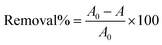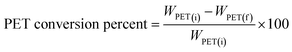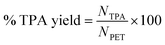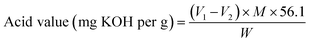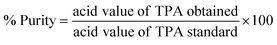Open Access Article
Open Access ArticleA facile approach towards recycling of polyurethane coated PET fabrics†
Meenakshisundaram
Vaishali
ab,
Sathyaraj
Gopal
a and
Kalarical Janardhanan
Sreeram
 *a
*a
aCentre for Analysis, Testing, Evaluation and Reporting Services (CATERS), CSIR – Central Leather Research Institute, Adyar, Chennai 600 020, India. E-mail: kjsreeram@clri.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India
First published on 10th June 2024
Abstract
The apparel industry widely uses polyurethane coated fabrics for their durability, comfort, style, and versatility. Due to the presence of multiple layers of polymers, recycling such fabrics results in low efficiency and poor yield; thus predominantly they are disposed of in landfills, resulting in severe environmental pollution. Herein we achieved a remarkably precise separation of the polyurethane (PU) coating as a neat film from the polyethylene terephthalate (PET) fabric through a surfactant-aided alkali treatment of the adhesive at room temperature. Furthermore, the dye from the fabric was continuously extracted through Soxhlet extraction resulting in 93% dye removal from the material. The PET fabric as obtained was hydrolyzed through an alkaline hydrolysis procedure with a maximum terephthalic acid (TPA) yield above 80% and purity above 90%. Dye removal from the fabric proved to be a crucial step in recycling PET fabrics as we found a notable reduction in the purity and yield of dyed PET fabrics. This work is the first to study the delamination of polymer coatings as neat films from PET fabrics commonly used in the apparel industry. It will provide useful insight and direction for recycling other such polymer-coated PET fabrics.
Sustainability spotlightThe footwear industry is embracing composite polymers to meet rising consumer fashion preferences, aiming to deliver enhanced durability, comfort, and style in footwear designs. Among the various composite polymers utilized in footwear production, PU-coated PET fabric stands out as a prominent choice. However, the industry faces a significant challenge with the substantial waste generated in manufacturing units, predominantly PU-coated PET fabrics that frequently end up in landfills. This disposal method not only contributes to environmental pollution but also significantly increases the carbon footprint associated with footwear production. In this study, we sought to valorize PU-coated PET fabrics by employing a systematic three-step approach. This method involved the precise separation of the PU coating from the PET fabric, extraction of dye from the PET fabric, and conversion into terephthalic acid. As a result, our research aligns with several UN Sustainable Development Goals, including affordable and clean energy (SDG 7), sustainable cities and communities (SDG 11), responsible consumption and production (SDG 12), and climate action (SDG 13). |
1. Introduction
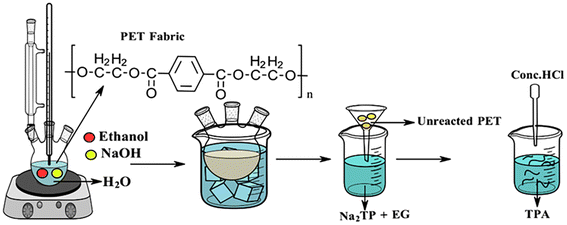
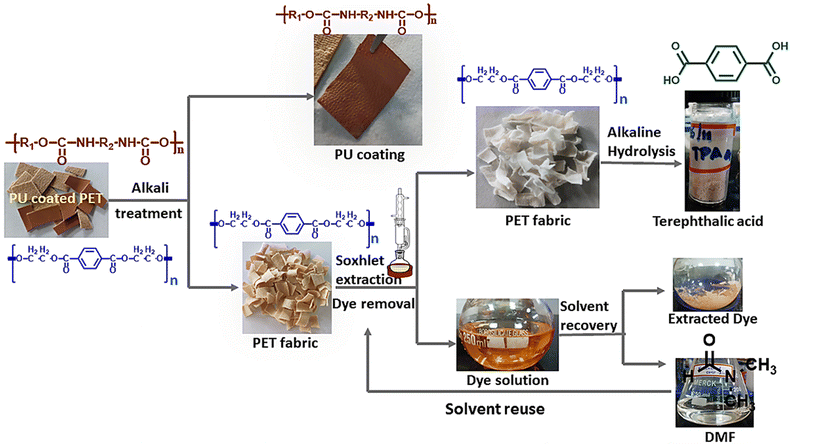
Post-consumer waste recycling and reuse are deficient across the globe. Compounded with growing consumption and poor biodegradability, polymeric waste is of serious concern.1 The textile, clothing, and footwear industries are growing significantly, with the footwear market expected to reach $530.3 billion by 2030.2 The materials used in footwear have undergone massive changes from dry and rugged feel to comfortable and soft. This change has been due to composite materials that provide specific characteristics and performance parameters.3 It is estimated that over 40 materials, ranging from leather, rubber, foam, textile, thermoplastic polyurethane (TPU), ethyl vinyl acetate (EVA), polyvinyl chloride (PVC), etc., are used either alone or in combination in footwear. Mechanical recycling by crushing, air-based separation of granulates into fractions,3 blending the polymeric waste and manufacturing sheets, and additives for concrete have been considered for waste management.2 The heterogeneity and poor mechanical properties of such materials have hindered the widespread use of recycled materials, and landfills remain the favoured disposal route for manufacturers and consumers.3 Incineration of these wastes results in the release of poisonous gases.Among fabric types utilized in the manufacture of non-leather footwear, PU-coated PET (polyurethane coated polyethylene terephthalate) fabrics are the most popular and have three major components, viz., a polymeric film of 0.025–0.05 mm thickness, a tie coat, or an adhesive layer that binds the PU film to the fabric, and a fabric substrate (polyester, polyester – cotton blend, cotton, etc.). The PU film provides for the reduction of liquid, gas, and dust permeability, abrasion resistance, and any other properties that are part of consumer requirements. The fabric layer provides strength character (tensile and tear), dimensional stability, elongation, etc.4
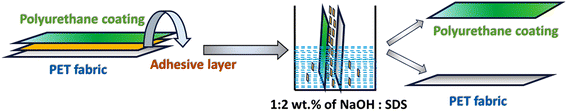
To enable recycling as a valorization option, the requisite separation of the top polyurethane (PU) coat and the underlying fabric, interconnected by a tie coat, is essential to facilitate the efficient processing of both constituents. This segregation facilitates the potential for functional upcycling of the individual layers, thereby converting them into value-added products.5 Conversely, the absence of such layer separation necessitates resorting to depolymerization as the preferred route for recycling.6–8 The adhesive between these two layers would require a chemical treatment, as the mechanical separation, such as through the froth flotation process, is difficult due to similar densities (in the range of 1.8–2.4 g cm−3).9,10
Coated PU fabrics, such as polyester, carry disperse dyes, which require removal before recycling. While there are several methods to treat wastewater containing disperse dyes,11–13 extraction of the same from the fabric is still a challenge. Solvent extraction, a commonly reported method, is one where an equilibrium in the concentration of dye between the inside and outside of the fabric is established; it requires closed-loop process methodologies that ensure that the concentration of dye in the fabric is always higher than that of the solvent medium, for maximizing the extraction process.14,15
The base PET fabric can be recycled in a manner similar to PET bottles, where PET is turned into terephthalic acid (TPA) under acidic,16–18 alkaline19–21 or neutral conditions.22–24 The use of ionic liquids as green catalysts for the depolymerisation of PET has also been extensively studied.19,25–27 There have been efforts to improve the yield of TPA through alkaline hydrolysis of PET, such as the use of ethylene glycol and tetrahydrofuran as solvents.28
The literature on recycling of PET fabrics from PU-coated materials is notably limited. However various studies have explored innovative methods in related areas. For instance, alkaline hydrolysis of PVC-coated PET fabrics has been studied for the simultaneous recovery of PVC and TPA efficiently.8 Another approach involves the solvent-targeted recovery of polymers from multilayer plastic packaging materials, where specific solvents are employed to dissolve each component, followed by precipitation and separation of polymer layers.29 Additionally, delamination of polyamide/polyolefin multilayer films via selective glycolysis of polyurethane adhesive has proven effective in producing pure split films.10 Similarly, investigations have been conducted into the recovery of polyols and amines from bio-based PU-coated fabrics through selective solvolysis. This approach facilitates the depolymerisation of PU coatings, resulting in the effortless separation of coatings from the fabric.30 Lastly, selective disassembly of polyurethane coatings and elastane fibers via solvolysis offers a promising solution for recycling blended fabrics.6
A significant limitation observed in these studies is the prevalent reliance on the depolymerisation of one of the components to facilitate separation between the layers. For example, common approaches such as selective glycolysis and solvent-targeted methods often entail depolymerizing one component, leading to loss of polymer integrity. This presents a substantial challenge in utilizing the recovered polymers directly for producing value-added products through functional upcycling. While there is literature on stripping polyurethane coatings from metal surfaces using compositions containing alkali solutions, these are limited to applications such as paint stripping from various metal bodies.31,32 A detailed study on the development of a methodology to strip PU films from fabric bases without the loss of integrity of the polymers has not been carried out before.
The scientific literature on PET fabric recycling focuses on the chemical depolymerisation of colourless PET fabrics, neglecting exploration possibilities of the recycling of predominantly available colored PET fabrics.33,34 While technology facilitates the recycling of PET bottles, the same approach does not apply to PET fabrics, primarily because of the dye content.
Thus herein, we report a novel approach for recycling PU-coated PET fabrics focusing on precisely controlling the alkali concentration to achieve targeted hydrolysis of the adhesive layer. Through this meticulous approach, we successfully separated the PU coating and PET fabric intact. Our work underscores the importance of selective hydrolysis of the adhesive in preserving the integrity of both components. Additionally, our process includes a solvent-assisted dye removal step to further enhance fabric recyclability. Finally, alkaline hydrolysis of the PET fabric allows for the extraction of TPA, highlighting the comprehensive nature of our recycling strategy.
2. Experimental
2.1 Materials
Three coloured PU-coated PET fabrics and one uncoloured fabric of 0.5–0.8 mm thickness were procured from a leading supplier of PU-coated fabrics in India. The chemicals and reagents employed in this work were of analytical reagent grade and procured from M/s. TCI Chemicals. Disperse blue 1 (C.I 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) and terephthalic acid were purchased from M/s. Merck India Ltd.
500) and terephthalic acid were purchased from M/s. Merck India Ltd.
2.2 Removal of the PU coating from the PET fabric
The procured fabrics were cut into rectangular strips (2 × 4 cm) and weighed. The cut fabric strips of known weight were independently soaked in a sodium hydroxide (NaOH) bath containing sodium dodecyl sulfate (SDS) and separated at varying temperatures under stirring at 250 rpm. The concentration of NaOH and SDS and the temperature of the medium were optimized to obtain delaminated fabrics. After delamination, the fabric and the PU film were washed and dried in an air oven at 60 °C. The mass of the dried film and fabric was measured gravimetrically. Experiments were carried out in triplicate and reported with a standard deviation of 0.5–2%.2.3 Removal of the dye from the fabric
The dye in the PET fabric after the PU coating removal was stripped using dimethylformamide (DMF) through a Soxhlet extraction method. A fixed time of 3 h and a temperature of 90 °C were employed for the extraction. The fabric after dye extraction was dried in an air oven at 40 °C.2.4 Alkaline hydrolysis of the PET fabric
A known method of alkaline hydrolysis of PET was adopted with minor modifications.35 The experiments were performed in a three-necked round bottom flask fitted with a condenser and the temperature was controlled through an oil bath. The schematic of the reaction design is presented in Fig. 1. Definite quantities of sodium hydroxide, water, and ethanol were added to the flask, and the contents were heated to the desired temperature by immersing the reaction vessel in an oil bath. PET fabric (dyed and dye stripped) was charged into the flask once the desired temperature was reached, and the hydrolysis reaction was run for 3 hours. After 3 hours of reaction, the alkaline hydrolysis reaction was quenched by placing the flask in an ice bath. The reaction mixture was then filtered through a G4 Whatman filter paper to remove unreacted PET fibers. To the filtrate, conc. HCl was added dropwise to reduce the pH to 2.5 while a white precipitate of terephthalic acid settled down at the bottom. The precipitate was washed several times with water and dried in a hot air oven at 40 °C.2.5 Quantification of dye removal
To optimize the dye removal, it is essential to know the concentration of the dye in the fabric. As the nature and characteristic features of the dye in the commercial samples were unknown, an undyed fabric was dyed with 1% wt/wt of C.I disperse blue 1. A conventional disperse dyeing technique was employed.36 In short, the methodology involved carrying out the dyeing in a dye solution (prepared using 0.5 mL acetic acid, 1% phenol solution (carrier), 15 mL sodium sulfate (1 g L−1), and 1% dye (based on the weight of the fabric)). The volume of the carrier solution was taken such that the weight of the fabric taken to that of the solution was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. The dye solution was stirred until a clear solution was obtained, following which the temperature of the dye solution was raised to 100 °C slowly. On attaining the temperature, the fabric was added and the stirring continued for 20 min. The temperature was gradually reduced to room temperature, and the dyed fabric was removed from the reaction vessel and washed with deionized water to remove the excess dye from the fabric surface.
30. The dye solution was stirred until a clear solution was obtained, following which the temperature of the dye solution was raised to 100 °C slowly. On attaining the temperature, the fabric was added and the stirring continued for 20 min. The temperature was gradually reduced to room temperature, and the dyed fabric was removed from the reaction vessel and washed with deionized water to remove the excess dye from the fabric surface.
2.6 Characterization
The absorbance of the undyed PET fabric, C.I disperse blue-dyed PET fabric, and dye stripped PET fabric was measured using a UV-Vis-NIR (Ultraviolet-Visible-Near Infrared) diffuse reflectance spectrophotometer (DRS) (Cary 5000, Agilent Technologies). The efficiency of dye removal was determined for the C.I disperse blue dye stripped fabric through a comparison of the absorption of the dye before and after the reaction at λmax = 603 nm. The dye removal was quantified by employing the simple formulawhere A0, and A are the absorbance of the PET fabric before and after dye removal respectively. The absorbance of the three PET samples obtained after PU coating detachment was also measured.
The PET fabric obtained after the dye stripping process was solubilized in 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 phenol
40 phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,1,2,2-tetrachloroethane and the viscosity was measured. The molecular weight of the solubilized product was determined using the Mark–Houwink equation.37
1,1,2,2-tetrachloroethane and the viscosity was measured. The molecular weight of the solubilized product was determined using the Mark–Houwink equation.37
The alkaline solution was analysed using matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) (model – Bruker micro flex. LRF, and software – Flexcontrol Microflex) both before and after the soaking process.
The % PET conversion and yield of TPA obtained were also determined using the simple equation35
The yield of TPA obtained was calculated using the equation
The acid value of TPA and thus the purity were determined as per ISO 15030:2001; About 1 g of TPA was dissolved in 25 mL of pyridine solvent, and the solution was further diluted in 20 mL of water. This solution was titrated against 0.5 M sodium hydroxide solution to the phenolphthalein endpoint. A blank titration was also carried out excluding the material.38
The acid value of the TPA standard purchased from Merck was also determined through the same method to determine the purity of the TPA obtained. The % purity was calculated using the formula
To understand the influence of various reactions carried out on the surface features of the fabric, the contact angle was measured by the Sessile drop technique using a HOLMORC opto-mechatronics contact angle meter (HO-IAD-Cam-01B). All Fourier transform infrared spectra (FTIR) were collected using a JASCO-4700 model spectrometer (JASCO Co., Tokyo Japan). The PET samples were mounted on the attenuated total reflectance (ATR) crystal (the crystal used was zinc selenide). The FTIR measurements of the adhesive and terephthalic acid were performed against the KBr standard. All FTIR data were collected at a resolution of 4 cm−1, employing 40 scans in the wavelength range of 400–4000 cm−1. The morphology of PET surfaces was examined using a CLARA GMU field emission scanning electron microscope (FE-SEM) operating in ultra-high resolution mode from M/s. TESCAN, based in Brno, Czech. The specimens underwent gold coating via a sputter coater (from M/s. Quorum Technologies) for surface preparation. To determine the elemental composition of the PET fabric, energy dispersive X-ray analysis (EDAX) was performed using an EDAX Octane Plus Probe. The microscopic images of the adhesive molecules were captured using a modular stereo microscope model: DZ.5040 from Euromex, Holland.
The DSC analysis (performed under a nitrogen atmosphere, in the temperature range of 25–350 °C with a heating ramp rate of 5 °C min−1, using a Q200 spectrometer, M/s. TA Instruments, UK) provided clues to the changes in crystallinity of the fabric on stripping off the dye. The thermogravimetric analysis was performed on a thermogravimetric analyzer (TGA, M/s SETARAM Themys One+) with an N2 flow of 20 mL min−1 in the temperature range of 25–1000 °C with a heating rate of 5 °C min−1. The terephthalic acid obtained from the PET fabric and TPA standard was subjected to X-ray diffraction (XRD) analysis (Malvern PANalytical, Empyrean Series 3, UK) using Ni-filtered Cu Kα radiation (λ = 1.5418 Å, 40 kV and 30 mA) in the range of 10–80° with a scan rate of 4° min−1. Nuclear magnetic resonance (NMR) spectra of the samples were recorded using DMSO-d6 on a Bruker Avance III HD, 400 MHz narrow bore FT-NMR spectrometer equipped with a broadband diffusion probe (Diff-BB) to verify the chemical structure of the obtained TPA.
3. Results and discussion
3.1 Removal of the coating from the fabric
PU-coated PET fabrics contain a PU adhesive that binds the two layers together. Upon immersion of the PU-coated PET fabric in an alkaline solution for varying durations, we observed the separation of the PU coating as a neat film from the PET fabric. This is due to the hydrolysis of the adhesive, resulting in its degradation and subsequent loss of adhesion to the PET fabric.39 However, due to the inadequate wettability of the PU-coated PET fabric by the alkali solution, the process typically exceeded 50 minutes for 10 wt% NaOH (Table S1†). Thus we introduced a surfactant into the system to enhance the wettability and thereby reduce the processing time. We observed a significant acceleration in the process (Table 1). We subsequently optimized the concentration of the surfactant relative to the alkali concentration. These findings were further supported by contact angle measurements. Good wetting of the coated fabric with alkaline water helps the alkali to penetrate through the hydrophobic surface of PET thus hydrolysing the PU adhesive and binding the PU to the PET fabric. The process is schematically presented in Fig. 2.| Entry | NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDSa (wt%) SDSa (wt%) |
Contact angle on PET surface | Time taken to remove the coating from the fabric (minutes) |
|---|---|---|---|
a The SDS concentration was increased from 0.5 to 20 wt% so as to vary the NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS ratio as 1 SDS ratio as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1 0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1 1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in entries # 1 to 4 respectively. 2 in entries # 1 to 4 respectively.
|
|||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
114 ± 2° | 540 |
| 2 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
106 ± 2° | 120 |
| 3 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
101 ± 3° | 90 |
| 4 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
92 ± 2° | 10 |
The contact angle between water and the coated fabric is expected to change as a function of alkali and surfactant concentration.40 As can be seen from Table 1, a decrease in the water contact angle was observed with an increase in the concentration of the NaOH/SDS mixture. Notably at a NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS weight ratio of 10
SDS weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
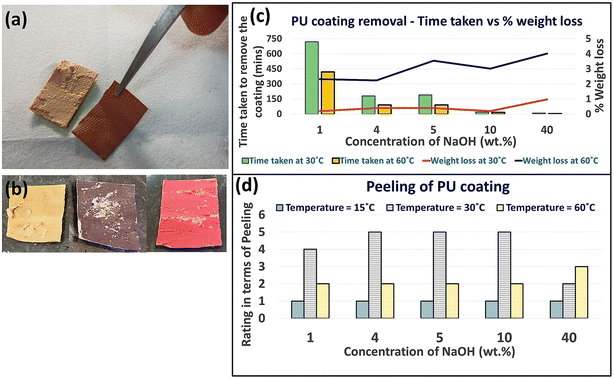
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, we achieved the lowest contact angle value of 92 ± 2°. Thus, the alkali–surfactant system lowers the surface tension on the polymer surface facilitating the penetration of alkali through the fabric.41 Even though the contact angles remained above 90°, potentially due to the increased hydrophobic nature of the PET fabric, this was sufficient to produce the required wettability for our system as the PU coating was removed within 10 minutes of soaking at this weight ratio at 30 °C (Fig. 3(a)). Continuing to extend the soaking duration or increasing the alkaline concentration resulted in the hydrolysis of not only the adhesive but also the PU top coating, as depicted in Fig. 3(b).
20, we achieved the lowest contact angle value of 92 ± 2°. Thus, the alkali–surfactant system lowers the surface tension on the polymer surface facilitating the penetration of alkali through the fabric.41 Even though the contact angles remained above 90°, potentially due to the increased hydrophobic nature of the PET fabric, this was sufficient to produce the required wettability for our system as the PU coating was removed within 10 minutes of soaking at this weight ratio at 30 °C (Fig. 3(a)). Continuing to extend the soaking duration or increasing the alkaline concentration resulted in the hydrolysis of not only the adhesive but also the PU top coating, as depicted in Fig. 3(b).
As the temperature of the medium increased from 30 °C to 60 °C, for a given concentration of NaOH, the time taken for detachment decreased (Fig. 3(c)). However, a visual assessment of the fabric indicated a shape distortion. To optimize the temperature and the extent of detachment with variable concentration of NaOH (at 20 wt% SDS concentration) visual assessment of the fabric was carried out and the results are presented in Fig. 3(d). Further confirmatory evidence for the role of temperature was obtained from an assessment of weight loss of the PU-coated PET fabric, which varied from 0.19% to 4% (Fig. 3(c)). Thus, definite contact time, temperature, and concentration of alkali ensure selective hydrolysis of the adhesive layer alone while leaving the PU coating and PET fabric intact.
We observed traces of the adhesive on the surface of the PET fabric when hydrolysis was carried out at 5 wt% NaOH (Fig. S1(b)†); thus we confirmed the nature of the adhesive through FTIR analysis. Bands corresponding to –NH, –C–N, and –C–O stretching frequencies observed in the spectra were attributed to the PU adhesive42,43 (Fig. S1(a)†).
As this study represented a pioneering effort in comprehensively investigating the separation of the PU coating from the PET fabric via adhesive disruption, it was imperative to validate the process's underlying mechanism. MALDI-TOF mass spectrometry analysis was conducted to achieve a thorough understanding and obtain a clear depiction of the process.44 Specifically, the alkali solution underwent MALDI-TOF analysis both before and after the hydrolysis reaction. Before soaking the fabric in sodium hydroxide solution, the mass spectrum of the solution did not reveal any discernible mass-to-charge (m/z) peaks (data not shown), which is attributed to the inherently low molecular weight of the analyte. Post-soaking, the alkali solution exhibited well-defined m/z peaks within the range of 700, characterized by minimal background noise as shown in Fig. 4(b). The possible molecules responsible for the observed mass values in MALDI-TOF from the PU hydrolysis are also shown in Fig. 4(b). The emergence of distinct m/z peaks in the post-soaking alkali solution strongly supports the hydrolysis process of the PU adhesive. Urethanes, known for their low hydrolytic stability, undergo pronounced hydrolysis in the presence of a potent alkali, augmented by the surfactant resulting in the formation of carbamic acid and alcohol end terminal oligomeric species (Fig. 4(a)).39,45
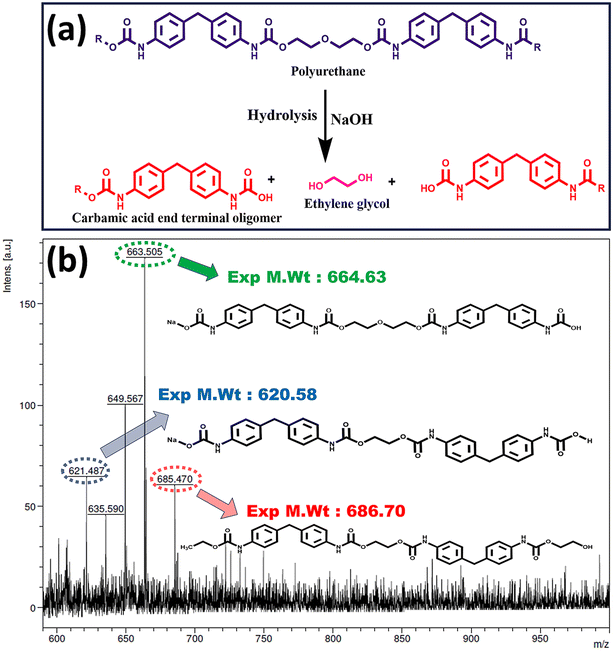 | ||
| Fig. 4 (a) Hydrolysis mechanism of the PU adhesive and (b) MALDI-TOF MS of NaOH solution after soaking the PUCF material. | ||
The concerted action of sodium hydroxide and the surfactant facilitates the efficient hydrolysis of the PU adhesive, resulting in the dissolution of hydrolysates in the solution.46 This observation provides valuable insights into the chemical processes underlying the detachment of the PU coating from the PET fabric, underscoring the significance of alkali-induced hydrolysis in this system.
3.2 Dye separation from the fabric
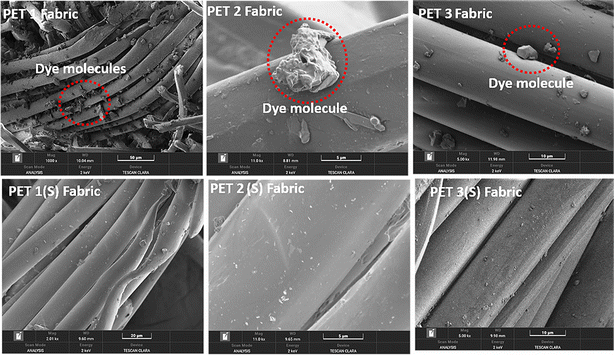
Soxhlet extraction of the PET fabric after the detachment of the PU coating in a DMF solution at 90 °C for a period of 3 h led to the complete extraction of the dye as can be seen in Fig. S3.† The three different coloured PET samples were labelled as PET 1, 2, and 3. The dye-extracted fabrics were further labelled as PET 1(S), 2(S), and 3(S). DMF was chosen as a solvent based on its high boiling point and solubility of disperse dyes.47 The ATR-FTIR spectra of the fabric before and after the extraction process show that the characteristic PET stretching vibrations are intact for all three fabrics (Fig. S4†). The –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency is observed in all three cases at around 1705–1712 cm−1,48 while the –C–C phenyl ring stretching frequency is at around 1400–1410 cm−1. The –CH phenyl group stretching vibrations were observed at 1013–1014 cm−1. The –CH2 wagging vibration was noted around 1337–1339 cm−1. The ester (–COO) group shows the stretching frequency at around 1236–1239 cm−1. The –C–O stretching vibration of the gauche conformer was at 1085 cm−1. The –CH phenyl ring stretching was observed at 1013–1014 cm−1.49 The FTIR spectra thus confirm that the PET remained intact on treatment with DMF, with the dye alone being extracted. Similar observations have been made through microscopic analysis (Fig. 5). Before extraction, dye molecules were observed adhering to the structures of the PET fabric in all three cases. SEM-EDX analysis of the PET fabric revealed distinct nitrogen peaks in specific regions, signifying the presence of dye molecules (Fig. S5(a)†). Meanwhile, smooth fabric areas exhibited carbon and oxygen peaks, indicative of the PET surface (Fig. S5(b)†). To further confirm this, the dye molecules extracted from the fabric were analysed using FTIR spectroscopy (Fig. S5(c)†). The –N
O stretching frequency is observed in all three cases at around 1705–1712 cm−1,48 while the –C–C phenyl ring stretching frequency is at around 1400–1410 cm−1. The –CH phenyl group stretching vibrations were observed at 1013–1014 cm−1. The –CH2 wagging vibration was noted around 1337–1339 cm−1. The ester (–COO) group shows the stretching frequency at around 1236–1239 cm−1. The –C–O stretching vibration of the gauche conformer was at 1085 cm−1. The –CH phenyl ring stretching was observed at 1013–1014 cm−1.49 The FTIR spectra thus confirm that the PET remained intact on treatment with DMF, with the dye alone being extracted. Similar observations have been made through microscopic analysis (Fig. 5). Before extraction, dye molecules were observed adhering to the structures of the PET fabric in all three cases. SEM-EDX analysis of the PET fabric revealed distinct nitrogen peaks in specific regions, signifying the presence of dye molecules (Fig. S5(a)†). Meanwhile, smooth fabric areas exhibited carbon and oxygen peaks, indicative of the PET surface (Fig. S5(b)†). To further confirm this, the dye molecules extracted from the fabric were analysed using FTIR spectroscopy (Fig. S5(c)†). The –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– stretching frequency, characteristic of azo groups in dye molecules, was observed at 1470 cm−1,50 the –C
N– stretching frequency, characteristic of azo groups in dye molecules, was observed at 1470 cm−1,50 the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– stretching frequency was observed at 1703 cm−1, the C–N stretching vibrations were observed at 1220 cm−1, and the –OH stretching frequency was observed at 3320 cm−1. Notably, the surface of the PET fabric displayed a rough and uneven texture before undergoing the extraction process. Also, the cylindrical rod-like structure of the PET fabric remains unchanged during the dye removal process thus indicating that there was no physical damage to the fabric during the extraction process.
O– stretching frequency was observed at 1703 cm−1, the C–N stretching vibrations were observed at 1220 cm−1, and the –OH stretching frequency was observed at 3320 cm−1. Notably, the surface of the PET fabric displayed a rough and uneven texture before undergoing the extraction process. Also, the cylindrical rod-like structure of the PET fabric remains unchanged during the dye removal process thus indicating that there was no physical damage to the fabric during the extraction process.
The intrinsic viscosity of the PET fabric was determined and from that the molecular weight of the fabric was calculated. There was no notable change in the molecular weight of the fabrics (Fig. S4†) before and after extraction of the dye, which indicated that the DMF selectively disturbed the intermolecular interactions between the dye and fibre structure, leaving the macromolecule backbone of the PET fabric intact.51
During the dye stripping process, the degree of crystallinity of the PET fabric exhibited a slight decrease (Table 2). This can be attributed to the interruption of dye–fabric interactions in the amorphous regions of the fabric by the DMF solvent, while the crystalline regions almost remained unaffected. Consequently, these dye-stripped fabrics have the potential to be mechanically melt-spun, allowing to produce new fibres.52
| Sample | T f (°C) | ΔHf (J g−1) | X c (%) |
|---|---|---|---|
| a X c – degree of crystallinity (%). | |||
| PET 1 | 252.53 | 69.88 | 49.9 |
| PET 1(S) | 254.53 | 59.68 | 42.6 |
| PET 2 | 252.35 | 50.05 | 35.7 |
| PET 2(S) | 252.4 | 38.39 | 27.4 |
| PET 3 | 253.33 | 51.57 | 36.8 |
| PET 3(S) | 253.03 | 45.59 | 32.5 |
3.3 Efficiency of dye removal
In the DRS spectra, the C.I disperse blue dyed PET had a maximum absorbance at 603 nm, which reduced to almost the same levels as that of the undyed fabric (Fig. 6). The colour removal was estimated to be 93.4%, after extraction using DMF for a duration of 3 h.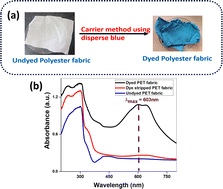 | ||
| Fig. 6 (a) Image of undyed PET fabric dyed using C.I disperse blue dye through the carrier method. (b) Absorbance of dyed PET fabric, dye stripped and undyed PET fabric. | ||
3.4 Hydrolysis of PET
The yield and purity of the terephthalic acid obtained from the dyed and dye-stripped PET fabrics at 80 °C are tabulated in Table 3. In the absence of a dye-stripping process, the yield of terephthalic acid is low (maximum of 52.4%), with a low purity of 53.4%. The dye-stripping process increases the yield and purity of the acid, irrespective of the dye used.| S. no. | PET label | TPA label | TPA yield (%) | TPA purity (%) | Remarks |
|---|---|---|---|---|---|
| 1 | PET 1 | TPA1 | 48.85 | 57.2 | TPA – pink solid |
| 2 | PET 1(S) | TPA2 | 82.78 | 92.19 | TPA white solid |
| 3 | PET 2 | TPA3 | 47.63 | 56.08 | TPA rosy brown solid |
| 4 | PET 2(S) | TPA4 | 76.33 | 91.43 | TPA white solid |
| 5 | PET 3 | TPA5 | 52.34 | 53.74 | TPA cream yellow solid |
| 6 | PET 3(S) | TPA6 | 82.41 | 91.18 | TPA white solid |
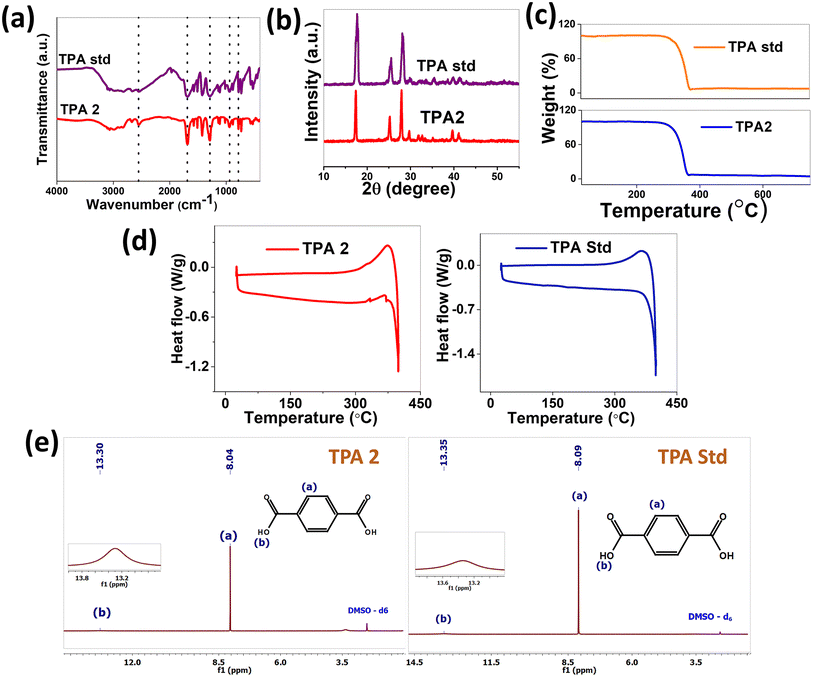
To confirm the purity of the extracted terephthalic acid, a range of physicochemical characterization experiments were conducted on the obtained compound. These supplementary analyses serve to enhance the assessment of purity beyond the scope of the ISO standard methodology, offering a comprehensive understanding of the compound's properties. The FTIR spectra of TPA 2 (having maximum yield and purity) were compared with those of the terephthalic acid standard (TPA Std). All the IR stretching bands of the obtained terephthalic acid match with those of the standard. A broad –OH stretching is seen in the range of 2500–3000 cm−1. Aromatic –CH stretching is observed at 3061 cm−1. The –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the acid group is found at 1688 cm−1. The –C–O stretching frequency is at 1282 cm−1.53 The –OH wagging is observed between 700 and 800 cm−1 (ref. 54) (Fig. 7(a)). The thermogravimetric analysis showed that the mass losses for TPA 2 obtained from the PET fabric and TPA standard followed similar trends (Fig. 7(b)). There was a rapid degradation (measured in terms of weight loss) beyond 300 °C in TPA 2 which was also seen in the TPA standard.54 The XRD pattern of the synthesized terephthalic acid also matches well with that of the terephthalic acid standard as shown in Fig. 7(c).
O stretching of the acid group is found at 1688 cm−1. The –C–O stretching frequency is at 1282 cm−1.53 The –OH wagging is observed between 700 and 800 cm−1 (ref. 54) (Fig. 7(a)). The thermogravimetric analysis showed that the mass losses for TPA 2 obtained from the PET fabric and TPA standard followed similar trends (Fig. 7(b)). There was a rapid degradation (measured in terms of weight loss) beyond 300 °C in TPA 2 which was also seen in the TPA standard.54 The XRD pattern of the synthesized terephthalic acid also matches well with that of the terephthalic acid standard as shown in Fig. 7(c).
The heating and cooling profiles of TPA2 closely resemble those of the TPA Std (Fig. 7(d)), with the exception of a minor transition at 370 °C. This anomaly, inconsistent with a melting point, is likely attributable to either impurities or an instrumental artifact. Overall, TPA2 demonstrates a strong correlation with the TPA Std, suggesting high purity and consistency in its synthesis. The 1H-NMR of synthesised terephthalic acid exhibited a singlet at around 8 ppm corresponding to aromatic CH protons. One broad singlet was observed at around 13.2 ppm corresponding to the OH proton of the carboxylic group, which clearly matches with that of the standard55 (Fig. 7(e)).
4. Conclusions
The overall process described is one for value addition to a significant quantity of PU-coated PET fabric which is used predominantly in the construction of non-leather footwear and is currently disposed of in landfills. At a temperature of 30 °C and a NaOH concentration of 10% with a NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS ratio of 1
SDS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and contact time of 10 min, the PU coating was detached from the dyed PET fabric. Anionic surfactants do aid the detachment process by way of reducing the time and alkali concentration. A DMF-based extraction of disperse dyes from the PET fabric was found essential to improve the yield and purity of the terephthalic acid targeted. Despite its environmental impact, DMF emerges as the optimal solvent for the removal of disperse dyes from the polyethylene terephthalate (PET) fabric, exhibiting an exceptional colour removal efficiency of 93%. The environmental concerns associated with DMF can be mitigated through strategic solvent management, specifically through solvent recycling methodologies. Notably, we achieved successful recycling of DMF via distillation under reduced pressure, rendering the solvent reusable for subsequent disperse dye extraction processes (Fig. 8). Alkaline hydrolysis of the dye stripped PET resulted in terephthalic acid, with a yield of above 80% and purity of above 90%.
2 and contact time of 10 min, the PU coating was detached from the dyed PET fabric. Anionic surfactants do aid the detachment process by way of reducing the time and alkali concentration. A DMF-based extraction of disperse dyes from the PET fabric was found essential to improve the yield and purity of the terephthalic acid targeted. Despite its environmental impact, DMF emerges as the optimal solvent for the removal of disperse dyes from the polyethylene terephthalate (PET) fabric, exhibiting an exceptional colour removal efficiency of 93%. The environmental concerns associated with DMF can be mitigated through strategic solvent management, specifically through solvent recycling methodologies. Notably, we achieved successful recycling of DMF via distillation under reduced pressure, rendering the solvent reusable for subsequent disperse dye extraction processes (Fig. 8). Alkaline hydrolysis of the dye stripped PET resulted in terephthalic acid, with a yield of above 80% and purity of above 90%.
The novelty of the work is rooted in its capability to completely remove the polyurethane (PU) coating from the PU-coated PET fabric while preserving its integrity. This facilitates the conversion of the PU coating into alternative value-added products through functional modifications, diverging from conventional approaches reliant on depolymerization. The continuous extraction of dye through Soxhlet extraction was found to enhance the value of resulting terephthalic acid, giving way for extending the process methodology to other multi-layered fabrics.
Data availability
The data supporting the article titled – A facile approach towards recycling of polyurethane coated PET fabrics have been included as a part of the ESI.†Author contributions
Vaishali Meenakshisundaram – conceptualization, methodology, implementation of work, software analysis, and writing – original draft. Sathyaraj Gopal – methodology and formal analysis. Kalarical Janardhanan Sreeram – conceptualization, supervision, project administration, and writing – review and editing.Conflicts of interest
The authors affirm that they have no identifiable competing financial interests or personal relationships that may have influenced the research documented in the paper. All authors confirm the absence of competing interests.Acknowledgements
VM acknowledges the funding agency Council for Scientific and Industrial Research (CSIR) for Junior/Senior research fellowship. Support of Mr L. Murugan (Shoe & Product Design Centre – CSIR – CLRI) in the identification of material and understanding of the industry scenario is acknowledged. Research funding under Theme 2: Materials for footwear (OLP – 2302) is also acknowledged. CSIR – Central Leather Research Institute communication number – 1958.References
- M. Geissdoerfer, P. Savaget, N. M. P. Bocken and E. J. Hultink, J. Cleaner Prod., 2017, 143, 757–768 CrossRef
.
- X. Yang, J. Liu, B. Zhou and X. Zhang, Compos. Commun., 2023, 41, 101646 CrossRef
.
- M. J. Lee and S. Rahimifard, Resour., Conserv. Recycl., 2012, 69, 90–99 CrossRef
.
- D. De Smet, M. Wéry, W. Uyttendaele and M. Vanneste, Polymers, 2021, 13, 4229 CrossRef CAS PubMed
.
- O. Guselnikova, O. Semyonov, E. Sviridova, R. Gulyaev, A. Gorbunova, D. Kogolev, A. Trelin, Y. Yamauchi, R. Boukherroub and P. Postnikov, Chem. Soc. Rev., 2023, 52, 4755–4832 RSC
.
- M. B. Johansen, B. S. Donslund, M. L. Henriksen, S. K. Kristensen and T. Skrydstrup, Green Chem., 2023, 25, 10622–10629 RSC
.
- D. De Smet, J. Verjans and M. Vanneste, Polymers, 2022, 14, 5452 CrossRef CAS PubMed
.
- S. Kumagai, S. Hirahashi, G. Grause, T. Kameda, H. Toyoda and T. Yoshioka, J. Mater. Cycles Waste Manage., 2018, 20, 439–449 CrossRef CAS
.
- G. A. Marques and J. A. S. Tenório, Waste Manage., 2000, 20, 265–269 CrossRef CAS
.
- G. O'Rourke, M. Houbrechts, M. Nees, M. Roosen, S. De Meester and D. De Vos, Green Chem., 2022, 24, 6867–6878 RSC
.
- S. Ong, E. Toorisaka, M. Hirata and T. Hano, J. Environ. Sci., 2008, 20, 952–956 CrossRef CAS PubMed
.
- X. Fei, J. Yao, J. Du, C. Sun, Z. Xiang and C. Xu, Cellulose, 2015, 22, 1379–1388 CrossRef CAS
.
- H. R. Dihom, M. M. Al-Shaibani, R. M. S. R. Mohamed, A. A. Al-Gheethi, A. Sharma and M. H. B. Khamidun, J. Water Process Eng., 2022, 47, 102705 CrossRef
.
- X. Fei, H. S. Freeman and D. Hinks, J. Cleaner Prod., 2020, 254, 120027 CrossRef CAS
.
- Z. Chen, H. Sun, W. Kong, L. Chen and W. Zuo, Green Chem., 2023, 25, 4429–4437 RSC
.
- W. Yang, R. Liu, C. Li, Y. Song and C. Hu, Waste Manage., 2021, 135, 267–274 CrossRef CAS PubMed
.
- D. S. Achilias and G. P. Karayannidis, Water, Air, Soil Pollut.: Focus, 2004, 4, 385–396 CrossRef CAS
.
- S. D. Mancini and M. Zanin, Polym.-Plast. Technol. Eng., 2007, 46, 135–144 CrossRef CAS
.
- W. Chen, Y. Yang, X. Lan, B. Zhang, X. Zhang and T. Mu, Green Chem., 2021, 23, 4065–4073 RSC
.
- G. Karayannidis, A. Chatziavgoustis and D. Achilias, Adv. Polym. Technol., 2002, 21, 250–259 CrossRef CAS
.
- A. Barredo, A. Asueta, I. Amundarain, J. Leivar, R. Miguel-Fernández, S. Arnaiz, E. Epelde, R. López-Fonseca and J. I. Gutiérrez-Ortiz, J. Environ. Chem. Eng., 2023, 11, 109823 CrossRef CAS
.
- D. A. S. Ravens and I. M. Ward, Trans. Faraday Soc., 1961, 57, 150–159 RSC
.
- H. Zimmerman and N. T. Kim, Polym. Eng. Sci., 1980, 20, 680–683 CrossRef CAS
.
- S. D. Mancini and M. Zanin, Prog. Rubber, Plast. Recycl. Technol., 2004, 20, 117–132 CrossRef CAS
.
- S. Marullo, C. Rizzo, N. T. Dintcheva and F. D'Anna, ACS Sustain. Chem. Eng., 2021, 9, 15157–15165 CrossRef CAS
.
- Y. Liu, X. Yao, H. Yao, Q. Zhou, J. Xin, X. Lu and S. Zhang, Green Chem., 2020, 22, 3122–3131 RSC
.
- H. Wang, Z. Li, Y. Liu, X. Zhang and S. Zhang, Green Chem., 2009, 11, 1568–1575 RSC
.
- S. Zhang, W. Xu, R. Du, X. Zhou, X. Liu, S. Xu and Y.-Z. Wang, Green Chem., 2022, 24, 3284–3292 RSC
.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef PubMed
.
- D. De Smet, J. Verjans and M. Vanneste, Polymers, 2022, 14, 5452 CrossRef CAS PubMed
.
-
H. A. Goldsmith and L. Salavarrieta, California Pat., 3410805, 1964 Search PubMed
.
-
E. J. Belack and F. Corcoran, US Pat., 3954648, 1976 Search PubMed
.
- S. R. Shukla, A. M. Harad and L. S. Jawale, Waste Manage., 2008, 28, 51–56 CrossRef CAS PubMed
.
- A. Palme, A. Peterson, H. de la Motte, H. Theliander and H. Brelid, Text. Clothing Sustainability, 2017, 3, 4 CrossRef
.
- S. Ügdüler, K. M. Van Geem, R. Denolf, M. Roosen, N. Mys, K. Ragaert and S. De Meester, Green Chem., 2020, 22, 5376–5394 RSC
.
- Z. F. Cai, Z. H. Niu, J. X. Ren, J. Y. Li, M. Niu, J. M. Dai and W. S. Hou, Adv. Mater. Res., 2012, 627, 200–204 Search PubMed
.
- N. B. Sanches, M. L. Dias and E. B. A. V. Pacheco, Polym. Test., 2005, 24, 688–693 CrossRef CAS
.
- S. Mishra, V. S. Zope and A. S. Goje, Polym. Int., 2002, 51, 1310–1315 CrossRef CAS
.
- M. Somani, S. Mukhopadhyay and B. Gupta, J. Appl. Polym. Sci., 2022, 139, e52528 CrossRef CAS
.
- M. Y. Khan, A. Samanta, K. Ojha and A. Mandal, Asia-Pac. J. Chem. Eng., 2008, 3, 579–585 CrossRef CAS
.
- M. Y. Khan, A. Samanta, K. Ojha and A. Mandal, Asia-Pac. J. Chem. Eng., 2008, 3, 579–585 CrossRef CAS
.
- A. Sengupta and H. P. Schreiber, J. Adhes. Sci. Technol., 1991, 5, 947–957 CrossRef CAS
.
- N. V. Gama, B. Soares, C. S. Freire, R. Silva, A. Ferreira and A. Barros-Timmons, J. Cell. Plast., 2018, 54, 633–649 CrossRef CAS
.
- M. Grdadolnik, A. Drinčić, A. Oreški, O. C. Onder, P. Utroša, D. Pahovnik and E. Žagar, ACS Sustain. Chem. Eng., 2022, 10, 1323–1332 CrossRef CAS PubMed
.
- B. S. Kim and B. K. Kim, J. Appl. Polym. Sci., 2005, 97, 1961–1969 CrossRef CAS
.
- S. Cui, X. Luo and Y. Li, Int. J. Adhes. Adhes., 2017, 79, 67–72 CrossRef CAS
.
- A. Datyner, J. Soc. Dyers Colour., 1978, 94, 256–260 CrossRef CAS
.
- S. M. Burkinshaw and J. G. Lu, Dyes Pigm., 1993, 21, 185–203 CrossRef CAS
.
- A. Gore and A. Venkataraman, Indian J. Fibre Text. Res., 1998, 23, 165–169 CAS
.
- J. Safari and Z. Zarnegar, RSC Adv., 2015, 5, 17738–17745 RSC
.
- B. Mu and Y. Yang, Chem. Eng. J., 2022, 427, 131570 CrossRef CAS
.
- T. Brueckner, A. Eberl, S. Heumann, M. Rabe and G. M. Guebitz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6435–6443 CrossRef CAS
.
- C. A. Téllez S, E. Hollauer, M. A. Mondragon and V. M. Castaño, Spectrochim. Acta, Part A, 2001, 57, 993–1007 CrossRef PubMed
.
- S. A. Ravichandran, V. P. Rajan, P. V. Aravind, A. Seenivasan, D. G. Prakash and K. Ramakrishnan, Macromol. Symp., 2016, 361, 30–33 CrossRef CAS
.
- H. L. Lee, C. W. Chiu and T. Lee, Chem. Eng. J. Adv., 2021, 5, 100079 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00136b |
| This journal is © The Royal Society of Chemistry 2024 |