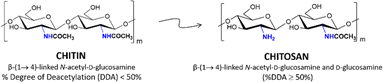Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionic liquid strategy for chitosan production from chitin and molecular insights†
Van Minh
Dinh
 *a,
Santosh Govind
Khokarale
*a,
Santosh Govind
Khokarale
 a,
Pedro Ojeda
May
b,
Tobias
Sparrman
a,
Knut
Irgum
a and
Jyri-Pekka
Mikkola
*ac
a,
Pedro Ojeda
May
b,
Tobias
Sparrman
a,
Knut
Irgum
a and
Jyri-Pekka
Mikkola
*ac
aWallenberg Wood Science Center, Technical Chemistry, Department of Chemistry, Chemical-Biological Centre, Umeå University, SE-90187, Umeå, Sweden. E-mail: van.dinh@umu.se; jyri-pekka.mikkola@umu.se
bHigh Performance Computing Center North (HPC2N), Faculty of Science and Technology, Umeå University, SE-90187, Umeå, Sweden
cIndustrial Chemistry & Reaction Engineering, Department of Chemical Engineering, Process Chemistry Centre, Åbo Akademi University, FI-20500, Åbo-Turku, Finland
First published on 12th March 2024
Abstract
The production of chitosan is an interesting research topic. Chitosan is an important polysaccharide in terms of its various applications in industries and is produced from chitin, an abundant biopolymer in crustacean shell biomass wastes. Traditional processes for chitosan manufacture are commonly based on highly concentrated alkaline or acid solutions, which are, however, severely corrosive and harmful to the environment. In this study, we have described a ‘greener’ method using 1-ethyl-3-methylimidazolium acetate, [Emim][OAc] ionic liquid (IL), for decrystallization of shrimp crystalline chitin flakes followed by a microwave-mediated NaOH or tetrabutylammonium hydroxide, [TBA][OH], solution-based deacetylation step for chitosan production. The decrease in crystallinity in IL-pretreated chitin was confirmed by XRD and SEM analysis, which subsequently benefited chitosan production with up to 85% degree of deacetylation (% DDA) in shorter time periods (1–2 hours) and lower alkaline concentrations (20–40 wt%). The % DDA in chitin/chitosan was estimated using FT-IR and NMR spectroscopies. Notably, we could regenerate the ionic liquids: in the case of [Emim][OAc] 97 wt% and in the case of [TBA][OH] 83 wt% could be reused. Roles of ionic liquids in the process were discussed. Molecular dynamics (MD) simulations showed the roles of [TBA]+ cations in the molecular driving forces of the [TBA][OH]-induced deacetylation mechanism. The strategy promises a sustainable and milder approach compared to the existing highly corrosive alkaline- or acid-involved processes for chitosan production.
Sustainability spotlightMaximizing biomass waste utilization is a societal priority. Of marine biomass byproducts, chitin is found abundantly and holds importance for chitosan production in diverse industries. Traditional chitosan manufacturing methods rely on harsh alkaline/acidic solutions, detrimental to water ecosystems. We have proposed an energy-efficient, sustainable chitosan production method, replacing even harmful reagents. Shrimp chitin was pretreated with [Emim][OAc] ionic liquid and subjected to microwave-mediated deacetylation using [TBA][OH] or NaOH solution. This synergistic approach improved chitosan production, reaching 85% DDA in two hours (40 wt%-NaOH) and 71% DDA (40 wt%-[TBA][OH]), surpassing traditional methods. Both [Emim][OAc] and [TBA][OH] could be regenerated, with 97% and 83%, respectively. Our research supports UN-Sustainable Development Goals: industry, innovation, and infrastructure (SDG-9), responsible consumption/production (SDG-12), and life below water (SDG-14). |
Introduction
Deacetylation of chitin into chitosan is a key industrial process. Owing to being less acetylated and crystalline, chitosan has various technical applications compared to chitin.1,2 Besides, the deacetylation process makes use of chitin – a main component (15–40 wt%) of crustacean shell biomass wastes, which are produced every year in millions of tons.1,3,4 While chitin valorization is highly demanded, chitosan, being biocompatible, biodegradable, less toxic and having antibacterial properties, has important industrial roles in diverse fields. Examples include drug delivery in medicine, coating films in pharmaceuticals, carriers in agriculture, membranes for water purification, and so forth.5–8 Additionally, chitosan is also explored as a nanosupport for heterogeneous reactions9 as well as a precursor for the synthesis of low molecular weight chemicals such as glucosamine and hydroxymethylfurfural.10–12The chemistry of deacetylation comprises cleavage of acetyl groups in chitin for production of chitosan with free –NH2 groups (Fig. 1), where to be called chitosan needs at least 50% degree of deacetylation (% DDA or molar fraction of non-acetylated D-glucosamine unit).13 Thermal treatment of chitin with the use of alkaline solution of NaOH is one of the traditional and industrially applied methods for chitosan production.14 However, this method is time- and energy-consuming (up to days and/or at high temperatures and pressures) when, for instance, crystalline chitin microfibrils are used.14 Besides, the method needs also large amounts of NaOH (60–70 wt% solutions), which is a highly corrosive reagent, affecting not only the reaction equipment but also the environment. Recently, some methods have been introduced to improve the chitin deacetylation process, for example, deep eutectic solvents,15 alkali treatment at high temperature with intermittent washing with water,16 mechanochemistry with repeated ball milling cycles,17 ultrasound-assisted alkali treatment,18 alkali deacetylation at high temperature in the presence of glycerol,19 or use of water-miscible organic solvents together with alkali solution.20 However, a few challenges still exist in terms of processing time, energy efficiency, or highly corrosive chemicals, which we aim to deal with.
Chitin has a highly crystallized and rigid structure due to an extended intra- and intermolecular hydrogen bonding network, which is considered as a bottleneck for its direct dissolution as well as its processing in common solvents or reagents.21,22 We hypothesize that when being less crystalline, chitin might efficiently interact with various reagents as well as solvents, allowing for desired application-oriented structural modifications. In order to diminish the crystalline nature of chitin, several aqueous as well as non-aqueous solvent media have been used for chitin pretreatment, including alkali aqueous solvents,23,24 inorganic salt aqueous solvents, ionic liquids (IL), deep eutectic solvents (DES), etc.13,25 Out of these studied solvent systems, IL gained considerable attention for dissolution and derivatization of chitin. Generally being liquids below 100 °C, ILs are special non-aqueous solvent media commonly composed of an organic cation and an organic/inorganic anion.13,25–27 Owing to their special solvent properties such as high thermal stability, low volatile nature, negligible vapour pressure, high ionic conductivity and large window of liquid phase, ILs have been extensively used as a solvent medium for organic transformations and biopolymer processing (cellulose, lignin, and polyhydroxyalkanoate) as well as an electrolyte in batteries, etc.28–31 In case of chitin processing, some types of ILs including different types of cations and anions in their composition have been previously applied to obtain a semi-crystalline or amorphous material via its dissolution under thermal treatments.32,33 In this report, we used an IL 1-ethyl-3-methylimidazolium acetate, [Emim][OAc] (Fig. 2), for the pretreatment of shrimp crystalline chitin and subsequently employed the obtained pretreated chitin for further reaction. Amongst common ILs, [Emim][OAc] is popularly known for the pretreatment of biopolymers considering its highly basic nature and short cationic alkyl chains, which effectively access the crystalline phase by entering the gap between the polymeric chains.34
The main step, which is chitin deacetylation to chitosan, requires energy for cleaving off acetyl groups. A method that can provide energy for alkaline deacetylation of chitin is microwave irradiation19 – a rapidly emerging energy efficient technique applied for various types of organic reactions as well as biomass processing.35,36 We hypothesize that the alkaline NaOH-induced deacetylation system can use energy from microwave irradiation more effectively than from conventional heating. It was reported that aqueous ions are excellent candidates that absorb highly the microwave irradiation, and thus can use most of the energy from microwave irradiation.37 In addition to NaOH, aqueous solution of tetrabutylammonium hydroxide, [TBA][OH], was also observed to be able to cleave off the acetyl groups of chitin.38 However, the potential for [TBA][OH] to be applied for chitosan production under microwave mediation has not been investigated. We therefore wished to study also the ability of [TBA][OH] in chitosan production, and later employ molecular dynamics (MD) simulation to understand the driving forces of [TBA][OH] in the chitin deacetylation process.
With these motivations, we aim in this study to (a) test whether combining IL pretreatment and microwave mediation improves the chitin deacetylation and (b) investigate the potential to use [TBA][OH] in chitosan production as well as discuss the [TBA][OH]-related mechanism using the MD simulation.
Experimental
Chemicals
Practical grade shrimp chitin flakes were received and air-dried at room temperature overnight before further processing. Water was filtered and deionized before use (Milli-Q water). NaOH micropearls were purchased from Sigma-Aldrich Co. LLC (Sweden) and were ground before each single use. Amberlite IRN-78 (hydroxide form) was purchased from Supelco, Sigma-Aldrich Co. LLC (Sweden). Deuterium oxide (99.9% D), deuterium chloride (99.5% D), dimethyl sulfoxide-d6 (99.9% D), chloroform-d (99.9% D), [Emim][OAc] ionic liquid, 40 wt% aqueous [TBA][OH], LiCl, dimethylacetamide, glacial acetic acid and NaCl were purchased from Sigma-Aldrich Co. LLC (Sweden). Acetone and ethanol were purchased from VWR Co. LLC (Sweden). All these purchased chemicals were used without further purification.Ionic liquid pretreatment of chitin
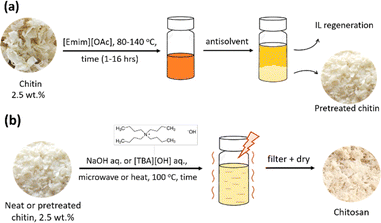
For the pretreatment, 2.5 wt% chitin (∼500 mg) was added in a round-bottom flask containing 20 mL [Emim][OAc] IL and heated at 120 °C in 1 hour under magnetic stirring. When complete, the mixture was cooled down to ambient temperature and water was added to precipitate the dissolved chitin out of the IL. The obtained mixture was stirred at 60 °C in 20 minutes and chitin was separated by filtration. The washing process was repeated twice, followed by a final washing step with acetone. The collected chitin was dried at 40 °C under vacuum for 2 hours to obtain a yield of 98 wt% and stored in a desiccator for further deacetylation experiments (Fig. 3a). The recovery of chitin was calculated using eqn (1).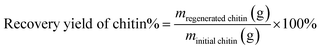 | (1) |
 | ||
| Fig. 3 (a) Ionic liquid pretreatment of chitin in [Emim][OAc] at different temperatures and time and (b) deacetylation of chitin into chitosan under various conditions. | ||
The pretreatment of chitin using the IL was also explored at different temperatures and processing times. Specifically, the mixture with 2.5 wt% chitin (∼50 mg) in a glass vial containing 2 mL [Emim][OAc] IL was treated at different temperatures (80 °C, 100 °C, 120 °C, and 140 °C) for 1 to 16 hours. The recovery of the chitin was carried out by the same process as above.
To recover the [Emim][OAc] IL, the liquid fractions after the pretreatment were collected to remove water using a rotary evaporator and dried under vacuum at 40 °C to regenerate the ionic liquid. The regeneration yield of [Emim][OAc] was 97 wt%. The recovery of the IL was calculated using eqn (2).
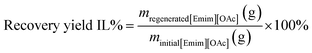 | (2) |
Chitosan production by deacetylation of chitin
To investigate the chitosan production, 75 mg chitin (IL-pretreated or non-pretreated) was added in 3 mL of 20, 40, or 60 wt% aqueous NaOH solution. The reaction proceeded under stirring at 100 °C either by conventional heating in 24 hours or by microwave mediation in 20 to 120 minutes (Microwave Initiator, Sweden, ∼50 W). When complete, the reaction mixture was cooled down to ambient temperature and filtered to separate out the solid. The obtained solid (chitin/chitosan) was washed with water (60 mL × 3 times) until neutral pH, dried at 40 °C under vacuum for 2 hours (Fig. 3b), and stored in a desiccator for further characterization.Chitin deacetylation with [TBA][OH] solution was also studied using the same microwave irradiation method. Typically, 75 mg of IL-pretreated chitin was mixed with 3 mL of 40 wt% aqueous [TBA][OH] solution. The reaction proceeded for 20–120 minutes under microwave mediation at 100 °C (∼50 W) and stirring. When the reaction completed, the product was obtained following the same procedure as described above.
To regenerate the [TBA][OH], the aqueous fractions after the reaction were collected to perform acetate exchange with hydroxide anions using an ion-exchange resin column. Specifically, 60 g of resin Amberlite IRN-78 (hydroxide form) was dispersed in Milli-Q water and loaded into a glass column (3 cm internal diameter), giving a bed volume of 120 mL after equilibration. The column resin was washed with one column volume (CV) of water to remove any associated impurity prior to use. Next the collected aqueous solution of [TBA][OH] after reaction (20 mL) was then applied onto the column with a flow rate of around 5 mL min−1 (≈3 drops per second). Water was then added to elute the sample using a flow rate of 2 mL min−1 (slowly ≈1–2 drops per second). Fractions were collected, evaporated and dried overnight at 60 °C under vacuum to remove the water. Meanwhile, the resin column was rejuvenated with 1 M aqueous NaOH solution. The regeneration yield of [TBA][OH] was 83% according to eqn (3).
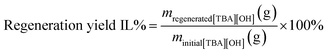 | (3) |
Analysis and characterization
For chitin and chitosan samples, 1H NMR was used to determine the degree of deacetylation according to eqn (4).39 In this case, the samples were dissolved in 20 wt% DCl/D2O at 70 °C in 30 minutes, and measured at 400 MHz, 80 °C.
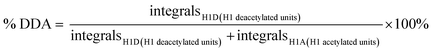 | (4) |
Besides, 13C cross-polarization magic angle spinning NMR (13C CP-MAS-NMR) was employed for solid chitin/chitosan samples. NMR spectra were recorded at 500 MHz using a Bruker Avance III 500 MHz spectrometer with 4 mm zirconia rotors spun at a magic angle of 10 kHz and 25 °C. All the NMR spectra were assigned using Bruker's Topspin (4.0.9) software.
| [η] = K × Mva | (5) |
Chitin samples were prepared similarly by dissolving 10 mg chitin in 20 mL of 5% (w/w) LiCl/dimethylacetamide.42 The measurements were performed at 30 °C and Mark–Houwink constant values for chitin samples were taken as a = 0.95 and K = 7.60 × 10−3 mL g−1.
Computational simulations
To observe the initial arrangements of the [TBA][OH]–chitin system prior to the chitin deacetylation, short-time scale MD simulations were performed. A chitin model (6-mer × 4-chain) was built for β-chitin based on experimental crystallographic data, showing regularly arranged chito-oligomers.43,44 The model was introduced in a periodic cubic box of 45 Å side length containing a total of 45 ion pairs [TBA][OH] solvated with water molecules. [TBA]+ and [OH]− ions were optimized using the B3LYP method and the 6-311G** basis set with Maestro 11.2 (Schrödinger, LLC).45 Chitin and ionic liquid systems were generated using the Packmol package (v20.3.3).46 All MD calculations for the system were carried out in the MOE platform (v2018.01, Chemical Computing Group ULC) using the Nosé–Poincaré–Andersen (NPA) method for the thermostat and the barostat simulator and together with the Amber10: EHT force field.47,48 An initial equilibration step of 100 ps was performed and followed by data production with further 200 ps at 373 K. Molecular graphics were constructed with the MOE platform and VMD 1.9.3 software.49Results and discussion
Ionic liquid pretreatment and its regeneration
The [Emim][OAc] IL has an ability to dissolve various types of rigid and crystalline biopolymers.50,51 Besides, its water-tolerant nature is a benefit considering a certain level of moisture in naturally occurring polymers. Here, chitin was pretreated in the [Emim][OAc] IL at different temperatures and time, and the crystallinity of chitin was recorded using XRD measurements (Fig. 4). The XRD patterns of neat chitin showed three intense reflections at 2θ 9.7° (020), 19.8° (110), and 26.8° (013) and minor reflections at 12.9° (021) and 23.9° (130), corresponding mostly to α-chitin.52 After IL-pretreatment at increasing temperatures from 80 to 100, 120 and 140 °C in 1 hour (Fig. 4a), the characteristic peaks of chitin were steadily shortened and broadened, implicative of the decreased crystallinity and more amorphous nature of the sample. It is also noticeable that a complete dissolution of chitin was obtained at 120 °C and above (ESI 1†), while a partly dissolved mixture occurred at 80 and 100 °C. This depicts a relationship between the processing temperature, chitin dissolution and the crystallinity of the material. Further, it was recorded that the processing time also had a significant influence on the IL-pretreatment of chitin (Fig. 4b). Observing the progress of the IL-pretreatment at 100 °C for varying time from 1 hour to 16 hours, the intensity of the characteristic peaks of chitin decreased from 1 to 8 hours and even disappeared upon further pretreatment, indicating the decrystallization of chitin during the process. Considering the obtained results, we therefore chose the IL pretreatment condition as 120 °C and 1 hour since it provided the treated material with significantly amorphous nature in a short processing time. The approach of chitin pretreatment using imidazolium-based ILs was also reported previously. Xie and coworkers described the dissolution of pure chitin using 1-butyl-3-methylimidazolium chloride [Bmim][Cl] at 110 °C in 5 hours.53 1-Allyl-3-methylimidazolium bromide [Amim][Br] was also used by Yamazaki et al. to obtain a similar solubility at 100 °C in 24 hours.54 More recently, Jaworska et al. used [Emim][OAc] to achieve chitin dissolution at 105 °C in 48 hours.55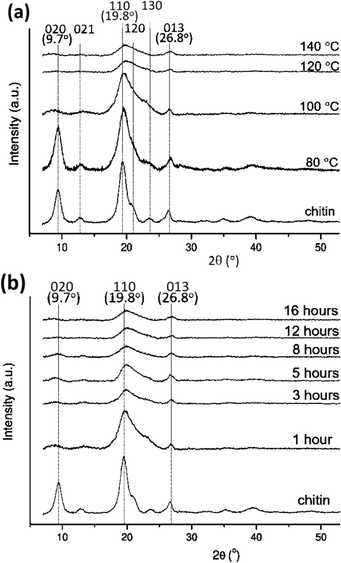 | ||
| Fig. 4 XRD shows a decrease of chitin crystallinity when treated with [Emim][OAc] IL over (a) temperatures (80–140 °C, 1 hour) and (b) time (1–16 hours, 100 °C). | ||
Upon the IL-pretreatment of chitin under the chosen conditions of 120 °C and 1 hour, SEM analysis was performed to compare the IL-pretreated chitin and the neat sample. As shown in Fig. 5, we observed a major change in the surface morphology of the samples. While the neat chitin presented a well-organized surface, the IL-pretreated sample exhibited a more irregular and damaged structure. The damage in the IL-pretreated chitin may represent the amorphous nature and the breakage of the intramolecular or intermolecular hydrogen bond networks in chitin.
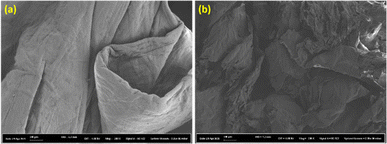 | ||
| Fig. 5 SEM imaging (at 200× magnification) illustrates chitin being more amorphous during IL pretreatment: (a) non-pretreated and (b) IL-pretreated at 120 °C, 1 hour. | ||
Considering the alkaline characteristics of the [Emim][OAc] IL, we also studied whether there happened any deacetylation of chitin during the IL-pretreatment process. By using FT-IR, structural changes of chitin after the pretreatment at 100 °C were recorded for various reaction times. ESI 2† shows the common peaks of chitin and chitosan at 3450 and 3300 cm−1, attributed to the O–H and N–H stretching vibrations and the extensive inter- and intra-molecular hydrogen bonding network. The bands in the region of 3000–2800 cm−1 correspond to the –CH2 symmetric and asymmetric stretching vibrations of polysaccharides. Chitin exhibits the doublet amide I band at 1655 and 1637 cm−1, representing the presence of H-bonding in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group with the NH group of the adjacent chain and the O–H group of the inter-chain. The amide II band is located at 1560 cm−1. The peak at 1320 cm−1 corresponds to the amide III band (in-plane mode of CONH group). The vibrations at around 1070 cm−1 are attributed to the C–O stretching of the polysaccharide. Upon deacetylation, the acetyl groups in chitin are cleaved, leaving the chitosan structure with a decreased intensity of amide group signals at 1320 and 1560 cm−1. By calculations we observed that the [Emim][OAc] IL was not able to induce the deacetylation of chitin samples, where % DDA was almost unchanged during the process i.e. 11–12% DDA (ESI 3†).
O group with the NH group of the adjacent chain and the O–H group of the inter-chain. The amide II band is located at 1560 cm−1. The peak at 1320 cm−1 corresponds to the amide III band (in-plane mode of CONH group). The vibrations at around 1070 cm−1 are attributed to the C–O stretching of the polysaccharide. Upon deacetylation, the acetyl groups in chitin are cleaved, leaving the chitosan structure with a decreased intensity of amide group signals at 1320 and 1560 cm−1. By calculations we observed that the [Emim][OAc] IL was not able to induce the deacetylation of chitin samples, where % DDA was almost unchanged during the process i.e. 11–12% DDA (ESI 3†).
Roles of the [Emim][OAc] IL in dissolution and decrystallization of chitin were reported.56,57 In contact with chitin microfibrils, anionic acetate [OAc]− species, as a small-sized component with the ability to form H-bonds with –OH and –NHCO− groups in chitin, penetrate easily into the crystalline structure and disturb the highly ordered intra- and intermolecular hydrogen bonding network of the chitin. The [Emim]+ species, following the acetate anions via ionic interactions, can also access inside the chitin lattice. With its large size and highly basic property, [Emim][OAc] limits the re-bonding of intermolecular H-bonds in chitin microfibrils, enabling the chitin to be decrystallized and become porous, less crystalline with more open structural sites. This open and reactive state of chitin can be beneficial for reagents to enter the lattice, creating more interactions with the functional groups –NHCOCH3 on chitin.
Another interesting point of this pretreatment process is the ability to regenerate the [Emim][OAc] IL. We were able to regenerate up to 97% yield of [Emim][OAc] for reuse. The IL structure remained intact during the process (confirmed by NMR analysis). 1H and 13C NMR results (Fig. 6) demonstrated spectroscopic features between fresh and regenerated ionic liquids. Signals of [Emim]+ and [OAc]− species remained the same, while the only difference is the water peak at 4.55 ppm (singlet) in the 1H NMR spectrum of the regenerated sample.58–60 Success of this regeneration step implies the ability to reuse the chemical and diminish effects on the environment, making the ionic liquid a ‘green’ solvent.
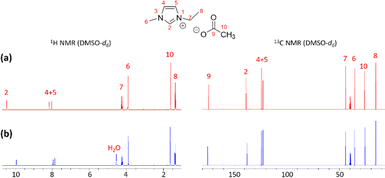 | ||
| Fig. 6 Recovery of [Emim][OAc] IL was confirmed by spectroscopic data (1H and 13C NMR) with (a) fresh IL and (b) recovered IL. | ||
Deacetylation of chitin
Chitin deacetylation proceeded while 1H NMR and 13C CP-MAS-NMR analysis were used to study the formation of chitosan (Fig. 7 and ESI 4†) and estimate % DDA according to equation.4 One benefit of using DCl as the solvent in 1H NMR measurements is that the resonance of the solvent (HDO) does not interfere with any carbohydrate protons. Fig. 7 shows the characteristic resonances of the acetylated α and β-H1 anomers at 5.84 and 5.38 ppm, respectively. Besides, H2 of the acetylated α and β-anomeric units appear, respectively, at 3.74 and 3.46 ppm. Meanwhile, H1 of deacetylated units resonates at 5.36 ppm and H2 resonates at 3.61 ppm. Acetyl-protons appear at 2.42 ppm, while the remaining ring protons appear between 3.8 and 4.5 ppm. It can be seen that after deacetylation, signals of acetyl groups and acetylated units decreased, while signals of deacetylated units arose.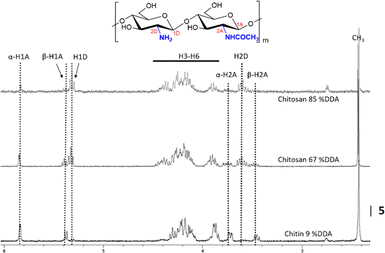 | ||
| Fig. 7 1H NMR analysis gave structural information and the degree of deacetylation in chitin/chitosan samples. | ||
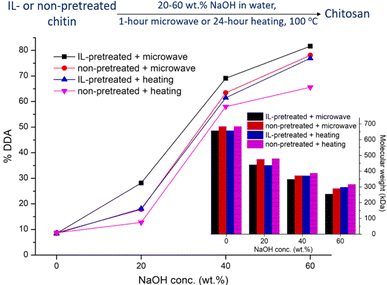
For the deacetylation, two types of chitin (IL-pretreated or non-pretreated) were processed at different conditions (microwave irradiation or conventional heating) with different concentrations of aqueous NaOH solution from 20 to 60 wt% (Fig. 8). It was observed that with increasing NaOH concentration, % DDA increased accordingly. NaOH is the reagent that gave rise to the deacetylation by breaking acetyl groups out of chitin. Comparing IL-pretreated samples with non-pretreated ones, it can be seen that the IL-pretreatment accelerated the deacetylation reaction. This is in agreement with the discussion about IL-pretreatment mentioned above, where the decrease in crystallinity of chitin allowed for more accessible sites towards the reagent. Regarding the heating methods, the microwave-assisted reactions were speeded up compared to the traditional heating. The combination of IL pretreatment and microwave mediation allowed the reduction of time to one hour with less amount of NaOH needed (40 wt% NaOH) while giving a higher % DDA (approximately 70% DDA), compared to around 60% DDA for non-treated chitin with 60 wt% NaOH in 24 hours of conventional heating. Besides, microwave mediated deacetylation of IL-pretreated chitin was studied over time to observe changes in % DDA (ESI 5†). Two hours of microwave-mediated reaction in 40 wt% aqueous NaOH solution could give up to 85% DDA. The microwave irradiation method for chitin deacetylation was also previously reported, where 82–85% DDA was obtained using 40% w/v NaOH solution at 150 °C in a microwave chamber for 3 to 6 hours, conducted by Lertwattanaseri and coworkers.61 However in our study, IL-pretreatment allowed for the chitin deacetylation to be achieved under even milder conditions (100 °C, ∼50 W, 2 hours).
Molecular weights (MW) of chitin/chitosan were also of interest and estimated using viscosity measurements and the Mark–Houwink equation. Since viscometric analysis requires the polymers to be soluble in a medium, we used acetic acid/NaCl/water for dissolving chitosan and LiCl/dimethylacetamide for chitin. By measuring viscometric values we could determine the intrinsic viscosity of the samples before calculating their molecular weights. It can be seen that the molecular weight of the obtained materials decreased corresponding to an increase in concentration of aqueous NaOH solution or reaction time, irrespective of microwave or conventional heating (Fig. 8 and ESI 5†). Additionally, we noted that chitosan produced by the microwave irradiation method gave a more extensive decrease in molecular weight than the one from conventional heating. It was described in the previous reports that the removal of acetyl groups (primary reaction) and the cleavage of glycoside bonds (side reaction, depolymerization) due to energy impact (microwave irradiation or heating), and chemicals such as NaOH and [Emim][OAc] induced a decrease in molecular weight of the obtained products.62–64 The deacetylation process gave a medium to high molecular weight (∼250–500 kDa and above), depending on the reaction condition involved. Hence this method can be useful for the synthesis of medium to high molecular weight chitosan materials with mild reaction conditions.65
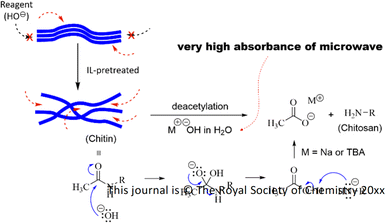
Besides IL-pretreatment, the use of microwave heating was a benefit to the chitin deacetylation. Microwave irradiation, which has its energy at the molecular level, interacts strongly with ionic molecules (conduction mechanism) by collisions and polar molecules (dipolar polarization mechanism) by rotations.66,67 Hence these molecules will absorb very well the microwave irradiation to convert it into heat energy. When using an aqueous solution of NaOH, polar water molecules and ionic features of Na+ and OH− were being taken advantage of, allowing for the reagent to use most of the energy from microwave irradiation. Hence OH− anions could rapidly attack the acetamido groups in chitin and then effectively cleave off the acetyl groups upon the deacetylation reaction (Fig. 9). Meanwhile, conventional heating requires a regular energy transfer that distributes evenly to the whole system, implicative of energy waste towards unnecessary sites during the reaction, for example, the reactor jacket.
[TBA][OH] in chitin deacetylation and possible molecular roles
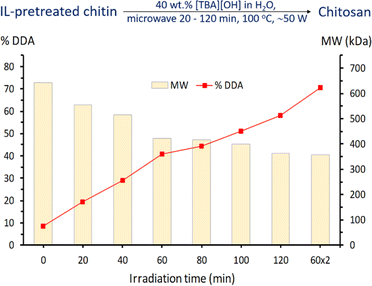
With the benefits of IL pretreatment and microwave irradiation over conventional heating, we continued studying the microwave-assisted deacetylation of the IL-pretreated chitin over time using 40 wt% aqueous [TBA][OH] solution as the reagent (Fig. 10). The reaction was investigated up to two hours at 100 °C and % DDA was estimated from 1H NMR analysis. It can be seen that an increased deacetylation was obtained over time to 59% DDA in 120 minutes and reached up to 71% DDA when performed twice on the same chitin (60 minutes × 2 times reaction with fresh 40 wt% aqueous [TBA][OH] solution). Although the % DDA is still slightly worse than in the case of aqueous NaOH (85% DDA), it is higher than that of conventional heating (60% DDA, 24 hours) and similar to that of commercial chitosan (69% DDA). A decreasing trend in molecular weight was also observed when using aqueous [TBA][OH] solution in the deacetylation process over time (Fig. 10).It was also noticeable that [TBA][OH] could be regenerated with 83% yield using ion exchange resin, making it promising compared to highly corrosive NaOH solutions at elevated temperature. Spectroscopic results of the recovered [TBA][OH] are shown in Fig. 11, confirming the success of regeneration. The 1H and 13C NMR spectra demonstrate that [TBA][OH] was recovered with high purity. For the liquid after deacetylation reaction, we could also observe the presence of acetate anion signals at 1.54 ppm (–CH3, singlet) on 1H NMR and 25.6 ppm (–CH3) and 173.5 ppm (–CO–) on 13C NMR. These peaks disappeared when [TBA][OH] was regenerated.
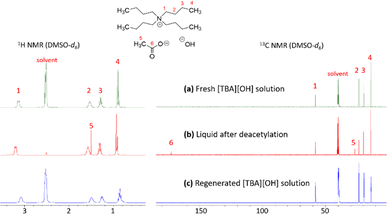 | ||
| Fig. 11 Regeneration of [TBA][OH] was confirmed by 1H and 13C NMR analysis of (a) fresh solution, (b) liquid after the reaction, and (c) recovered solution. | ||
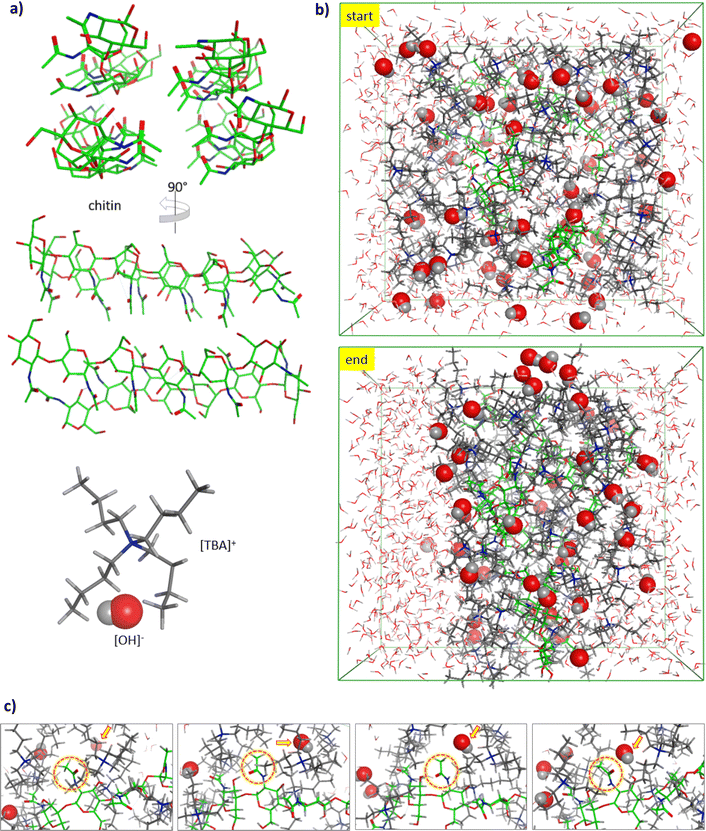
To acquire insights into the roles of [TBA]+ and [OH]− ions, MD simulations were performed to visualize the initial arrangements and motions of the system prior to the chitin deacetylation. As shown in Fig. 12, we constructed a model of chitin (6-mer × 4-chain) featuring regularly arranged chito-oligomers as well as [TBA]+ and [OH]− ions (Fig. 12a). To represent the amorphous state of IL-pretreated chitin, polymer chains were introduced randomly into the MD simulation box. Although the importance of [OH]− ions in cleaving off acetyl groups in chitin is already known, from experimental and theoretical perspectives,68–70 the roles of [TBA]+ cations in the system are still pending. Through simulations, we observed a notable preference of [TBA]+ species to selectively bind to the chitin surface. As depicted in Fig. 12b and c, [TBA]+ cations, being the larger constituents of the ionic liquid, exhibited a tendency to aggregate around the chitin fiber, attracting [OH]− anions of opposite charge. This electrostatic attraction facilitated the penetration of [OH]− anions into the previously inaccessible regions of the chitin structure for further deacetylation reaction.
Intermolecular bonding involving [TBA]+, [OH]−, and chitin can be attributed to (1) electrostatic attraction among quaternary nitrogen of the cation and oxygen of hydroxyl or acetamido groups in chitin, (2) hydrophobic interactions among butyl groups and carbohydrate backbone, and (3) hydrogen bonds between hydroxyl or acetamido groups on chitin and [OH]− anions.71,72 These interactions are likely responsible for maintaining proximity between [OH]− anionic reagents and the chitin surface. This spatial closeness provides numerous opportunities for effective collisions between the [OH]− anions and acetamido groups during the deacetylation process, as illustrated in Fig. 12c. The theoretical insights gained from these observations offer a rationale for understanding how the [TBA][OH] solution can effectively induce the deacetylation of chitin without requiring additional NaOH reagent.
Conclusions
In this study, we succeeded in improving the deacetylation of crystalline chitin for chitosan production using milder conditions. The developed method was composed of two steps: ionic liquid pretreatment and microwave-mediated deacetylation. The [Emim][OAc] IL pretreatment effectively decreased the crystallinity of chitin by disturbing the intermolecular hydrogen bonding among its polymeric chains, confirmed by XRD analysis. At 120 °C in 1 hour of pretreatment, amorphous/semi-crystalline chitin was obtained for use in further deacetylation reactions. Upon the chitin deacetylation, the combination of IL pretreatment and microwave mediation proved to increase significantly the % DDA of chitosan products up to 85% in two hours of reaction time using 40 wt% NaOH solution. In comparison, non-pretreated chitin with the conventional heating method gave merely 60% DDA chitosan in 24 hours of reaction time and 60 wt% NaOH solution. For the deacetylation of IL-pretreated chitin with 40 wt% [TBA][OH] solution under microwave irradiation, chitosan products were obtained with 59–71% DDA. Even though [TBA][OH] solution is slightly less effective compared to NaOH solution, the % DDA of the obtained chitosan was better compared with the conventional heating approach. Noticeably, both the [Emim][OAc] IL and the reagent [TBA][OH] were regenerated with 97 wt% and 83 wt%, respectively. MD simulations revealed the molecular driving forces and motions of [TBA][OH] in contact with chitin before the deacetylation process. By intermolecular interactions in the system, OH− anions remained close to the chitin polymeric chains, enabling the deacetylation of chitin through the cleavage of acetyl groups. With the ‘greener’, milder, and more sustainable approach in this report, we hope it can further more explorations for scalable production of chitosan considering the recoverability of the solvent/reagent systems and industrially feasible reaction parameters.Author contributions
Van Minh Dinh: investigation, methodology, software, data curation, writing – original draft. Santosh Khokarale: investigation, writing – review & editing. Pedro Ojeda May: software, methodology, writing – review & editing. Tobias Sparrman: methodology, software, data curation. Knut Irgum: conceptualization, methodology. Jyri-Pekka Mikkola: conceptualization, writing – review & editing, supervision, funding acquisition.Conflicts of interest
The authors declare that no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is part of activities at the Technical Chemistry, Department of Chemistry, Chemical-Biological Centre, Umeå University, Sweden as well as the Johan Gadolin Process Chemistry Centre at Åbo Akademi University in Finland. The research was funded by the Bio4Energy programme, Kempe Foundations and Wallenberg Wood Science Center under auspices of Alice and Knut Wallenberg Foundation. The FT-IR measurements were performed at the Vibrational Spectroscopy Core Facility (ViSp), Chemical Biological Centre (KBC), Umeå University. The Swedish NMR centre at Umeå University is acknowledged for technical support. The Umeå Core Facility for Electron Microscopy (UCEM-NMI node) at the Chemical Biological Centre (KBC), Umeå University, is gratefully acknowledged. The computations were supported by resources provided by the Swedish National Infrastructure for Computing (SNIC) at High Performance Computing Center North (HPC2N). Thanks also to Thai Q. Bui and Cheng Choo Lee for technical support.Notes and references
- T. Maschmeyer, R. Luque and M. Selva, Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials, Chem. Soc. Rev., 2020, 49(13), 4527–4563, 10.1039/c9cs00653b.
- I. Younes and M. Rinaudo, Chitin and chitosan preparation from marine sources. Structure, properties and applications, Mar. Drugs, 2015, 13(3), 1133–1174 CrossRef CAS PubMed.
- M. J. Hülsey, Shell biorefinery: a comprehensive introduction, Green Energy Environ., 2018, 3(4), 318–327 CrossRef.
- E. C. Achinivu, J. L. Shamshina and R. D. Rogers, Chitin extracted from various biomass sources: it's not the same, Fluid Phase Equilib., 2022, 552, 113286 CrossRef CAS.
- X. Xiong, I. K. M. Yu, D. C. W. Tsang, N. S. Bolan, Y. Sik Ok and A. D. Igalavithana, et al., Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects, Chem. Eng. J., 2019, 375, 121983, DOI:10.1016/j.cej.2019.121983.
- N. Adiera, H. Rosli, K. S. Loh, W. Y. Wong, R. M. Yunus and T. K. Lee, et al., Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids, Int. J. Mol. Sci., 2020, 21, 632 CrossRef PubMed.
- T. Philibert, B. H. Lee and N. Fabien, Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers, Appl. Biochem. Biotechnol., 2017, 181(4), 1314–1337 CrossRef CAS PubMed.
- A. Khezrian and Y. Shahbazi, Application of nanocomposite chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel's meat, Int. J. Biol. Macromol., 2018, 106, 1146–1158, DOI:10.1016/j.ijbiomac.2017.08.117.
- E. K. Abdelkrim, Chitosan as a sustainable organocatalyst: a concise overview, ChemSusChem, 2015, 8(2), 217–244 CrossRef PubMed.
- K. W. Omari, J. E. Besaw and F. M. Kerton, Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation, Green Chem., 2012, 14(5), 1480–1487 RSC.
- W. Hou, Q. Zhao and L. Liu, Selective conversion of chitin to levulinic acid catalyzed by ionic liquids: distinctive effect of: N-acetyl groups, Green Chem., 2020, 22(1), 62–70 RSC.
- H. Zhang, Y. Lu, Y. Wang, X. Zhang and T. Wang, D-Glucosamine production from chitosan hydrolyzation over a glucose-derived solid acid catalyst, RSC Adv., 2018, 8(10), 5608–5613 RSC.
- J. Pohling, K. Hawboldt and D. Dave, Comprehensive review on pre-treatment of native, crystalline chitin using non-toxic and mechanical processes in preparation for biomaterial applications, Green Chem., 2022, 24(18), 6790–6809 RSC.
- G. Lamarque, G. Chaussard and A. Domard, Thermodynamic aspects of the heterogeneous deacetylation of β-chitin: reaction mechanisms, Biomacromolecules, 2007, 8(6), 1942–1950 CrossRef CAS PubMed.
- F. A. Vicente, M. Huš, B. Likozar and U. Novak, Chitin Deacetylation Using Deep Eutectic Solvents: Ab Initio-Supported Process Optimization, ACS Sustain. Chem. Eng., 2021, 9(10), 3874–3886 CrossRef CAS PubMed.
- S. Mima, M. Miya, R. Iwamoto and S. Yoshikawa, Highly deacetylated chitosan and its properties, J. Appl. Polym. Sci., 1983, 28(6), 1909–1917 CrossRef CAS.
- T. Di Nardo, C. Hadad, A. Nguyen Van Nhien and A. Moores, Synthesis of high molecular weight chitosan from chitin by mechanochemistry and aging, Green Chem., 2019, 21(12), 3276–3285 RSC.
- W. G. Birolli, J. A. D. M. Delezuk and S. P. Campana-Filho, Ultrasound-assisted conversion of alpha-chitin into chitosan, Appl. Acoust., 2016, 103, 239–242, DOI:10.1016/j.apacoust.2015.10.002.
- C. Liu, G. Wang, W. Sui, L. An and C. Si, Preparation and Characterization of Chitosan by a Novel Deacetylation Approach Using Glycerol as Green Reaction Solvent, ACS Sustain. Chem. Eng., 2017, 5(6), 4690–4698 CrossRef CAS.
- I. Batista and G. A. F. Roberts, A novel, facile technique for deacetylating chitin, Die Makromol Chemie, 1990, 191(2), 429–434 CrossRef CAS.
- J. L. Shamshina, P. S. Barber, G. Gurau, C. S. Griggs and R. D. Rogers, Pulping of crustacean waste using ionic liquids: to extract or not to extract, ACS Sustain. Chem. Eng., 2016, 4(11), 6072–6081 CrossRef CAS.
- F. Hajiali, J. Vidal, T. Jin, L. C. de la Garza, M. Santos and G. Yang, et al., Extraction of Chitin from Green Crab Shells by Mechanochemistry and Aging, ACS Sustain. Chem. Eng., 2022, 10(34), 11348–11357 CrossRef CAS.
- Y. Zhong, X. Zhang, Q. Zhang and J. Cai, Rapid dissolution of chitin and chitosan with degree of deacetylation less than 80% in KOH/urea aqueous solution, Green Chem., 2023, 25(21), 8593–8605 RSC.
- J. Huang, Y. Zhong, P. Wei and J. Cai, Rapid dissolution of β-chitin and hierarchical self-assembly of chitin chains in aqueous KOH/urea solution, Green Chem., 2021, 23(8), 3048–3060 RSC.
- S. S. Silva, J. F. Mano and R. L. Reis, Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications, Green Chem., 2017, 19(5), 1208–1220 RSC.
- J. Guo, Z. D. Tucker, Y. Wang, B. L. Ashfeld and T. Luo, Ionic liquid enables highly efficient low temperature desalination by directional solvent extraction, Nat. Commun., 2021, 12(1), 437 CrossRef CAS PubMed.
- S. K. Shukla, S. G. Khokarale, T. Q. Bui and J.-P. T. Mikkola, Ionic liquids: potential materials for carbon dioxide capture and utilization, Front. Mater., 2019, 6, 42 CrossRef.
- P. Jablonski, D. Nikjoo, J. Warna, K. Irgum, J.-P. Mikkola and S. G. Khokarale, Sustainable, highly selective, and metal-free thermal depolymerization of poly-(3-hydroxybutyrate) to crotonic acid in recoverable ionic liquids, Green Chem., 2022, 24(10), 4130–4139 RSC.
- P. Mäki-Arvela, I. Anugwom, P. Virtanen, R. Sjöholm and J.-P. Mikkola, Dissolution of lignocellulosic materials and its constituents using ionic liquids—a review, Ind. Crops Prod., 2010, 32(3), 175–201 CrossRef.
- H. Li, R. Meng, Y. Guo, B. Chen, Y. Jiao and C. Ye, et al., Reversible electrochemical oxidation of sulfur in ionic liquid for high-voltage Al–S batteries, Nat. Commun., 2021, 12(1), 5714 CrossRef CAS PubMed.
- A. Basile, A. I. Bhatt and A. P. O'Mullane, Stabilizing lithium metal using ionic liquids for long-lived batteries, Nat. Commun., 2016, 7(1), ncomms11794 CrossRef CAS PubMed.
- J. Kadokawa, Dissolution, derivatization, and functionalization of chitin in ionic liquid, Int. J. Biol. Macromol., 2019, 123, 732–737, DOI:10.1016/j.ijbiomac.2018.11.165.
- A. Takegawa, M. Murakami, Y. Kaneko and J. Kadokawa, Preparation of chitin/cellulose composite gels and films with ionic liquids, Carbohydr. Polym., 2010, 79(1), 85–90, DOI:10.1016/j.carbpol.2009.07.030.
- M. M. Jaworska, T. Kozlecki and A. Gorak, Review of the application of ionic liquids as solvents for chitin, J. Polym. Eng., 2012, 32(2), 67–69 CAS.
- P. K. Raul, A. Mahanta, R. K. Borah, U. Bora, A. J. Thakur and J.-P. Mikkola, et al., Microwave assisted and in situ generated palladium nanoparticles catalysed desulfitative synthesis of cross-biphenyls from arylsulfonyl chlorides and phenylboronic acids, Results Chem., 2021, 3, 100181 CrossRef.
- A. F. Aguilera, P. Tolvanen, S. Heredia, M. G. Muñoz, T. Samson and A. Oger, et al., Epoxidation of fatty acids and vegetable oils assisted by microwaves catalyzed by a cation exchange resin, Ind. Eng. Chem. Res., 2018, 57(11), 3876–3886 CrossRef CAS.
- A. Sahu, P. Goswami and U. Bora, Microwave mediated rapid synthesis of chitosan, J. Mater. Sci.: Mater. Med., 2009, 20(1), 171–175 CrossRef CAS PubMed.
- Q. Ma, X. Gao, X. Bi, Q. Han, L. Tu and Y. Yang, et al., Dissolution and deacetylation of chitin in ionic liquid tetrabutylammonium hydroxide and its cascade reaction in enzyme treatment for chitin recycling, Carbohydr. Polym., 2020, 230, 115605, DOI:10.1016/j.carbpol.2019.115605.
- R. Czechowska-Biskup, D. Jarosińska, B. Rokita, P. Ulański and J. M. Rosiak, Determination of degree of deacetylation of chitosan – comparision of methods, Prog. Chem. Appl. Chitin Its Deriv., 2012, 2012, 5–20 Search PubMed.
- M. Shimo, M. Abe and H. Ohno, Functional comparison of polar ionic liquids and onium hydroxides for chitin dissolution and deacetylation to chitosan, ACS Sustain. Chem. Eng., 2016, 4(7), 3722–3727 CrossRef CAS.
- G. A. F. Roberts and J. G. Domszy, Determination of the viscometric constants for chitosan, Int. J. Biol. Macromol., 1982, 4(6), 374–377 CrossRef CAS.
- M. Poirier and G. Charlet, Chitin fractionation and characterization in N,N-dimethylacetamide/lithium chloride solvent system, Carbohydr. Polym., 2002, 50(4), 363–370 CrossRef CAS.
- P. Sikorski, R. Hori and M. Wada, Revisit of α-chitin crystal structure using high resolution X-ray diffraction data, Biomacromolecules, 2009, 10(5), 1100–1105 CrossRef CAS PubMed.
- D. Sawada, Y. Nishiyama, P. Langan, V. T. Forsyth, S. Kimura and M. Wada, Water in crystalline fibers of dihydrate β-chitin results in unexpected absence of intramolecular hydrogen bonding, PLoS One, 2012, 7(6), 4–11 Search PubMed.
- L. L. C. Schrödinger, Maestro, version 9.1., New York, NY, 2010 Search PubMed.
- L. Martínez, R. Andrade, E. G. Birgin and J. M. Martínez, PACKMOL: a package for building initial configurations for molecular dynamics simulations, J. Comput. Chem., 2009, 30(13), 2157–2164 CrossRef PubMed.
- P. Labute, Molecular Operating Environment, Chemical Computing Group. Inc, Montr, 2008 Search PubMed.
- J. B. Sturgeon and B. B. Laird, Symplectic algorithm for constant-pressure molecular dynamics using a Nosé-Poincaré thermostat, J. Chem. Phys., 2000, 112(8), 3474–3482 CrossRef CAS.
- N. Mathews, VMD User's Guide Version 1.9.3, NIH Biomed Res Cent Macromol Model Bioinforma, 2016, pp. 1–265, available from: https://www.ks.uiuc.edu/Research/vmd/current/ug.pdf Search PubMed.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Distillable acid–base conjugate ionic liquids for cellulose dissolution and processing, Angew. Chem., Int. Ed., 2011, 50(28), 6301–6305 CrossRef CAS PubMed.
- L. Kyllönen, A. Parviainen, S. Deb, M. Lawoko, M. Gorlov and I. Kilpeläinen, et al., On the solubility of wood in non-derivatising ionic liquids, Green Chem., 2013, 15(9), 2374–2378 RSC.
- R. S. Dassanayake, S. Acharya and N. Abidi, Biopolymer-based materials from polysaccharides: Properties, processing, characterization and sorption applications, Adv. Sorption Process Appl., 2018, 1–24 Search PubMed.
- H. Xie, S. Zhang and S. Li, Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2, Green Chem., 2006, 8(7), 630–633 RSC.
- S. Yamazaki, A. Takegawa, Y. Kaneko, J. Kadokawa, M. Yamagata and M. Ishikawa, An acidic cellulose–chitin hybrid gel as novel electrolyte for an electric double layer capacitor, Electrochem. Commun., 2009, 11(1), 68–70 CrossRef CAS.
- M. M. Jaworska, A. Górak and J. Zdunek, Modification of chitin particles with ionic liquids containing ethyl substituent in a cation, Adv. Mater. Sci. Eng., 2017, 2017, 3961318 Search PubMed.
- T. Uto, S. Idenoue, K. Yamamoto and J. I. Kadokawa, Understanding dissolution process of chitin crystal in ionic liquids: theoretical study, Phys. Chem. Chem. Phys., 2018, 20(31), 20669–20677 RSC.
- J. Li, W.-C. Huang, L. Gao, J. Sun, Z. Liu and X. Mao, Efficient enzymatic hydrolysis of ionic liquid pretreated chitin and its dissolution mechanism, Carbohydr. Polym., 2019, 211, 329–335 CrossRef CAS PubMed.
- Y. Chen, S. Li, Z. Xue, M. Hao and T. Mu, Quantifying the hydrogen-bonding interaction between cation and anion of pure [EMIM][Ac] and evidencing the ion pairs existence in its extremely diluted water solution: via13C, 1H, 15N and 2D NMR, J. Mol. Struct., 2015, 1079, 120–129, DOI:10.1016/j.molstruc.2014.09.023.
- D. C. Dibble, C. Li, L. Sun, A. George, A. Cheng and Ö. P. Çetinkol, et al., A facile method for the recovery of ionic liquid and lignin from biomass pretreatment, Green Chem., 2011, 13(11), 3255–3264 RSC.
- S. P. Magalhães Da Silva, A. M. Da Costa Lopes, L. B. Roseiro and R. Bogel-Łukasik, Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids, RSC Adv., 2013, 3(36), 16040–16050 RSC.
- T. Lertwattanaseri, N. Ichikawa, T. Mizoguchi, Y. Tanaka and S. Chirachanchai, Microwave technique for efficient deacetylation of chitin nanowhiskers to a chitosan nanoscaffold, Carbohydr. Res., 2009, 344(3), 331–335, DOI:10.1016/j.carres.2008.10.018.
- A. Sahu, P. Goswami and U. Bora, Microwave mediated rapid synthesis of chitosan, J. Mater. Sci.: Mater. Med., 2009, 20, 171–175 CrossRef CAS PubMed.
- G. Gözaydın, S. Song and N. Yan, Chitin hydrolysis in acidified molten salt hydrates, Green Chem., 2020, 22(15), 5096–5104 RSC.
- Y. Wang, J. Kou, X. Wang and X. Chen, Acid hydrolysis of chitin in calcium chloride solutions, Green Chem., 2023, 25(7), 2596–2607 RSC.
- J. Lv, X. Lv, M. Ma, D.-H. Oh, Z. Jiang and X. Fu, Chitin and chitin-based biomaterials: a review of advances in processing and food applications, Carbohydr. Polym., 2023, 299, 120142 CrossRef CAS PubMed.
- J. Sun, W. Wang and Q. Yue, Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies, Materials, 2016, 9(4), 231 CrossRef PubMed.
- P. Lidström, J. Tierney, B. Wathey and J. Westman, Microwave assisted organic synthesis—a review, Tetrahedron, 2001, 57(45), 9225–9283 CrossRef.
- K. L. B. Chang, G. Tsai, J. Lee and W. R. Fu, Heterogeneous N-deacetylation of chitin in alkaline solution, Carbohydr. Res., 1997, 303(3), 327–332 CrossRef CAS.
- D. Zahn, Car-Parrinello molecular dynamics simulation of base-catalyzed amide hydrolysis in aqueous solution, Chem. Phys. Lett., 2004, 383(1–2), 134–137 CrossRef CAS.
- D. Bakowies and P. A. Kollman, Theoretical study of base-catalyzed amide hydrolysis: gas- and aqueous-phase hydrolysis of formamide, J. Am. Chem. Soc., 1999, 121(24), 5712–5726 CrossRef CAS.
- M. A. Khapre and R. M. Jugade, Tetrabutylammonium Impregnated Chitosan for Adsorptive Removal of Harmful Carcinogenic Dyes from Water-Bodies, Chem. Afr., 2021, 4(4), 993–1005, DOI:10.1007/s42250-021-00281-5.
- M. A. Behrens, J. A. Holdaway, P. Nosrati and U. Olsson, On the dissolution state of cellulose in aqueous tetrabutylammonium hydroxide solutions, RSC Adv., 2016, 6(36), 30199–30204 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00053f |
| This journal is © The Royal Society of Chemistry 2024 |