Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionometallurgy: an academic exercise or promising approach?
Janine
Richter
a and
Michael
Ruck
 *ab
*ab
aFaculty of Chemistry and Food Chemistry, TU Dresden, 01062 Dresden, Germany. E-mail: michael.ruck@tu-dresden.de
bMax Planck Institute for Chemical Physics of Solids, 01187 Dresden, Germany
First published on 20th March 2024
Abstract
Metals and functional metal-containing compounds are indispensable mass products. Metals are produced from low-reactive resources that are activated by very high temperatures (pyrometallurgy) or aggressive chemicals (hydrometallurgy). These traditional and highly optimized processes are often environmentally problematic because they require a lot of energy and generate large quantities of waste. Nowadays, numerous metal oxides and sulfides can be dissolved in ionic liquids (ILs) or deep eutectic solvents (DESs) at moderate temperatures below 200 °C, at least on a laboratory scale. Some of these solutions are thermally and electrochemically stable enough to enable galvanic metal deposition. Moreover, valuable metal-containing compounds can by synthesized from them. This review discusses whether such ionometallurgical metal extraction could be a viable and sustainable alternative to conventional processes, focusing on the examples of copper, cobalt and aluminum. Among the various factors that are crucial for the sustainability and economic feasibility of ionometallurgical processes, we mainly focus on the chemical aspects, but also take a look at the availability, environmental impact and reusability of the new solvents. Although the challenges associated with ionometallurgy are great, especially when it comes to upscaling, ionometallurgy could contribute to a more sustainable production of metals and metal compounds and also provide rewarding results in other areas, e.g. battery applications.
Sustainability spotlightThe large-scale production of metals and metal compounds is among the most energy-intensive industrial processes, requiring high reaction temperatures and generating considerable CO2 emissions. This review article investigates critical elements of conventional metal production and discusses the feasibility of ionometallurgy as a more sustainable alternative. The application of ionic liquids and deep eutectic solvents could enable metal production at moderate temperatures, reducing its climate impact. While academic research is keen to explore this topic, industrial implication is still lacking. Therefore, the potential and obstacles for the transfer of ionometallurgy from lab experiments to large-scale processes are critically investigated. Research in this field aligns with SDGs 9 (industry, innovation and infrastructure), 12 (responsible consumption and production) and 13 (climate action). |
Introduction
Metals and valuable metal compounds are important for our everyday lives with applications ranging from aluminum foil over circuit boards to high-performance alloys for engineering and building construction. Large-scale metal production processes provide access to metals contained in numerous naturally occurring ores, earths and minerals and should be considered one of the major drivers of industrialization, leading to a continuous increase in living standards. As the global society undertakes significant efforts to become carbon neutral, the demand for metals will increase. For example, electromobility is inconceivable without copper and aluminum for the supply of electrical energy in a large-scale network of charging stations.1 The increasing demand for metals is exacerbating the problems associated with their production, particularly with regard to the energy required and the waste generated.Most metals are extracted from their oxides or sulfides. These are stable compounds with low reactivity that need to be activated for processing, which usually means reduction to the element. For activation either high temperatures or aggressive chemicals are needed. This makes metal extraction energy-intensive and often ecologically problematic. From a scientific and engineering point of view, the impressive efficiency of the current highly optimized large-scale metal production processes must be acknowledged. However, this assessment depends heavily on the underlying conditions, which are currently changing dramatically. With environmental aspects being increasingly taken into account and priced, it seems imperative to find alternatives that are more resource-efficient and environmentally friendly. The production of two industrially most relevant metals, iron (or steel) and aluminum, alone accounts for about 10% of the global CO2 emissions with little room for further efficiency improvement in current processes.2,3 A large share of these emissions originates from the immense amount of energy required to keep these processes running, as fossil fuels continue to cover the major part of the global energy demand (approx. 80%).4 In addition, pollution from by-products and waste threatens the well-being of people and their environment, especially in regions where environmental protection plays hardly any role.
In view of the urgent problems concerning climate change, in 2015, the United Nations General Assembly adopted the 17 Sustainable Development Goals (SDGs) to be implemented by 2030 through global efforts in scientific and industrial innovations as well as political commitments.5 Particularly SDG 12 “Responsible consumption and production” directly addresses the energy-intensive metal industry. Their processes must achieve a significant reduction of greenhouse gas emissions, implement recycling schemes with the ultimate goal of complete circularity and conserve valuable resources in every respect. Due to the hardly conquerable limits of conventional metallurgical processes, true “responsible consumption and production” requires completely new approaches for metal production. Energy consumption and, therefore, CO2 emissions need to be reduced by using lower reaction temperatures. At the same time, resource demand and waste should be minimized by efficient circular processes. In this case, we need to treat end-of-life products as new resources that require effective recycling strategies. Such a disruptive innovation could be the so-called ionometallurgy, i.e., metal extraction by means of ionic liquids (ILs) or deep eutectic solvents (DESs).
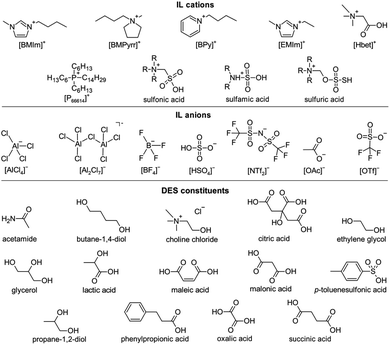
ILs, per definition, are salts with a melting point below 100 °C. Typically, ILs consist of sterically demanding organic cations and organic or inorganic anions. Due to their conductivity and usually wide electrochemical windows, ILs were first considered promising electrolytes for battery or electrolysis applications. Eventually, the negligible volatility, good thermal and chemical stability, a wide liquid range and excellent dissolution properties for various substances of many ILs6 contributed to their utilization, e.g., as lubricants,7 catalysts,8 solvents or reaction media for organic and inorganic syntheses.9,10 By the variation of cations or anions, IL properties can be tailored to fit specific applications, which, on the other hand, involve elaborate synthesis methods, making many ILs relatively expensive materials.11 However, the classes of ILs and DESs have numerous diverse representatives and there are exceptions to each of these statements. Generalizations such as the previous ones, therefore, refer to general tendencies.
In 2002, Abbott introduced DESs as liquids with properties similar to those of ILs, such as high conductivity, low volatility and good solubility for various materials, but with much simpler preparation.12 DESs are eutectic mixtures of two or more molecular compounds with a melting point below that of the individual components.13 Complex organic synthesis of IL ions is replaced by the straightforward mixing of the reagents in specific ratios. The usage of natural substances, especially, has great potential making these DESs allegedly “green” and easy to produce.14
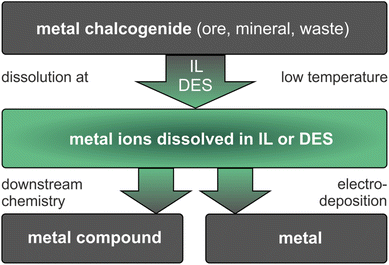
In ionometallurgy, ILs and DESs are applied in a seemingly simple two-step reaction, as shown in Fig. 1. In the first step, metal compounds are dissolved in an IL or DES at moderate temperature. Thereby, the excellent and tailorable dissolution properties of ILs and DESs make even poorly soluble metal compounds accessible. In the second step, either metals are electrodeposited directly from the resulting solutions or valuable metal compounds are produced by downstream chemistry. The separation of mixtures is possible, for example, by selective dissolution, extraction or deposition. These reactions require ILs and DESs that are thermally and (electro)chemically stable under the conditions of use.15–17
Although ILs and DESs generated considerable scientific interest in the last few decades, they are still lacking industrial application in metallurgy, as a recent opinion article pointed out.18 Reasons include their limited stability and the high costs, hindering competitiveness to current hydrometallurgical processes. While ILs consist of often expensively synthesized discrete cations and anions, various ionic and molecular species are present in DESs, mainly interacting through hydrogen bonding. It became apparent with ongoing research that this has significant implications for their properties, distinguishing them from ILs. For example, the vapor pressure of DESs is not negligible although it lies several magnitudes below that of typical single-compound organic solvents. This can even affect the composition of the DES if its components feature differing volatility. Furthermore, many DESs were shown to be significantly less stable than ILs with numerous decomposition reactions taking place at room temperature and even more at elevated temperatures, as shown in Fig. 2.19 More importantly, the properties of the solvents change when dissolving salts or other substances. This applies in particular to DESs, as their hydrogen bonding system is severely impaired by solvated ions. Thus, information on the properties of pure ILs and DESs is only of limited predictive power as far as the behavior of solutions is concerned. It must therefore be checked on a case-by-case basis whether ILs or DESs are suitable as green solvents for a particular process.
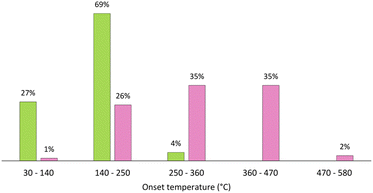 | ||
| Fig. 2 Distributions of onset temperatures of thermal decomposition of ILs (pink) and eutectic solvents (green). Reproduced from ref. 19, copyright 2023. | ||
In this review, we critically discuss whether ILs and DESs should indeed no longer be considered for use in metallurgy as recently stated.18 Initially, critical aspects of metal production are highlighted in a brief overview for the examples of copper, cobalt and aluminum. The results of research in the field of ionometallurgy are subsequently presented, covering both basic research and application-oriented results. This review does not mean to give an exhaustive summary about the entirety of the performed research in this field but shows general findings and common tendencies. We want to show the potential of ILs and DESs, but also the current deficits, and emphasize that ionometallurgy should not be written off prematurely.
Conventional metal production
The birth of many large-scale metal production processes can be traced back to ancient times, such as the pyrometallurgical production of copper, tin and iron during the Bronze and Iron ages.20 The much more recent Bayer and Hall–Héroult processes for aluminum production were also developed at the end of the 19th century.21 Although these industrial processes were optimized to have higher and higher efficiencies, especially after industrialization,22 at the core, they continue to remain the same. At that time, considerations about environmental pollution and climate change hardly played a role. As a result, conventional metal production today contributes significantly to the annual production of greenhouse gases amounting to 55 billion tons of CO2 equivalents.23 CO2 equivalents are a metric that converts the global warming potential of various greenhouse gases over 100 years into the respective mass of CO2. Thus, a more balanced picture of the impact on climate change emerges with greenhouse gases such as methane being taken into account rather than by just considering CO2.24 In the following section, three examples, namely copper, cobalt and aluminum, are briefly discussed to illustrate the complexity and problematic aspects of conventional metal production processes. A schematic representation of the prevalent production processes is given in Fig. 3.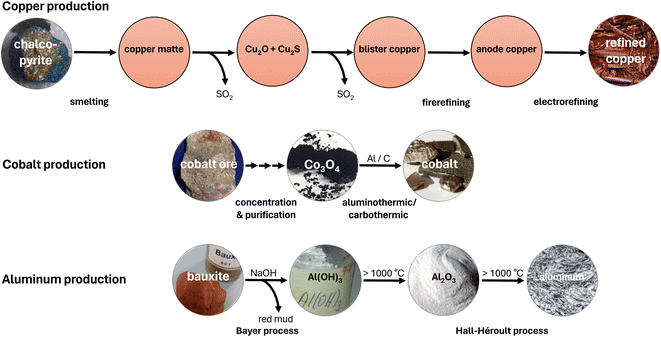 | ||
| Fig. 3 Schematic representation of the prevalent production processes of copper, cobalt and aluminum. | ||
Copper
Due to its excellent electrical and thermal conductivity, copper is a widely used material in electric and electronic devices or heat exchangers with an annual mine production of 22 million tons.25 As private, public and industrial energy consumers switch to sustainable sources and thus to electricity, the demand for copper will increase sharply again in the near future. On exposure to air, copper forms a durable patina that protects the subjacent material from further corrosion. This also makes copper a favorable material for building construction and protective coatings.21Although copper is one of the few metals that occurs in the elemental form, the majority of the 0.006 wt% copper in the earth's crust26 is bound in ores. The main industrial relevance belongs to the sulfuric minerals chalcopyrite (CuFeS2), bornite (Cu5FeS4) and chalcocite (Cu2S). Oxidic minerals, such as tenorite (CuO) or cuprite (Cu2O), on the other hand, are rare.21 Since these minerals, typically, are present in naturally occurring ores with a low copper content (≤2%) and many applications require copper of high purity, the predominant part of this metal is produced by a complex, multi-step pyrometallurgical process.27 Although the exothermic nature of the process makes additional heating on a large scale obsolete,21 copper is placed among the top ten metals regarding greenhouse gas emissions, that amount to approximately 66 million tons of CO2 equivalents per year.28
Initially, the mined copper ores undergo a flotation step in order to increase the copper concentration. This process has a high water demand and generates large quantities of sewage.29 Subsequently, the accompanying iron is removed in a high-temperature reaction yielding copper matte, consisting of Cu2S. Keeping temperatures above 1000 °C, two thirds of the copper matte is converted to Cu2O followed by the formation of blister copper. In a subsequent fire refinement, impurities are removed from 98% pure blister copper. The product is then suitable to be used as an anode in electrorefining. Typically, acidic CuSO4 electrolyte baths are used for bulk copper refinement.27 However, this is unsuitable for many electroplating applications as base metals are attacked by the acidic electrolyte. Alkaline cyanide baths are a common alternative, but due to their high toxicity, they pose a significant safety risk during handling and wastewater treatment.30
An alternative or complementation to pyrometallurgical copper processing is hydrometallurgical solvent extraction and electrowinning (SX/EW process), which accounts for approximately 20% of the total copper production. Thereby, copper is extracted from mine wastes or ores by using diluted sulfuric acid and separated from other metal impurities by extraction with an organic solvent. Another extraction step with a strongly acidic aqueous solution provides the electrolyte in the subsequent electrowinning stage. Although the individual process conditions differ widely, hydrometallurgical copper production is considered to have an overall lower energy consumption compared to the pyrometallurgical approach.31 However, the recovery rates for flotation are typically higher than those for solvent extraction; thus, the hydrometallurgical process is primarily applied to low-grade ores.32,33 Since chalcopyrite ore grades are decreasing, hydrometallurgical processing gains more and more interest. Process optimization includes research on oxidation leaching, coordination leaching and bioleaching.34
Cobalt
One of the most important uses of metallic cobalt is its numerous alloys. Among them, superalloys are employed in turbines due to their excellent high-temperature strength.35 Cobalt-containing magnetic alloys, such as SmCo5, are used as permanent magnets in various applications.36,37 Especially with electric mobility on the rise, oxide materials also, such as LiCoO2, acquire greater importance as cathode materials in lithium-ion batteries.38Common cobalt sources are widespread ores with cobalt contents usually below 0.01 wt%, accompanied by nickel, copper and other metal compounds. Therefore, cobalt primarily is a byproduct of the mining of these metals. Depending on the actually present arsenide, sulfide or oxide minerals, different processes are applied to produce cobalt. Typically, initial froth flotation or gravity methods concentrate cobalt in the source material.37 Subsequent roasting, leaching and fractional precipitation allow for the removal of arsenic and sulfur as well as the separation from accompanying metal compounds, yielding Co3O4. This cobalt oxide is then reduced either carbothermically or aluminothermically to give the metal powder.21 The annual mine production of cobalt amounts to 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.25
000 tons.25
The final reduction step significantly contributes to annual greenhouse gas emissions by cobalt production amounting to approximately 1 million tons of CO2 equivalents.28 The carbothermal reduction of Co3O4 in a blast furnace yields carbon monoxide and CO2 as the reaction products. Although this is not the case for the aluminothermic reaction, the production of aluminum in the first place involves massive environmental pollution and greenhouse gas emissions, as shown in the following section.
The numerous applications of cobalt counteract its low abundance of only 0.0025 wt% in the earth's crust.26 Therefore, besides ore mining, recycling schemes increasingly move to the center of attention. This is especially relevant for lithium-ion batteries that have a short life time of only a few years.39–41 Cobalt is present in cathode materials of strongly varying compositions ranging from ternary LiCoO2 (LCO) to more complex LiNixMnyCo1−x−yO2 (NMC) or LiNi1−x−yCoxAlyO2 (NCA).42 Since a LiCoO2 cathode accounts for 25–30 wt% of a lithium-ion battery, cobalt concentrations are significantly higher compared to that of naturally occurring ores, and their recovery is worthwhile.43–45 Just as lithium-ion batteries are still refined and optimized, their recycling is also a topic of ongoing research. Current processes apply mechanical, hydrothermal or pyrometallurgical recycling strategies as well as a combination thereof. Mechanical methods are particularly employed for pretreatment in order to remove solid constituents, e.g., metal parts. Further pyrometallurgical recycling recovers only metal compounds, while organic components are decomposed at approximately 1000 °C. Hydrometallurgy, on the other hand, leaches valuable components with the help of organic or inorganic solvents below 100 °C for subsequent recovery. While pyrometallurgical treatment requires large amounts of energy, hydrometallurgical approaches consume great volumes of solvents, and toxic byproducts are a drawback of both methods.43,44,46,47
Aluminum
Aluminum is the most abundant metal in the earth's crust (8.23 wt%)26 and its favorable properties made it the most produced non-ferrous metal with 69 million tons annually.25,48 Its high abundance leads to relative cheapness that even allows for usage in disposable items, such as aluminum foil or beverage cans. Furthermore, various aluminum alloys with optimized characteristics, such as high hardness, are popular lightweight construction materials for buildings and transportation. As a good electrical conductor, aluminum is also widely employed in power lines. In air, it forms a passivating oxide layer, which serves as corrosion protection and makes aluminum an attractive protective coating.21Due to its high oxygen affinity, aluminum only occurs in the form of oxidic minerals, with the ore bauxite being of exclusive industrial relevance. In bauxite, Al(OH)3 and AlOOH minerals are mainly accompanied by iron, silicon and calcium compounds. Since further processing relies on pure Al2O3 as the starting material, the Bayer process provides the initial purification. The reaction with 35–38% NaOH solution at 140–250 °C and 5–7 bar extracts aluminum as a soluble hydroxo complex and allows for the separation from the undissolved iron- and silicon-rich residues. This residual highly alkaline “red mud” is dumped in giant basins, causing severe problems for soil and life in the surroundings. The remaining fraction of the NaOH solution is recirculated after the precipitation and separation of Al(OH)3. The hydroxide is then thermally dehydrated to Al2O3 in a calcination reaction at about 1200 °C.21
In the subsequent Hall–Héroult process, Al2O3 is molten together with about a fourfold quantity of (synthetic) cryolite (Na3AlF6) and additional additives, such as AlF3, CaF2, LiF or MgF2. This allows the reduction of the melting point from 2045 °C for pure Al2O3 to about 950 °C for the mixture. In molten salt electrolysis, aluminum is then electrodeposited at the cathode. Concomitantly, CO2 is formed at the carbon anode, resulting in the total formation of aluminum according to eqn (1).21,27,49
| 2Al2O3 + 3C → 4Al + 3CO2 | (1) |
The high process temperatures for calcination and electrolysis require massive amounts of electrical energy with only a quarter produced by hydro-power and renewable resources worldwide. Thereby, aluminum production generates annual greenhouse gas emissions of 732 million tons of CO2 equivalents,28 accounting for 1.3% of the total global emissions. Taking only CO2 into account, aluminum production was responsible for 3% of direct industrial CO2 emissions in 2021.2 Due to the electrolytic bath composition in the Hall–Héroult process, fluorinated hydrocarbons, especially, play a role as additional greenhouse gases. Attempts to avoid their release, on the other hand, generate large quantities of waste water.49
Ionometallurgy
Ionic liquids for ionometallurgy
Leaching of metal ions: ionometallurgy relies on the initial accessibility of metal ions by the chemical dissolution of metal salts, such as metal oxides (MOs) or metal sulfides (MSs). Since MOs appear to be more the focus of scientific attention than MSs, the following discussion will only briefly cover the latter. Fig. 4 presents the constituents of ILs and DESs discussed in this manuscript.In the early days of this research, chloridoaluminate ILs, also known as first-generation ILs, were used. They can be easily produced by combining an organic chloride salt, e.g., an imidazolium chloride, with AlCl3. By variation of the molar ratio between the organic chloride salt and AlCl3, the Lewis acidity of the solution changes. Chloride and chloridoaluminate anions in these ILs provide strong interactions with metal ions, dissolving, e.g., Bi2O3,50 V2O3,51 V2O5 (ref. 50 and 51) and UO3.52 However, these ILs are very sensitive to moisture, which severely limits their practical applicability.11
ILs with stable anions, such as tetrafluoridoborate ([BF4]−), triflate ([OTf]−) or bis(trifluoromethylsulfonyl)imide ([NTf2]−), allow for handling in air as well as mixing with aqueous solutions at least to some extent. Due to the hydrophobicity of the [NTf2]− anion, such ILs generally only exhibit low water miscibility. Since these anions are usually weakly coordinating, the dissolution of MOs is mainly driven by interactions with the IL cations. One example is imidazolium cations, which have an acidic proton. Their acidity enables the dissolution of Ag2O, CuO, NiO and ZnO, with the formation of water and carbenes.53 Similarly, several rare-earth oxides RE2O3 (RE = La–Ho) dissolve in 1-butyl-3-methylimidazolium acetate ([BMIm][OAc]) via the formation of N-heterocyclic carbenes. Since the latter can be regenerated to the initial IL with acetic acid, the overall process comes down to the dissolution of RE2O3 in anhydrous acetic acid, a reaction that does not proceed directly.54
In addition, mixtures of water-stable ILs with aqueous mineral acids, such as HCl or HNO3, are able to dissolve CaO, CoO, CuO, Fe2O3, MnO, NiO, ZnO,55 Eu2O3, Nd2O3, Pr6O11, UO2 and UO3,56 but the solubility does not significantly exceed that of the pure mineral acids.
A breakthrough has been achieved through the use of task-specific ILs, i.e., ILs with a functional group tailored to a specific purpose. For the dissolution of MOs, Brønsted-acidic groups serve this purpose excellently: their acidic protons form water together with the oxide ions of a MO and the deprotonated corresponding base is a ligand for the metal cations.57 Since the dissolution comes down to an acid–base reaction, especially basic or amphoteric metal oxides can be dissolved in this IL. The complex formation in combination with the evaporation of the reaction water at elevated temperatures drives the dissolution. Sulfonic,58 sulfamic59 and alkylsulfuric60 acid ILs were shown to have outstanding dissolution properties for numerous MOs. A research focus lies on the carboxylic acid IL betainium bis(trifluoromethylsulfonyl)imide ([Hbet][NTf2]) due to several main reasons: first, the cation is easily accessible, as betaine is a natural byproduct of sugar production.61 Second, the hydrophobicity of the IL supports anhydrous reaction conditions.57 Third, the anion is weakly coordinating, making betaine with its carboxylate group the preferred ligand for the metal cations. As the zwitterion betaine has no effective charge, the complexes are cationic, which is important for subsequent electrodeposition of the metal. However, since betaine is a sterically demanding ligand, small molecules, such as water, were found to promote MO dissolution by saturating all coordination sites. Numerous oxides of metals all across the periodic table can be dissolved in aqueous solutions of [Hbet][NTf2],57,62–66 while Al2O3,57,67,68 Fe2O3,67,68 MnO2,57 TiO2,68 cobalt oxides57 and U3O8 (ref. 64) have negligible solubility. These differing solubilities make [Hbet][NTf2] a suitable solvent for the leaching and separation of valuable metals from recycling waste, such as rare-earth metals from bauxite residues,68 yttrium from lamp phosphor waste,69 or neodymium, cobalt and dysprosium from roasted NdFeB magnets.65 Hereby, the leaching efficiencies for specific metals can be tuned by varying the reaction temperature and water content, e.g., allowing for the separation of light from heavy rare earths.70
The dissolution of MOs in aqueous ILs also has some disadvantages. Some metals are difficult to release from stable aqua complexes for downstream chemistry, and the presence of water hinders electrochemical processing. Similar to water, chloride acts as an additional small ligand, promoting the otherwise slow dissolution of MOs in water-free [Hbet][NTf2].71 This was confirmed by comparative dissolution experiments on 30 metal oxides in water-free [Hbet][NTf2] and [Hbet]2[NTf2]Cl, i.e., an equimolar mixture of [Hbet][NTf2] and [Hbet]Cl. Even small amounts of chloride can significantly promote dissolution. Thus, chloride ions can catalyze dissolution but also act as a ligand to the dissolved metal ions.72 In the case of CoO and Co3O4, chloride was even found to be reaction-driving due to the high affinity of cobalt(II) to chloride.73
Metal leaching from MSs, typically, proceeds less readily than that from MOs, because often additional oxidizing agents are required for efficient dissolution through the conversion of sulfide into elemental sulfur or sulfate.74,75 A recent review summarizes numerous examples where metals were leached from different sulfide ores by using aqueous solutions of ILs and common oxidants.34 A non-aqueous approach is the selective extraction of gold(I) and silver(I) from a complex ore by using 1-butyl-3-methyl-imidazolium hydrogensulfate ([BMIm][HSO4]) with additional Fe2(SO4)3 or thiourea as oxidants. After metal stripping by activated carbon, the IL was shown to be reusable at least four times.76 Furthermore, Bi2S3, which requires harsh reaction conditions to be dissolved conventionally, can be dissolved in [BMIm]Cl·4AlCl3 without sulfur oxidation.10,77–80 Also, Sb2S3 readily dissolves in [BMImCl]/4.4AlCl3 (ref. 81) and, to a smaller extent, can also be accessed with the ILs [P66614]Cl or [P66614][OAc].82
Bulk electrodeposition of metals from ILs is not trivial and affected by more factors than the electrochemical window, whereby complex speciation can have a major influence. Even the electrodeposition of the most ignoble metal lithium was reported, but only from neutral and slightly Lewis-basic solutions of 1-ethyl-3-methylimidazolium chloride/AlCl3 ([EMIm]Cl/AlCl3) or 1-butylpyridinium chloride/AlCl3 ([BPy]Cl/AlCl3). In acidic mixtures, aluminum is deposited at a less negative potential than lithium, i.e., the electrochemical window of the IL is smaller. Basic mixtures, on the other hand, provide an excess of chloride, which interferes with lithium reduction by acting as a ligand.85 The influence of complex speciation on the electrodeposition of neodymium from the IL trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)-imide ([P66614][NTf2]) was shown by the addition of small, defined amounts of water. A water content of 0.4 wt% enhanced neodymium reduction by the reorganization of the coordination environment of the metal ion by means of a varied cis/trans coordination ratio, with [NTf2]− rather acting as a monodentate ligand and partial [NTf2]− replacement.86,87
The electrochemical reduction of lithium being possible in IL, however, does not mean that the electrodeposition of nobler metals is straightforward. For example, the bulk electrodeposition of titanium from various ILs remains a challenge, as titanium forms stable intermediates, such as subhalides, precipitating from the IL.88–93
Since the first report about electrodeposition of aluminum from acidic [BPy]Cl/AlCl3,84,94–96 the procedure attracted considerable interest due to the industrial relevance of aluminum coatings. Chloridoaluminate ILs appear to be especially well-suited for this purpose because of their high aluminum concentrations and good conductivities. From these ILs, aluminum is only deposited under Lewis-acidic conditions according to eqn (2) with [Al2Cl7]− being the electro-active species.95
| 4[Al2Cl7]− + 3e− → Al + 7[AlCl4]− | (2) |
Since this type of aluminum electrodeposition typically uses AlCl3 as the starting material, which is produced by the chlorination of aluminum metal,21 this approach can be useful for applying aluminum coatings with specific morphologies, but it is not a reasonable way for aluminum bulk production. Furthermore, the adverse potential gradient causes the transport of the anionic electroactive species [Al2Cl7]− to the negatively charged cathode to be based on diffusion rather than electrolytic migration.97
In contrast to aluminum, the nobler metals copper and cobalt do not rely on air- and water-sensitive chloridoaluminate ILs. Although investigations were performed in these ILs, they were primarily used for mechanistic studies or alloy formation,98 while application-oriented approaches for pure metal synthesis generally make use of the easier handling and experimental set-up of air-stable ILs. Although copper can also be reduced electrochemically in water, ILs can be useful for tuning the morphology of copper coatings.83 Cobalt electrodeposition from aqueous electrolytes involves water electrolysis and the simultaneous deposition of cobalt(II) hydroxide due to an increase in the local pH.99,100 In ILs, cobalt electrodeposition often requires a high overpotential, as negatively charged complexes with anionic ligands, such as chloride, are formed, whose access to the cathode is hindered. Uncharged additives, such as acetone or thiourea were shown to decrease the deposition potential by the formation of cationic cobalt complex species.84,101,102 Although readily soluble cobalt compounds, e.g., CoCl2, are usually used as starting materials for electrodeposition, one study employed CoO. The cobalt oxide was dissolved in a mixture of 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([BMPyrr][NTf2]) and [BMPyrr]Cl with the help of perchloroethane (C2Cl6). However, the disadvantage of this approach is the toxicity and the electrochemical activity of C2Cl6, requiring its removal before electrodeposition.103
Easier to handle electrolytes are solutions of metal oxides in [Hbet][NTf2]. This IL with a cathodic stability up to −2.0 V allows for the electrodeposition of lead,104–106 plutonium,107 copper,106,108 zinc104,106 and cobalt109 at a maximum of 90 °C. In the latter case, the ionometallurgical work-up of LiCoO2 also demonstrates a promising approach for the recycling of spent lithium-ion batteries. As mentioned above, betaine is a zwitterion and thus an uncharged ligand that retains the positive charge of the cation in the complex, which enables its efficient migration to the cathode.
These examples illustrate that electrodeposition from ILs remains complex despite their large electrochemical windows and high conductivity. With regard to the metals and the IL, individual approaches have to be found for various challenges.
Ionometallurgy in deep eutectic solvents
DESs, first reported in 2002,12 are a younger class of materials compared to ILs and, therefore, less investigated. However, in the last few years, a shift of scientific attention from ILs to DESs could be observed. This might be ascribed to the discovery of an increasing number of DESs with cheap and facile syntheses from mostly natural resources, showing excellent solubility for metal oxides. However, it has to be taken into account that DESs rely on hydrogen bonding, which is strongly affected by the dissolution of metal salts, ores or minerals (in high concentrations) and other substances, such as water or impurities. Thus, the (beneficial) properties reported for the pure DES change and might be lost.19,110,111 This effect is less pronounced for ILs.The ionometallurgical production of several metal coatings proceeding from MOs is well-established. Cu2O,112 CuO,113 Ni2O3,114 PbO115–117 and ZnO113,118–122 were dissolved in various DESs before the electrodeposition of the respective metal or an alloy. Furthermore, some dissolution of MnO2,17 NiO,17 MoO3 (ref. 123) and V2O5 (ref. 124) was reported. Among these DESs is the popular and well-investigated representative choline chloride/urea (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, ChCl/2U) that was shown to dissolve MOs via the coordination of urea.17,123,125
2, ChCl/2U) that was shown to dissolve MOs via the coordination of urea.17,123,125
Similar to ILs, DESs that contain Brønsted acids are particularly suitable for the dissolution of MOs. Various combinations of alcohols (e.g., choline chloride, ethylene glycol, propane-1,2-diol, glycerol or butane-1,4-diol) and carboxylic acids (e.g., malonic, citric, maleic, oxalic, phenylpropionic or succinic acid) were investigated.13,126 Among them, the DES ethylene glycol/maleic acid proved suitable for the separation of light rare-earths from the heavy ones.126 Although these results sound promising, MO solubility usually does not exceed the solubility in aqueous hydrochloric acid. This could be due to the low thermal stability of many organic constituents, e.g., urea decomposing at 133 °C,127 limiting the reaction temperatures to a maximum of approximately 100 °C.13,17,112–122,124,126 A better solubility of many MOs was found in the DES choline chloride/p-toluenesulfonic acid in varying molar ratios. Thereby, changing the DES composition affects the solubility of MOs in different ways, providing possibilities for the separation of mixtures.128 This approach was tested for the leaching of neodymium, dysprosium, praseodymium and gadolinium from roasted doped NdFeB magnets. While mixtures of choline chloride and twice the amount of urea or ethylene glycol showed a low performance, positive results were found for the respective DES consisting of choline chloride and lactic acid.129
Extensive investigations regarding the dissolution of metal oxides and the subsequent electrodeposition were also performed with a DES consisting of [Hbet]Cl, urea and glycerol. The ionometallurgical synthesis of metals starting from their oxides was shown for cobalt, copper, zinc, tin, lead, and even small amounts of vanadium. However, the DES consisting of [Hbet]Cl, urea and glycerol (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, [Hbet]Cl/4U/2.5GLY) already decomposes at 60 °C, impeding circular application in ionometallurgy. Specifically ammonia evolution during decomposition can have significance in the solubility of MOs.111
2.5, [Hbet]Cl/4U/2.5GLY) already decomposes at 60 °C, impeding circular application in ionometallurgy. Specifically ammonia evolution during decomposition can have significance in the solubility of MOs.111
This shows that although DESs appear to be suitable solvents at first glance, their stability over the entire chemical process must be taken into account. Decomposition reactions are not only a problem regarding the recycling of the DES (or IL), but toxic byproducts also pose a risk for safe handling and waste management. The higher costs related with this impede industrial application.
In the dissolution of sulfide minerals, high chloride concentrations in DESs were shown to be beneficial for metal leaching by the formation of chloridometallate complexes. Thus, the ionometallurgical processing of chalcopyrite (CuFeS2) was shown in a DES consisting of choline chloride and ethylene glycol in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (ChCl/2 EG). Metal separation was achieved by Fe(OH)3 precipitation by the addition of water and the subsequent recovery of copper metal by electrodeposition or cementation.130
2 (ChCl/2 EG). Metal separation was achieved by Fe(OH)3 precipitation by the addition of water and the subsequent recovery of copper metal by electrodeposition or cementation.130
However, especially for sulfides131–133 but also for other chalcogenides,133–135 electrolytic mineral dissolution appears to be an even more promising reaction route. Thereby, a slurry of the mineral and the DES is typically paint-cast on the anode. Electrolytic dissolution of the mineral proceeds by the oxidation of the chalcogenide anion.131–135 The dissolved metal ions can subsequently be directly reduced at the cathode.131,132,134,135Fig. 5 shows an example set-up of this approach.
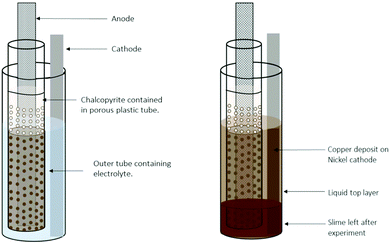 | ||
| Fig. 5 Cell design for copper electrodeposition from a chalcopyrite paint-cast anode. Reproduced from ref. 131, copyright 2019. | ||
DESs are also being considered for the ionometallurgical recycling of elemental metals. In this process, the metals are oxidatively dissolved in DESs and electrolyzed directly. This could be a promising approach for the recycling of printed circuit boards.136,137
On average, the electrochemical windows of DESs are significantly smaller than those of ILs, but are still sufficient to reduce many dissolved metals.138,139 As already mentioned above, metals with relatively high reduction potentials, such as copper, chromium, nickel, lead, silver or zinc, are commonly electrodeposited from various DESs.139 Even the ignoble aluminum is accessible by mixing AlCl3 with urea,140 acetamide141 or amidine salts.142 Although the preparation and handling of these electrolytes seems easier than those of chloridoaluminate ILs, the utilization of hygroscopic AlCl3 still requires inert working conditions, complicating large-scale application.
Synthesis of valuable metal compounds
The previous sections dealt with the electrodeposition of metals, but valuable metal-containing compounds can also be synthesized from solutions of metal cations in ILs or DESs. In recent years, the ionometallurgical application of DESs got increasingly popular in the field of lithium-ion battery recycling. Valuable metal cations were leached from lithium-ion battery cathode materials with high efficiencies using mixtures of choline chloride and urea,143 ethylene glycol,144–146 carboxylic acids147–154 or p-toluenesulfonic acid.155 For the recovery of these metals, typically, precipitation strategies are applied to obtain, e.g., oxalates,145,149,151–154,156 tartrates148 or carbonates.144,150,156 From these precipitates, new lithium-ion battery cathode materials can be produced.145,148,153Another example is the one-step synthesis of anhydrous trichlorides of rare-earth elements (RE = La–Nd, Sm–Dy) starting from the oxides in the IL [BMIm]Cl/3AlCl3 at 175 °C.157 The easy-to-perform process provides a reliable and scalable alternative to the production of these difficult-to-access anhydrous chlorides. In contrast to previous methods, neither high temperatures nor highly toxic substances are involved.158–161
Although ionometallurgy has not yet reached the stage of industrial application, there are already promising applications in other areas. For example, in the course of investigations on the ionometallurgical production of zinc104 from ZnO in [Hbet][NTf2], the obtained solutions were identified as high-performance electrolytes for rechargeable zinc-ion batteries and ion capacitors, which enable energy-storage devices with excellent power and energy densities.106,162,163
Sustainability considerations
Ionometallurgy is expected to require only moderate reaction temperatures and to produce significantly smaller amounts of greenhouse gases or contaminated (solvent) waste compared to conventional pyrometallurgical and hydrometallurgical processes. Furthermore, a reasonable recycling of the ILs or DESs should minimize resource demands. For these reasons, ionometallurgy could be a sustainable alternative to the conventional processing of metal chalcogenides. However, valid statements about energy consumption and general environmental impact will require ongoing research on feasibility and upscaling as well as life cycle assessments.In electrochemical processes, successful large-scale synthesis has been shown to be achievable by two approaches: building larger electrochemical reactors (scaling up) or running more electrochemical reactors in parallel (numbering up). Scaling up usually involves challenges in heat and mass transfer that require an expensive optimization of reactor design on every larger scale.164 Electro-organic syntheses provide several examples of the upscaling of electrochemical reactors; for example, a spinning electrode electrochemical reactor was shown to produce milligrams to multi-hundred grams of the product in batch as well as continuous flow mode.165 However, heat and mass transfer, fluid dynamics and electrode processes are placing limits on ever larger electrochemical reactors. Thus, multiple cells run in parallel are a common alternative approach in industrial electrochemistry. One of the most prominent examples is the Hall–Héroult process, where aluminum is electrodeposited by a molten salt electrolysis. Commonly, numerous baths of a volume of 27 to 72 m3 are run in parallel at one production site.166 An equally important example is the chloralkali process, where NaCl electrolysis runs in multiple parallel cells situated in large cell rooms.167 Especially continuous flow microreactors exploit the advantages of numbering up to its maximum. Besides a low electrode distance that reduces ohmic resistance and electrolyte volume, numbering up of small cells can be implemented quickly since no additional optimization of the reaction parameters is required.168 Although these examples show that numbering up is a common practice in industrial electrochemistry, ionometallurgical processes are expected to involve significant upscaling effort beforehand. Since these processes should yield many tons of metal products, parallel cells are expected to require a larger size by several orders of magnitude compared to current laboratory set-ups. Altogether, industrial processes as well as research literature provide numerous approaches for the design of large-scale electrochemical reactions. This could save significant amounts of exploratory work when it comes to ionometallurgy. However, only little work has been performed with ionic liquids in this context.
Focusing on the applied ILs and DESs, in a relatively naive approach, one could argue that they are green solvents because they are intended to contribute to sustainable processes. This was a common view in the initial decades of IL research. At that time, especially the low volatility was taken into account, reducing safety risks of the inhalation of potentially toxic fumes, flammability or the evaporation of potential greenhouse gases.169 However, the classification of solvents as green is still under debate with different solvent selection guides proposing varying criteria or weighing of criteria.169–171 According to the 12 principles of green chemistry,172 general requirements for ILs and DESs to be considered green solvents were determined to be the renewability of starting materials, an atom-economic synthesis, non-toxicity, high stability, degradability, the safety of handling and a low price.169 Therefore, the greenness of ILs and DESs cannot be generalized but has to be evaluated individually.
For ionometallurgy, the circular application of ILs or DESs is key for the design of an efficient process. Negligible solvent losses throughout the production of large amounts of the product make higher initial investments more tolerable. This also reduces the amount of waste produced, provided that recycling does not generate (much) additional waste. For ILs and DESs to be reusable, close attention has to be paid to their chemical, electrochemical and thermal stability. For the example of [Hbet][NTf2], the long-term stability at 175 °C71 and a low vapor pressure contribute to safe handling under suitable reaction conditions. However, it has been shown that chloride can affect this stability, leading to decomposition products that can, for example, increase toxicity and should therefore be avoided.108,109 Thus, the chloride content has a direct impact on the process in which [Hbet][NTf2] can be used, e.g., the reaction temperature should not exceed 150 °C.109
For some DESs, it became apparent only recently that their stability is significantly lower than anticipated.19 The formation of toxic byproducts compromises the greenness of ILs and DESs due to reduced recyclability as well as safety and waste treatment concerns.128,173 In order to assess stability, the suitability of the analytical methods used should be evaluated more critically, whereby not only the pure DES, but the DES in the specific application including all reactants contained therein should be examined. For example, thermal analysis with low heating rates can obscure the true long-term thermal stability. Investigations under isothermal conditions should therefore be performed as a routine when evaluating the stability of ILs and DESs.111 NMR spectroscopy can be a suitable, fast and convenient method to identify decomposition products. However, it has to be taken into account, that the detection limit is relatively high and that especially the presence of paramagnetic metal ions significantly affects the quality of the spectra.174–176 Mass spectrometry is a suitable addition that allows for the identification of traces of compounds that potentially accumulate during repeated IL or DES usage. This will contribute to a critical assessment of newly developed ILs and DESs from the beginning.
This also implies that the synthesis of ILs and DESs from renewable starting materials should further move to the center of attention. [Hbet][NTf2] is a good example to discuss this. While betaine is an easily accessible biomolecule,61 H[NTf2] must be synthesized from toxic chemicals in a multi-step reaction.177,178 Due to the associated economic challenges and potentially negative environmental impact, the [NTf2]− anion should be replaced by a cheaper, more benign alternative, which is yet to be found.
Conclusions
Based on current literature and our own experience, we have critically discussed the promise of ionometallurgy as an approach for more sustainable metal production. The process-inherent problems of conventional metal production processes show that a disruptive change is urgently needed to mitigate climate change. We are convinced that the seemingly simple idea of low-temperature dissolution of metal oxides or metal sulfides in an IL or DES and subsequent electrodeposition of the respective metal could become such a sustainable technology. Research into this has only been conducted for a few years and has not yet progressed beyond the laboratory scale. A direct comparison with the established large-scale processes that have been optimized over centuries or demands for an immediate solution to all unsolved problems of the new approach are therefore unrealistic and demotivate further development.18 Moreover, the environmental impact of the current processes, which has not yet been priced, must be taken into account for a fair cost comparison.However, these critical remarks make us aware that the technological implementation of ionometallurgy is very complex and that research into its specific aspects should include the most comprehensive knowledge possible of the entire process. For example, investigating the dissolution process for a solvent that is clearly not suitable for the electrodeposition step (e.g., due to chemical or electrochemical instability, too high viscosity, too stable metal complexes, etc.) makes little sense. Also, the IL or DES should not be consumed or degraded but must be reusable either directly or after a reasonable recycling process. Then also more expensive task-specific solvents can be taken into consideration.
According to the current state of scientific knowledge, it can be stated that numerous metal chalcogenides with low reactivity can be activated by dissolving them in ILs and DESs at temperatures below 200 °C. This consumes less energy and releases less CO2 than pyrometallurgical processes and requires less toxic and corrosive chemicals and produces less wastewater than hydrometallurgical processes. These improvements have not yet led to optimal dissolution processes that meet all technical, economic and ecological requirements. The electrodeposition of metals directly from these solutions has been demonstrated for several important metals. However, the electrochemical processes must be made much more efficient, which requires efforts at both the chemical and process engineering level. There are also other useful applications of metal-containing IL and DES solutions, e.g., for the production of valuable chemicals and functional materials or as high-current stable electrolytes in metal-ion batteries and capacitors.
The suitability of ILs or DESs for ionometallurgy should be evaluated rather critically by applying complementary analytical methods. New ILs and DESs probably need to be developed to meet the different process requirements, but also to obtain low toxicity solvents from renewable resources. A suitable IL or DES for ionometallurgy should have at least the following properties:
- Thermal, chemical and electrochemical long-term stability under process conditions.
- Good and preferably selective solubility for the desired metal ores.
- Sufficiently low viscosity.
- Direct reusability or reprocessing at reasonable effort.
- Ideally producible from renewable raw materials.
- Low toxicity and environmental compatibility.
- Reasonable price.
However, continued research and development of ionometallurgy should not fail because there is no perfect solvent at hand, as compromise solutions are acceptable, at least for the time being. Further process steps may also need to be incorporated, such as changing the solvent by extraction from the primary solution. Furthermore, it is not absolutely necessary or useful to start ionometallurgy with a raw ore. Intermediate products that have already been processed, e.g., using traditional methods, may be more suitable. The transition to ionometallurgy for subsequent processing should still have significant ecological advantages. Given the potential benefits of more sustainable metal production, it is worth tackling the challenges associated with ionometallurgy with vigor.
Author contributions
JR: conceptualization, data curation, visualization, writing – original draft, writing – review & editing. MR: conceptualization, funding acquisition, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Matthias Smuda and Maike Oltermann for discussion.Notes and references
- M. Bhar, S. Ghosh, S. Krishnamurthy, Y. Kaliprasad and S. K. Martha, RSC Sustainability, 2023, 1, 1150–1167 RSC.
- International Energy Agency, Aluminium, IEA, Paris, 2022 Search PubMed.
- International Energy Agency, Iron and Steel, IEA, Paris, 2022 Search PubMed.
- International Energy Agency, World Energy Outlook 2022, IEA, Paris, 2022 Search PubMed.
- UN General Assembly, Transforming Our World: The 2030 Agenda for Sustainable Development, 2015 Search PubMed.
- Ionic Liquids in Synthesis, ed. P. Wasserscheid, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- K. Oster, C. Hardacre, J. Jacquemin, A. P. C. Ribeiro and A. Elsinawi, Pure Appl. Chem., 2019, 91, 1309–1340 CrossRef CAS.
- H. K. Timken, H. Luo, B.-K. Chang, E. Carter and M. Cole, in Commercial Applications of Ionic Liquids, ed. M. B. Shiflett, Springer International Publishing, Cham, Switzerland, 2020, pp. 33–47 Search PubMed.
- C. W. Lee, Tetrahedron Lett., 1999, 40, 2461–2464 CrossRef CAS.
- M. Knies, M. F. Groh, T. Pietsch, M. Lê Anh and M. Ruck, ChemistryOpen, 2021, 10, 110–116 CrossRef CAS PubMed.
- P. Wasserscheid and H. Waffenschmidt, J. Mol. Catal. A: Chem., 2000, 164, 61–67 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- A. P. Abbott, G. Frisch, S. J. Gurman, A. R. Hillman, J. Hartley, F. Holyoak and K. S. Ryder, Chem. Commun., 2011, 47, 10031 RSC.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and P. Shikotra, Inorg. Chem., 2005, 44, 6497–6499 CrossRef CAS PubMed.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2023, 9, 423–438 CrossRef.
- J. Afonso, A. Mezzetta, I. M. Marrucho and L. Guazzelli, Green Chem., 2023, 25, 59–105 RSC.
- D. E. Miller and N. J. Van Der Merwe, J. Afr. Hist., 1994, 35, 1–36 CrossRef.
- A. F. Holleman, E. Wiberg, N. Wiberg and G. Fischer, Anorganische Chemie, De Gruyter, Berlin, Germany, 103rd edn, 2017 Search PubMed.
- J. Yang, Y. Yu, T. Ma, C. Zhang, Q. Wang and C. J. Popul, Resour. Environ., 2021, 19, 256–264 Search PubMed.
- M. W. Jones, et al., in CO2 and Greenhouse Gas Emissions, ed. H. Ritchie, M. Roser and P. Rosado, published online at https://OurWorldInData.org, 2023 Search PubMed.
- E. G. Hertwich, Nat. Geosci., 2021, 14, 151–155 CrossRef CAS.
- U. S. Geological Survey, Mineral Commodity Summaries 2023, U.S. Geological Survey, Reston, Virginia, USA, 2023 Search PubMed.
- CRC Handbook of Chemistry and Physics, ed. J. R. Rumble, CRC Press/Taylor and Francis, Boca Raton, Florida, USA, 103rd edn, 2022 (internet version) Search PubMed.
- Kirk-Othmer Encyclopedia of Chemical Technology, ed. K.-Othmer, Wiley-VCH, New York, USA, 1st edn, 2001 Search PubMed.
- M. Azadi, S. A. Northey, S. H. Ali and M. Edraki, Nat. Geosci., 2020, 13, 100–104 CrossRef CAS.
- Bundesanstalt für Geowissenschaften und Rohstoffe, Copper - Sustainability Information, 2020 Search PubMed.
- J. K. Horner, Plat. Surf. Finish., 1997, 84, 16–18 Search PubMed.
- W. H. Dresher, How Hydrometallurgy and the SX/EW Process Made Copper the “Green” Metal, Copper Development Association Inc., 2001 Search PubMed.
- M. Mokmeli and M. T. Parizi, Hydrometallurgy, 2022, 211, 105885 CrossRef CAS.
- P. J. Bartos, Resour. Policy, 2002, 28, 85–94 CrossRef.
- G. Tian and H. Liu, Miner. Process. Extr. Metall. Rev., 2022, 1–24 Search PubMed.
- D. Coutsouradis, A. Davin and M. Lamberigts, Mater. Sci. Eng., 1987, 88, 11–19 CrossRef CAS.
- A. E. Sarnat, J. Dent., 1983, 11, 324–333 CrossRef CAS PubMed.
- J. D. Donaldson and D. Beyersmann, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 1st edn, 2005 Search PubMed.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- H. Beltran, P. Ayuso and E. Pérez, Energies, 2020, 13, 568 CrossRef CAS.
- S. S. Rangarajan, S. P. Sunddararaj, A. Sudhakar, C. K. Shiva, U. Subramaniam, E. R. Collins and T. Senjyu, Clean Technol., 2022, 4, 908–930 CrossRef.
- M. Cordella, F. Alfieri, C. Clemm and A. Berwald, J. Cleaner Prod., 2021, 286, 125388 CrossRef PubMed.
- D. L. Thompson, J. M. Hartley, S. M. Lambert, M. Shiref, G. D. J. Harper, E. Kendrick, P. Anderson, K. S. Ryder, L. Gaines and A. P. Abbott, Green Chem., 2020, 22, 7585–7603 RSC.
- A. Boyden, V. K. Soo and M. Doolan, Procedia CIRP, 2016, 48, 188–193 CrossRef.
- J. Xu, H. R. Thomas, R. W. Francis, K. R. Lum, J. Wang and B. Liang, J. Power Sources, 2008, 177, 512–527 CrossRef CAS.
- T. Georgi-Maschler, B. Friedrich, R. Weyhe, H. Heegn and M. Rutz, J. Power Sources, 2012, 207, 173–182 CrossRef CAS.
- E. Mossali, N. Picone, L. Gentilini, O. Rodrìguez, J. M. Pérez and M. Colledani, J. Environ. Manage., 2020, 264, 110500 CrossRef PubMed.
- I. A. Popescu, S.-A. Dorneanu, R. M. Truta and P. Ilea, Studia UBB Chemia, 2022, 67, 257–280 CrossRef CAS.
- N. E. Idoine, E. R. Raycraft, F. Price, S. F. Hobbs, E. A. Deady, P. Everett, R. A. Shaw, E. J. Evans and A. J. Mills, World Mineral Production 2017-21, British Geological Survey, Nottingham, UK, 2023 Search PubMed.
- W. B. Frank, W. E. Haupin, H. Vogt, M. Bruno, J. Thonstad, R. K. Dawless, H. Kvande and O. A. Taiwo, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 1st edn, 2009 Search PubMed.
- P. Mahjoor and S. E. Latturner, Cryst. Growth Des., 2009, 9, 1385–1389 CrossRef CAS.
- R. C. Bell, A. W. Castleman and D. L. Thorn, Inorg. Chem., 1999, 38, 5709–5715 CrossRef CAS.
- S. Dai, Y. S. Shin, L. M. Toth and C. E. Barnes, Inorg. Chem., 1997, 36, 4900–4902 CrossRef CAS PubMed.
- S. Wellens, N. R. Brooks, B. Thijs, L. V. Meervelt and K. Binnemans, Dalton Trans., 2014, 43, 3443–3452 RSC.
- S. Shah, T. Pietsch, M. A. Herz, F. Jach and M. Ruck, Chemistry, 2023, 5, 1378–1394 CrossRef CAS.
- S. Wellens, T. V. Hoogerstraete, C. Möller, B. Thijs, J. Luyten and K. Binnemans, Hydrometallurgy, 2014, 144–145, 27–33 CrossRef CAS.
- I. Billard, C. Gaillard and C. Hennig, Dalton Trans., 2007, 4214–4221 RSC.
- P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. Van Hecke, L. Van Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110, 20978–20992 CrossRef CAS PubMed.
- D. Dupont, S. Raiguel and K. Binnemans, Chem. Commun., 2015, 51, 9006–9009 RSC.
- D. Dupont, E. Renders, S. Raiguel and K. Binnemans, Chem. Commun., 2016, 52, 7032–7035 RSC.
- D. Dupont, E. Renders and K. Binnemans, Chem. Commun., 2016, 52, 4640–4643 RSC.
- P. Mäkelä, Sugar Tech, 2004, 6, 207–212 CrossRef.
- K. Jayachandran, R. Gupta, K. R. S. Chandrakumar, D. Goswami, D. M. Noronha, S. Paul and S. Kannan, Chem. Commun., 2019, 55, 1474–1477 RSC.
- H.-W. Yeh, Y.-H. Tang and P.-Y. Chen, J. Electrochem. Soc., 2018, 811, 68–77 CAS.
- C. J. Rao, K. A. Venkatesan, K. Nagarajan and T. G. Srinivasan, Radiochim. Acta, 2008, 96, 403–409 CrossRef CAS.
- D. Dupont and K. Binnemans, Green Chem., 2015, 17, 2150–2163 RSC.
- F.-L. Fan, Z. Qin, S.-W. Cao, C.-M. Tan, Q.-G. Huang, D.-S. Chen, J.-R. Wang, X.-J. Yin, C. Xu and X.-G. Feng, Inorg. Chem., 2019, 58, 603–609 CrossRef CAS PubMed.
- P. Davris, D. Marinos, E. Balomenos, A. Alexandri, M. Gregou, D. Panias and I. Paspaliaris, Hydrometallurgy, 2018, 175, 20–27 CrossRef CAS.
- P. Davris, E. Balomenos, D. Panias and I. Paspaliaris, in Light Metals 2018, ed. O. Martin, Springer International Publishing, Cham, Switzerland, 2018, pp. 149–156 Search PubMed.
- D. Dupont and K. Binnemans, Green Chem., 2015, 17, 856–868 RSC.
- N. Schaeffer, S. Grimes and C. Cheeseman, Inorg. Chim. Acta, 2016, 439, 55–60 CrossRef CAS.
- M. Orefice, K. Binnemans and T. V. Hoogerstraete, RSC Adv., 2018, 8, 9299–9310 RSC.
- J. Richter, M. Knies and M. Ruck, ChemistryOpen, 2021, 10, 97–109 CrossRef CAS PubMed.
- J. Richter, T. Pietsch and M. Ruck, ChemSusChem, 2023, 16, e202300090 CrossRef CAS PubMed.
- J. E. Dutrizac, Metall. Trans. B, 1978, 9, 431–439 CrossRef.
- R. P. Hackl, D. B. Dreisinger, E. Peters and J. A. King, Hydrometallurgy, 1995, 39, 25–48 CrossRef CAS.
- J. A. Whitehead, G. A. Lawrance and A. McCluskey, Green Chem., 2004, 6, 313–315 RSC.
- M. Knies and M. Ruck, ChemistryOpen, 2022, 11, e202100145 CrossRef CAS PubMed.
- M. Knies, P. Nawroth, P. Golub, A. Isaeva and M. Ruck, Z. Anorg. Allg. Chem., 2021, 647, 2055–2060 CrossRef CAS.
- M. F. Groh, M. Knies, A. Isaeva and M. Ruck, Z. Anorg. Allg. Chem., 2015, 641, 279–284 CrossRef CAS.
- M. F. Groh, A. Isaeva, U. Müller, P. Gebauer, M. Knies and M. Ruck, Eur. J. Inorg. Chem., 2016, 2016, 880–889 CrossRef CAS.
- M. A. Grasser, K. Finzel and M. Ruck, Z. Anorg. Allg. Chem., 2022, 648, e202200021 CrossRef CAS.
- M. A. Grasser, T. Pietsch, E. Brunner and M. Ruck, Dalton Trans., 2022, 51, 4079–4086 RSC.
- Electrodeposition from Ionic Liquids, ed. F. Endres, A. Abbott and D. MacFarlane, Wiley-VCH, Weinheim, Germany, 2017 Search PubMed.
- J. G. dos, R. da Costa, J. M. Costa and A. F. de Almeida Neto, Metals, 2022, 12, 2095 CrossRef.
- M. Lipsztajn and R. A. Osteryoung, Inorg. Chem., 1985, 24, 716–719 CrossRef CAS.
- L. Sanchez-Cupido, J. M. Pringle, A. L. Siriwardana, A. Unzurrunzaga, M. Hilder, M. Forsyth and C. Pozo-Gonzalo, J. Phys. Chem. Lett., 2019, 10, 289–294 CrossRef CAS PubMed.
- G. S. Dobhal, L. N. Pham, S. A. Tawfik, C. Pozo-Gonzalo and T. R. Walsh, ACS Sustainable Chem. Eng., 2023, 11, 14614–14621 CrossRef CAS.
- F. Endres, Chem. Ing. Tech., 2011, 83, 1485–1492 CrossRef CAS.
- X.-Y. Zhang, Y.-X. Hua, C.-Y. Xu, Q.-B. Zhang, X.-B. Cong and N. Xu, Electrochim. Acta, 2011, 56, 8530–8533 CrossRef CAS.
- F. Endres, S. Z. E. Abedin, A. Y. Saad, E. M. Moustafa, N. Borissenko, W. E. Price, G. G. Wallace, D. R. MacFarlane, P. J. Newman and A. Bund, Phys. Chem. Chem. Phys., 2008, 10, 2189–2199 RSC.
- C. A. Berger, M. Arkhipova, A. Farkas, G. Maas and T. Jacob, Phys. Chem. Chem. Phys., 2016, 18, 4961–4965 RSC.
- Y. Andriyko, U. Fastner, H. Kronberger and G. E. Nauer, Z. Naturforsch., A: Phys. Sci., 2007, 62, 529–539 CrossRef CAS.
- Y. Andriyko and G. E. Nauer, Electrochim. Acta, 2007, 53, 957–962 CrossRef CAS.
- J. Robinson and R. A. Osteryoung, J. Electrochem. Soc., 1980, 127, 122–128 CrossRef CAS.
- G. Tian, in Materials Research Foundations, Materials Research Forum LLC, 1st edn, 2019, vol. 54, pp. 249–293 Search PubMed.
- K. K. Maniam and S. Paul, Coatings, 2021, 11, 80 CrossRef CAS.
- C. Wagner, J. Electrochem. Soc., 1954, 101, 181–184 CrossRef CAS.
- F. Endres, ChemPhysChem, 2002, 3, 144–154 CrossRef CAS.
- S. Banbur-Pawlowska, K. Mech, R. Kowalik and P. Zabinski, Appl. Surf. Sci., 2016, 388, 805–808 CrossRef CAS.
- P. Sebastián, V. Climent, J. M. Feliu and E. Gómez, in Encyclopedia of Interfacial Chemistry, Elsevier, 2018, pp. 690–700 Search PubMed.
- K. Motobayashi, Y. Shibamura and K. Ikeda, J. Phys. Chem. Lett., 2020, 11, 8697–8702 CrossRef CAS PubMed.
- L. A. da S. Ries, H. A. de Brito, F. P. Gasparin and I. L. Muller, J. Mol. Liq., 2021, 325, 114787 CrossRef CAS.
- H.-W. Yeh, N. Serizawa and Y. Katayama, J. Electrochem. Soc., 2021, 168, 082502 CrossRef CAS.
- P. Chen, J. Richter, G. Wang, D. Li, T. Pietsch and M. Ruck, Small, 2021, 17, 2102058 CrossRef CAS PubMed.
- H.-W. Yeh, Y.-H. Tang and P.-Y. Chen, J. Electrochem. Soc., 2018, 811, 68–77 CAS.
- P. Chen, X. Wang, D. Li, T. Pietsch and M. Ruck, ChemSusChem, 2022, 15, e202200039 CrossRef CAS PubMed.
- K. Jayachandran, R. Gupta, K. R. S. Chandrakumar, D. Goswami, D. M. Noronha, S. Paul and S. Kannan, Chem. Commun., 2019, 55, 1474–1477 RSC.
- J. Richter, M. Knies and M. Ruck, ChemistryOpen, 2021, 10, 97–109 CrossRef CAS PubMed.
- J. Richter, T. Pietsch and M. Ruck, ChemSusChem, 2023, e202300090 CrossRef CAS PubMed.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed.
- J. Richter, T. Pietsch, N. Elsner and M. Ruck, ChemistryOpen, 2023, 12, e202300114 CrossRef CAS PubMed.
- T. Tsuda, L. E. Boyd, S. Kuwabata and C. L. Hussey, J. Electrochem. Soc., 2010, 157, F96–F103 CrossRef CAS.
- X. Xie, X. Zou, X. Lu, Q. Xu, C. Lu, C. Chen and Z. Zhou, J. Appl. Electrochem., 2017, 47, 679–689 CrossRef CAS.
- P. Huang and Y. Zhang, Int. J. Electrochem. Sci., 2018, 13, 10798–10808 CrossRef CAS.
- J. Ru, Y. Hua, C. Xu, J. Li, Y. Li, D. Wang, C. Qi and Y. Jie, Appl. Surf. Sci., 2015, 335, 153–159 CrossRef CAS.
- J. Ru, Y. Hua, C. Xu, J. Li, Y. Li, D. Wang, C. Qi and K. Gong, Russ. J. Electrochem., 2015, 51, 773–781 CrossRef CAS.
- H. Yang and R. G. Reddy, J. Electrochem. Soc., 2014, 161, D586–D592 CrossRef CAS.
- H. Yang and R. G. Reddy, Electrochim. Acta, 2015, 178, 617–623 CrossRef CAS.
- H. Yang and R. G. Reddy, Electrochim. Acta, 2014, 147, 513–519 CrossRef CAS.
- A. Liu, Z. Shi and R. G. Reddy, J. Electrochem. Soc., 2017, 164, D666–D673 CrossRef CAS.
- Y. Zheng, K. Dong, Q. Wang, S. Zhang, Q. Zhang and X. Lu, Sci. China: Chem., 2012, 55, 1587–1597 CrossRef CAS.
- W. He, L. Shen, Z. Shi, B. Gao, X. Hu, J. Xu and Z. Wang, Electrochem., 2016, 84, 872–877 CrossRef CAS.
- S. M. Shuwa, R. S. Al-Hajri, B. Y. Jibril and Y. M. Al-Waheibi, Ind. Eng. Chem. Res., 2015, 54, 3589–3601 CrossRef CAS.
- P. Billik, P. Antal and R. Gyepes, Inorg. Chem., 2015, 60, 37–40 CAS.
- J. M. Rimsza and L. R. Corrales, Comput. Theor. Chem., 2012, 987, 57–61 CrossRef CAS.
- W. Chen, J. Jiang, X. Lan, X. Zhao, H. Mou and T. Mu, Green Chem., 2019, 17, 4748–4756 RSC.
- RÖMPP-Redaktion, F. Geldsetzer and Harnstoff, in RD-08-00427, ed. F. Böckler, B. Dill, G. Eisenbrand, F. Faupel, B. Fugmann, T. Gamse, P. Heretsch, R. Matissek, G. Pohnert, A. Rühling, S. Schmidt, G. Sprenger, RÖMPP, Georg Thieme Verlag, Stuttgart, 2019, https://roempp.thieme.de/lexicon/RD-08-00427 Search PubMed.
- N. R. Rodriguez, A. van den Bruinhorst, L. J. B. M. Kollau, M. C. Kroon and K. Binnemans, ACS Sustainable Chem. Eng., 2019, 7, 11521–11528 CrossRef.
- S. Riaño, M. Petranikova, B. Onghena, T. V. Hoogerstraete, D. Banerjee, M. R. S. Foreman, C. Ekberg and K. Binnemans, RSC Adv., 2017, 7, 32100–32113 RSC.
- C. Carlesi, R. C. Harris, A. P. Abbott and G. R. T. Jenkin, Minerals, 2022, 12, 65 CrossRef CAS.
- S. Anggara, F. Bevan, R. C. Harris, J. M. Hartley, G. Frisch, G. R. T. Jenkin and A. P. Abbott, Green Chem., 2019, 21, 6502–6512 RSC.
- W. Zhang, J. Ru, Y. Hua and X. Geng, J. Electrochem. Soc., 2021, 168, 082505 CrossRef CAS.
- J. M. Hartley, A. Z. M. Al-Bassam, R. C. Harris, G. Frisch, G. R. T. Jenkin and A. P. Abbott, Hydrometallurgy, 2020, 198, 105511 CrossRef CAS.
- F. Bevan, H. Galeb, A. Black, I. M. Pateli, J. Allen, M. Perez, J. Feldmann, R. Harris, G. Jenkin, A. Abbott and J. Hartley, ACS Sustainable Chem. Eng., 2021, 9, 2929–2936 CrossRef CAS.
- I. M. Pateli, A. P. Abbott, G. R. T. Jenkin and J. M. Hartley, Green Chem., 2020, 22, 8360–8368 RSC.
- J. M. Hartley, S. Scott, Z. Dilruba, A. J. Lucio, P. J. Bird, R. C. Harris, G. R. T. Jenkin and A. P. Abbott, Phys. Chem. Chem. Phys., 2022, 24, 24105–24115 RSC.
- A. P. Abbott, R. C. Harris, F. Holyoak, G. Frisch, J. Hartley and G. R. T. Jenkin, Green Chem., 2015, 17, 2172–2179 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- R. Bernasconi, G. Panzeri, A. Accogli, F. Liberale, L. Nobili and L. Magagnin, in Progress and Developments in Ionic Liquids, ed. S. Handy, InTech, 2017 Search PubMed.
- A. P. Abbott, R. C. Harris, Y.-T. Hsieh, K. S. Ryder and I.-W. Sun, Phys. Chem. Chem. Phys., 2014, 16, 14675–14681 RSC.
- H. M. A. Abood, A. P. Abbott, A. D. Ballantyne and K. S. Ryder, Chem. Commun., 2011, 47, 3523 RSC.
- A. J. Lucio, I. Efimov, O. N. Efimov, C. J. Zaleski, S. Viles, B. B. Ignatiuk, A. P. Abbott, A. R. Hillman and K. S. Ryder, Chem. Commun., 2021, 57, 9834–9837 RSC.
- S. Wang, Z. Zhang, Z. Lu and Z. Xu, Green Chem., 2020, 22, 4473–4482 RSC.
- M. K. Tran, M.-T. F. Rodrigues, K. Kato, G. Babu and P. M. Ajayan, Nat. Energy, 2019, 4, 339–345 CrossRef CAS.
- P. G. Schiavi, P. Altimari, M. Branchi, R. Zanoni, G. Simonetti, M. A. Navarra and F. Pagnanelli, Chem. Eng. J., 2021, 417, 129249 CrossRef CAS.
- K. Wang, T. Hu, P. Shi, Y. Min, J. Wu and Q. Xu, ACS Sustainable Chem. Eng., 2022, 10, 1149–1159 CrossRef CAS.
- X. Chang, M. Fan, C. Gu, W. He, Q. Meng, L. Wan and Y. Guo, Angew. Chem., Int. Ed., 2022, 61, e202202558 CrossRef CAS PubMed.
- C. Ma, M. Svärd and K. Forsberg, Resour. Conserv. Recycl., 2022, 186, 106579 CrossRef CAS.
- Q. Lu, L. Chen, X. Li, Y. Chao, J. Sun, H. Ji and W. Zhu, ACS Sustainable Chem. Eng., 2021, 9, 13851–13861 CrossRef CAS.
- L. Chen, Y. Chao, X. Li, G. Zhou, Q. Lu, M. Hua, H. Li, X. Ni, P. Wu and W. Zhu, Green Chem., 2021, 23, 2177–2184 RSC.
- R. Morina, D. Callegari, D. Merli, G. Alberti, P. Mustarelli and E. Quartarone, ChemSusChem, 2022, 15, e202102080 CrossRef CAS PubMed.
- D. L. Thompson, I. M. Pateli, C. Lei, A. Jarvis, A. P. Abbott and J. M. Hartley, Green Chem., 2022, 24, 4877–4886 RSC.
- T. Li, Y. Xiong, X. Yan, T. Hu, S. Jing, Z. Wang and X. Ge, J. Energy Chem., 2022, 72, 532–538 CrossRef CAS.
- N. Peeters, K. Binnemans and S. Riaño, Green Chem., 2020, 22, 4210–4221 RSC.
- M. J. Roldán-Ruiz, M. L. Ferrer, M. C. Gutiérrez and F. del Monte, ACS Sustainable Chem. Eng., 2020, 8, 5437–5445 CrossRef.
- T. Hanada and M. Goto, Green Chem., 2022, 24, 5107–5115 RSC.
- S. Shah, T. Pietsch and M. Ruck, Angew. Chem., Int. Ed., 2023, e202317480 Search PubMed.
- H. Chen, P. Yang, C. Zhou, C. Jiang and J. Pan, Cryst. Growth Des., 2006, 6, 809–811 CrossRef CAS.
- G. Meyer, Prog. Solid State Chem., 1982, 14, 141–219 CrossRef CAS.
- N. H. Kiess, J. Res. Natl. Bur. Stand., Sect. A, 1963, 67A, 343–345 CrossRef PubMed.
- J. H. Freeman and M. L. Smith, J. Inorg. Nucl. Chem., 1958, 7, 224–227 CrossRef CAS.
- P. Chen, X. Sun, T. Pietsch, B. Plietker, E. Brunner and M. Ruck, Adv. Mater., 2023, 35, 2207131 CrossRef CAS PubMed.
- P. Chen, X. Sun, T. Pietsch, B. Plietker, E. Brunner and M. Ruck, Adv. Mater., 2023, 35, 2207131 CrossRef CAS PubMed.
- K. Lovato, P. S. Fier and K. M. Maloney, Nat. Rev. Chem, 2021, 5, 546–563 CrossRef CAS PubMed.
- N. Petrović, B. K. Malviya, C. O. Kappe and D. Cantillo, Org. Process Res. Dev., 2023, 27, 2072–2081 CrossRef.
- W. B. Frank, W. E. Haupin, H. Vogt, M. Bruno, J. Thonstad, R. K. Dawless, H. Kvande and O. A. Taiwo, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 1st edn, 2009 Search PubMed.
- P. Schmittinger, T. Florkiewicz, L. C. Curlin, B. Lüke, R. Scannell, T. Navin, E. Zelfel and R. Bartsch, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 1st edn, 2011 Search PubMed.
- G. Laudadio, W. De Smet, L. Struik, Y. Cao and T. Noël, J. Flow Chem., 2018, 8, 157–165 CrossRef PubMed.
- Y. Chen and T. Mu, Green Chem. Eng., 2021, 2, 174–186 CrossRef.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. R. McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- N. Peeters, K. Janssens, D. de Vos, K. Binnemans and S. Riaño, Green Chem., 2022, 24, 6685–6695 RSC.
- A.-S. Felten, N. Pellegrini-Moïse, K. Selmeczi, B. Henry and Y. Chapleur, Eur. J. Org Chem., 2013, 2013, 5645–5654 CrossRef CAS.
- R. Febrian, J. P. Roddy, C. H. Chang, C. T. Devall and P. J. Bracher, ACS Omega, 2021, 6, 14727–14733 CrossRef CAS PubMed.
- P. Faller, C. Hureau, P. Dorlet, P. Hellwig, Y. Coppel, F. Collin and B. Alies, Coord. Chem. Rev., 2012, 256, 2381–2396 CrossRef CAS.
- J. Foropoulos and D. D. DesMarteau, Inorg. Chem., 1984, 23, 3720–3723 CrossRef CAS.
- W. Zhao and J. Sun, Chem. Rev., 2018, 118, 10349–10392 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |