Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, evaluation and modification of heterofunctional polyester dendrimers with internally queued bromide groups†
Arunika
Singh
 ,
Daniel J.
Hutchinson
,
Daniel J.
Hutchinson
 ,
Maria Isabel
Montañez
,
Natalia
Sanz del Olmo
,
Maria Isabel
Montañez
,
Natalia
Sanz del Olmo
 * and
Michael
Malkoch
* and
Michael
Malkoch
 *
*
Division of Coating Technology, Fiber and Polymer Technology, KTH, Teknikringen 48, Stockholm, SE-10044, Sweden. E-mail: natsdo@kth.se; malkoch@kth.se
First published on 13th September 2024
Abstract
Heterofunctional polyester dendrimers up to the third generation, containing 21 internally queued bromine atoms, have been successfully synthesized for the first time using a divergent growth approach. Direct azidation reactions enabled the conversion of the bromide groups to clickable azide pendant functionalities. Therapeutic and chemical moeities could then be coupled to the internal azide or bromide functionalities and external hydroxyl groups of the heterofunctional dendrimers through CuAAC, thiol-bromo click and esterification reactions, expanding their potential for biomedical applications.
Dendrimers are the only monodisperse polymers that can be synthesized in a controlled manner to render nanoscale sized highly branched macromolecules with unique properties such as a compact globular topology, the presence of internal cavities and multiple functional groups at the periphery.1 These functional groups can then be utilized for covalently linking large numbers of therapeutic entities in order to enhance their solubility, bioavailability and cell selectivity for drug delivery applications. Promising instances of dendritic families reaching the pre-clinical and clinical stage as delivery carriers include poly(amidoamine)2 (PAMAM), polylysine3 (e.g., Vivagel), and 2,2-bis(methylol)propionic acid4 (bis-MPA) polyester scaffolds. Polyester dendrimers have attracted significant attention in the biomedical field due to their biocompatible, non-toxic and biodegradable nature when compared to the more researched PAMAM dendrimers that possess inherent toxicity due to their large concentration of surface amine groups.5
The location and nature of the chemical functionalities present in these dendritic scaffolds also play a crucial role in their use within nanomedicine. Dendrimers are commonly synthesized with an active exterior, containing peripheral groups available for further manipulation, and a dormant interior. The dendritic polymers may be homo- or heterofunctional, with the latter including two or more distinctly different functionalities.4 Most reports on polyester dendrimers depict distinctly different peripheral functionalities arranged in an alternating manner6 or Janus configuration7 at the exterior with instances of modifications with drugs such as doxorubicin and polyethylene oxide.8 However, when placing them in the context of biomedical applications, their cargo carrying capacity and selectivity towards different therapeutic entities could be greatly enhanced by including active interior functionalities throughout the polymer skeleton. There are very few research studies that have successfully shown the introduction of active interior groups in the dendritic framework. A recent report focused on synthesizing multifunctional polyester dendrimers from bis-MPA based AB2C monomers with interior and exterior functionalities for use as versatile multipurpose platforms.9 Another paper highlighted the synthesis of bifunctional polyester dendrimers based on a 2-(bromomethyl)-2-(hydroxymethyl) propane-1,3 diol (BHP-diol) building block with internal azide functionalities and peripheral hydroxyl groups.10 Both the studies capitalized on AB2C monomers displaying different orthogonal functionalities for building their respective dendritic libraries where the A(COOH) and B(OH) functions were used for dendrimer growth while the C (alkyne/alkene/azide) function remained dormant throughout the growth steps.9,10 The highly selective interior C functionality makes the dormant dendritic interior more accessible and can be precisely functionalized with therapeutic entities through click chemistry reactions. The BHP-diol based AB2C monomer presents advantages in terms of its straightforward and facile synthesis with simpler purification protocols when compared to the bis-MPA based AB2C monomer, which involves tedious and time-consuming synthesis with intensive purification steps.
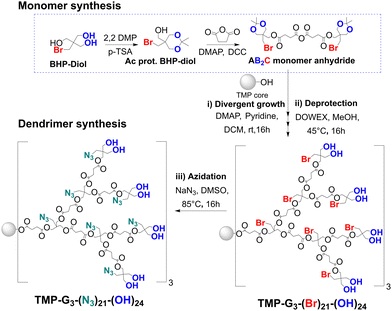
Herein, we demonstrate the synthesis of heterofunctional polyester dendrimers (HFDs) with internal halides for the first time, from BHP-diol based AB2C monomer with bromide as the C functionality, following a divergent growth strategy through anhydride-based esterification reactions (Scheme 1). Bromides are well known as good leaving groups and can be replaced by chemical entities containing stronger nucleophiles such as azides and thiols.10,11 The azide groups are shown to possess high selectivity for alkynes and participate efficiently in copper-catalyzed azide–alkyne cycloaddition (CuAAC) reactions.12,13 These features are of interest for post-functionalizing azide-bearing dendrimers with alkyne modified therapeutic moieties. In this regard, an alternative and a more stable approach has been explored to synthesize azide-based dendrimers via direct azidation of bromide HFDs (Scheme 1) which takes the labile azide groups into consideration as opposed to a previously described divergent growth route using an azide-based AB2C monomer.10 The direct azidation approach for the conversion of bromide HFDs into azide derivatives avoids iterative dendrimer growth sequences and exposure to acidic conditions during esterification and deprotection reactions including various purification techniques such as NaHSO4 washes and silica column chromatography to prevent decomposition of the less stable azide groups.14,15 Furthermore, robust reactions based on CuAAC and esterification have been utilized for the successful post-functionalization of the internal azides and peripheral hydroxyl groups with therapeutic moieties containing alkyne and carboxylic acid groups, respectively. The thiol-bromo click reaction11,16 has also been explored as a means for selectively post-functionalizing the internal bromide groups, resulting in thiol functionalities linked to the dendrimer interior via stable thioether bonds, thus expanding the scope of the bromide HFDs. The synthesized constructs have been evaluated for biostability and in vitro cytotoxicity, to assess their role in the context of nanomedicine.
 | ||
| Scheme 1 Synthetic route towards bromide and azide based heterofunctional polyester dendrimers using a BHP-diol based AB2C monomer containing bromine functionality. | ||
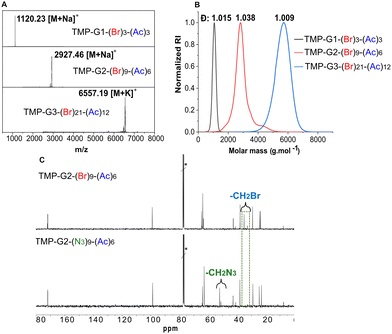
The synthetic route towards bromide HFDs began with preparation of a bromide based AB2C monomer constituting a BHP-diol unit following acetonide protection and esterification steps (Scheme 1). After the bromide-functional AB2C monomer was synthesized, its anhydride was formed with N,N′-dicyclohexylcarbodiimide (DCC) as the dehydration agent. The AB2C monomer anhydride was reacted in situ with trimethylolpropane (TMP), in the presence of pyridine as a base and DMAP as a catalyst. After an overnight reaction, and consequent purification methods consisting of washes and column chromatography, the first-generation dendrimer TMP-G1-(Br)3-(Ac)3 was isolated in near quantitative yields. Subsequent deprotection using DOWEX in MeOH for 16 h at 45 °C yielded TMP-G1-(Br)3-(OH)3. Based on the same repeated reaction sequences, a library of HFDs with internal bromides were synthesized until generation 3 within 9 reaction steps, including monomer synthesis. The molecular weights of the dendrimers ranged from 1120.23 to 6548.74 Da (Fig. 1A) and all had low polydispersity values (∼1.0) (Fig. 1B) as indicated by both MALDI-TOF and SEC, respectively. Due to the additional C functionality, these bromide HFDs (G1–G3) showed almost twice the number of functionalities when compared with the equivalent traditional G1–G3 homofunctional AB2 bis-MPA polyester dendrimers,5 despite containing the same TMP core and peripheral hydroxyl groups (TMP-G3-(Br)21-(OH)24: 21Br + 24OH groups > TMP-G3-(OH)24: 24OH groups).
As a next step, the feasibility of replacing the internal bromides in the dendrimers with azide functionalities via direct azidation reaction was explored. In general, acetonide protected bromide dendrimers (G1–G3 Br) were converted to their azide derivatives (G1–G3 N3) overnight at 85 °C by performing the azidation reaction in DMSO with NaN3. The crude derivatives were extracted in ether (G1–G2 N3) or ethyl acetate (G3 N3) and washed with water for purification. The completion of the azidation reaction was corroborated by 13C-NMR spectroscopy due to the shift in the carbon of the methylene moiety next to the bromine functionality from 32.5–34.8 ppm to 51.2–52.0 ppm (Fig. 1C). The acetonide protected azide based dendrimers were deprotected in the same manner as the bromide intermediates.
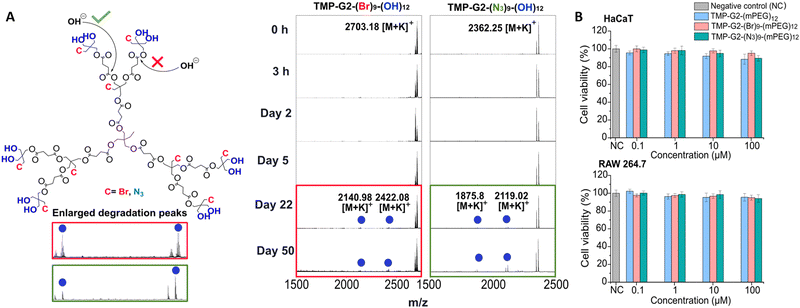
The stability and in vitro cytotoxicity of the HFDs were evaluated to place them in the context of biomedical applications. It is essential to determine the stability of dendritic nanocarriers as their degradation profile can greatly impact the pharmacokinetics of the therapeutic cargo as well as its bioavailability.17 Moreover, the influence of varying pH in different parts of the body is important to consider when assessing their hydrolytic degradation. Generation 2 bromide and azide-based HFDs along with the reference TMP-G2-(OH)1218 dendrimer, were dissolved in 10% DMSO before the addition of different pH buffers (4.4–7.4) to obtain a homogeneous system for the synthesized hydrophobic dendritic constructs. In these conditions, both TMP-G2-(Br)9-(OH)12 and TMP-G2-(N3)9-(OH)12 exhibited high hydrolytic stability up to 50 days when compared with the reference TMP-G2-(OH)12 dendrimer as demonstrated by MALDI-TOF (Fig. S19–S21, ESI†). Only after 22 days did the G2 bromide and azide HFDs start to exhibit slight degradation peaks with very low intensity at physiological pH (pH 7.4) (Fig. 2A). The presence of signals corresponding to a loss of two monomeric repetitive units suggested that the hydrolytic attack begun from the more hindered internal ester bonds present in the second layer of the dendrimer despite the better availability of the external ester linkages (Fig. 2A). The hydrolytic stability of the bromide and azide dendrimers was found to be far higher than the previously reported bis-MPA dendrimers,5 which showed significant hydrolytic degradation after 15 days at physiological pH (Fig. S19, ESI†). This durability is desirable to prevent premature release of the linked therapeutic entity until its targeted delivery. The enhanced hydrolytic stability could be attributed to the hydrophobic nature of the synthesized HFDs composed of a BHP-diol based AB2C monomer containing a longer succinic anhydride chain with greater number of hydrophobic carbon atoms as opposed to the small AB2 type bis-MPA monomer in TMP-G2-(OH)12. Furthermore, the presence of internal bromide and azide groups in HFDs could minimize the accessibility of OH-ions to the ester linkages when compared with the more accessible bis-MPA dendrimer lacking internal functionalities. For the purpose of cytotoxicity evaluation, both the second-generation bromide and azide derivatives were PEGylated with methoxy polyethylene glycol-propionic acid (mPEG) to yield TMP-G2-(Br)9-(mPEG)12 and TMP-G2-(N3)9-(mPEG)12, in order to enhance their aqueous solubility for dissolution in cell culture medium. A PEGylated second generation bis-MPA dendrimer (TMP-G2-(mPEG)12)19 was chosen as the reference compound since traditional bis-MPA dendrimers are known to be highly biocompatible. The results from in vitro cytotoxicity studies (Fig. 2B) showed that both TMP-G2-(Br)9-(mPEG)12 and TMP-G2-(N3)9-(mPEG)12 were non-toxic towards human keratinocytes (HaCaT) and mouse monocyte (RAW 264.7) cell lines up to high concentrations of 100 μM after 24 h. These findings were in line with the cytotoxicity results exhibited by the homofunctionalized TMP-G2-(mPEG)12.
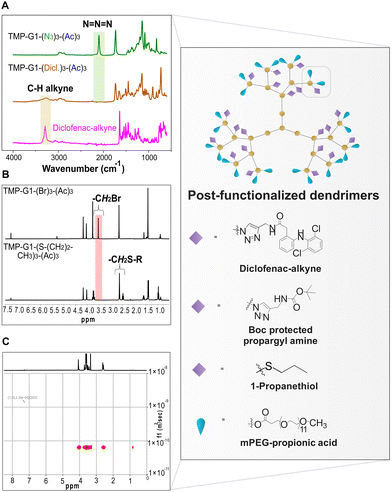
Lastly, the versatility of the newly synthesized HFDs displaying chemoselective groups was showcased through post-functionalization based on CuAAC, thiol-bromo click and esterification reactions. Diclofenac-alkyne, di-tert-butyl dicarbonate protected propargyl amine (Boc-PA) and mPEG were selected as therapeutically relevant substrates to modify the selected azide-based dendrimers and in turn represent their utility as multipurpose scaffolds for biomedical applications. Diclofenac-alkyne was chosen as an anti-inflammatory reagent for treatment of diseases such as rheumatoid arthritis.20 Boc-PA was selected to introduce cationic charges for application towards antibacterial activity after its deprotection.21 The addition of mPEG allows control over size of the carrier as well as blood circulation half-time.22 Additionally, 1-propanethiol was selected as the chemical moiety to modify the internal bromide groups of a bromide-based HFD and showcase as proof of concept the possibility of post-functionalization with biologically interesting thiol-based molecules in the future. The structural integrity and purity of all the post-functionalized dendrimers was verified with a set of complimentary techniques including MALDI, FTIR, SEC and NMR spectroscopy. Fig. 3 illustrates a few examples of the above-mentioned characterization techniques indicating successful post-functionalization of the HFDs. Generation 1 and 2 azide HFDs were selected and post-functionalized with diclofenac-alkyne and Boc-PA, respectively in a THF/H2O mixture with sodium ascorbate as a reducing agent and CuSO4 salt to demonstrate the modular approach of CuAAC. Functionalization with diclofenac-alkyne was evidenced by FTIR, with the disappearance of the azide stretch from the dendritic precursor at 2103 cm−1 and the C–H stretch of the terminal alkyne from the free drug at 3291 cm−1 (Fig. 3A). The addition of 9 Boc-PA units in the generation 2 azide dendrimer was confirmed by MALDI-TOF with a shift in mass of the dendritic precursor from 2589.26 to 4007.93 Da after complete functionalization (Fig. S31, ESI†). A generation 1 bromide HFD was modified with 1-propanethiol in acetonitrile with cesium fluoride (CsF) as a catalyst. Post-functionalization with 1-propanethiol to render sulphide functionalities was indicated with a significant shift in the protons of the methylene moiety next to the bromide functionality from 3.53 ppm to 2.62 ppm (Fig. 3B). Next, anhydride-based esterification was employed to post-functionalize peripheral hydroxyl groups of a generation 2 azide HFD with mPEG. The DOSY NMR indicated the diffusion coefficients of one specie with signals from mPEG in alignment with the HFD, representing its complete functionalization (Fig. 3C). The post-functionalized dendritic constructs obtained from CuAAC and esterification reactions were purified by bio-beads S-X8 and Sephadex LH-20 columns, respectively.
In conclusion, a library of HFDs with interior bromine atoms was successfully synthesized via anhydride chemistry. The direct azidation of these bromide HFDs to form azide analogues was demonstrated as a more stable route to forming dendrimers with high concentrations of nitrogen. The multifunctional HFDs showed enhanced hydrolytic stability and comparable non-toxicity when compared to bis-MPA dendrimers. The internal azide and bromide groups along with peripheral hydroxyl functionalities of the HFDs were successfully post-functionalized through CuAAC, thiol-bromo click and anhydride chemistry with relevant therapeutic and chemical probes showcasing their chemoselectivity and potential for future biomedical applications.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge support from the Swedish Research Council (VR) grant (2020-04339), the European Union’s Horizon 2020 research and innovation programme under grant agreement No 952150, Knut and Alice Wallenberg Foundation, Grant KAW (2017.0300), and the European Union’s Horizon Europe research and innovation programme under grant agreement No 101064084.Notes and references
- C. C. Lee, J. A. MacKay, J. M. J. Fréchet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517–1526 CrossRef CAS PubMed.
- D. A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J., 1985, 17, 117–132 CrossRef CAS.
- R. J. Mumper, M. A. Bell, D. R. Worthen, R. A. Cone, G. R. Lewis, J. R. A. Paull and T. R. Moench, Drug Dev. Ind. Pharm., 2009, 35, 515–524 CrossRef CAS PubMed.
- A. Carlmark, E. Malmström and M. Malkoch, Chem. Soc. Rev., 2013, 42, 5858 RSC.
- N. Feliu, M. V. Walter, M. I. Montañez, A. Kunzmann, A. Hult, A. Nyström, M. Malkoch and B. Fadeel, Biomaterials, 2012, 33, 1970–1981 CrossRef CAS PubMed.
- A. P. Goodwin, S. S. Lam and J. M. J. Fréchet, J. Am. Chem. Soc., 2007, 129, 6994–6995 CrossRef CAS PubMed.
- E. R. Gillies and J. M. J. Fréchet, J. Am. Chem. Soc., 2002, 124, 14137–14146 CrossRef CAS PubMed.
- C. C. Lee, E. R. Gillies, M. E. Fox, S. J. Guillaudeu, J. M. J. Fréchet, E. E. Dy and F. C. Szoka, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 16649–16654 CrossRef CAS PubMed.
- S. García-Gallego, P. Stenström, P. Mesa-Antunez, Y. Zhang and M. Malkoch, Biomacromolecules, 2020, 21, 4273–4279 CrossRef PubMed.
- P. Antoni, Y. Hed, A. Nordberg, D. Nyström, H. Von Holst, A. Hult and M. Malkoch, Angew. Chem., Int. Ed., 2009, 48, 2126–2130 CrossRef CAS PubMed.
- B. M. Rosen, G. Lligadas, C. Hahn and V. Percec, J. Polym. Sci., 2009, 47, 3931–3939 CrossRef CAS.
- L. Liang and D. Astruc, Coord. Chem. Rev., 2011, 255, 2933–2945 CrossRef CAS.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed.
- S. Bräse, C. Gil, K. Knepper and V. Zimmermann, Angew. Chem., Int. Ed., 2005, 44, 5188–5240 CrossRef PubMed.
- A. Carlmark, C. Hawker, A. Hult and M. Malkoch, Chem. Soc. Rev., 2009, 38, 352–362 RSC.
- S. T. A. Shah, K. M. Khan, H. Hussain, S. Hayat and W. Voelter, Chem. Mon., 2005, 136, 1583–1589 CrossRef CAS.
- P. M. Perrigue, R. A. Murray, A. Mielcarek, A. Henschke and S. E. Moya, Pharmaceutics, 2021, 13, 770 CrossRef CAS.
- S. García-Gallego, D. Hult, J. V. Olsson and M. Malkoch, Angew. Chem., Int. Ed., 2015, 54, 2416–2419 CrossRef PubMed.
- Y. Zhang, P. Mesa-Antunez, L. Fortuin, O. C. J. Andrén and M. Malkoch, Biomacromolecules, 2020, 21, 4294–4301 CrossRef CAS.
- A. Amanullah, A. Upadhyay, R. Dhiman, S. Singh, A. Kumar, D. K. Ahirwar, R. K. Gutti and A. Mishra, Cancers, 2022, 14, 4385 CrossRef CAS PubMed.
- F. Namata, N. Sanz Del Olmo, N. Molina and M. Malkoch, Biomacromolecules, 2023, 24, 858–867 CrossRef CAS PubMed.
- P. L. Turecek, M. J. Bossard, F. Schoetens and I. A. Ivens, J. Pharm. Sci., 2016, 105, 460–475 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization. See DOI: https://doi.org/10.1039/d4sm00849a |
| This journal is © The Royal Society of Chemistry 2024 |