Open Access Article
Open Access ArticleMycobacterium smegmatis acyltransferase catalyzes the synthesis of esters and polyesters†
Filippo
Fabbri
 ab,
Ilaria
Vergani
ab,
Ilaria
Vergani
 a,
Silvia
Donoso
a,
Silvia
Donoso
 c,
Luca
Nespoli
c,
Luca
Nespoli
 d,
Valeria Marisa
Rocca
d,
Valeria Marisa
Rocca
 c,
Lisa
Moni
c,
Lisa
Moni
 c,
Georg M.
Guebitz
c,
Georg M.
Guebitz
 ab,
Martina Letizia
Contente
ab,
Martina Letizia
Contente
 d and
Alessandro
Pellis
d and
Alessandro
Pellis
 *c
*c
aUniversity of Natural Resources and Life Sciences Vienna, Department of Agrobiotechnology IFA-Tulln, Institute of Environmental Biotechnology, Konrad Lorenz Strasse 20, 3430, Tulln an der Donau, Austria
bAustrian Centre of Industrial Biotechnology, Konrad Lorenz Strasse 20, 3430, Tulln an der Donau, Austria
cUniversity of Genova, Department of Chemistry and Industrial Chemistry, Via Dodecaneso 31, 16146, Genova, Italy. E-mail: alessandro.pellis@unige.it
dUniversity of Milan, Department of Food, Environmental and Nutritional Sciences (DeFENS), Via Celoria 2, 20133, Milan, Italy
First published on 15th April 2024
Abstract
In the present work, Mycobacterium smegmatis acyltransferase (MsAcT) was successfully immobilized onto polypropylene beads and the selectivity was investigated both in esterification and polycondensation reactions. All the syntheses were carried out under solventless conditions at room temperature to better comply with today's green chemistry principles. Therefore, ester synthesis was performed according to the planned full-factorial design of experiments (DoE) investigation to study MsAcT selectivity towards ester functional groups (vinyl, ethyl, and methyl), alcohol carbon chain length (C4, C8, C12) and ester carbon chain length (C2, C4, C6). The results clearly showed MsAcT selectivity towards vinyl esters (vinyl- > ethyl- > methyl-) and short-chain compounds (C2 esters and C4 alcohol). Moreover, to confirm the obtained DoE model in ester synthesis, the immobilized MsAcT formulation was used to perform polycondensation reactions using bio-based diesters and diols. Higher conversion rates were obtained using ethylene glycol when compared to 1,4-butanediol in polyester synthesis with divinyl adipate as the diesters and at different time-lengths of applied vacuum (20 mbar). This resulted in agreement with the selectivity of MsAcT in esterification reactions. Furthermore, divinyl succinate (DVS) was used in polycondensations and all the resulting oligomers were analyzed via GPC and LC-MS.
Sustainability spotlightThe need for greener sustainable technologies to produce both small molecules and polymers highlighted once again how biocatalysts can play a pivotal role in humanity's fight against climate change. In this work, the acyltransferase from Mycobacterium smegmatis was for the first time used to prepare short esters (that could be used as additives or flavoring ingredients) and short oligoesters from biomass-derived monomers using a solventless synthetic approach. The work relates to the UN sustainability goal numbers 4, 9, 12 and 13. |
1 Introduction
Since the very first in vitro enzymatically catalyzed polyester synthesis carried out originally in 1984 by Okumura et al.1 and then in 1993 by two independent groups,2,3 lipases, and in general α/β hydrolases, have always been representing the most sought-after choice for polycondensation and ring-opening polymerization (ROP) reactions.4 Furthermore, the same superfamily of enzymes, which includes proteases and esterases, have been widely investigated in the past decades and found exceptional for the synthesis of different short-esters and flavor esters.5–7Recently, a new and promising acyltransferase from Mycobacterium smegmatis (MsAcT) has been fully characterized,8 attracting a lot of interest for its outstanding activity in an extensive range of reactions, shedding light on the possibility of becoming the next big player in the biotransformation scenario.9
This enzyme possesses a catalytic triad (Ser11, Asp192 and His195) common to the α/β hydrolase superfamily, but has unusual architecture that allows its two-step catalytic mechanism to take place in water. In fact, MsAcT, unlike many cofactor-dependent acyltransferases, is able to hydrolyze both the acyl donor and the final product therefore favoring the condensation reaction over the hydrolysis.10
Many researchers have exploited this extraordinary feature exploring methods to synthesize short esters,11N-acylation of amines and trans-amidation reaction12 utilizing both batch11,13 and flow systems.14 Moreover, several immobilization strategies such as those on acid-functionalized multiwalled carbon nanotubes,15 single-walled carbon nanotubes,16 and on activated glyoxyl agarose14 were also developed to further enhance MsAcT's stability and reusability.
Interestingly, many groups have recently aimed their effort at engineered MsAcT with different purposes: among others, Finnveden and co-workers17 designed single point (L12A) and double point (T93A/F154A) mutants to expand the acyl donor specificity, enabling longer substrates to accommodate the active site; first Godehard et al.18 and then Jost et al.19 successfully created libraries of single and double variants with different specificities and selectivities, obtaining biocatalysts with increased acyl transferase to hydrolysis ratio. Moreover, Contente and colleagues20 reported a strategic single point mutation (S11C) in the catalytic triad able to extend the enzyme activity towards a wider set of substrates, enabling the acceptance of thiols and secondary amines (while vinyl ester was employed as the acylating agent).
In this work, the goal was to investigate transesterification reactions catalyzed by MsAcT in the absence of water based on a design of experiments (DoE). This strategy was led by the fact that most organic compounds employed for industrial applications are not water soluble and therefore some alternative enzymatic synthetic strategies might be of interest to produce more hydrophobic structures. Furthermore, the possibility of performing synthesis at room temperature and in bulk (i.e., solventless) fulfils two of today's green chemistry principles21 (number 5 and 6) regarding a more sustainable way of catalyzing condensation reactions, hence reducing the process' environmental impact by lowering energy consumption and avoiding the use of common petrol-based solvents such as hexane or toluene.22
To the best of our knowledge, this work represents the first study where MsAcT was investigated related to the synthesis of short oligoesters. As already pointed out by Cannazza et al.,9 in the past, only a few efforts were made in using this biocatalyst for the biotransformation/synthesis of macromolecules. The study by Finnveden et al.17 probably represents the only available example in the literature connecting MsAcT to polymer biotechnology since it reports a method for the selective mono-substitution of symmetric dicarboxylic esters (divinyl adipate), having as a scope the possibility of producing multifunctional vinyl ester monomers.
2 Results and discussion
2.1 MsAcT immobilization and characterization
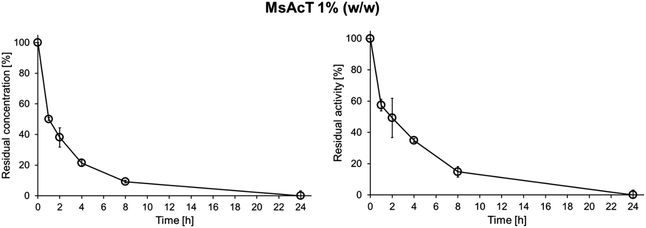
MsAcT was immobilized onto the Accurel MP 1000 polypropylene beads following an already established protocol (see the ESI, Section 2.3†). The final immobilized formulation consisted of 1% w/w of the biocatalyst on the amount of solid support, according to the following formula:Therefore, the enzyme derived from the purification was diluted to a concentration of 0.286 mgMsAcT mL−1 in 0.1 M NaH2PO4/Na2HPO4 buffer having a pH of 8.0 (in a total volume of 35 mL). The enzyme dilution was stirred for 24 h at 25 °C using a blood rotator set at 20 rpm with 1 g of the activated Accurel MP 1000 to ensure proper adsorption of the acyltransferase on the hydrophobic polypropylene beads. The results of the immobilization, given as residual concentration and activity (%), were calculated on the supernatant by dividing every timepoint by the starting point of the immobilization procedure (0 h timepoint). The outcomes are shown in Fig. 1 (≥99% bound protein, ≥99% lost activity of the supernatant).
Already after 8 h of MsAcT immobilization, less than 10% of residual protein concentration was detected in the supernatant, meaning that >90% of the enzyme was successfully adsorbed onto the solid support. Almost the same percentage was found for the residual activity, with around 15% of activity left, confirming the 85–90% enzyme immobilization rate after 8 h. Finally, after 24 h, the percentage of immobilized biocatalyst increased to ≥99%, according to both the protein concentration and enzyme activity, indicating that all the acyltransferase was properly adsorbed onto the polypropylene beads. This result confirms once more MsAcT's ease of immobilization and versatility, since from its first immobilization in 2010 on carbon nanotubes,15,16 it has been successfully immobilized onto various solid supports by many different groups.14,23 Moreover, the immobilization profile of MsAcT 1% w/w aligns very well with other 1% immobilization formulations on propylene beads (Accurel MP 1000) reported by the Pellis' group,24,25 where most of the tested lipases and cutinases appeared to be >90% adsorbed onto the solid support after 8 h, while the complete immobilization was achieved after 24 h. These data suggest an enzyme immobilization rate on polypropylene beads that seems to be independent of their hydrophobicity (Gravy index of MsAcT +0.147 and CaLB +0.037 polar, HiC −0.034 non-polar) and of their molecular weight (CaLB 33 kDa, HiC 22 kDa, MsAcT 23 kDa). The obtained MsAcT formulation was further tested for ester and polyester synthesis.
2.2 Enzymatic synthesis of esters
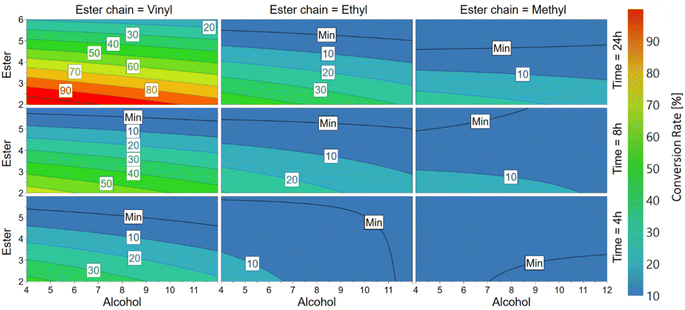
Upon immobilization, the MsAcT preparation was used to carry out the full factorial DoE (detailed in the ESI, Table S1†) with the aim of elucidating the selectivity of the enzyme related to: (1) the ester functional group (vinyl, ethyl, or methyl), (2) the ester chain length (from C2 to C6) and (3) the alcohol chain length (from C4 to C12). The optimal temperature for this enzyme was not further investigated in the DoE study, since the peculiarity of being active at room temperature (25 °C) was already reported by several groups for transesterification or transacylation reactions on small molecules.8,26,27 Moreover, the possibility of performing such reactions in bulk and at low operational temperatures fully aligns with the green chemistry principles of a more sustainable way of catalyzing condensation reactions, both in terms of energy consumption and avoidance of toxic solvents. Therefore, the reactions were carried out for 24 h at 25 °C and 400 rpm in bulk, with timepoint withdrawals at 4, 8 and 24 h. The response measured for every experiment was the conversion (%) of the ester (calculated by GC-FID, see ESI Section 2.9†). After performing each reaction from the DoE, the data were elaborated into a 4D contour response model, where the factors are shown in the axis while the response is represented by different colors in the plot (Fig. 2).Quite expectedly, regarding the preferred ester functional group, MsAcT showed high conversion when a vinyl ester was used, leading to conversions up to 4-times higher when compared to the ethyl ester (100% vs. 24% for the reaction of vinyl acetate and ethyl acetate with 1-octanol after 24 h, respectively). The advantage of using vinyl esters was already reported by several authors that observed enhanced enantioselectivities and reaction rates when using the lipase B from Candida antarctica (in apolar solvents such as n-hexane and toluene) for catalyzing the resolution of aryl aliphatic carboxylic acids.28 Moreover, de Leeuw et al.29 observed higher conversion rates for MsAcT in the synthesis of aromatic cyanohydrins using vinyl acetate rather than ethyl acetate as the acyl donor, confirming the reactivity profile. On the other hand, the enzyme activity appeared to be very limited towards methyl esters, showing the best conversion with methyl acetate and 1-butanol (25% after 24 h reaction). These results align well with the literature, since in the work performed by Finnveden et al.,17 only very limited (<4%) conversion yields of methyl esters with 1-octanol catalyzed by wild-type MsAcT were observed after 6 h of reaction time.
Regarding the selectivity towards monomers with various carbon chain lengths, MsAcT showed a higher conversion rate towards short-chain alcohols and esters. This can be easily observed in the top part of the 4D-contour plot, where the highest conversions were obtained for all the different ester chains with the smallest carbon compound (acetate, C2). Furthermore, regarding alcohols, the conversions for vinyl acetate and 1-butanol, 1-octanol and 1-dodecanol after 8 h reaction clearly showed the enzyme selectivity towards short-chained alcohols, since 77%, 49% and 47% conversions were obtained, respectively. The same preference pattern of 1-butanol > 1-octanol > 1-dodecanol was maintained in the conversions after 8 h both with ethyl acetate (46%, 17%, 16%) and methyl acetate (22%, 15%, 14%), confirming the enzyme's preference towards short-chained alcohols and esters. The same trend was observed once more by Finnveden et al.,17 where the highest conversion for MsAcT wild-type in divinyl adipate transacylation reactions was reported with butanediol vinyl ether (C4) as the acyl acceptor, reaching around 80% of DVA conversion after 28 h, while a lower conversion of around 60% was obtained with 1-octanol (C8), confirming this enzyme selectivity towards small chain compounds. Notably, in all the reactions carried out with a C6 ester (namely vinyl, ethyl or methyl hexanoate), no conversion was observed after 24 h, except for vinyl hexanoate and 1-butanol where only a meagre 5% was obtained, highlighting once more MsAcT poor reactivity with long chain esters. These results are in good agreement with the work reported by Hendil-Forssell,30 who observed that MsAcT wild-type is able to accommodate esters only up to C4 as the acyl donors in its active site and by Godehard et al.,18 who detected no MsAcT wild-type activity using p-nitrophenyl hexanoate as the acyl donor with 10 mM benzyl alcohol as the acyl acceptor.
In this full-factorial design investigation, our model showed that MsAcT expressed the highest conversion using vinyl acetate (C2) and 1-butanol (C4), therefore leading to the conclusion that this biocatalyst selectivity is towards vinyl esters (vinyl > ethyl > methyl) and short-chained alcohols and esters (C2 > C4 > C6 for esters, C4 > C8 > C12 for alcohols), while poor results were achieved using long-chain compounds (no conversion observed with almost all C6 esters).
2.3 Polyester synthesis
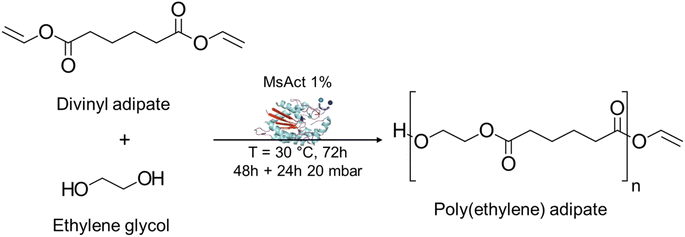
To verify and confirm the previously obtained MsAcT 4D contour model in the short-ester synthesis, polycondensation reactions were carried out using various bio-based monomers as the building blocks. Divinyl adipate was adopted as the model diester as it is commercially available, and a first screening was carried out to investigate MsAcT selectivity towards diols. The immobilized enzyme (1% w/w MsAcT per beads) concentration in the reaction mixture was first tested at 10% (w/w) on the total weight of monomers but very limited conversions were obtained (see ESI Table S3†). Therefore, a higher concentration of 30% was adopted for the polyester synthesis. To elucidate whether MsAcT accommodates better short-chained diols or not, polycondensations of divinyl adipate with ethylene glycol (EG, C2) and 1,4-butanediol (BDO, C4) were carried out at 30 °C, as it is shown in Table 1 (entries 1 and 5). Moreover, different time-lengths of applied vacuum (20 mbar) were used to investigate the best reaction conditions for MsAcT in polyester synthesis (Fig. 3).| N° | Diol | Reaction time | Vacuum | M n | M w | Đa | M o | DPc | Conv.b [%] |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated via GPC calibrated with low molecular weight polystyrene standards 250–70000 Da. b Calculated via1H-NMR by comparing the ratio between the signal methylene groups adjacent to –OH of EG/BDO and the methylene groups of DVA (assumed as constant). c Degree of polymerization (DP) = Mn/molecular weight of the repeating unit (Mo). | |||||||||
| 1 | EG | 72 h | — | 500 | 500 | 1.01 | 172.2 | 2.93 | 48 |
| 2 | 24 h | 500 | 550 | 1.01 | 3.02 | 57 | |||
| 3 | 48 h | 400 | 400 | 1.04 | 2.18 | 15 | |||
| 4 | 168 h | — | 400 | 450 | 1.08 | 2.18 | 58 | ||
| 5 | BDO | 72 h | — | 500 | 500 | 1.01 | 200.2 | 2.59 | 27 |
| 6 | 24 h | 500 | 550 | 1.18 | 2.41 | 42 | |||
| 7 | 48 h | 400 | 400 | 1.11 | 1.84 | 14 | |||
The conversion of the diol was higher in polycondensations with DVA with EG than for BDO (48% and 27%, respectively) after 72 h and without applying a vacuum (reactions 1 and 5). The same trend was observed in all the other reactions with the same vacuum settings: with 24 h of applied vacuum, 57% and 42% conversions for EG and BDO were observed, respectively (Mw = 550 Da for both entries 2 and 6), while after 48 h vacuum, meagre values of 15% and 14% were achieved for EG and BDO (Mw = 400 Da for both entries 3 and 7), confirming MsAcT selectivity towards short-chained compounds. The molecular masses of the resulting polymers partially confirmed the trend, since the highest Mw and degree of polymerization were obtained using EG after 24 h of vacuum (Mw = 550 Da, DP = 3.02, reaction 2), while a slightly lower DP was shown with BDO (DP = 2.41, reaction 6). In order to verify if a longer reaction time could result in higher yields, a 168 h reaction was performed at 1000 mbar (entry 4), achieving 58% conversion and, as expected, a low molecular polyester (Mw = 450). These results, obtained using MsAcT as the biocatalyst, are somehow limited when compared to previous studies in which well-established lipases were used. For instance, Uyama et al.31 reported the synthesis of poly(ethylene adipate) starting from DVA and EG using the lipase from Pseudomonas cepacia at 45 °C for 48 h in diisopropyl ether, obtaining a polyester with Mn = 6000 Da. Moreover, Russell and co-workers32 used Novozym-435 (CaLB immobilized on acrylic resin beads) for the solventless reaction of DVA and BDO at 50 °C, resulting in a polymer having a Mw of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Da. In this study, since one of MsAcT's advantages lies in the ability to be active at room temperature (25–30 °C), the polycondensations were performed at 30 °C in the bulk, thus presenting very interesting reaction conditions for the enzymatic polyester synthesis.
200 Da. In this study, since one of MsAcT's advantages lies in the ability to be active at room temperature (25–30 °C), the polycondensations were performed at 30 °C in the bulk, thus presenting very interesting reaction conditions for the enzymatic polyester synthesis.
As the observed differences in molecular masses of the polyesters catalyzed by MsAcT were minimal and the obtained molecular weights were rather low, an LC-MS analysis was carried out. These analyses confirmed that prolonging the reaction time (see the ESI, Fig. S3 and S5†) and applying a vacuum (see the ESI, Fig. S4 and S6†) are useful to obtain higher molecular weight oligomers (up to trimers) while for the other reactions only shorter oligomers were obtained. When changing the divinyl ester from DVA to divinyl succinate (DVS, see ESI Table S4†) a monomer conversion drop (<14% under the best reaction conditions) was observed. This lower reactivity of DVS might be due to the proximity of the two carbonyl groups that might somehow hinder the accessibility and interaction of the monomer with the enzyme's active site.
3 Conclusions
Mycobacterium smegmatis acyltransferase (MsAcT) was successfully immobilized via adsorption onto polypropylene beads. To study its selectivity in ester synthesis, a full-factorial design of experiment (DoE) was implemented. The results showed the acyltransferase selectivity towards vinyl esters (vinyl- > ethyl- > methyl-) and short-chained alcohols and esters (C2 > C4 > C6 for esters, C4 > C8 > C12 for alcohols). In particular, the results obtained after 8 h using vinyl acetate (C2 ester) and 1-butanol, 1-octanol and 1-dodecanol clearly underlined the selectivity trend, since 77%, 49% and 47% conversions were obtained, respectively. Moreover, MsAcT showed poor results using long-chain compounds (no conversion observed with almost all C6 esters) thus confirming its preference for short-chained compounds. Divinyl adipate (DVA) and two different diols, ethylene glycol (EG) and 1,4-butanediol (BDO), were used as bio-based building blocks for MsAcT-catalyzed polycondensation reactions. MsAcT showed a higher conversion when using EG rather than BDO, achieving 47% and 27% conversion respectively, confirming the previously observed short ester synthesis trend. The best results were obtained after 24 h at 20 mbar using EG, achieving 57% conversion and Mw = 550 Da, DP = 3.02, while with BDO, 42% conversion, MW = 550 Da and a slightly lower DP = 2.41 were achieved. LC-MS analysis confirmed the obtained results that a longer reaction period and reduced pressure were suitable to obtain longer oligomers, up to trimers. On the other side, polycondensation reactions carried out using divinyl succinate (DVS) as the diesters showed very limited results (only 14% conversion under the best conditions), hence revealing a potential steric hindrance by the proximity of the carbonyl carbons of DVS towards the MsAcT active site. In conclusion, this work represents the first study in which MsAcT was directly used to catalyze a polycondensation reaction, shedding light on the possibility of using this biocatalyst in the polymer biotechnology field.Author contributions
F. F. and I. V. immobilized the enzyme, carried out the ester and polyester synthesis and performed the NMR and the GPC analysis. S. D. carried out the divinyl succinate syntheses. L. N. expressed and purified the enzyme. V. M. R. carried out the LC-MS analysis. F. F. and A. P. wrote the manuscript. M. L. C., L. M., G. M. G. and A. P. supervised the work. M. L. C., L. M. and A. P. acquired the funding. The manuscript was revised and approved by all authors before submission.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The TURBOPACK project (P2022CSXLM) received funding from the European Union Next-Generation EU through the PRIN (Progetti di Ricerca di Rilevante Interesse Nazionale) PNRR (Piano Nazionale di Ripresa e Resilienza) 2022 call from the Italian Ministry of Education and Research (MUR).References
- S. Okumura, M. Iwai and Y. Tominaga, Agric. Biol. Chem., 1984, 48, 2805–2808 CAS
.
-
D. Knani, Enzymatic Polyesterification in Organic Media, 1993, vol. 31, pp. 1221–1232 Search PubMed
.
- H. Uyama and S. Kobayashi, Chem. Lett., 1993, 22, 1149–1150 CrossRef
.
- S. Kobayashi, Proc. Jpn. Acad. B: Phys. Biol., 2010, 86, 338–365 CrossRef CAS PubMed
.
- S. Hari Krishna and N. G. Karanth, Catal. Rev.: Sci. Eng., 2002, 44, 499–591 CrossRef
.
- N. R. Khan and V. K. Rathod, Process Biochem., 2015, 50, 1793–1806 CrossRef CAS
.
- A. G. A. SÁ, A. C. de Meneses, P. H. H. de Araújo and D. de Oliveira, Trends Food Sci. Technol., 2017, 69, 95–105 CrossRef
.
- I. Mathews, M. Soltis, M. Saldajeno, G. Ganshaw, R. Sala, W. Weyler, M. A. Cervin, G. Whited and R. Bott, Biochemistry, 2007, 46, 8969–8979 CrossRef CAS PubMed
.
- P. Cannazza, S. Donzella, A. Pellis and M. L. Contente, Biotechnol. Adv., 2022, 59, 107985 CrossRef CAS PubMed
.
- M. Kazemi, X. Sheng, W. Kroutil and F. Himo, ACS Catal., 2018, 8, 10698–10706 CrossRef CAS
.
- L. Wiermans, S. Hofzumahaus, C. Schotten, L. Weigand, M. Schallmey, A. Schallmey and P. D. De María, ChemCatChem, 2013, 5, 3719–3724 CrossRef CAS
.
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed
.
- M. L. Contente, A. Pinto, F. Molinari and F. Paradisi, Adv. Synth. Catal., 2018, 360, 4814–4819 CrossRef CAS
.
- M. L. Contente, S. Farris, L. Tamborini, F. Molinari and F. Paradisi, Green Chem., 2019, 21, 3263–3266 RSC
.
- C. Z. Dinu, G. Zhu, S. S. Bale, G. Anand, P. J. Reeder, K. Sanford, G. Whited, R. S. Kane and J. S. Dordick, Adv. Funct. Mater., 2010, 20, 392–398 CrossRef CAS
.
- C. Z. Dinu, I. V. Borkar, S. S. Bale, A. S. Campbell, R. S. Kane and J. S. Dordick, J. Mol. Catal. B: Enzym., 2012, 75, 20–26 CrossRef CAS
.
- M. Finnveden, S. Semlitsch, O. He and M. Martinelle, Catal. Sci. Technol., 2019, 9, 4920–4927 RSC
.
- S. P. Godehard, C. P. S. Badenhorst, H. Müller and U. T. Bornscheuer, ACS Catal., 2020, 10, 7552–7562 CrossRef CAS
.
- E. Jost, M. Kazemi, V. Mrkonjić, F. Himo, C. K. Winkler and W. Kroutil, ACS Catal., 2020, 10, 10500–10507 CrossRef CAS
.
- M. L. Contente, D. Roura Padrosa, F. Molinari and F. Paradisi, Nat. Catal., 2020, 3, 1020–1026 CrossRef CAS
.
-
P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, 2000 Search PubMed
.
- A. Pellis, F. P. Byrne, J. Sherwood, M. Vastano, J. W. Comerford and T. J. Farmer, Green Chem., 2019, 21, 1686–1694 RSC
.
- M. H. Sipponen, M. Farooq, J. Koivisto, A. Pellis, J. Seitsonen and M. Österberg, Nat. Commun., 2018, 9, 1–7 CrossRef CAS PubMed
.
- F. Fabbri, F. A. Bertolini, G. M. Guebitz and A. Pellis, Int. J. Mol. Sci., 2021, 22(16), 8493 CrossRef CAS PubMed
.
- S. Weinberger, A. Pellis, J. W. Comerford, T. J. Farmer and G. M. Guebitz, Catalysts, 2018, 8(9), 369 CrossRef
.
- I. C. Perdomo, S. Gianolio, A. Pinto, D. Romano, M. L. Contente, F. Paradisi and F. Molinari, J. Agric. Food Chem., 2019, 67, 6517–6522 CrossRef CAS PubMed
.
- K. Szymańska, K. Odrozek, A. Zniszczoł, G. Torrelo, V. Resch, U. Hanefeld and A. B. Jarzębski, Catal. Sci. Technol., 2016, 6, 4882–4888 RSC
.
- H. Yang, E. Henke and U. T. Bornscheuer, J. Org. Chem., 1999, 64, 1709–1712 CrossRef CAS PubMed
.
- N. de Leeuw, G. Torrelo, C. Bisterfeld, V. Resch, L. Mestrom, E. Straulino, L. van der Weel and U. Hanefeld, Adv. Synth. Catal., 2018, 360, 242–249 CrossRef CAS
.
-
P. Hendil-forssell, Rational Engineering of Esterases for Improved Amidase Specificity in Amide Synthesis and hydrolysis Stockholm 2016, 2016 Search PubMed
.
- H. Uyama, S. Yaguchi and S. Kobayashi, Polym. J., 1999, 31, 380–383 CrossRef CAS
.
- A. K. Chaudhary, B. J. Kline, E. J. Beckman and A. J. Russell, Am. Chem. Soc., Div. Polym. Chem., Prepr., 1997, 38, 396–397 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00038b |
| This journal is © The Royal Society of Chemistry 2024 |