Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biocatalytic PEI–PSS membranes through aqueous phase separation: influence of casting solution pH and operational temperature†
Lijie
Li
 ab,
Muhammad Irshad
Baig
ab,
Muhammad Irshad
Baig
 b,
Wiebe M.
de Vos
b,
Wiebe M.
de Vos
 b and
Saskia
Lindhoud
b and
Saskia
Lindhoud
 *a
*a
aFaculty of Science and Technology, Department of Molecules & Materials, MESA+ Institute for Nanotechnology, University of Twente, Enschede 7500 AE, The Netherlands. E-mail: s.lindhoud@utwente.nl
bDepartment of Membrane Science and Technology, MESA+ Institute for Nanotechnology, University of Twente, Enschede 7500 AE, The Netherlands
First published on 21st June 2024
Abstract
Biocatalytic membranes combine the separation properties of membranes and the catalytic abilities of enzymes, holding great promise for industries where both purification and conversion are required. In this work, polyelectrolyte complex membranes incorporated with lysozyme were prepared using polyethyleneimine (PEI) and poly(sodium 4-styrenesulfonate) (PSS) through a one-step and mild pH shift aqueous phase separation (APS) approach. The effects of lysozyme addition and casting solution pH on the membrane properties were studied. All the membranes, both with and without added lysozyme, exhibited asymmetric structures with relatively dense top surfaces and porous cross-sections with finger-like macrovoids. The incorporation of lysozyme did not significantly influence the structure and permeability of the formed membranes. The PEI–PSS biocatalytic membranes exhibited temperature dependent enzymatic activity. The activity strongly increased with increased operational temperature, with the highest activity of 4.30 ± 0.15 U cm−2 at 45 °C. This indicates a responsive effect, where a higher temperature leads to some swelling of the polyelectrolyte complex membrane, making the enzyme more accessible to the used substrate. Moreover, the biocatalytic membranes demonstrate desirable enzymatic stability, maintaining 60% activity even after 60 days of storage. This study validates the potential of the water-based APS process as a straightforward approach for integrating enzymes into responsive biocatalytic membranes.
Introduction
Enzymes are biological catalysts that are capable of catalyzing chemical reactions with high specificity and efficiency. Enzyme technology is commonly used for applications in biology, chemistry, and medicine, but can also be found in consumer products such as laundry detergents.1–3 For these applications, a certain stability, e.g., towards changes in temperature or acidity, is desirable.4,5 One strategy to improve the stability of enzymes is to immobilize them on suitable support materials, like membranes.6,7 Membranes are widely used materials in industrial, agricultural, and medical fields for their outstanding separation and purification performance.8–10Biocatalytic membrane reactors (BMRs) are membranes with immobilized enzymes, which combine the separation functions of the membranes with the catalytic abilities of the enzymes.11–13 Various approaches have been employed to prepare BMRs, including covalent bonding, adsorption, or encapsulation.12,14,15 These approaches often require complex activation or modification steps to allow sufficient enzyme immobilization in the membranes. Furthermore, harsh treatment processes may lead to membrane damage or require the use of harmful organic solvents. Therefore, more safe and sustainable approach to prepare biocatalytic membranes is necessary. Water, the most abundant solvent in nature, is an ideal green and environment-friendly solvent. One way to utilize water as a solvent for membrane preparation is using polyelectrolytes.16 Polyelectrolytes are polymers with positively or negatively charged repeating units. It has been shown that enzymes can be simply incorporated into polyelectrolyte multilayers via alternating layer-by-layer coating of polyelectrolytes and enzymes.17,18 Although this method can allow the preparation of BMRs in water, the build-up of these layers is a time-consuming process. Recently, a one-step aqueous phase separation (APS) approach was proposed to prepare biocatalytic membranes.19
The APS approach is inspired by the traditional non-solvent induced phase separation (NIPS) process, where the solvent and non-solvent exchange leads to the formation of pores and usually shows asymmetric structure.20,21 NIPS is the dominant approach for the preparation of commercial membranes, but one major disadvantage of NIPS is the need for environmentally harmful organic solvents.20 In an APS process, polyelectrolyte membranes are obtained via pH change22–24 or salinity change25,26 induced complexation. Like NIPS, the APS process contains two steps, the casting of a polyelectrolyte solution and the subsequent phase inversion (through polyelectrolyte complexation) in a coagulation bath. For pH-change induced complexation, the casting solution is prepared at a pH where a weak polyelectrolyte is uncharged, preventing complexation with the other polyelectrolyte. Subsequent immersion in a coagulation bath with another pH, leads to charging of the weak polyelectrolyte and complexation between the oppositely charged polyelectrolytes. For salinity-change induced complexation, a homogenous polyelectrolyte casting solution is prepared at high salt concentration, where the charges on both polyelectrolytes are screened by the excess salt ions and the complexation is prevented. After casting, the membrane is formed by immersion in a low salinity coagulation bath where complexation is favored. The pore structure of the APS-produced membranes can be tuned by the composition of the casting solution and coagulation bath.23,25
In APS, membranes are formed in water, which not only makes the whole process safer and more sustainable, but also allows for easy enzyme incorporation in the membranes. Recently, van Lente et al. successfully prepared biocatalytic poly(allylamine hydrochloride) (PAH) and poly(sodium 4-styrenesulfonate) (PSS) membranes via pH-change induced phase separation. The obtained PAH–PSS membranes functionalized with lysozyme showed effective enzymatic activity that remained for at least one week.19 Restrepo et al. also confirmed the possibility to prepare self-supporting biocatalytic hollow fiber membranes from poly(diallyl dimethylammonium chloride) (PDADMAC) and PSS via salt-induced APS.27
A downside of the above approaches is that rather extreme changes in water chemistry were required for phase inversion. Production of PAH–PSS membranes via APS requires a pH change from 14 to 1, while the PDADMAC–PSS membranes required a shift in salinity from 2 mol L−1 to 0 mol L−1. These extreme pH and salinity changes could affect the stability of enzymes. It would thus be beneficial to explore APS approaches where relatively mild conditions of pH or salinity are used. Here we use polyethyleneimine (PEI) and PSS to prepare biocatalytic membranes via APS. According to previous work, the pH shift between the casting solution and coagulation bath for PEI–PSS polyelectrolyte system is much narrower (pH ∼12 to pH ∼4) compared to the PAH–PSS system.28,29 It has also been demonstrated in other work that both PEI and PSS are very suitable polyelectrolytes for enzyme incorporation.30,31 In this work, lysozyme is used as model enzyme since its properties are well known and its enzymatic activity can be easily measured. Lysozyme is an abundant enzyme that is found in hen egg white, human saliva, and tears.15 This enzyme is often used as an antimicrobial agent in food industry and medicine application. It can kill bacteria, especially Gram-positive bacteria, via hydrolysis of specific peptidoglycan bonds in bacterial cell walls.32,33
The aim of this work is to prepare biocatalytic PEI–PSS complex membranes with incorporated lysozyme via a one-step pH-change induced APS approach under a milder pH condition. In this work, casting solutions with different pH values (11.4, 10.9, and 10.5) were prepared by adding different amount of HCl during casting solution preparation, to further reduce the width of the required pH shift. The solutions were cast and immersed in an acetate buffer bath (pH ∼4) to induce phase separation. We investigated the influence of casting solution pH on the membrane structures and pure water permeability. Besides, the enzymatic activities of the obtained membranes were studied by measuring their activity to lyophilized micrococcus lysodeikticus. The activity measurements were performed at different temperatures (25, 35, and 45 °C) to investigate the effects of temperature on the enzymatic activities. The APS-produced biocatalytic PEI–PSS membranes demonstrated the potential to be utilized as porous antibacterial materials.
Experimental
Materials
Branched polyethyleneimine (PEI, Mw ∼750 kDa, 50 wt% aqueous solution), poly(sodium 4-styrene sulfonate) (PSS, Mw ∼1000 kDa, powder), sodium acetate, acetic acid (glacial, ACS reagent, ≥99%), hydrochloric acid (HCl, ACS reagent, 37%), glycerol solution (86–89%), lyophilized hen-egg lysozyme (product number L6876), and lyophilized micrococcus lysodeikticus (product number M3770) were all purchased from Sigma-Aldrich, The Netherlands. Ultrapure deionized water was obtained from the Advantage A10 purification system (Millipore). All the chemicals were used as received.Preparation of casting solutions
To prepare the casting solutions, single polyelectrolyte solutions were prepared first. PSS powder was dissolved in deionized water to prepare a 30 wt% stock solution, and the PEI solution from Sigma (50 wt%) was diluted with water to obtain a 35 wt% stock solution. A lysozyme solution (3 g L−1) was prepared by directly dissolving the powder in deionized water. The 37% HCl solution was diluted with water to obtain a 10% solution. Then, the PEI, PSS solutions and water were mixed to obtain a 24 wt% polyelectrolyte casting solution. The monomer ratio between PEI and PSS was 1.7 to 1, calculated by the molecular weights of their monomers, i.e. PSS ∼206.21 g mol−1 and PEI ∼43.04 g mol−1, based on previous work.28Meanwhile, casting solutions with lysozyme were prepared via direct mixing of the polyelectrolyte solutions and the lysozyme solution. The added amount of lysozyme was fixed at 0.024 wt% in the casting solution, this is comparable with the reported biocatalytic PAH–PSS membranes.19 In a previous study, the PEI–PSS polyelectrolyte casting solution was prepared at a pH of around 12.28 However, as lysozyme is a biological catalyst, starting from an even milder pH before adding lysozyme would be more beneficial. Therefore, to create a milder condition, we first added different amounts of HCl to the PEI solutions to decrease the pH. Adding HCl, also brings the polyelectrolyte solution closer to the pH where it would form a solid precipitate (onset of phase separation) and would thus also be expected to influence the membrane structure. The weight ratio of HCl to PEI were set as 0, 0.05, and 0.1, and the obtained casting solutions showed pHs around 11.4, 10.9, and 10.5 respectively (measured by pH meter), as shown in Table 1. For all the casting solutions, the final polyelectrolyte concentration was kept at 24 wt%. All the mixtures were stirred until homogeneous solutions were obtained.
| Composition | PEI (wt%) | PSS (wt%) | HCl (wt%) | Lysozyme (wt%) | Water (wt%) | Final pH of solutions |
|---|---|---|---|---|---|---|
| Notes: the solutions of PEI, PSS, and HCl were weighed by an analytical balance with an accuracy of 0.1 mg; the lysozyme solution and water were added using pipette (volume of 100–1000 μL) with an accuracy about 0.5%. | ||||||
| M-pH11.4 | 6.3 | 17.7 | 0 | 0 | 76.00 | 11.40 ± 0.05 |
| M-pH10.9 | 6.3 | 17.7 | 0.31 | 0 | 75.69 | 10.85 ± 0.11 |
| M-pH10.5 | 6.3 | 17.7 | 0.63 | 0 | 75.37 | 10.47 ± 0.10 |
| M-L-pH11.4 | 6.3 | 17.7 | 0 | 0.024 | 75.98 | 11.42 ± 0.05 |
| M-L-pH10.9 | 6.3 | 17.7 | 0.31 | 0.024 | 75.66 | 10.88 ± 0.04 |
| M-L-pH10.5 | 6.3 | 17.7 | 0.63 | 0.024 | 75.35 | 10.52 ± 0.04 |
Preparation of biocatalytic complex membranes
The casting solutions were cast on glass plates using a casting bar (gap ∼0.5 mm). The glass plates were then immediately immersed in an acetic acid–sodium acetate buffer (pH ∼4, 0.5 mol L−1) bath to induce phase separation. After 1 hour, the resultant membranes were taken out, washed thoroughly, and stored in water for further characterization. The composition of all the casting solutions and the name of the final membranes are shown in Table 1.Characterization
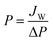 | (1) |
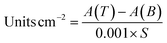 | (2) |
Results and discussion
Biocatalytic membranes possess not only the separation abilities of membranes but also the catalytic activity of the embedded enzymes.34,35 Here, we study a sustainable, mild, one-step approach to prepare biocatalytic membranes with lysozyme. PEI–PSS membranes with and without lysozyme were prepared via aqueous phase separation. Fig. 1 shows the preparation process of the biocatalytic membranes. Homogeneous casting solutions were prepared via the direct mixing of PEI solution (with different amount HCl) and PSS solutions with the lysozyme solution. Then the obtained solution was cast on a glass plate, and then put in an acetate buffer bath to induce phase separation. In the bath, PEI became positively charged and therefore formed polyelectrolyte complexes with the negatively charged PSS. After removing the complex membrane from the glass plate, a free-standing and mechanically stable membrane was obtained. In this work, the influences of lysozyme addition and casting solution pH on the membrane structure and permeability were first investigated. In the second section, we investigated the enzymatic activities of the biocatalytic membranes at different temperatures, where the influence of operational temperature will be discussed. Finally, the stability of the enzymatic activity was evaluated by measuring the activity after 7 and 60 days of storage. This work thus intends to demonstrate the possibility of utilizing the APS approach for preparing biocatalytic PEI–PSS membranes under a mild pH condition, while revealing the potential application of the biocatalytic membranes as antibacterial materials with temperature controlled enzymatic activity.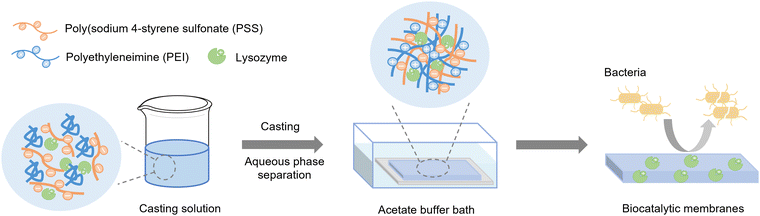 | ||
| Fig. 1 Schematic illustration of the preparation process of biocatalytic PEI–PSS membranes through the APS method. | ||
Structure and permeability of the biocatalytic membranes
To obtain the biocatalytic PEI–PSS membranes via the APS approach, homogenous casting solutions need to be prepared first. Previous results showed that homogenous solutions could be obtained with monomer mixing ratios of PEI to PSS from 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.28,36 In this work, homogenous casting solutions were prepared using a monomer ratio of 1.7
1.28,36 In this work, homogenous casting solutions were prepared using a monomer ratio of 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The aim of this work is to prepare membranes with biocatalytic abilities. Therefore, we need to create a more mild environment for the lysozyme incorporation, for example, suitable pH and temperature.37
1. The aim of this work is to prepare membranes with biocatalytic abilities. Therefore, we need to create a more mild environment for the lysozyme incorporation, for example, suitable pH and temperature.37
Different amounts of HCl were added to adjust the pH of casting solutions (see Table 1). The pH of the casting solution without added lysozyme and HCl was 11.40 ± 0.05, which decreased to 10.85 ± 0.11 and 10.47 ± 0.10 upon addition of HCl. For comparison, the pH of the casting solution with lysozyme demonstrated the same pH decrease with the addition of HCl. Lysozyme was thus not found to have a significant effect on the casting solution pH. Similarly, the addition of lysozyme did not significantly influence the viscosity of the casting solutions. On the other hand, the relatively small pH changes demonstrated a significant influence on the viscosity of casting solutions.
As shown in Fig. 2, the original casting solutions with and without lysozyme (pH ∼11.4) showed almost similar dynamic viscosities of 1533 ± 21 and 1527 ± 85 mPa s. However, the addition of small amounts of HCl (weight ratio of HCl/PEI ∼0.05) increased the viscosity of the casting solution by more than twice to 3783 ± 32 and 3793 ± 85 mPa s. Further increasing the HCl ratio to 0.1 resulted in casting solutions with viscosities of 8597 ± 273 and 9017 ± 119 mPa s. This significant influence of pH on the casting solution viscosity likely stems from its effects on PEI. PEI consists of three kinds of amines, i.e. primary, secondary, and tertiary, which have different pKa values of 4.5, 6.7, and 11.6 respectively.38,39 In the original casting solution (without HCl) with a pH of 11.4, the pH was close to the pKa of tertiary amines, which meant the tertiary amines in the PEI were only partly charged. When more HCl was added to the casting solution, the pH decreased to values that were lower than the pKa of tertiary amines, leading to charging up of PEI. Increased charges on PEI can result in some ionic interactions between PEI and PSS, and thus casting solution with higher dynamic viscosity. We stress that no real polyelectrolyte complexes are formed as PEI is far from fully charged, the secondary and primary amines have much lower pKa values and will thus be uncharged at these pH values. As such, the obtained casting solutions remained transparent and homogeneous, and could be used for membrane preparation.
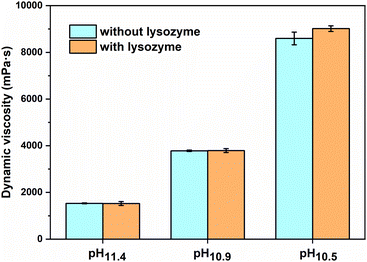 | ||
| Fig. 2 Effect of casting solution pH and lysozyme on the dynamic viscosity of the polyelectrolyte casting solutions. | ||
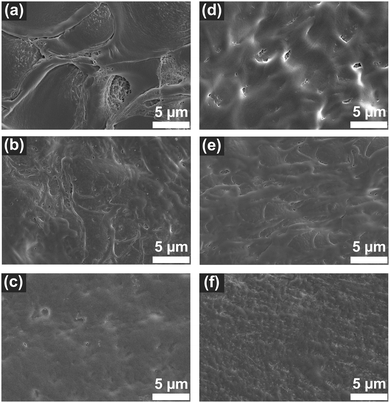
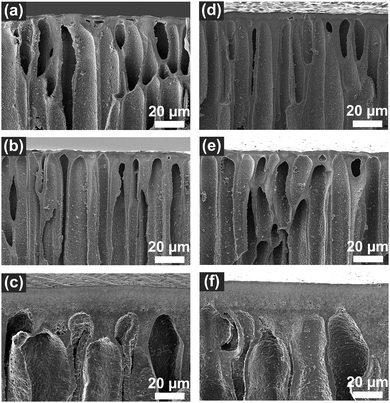
The PEI–PSS membranes were prepared in acetate buffer coagulation bath (see Fig. S1, ESI†). When the casting film (pH ∼11.4 to 10.5) was immersed in the acetate buffer bath (pH ∼4), the low pH buffer bath acted as non-solvent. Due to the change of pH, the uncharged PEI immediately became positively charged, therefore the phase separation/polyelectrolyte complexation started at the surface of the film and moved towards the bottom. The SEM images presented in Fig. 3 and Fig. 4 show asymmetric membranes with relatively dense top surfaces and porous cross-sections with finger-like macrovoids. Similar structures were observed in the previous APS-produced PEI–PSS membranes.28,36
Fig. 3a–c are the top-surfaces of membranes prepared with different casting solution pHs without lysozyme. M-pH11.4 showed a rather inhomogeneous top-surface with some patterns. When the casting solution pH decreased, the top-surfaces of M-pH10.9 and M-pH10.5 became smoother. This was most likely due to the faster precipitation of M-pH11.4, connected to the lower viscosity of the cast solution, something also observed on PEI–PSS membranes prepared in different pH coagulation baths.28 The large pH difference between the casting solution and coagulation bath causes fast complexation, but too fast complexation could lead to inadequate time for polyelectrolyte chains to rearrange, leading to the inhomogeneity in M-pH11.4. Decreasing the casting solution pH slowed down the complexation rate, due to the higher viscosity, reducing the inhomogeneity. Moreover, decreasing the pH would also increase the onset of phase separation and in that way allow for more porous and smooth surfaces of M-pH10.9 and M-pH10.5.40Fig. 3d–f shows the surface SEM images of the membranes prepared with incorporated lysozyme. Although there was slight difference between M-pH11.4 and M-L-pH11.4, the addition of lysozyme did not have a significant influence on the surface morphology. This is expected because the casting solution pH and viscosity, which determine the rate of phase separation, were not influenced by lysozyme (see Table 1 and Fig. 1), thus the membranes with and without lysozyme showed similar structure.8,23 We do expect that at higher lysozyme concentrations this could change. But in this work, enzymes can be added to the PEI/PSS system without influencing the membrane surface structures.
The influence of casting solution pH and lysozyme addition on the cross-section structures are shown in Fig. 4 and Fig. S2 (ESI†). Asymmetric structures were observed in all the membranes with dense top-layers and porous cross-sections with finger-like structures. The finger-like structure indicated the phase separation of all the membranes was instantaneous demixing.40,41 The membranes prepared with pH11.4 and pH10.9 casting solutions without and with lysozyme showed similar size macrovoids. However, when the solution pH was decreased to 10.5, less finger-like macrovoids and thicker top-layers formed, as observed in Fig. 4c and f. We also observed M-pH10.5 and M-L-pH10.5 exhibited lower porosity (67.1% and 66.0%) while membranes prepared with higher casting solution pH showed a porosity over 80% (Table S1, ESI†). We expect that this is the result of the decrease of precipitation rate and increase of viscosity of casting solution, leading to less effect of macrovoids formation in M-pH10.5 and M-L-pH10.5.40,42,43
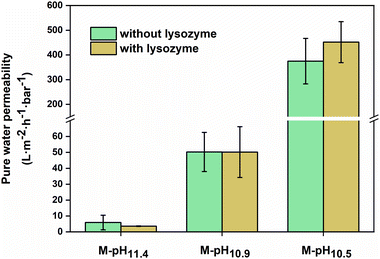
The pure water permeability of the prepared membrane was measured using a dead-end filtration set-up and the results are shown in Fig. 5. All the measurements were performed under 1 bar of applied pressure. The membranes prepared using pH ∼11.4 casting solutions without and with lysozyme had a low pure water permeability around 5.9 ± 4.6 and 3.6 ± 0.2 L m−2 h−1 bar−1. When the casting solution pHs decreased to 10.9, M-pH10.9 and M-L-pH10.9 showed increased pure water permeability of 50 ± 12 and 50 ± 16 L m−2 h−1 bar−1. Here the very low permeabilities at higher pH values indicate dense membranes, in line with expected values for nanofiltration. However, membranes prepared with pH ∼10.5 casting solutions without and with lysozyme showed significantly increased water permeability to 375 ± 92 and 452 ± 83 L m−2 h−1 bar−1 respectively, indicating a membrane in the ultrafiltration range. For membranes with asymmetric structures consisting of dense top layer (skin layer) and porous substructure (support layer), the skin layer would determine the permeability. Although M-pH10.5 and M-L-pH10.5 showed lower porosity and less finger-like structures, the more porous and smooth surfaces led to the higher permeability.40,44
Clearly, the incorporation of lysozyme did not significantly influence the membrane structures and permeability. This was also shown in the biocatalytic PAH–PSS membranes incorporated with lysozyme prepared via APS.19 Membranes can be prepared through APS of PEI and PSS using only a very mild pH shift, and lysozyme could be added without affecting the membrane structure, showing that the phase inversion of PEI/PSS remains dominant. But for biocatalytic membranes, it then becomes important to study the resulting enzymatic activity.
Enzymatic activity of the biocatalytic membranes
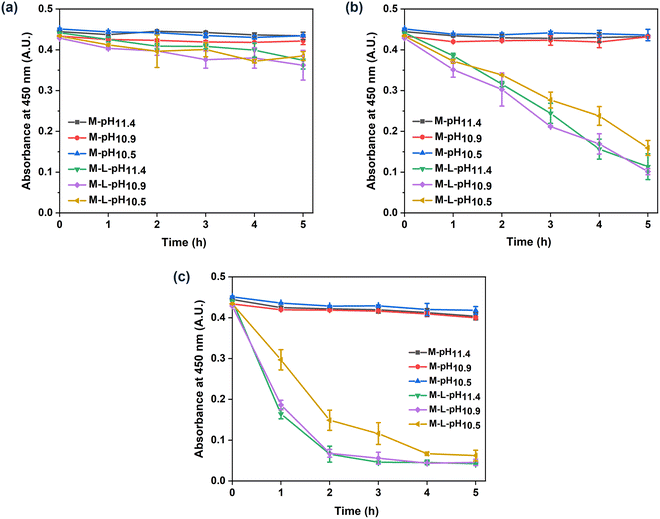
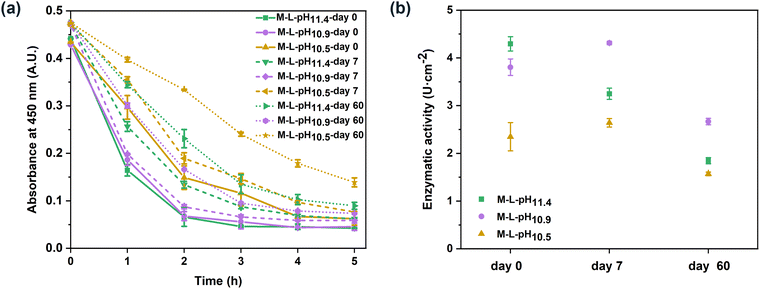
The biocatalytic PEI–PSS membranes were prepared via the one-step incorporation of lysozyme. According to the lysozyme concentration in the casting solutions and the mass of the dried membranes, the maximal lysozyme loading of the prepared PEI–PSS biocatalytic membranes was 7.5 μg cm−2. Lysozyme is an antibacterial enzyme that can kill bacteria via the hydrolysis of the β-1,4-glycosidic bonds in the polysaccharide backbone of their cell walls.45 In this work, to evaluate the enzymatic activity of the biocatalytic PEI–PSS membranes, we used lyophilized micrococcus lysodeikticus, a typical substrate for the assay of lysozyme.46,47 Lysozyme can hydrolyse the cell walls of micrococcus lysodeikticus, which causes a decrease in the turbidity of the substrate suspension. The prepared membranes with and without lysozyme were cut into 1 cm2 squares and put in the substrate suspension to incubation, the absorbance at 450 nm was measured to evaluate the enzymatic activity.Fig. 6 shows the enzymatic activity of all the prepared membranes at different temperatures. Membranes without lysozyme did not demonstrate any catalytic activity, while the membranes with lysozyme showed increased enzymatic activity with the increase of temperature. In Fig. 6a, the turbidity of the substrate containing membranes with lysozyme showed a slight decrease after 5 hours, indicating a low enzymatic activity of the obtained biocatalytic membranes at room temperature. When the temperature increased to 35 °C, the biocatalytic membranes showed much increased enzymatic activity. The absorbance at 450 nm exhibited a nearly linear decrease during the studied 5 hours, resulting in enzymatic activities of 1.50 ± 0.18, 1.53 ± 0.78 and 1.25 ± 0.22 U cm−2 respectively towards the micrococcus lysodeikticus suspension. In addition, when the temperature increased to 45 °C, the enzymatic activity of all the biocatalytic membranes further improved, as shown in Fig. 6c. In the first 2 hours, the enzymatic process was almost completed. The calculated highest activities of M-L-pH11.4, M-L-pH10.9, and M-L-pH10.5 were 4.29 ± 0.15, 3.80 ± 0.17, and 2.35 ± 0.30 U cm−2 respectively. The enzymatic activity of the free lysozyme at different temperatures is shown in Fig. S4 (ESI†). Lysozyme showed a bit higher activity at 35 and 45 °C, but this could not explain the increased activity of the biocatalytic membranes. The increase of activity might come from a structural change of the membranes. It has been shown that polymer membranes can exhibit a thermal expansion and improved water flux at higher temperatures.48,49 More specifically for polyelectrolyte complexes, a higher temperature has been associated with a larger degree of swelling.50,51 That could make the lysozyme more accessible to the substrate, leading to a more increased activity. Alternatively, some lysozyme trapped in the membranes could become free lysozyme and diffuse into the substrate suspension. Here, we also observed that M-L-pH10.5 showed a slightly lower enzymatic activity compared to M-L-pH11.4 and M-L-pH10.9. In this work, HCl was added to the casting solutions to decrease the solution pH, as a milder pH would be more suitable for lysozyme. However, membranes prepared from the casting solutions with decreased pH showed a relatively low enzymatic activity. The slightly decreased enzymatic activity of M-L-pH10.5, might due to the fewer macrovoids in the cross-section structure, which could lead to less contact between the lysozyme and the rather large substrate. As the decrease of pH from 11.4 to 10.5 did not improve the activity of lysozyme, it demonstrates that lysozyme could well maintain its activity at pH ∼11.4 casting solution.19 While the lower pH was thus needed for a stable enzyme like lysozyme, we are convinced that the ability to form membranes with a mild pH shift, as shown here, will be essential for the incorporation of less stable enzymes.
To investigate the reason for the increased enzymatic activity at higher temperatures, we performed a lysozyme release measurement at different temperatures. Membranes with lysozyme were cut into 1 cm2 squares and put in the micrococcus lysodeikticus suspension. The membrane samples were incubated at 25, 35, and 45 °C respectively. After 1 hour, the samples were taken out, and the change of absorbance at 450 nm of the remaining substrate suspension was detected at room temperature. As shown in Fig. S5 (ESI†), the absorbance of the remaining suspension from the membranes incubated at 25 and 35 °C did not show obvious decrease, while the remaining suspension from 45 °C treated membranes exhibited a decreased absorbance at 450 nm. This indicated that there was more lysozyme released from the membranes at 45 °C, but certainly not enough to explain the observed increase in enzymatic activity. To evaluate if the incubated membranes still retained their enzymatic activity, the samples taken out from the substrate suspension after 1-hour treatment at different temperatures were placed into new micrococcus lysodeikticus substrate suspension, and the absorbance at 450 nm was measured. The results in Fig. S6 (ESI†) showed that the treated biocatalytic membranes still maintained their enzymatic activities. This also indicated limited release of lysozyme at high temperatures. These interesting effects of temperature could be beneficial for certain applications. Indeed, the biocatalytic membranes prepared in this work could be potentially used as antibacterial materials for wound dressing. When there is inflammation, the increased body temperature could stimulate an increased lysozyme activity, or even some release at even higher temperatures to kill any adhered bacteria. Also, for separation membranes in complex waste waters, where adhered bacteria could be killed by a small increase in temperature could be very relevant.
To study the stability of the lysozyme inside the biocatalytic membranes, the prepared PEI–PSS membranes were cut into 1 cm2 squares, and each piece of membrane was stored in 2 mL water at 4 °C for 1 week. Then the membranes were taken out from the water, and the absorbance of the supernatant water at 281.5 nm was measured to detect the release of lysozyme. However, both the membranes without and with lysozyme demonstrated absorbance at 281.5 nm (Fig. S7, ESI†). It has been shown that soluble polyelectrolyte complexes showed absorbance at 281.5 nm.19 Besides, the immobilized amount of lysozyme inside the membranes was very low (maximal 7.5 ug cm−2), therefore we could not detect any released lysozyme in the supernatant. Then, the enzymatic activity at 45 °C of the biocatalytic membranes were measured after 7 days and 60 days storage.
As shown in Fig. 7, M-L-pH10.9 and M-L-pH10.5 demonstrated activities of 4.31 ± 0.03 and 2.64 ± 0.09 U cm−2 respectively on day 7. We believe that over time two kinetic processes take place, possibly also leading to the larger error bars on day 0, rearrangement of the polyelectrolyte complexes and denaturing of the lysozyme. The slight increase of activity on day 7 compared to the freshly prepared biocatalytic membranes may relate to chain rearrangement of the polyelectrolyte complexes, which could allow some lysozyme to be better accessible to the substrate during activity measurement.52 After 60 days, the enzymatic process slowed down. The enzymatic activities o of M-L-pH11.4, M-L-pH10.9, and M-L-pH10.5 were 1.85 ± 0.07, 2.67 ± 0.07, and 1.57 ± 0.04 U cm−2 respectively. This meant that M-L-pH10.9 and M-L-pH10.5 could maintain 62% and 59% of the enzymatic activity, which were higher than M-L-pH11.4 (43%). Therefore, the decrease of casting solution pH increased the enzymatic stability of the biocatalytic membranes. The stable enzymatic activity again makes the biocatalytic PEI–PSS membranes interesting candidates for antibacterial porous materials.
Compared with the APS-produced biocatalytic PAH–PSS membranes incorporated with lysozyme,19 our PEI–PSS membranes were prepared via a less extreme pH change process, and demonstrated a temperature-dependent activity, with a highest activity of 4.29 ± 0.15 U cm−2. The membranes also showed a higher stability of activity with the same level of incorporated lysozyme. The detailed comparison was shown in Table S2 (ESI†). This work confirmed the versatility of the APS method on the functional membrane preparation. In the future, we can further study the influence other parameters, for example, lysozyme concentration, to improve the enzymatic activity of the PEI–PSS membranes.
Conclusions
In this work, biocatalytic PEI–PSS polyelectrolyte membranes were successfully prepared through a one-step APS approach. The membranes were obtained under mild pH change and completely aqueous conditions, which allowed easy incorporation of lysozyme. We first investigated the influences of lysozyme addition and casting solution pH on the membrane structure and water permeability. The results showed that with a decrease of casting solution pH, the solution viscosity increased significantly, stemming from a small amount of charge interactions between PSS and PEI. The SEM images demonstrated all the membranes had asymmetric structures with relatively dense top surfaces and porous cross-sections with finger-like structures. However, with the decrease of casting solution pH, the membranes with and without lysozyme showed more homogenous and smooth surfaces and less finger-like structures. Besides, the pure water permeability increased from 3.6 ± 0.2 L m−2 h−1 bar−1 (M-L-pH11.4) to 452 ± 83 L m−2 h−1 bar−1 (M-L-pH10.5), also in line with an expected more porous top layer at lower pH. Meanwhile, the incorporation of lysozyme did not show distinct effects on the final membrane structure and permeability. The membrane structure is thus still dominated by the phase inversion of PEI–PSS, and lysozyme can be added without altering the structure.The enzymatic activities of the prepared biocatalytic membranes exhibited responsiveness to the operational temperature. The membranes showed low enzymatic activity at room temperature, and much improved activity when the temperature increased. The activity of M-L-pH10.9 increased from 1.53 ± 0.78 to 3.80 ± 0.17 U cm−2 when the operational temperature increased from 35 °C to 45 °C, with a lysozyme loading of 7.5 μg cm−2. Besides, the biocatalytic membranes demonstrated stable enzymatic activities. M-L-pH10.9 showed comparable activity at day 0 and day 7, and could maintain 62% of its activity after 60 days. The response of enzymatic activity towards operational temperature and the stability of the PEI–PSS biocatalytic membranes makes them capable of being used as porous antibacterial materials. What's more, the here developed water-based mild pH shift APS process is expected to work for a large range of enzymes and could allow the preparation of many functional biocatalytic membranes.
Author contributions
Lijie Li: conceptualization, data curation, investigation, methodology, writing – original draft. Muhammad Irshad Baig: methodology, writing – review & editing. Wiebe M. de Vos: conceptualization, project administration, supervision, writing – review & editing. Saskia Lindhoud: conceptualization, project administration, supervision, writing – review & editing.Data availability
The data of this article are available in 4TU. Research Data at https://doi.org/10.4121/4458ff76-1691-4f14-81ca-292fc0307b92.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Lijie Li appreciates the China Scholarship Council (CSC) for providing a scholarship. The authors acknowledge Mia Jurković, Jéré J. van Lente and Jiaying Li for the support in this work.References
- O. Kirk, T. V. Borchert and C. C. Fuglsang, Curr. Opin. Biotechnol, 2002, 13, 345–351 CrossRef CAS PubMed
.
- J. B. van Beilen and Z. Li, Curr. Opin. Biotechnol, 2002, 13, 338–344 CrossRef CAS PubMed
.
- H. S. Olsen and P. Falholt, J. Surfactants Deterg., 1998, 1, 555–567 CrossRef CAS
.
- N. A. Turner and E. N. Vulfson, Enzyme Microb. Technol., 2000, 27, 108–113 CrossRef CAS PubMed
.
- P. V. Iyer and L. Ananthanarayan, Process Biochem., 2008, 43, 1019–1032 CrossRef CAS
.
- C. Garcia-Galan, Á. Berenguer-Murcia, R. Fernandez-Lafuente and R. C. Rodrigues, Adv. Synth. Catal., 2011, 353, 2885–2904 CrossRef CAS
.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS
.
-
R. W. Baker, Membrane Technology and Applications, John Wiley & Sons, 2023 Search PubMed
.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS
.
- S. Madaeni, Water Res., 1999, 33, 301–308 CrossRef CAS
.
- P. Jochems, Y. Satyawali, L. Diels and W. Dejonghe, Green Chem., 2011, 13, 1609–1623 RSC
.
- J. Luo, S. Song, H. Zhang, H. Zhang, J. Zhang and Y. Wan, Eng. Life Sci., 2020, 20, 441–450 CrossRef CAS PubMed
.
- S. S. Ozdemir, M. G. Buonomenna and E. Drioli, Appl. Catal., A, 2006, 307, 167–183 CrossRef CAS
.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Biotechnol. Adv., 2012, 30, 489–511 CrossRef CAS PubMed
.
- P. T. Anastas, A. Rodriguez, T. M. de Winter, P. Coish and J. B. Zimmerman, Green Chem. Lett. Rev., 2021, 14, 302–338 CrossRef CAS
.
- E. N. Durmaz, S. Sahin, E. Virga, S. De Beer, L. C. De Smet and W. M. De Vos, ACS Appl. Polym. Mater., 2021, 3, 4347–4374 CrossRef CAS PubMed
.
- O. S. Sakr and G. Borchard, Biomacromolecules, 2013, 14, 2117–2135 CrossRef CAS PubMed
.
- A. Popkov, Z. Su, S. B. Sigurdardóttir, J. Luo, M. Malankowska and M. Pinelo, Biochem. Eng. J., 2023, 193, 108838 CrossRef CAS
.
- J. J. van Lente, M. I. Baig, W. M. de Vos and S. Lindhoud, J. Colloid Interface Sci., 2022, 616, 903–910 CrossRef CAS PubMed
.
- G. R. Guillen, Y. Pan, M. Li and E. M. Hoek, Ind. Eng. Chem. Res., 2011, 50, 3798–3817 CrossRef CAS
.
- D.-M. Wang and J.-Y. Lai, Curr. Opin. Chem. Eng., 2013, 2, 229–237 CrossRef
.
- J. D. Willott, W. M. Nielen and W. M. de Vos, ACS Appl. Polym. Mater., 2019, 2, 659–667 CrossRef PubMed
.
- M. I. Baig, E. N. Durmaz, J. D. Willott and W. M. de Vos, Adv. Funct. Mater., 2020, 30, 1907344 CrossRef CAS
.
- L. Li, M. I. Baig, W. M. de Vos and S. Lindhoud, ACS Appl. Polym. Mater., 2023, 5, 1810–1818 CrossRef CAS
.
- E. N. Durmaz, M. I. Baig, J. D. Willott and W. M. de Vos, ACS Appl. Polym. Mater., 2020, 2, 2612–2621 CrossRef CAS PubMed
.
- J. Kamp, S. Emonds, J. Borowec, M. A. R. Toro and M. Wessling, J. Membr. Sci., 2021, 618, 118632 CrossRef CAS
.
- M. A. Restrepo, S. Emonds, A. Zhao, F. Karakas, J. Kamp, H. Roth and M. Wessling, J. Membr. Sci., 2024, 689, 122157 CrossRef CAS
.
- M. I. Baig, P. P. I. Sari, J. Li, J. D. Willott and W. M. de Vos, J. Membr. Sci., 2021, 625, 119114 CrossRef CAS
.
- M. I. Baig, J. D. Willott and W. M. de Vos, J. Membr. Sci., 2020, 615, 118502 CrossRef CAS
.
- S. B. Sigurdardóttir, J. Lehmann, S. Ovtar, J. C. Grivel, M. D. Negra, A. Kaiser and M. Pinelo, Adv. Synth. Catal., 2018, 360, 2578–2607 CrossRef
.
- Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, J. Am. Chem. Soc., 1995, 117, 6117–6123 CrossRef CAS
.
- R. Cegielska-Radziejewska, G. Lesnierowski and J. Kijowski, Pol. J. Food Nutr. Sci., 2008, 58 Search PubMed
.
- G. Leśnierowski and R. Cegielska-Radziejewska, Acta Sci. Pol., Technol. Aliment., 2012, 11, 223–230 Search PubMed
.
- E. Drioli, A. Criscuoli and E. Curcio, Chem. Eng. Technol., 2003, 26, 975–981 CrossRef CAS
.
- N. H. Barbhuiya, U. Misra and S. P. Singh, Chemosphere, 2022, 286, 131757 CrossRef CAS PubMed
.
- M. M. H. Mizan, M. Rastgar, S. A. Aktij, A. Asad, P. Karami, A. Rahimpour and M. Sadrzadeh, J. Membr. Sci., 2023, 668, 121197 CrossRef
.
- S. Venkataramani, J. Truntzer and D. R. Coleman, J. Pharm. BioAllied Sci., 2013, 5, 148 CrossRef PubMed
.
- K. D. Demadis, M. Paspalaki and J. Theodorou, Ind. Eng. Chem. Res., 2011, 50, 5873–5876 CrossRef CAS
.
- A. von Harpe, H. Petersen, Y. Li and T. Kissel, J. Controlled Release, 2000, 69, 309–322 CrossRef CAS PubMed
.
- H. Strathmann and K. Kock, Desalination, 1977, 21, 241–255 CrossRef CAS
.
- C. Smolders, A. Reuvers, R. Boom and I. Wienk, J. Membr. Sci., 1992, 73, 259–275 CrossRef CAS
.
- G. Bakeri, A. F. Ismail, M. Shariaty-Niassar and T. Matsuura, J. Membr. Sci., 2010, 363, 103–111 CrossRef CAS
.
- A. K. Hołda, B. Aernouts, W. Saeys and I. F. Vankelecom, J. Membr. Sci., 2013, 442, 196–205 CrossRef
.
- W. Albrecht, T. Weigel, M. Schossig-Tiedemann, K. Kneifel, K.-V. Peinemann and D. Paul, J. Membr. Sci., 2001, 192, 217–230 CrossRef CAS
.
- P. Ferraboschi, S. Ciceri and P. Grisenti, Antibiotics, 2021, 10, 1534 CrossRef CAS PubMed
.
- P. Mörsky, Anal. Biochem., 1983, 128, 77–85 CrossRef PubMed
.
- K. Hanušová, L. Vápenka, J. Dobiáš and L. Mišková, Open Chem., 2013, 11, 1066–1078 CrossRef
.
- J. Wei, X. Jian, C. Wu, S. Zhang and C. Yan, J. Membr. Sci., 2005, 256, 116–121 CAS
.
- G. Zhang, G. Yang, S. Li, Q. Shen, H. Wang, Z. Li, Y. Zhou and W. Ye, Membranes, 2021, 11, 695 CrossRef CAS PubMed
.
- K. Büscher, K. Graf, H. Ahrens and C. A. Helm, Langmuir, 2002, 18, 3585–3591 CrossRef
.
- M. Schönhoff, J. Phys.: Condens. Matter, 2003, 15, R1781 CrossRef
.
- S. Lindhoud, W. Norde and M. A. Cohen Stuart, J. Phys. Chem. B, 2009, 113, 5431–5439 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm00311j |
| This journal is © The Royal Society of Chemistry 2024 |