched active sites, and optimized reaction conditions facilitated by heterostructure design.83 Based on the position of the conduction band (CB) and valence band (VB), these mechanisms are often categorized into five groups: p–n junction, type I, type II, Z-scheme and S-scheme.7,8,84,85 Notably, the charge transfer mechanism differs when incorporating carbon-based materials into the photo-electrocatalyst heterostructure.68 For instance, when a graphene-based heterostructure is irradiated with light, the semiconductor generates charge carriers, which then transfer electrons from the CB of the semiconductor to the graphene sheets, resulting in a reduction reaction. Meanwhile, the remaining holes in the VB of the semiconductor participate in oxidation reactions. A schematic of the charge transfer mechanism of graphene-semiconductors is available in ESI, Fig. S3d.†
In (photo-)electrocatalysis, heterostructure design provides the (photo-)electrocatalyst with high surface area and strong interfacial contact. In this context, the charge carriers generated during the reaction can be quickly and efficiently transported away from the active sites, thus enhancing the overall reaction rate. As a prime example, Sun et al.86 investigated the coupling of the HMFOR and HER using a heterostructured nanorod array electrocatalyst deposited on Ni foam (Ni/Ni0.2Mo0.8N/NF), which demonstrated excellent activity toward both the HMFOR and HER. Remarkably, the hybrid water electrolysis system employing the bifunctional Ni/Ni0.2Mo0.8N/NF electrode only required 1.51 V for a current density of 50 mA cm−2. And product analysis showed 98.5% yield for FDCA. This superior performance was attributed to electronic structure modulation and robust contact between the Ni foam support and Ni0.2Mo0.8N. In another example by Zhong et al.,87 NixSey nanowire arrays covered with NiFe LDH nanosheets (named NixSey–NiFe LDH@NF) were applied for the HMFOR coupled with the HER on Pt sheets. In the reported heterostructured NixSey–NiFe LDH@NF, a NixSey nanowire core facilitated fast charge transfer while NiFe LDH nanosheets provided a large surface area with abundant active sites (Fig. 5a and b). To this end, the HMFOR activity of NixSey–NiFe LDH@NF was significantly promoted, leading to substantially reduced overpotentials as compared to the presented reference materials (Fig. 5c). In addition, a high yield of 99.3% and FE of 98.9% were observed for the HMFOR to FDCA at 1.42 V vs. RHE. Moreover, Deng et al.88 modified copper sulfide core nanowires grown on Cu foam with NiCo-layered double hydroxide outer-shell nanoarrays (CuxS@Ni0.75Co0.25OxHy/CF) for simultaneous HMFOR and HER (Fig. 5d–f). The hybrid electrolysis system only required a cell voltage of 1.34 V to reach 10 mA cm−2, which is lower than that of the HMF-absent system by 0.27 V (Fig. 5g). Notably, CuxS@Ni0.75Co0.25OxHy/CF achieved complete HMF conversion to FDCA with 100% FE at 1.3 V vs. RHE and a unity FE for the HER. The open core–shell structure of nanoarrays provided a large number of active sites, which facilitated mass transfer. Furthermore, the incorporation of Co adjusted the electronic structure of Ni to a higher content of Ni3+, which accelerated the adsorption of reactants and intermediates. The synergetic effect between the designed specific nanostructure and modulated electronic structure is beneficial for the improved performance.
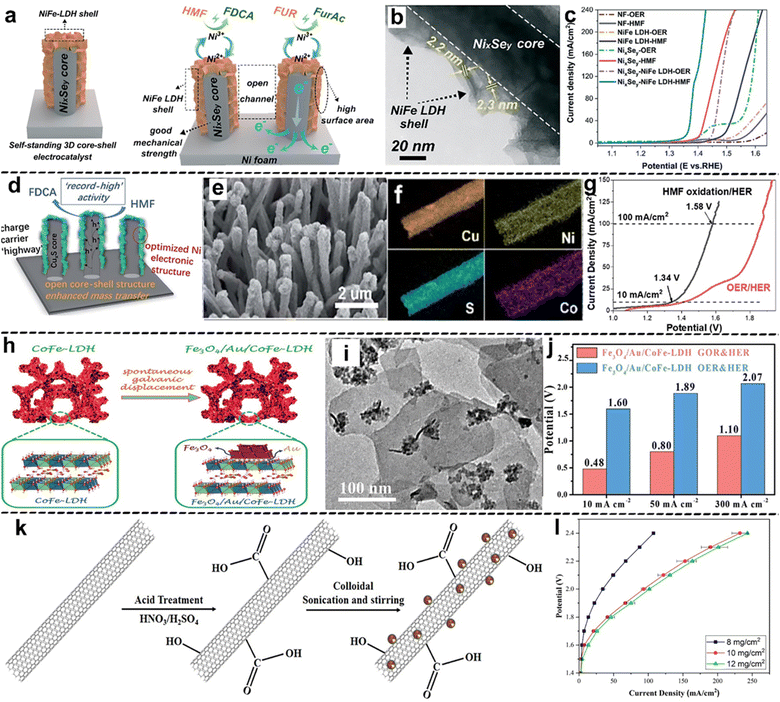 | ||
| Fig. 5 Selected examples for the Construction of heterostructures subsection: (a) the schematic structure and the mechanism proposed for the electrooxidation of HMF and furfural (FUR) on the NixSey–NiFe LDH@NF electrode, (b) TEM images of the NixSey–NiFe LDH core–shell structure, (c) LSV curves of NF, NiFe LDH@NF, NixSey@NF and NixSey–NiFe LDH@NF (these figures have been reproduced from ref. 87 with permission from Royal Society of Chemistry, copyright 2022), (d) schematic illustration, (e) SEM image and (f) elemental mapping of CuxS@Ni0.75Co0.25OxHy/CF, (g) LSV curves for the OER/HER and HMFOR/HER over the bifunctional CuxS@Ni0.75Co0.25OxHy/CF electrode (these figures have been reproduced from ref. 88 with permission from Royal Society of Chemistry, copyright 2020), (h) synthetic route and (i) TEM image of CoFe-LDH and Fe3O4/Au/CoFe-LDH, (j) cell potential of the GOR/HER and OER/HER at different current densities with iR-compensation (these figures have been reproduced from ref. 89 with permission from Wiley, copyright 2021), (k) synthetic approach for PbO2/MWNTs for the LOR, and (l) LSV curve from different PbO2/MWNT catalyst loading samples (these figures have been reproduced from ref. 90 with permission from IOP Publishing, copyright 2019). | ||
In addition, Sun et al.89 designed hetero-nanoparticles (Fe3O4/Au) distributed on the basal plane of CoFe-LDH, leading to Fe3O4/Au/CoFe-LDH with heterostructures for efficient GOR coupled with the HER (Fig. 5h and i). This study highlighted that the presence of Fe3O4 and CoFe-LDH in the sandwich structure boosted the electrochemical activity of Au nanoparticles in the GOR by regulating its electronic structure and providing a large surface area. In this context, the Fe3O4/Au/CoFe-LDH heterostructures displayed significantly reduced cell voltages for the GOR/HER compared to the OER/HER (Fig. 5j). In particular, a current density of 50 mA cm−2 led to full conversion of glucose to gluconate and the HER with a FE of ca. 99.6%. Moreover, Bateni et al.90 integrated PbO2 nanoparticles into high surface area multi-walled carbon nanotubes (PbO2/MWNTs) via the impregnation method for the electrochemical LOR (Fig. 5k), which showed boosted rates in terms of both the LOR and HER (on a Pt black cathode) in a lignin electrolysis cell. Additionally, the comparison of current densities at the same potential shows that increasing the catalyst loading from 8 to 10 mg cm−2 significantly enhanced the performance due to the change in the number of accessible active sites (Fig. 5l), while a further increase in catalyst loading (12 mg cm−2) cannot yield higher performance. In another study, the electroactivity of a NiCo electrocatalyst was improved towards lignin-assisted water electrolysis by fabricating a NiCo/TiO2 heterostructure electrocatalyst as an anode and Pt nanoparticles loaded on a carbon cloth as a cathode.91 The results showed that at a cell voltage of 1.6 V, the current density and the FE of H2 evolution reached 10 mA cm−2 and 99%, respectively. Furthermore, the gas product analysis revealed 100% purity of H2.
For the applications of the surface engineering design strategy, Deng et al.96 reported hexagonal nanoplatelet-on-nanoarray NiCo hydroxide-based electrocatalysts (t-NiCo-MOF) for the HMFOR coupled with the HER on a MoNi4 cathode (Fig. 6a and b). To achieve a current density of 100 mA cm−2, the t-Ni1Co1-MOF‖MoNi4 hybrid electrolysis system requires a low cell voltage of 1.392 V, while the HMF-free system requires 1.7 V (Fig. 6c). The product quantification highlighted complete conversion of HMF to FDCA with a high FE of 98%. The high activity and selectivity observed can be attributed to the reasonable morphology design, providing increased interfacial contact and facilitating mass transfer. In a related study, the Wang group97 developed a bifunctional superhydrophilic/superaerophobic surface by depositing NiS quantum dots (QDs) onto wrinkled N,O,S-tri-doped carbon porous nanosheets (NiS@NOSC) for simultaneous HMFOR and HER (Fig. 6d). Static-water-droplet contact angles and underwater air-bubble contact angles (Fig. 6e) revealed the superhydrophilic and superaerophobic properties of the NiS@NOSC surface, accelerating electrolyte penetration and bubble release, respectively. As compared to the OER/HER system, the HMFOR/HER system exhibited a considerably reduced cell voltage, and only 1.51 V was required for reaching 50 mA cm−2 current density (Fig. 6f). Remarkably, 100% conversion of HMF to FDCA was observed with a FE of ca. 99.6% and a 100% FE for the HER. In another example, Zhou et al.98 employed pine needle-like Co3O4 nanowires on Ni foam (CoNW/NF, Fig. 6g) as an efficient bifunctional electrocatalyst for the HMFOR and HER. Benefiting from the porous nanorod-like leaf surface morphology (Fig. 6h), abundant active sites can be exposed. This led to a reduced cell voltage for the HMF-present system (Fig. 6i). Specifically, after 5.73 h of the HMFOR, complete conversion into FDCA with a yield of 96.8% and a FE of 96.6% was observed, together with a 100% FE for the HER. The outstanding performance is attributed to the open nanowire structures that facilitated mass transfer, while the interconnected structures enhanced the charge transfer process.
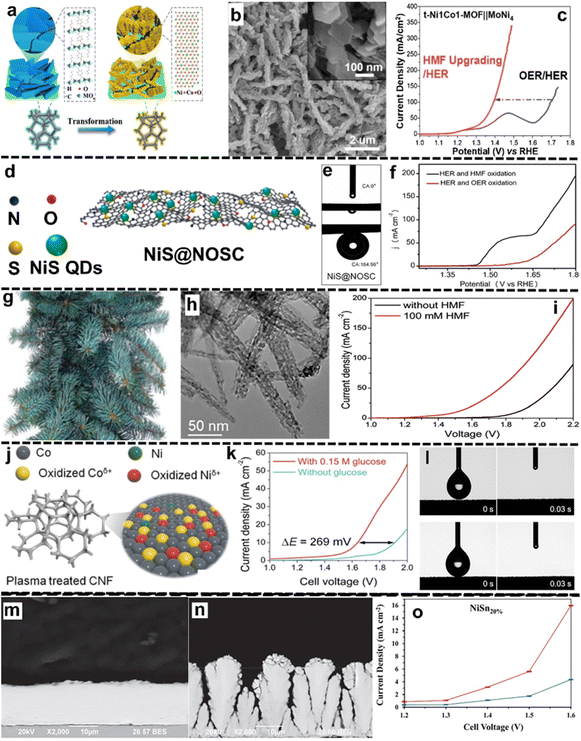 | ||
| Fig. 6 Selected examples for the Surface engineering subsection: (a) the illustration of a nanoplatelet-on-nanoarray t-NiCo-MOF electrocatalyst deposited on NFs, (b) SEM images of the transformed Ni1Co1-MOF (t-Ni1Co1-MOF), and (c) LSV curve of the t-Ni1Co1-MOF(anode)‖MoNi4(cathode) system for the OER/HER and HMFOR/HER (these figures have been reproduced from ref. 96 with permission from Elsevier, copyright 2020), (d) illustration of the synthesis procedure of NiS@NOSC, (e) static-water-droplet contact angles for under-water air-bubble contact angles on the surface of NiS@NOSC, (f) LSV curves of NiS@NOSC‖NiS@NOSC for the OER/HER and HMFOR/HER (these figures have been reproduced from ref. 97 with permission from Elsevier, copyright 2022), (g) photo of a pine needle, (h) TEM image of morphological features of the exfoliated CoNWs, (i) LSV curves of the two-electrode system (CoNW/NFs‖CoNW/NFs) with and without HMF (these figures have been reproduced from ref. 98 with permission from Royal Society of Chemistry, copyright 2019), (j) schematic illustration of the modification of CNFs via O2 plasma treatment, (k) LSV curve of the CNF-60‖CNF-60 system for the GOR coupled with the HER, (l) changes in the wetting behavior of the surface: (top row) pristine CNFs and (down row) CNFs after 60 minutes (CNF-60) of plasma exposure (these figures have been reproduced from ref. 99 with permission from Royal Society of Chemistry, copyright 2023), (n) cross-section image of Ni (m) and NiSn20%, and (o) current density as a function of cell voltages for NiSn20% in 1 M NaOH solution (absence of lignin: blue plot and presence of lignin: red plot) (these figures have been reproduced from ref. 100 with permission from IOP publishing, copyright 2020). | ||
Additionally, the Augustynski group101 proposed a nanostructured WO3 photoanode for the GOR coupled with the HER, resulting in a variety of valuable products such as GLA, GUA, erythrose and arabinose. In this study, simultaneous GOR and HER at 1.23 V vs. RHE demonstrated a high photocurrent density of ca. 6.5 mA cm−2 under stimulating AM 1.5G illumination. In another example, Wang et al.99 employed ultrafast oxygen plasma to modify commercial Co–Ni foam (CNF) to give CNF-60 a superhydrophilic surface (Fig. 6j) for the GOR and HER. Compared to the pristine CNF, CNF-60 yielded higher current density at applied potentials higher than 1.45 V, suggesting more favorable mass transfer of glucose. In the two-electrode (CNF-60‖CNF-60) cell, reduced cell voltages can be observed in the presence of glucose, for example, by 269 mV at a current density of 10 mA cm−2 (Fig. 6k). Moreover, water contact angle evaluation of the modified surface and pristine conditions (Fig. 6l) suggested that both materials possess superhydrophilic features. This indicates that the improved electrochemical performance is attributed to elemental changes resulted from the plasma treatment. Furthermore, Ghahremani et al.100 reported the LOR coupled with the HER by co-electrodepositing Ni and Sn on a Ti substrate, leading to the NiSn20% alloy electrode showing cauliflower-like morphology with increased porosities and channels (Fig. 6m and n). Compared to the lignin-free condition, the obtained NiSn20% demonstrated higher performance in the lignin-present system (Fig. 6o). Additionally, it delivered a high H2 production rate of 72 ml h−1 at a cell voltage of 1.6 V, while a remarkable target vanillin production rate of 300 g kg per lignin per min was observed at a cell voltage of 1.4 V.
For example, Chen and coworkers111 proposed electrochemically tuning nonporous CoSx deposited on Co foam to Co3O4 (Co3O4/CF, Fig. 7a) for the HMFOR and HER. The obtained Co3O4/CF exhibits a hierarchical structure consisting of microspheres and nanosheets distributed on the surface (Fig. 7b). The cell voltage was significantly reduced when HMF was present in the hybrid electrolysis system (Fig. 7c). Attributed to defective structures and abundant electroactive sites distributed on the 3D Co foam support, bifunctional Co3O4/CF achieved complete conversion of HMF to FDCA with a high yield (93.2%), as well as high FEs for both the HMFOR and HER (92.9% and 99.8%, respectively). Moreover, the Zhao group112 synthesized a sheet-like cobalt hydroxide-cerium dioxide composite catalyst on carbon fiber paper (Co(OH)2–CeO2) for the HMFOR coupled with the HER (Fig. 7d and e). Through two-electron oxidation, HMF was converted to 2-furancarboxylic acid (HMFCA) with a selectivity of 89.4% and a yield of 85.8%. Promising hybrid water electrolysis was carried out at 1.4 V with an optimized HMF conversion rate (96%) and boosted H2 production rate (114.39 μmol cm−2 h−1, Fig. 7f). In addition, Xin et al.113 anchored bifunctional size-controllable Co/Ni-co-doped carbon on Cu foam (CF@CoNC-xT, x represents the crystallization times) for the GOR and HER (Fig. 7g). The obtained CF@CoNC-2T electrode shows a rough surface with Co nanoparticles distributed (Fig. 7h). The two-electrode cell showed a cell voltage of only 0.9 V, which is 0.88 V lower than that of the glucose-free system (Fig. 7i). Additionally, a FE of 100% for the HER and value-added products (GNA as well as GRA) was obtained. In another study by Liu and colleagues,115 NiFeOx and NiFeNx were deposited on 3D nickel foam for the GOR and HER, respectively. The optimized electrodes were able to deliver a current density of 200 mA cm−2 at a low cell voltage of 1.48 V. Meanwhile, the efficient conversion of glucose to GRA was observed with a high FE of 87% and yield of 83%.
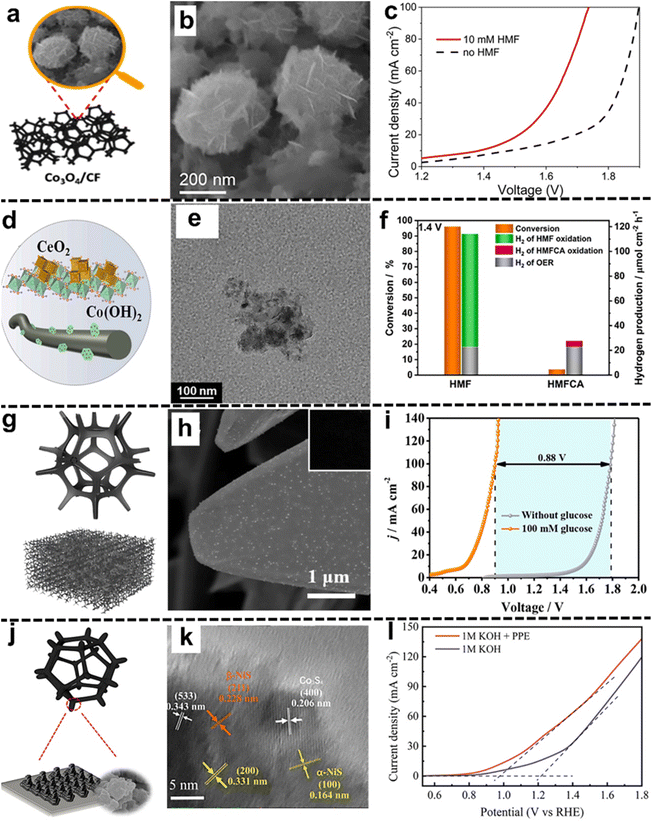 | ||
| Fig. 7 Selected examples for the 2D nanomaterials integrated into 3D matrices subsection: (a) illustration and (b) SEM image of the fabricated Co3O4/CF, (c) LSV curves of the Co3O4/CF‖Co3O4/CF system in the presence and absence of 10 mM HMF (these figures have been reproduced from ref. 111 with permission from Elsevier, copyright 2022), (d) a schematic illustration and (e) TEM images of the Co(OH)2–CeO2 electrode, (f) the conversion of HMF and HMFCA and corresponding H2 production at a potential of 1.4 V using the Co(OH)2–CeO2 electrode (these figures have been reproduced from ref. 112 with permission from Elsevier, copyright 2023), (g) a schematic illustration and (h) SEM image (inset: optical photograph of the sample) of CF@CoNC-2T, (i) LSV curves of a two-electrode cell consisting of CF@CoNC-2T‖CF@CoNC-2T with and without glucose (these figures have been reproduced from ref. 113 with permission from Royal society of chemistry, copyright 2022), (j) a schematic structure and (k) TEM image of NF@Co3S4/(α,β)-NiS, and (l) LSV curves of NF@Co3S4/(α,β)-NiS in 1.0 M KOH with and without 1.0 mM PPE (these figures have been reproduced from ref. 114 with permission from Elsevier, copyright 2022). | ||
Recently, Ni-based porous electrodes have been extensively developed for the LOR, owing to their low cost, high catalytic activity, and robust anti-corrosion stability.116 Notably, under oxidative operation, Ni electrodes always form a catalytically active surface NiOOH layer, leading to enhanced lignin conversion performance.117 In this regard, the Waldvogel group reported a series of studies on the anodic depolymerization of lignin on 2D planar or 3D porous Ni-based electrodes.118–121 It is reported that the repeated use of Ni foam electrodes for the LOR significantly increased vanillin production, indicating an activation phenomenon from the in situ deposition of a modified layer on the electrode surface (ESI, Fig. S4†).117 In addition, Wang et al.114 fabricated self-supported Co and Ni bimetallic sulfides on Ni foam (NF@Co3S4/(α,β)-NiS, Fig. 7j) for the oxidation of 2-phenoxy-1-phenylethanol (PPE), a typical β-O-4 lignin model compound coupled with the Pt catalyzed HER. The structural analysis demonstrates that Co3S4 is stacked in a lamellar structure with pore channels which facilitate mass transfer rate (Fig. 7k). At the same current density (i.e., 10 mA cm−2), the potential of PPE oxidation (0.954 V vs. RHE) was considerably lower than that of the PPE-free system (i.e., 1.107 V vs. RHE, Fig. 7l). Meanwhile, a large amount of H2 bubbles produced at the cathode was observed.
3. Current challenges and emerging strategies
This review reports on recently developed design strategies for 2D nanomaterial based (photo-)electrocatalysts for biomass valorization coupled with H2 evolution, of which HMF, glucose and lignin have been exemplified as promising representatives. The long-standing benefits as well as existing limitations of reviewed designed strategies are presented in Table 1. In addition, Table S2† concretely summarizes how a specific design strategy improves the performance of the investigated hybrid water electrolysis systems, in terms of reduced cell voltage, enhanced H2 production efficiency, and simultaneous access to value-added products. In spite of the progress achieved, bridging the current-generation hybrid water electrolysis systems to the future-advanced ones still requires more efforts. Hence, the following section highlights the current research challenges, as well as pioneering examples of emerging strategies for developing the next generation of robust hybrid water electrolysis systems.| Design strategies | Benefits | Limitations | Complementary strategies |
|---|---|---|---|
| Host structure remodeling122–127 | |||
| Heteroatom doping | • Enhance catalytic activity | • Difficult to control the distribution of heteroatoms uniformly | • Surface engineering and integration of 2D nanomaterials into 3D matrices: ensure uniform dispersion of doped heteroatoms |
| • Tailor electronic properties and surface chemistry | • Complex synthesis processes | • Defect/vacancy engineering: simplifies the synthesis process as defect/vacancy sites can facilitate the incorporation of heteroatoms | |
| Defect/vacancy engineering | • Creates more active sites | • Excessive defects may compromise structural integrity | • Heteroatom doping: mitigates the risk of compromising integrity by stabilizing the structure |
| • Improves reaction kinetics | • Difficult to control defect density | • Construction of heterostructures: reduces the impact of excessive defects by providing additional mechanical support | |
| • Surface engineering: maintains the uniformity of defect density by functionalizing the surface with specific properties | |||
| Co-catalyst engineering | • Enables synergistic effects with the host material | • Possible leaching of co-catalysts | • Heteroatom doping and defect/vacancy engineering: reduces the risk of leaching by creating more robust active sites |
| • Enhances selectivity and efficiency | • Higher cost due to multiple components | • Construction of heterostructures: simplifies the integration process by providing a more stable structure | |
| • Complexity of selection and integration of suitable co-catalysts | • Integration of 2D nanomaterials into 3D matrices: addresses the complexity of selection and integration by providing a more stable structure | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Structure assembly design38,128–131 | |||
| Construction of heterostructures | • Enhances charge separation | • Complex fabrication | • Surface engineering: achieves uniform integration and simplifies the fabrication process |
| • Improves catalytic performance | • Potential stability issues under specific reaction conditions | • Heteroatom doping and defect/vacancy engineering: addresses stability issues by improving the interaction between different components | |
| • Synergistic effects between different components | • Integration of 2D nanomaterials into 3D matrices: increases stability by improving the mechanical stability | ||
| Surface engineering | • Improves catalytic selectivity | • Requires precise control | • Heteroatom doping and defect/vacancy engineering: achieves uniform surface coverage and reduces contamination by stabilizing functional groups as anchoring points and improving the interaction with reactants |
| • Increases surface area | • Potential for surface contamination | • Construction of heterostructures and integration of 2D nanomaterials into 3D matrices: reduces the risk of contamination and improves uniform surface coverage | |
| • Enhances interaction with reactants | • Ununiform surface coverage | ||
| Integration of 2D nanomaterials into 3D matrices | • High surface area | • Unstable immobilization | • Construction of heterostructures and surface engineering: facilitates better immobilization and reduces aggregation by improving the interaction ability |
| • Unique electronic properties | • Potential aggregation issues | • Heteroatom doping and defect/vacancy engineering: achieves uniform dispersion and reduces aggregation by introducing more reactive sites on the 2D nanomaterials | |
| • Enables efficient charge transport and mass transfer | • Ununiform dispersion and alignment of 2D materials in a 3D matrix | • Co-catalysts: improves alignment within the 3D matrices by the synergistic effect with the 2D nanomaterials | |
(i) Multiple design strategies for advanced 2D nanomaterials: to date, single design strategies have been widely employed for 2D nanomaterial modification, targeting the promoted activity, selectivity or durability, respectively. To comprehensively improve the catalytic performance of developed 2D nanomaterials, employing multiple design strategies on one material is considered to be a more versatile technique. Here, we have summarized the complementation of reviewed design strategies (Table 1) to guide smarter and more efficient 2D nanomaterial design. In addition, selective design strategies for anode and cathode materials should be applied in a compatible manner so as to boost biomass valorization as well as H2 production simultaneously, while bifunctional design strategies are highly desired to simplify the material fabrication and avoid cross-contamination under oxidative/reductive conditions.
(ii) Comprehensive investigation of catalyst interfaces under operation: it is well known that catalyst interfaces play an important role in governing the catalytic performance in terms of reactivity, selectivity and stability. In this context, a clear understanding of catalyst interfacial processes such as dynamic chemical/structural/electronic evolution, formation/interaction of key electroactive intermediates, and dominant reaction pathways, is required to rationalize the selection of design strategies and to drive the knowledge-based development of next-generation biomass valorization/HER electrocatalysts.
Combination of (photo-)electrochemical and cutting-edge in situ characterization techniques under operation can offer valuable insights at atomic- and molecular-levels to this end, leading to the emergence of a wide range of in situ microscopic and spectroscopic methods, such as surface interrogation-scanning electrochemical microscopy (SI-SECM) for quantitative information of surface adsorbates,132–134 transmission electron microscopy (TEM) for dynamic structural and morphological evolution at the nano-/atomic-scale,135 Fourier transform infrared spectroscopy (FTIR)63,136,137 and Raman spectroscopy138–141 for delivering structural information on surface-formed species, surface-bound adsorbates and species in the electrochemical double-layer, vibrational sum-frequency generation (vSFG) for accessing the structure of water molecules at the catalyst/water interface without bulk signal contribution,142–144 X-ray absorption spectroscopy (XAS) for capturing the transformations of the local chemistry of the probed element, e.g., precise information on the electronic structure, bond length, oxidation state, and coordination environment under operation,145–148 X-ray diffraction (XRD) for tracking crystal structure and phase transitions,149,150 and X-ray photoelectron spectroscopy (XPS) for surface elemental composition and oxidation state alteration under operation.151,152
In addition to these milestones, facilitating the use of machine-learning methods for theoretical calculation is a versatile approach for in-depth kinetic modeling of the focused catalyst interfaces, which can be used to predict the reaction mechanism by calculating the energy levels of the electrons in the catalytic material and the interactions between the electrons and the ions in the electrolyte.153–156
(iii) (Photo-)electrocatalyst recycling and regeneration: under harsh catalytic conditions, the degradation of catalytic performance in terms of activity, selectivity and durability, is always difficult to predict and/or prevent. Even the improper handling of the deactivated (photo-)electrocatalysts has caused serious implications, including waste generation and resource depletion.157 To this end, reactive element recycling and sustained performance regeneration of the aging catalyst are essential for environmental and economic relevance, which is however limited not only by high cost, but also high energy consumption.158–161 Notably, the lifespan of 2D nanomaterial-based (photo-)electrocatalysts can be extended in more eco-friendly modes,162,163 like employing green synthesis methods (e.g. grinding, milling, and laser-assisted methods) or an electrochemical activation approach. More remarkably, to re-attain superior performance, they can be re-modified by considerate design strategies164 summarized in this review.
(iv) Strategically bridging the lab-scale to the industrial-scale: despite significant progress in the design and fabrication of highly efficient 2D nanomaterials, there is still a gap between the laboratory scale and industrial scale for advanced biomass valorization coupled with H2 production.165,166 On one hand, low-cost manufacturing and scaling up approaches of 2D nanomaterials should be developed to this end.167 As an instructive example, roll-to-roll chemical vapor deposition (CVD) reactors are emerging as a prominent tool to produce continuous and uniform films of 2D nanomaterials on various substrates (e.g. steel sheet, titanium sheet, and carbon foam) for industrial demands.168 On the other hand, integration of the obtained cost-efficient and activity-intensive 2D nanomaterial into a suitable electrolyzer is considered to be another wise strategy to meet industrial demands. In this regard, a flow electrolyzer shows enormous potential for the continuous production of H2 and valuable chemicals, benefitting from a number of significant advantages over the batch cell including: (1) higher productivity and yield, (2) better control and optimization of the reaction parameters, (3) easier separation and purification of the products, and (4) lower energy consumption and waste generation.169 More promisingly, employment of several flow electrolyzers in a row will offer a great opportunity to improve the efficiency, selectivity, and scalability of targeting reactions.
Considering the aforementioned challenges and emerging solutions, a schematic representation of the four-gear concept is depicted in Fig. 8, demonstrating how the four interrelated, yet independent parts can form a whole.
4. Conclusion
In summary, this review highlights the design and application of versatile 2D nanomaterials in hybrid water electrolysis technology, which is able to simultaneously produce green H2 fuels and valuable chemicals/intermediates from naturally abundant biomass feedstocks. Specifically, this review provides a comprehensive summary of recently developed design strategies (including benefits, limitations and complementary strategies) for functional nanostructured 2D (photo-)electrocatalysts for the HMFOR, GOR or LOR coupled with the HER. A thorough literature survey reveals that, benefiting from rational design strategies, the enhanced physical and chemical properties of 2D nanomaterials largely facilitate hybrid water electrolysis performance. Notably, it is crucial to address the current challenges related to intelligent design toward next-generation 2D nanostructured electrocatalysts, insightful (photo-)electrochemical surface investigation under operation, sustainable recovery and regeneration of active species after service, and industrially relevant scaling-up applications. Addressing these practical issues provides a great opportunity to advance hybrid water (photo-)electrolysis technology towards higher efficiency and lower cost.Data availability
This review article does not present any new data analyzed by the authors. All data discussed in this review are derived from previously published studies with the respective citation in the text. Additional ESI data† and comparison tables have been included, which are cited accordingly within the ESI.†Author contributions
B. F. M. and D. G. conceived the idea for the review. The manuscript was written and reviewed through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interests.Acknowledgements
B. F. M. acknowledges the Alexander-von-Humboldt-Foundation for a postdoctoral fellowship (project no. 1231127). Johannes Gutenberg University Mainz is gratefully acknowledged for financial support. D. G. acknowledges the Deutsche Forschungsgemeinschaft (DFG) for a Walter Benjamin Fellowship (project no. 510966757). D. G. gratefully acknowledges the financial support by the Carl Zeiss Foundation (Halocycles no P2021-10-007). D. G. gratefully acknowledges funding from the Top Level Research Area SusInnoScience of the federal state of Rheinland-Pfalz.Notes and references
- M. Faraji, M. Yousefi, S. Yousefzadeh, M. Zirak, N. Naseri, T. H. Jeon, W. Choi and A. Z. Moshfegh, Energy Environ. Sci., 2019, 12, 59–95 RSC.
- Y. S. Park, J. Yang, J. Lee, M. J. Jang, J. Jeong, W.-S. Choi, Y. Kim, Y. Yin, M. H. Seo and Z. Chen, Appl. Catal., B, 2020, 278, 119276 CrossRef.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef.
- A. Raveendran, M. Chandran and R. Dhanusuraman, RSC Adv., 2023, 13, 3843–3876 RSC.
- J. E. Lee, K.-J. Jeon, P. L. Show, S.-C. Jung, Y. J. Choi, G. H. Rhee, K.-Y. A. Lin and Y.-K. Park, Fuel, 2022, 308, 122048 CrossRef.
- L. Wang, Y. Tong, J. Feng, J. Hou, J. Li, X. Hou and J. Liang, Sustainable Mater. Technol., 2019, 19, e00089 CrossRef CAS.
- X. Li, R. Shen, S. Ma, X. Chen and J. Xie, Appl. Surf. Sci., 2018, 430, 53–107 CrossRef CAS.
- J. Yi, W. El-Alami, Y. Song, H. Li, P. M. Ajayan and H. Xu, Chem. Eng. J., 2020, 382, 122812 CrossRef CAS.
- H. Zhou, F. Yu, Q. Zhu, J. Sun, F. Qin, L. Yu, J. Bao, Y. Yu, S. Chen and Z. Ren, Energy Environ. Sci., 2018, 11, 2858–2864 RSC.
- T. Naito, T. Shinagawa, T. Nishimoto and K. Takanabe, Inorg. Chem. Front., 2021, 8, 2900–2917 RSC.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef PubMed.
- X. Xie, L. Du, L. Yan, S. Park, Y. Qiu, J. Sokolowski, W. Wang and Y. Shao, Adv. Funct. Mater., 2022, 32, 2110036 CrossRef.
- X. Liu, Y. Ji, G. Chen, X. Peng, L. Yi, J. Chen, X. Feng and Z. Wen, Small Struct., 2021, 2, 2100121 CrossRef.
- B. You, X. Liu, X. Liu and Y. Sun, ACS Catal., 2017, 7, 4564–4570 CrossRef.
- K. Xiang, D. Wu, X. Deng, M. Li, S. Chen, P. Hao, X. Guo, J. L. Luo and X. Z. Fu, Adv. Funct. Mater., 2020, 30, 1909610 CrossRef CAS.
- W.-J. Liu, L. Dang, Z. Xu, H.-Q. Yu, S. Jin and G. W. Huber, ACS Catal., 2018, 8, 5533–5541 CrossRef CAS.
- B. You, X. Liu, N. Jiang and Y. Sun, J. Am. Chem. Soc., 2016, 138, 13639–13646 CrossRef PubMed.
- C. Lamy, A. Devadas, M. Simoes and C. Coutanceau, Electrochim. Acta, 2012, 60, 112–120 CrossRef.
- W. Guo, L. Li, L. Li, S. Tian, S. Liu and Y. Wu, Int. J. Hydrogen Energy, 2011, 36, 9415–9419 CrossRef.
- Y. Huang, X. Chong, C. Liu, Y. Liang and B. Zhang, Angew. Chem., Int. Ed., 2018, 130, 13347–13350 CrossRef.
- S. Xue, S. Watzele, V. Čolić, K. Brandl, B. Garlyyev and A. S. Bandarenka, ChemSusChem, 2017, 10, 4812–4816 CrossRef.
- W. Peng, L. Xiao, B. Huang, L. Zhuang and J. Lu, J. Phys. Chem. C, 2011, 115, 23050–23056 CrossRef.
- J. Huang, J. Cai and J. Wang, ACS Appl. Energy Mater., 2020, 3, 4108–4113 CrossRef.
- J. Y. Zhang, H. Wang, Y. Tian, Y. Yan, Q. Xue, T. He, H. Liu, C. Wang, Y. Chen and B. Y. Xia, Angew. Chem., Int. Ed., 2018, 57, 7649–7653 CrossRef PubMed.
- P. Babar, A. Lokhande, V. Karade, I. J. Lee, D. Lee, S. Pawar and J. H. Kim, J. Colloid Interface Sci., 2019, 557, 10–17 CrossRef.
- X. Zhao, L. Dai, Q. Qin, F. Pei, C. Hu and N. Zheng, Small, 2017, 13, 1602970 CrossRef PubMed.
- J. Zheng, X. Chen, X. Zhong, S. Li, T. Liu, G. Zhuang, X. Li, S. Deng, D. Mei and J. G. Wang, Adv. Funct. Mater., 2017, 27, 1704169 CrossRef.
- Z. Fan, W. Zhang, L. Li, Y. Wang, Y. Zou, S. Wang and Z. Chen, Green Chem., 2022, 24, 7818–7868 RSC.
- H. Luo, J. Barrio, N. Sunny, A. Li, L. Steier, N. Shah, I. E. Stephens and M. M. Titirici, Adv. Energy Mater., 2021, 11, 2101180 CrossRef.
- Z. Sun, B. Fridrich, A. De Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef.
- C. Liu, S. Wu, H. Zhang and R. Xiao, Fuel Process. Technol., 2019, 191, 181–201 CrossRef.
- M.-I. Jamesh and X. Sun, J. Power Sources, 2018, 400, 31–68 CrossRef.
- C. Deng, C. Y. Toe, X. Li, J. Tan, H. Yang, Q. Hu and C. He, Adv. Energy Mater., 2022, 2201047 CrossRef.
- S. Bellani, A. Bartolotta, A. Agresti, G. Calogero, G. Grancini, A. Di Carlo, E. Kymakis and F. Bonaccorso, Chem. Soc. Rev., 2021, 11870–11965 RSC.
- D. Scarano and F. Cesano, Materials, 2021, 14, 7108 CrossRef.
- C. S. Bongu, S. Tasleem, M. R. Krishnan and E. H. Alsharaeh, Sustainable Energy Fuels, 2024, 4039–4070 RSC.
- H. He, L. Guan and H. Le Ferrand, J. Mater. Chem. A, 2022, 19129–19168 RSC.
- M. H. Kalantari and X. Zhang, Nanomaterials, 2022, 13, 117 CrossRef PubMed.
- B. Zheng, S. Yu, Z. Chen and Y.-X. Huo, Front. Microbiol., 2022, 13, 933882 CrossRef.
- A. Zhang, Y. Liang, H. Zhang, Z. Geng and J. Zeng, Chem. Soc. Rev., 2021, 9817–9844 RSC.
- H. Zhu, X. Gan, A. McCreary, R. Lv, Z. Lin and M. Terrones, Nano Today, 2020, 30, 100829 CrossRef.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS PubMed.
- Z. Ning, S. Gan, H. Xu, C. Gu, P. Zhu, J. Zhou and G. Xiao, J. Mater. Chem. A, 2024, 12, 5885–5897 RSC.
- M. Gao, L. Zhu, C. K. Peh and G. W. Ho, Energy Environ. Sci., 2019, 12, 841–864 RSC.
- J. Li and N. Wu, Catal. Sci. Technol., 2015, 5, 1360–1384 RSC.
- D. N. Tafen, J. Wang, N. Wu and J. P. Lewis, Appl. Phys. Lett., 2009, 94, 093101 CrossRef.
- H. P. Maruska and A. K. Ghosh, Sol. Energy Mater., 1979, 1, 237–247 CrossRef CAS.
- J. M. Luther, P. K. Jain, T. Ewers and A. P. Alivisatos, Nat. Mater., 2011, 10, 361–366 CrossRef CAS PubMed.
- T. Wei, W. Liu, S. Zhang, Q. Liu, J. Luo and X. Liu, Chem. Commun., 2023, 59, 442–445 RSC.
- J. Zhang, P. Yu, G. Zeng, F. Bao, Y. Yuan and H. Huang, J. Mater. Chem. A, 2021, 9, 9685–9691 RSC.
- D. Li, Y. Huang, Z. Li, L. Zhong, C. Liu and X. Peng, J. Chem. Eng., 2022, 430, 132783 CrossRef CAS.
- D. Li, Z. Li, R. Zou, G. Shi, Y. Huang, W. Yang, W. Yang, C. Liu and X. Peng, Appl. Catal., B, 2022, 307, 121170 CrossRef CAS.
- T. Cui, L. Ma, S. Wang, C. Ye, X. Liang, Z. Zhang, G. Meng, L. Zheng, H.-S. Hu and J. Zhang, J. Am. Chem. Soc., 2021, 143, 9429–9439 CrossRef CAS PubMed.
- X. Hao, Y. Quansheng, S. Dan, Y. Honghui, L. Jidong, F. Jiangtao and Y. Wei, J. Hazard. Mater., 2015, 286, 509–516 CrossRef CAS.
- C. Zhao, Z. Li, T. Fan, C. Xiao and Y. Xie, Research, 2020, 2020, 652749 Search PubMed.
- X. Yan, L. Zhuang, Z. Zhu and X. Yao, Nanoscale, 2021, 13, 3327–3345 RSC.
- T. Tang, Z. Wang and J. Guan, Chin. J. Catal., 2022, 43, 636–678 CrossRef CAS.
- D. Maarisetty and S. S. Baral, J. Mater. Chem. A, 2020, 8, 18560–18604 RSC.
- J. Yan, G. Wu, N. Guan, L. Li, Z. Li and X. Cao, Phys. Chem. Chem. Phys., 2013, 15, 10978–10988 RSC.
- M. Xu, X. Ruan, D. Meng, G. Fang, D. Jiao, S. Zhao, Z. Liu, Z. Jiang, K. Ba, T. Xie, W. Zhang, J. Leng, S. Jin, S. K. Ravi and X. Cui, Adv. Funct. Mater., 2024, 2402330 CrossRef CAS.
- B. Zhang, Z. Yang, C. Yan, Z. Xue and T. Mu, Small, 2023, 19, 2207236 CrossRef CAS.
- B. Zhu, Y. Qin, J. Du, F. Zhang and X. Lei, ACS Sustain. Chem. Eng., 2021, 9, 11790–11797 CrossRef CAS.
- Z. Tian, Y. Da, M. Wang, X. Dou, X. Cui, J. Chen, R. Jiang, S. Xi, B. Cui and Y. Luo, Nat. Commun., 2023, 14, 142 CrossRef CAS.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900–1909 CrossRef CAS PubMed.
- M. Kumar, B. Meena, P. Subramanyam, D. Suryakala and C. Subrahmanyam, NPG Asia Mater., 2022, 14, 88 CrossRef CAS.
- X. T. Xu, L. Pan, X. Zhang, L. Wang and J. J. Zou, Adv. Sci., 2019, 6, 1801505 CrossRef.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- H. Hou, X. Zeng and X. Zhang, Sci. China Mater., 2020, 63, 2119–2152 CrossRef CAS.
- S. Liang, L. Pan, T. Thomas, B. Zhu, C. Chen, J. Zhang, H. Shen, J. Liu and M. Yang, J. Chem. Eng., 2021, 415, 128864 CrossRef CAS.
- S. Wang, X. Gao, X. Hang, X. Zhu, H. Han, X. Li, W. Liao and W. Chen, J. Am. Chem. Soc., 2018, 140, 6271–6277 CrossRef CAS.
- D. Rauber, T. K. Dier, D. A. Volmer and R. Hempelmann, Z. Phys. Chem., 2018, 232, 189–208 CrossRef CAS.
- J. Miao, Y. Ma, X. Wang, Y. Li, H. Wang, L. Zhang, J. Zhang, Y. Qin and J. Gao, Appl. Catal., B, 2023, 122937 CrossRef CAS.
- H. Wang, W. Fu, X. Yang, Z. Huang, J. Li, H. Zhang and Y. Wang, J. Mater. Chem. A, 2020, 8, 6926–6956 RSC.
- X. Ruan, S. Li, C. Huang, W. Zheng, X. Cui and S. K. Ravi, Adv. Mater., 2024, 36, 2305285 CrossRef CAS.
- P. Solís-Fernández, M. Bissett and H. Ago, Chem. Soc. Rev., 2017, 46, 4572–4613 RSC.
- X. Du, J. Huang, J. Zhang, Y. Yan, C. Wu, Y. Hu, C. Yan, T. Lei, W. Chen and C. Fan, Angew. Chem., Int. Ed., 2019, 58, 4484–4502 CrossRef CAS.
- X. Wu, Y. Wang and Z.-S. Wu, Chem, 2022, 13170–13189 Search PubMed.
- J. Low, S. Cao, J. Yu and S. Wageh, Chem. Commun., 2014, 50, 10768–10777 RSC.
- J. Su, G. D. Li, X. H. Li and J. S. Chen, Adv. Sci., 2019, 6, 1801702 CrossRef PubMed.
- X. Li and J. Wang, InfoMat, 2020, 2, 3–32 CrossRef CAS.
- Q. Wei, F. Xiong, S. Tan, L. Huang, E. H. Lan, B. Dunn and L. Mai, Adv. Mater., 2017, 29, 1602300 CrossRef.
- Y. Li, M. Ma, D. Yi, Q. Zhou, J. Chen, Y. Bian, W. Niu, C. Yang, Z. Yin, Z. Wang and A. Tang, Adv. Funct. Mater., 2024, 2407271 CrossRef.
- L. Zhang, J. Zhang, H. Yu and J. Yu, Adv. Mater., 2022, 34, 2107668 CrossRef CAS.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6, 1543–1559 CAS.
- M. Sun, J. Yang, J. Huang, Y. Wang, X. Liu, Y. Qi and L. Zhang, Langmuir, 2023, 39, 3762–3769 CrossRef CAS.
- Y. Zhong, R.-Q. Ren, J.-B. Wang, Y.-Y. Peng, Q. Li and Y.-M. Fan, Catal. Sci. Technol., 2022, 12, 201–211 RSC.
- X. Deng, X. Kang, M. Li, K. Xiang, C. Wang, Z. Guo, J. Zhang, X.-Z. Fu and J.-L. Luo, J. Mater. Chem. A, 2020, 8, 1138–1146 RSC.
- F. Sun, Y. Zhou, Z. You, H. Xia, Y. Tuo, S. Wang, C. Jia and J. Zhang, Small, 2021, 17, 2103307 CrossRef CAS.
- F. Bateni, M. NaderiNasrabadi, R. Ghahremani and J. A. Staser, J. Electrochem. Soc., 2019, 166, F1037 CrossRef CAS.
- M. NaderiNasrabadi, F. Bateni, Z. Chen, P. B. Harrington and J. A. Staser, J. Electrochem. Soc., 2019, 166, E317 CrossRef CAS.
- Y. Hu, H. Huang, J. Feng, W. Wang, H. Guan, Z. Li and Z. Zou, Sol. RRL, 2021, 5, 2100100 CrossRef CAS.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- Y. Zhou, L. Zhang, L. Lin, B. R. Wygant, Y. Liu, Y. Zhu, Y. Zheng, C. B. Mullins, Y. Zhao and X. Zhang, Nano Lett., 2017, 17, 8012–8017 CrossRef CAS PubMed.
- M. Bae, Y. Kang, D. W. Lee, D. Jeon and J. Ryu, Adv. Energy Mater., 2022, 12, 2201452 CrossRef CAS.
- X. Deng, M. Li, Y. Fan, L. Wang, X.-Z. Fu and J.-L. Luo, Appl. Catal., B, 2020, 278, 119339 CrossRef CAS.
- C. Sun, D. Zhang, Y. Zhao, C. Song and D. Wang, Colloids Surf., A, 2022, 650, 129597 CrossRef CAS.
- Z. Zhou, C. Chen, M. Gao, B. Xia and J. Zhang, Green Chem., 2019, 21, 6699–6706 RSC.
- Y. Wang, W. Yan, M. Ni, C. Zhu and H. Du, Chem. Commun., 2023, 59, 2485–2488 RSC.
- R. Ghahremani, F. Farales, F. Bateni and J. A. Staser, J. Electrochem. Soc., 2020, 167, 043502 CrossRef CAS.
- K. Jakubow-Piotrowska, B. Witkowski and J. Augustynski, Commun. Chem., 2022, 5, 125 CrossRef CAS PubMed.
- Y. Xue, G. Zhao, R. Yang, F. Chu, J. Chen, L. Wang and X. Huang, Nanoscale, 2021, 13, 3911–3936 RSC.
- L. P. Lefebvre, J. Banhart and D. C. Dunand, Adv. Eng. Mater., 2008, 10, 775–787 CrossRef CAS.
- W. Zhu, R. Zhang, F. Qu, A. M. Asiri and X. Sun, ChemCatChem, 2017, 9, 1721–1743 CrossRef CAS.
- C. Körner and R. F. Singer, Adv. Eng. Mater., 2000, 2, 159–165 CrossRef.
- D. Gao, R. Liu, S. Liu, S. Greiner, M. Anjass, J. Biskupek, U. Kaiser, H. Braun, T. Jacob and C. Streb, ACS Appl. Mater. Interfaces, 2021, 13, 19048–19054 CrossRef CAS.
- D. Gao, R. Liu, J. Biskupek, U. Kaiser, Y. F. Song and C. Streb, Angew. Chem., Int. Ed., 2019, 58, 4644–4648 CrossRef CAS.
- F. Chen, C. Chen, Q. Hu, B. Xiang, T. Song, X. Zou, W. Li, B. Xiong and M. Deng, J. Chem. Eng., 2020, 401, 126145 CrossRef CAS.
- R. Zhang, J. Liu, H. Guo and X. Tong, Mater. Lett., 2015, 139, 55–58 CrossRef CAS.
- D. Gao, S. Liu, R. Liu and C. Streb, Chem.–Eur. J., 2020, 26, 11109–11112 CrossRef CAS PubMed.
- C. Chen, Z. Zhou, J. Liu, B. Zhu, H. Hu, Y. Yang, G. Chen, M. Gao and J. Zhang, Appl. Catal., B, 2022, 307, 121209 CrossRef CAS.
- Y. Xie, L. Sun, X. Pan, Z. Zhou and G. Zhao, Appl. Catal., B, 2023, 338, 123068 CrossRef CAS.
- Y. Xin, F. Wang, L. Chen, Y. Li and K. Shen, Green Chem., 2022, 24, 6544–6555 RSC.
- N. Wang, R. Xue, N. Yang, H. Sun, B. Zhang, Z. Ma, Y. Ma and L. Zang, J. Alloys Compd., 2022, 929, 167324 CrossRef CAS.
- W.-J. Liu, Z. Xu, D. Zhao, X.-Q. Pan, H.-C. Li, X. Hu, Z.-Y. Fan, W.-K. Wang, G.-H. Zhao, S. Jin, G. W. Huber and H.-Q. Yu, Nat. Commun., 2020, 11, 265 CrossRef CAS.
- M. Garedew, F. Lin, B. Song, T. M. DeWinter, J. E. Jackson, C. M. Saffron, C. H. Lam and P. T. Anastas, ChemSusChem, 2020, 13, 4214–4237 CrossRef CAS.
- M. Zirbes, D. Schmitt, N. Beiser, D. Pitton, T. Hoffmann and S. R. Waldvogel, ChemElectroChem, 2019, 6, 155–161 CrossRef CAS.
- Z. Fang, J. E. Jackson and E. L. Hegg, ACS Sustain. Chem. Eng., 2022, 10, 7545–7552 CrossRef CAS.
- D. Schmitt, C. Regenbrecht, M. Hartmer, F. Stecker and S. R. Waldvogel, Beilstein J. Org. Chem., 2015, 11, 473–480 CrossRef CAS.
- D. Schmitt, C. Regenbrecht, M. Schubert, D. Schollmeyer and S. R. Waldvogel, Holzforschung, 2017, 71, 35–41 CrossRef CAS.
- M. Breiner, M. Zirbes and S. R. Waldvogel, Green Chem., 2021, 23, 6449–6455 RSC.
- X. Wang, G. Sun, P. Routh, D.-H. Kim, W. Huang and P. Chen, Chem. Soc. Rev., 2014, 43, 7067–7098 RSC.
- B. F. Mohazzab, A. Akhundi, K. Rahimi, B. Jaleh and A. Z. Moshfegh, ACS Appl. Energy Mater., 2022, 5, 12283–12296 CrossRef CAS.
- S. Mansoor, M. Tayyab, M. Khan, Z. Akmal, L. Zhou, J. Lei, M. Anpo and J. Zhang, Res. Chem. Intermed., 2023, 49, 3723–3745 CrossRef CAS.
- A. Kumar, P. Raizada, A. Hosseini-Bandegharaei, V. K. Thakur, V.-H. Nguyen and P. Singh, J. Mater. Chem. A, 2021, 9, 111–153 RSC.
- V. Gurylev and V. Gurylev, Nanostructured Photocatalyst via Defect Engineering: Basic Knowledge and Recent Advances, 2021, pp. 37–72 Search PubMed.
- H. L. Chia, C. C. Mayorga-Martinez and M. Pumera, Adv. Funct. Mater., 2021, 31, 2102555 CrossRef CAS.
- H. Zheng, Y. Li, H. Liu, X. Yin and Y. Li, Chem. Soc. Rev., 2011, 40, 4506–4524 RSC.
- X. Sun, L. Shi, H. Huang, X. Song and T. Ma, Chem. Commun., 2020, 56, 11000–11013 RSC.
- L. Maggini and R. R. Ferreira, J. Mater. Chem. C, 2021, 9, 15721–15734 RSC.
- C. Feng, Z.-P. Wu, K.-W. Huang, J. Ye and H. Zhang, Adv. Mater., 2022, 34, 2200180 CrossRef CAS.
- Y. Wu and N. Liu, Chem, 2018, 4, 438–465 CAS.
- K. Zhu, X. Zhu and W. Yang, Angew. Chem., Int. Ed., 2019, 58, 1252–1265 CrossRef CAS PubMed.
- A. Preet and T.-E. Lin, Catalysts, 2021, 11, 594 CrossRef CAS.
- N. Ortiz Peña, D. Ihiawakrim, M. Han, B. Lassalle-Kaiser, S. Carenco, C. Sanchez, C. Laberty-Robert, D. Portehault and O. Ersen, ACS Nano, 2019, 13, 11372–11381 CrossRef.
- P. Su, V. Prabhakaran, G. E. Johnson and J. Laskin, Anal. Chem., 2018, 90, 10935–10942 CrossRef CAS.
- T. Faverge, B. Gilles, A. Bonnefont, F. Maillard, C. Coutanceau and M. Chatenet, ACS Catal., 2023, 13, 2657–2669 CrossRef CAS.
- D.-J. Chen and Y. Tong, Chem. Commun., 2015, 51, 5683–5686 RSC.
- S.-i. Ogino, T. Itoh, D. Mabuchi, K. Yokoyama, K. Motomiya, K. Tohji and Y. Sato, J. Phys. Chem. C, 2016, 120, 7133–7143 CrossRef CAS.
- Y.-H. Wang, S. Li, R.-Y. Zhou, S. Zheng, Y.-J. Zhang, J.-C. Dong, Z.-L. Yang, F. Pan, Z.-Q. Tian and J.-F. Li, Nat. Protoc., 2023, 18, 883–901 CrossRef CAS PubMed.
- D. Chen, Y. Ding, X. Cao, L. Wang, H. Lee, G. Lin, W. Li, G. Ding and L. Sun, Angew. Chem., Int. Ed., 2023, 62, e202309478 CrossRef CAS PubMed.
- N. Zhang, Y. Zou, L. Tao, W. Chen, L. Zhou, Z. Liu, B. Zhou, G. Huang, H. Lin and S. Wang, Angew. Chem., Int. Ed., 2019, 131, 16042–16050 CrossRef.
- R. De and B. Dietzek-Ivanšić, Chem.–Eur. J., 2022, 28, e202200407 CrossRef CAS.
- A. Kemna, N. García Rey and B. Braunschweig, ACS Catal., 2019, 9, 6284–6292 CrossRef CAS.
- J. Timoshenko and B. Roldan Cuenya, Chem. Rev., 2020, 121, 882–961 CrossRef.
- M. Risch, D. M. Morales, J. Villalobos and D. Antipin, Angew. Chem., Int. Ed., 2022, 61, e202211949 CrossRef CAS PubMed.
- S. Chen, L. Ma, Z. Huang, G. Liang and C. Zhi, Cell Rep. Phys. Sci., 2022, 100729 CrossRef CAS.
- N. Gupta, C. Segre, C. Nickel, C. Streb, D. Gao and K. D. Glusac, ACS Appl. Mater. Interfaces, 2024, 16, 35793–35804 CrossRef CAS.
- C.-J. Chang, Y. Zhu, J. Wang, H.-C. Chen, C.-W. Tung, Y.-C. Chu and H. M. Chen, J. Mater. Chem. A, 2020, 8, 19079–19112 RSC.
- Y. Zhu, T.-R. Kuo, Y.-H. Li, M.-Y. Qi, G. Chen, J. Wang, Y.-J. Xu and H. M. Chen, Energy Environ. Sci., 2021, 14, 1928–1958 RSC.
- K. A. Stoerzinger, W. T. Hong, E. J. Crumlin, H. Bluhm and Y. Shao-Horn, Acc. Chem. Res., 2015, 48, 2976–2983 CrossRef CAS.
- Y.-D. Cao, W.-X. Mu, M. Gong, L.-L. Fan, J. Han, H. Liu, B. Qi and G.-G. Gao, Dalton Trans., 2023, 7343–7350 Search PubMed.
- J. K. Nørskov, F. Abild-Pedersen, F. Studt and T. Bligaard, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 937–943 CrossRef.
- Y.-K. Lee, Catalysts, 2021, 11, 454 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- L. Ostervold, S. I. P. Bakovic, J. Hestekin and L. F. Greenlee, RSC Adv., 2021, 11, 31208–31218 RSC.
- M. G. Kibria, N. I. Masuk, R. Safayet, H. Q. Nguyen and M. Mourshed, Int. J. Environ. Res., 2023, 17, 20 CrossRef CAS.
- S. Cheng, L. Wei, X. Zhao and J. Julson, Catalysts, 2016, 6, 195 CrossRef.
- I. V. Yentekakis and F. Dong, Front. Environ. Chem., 2020, 5, 1–5 Search PubMed.
- C. H. Bartholomew and M. D. Argyle, Catalysts, 2015, 5, 949–954 CrossRef CAS.
- X. Zhang, S. Xu, J. Tang, L. Fu and H. Karimi-Maleh, Catalysts, 2022, 12, 818 CrossRef CAS.
- L. X. Chen, Z. W. Chen, M. Jiang, Z. Lu, C. Gao, G. Cai and C. V. Singh, J. Mater. Chem. A, 2021, 9, 2018–2042 RSC.
- K. Khan, A. K. Tareen, M. Aslam, R. U. R. Sagar, B. Zhang, W. Huang, A. Mahmood, N. Mahmood, K. Khan and H. Zhang, Nano-Micro Lett., 2020, 12, 1–77 CrossRef PubMed.
- N. Rohaizad, C. C. Mayorga-Martinez, M. Fojtů, N. M. Latiff and M. Pumera, Chem. Soc. Rev., 2021, 50, 619–657 RSC.
- H. Sun, X. Xu, H. Kim, W. Jung, W. Zhou and Z. Shao, Energy Environ. Mater., 2022, e12441 Search PubMed.
- W. Li, H. Tian, L. Ma, Y. Wang, X. Liu and X. Gao, Mater. Adv., 2022, 3, 5598–5644 RSC.
- S. H. Choi, S. J. Yun, Y. S. Won, C. S. Oh, S. M. Kim, K. K. Kim and Y. H. Lee, Nat. Commun., 2022, 13, 1484 CrossRef CAS PubMed.
- E. S. Polsen, D. Q. McNerny, B. Viswanath, S. W. Pattinson and A. John Hart, Sci. Rep., 2015, 5, 1–12 CrossRef.
- D. Pollok and S. R. Waldvogel, Chem. Sci., 2020, 11, 12386–12400 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se01034e |
| This journal is © The Royal Society of Chemistry 2024 |