Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cellulose modification for sustainable polymers: overcoming problems of solubility and processing†
Peter
McNeice
 a,
Gert H.
ten Brink
a,
Gert H.
ten Brink
 b,
Ulrik
Gran
c,
Leif
Karlson
c,
Rolf
Edvinsson
c and
Ben L.
Feringa
b,
Ulrik
Gran
c,
Leif
Karlson
c,
Rolf
Edvinsson
c and
Ben L.
Feringa
 *a
*a
aAdvanced Research Centre CBBC, Stratingh Institute for Chemistry, Faculty of Science and Engineering, University of Groningen, Nijenborgh 4, Groningen, 9747AG, The Netherlands. E-mail: b.l.feringa@rug.nl
bZernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, Groningen 9747AG, The Netherlands
cPerformance Formulations, Nouryon, SE-402 58 Göteborg, Sweden
First published on 4th January 2024
Abstract
Two new water-soluble cellulose derivatives were prepared by a two-step transformation with 1,3-propane sultone, followed by either maleic or succinic anhydride, thereby converting cellulose into a more easily processable form. It was found that the solubility was dependent on both the degree of substitution and the chemical properties of the substituents. The water-soluble cellulose has a molecular weight greater than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and both the morphology and molecular weight can be tuned by varying the reaction conditions. Furthermore, the flexible, two-step nature of the process allows for expansion of this methodology in order to prepare cellulose analogues for different applications.
000 g mol−1 and both the morphology and molecular weight can be tuned by varying the reaction conditions. Furthermore, the flexible, two-step nature of the process allows for expansion of this methodology in order to prepare cellulose analogues for different applications.
Sustainability spotlightAccording to UN Sustainability Development Goal 12, there is an urgent need to reduce our reliance on fossil feedstocks in order to produce materials. The use of natural starting materials would help achieve this goal. Cellulose has excellent potential for this, as it is the most abundant renewable polymer. However, due to the challenge of solubility, converting cellulose into useful materials currently involves the use of toxic solvents and produces harmful waste. We overcome these problems by modifying cellulose to make it water-soluble. This benign method allows cellulose to be used as a polymer feedstock to contribute to the UN Sustainability Development Goal 12, whilst bringing the processing in line with the Principles of Green Chemistry. |
Introduction
Reducing the reliance on fossil feedstocks will help achieve a more sustainable society, as set out in the UN's Sustainable Development Goal 12.1 Currently, 90% of the feedstock for the synthesis of polymers, and in particular plastics, is reliant on oil and gas.2 It is estimated that 4–8% of the total oil produced is used in the manufacture of plastics. The production of plastics has increased 20-fold since 1964 and is expected to almost quadruple by 2050. Therefore, to meet the demand for plastics and polymers, whilst reducing the consumption of oil and gas, new methods to synthesise these materials must be developed. One potential solution is to use biomass as a feedstock for polymer production.Cellulose is a clear choice as a future sustainable polymer feedstock. It is the most abundant biorenewable and biodegradable resource on earth, with an annual growth of 1.5 × 1012 tons.3,4 A major benefit of using cellulose as a feedstock rather than other biomass, is that it can be obtained from waste,3–5 and therefore does not compete with food production. Furthermore, highly pure nanocellulose can be produced by bacteria on an industrial scale,6,7 making cellulose an extremely promising green resource.
As shown by several recent reviews,8–15 the number and range of applications for cellulose based materials is expanding. These include food packaging,8 biomedical applications,9–11 water purification,12 photonic materials and pigments,13,16,17 and as an additive to alter the properties of polymer composites.14,15 However, a limiting factor to developing useful materials from cellulose is the difficulty in its processing.
Cellulose is a bio-polymer with up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 anhydroglucose (AGU) monomer units (Fig. 1, left),4 so it could be envisaged that the conversion of cellulose to other polymer materials would be facile. However, cellulose is insoluble in conventional solvents. Several factors account for this. Strong inter- and intra-molecular hydrogen bonding (Fig. 1, right), provides chain stiffness, and allows the linear chains to form sheet-like structures.4 Furthermore, hydrophobic interactions have been shown to have a significant contribution to cellulose–cellulose attraction, and therefore its insolubility.18–20 Dissolution and regeneration of cellulose is often necessary to produce fibres, films, and cellulose derivatives, as well as to assist in degradation for bio-refineries.18 However an alternate process is cellulose swelling. This is where a solvent causes changes in the molecular order of cellulose which can improve access to the fibres interior by increasing the surface area and porosity, and decreasing the crystallinity.3,19,21 One traditional industrial method for processing cellulose, the viscose process, relies on hazardous CS2, and produces waste in the form of H2S and SO2 (Fig. 2, top).4 The alternate, more environmental, Lyocell process involves regenerating cellulose from solutions of N-methylmorpholine-N-oxide by spinning.4 Drawbacks to this system are that the solvent is expensive and has poor thermal stability, with stabilisers required to prevent spontaneous exothermic reactions which would lead to cellulose degradation (Fig. 2, middle). Whilst ionic liquids have emerged as a milder way to dissolve and process cellulose,22–28 these have yet to find commercial use. Therefore, although using cellulose as a polymer feedstock can potentially improve sustainability, the use of toxic solvents, the production of waste, and the need for extra stabilisers means that current cellulose processing does not adhere to the Principles of Green Chemistry.29
000 anhydroglucose (AGU) monomer units (Fig. 1, left),4 so it could be envisaged that the conversion of cellulose to other polymer materials would be facile. However, cellulose is insoluble in conventional solvents. Several factors account for this. Strong inter- and intra-molecular hydrogen bonding (Fig. 1, right), provides chain stiffness, and allows the linear chains to form sheet-like structures.4 Furthermore, hydrophobic interactions have been shown to have a significant contribution to cellulose–cellulose attraction, and therefore its insolubility.18–20 Dissolution and regeneration of cellulose is often necessary to produce fibres, films, and cellulose derivatives, as well as to assist in degradation for bio-refineries.18 However an alternate process is cellulose swelling. This is where a solvent causes changes in the molecular order of cellulose which can improve access to the fibres interior by increasing the surface area and porosity, and decreasing the crystallinity.3,19,21 One traditional industrial method for processing cellulose, the viscose process, relies on hazardous CS2, and produces waste in the form of H2S and SO2 (Fig. 2, top).4 The alternate, more environmental, Lyocell process involves regenerating cellulose from solutions of N-methylmorpholine-N-oxide by spinning.4 Drawbacks to this system are that the solvent is expensive and has poor thermal stability, with stabilisers required to prevent spontaneous exothermic reactions which would lead to cellulose degradation (Fig. 2, middle). Whilst ionic liquids have emerged as a milder way to dissolve and process cellulose,22–28 these have yet to find commercial use. Therefore, although using cellulose as a polymer feedstock can potentially improve sustainability, the use of toxic solvents, the production of waste, and the need for extra stabilisers means that current cellulose processing does not adhere to the Principles of Green Chemistry.29
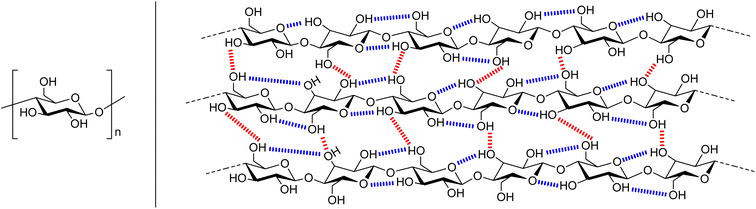 | ||
| Fig. 1 A representation of the structure of cellulose. Left is the anhydroglucose unit (AGU) and right shows the inter- (red) and intramolecular (blue) hydrogen bonds. | ||
Despite the environmental improvements offered by the Lyocell process, it is beneficial to have a form of cellulose which is itself soluble in conventional solvents, thereby enabling facile processing of cellulose for polymer synthesis. Derivatisation can be used to produce cellulose analogues,3,4 the most common of which, cellulose esters and ethers, are available commercially.30–32 Ethers are generally water soluble, and find use in the food and beverage, paint and coatings, textile, and construction industries as rheology modifiers,3,30–33 the mining industry to improve product yield,3,30 the oil and gas industry as drilling fluid,3,30,31 as well as being applied in tissue engineering3,33 and bio-sensing.33 Some esters, such as cellulose triacetate, can be water-soluble (depending on the degree of polymerisation) but many are not. Cellulose esters are used mainly to produce fibres, plastics, films, and coatings.3,34,35 Unfortunately, derivatisation processes can suffer from the same problems as described above for the viscose and Lyocell processes, namely the use of toxic or expensive solvents. New derivatisation methods to produce water-soluble cellulose in a more environmentally friendly manner are highly desirable to meet the UN's Sustainable Development Goals, whilst also following the Principles of Green Chemistry.
We present a simple, two-step modification of microcrystalline cellulose using organic compounds that render it water-soluble, whilst maintaining the backbone and polymer structure (Fig. 2, bottom). Analysis of these materials shows that both the degree of substitution and the nature of the substituent play a role in solubility, with a sulfonate group being essential. Furthermore, altering the second step allows the morphology (porous or plate-like) and molecular weight (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–243
000–243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) of the material to be varied, whilst the inclusion of carboxylic acid and alkene moieties open up the potential for further modifications, making this a truly versatile process.
000 g mol−1) of the material to be varied, whilst the inclusion of carboxylic acid and alkene moieties open up the potential for further modifications, making this a truly versatile process.
Results and discussion
As a major reason for the lack of solubility of cellulose is strong hydrogen bonding from the alcohol groups, we selected these sites for modification, aiming to improve the solubility by disrupting the hydrogen bonding interactions. We decided to specifically target water solubility, and therefore selected cyclic molecules which would be ring-opened via a reaction with cellulose hydroxyl groups to provide moieties with high water affinity. To this end we chose 1,3-propane sultone, maleic anhydride, and succinic anhydride. It is well-known that maleic anhydride36–40 and succinic anhydride41,42 can be derived from nature. 1,3-Propane sultone can also be derived from glycerol via allyl alcohol reacted with sodium sulfite.43–45 The toxicity of 1,3-propane sultone should not to be an issue as it is used as a reagent rather than solvent, and the final product is non-toxic.46 We adapted literature procedures to react these species with microcrystalline cellulose (Scheme 1, ESI Sections S2.2 and S2.3†).47,48 While the presence of water may lead to side reactions in the 1,3-propane sultone step, it has been reported to be essential for the reaction to proceed.47 A similar degree of substitution (DS) was obtained in each reaction, as determined by elemental analysis (ESI section S3†). The slightly higher DS from succinic anhydride (DS = 0.80) could be due to a preferential adsorption at the cellulose interface compared to maleic anhydride (DS = 0.57), as reported by Sehaqui et al.49 The maximum DS is 3, which represents all 3 OH groups in a glucose unit being substituted. We expect the primary alcohol to be substituted preferentially, but other substitution patterns have been reported using related methods.3 Despite a decrease in crystallinity (ESI Fig. S5†), in all three cases (Scheme 1) the cellulose derivatives remained insoluble in water.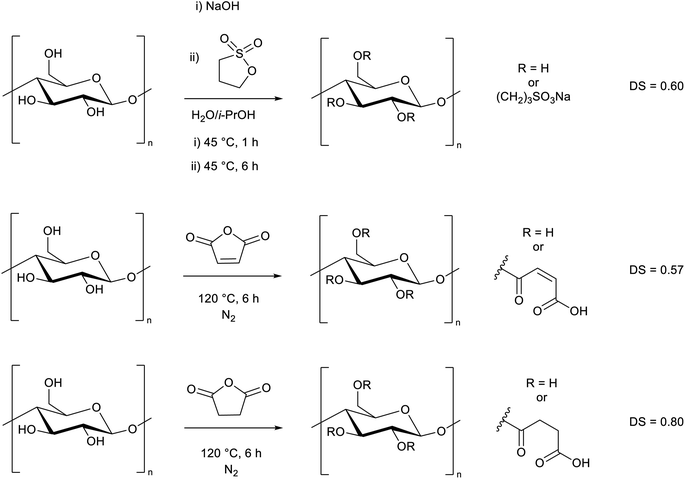 | ||
| Scheme 1 Modification of cellulose with 1,3-propane sultone, maleic anhydride, and succinic anhydride. The degree of substitution (DS) is shown (maximum DS = 3). | ||
Although the cellulose was insoluble, we noticed a different qualitative behavior after modification with 1,3-propane sultone. Whilst the unmodified cellulose could be easily dispersed in water, the sulfonated cellulose seemed at first to repel water, before slowly absorbing it to form a suspension (Fig. 3).
 | ||
| Fig. 3 The interaction of sulfonated cellulose with water. Left: water-repellent behaviour, and right: the formed suspension. | ||
Based on this observation, we used the sulfonated cellulose as a substrate for further reactions. Reacting this species with maleic or succinic anhydride, using the anhydride as both solvent and reagent, led to a DS greater than 1.1, and notably it allowed the cellulose to fully dissolve in water (Scheme 2, ESI Section S2.4†), thereby demonstrating a simple two-step process to prepare a water-soluble form of cellulose. We performed the reaction in this order to prevent removal of the anhydride group via cleavage of the ester linkage during the sultone reaction. 1H NMR spectra of these cellulose analogues clearly show the AGU unit and the sultone CH2 groups (ESI Section S5†). The maleic and succinic resonances are less distinct, presumably due to substitution at different alcohol groups along the chain.
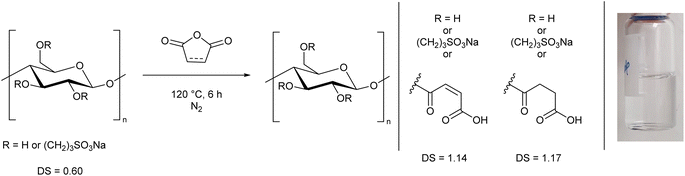 | ||
| Scheme 2 Synthesis of water-soluble cellulose derivatives from the reaction of sulfonated cellulose (Scheme 1) with either maleic or succinic anhydride. The DS and an example solution are shown. | ||
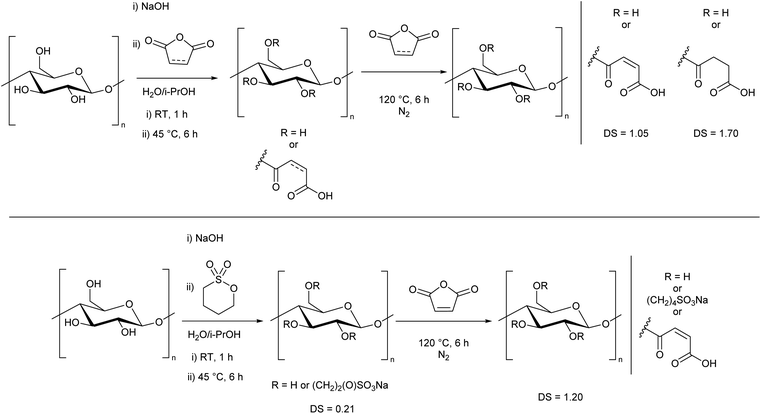
We then determined that the water solubility was related to both the DS and the nature of the substituent. Zero, or low sulfonate incorporation did not yield water-soluble cellulose, despite a high DS. By performing analogous syntheses where 1,3-propane sultone was replaced by either maleic or succinic anhydride, degree of substitutions of 1.05 and 1.70 were achieved, respectively (Scheme 3, top, ESI Section 2.5†). However, despite having similar DS as the water-soluble derivatives, these analogues were not water-soluble, showing the importance of the ionic sulfonate group. This also demonstrates that the treatment with NaOH in the first step is not a defining factor in the dissolution, but rather the means to facilitate the efficient reaction of cellulose with 1,3-propane sultone.
Furthermore, when 1,4-butane sultone was used in place of 1,3-propane sultone (Scheme 3, bottom, ESI Section S2.2†), despite a higher total DS of 1.20, the cellulose remained insoluble, presumably due to a low sultone incorporation (DS 0.21) which shows that a significant amount of sulfonate group is essential to produce water-soluble cellulose with this method.
Analysis of the cellulose analogues by XRD show that all species have reduced crystallinity compared to microcrystalline cellulose (Fig. 5), however, cellulose without a sulfonate group (Fig. 5, red), or with only a small amount of sulfonate (Fig. 5, grey), has a higher crystallinity than water-soluble cellulose (Fig. 5, blue). Higher crystallinity is associated with stronger hydrogen bonding, dispersion forces, and electrostatic attractions, which reduce access of reactants to the cellulose surface,50–52 thereby preventing cellulose solubility by hindering interaction with water molecules.
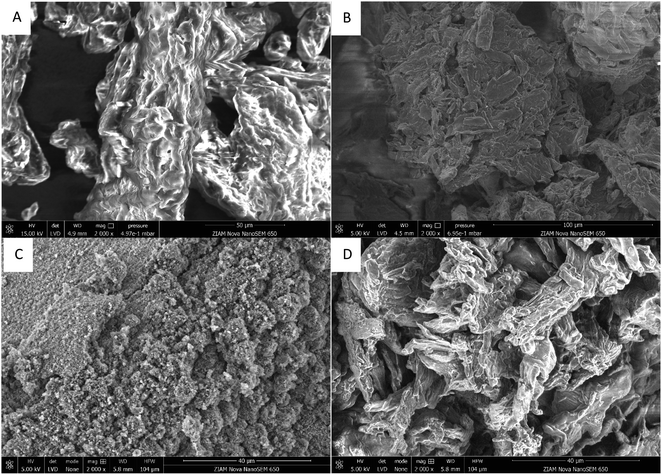
It has previously been reported that cellulose bearing sulfonate groups can be water-soluble,47,53–55 however, the solubility was strongly dependant on the reaction conditions (temperature, time, concentration, solvent, etc.). These derivatives have low importance commercially, with carboxymethylcellulose being much more widely used.3 A major benefit of our two-step process is that different properties can be conferred onto the final cellulose material depending on the second step. This is clearly seen in the SEM images showing the morphology of the modified cellulose (Fig. 4). A more porous material is formed when maleic anhydride is used in the second modification step compared to succinic anhydride, where the natural cellulose morphology is largely maintained (Fig. 4C and D). We believe this is due to the lower first pKa of maleic acid (1.8)56 compared to succinic acid (4.2).56 Further modulation of the polymer properties is possible by varying the reaction conditions (time and excess of anhydride). This is seen in the molecular weight obtained. For maleic anhydride, increasing the reaction time, or increasing the cellulose concentration in the maleic anhydride, reduces the molecular weight of the polymer (Table 1, entries 1–3). However, the opposite trend is observed with succinic anhydride (Table 1, entries 4–6). This could be due to a lower amount of cellulose decomposition when succinic anhydride is used as modifier, due to higher pKa of the corresponding acid, or some condensation reactions may occur with a longer reaction time or more concentrated reaction mixture,48 cross-linking the succinate groups and increasing the molecular weight. Using this method, we reach the remarkable value of 242![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1. The ability to control the molecular weight allows for the design of materials for different applications. Despite the different DS and molecular weights, there was little difference in thermal stability between the different modified celluloses (ESI Section S9†). All cellulose analogues containing a sulfonate group have a lower decomposition temperature than microcrystalline cellulose, which can be expected due to their lower crystallinity.57 However, they remained stable up to almost 200 °C, which is suitable for many applications, including traditional cellulose polymer processing.58 It should be emphasised that high temperature processing will not be necessary due to the water solubility of the cellulose. Furthermore, sulfonate modified cellulose did not decompose to 100% by mass, unlike unmodified cellulose, opening the possibility of these modified celluloses functioning as flame retardants.59
500 g mol−1. The ability to control the molecular weight allows for the design of materials for different applications. Despite the different DS and molecular weights, there was little difference in thermal stability between the different modified celluloses (ESI Section S9†). All cellulose analogues containing a sulfonate group have a lower decomposition temperature than microcrystalline cellulose, which can be expected due to their lower crystallinity.57 However, they remained stable up to almost 200 °C, which is suitable for many applications, including traditional cellulose polymer processing.58 It should be emphasised that high temperature processing will not be necessary due to the water solubility of the cellulose. Furthermore, sulfonate modified cellulose did not decompose to 100% by mass, unlike unmodified cellulose, opening the possibility of these modified celluloses functioning as flame retardants.59
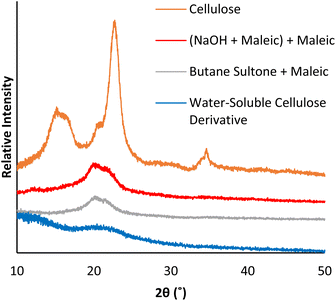 | ||
| Fig. 5 XRD patterns of cellulose, and differently modified cellulose analogues (from Schemes 2 and 3). | ||
| Entry | Reactant | Reaction conditions | DSa | M w (g mol−1) |
|---|---|---|---|---|
| a Determined via elemental analysis and back titration. b Determined via gel permeation chromatography using a Pullulan calibration. c Based on the molecular weight of the anhydroglucose unit (162.14 g mol−1) of cellulose. | ||||
| 1 | Maleic anhydride | Sulfonated cellulose (100 mg, 5 wt%), anhydride (2 g), 120 °C, 6 h | 1.14 | 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| 2 | Maleic anhydride | Sulfonated cellulose (100 mg, 5 wt%), anhydride (2 g), 120 °C, 16 h | 1.05 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
| 3 | Maleic anhydride | Sulfonated cellulose (500 mg), anhydride (1 g, 3 equiv.c), 120 °C, 6 h | 1.08 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
| 4 | Succinic anhydride | Sulfonated cellulose (100 mg, 5 wt%), anhydride (2 g), 120 °C, 6 h | 1.17 | 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| 5 | Succinic anhydride | Sulfonated cellulose (100 mg, 5 wt%), anhydride (2 g), 120 °C, 16 h | 1.02 | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| 6 | Succinic anhydride | Sulfonated cellulose (500 mg), anhydride (1 g, 3 equiv.c), 120 °C, 6 h | 1.08 | 242![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Outlook
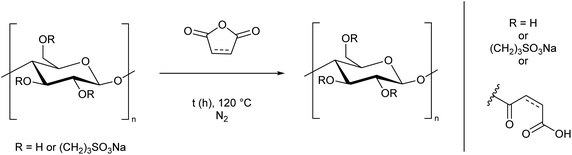
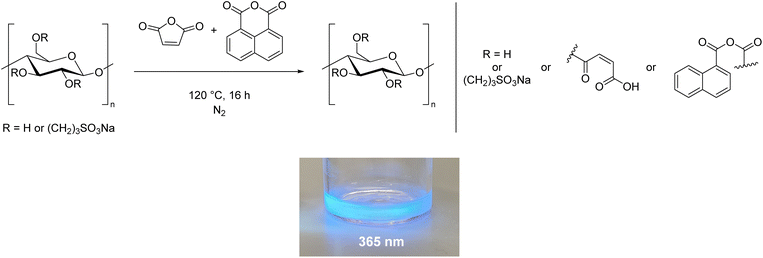
Using cellulose as a feedstock for polymers is in alignment with the UN's Sustainable Development Goals.1 However, as discussed in the introduction, processing cellulose is usually not environmentally benign. In this work we demonstrate a simple process which facilitates greener processing of cellulose. The water-soluble cellulose derivatives prepared here could be used in one of the many applications set out in the introduction. Aside from these applications, the water solubility will aid processing to develop new materials. For example, after cross-linking, the presence of sulfonate groups opens the possibility of preparing proton exchange membranes.60–64 Furthermore, the carboxylic acid and alkene moieties provided by the anhydrides offer scope for further reactivity, and studies along this line are in progress. This could lead to more functional groups being added to the cellulose in order to tune the physical properties. For example, cross-linking the maleic anhydride double bond could convert the cellulose from a polymer into a coating, as demonstrated for short chain oligomers of glucose with maleate substituents.65 Composite materials prepared using water-soluble cellulose could be biodegradable, which would contribute to their sustainability.3–5,8,66We believe an additional advantage to the modification method reported here is the flexibility offered by the unique two-step process, which allows the properties of cellulose to be tuned based on the substituent added in the second step. Cellulose is intrinsically chiral but it is difficult to transfer this into the bulk phase.67–69 The addition of chiral groups could aid this process. Replacing maleic and succinic anhydride with tartaric acid derivatives, or performing a ring-opening polymerisation with lactide70,71 would add chiral side chains to the cellulose. This could exploit the inherent chiral properties in solution, for instance, to create liquid crystals.67–69,72 Another potential use is to prepare fluorescent cellulose materials using our procedure. Including compounds such as 1,8-naphthalic anhydride, or perylene tetracarboxylic acid in the synthesis could impart fluorescent properties to the cellulose, as reported by Tian et al.,73 opening the possibility of applications in sensing, printing and anti-counterfeiting. Our preliminary results in this area demonstrate that it is possible to prepare fluorescent cellulose derivatives using the methodology developed here (Scheme 4, ESI Section 2.6†).
We therefore present not only two specific water-soluble cellulose derivatives, but also a methodology which we anticipate will allow for the preparation of further water-soluble cellulose analogues with tailored functionality.
Conclusions
We report a two-step modification of cellulose to produce a water-soluble polymer. Cellulose is first reacted with 1,3-propane sultone, and then with either maleic or succinic anhydride, making it water-soluble. Both the degree of substitution (DS) and the nature of the substituent were found to influence the water solubility, with a sulfonate group being essential in our process. The morphology of the final material depended on the treatment, with maleic anhydride producing a more porous structure, and succinic anhydride a plate-like structure. Remarkably, the molecular weight of the cellulose under our standard conditions was greater than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and could be increased or decreased depending on the reaction conditions (Table 1). Unlike current industrial methods to dissolve cellulose, we do not require toxic or unstable solvents. As well as the common uses for water-soluble cellulose, the methodology introduced here could be expanded, offering attractive prospects for different functionalities to be added in order to alter the properties, and the end use, of the cellulose.
000 g mol−1 and could be increased or decreased depending on the reaction conditions (Table 1). Unlike current industrial methods to dissolve cellulose, we do not require toxic or unstable solvents. As well as the common uses for water-soluble cellulose, the methodology introduced here could be expanded, offering attractive prospects for different functionalities to be added in order to alter the properties, and the end use, of the cellulose.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is part of the Advanced Research Center for Chemical Building Blocks, ARC CBBC (2021.036.A.RUG.1), which is co-founded and co-financed by the Dutch Research Council (NWO) and the Netherlands Ministry of Economic Affairs and Climate Policy. We thank Hans van der Velde, Jur van Dijken, and Gert-Jan Boer (all University of Groningen) for analytical support. We thank Annemarie Doze and David Grantz (University of Groningen) for laboratory assistance.Notes and references
- United Nations 17 Sustainable Development Goals, https://sdgs.un.org/goals, accessed April 2023.
- The New Plastics Economy: Rethinking the Future of Plastics, World Economic Forum, 2016, https://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf, accessed March 2023 Search PubMed.
- T. Heinze, O. A. El Seoud and A. Koschella, Cellulose Derivatives: Synthesis, Structure, and Properties, Springer International Publishing AG, Switzerland, 2019 Search PubMed.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS.
- R. Mori, RSC Sustainability, 2023, 1, 179–212 RSC.
- C. Campano, A. Balea, A. Blanco and C. Negro, Cellulose, 2016, 23, 57–91 CrossRef CAS.
- C. Zhong, Front. Bioeng. Biotechnol., 2020, 8, 605374 CrossRef PubMed.
- Y. Liu, S. Ahmed, D. E. Sameen, Y. Wang, R. Lu, J. Dai, S. Li and W. Qin, Trends Food Sci. Technol., 2021, 112, 532–546 CrossRef CAS.
- J. Li, R. Cha, K. Mou, X. Zhao, K. Long, H. Luo, F. Zhou and X. Jiang, Adv. Healthcare Mater., 2018, 7, 1800334 CrossRef PubMed.
- H. Seddiqi, E. Oliaei, H. Honarkar, J. Jin, L. C. Geonzon, R. G. Bacabac and J. Klein-Nulend, Cellulose, 2021, 28, 1893–1931 CrossRef CAS.
- K. Heise, E. Kontturi, Y. Allahverdiyeva, T. Tammelin, M. B. Linder, Nonappa and O. Ikkala, Adv. Mater., 2021, 33, 2004349 CrossRef CAS PubMed.
- R. Das, T. Lindström, P. R. Sharma, K. Chi and B. S. Hsiao, Chem. Rev., 2022, 122, 8936–9031 CrossRef CAS PubMed.
- B. Frka-Petesic and S. Vignolini, Nat. Photonics, 2019, 13, 365–367 CrossRef CAS PubMed.
- A. Sharma, M. Thakur, M. Bhattacharya, T. Mandal and S. Goswami, Biotechnol. Rep., 2019, 21, e00316 CrossRef PubMed.
- P. G. Gan, S. T. Sam, M. F. B. Abdullah and M. F. Omar, J. Appl. Polym. Sci., 2020, 137, 48544 CrossRef CAS.
- R. M. Parker, T. H. Zhao, B. Frka-Petesic and S. Vignoli, Nat. Commun., 2022, 13, 3378 CrossRef CAS PubMed.
- B. E. Droguet, H. L. Liang, B. Frka-Petesic, R. M. Parker, M. F. De Volder, J. J. Baumberg and S. Vignolini, Nat. Mater., 2022, 21, 352–358 CrossRef CAS PubMed.
- B. Lindman and B. Medronho, BioResources, 2015, 10, 3811–3814 CAS.
- B. Medronho and B. Lindman, Adv. Colloid Interface Sci., 2015, 222, 502–508 CrossRef CAS PubMed.
- B. Medronho, H. Duarte, L. Alves, F. Antunes, A. Romano and B. Lindman, Nord. Pulp Pap. Res. J., 2015, 30, 58–66 CrossRef CAS.
- L. A. Ramos, D. L. Morgado, F. Gessner, E. Frollini and O. A. El Seoud, ARKIVOC, 2011, 7, 416–425 Search PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS PubMed.
- Q. Zhang, M. Benoit, K. De Oliveira Vigier, J. Barrault and F. Jérôme, Chem.–Eur. J., 2012, 18, 1043–1046 CrossRef CAS PubMed.
- H. T. Vo, Y. J. Kim, E. H. Jeon, C. S. Kim, H. S. Kim and H. Lee, Chem.–Eur. J., 2012, 18, 9019–9023 CrossRef CAS PubMed.
- S. Thiemann, S. J. Sachnov, F. Pettersson, R. Bollström, R. Österbacka, P. Wasserscheid and J. Zaumseil, Adv. Funct. Mater., 2014, 24, 625–634 CrossRef CAS.
- J. Zhang, J. Wu, J. Yu, X. Zhang, J. He and J. Zhang, Mater. Chem. Front., 2017, 1, 1273–1290 RSC.
- S. Elsayed, J. Helminen, S. Hellsten, C. Guizani, J. Witos, M. Rissanen, A. H. Rantamäki, P. Hyväri, P. Varis, S. K. Wiedmer, I. Kilpeläinen and H. Sixta, ACS Sustainable Chem. Eng., 2020, 8, 14217–14227 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, pp. 29–56 Search PubMed.
- Nouryon Cellulose Ethers, https://www.nouryon.com/products/cellulose-ethers/?_gl=1*a260kf*_up*MQ%26gclid=EAIaIQobChMI4bu715T-_QIVRg0GAB1q0AQnEAAYASAAEgIxlfD_BwE, accessed April 2023 Search PubMed.
- DOW Cellulose Ether, https://www.dow.com/en-us/search.html#q=carboxymethyether%26t=All%26sort=relevancy, accessed April 2023 Search PubMed.
- Ashland Cellulosics, https://www.ashland.com/search?chemistry=cellulosics, accessed April 2023 Search PubMed.
- M. S. Rahman, M. S. Hasan, A. S. Nittai, S. Nam, A. K. Karmakar, M. S. Ahsan, M. J. A. Shiddiky and M. B. Ahmed, Polymers, 2021, 13, 1345 CrossRef CAS.
- K. J. Edgar, C. M. Buchanan, J. S. Debenham, P. A. Rundquist, B. D. Seiler, M. C. Shelton and D. Tindall, Prog. Polym. Sci., 2001, 26, 1605–1688 CrossRef CAS.
- V. Vatanpour, M. E. Pasaoglu, H. Barzeger, O. O. Teber, R. Kaya, M. Bastug, A. Khataee and I. Koyuncu, Chemosphere, 2022, 295, 133914 CrossRef CAS PubMed.
- Z. Du, J. Ma, F. Wang, J. Liu and J. Xu, Green Chem., 2011, 13, 554–557 RSC.
- H. Guo and G. Yin, J. Phys. Chem. C, 2011, 115, 17516–17522 CrossRef CAS.
- G. Pavarelli, J. Velasquez Ochoa, A. Caldarelli, F. Puzzo, F. Cavani and J.-L. Dubois, ChemSusChem, 2015, 8, 2250–2259 CrossRef CAS PubMed.
- W. Jia, Z. Si, Y. Feng, X. Zhang, X. Zhang, Y. Sun, X. Tang, X. Zeng and L. Lin, ACS Sustainable Chem. Eng., 2020, 8, 7901–7908 CrossRef CAS.
- J. G. H. Hermens, A. Jensma and B. L. Feringa, Angew. Chem., Int. Ed., 2022, 61, e202112618 CrossRef CAS PubMed.
- I. Bechthold, K. Bretz, S. Kabasci, R. Kopitzky and A. Springer, Chem. Eng. Technol., 2008, 31, 647–654 CrossRef CAS.
- B. Cok, I. Tsiropoulos, A. L. Roes and M. K. Patel, Biofuels, Bioprod. Biorefin., 2014, 8, 16–29 CrossRef CAS.
- R. G. Bergman, J. A. Ellman and E. A. Rebollo, Conversion of glycerol from biodiesel production to allyl alcohol, WO Pat., WO2008092115A1, 2008 Search PubMed.
- M. Wormann and M. E. Maier, RSC Adv., 2019, 9, 15314–15317 RSC.
- X. Zhang, D. Liu, Z. Yang, G. Lin and W. Li, Method for preparing 1,3-propanesultone, CN Pat., CN101456855B, 2008 Search PubMed.
- Sigma Aldrich Safety Data Sheet: 3-Hydroxypropane-1-Sulfonic Acid, https://www.sigmaaldrich.com/NL/en/sds/aldrich/56260, accessed April 2023 Search PubMed.
- G. Natus and E. J. Goethals, J. Macromol. Sci., Chem., 1968, 2, 489–499 CAS.
- J. C. P. de Melo, E. C. da Silva Filho, S. A. A. Santana and C. Airoldi, Colloids Surf., A, 2009, 346, 138–145 CrossRef CAS.
- H. Sehaqui, K. Kulasinski, N. Pfenninger, T. Zimmermann and P. Tingaut, Biomacromolecules, 2017, 18, 242–248 CrossRef CAS PubMed.
- J. A. Rollin, Z. Zhu, N. Sathitsuksanoh and Y.-H. PercivalZhang, Biotechnol. Bioeng., 2011, 108, 22–30 CrossRef CAS PubMed.
- S. P. S. Chundawat, G. Bellesia, N. Uppungundla, L. da Costa Sousa, D. Gao, A. M. Cheh, U. P. Agarwal, C. M. Bianchetti, G. N. Phillips Jr, P. Langan, V. Balan, S. Gnanakaran and B. E. Dale, J. Am. Chem. Soc., 2011, 133, 11163–11174 CrossRef CAS PubMed.
- M. C. Jarvis, Cellulose, 2023, 30, 667–687 CrossRef CAS.
- R. Kiesewetter, K. Szablikowski and W. Lange, Water soluble sulfoalkyl hydroxyalkyl derivatives of cellulose and their use in gypsum and cement compositions, EU Pat., EP0554749A2, 1993 Search PubMed.
- R. Doenges and H. Wurm, Water-soluble, sulfoalkyl-containing, hydrophobically modified cellulose ethers, process for preparing them, and their use as protective colloids in polymerizations, US Pat., US6515049B1, 1999 Search PubMed.
- F. Höhl, H. Schlesinger and R. Kiesewetter, Use of optionally substituted sulfoalkyl-modified cellulose ethers as non-associative thickeners for aqueous coating systems, WO Pat., WO2001009254A1, 2000 Search PubMed.
- S. Goswami, N. K. Das, D. Sen, G. Hazra, J. H. Goh, Y. C. Sing and H.-K. Fun, New J. Chem., 2011, 35, 2811–2819 RSC.
- M. E. Calahorra, M. Cortázar, J. I. Eguiazábal and G. M. Guzmán, J. Appl. Polym. Sci., 1989, 37, 3305–3314 CrossRef CAS.
- M. Poletto, H. L. Ornaghi Jnr and A. J. Zattera, Materials, 2014, 7, 6105–6119 CrossRef PubMed.
- M. N. A. M. Taib, T. S. Hamidon, Z. N. Garba, D. Trache, H. Uyama and M. H. Hussin, Polymer, 2022, 244, 124677 CrossRef.
- J. A. Seo, J. C. Kim, J. K. Koh, S. H. Ahn and J. H. Kim, Ionics, 2009, 15, 555–560 CrossRef CAS.
- A. Sriruangrungkamol and W. Chonkaew, Polym. Bull., 2021, 78, 3705–3728 CrossRef CAS.
- V. Guccini, A. Carlson, S. Yu, G. Lindbergh, R. W. Lindström and G. Salazar-Alvarez, J. Mater. Chem. A, 2019, 7, 25032–25039 RSC.
- O. Selyanchyn, R. Selyanchyn and S. M. Lyth, Front. Energy Res., 2020, 8, 596164 CrossRef.
- B. Mottet, B. Laborie and M. Kechadi, Device for producing energy by salinity gradient through a membrane based on crosslinked cellulose fibres, WO Pat., WO2021/234296A2, 2021 Search PubMed.
- A. Badía, M. Kastelijn, J. Scheerder and J. R. Leiza, Prog. Org. Coat., 2020, 147, 105708 CrossRef.
- Y. H. Jung, T.-H. Chang, H. Zhang, C. Yao, Q. Zheng, V. W. Yang, H. Mi, M. Kim, S. J. Cho, D.-W. Park, H. Jiang, J. Lee, Y. Qiu, W. Zhou, Z. Cai, S. Gong and Z. Ma, Nat. Commun., 2015, 6, 7170 CrossRef PubMed.
- M. Khandelwal and A. Windle, Carbohydr. Polym., 2014, 106, 128–131 CrossRef CAS PubMed.
- T. G. Parton, R. M. Parker, G. T. van de Kerkhof, A. Narkevicius, J. S. Haataja, B. Frika-Petesic and S. Vignolini, Nat. Commun., 2022, 13, 2657 CrossRef CAS PubMed.
- G. Fittolani, D. Vargová, P. H. Seeburger, Y. Ogawa and M. Delbianco, J. Am. Chem. Soc., 2022, 144(27), 12469–12475 CrossRef CAS PubMed.
- A. Córdova and J. Hafrén, Nord. Pulp Pap. Res. J., 2005, 20, 477–480 CrossRef.
- W. Yuan, J. Yuan, F. Zhang and X. Xie, Biomacromolecules, 2007, 8, 1101–1108 CrossRef CAS PubMed.
- A. A. Moud, ACS Omega, 2022, 7, 30673–30699 CrossRef PubMed.
- W. Tian, J. Zhang, J. Yu, J. Wu, H. Nawaz, J. Zhang, J. He and F. Wang, Adv. Opt. Mater., 2016, 4, 2044–2050 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General remarks, transformations of cellulose degree of substitution calculation, infrared spectra, nuclear magnetic resonance spectra, gel permeation chromatography, X-ray diffraction analysis, scanning electron microscopy, thermogravimetric analysis. See DOI: https://doi.org/10.1039/d3su00317e |
| This journal is © The Royal Society of Chemistry 2024 |